Introduction
In the last decade, acute myocardial infarction
(AMI) has become a common cardiovascular disease threatening human
health, among which ST-segment elevation myocardial infarction
(STEMI) is the most common type and accounts for ~30% of all acute
coronary syndromes (ACS) (1). The
clinical manifestations of STEMI are diverse, and closely related
to the characteristics of coronary atherosclerotic plaques
(2). Culprit lesions causing STEMI
are more likely to be plaque ruptures with larger rupture cavity,
and tend to form occlusive thrombi (3). Among patients with STEMI prior to
primary percutaneous coronary intervention (PCI), the incidence of
poor thrombolysis in myocardial infarction (TIMI) blood flow (grade
0/1) is as high as >60.0% (4-6).
A number of studies have confirmed that reduced initial TIMI blood
flow is highly associated with a worse clinical prognosis,
including coronary no-reflow after PCI, mechanical complications
(e.g., free wall rupture, papillary muscle rupture and ventricular
septal rupture), major adverse cardiovascular and cerebrovascular
events and even death (5,7-10).
Recently, optical coherence tomography (OCT) is a
high-resolution (10-20 µm) intravascular imaging technology, and
can accurately assess vessel and lumen geometry and discriminate
plaque characteristics of culprit lesions (11). To date, plaque rupture and plaque
erosion visualized by OCT imaging have been identified to be the
most common culprit lesion morphologies, which are responsible for
the most ACS events (12). In
addition, a healed plaque is considered to be a signature of prior
plaque destabilization, and is found at the culprit site in >1/4
of ACS patients (13). Shimokado
et al (14) assessed the
agreement between healed plaques when assessed using OCT
examination and histopathology, and confirmed that OCT is also a
useful intracoronary imaging for healed plaque detection. However,
the relationship between morphological characteristics of culprit
plaques and initial TIMI blood flow has not been fully evaluated.
Therefore, the present study aimed to use coronary angiographic
findings and OCT images to investigate this association in patients
with STEMI before PCI.
Materials and methods
Study population
A total of 243 consecutive patients (171 males and
72 females; mean age, 64.7 years) with STEMI who underwent coronary
angiography and OCT examination between January 2020 and September
2021 at Henan Provincial People's Hospital (Zhengzhou, China), were
retrospectively considered for inclusion in the present study.
Inclusion criteria was a diagnosis of STEMI according to the
following criteria: i) Met the diagnostic criteria for AMI
(2); ii) presented within 12 h of
symptom onset; and iii) had ST-segment elevation ≥0.1 mV in two or
more contiguous leads or new-onset left bundle branch block on
12-lead echocardiography (ECG) (15). Patients who met any of the
following criteria were excluded: i) Massive thrombus in the
coronary artery; ii) reperfusion blood flow was not achieved
despite thrombus aspiration; iii) spontaneous coronary dissection;
iv) acute thrombosis in coronary stents; v) poor image quality that
could not be analyzed; vi) cardiogenic shock; vii) OCT imaging
catheter could not pass through the lesion. For all included
patients, loading doses of aspirin 300 mg, ticagrelor 180 mg and
heparin 70-100 U/kg were administered preoperatively, and patients
received PCI within 12 h of symptom onset. The present study was
approved by the Ethics Committee of Henan Provincial People's
Hospital (approval no. HNSRMYY-2017-47), and written informed
consent for research purposes was obtained from all patients at
admission.
Data collection
The following data were collected: i) Demographic
characteristics (age, sex, body mass index, smoking status); ii)
previous disease history (hypertension, diabetes mellitus,
hyperlipidemia, myocardial infarction and previous PCI); iii) blood
biochemical indexes [levels of high-density
lipoprotein-cholesterol, low-density lipoprotein-cholesterol,
triglyceride, cardiac troponin I and creatine kinase myocardial
band (CKMB) and estimated glomerular filtration rate], as well as
left ventricular ejection fraction (LVEF) and total ischemic
time.
Coronary angiography and TIMI blood
flow grade
Coronary angiography was performed by two
experienced interventional physicians. Lesion-related variables
were evaluated according to the results of coronary angiography,
including infarct-related artery, number of diseased vessels and
TIMI blood flow grade. When the angiographic report was incomplete
or controversial, the present study manually reviewed the
angiographic image to confirm the accuracy of the data. Coronary
blood flow was assessed by TIMI grade, which is defined as no
perfusion (grade 0), incomplete filling (grade 1), slow-reflow but
complete filling (grade 2) or complete perfusion (grade 3)
(16). According to a previous
study, patients with pre-procedural TIMI grade 0/1 were considered
to have poor initial blood flow, therefore, patients were divided
into TIMI 0/1 (n=164) and TIMI 2/3 (n=58) groups (10).
The culprit vessel was clinically determined based
on coronary angiography, ECG or echocardiography. Quantitative
coronary angiography analysis was performed to measure minimum
lumen diameter (MLD), diameter stenosis, lesion length and
reference vessel diameter (RVD) of the culprit vessels, as well as
to determine the presence of severe calcification, multivessel
lesion and bifurcation lesion. Multivessel lesion was defined as
>2 culprit vessels with a significant diameter stenosis >50%,
and a bifurcation lesion was defined as having significant stenosis
in both the main branch and the side branch of the coronary artery
(17). The aspiration catheter
(Export®; Medtronic) was used to remove the thrombus in
patients with TIMI grade 0/1.
OCT examination
The intravascular OCT imaging system (model no.
C408661; OPTIS™ Mobile System; Abbott Medical) was used to analyze
the morphological characteristics of culprit plaques. All OCT
images were analyzed by two experienced researchers who were
blinded to the coronary angiography data and clinical
manifestations (HS and HY). When the opinions of the two
researchers differed, a third researcher was asked to evaluate such
research and reach a consensus through discussion (SD).
Quantitation of plaque composition included lipid plaque
(heterogenic, signal-poor, highly attenuating intimal regions with
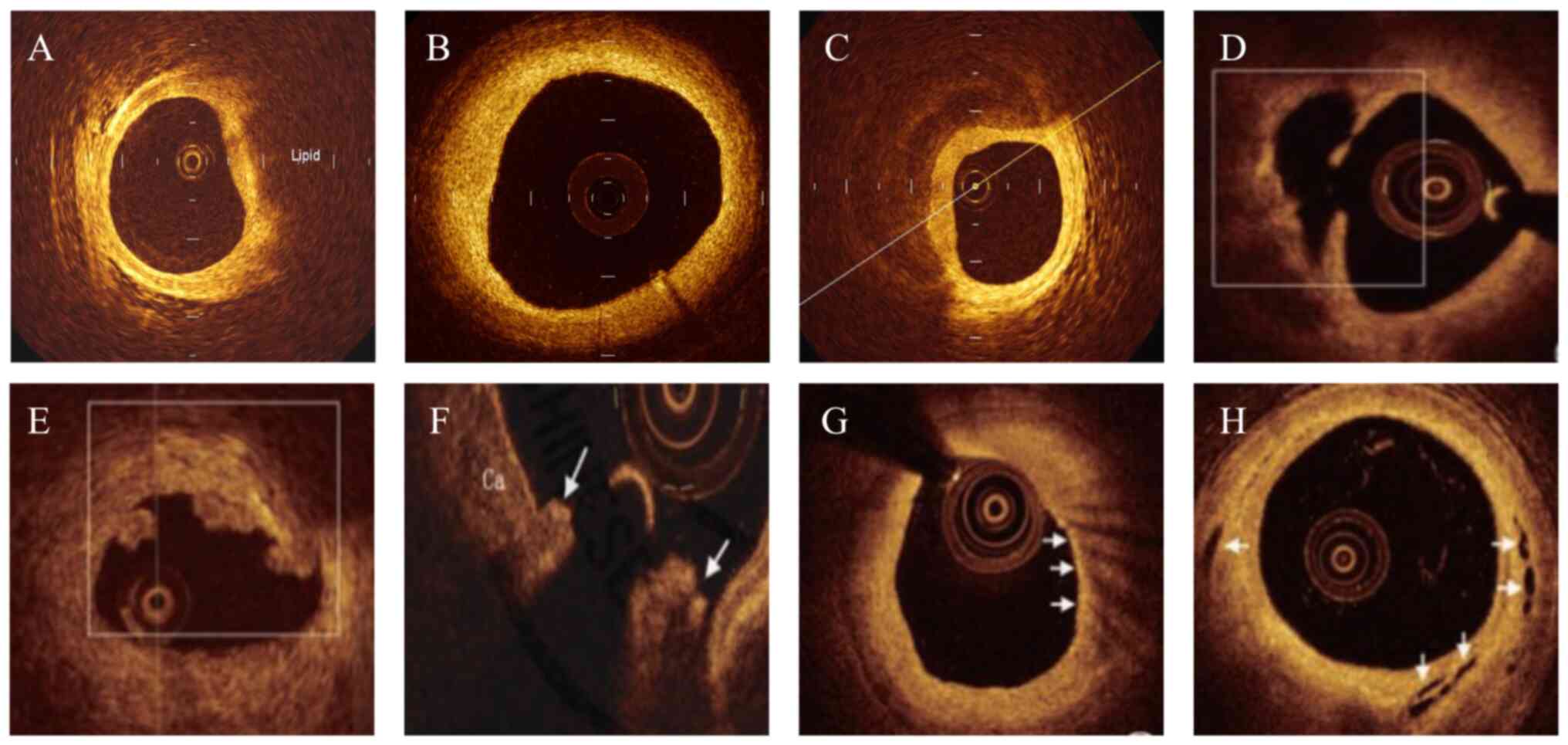
diffuse or poorly defined border; Fig.
1A), fibrous plaque (high backscattering and homogeneous
signal-rich region; Fig. 1B) and
calcified plaque (signal-poor or heterogeneous region with a
sharply delineated border; Fig.
1C) (18). In the present
study, the culprit lesion was categorized into plaque rupture,
plaque erosion or calcified nodule (19). Plaque rupture was defined as the
presence of fibrous cap discontinuity and cavity formation in the
plaque (Fig. 1D), plaque erosion
was characterized by a lesion with attached thrombus overlying an
intact fibrous cap (Fig. 1E) and
calcified nodule was defined as an accumulation of nodular
calcification with disruption of the fibrous cap on the calcified
plate (arrowheads, Fig. 1F).
The plaque features of vulnerability were also
evaluated, including lipid length, minimum fibrous cap thickness,
lipid arc, macrophage accumulation and microchannel (20). Lipid length was obtained on the
longitudinal view, minimum fibrous cap thickness was measured three
times at the thinnest part and the average value was calculated,
and lipid arc was measured at every 1 mm interval throughout the
entire length of lipid length. Subsequently, lipid index was
calculated as the product of lipid length and mean lipid arc, and
thin-cap fibroatheroma (TCFA) was defined as a lipid-rich plaque
with a minimum fibrous cap thickness <65 um. Macrophage
accumulation was characterized by increased signal intensity within
the fibrous cap, accompanied by heterogeneous backward shadows
(arrowheads, Fig. 1G), and
microchannel was defined as a black hole or tubular structure
within a plaque observed on ≥3 consecutive cross-sectional images
(arrowheads, Fig. 1H). For
patients with plaque rupture, the cross-sectional area (CSA) of the
lumen was measured at the largest plaque site. Healed plaques were
defined as plaques with ≥1 signal-rich layers of different optical
density (21).
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used for
statistical analysis. Continuous variables were expressed as mean ±
standard deviation (SD) or median (Q1, Q3)
according to data distribution. Differences between the two groups
were compared by the unpaired Student's t-test or Mann-Whitney U
test, as appropriate. Categorical variables were presented as
numbers (percentages) and compared using the χ2 test or
Fisher's exact test. Multivariate logistic regression analysis was
conducted to identify factors independently associated with poor
initial TIMI blood flow, and odds ratios (OR) with 95% confidence
interval (CI) were calculated. The significance level was set to
α=0.05, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Baseline characteristics
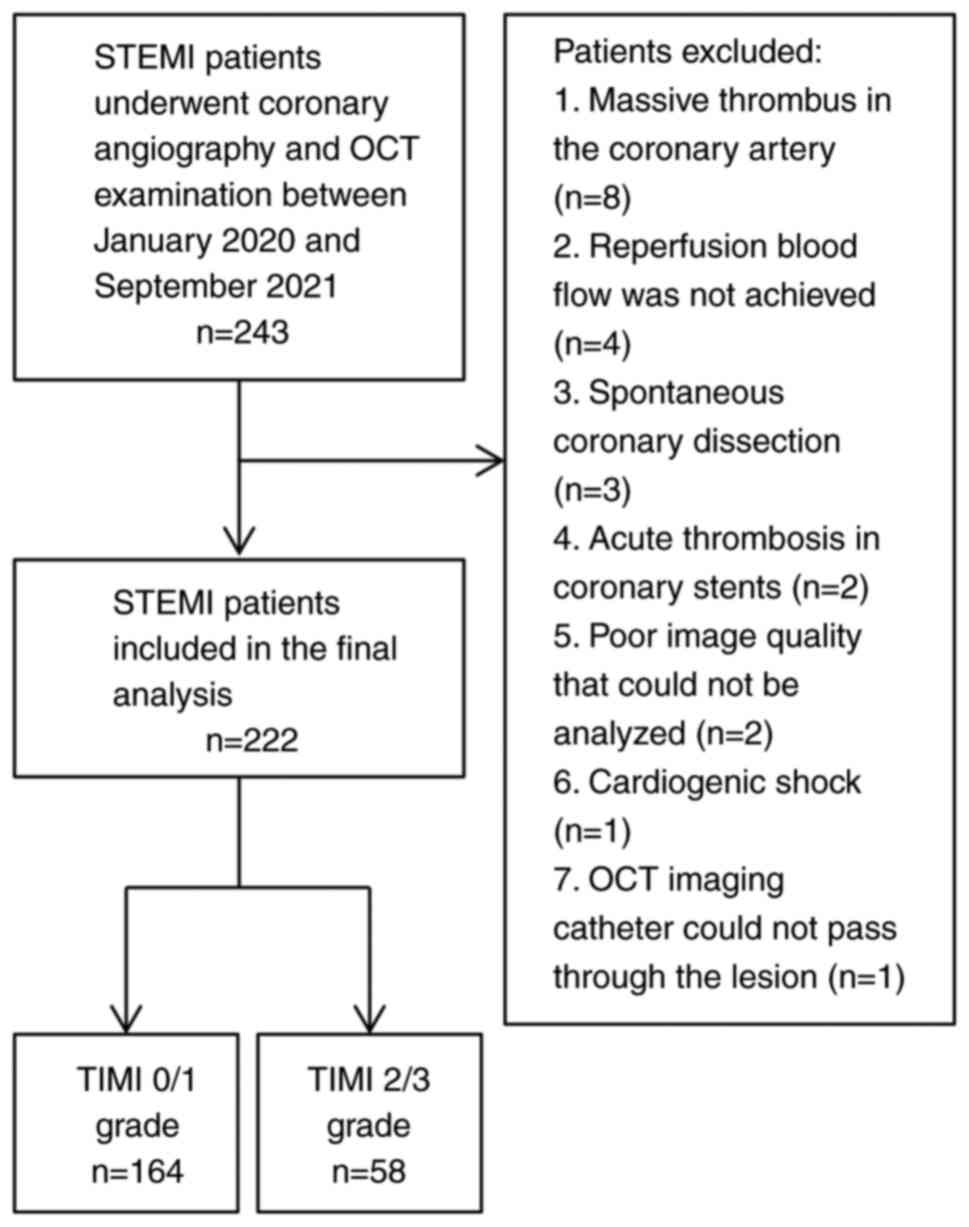
After applying the exclusion criteria (Fig. 2), 222 patients with STEMI were
included in the present study. The baseline characteristics of
patients are summarized in Table
I. Of these patients, 164 (73.9%) were TIMI blood flow grade
0/1, and 58 (26.1%) were TIMI grade 2/3. Compared with patients in
TIMI 2/3 group, patients in TIMI 0/1 group were older, had a higher
peak CKMB level and a lower LVEF (P<0.05). No significant
differences between the two groups were found in terms of other
demographic characteristics, previous disease history, blood
biochemical indexes and total ischemic time.
 | Table IComparison of baseline characteristics
between TIMI 0/1 and TIMI 2/3 group. |
Table I
Comparison of baseline characteristics
between TIMI 0/1 and TIMI 2/3 group.
| Characteristics | TIMI 0/1 group
(n=164) | TIMI 2/3 group
(n=58) | P-value |
|---|
| Demographic
characteristics | | | |
|
Age, years
(mean ± SD)a | 65.8±12.0 | 60.3±12.0 | 0.003 |
|
Male, n
(%)b | 120 (73.2) | 38 (65.5) | 0.269 |
|
BMI,
kg/m2 (mean ± SD)a | 24.7±4.0 | 24.2±4.0 | 0.415 |
|
Smoking
status, n (%)b | 87 (53.0) | 29 (50.0) | 0.815 |
| Previous disease
history, n (%)b | | | |
|
Hypertension | 103 (62.8) | 42 (72.4) | 0.186 |
|
Diabetes
mellitus | 36 (22.0) | 20 (34.5) | 0.059 |
|
Hyperlipidemia | 85 (51.8) | 35 (60.3) | 0.263 |
|
Myocardial
infarction | 21 (12.8) | 6 (10.3) | 0.622 |
|
Previous
PCI | 8 (4.9) | 0 (0) | 0.115 |
| Blood biochemical
indexes (mean ± SD)a | | | |
|
HDL-C,
mg/dl | 46.9±11.9 | 43.8±10.8 | 0.082 |
|
LDL-C,
mg/dl | 122.1±35.1 | 122.6±40.8 | 0.929 |
|
Triglyceride,
mg/dl [M (Q1, Q3)]c | 108.2 (74.6,
151.3) | 123.3 (80.5,
161.2) | 0.482 |
|
eGFR,
ml/min/1.73m2 (mean ± SD)a | 80.5±26.8 | 74.5±23.5 | 0.132 |
|
cTnI, ng/ml
[M (Q1, Q3)]c | 0.14 (0.04,
0.42) | 0.26 (0.10,
0.85) | 0.206 |
|
CKMB peak,
IU/l [M (Q1, Q3)]c | 353.2 (222.3,
565.5) | 109.3 (54.2,
217.6) | <0.001 |
| LVEF, % (mean ±
SD)a | 58.1±5.6 | 64.8±4.7 | <0.001 |
| Total ischemic
time, min [M (Q1, Q3)]c | 274.5 (198.2,
413.6) | 255.4 (203.2,
688.7) | 0.636 |
Coronary angiography findings
As presented in Table
II, the most common infarct-related artery in the TIMI 0/1 and
TIMI 2/3 group was the left anterior descending artery [82 (50.0%),
32 (55.2%)], followed by the right coronary artery [67 (40.9%), 22
(37.9%)] and left circumflex artery [15 (9.1%), 4 (6.9%)],
respectively. According to the results of quantitative coronary
angiography, TIMI 0/1 group had a significantly smaller MLD,
greater diameter stenosis and longer lesion length compared with
the TIMI 2/3 group (P<0.05). However, there were no significant
differences in the infarct-related artery (left anterior descending
artery, left circumflex artery and right coronary artery), RVD,
severe calcification, multivessel lesion and bifurcation
lesion.
 | Table IIComparison of coronary angiography
findings between TIMI 0/1 and TIMI 2/3 group. |
Table II
Comparison of coronary angiography
findings between TIMI 0/1 and TIMI 2/3 group.
| Parameter | TIMI 0/1 group
(n=164) | TIMI 2/3 group
(n=58) | P-value |
|---|
| Infarct-related
artery, n (%)a | | | |
|
Left
anterior descending artery | 82 (50.0) | 32 (55.2) | 0.498 |
|
Left
circumflex artery | 15 (9.1) | 4 (6.9) | 0.787 |
|
Right
coronary artery | 67 (40.9) | 22 (37.9) | 0.696 |
| Quantitative
coronary angiography findings | | | |
|
MLD, mm
(mean ± SD)b | 0.12±0.46 | 0.64±0.40 | <0.001 |
|
Diameter
stenosis, % (mean ± SD)b | 95.1±11.6 | 73.8±12.3 | <0.001 |
|
Lesion
length, mm (mean ± SD)b | 14.8±6.6 | 11.9±7.6 | 0.006 |
|
RVD, mm
(mean ± SD)b | 2.9±0.6 | 3.0±0.6 | 0.673 |
|
Severe
calcification, n (%)a | 8 (4.9) | 2 (3.4) | 0.999 |
|
Multivessel
lesion, n (%)a | 64 (39.0) | 28 (48.3) | 0.219 |
|
Bifurcation
lesion, n (%)a | 21 (12.8) | 12 (20.7) | 0.147 |
OCT results
Among these patients, plaque morphology was
classified according to the plaque composition: 38 (17.1%) were
fibrous plaque, 184 (82.9%) were lipid plaque and 141 (63.5%) were
calcified plaque (Table III).
Patients in the TIMI 0/1 group had a higher incidence of lipid
plaque compared with those in TIMI 2/3 group (P<0.05). In the
TIMI 0/1 and TIMI 2/3 group, plaque rupture [123 (75.0%), 36
(62.1%)] was the most prevalent finding, followed by plaque erosion
[38 (23.2%), 20 (34.5%)] and calcified nodule [3 (1.8%), 2 (3.4%)],
and no statistical difference was observed in the culprit lesion.
With respect to the plaque features of vulnerability, the TIMI 0/1
group had a significantly longer lipid length, maximum lipid arc,
lipid index, maximum CSA of plaque rupture, higher prevalence of
TCFA and healed plaque, as well as a lower proportion of
microchannel compared with the TIMI 2/3 group (P<0.05).
 | Table IIIComparison of optical coherence
tomography results between TIMI 0/1 and TIMI 2/3 group. |
Table III
Comparison of optical coherence
tomography results between TIMI 0/1 and TIMI 2/3 group.
| Parameter | TIMI 0/1 group
(n=164) | TIMI 2/3 group
(n=58) | P-value |
|---|
| Plaque composition,
n (%)a | | | |
|
Fibrous
plaque | 26 (15.9) | 12 (20.7) | 0.681 |
|
Lipid
plaque | 142 (86.6) | 42 (72.4) | 0.014 |
|
Calcified
plaque | 100 (61.0) | 41 (70.7) | 0.186 |
| Culprit lesion, n
(%)a | | | |
|
Plaque
rupture | 123 (75.0) | 36 (62.1) | 0.060 |
|
Plaque
erosion | 38 (23.2) | 20 (34.5) | 0.092 |
|
Calcified
nodule | 3 (1.8) | 2 (3.4) | 0.608 |
| Plaque features of
vulnerability, [M (Q1, Q3)]b | | | |
|
Lipid
length, mm | 12.6 (8.7,
16.8) | 9.2 (6.8,
13.2) | 0.027 |
|
Minimum
fibrous cap thickness, µm | 51.0 (40.2,
62.8) | 50.0 (40.3,
130.6) | 0.596 |
|
Maximum
lipid arc, ˚ | 348.0 (293.1,
360.8) | 270.0 (232.2,
360.7) | 0.032 |
|
Lipid
index | 3149.0 (1743.2,
3971.6) | 2630.0 (1188.3,
2694.7) | 0.015 |
| TCFA, n
(%)a | 118 (72.0) | 24(41.4) | <0.001 |
| Macrophage
accumulation, n (%)a | 133 (81.1) | 49 (84.5) | 0.564 |
| Microchannel, n
(%)a | 57 (34.8) | 35 (60.3) | <0.001 |
| Maximum CSA of
plaque rupture, mm2 (mean ± SD)c | 2.8±1.1 | 2.2±1.2 | 0.001 |
| Healed plaque, n
(%)a | 77 (47.0) | 17 (29.3) | 0.019 |
Multivariate logistic regression
analysis
The results of multivariate analysis demonstrated
that lipid plaque, lipid length, maximum lipid arc, lipid index,
TCFA, maximum CSA of plaque rupture and healed plaque were
significantly associated with poor initial TIMI blood flow
(P<0.05, Table IV).
 | Table IVMultivariate logistic regression
analysis of factors associated with poor initial thrombolysis in
myocardial infarction blood flow. |
Table IV
Multivariate logistic regression
analysis of factors associated with poor initial thrombolysis in
myocardial infarction blood flow.
| Parameter | OR | 95% CI | P-value |
|---|
| Lipid plaque | 1.48 | 1.06-2.07 | 0.032 |
| Lipid length,
mm | 1.61 | 1.18-2.29 | 0.003 |
| Maximum lipid arc,
˚ | 2.03 | 1.43-2.86 | 0.002 |
| Lipid index | 1.35 | 0.92-1.96 | 0.029 |
| TCFA | 1.03 | 1.01-1.21 | 0.001 |
| Maximum CSA of
plaque rupture, mm2 | 1.02 | 1.01-1.08 | 0.021 |
| Healed plaque | 1.41 | 1.09-2.05 | 0.036 |
Discussion
To the best of our knowledge, reduced preoperative
TIMI blood flow (grade 0/1) in patients with STEMI is associated
with a worse clinical prognosis (5,7-10).
Using coronary angiography for the assessment of blood flow, the
present study revealed that the incidence of poor initial TIMI
blood flow before PCI was as high as 73.9%, which was slightly
higher compared with that reported by Bauer et al (66.3%)
(4), and Bouisset et al
(66.5%) (5); however, it was
similar to the study by Kalinskaya et al (77.6%) (6). Consistent with a previous study
(22), the present study further
demonstrated that patients with STEMI with TIMI 0/1 had a smaller
MLD, greater diameter stenosis and longer lesion length detected by
coronary angiography when compared with those with TIMI 2/3. In
addition, OCT findings indicated that the TIMI 0/1 group had a
higher incidence of lipid plaque, TCFA and healed plaque, and a
lower incidence of microchannel, as well as a larger lipid length,
maximum lipid arc, lipid index and maximum CSA of plaque rupture
compared with the TIMI 2/3 group. Recently, a higher number of
lipid plaques (53.9 vs. 41.8%) have also been observed in patients
with STEMI with pre-procedural TIMI 0/1(23).
Moreover, the present study explored the
relationship between morphological characteristics of culprit
plaques and preoperative TIMI blood flow using multivariate
analysis, and revealed that lipid plaque, lipid length, maximum
lipid arc, lipid index, TCFA, maximum CSA of plaque rupture and
healed plaque were significantly associated with poor initial TIMI
blood flow. Consistent with the present findings, Yu et al
(24) reported that TIMI 0/1 flow
is more prone to the formation of in-stent plaque rupture in
patients with ACS, and lesions in the plaque rupture have more
cholesterol crystals, and multivariate analysis demonstrated that
lipidic neointima length has a 1.3-fold higher risk for occurrence
of in-stent plaque rupture. Majeed et al (25) also revealed that patients with
reduced blood flow (TIMI ≤2) after PCI have more plaques behind
stent struts that are detected by OCT, and that lipid arc is
significantly associated with abnormal TIMI flow in multivariate
logistic regression analysis (OR=1.29; 95% CI, 1.14-1.38).
Moreover, lipid plaques are not only associated with culprit
lesions but also with non-culprit lesions in patients with ACS
(26), and the large lipid index
is also considered as a critical morphological discriminator for
myocardial no-reflow (27).
The proposed mechanism for reduced TIMI blood flow
may be that the lipid core can be released after plaque rupture and
flows into the lumen, which has procoagulant properties that lead
to the release of procoagulant substances, and eventually results
in thrombosis and coronary artery lumen occlusion followed by
platelet adhesion, aggregation and activation (28). Another reason may be that plaque
rupture or erosion results in higher fibrinogen and lower platelet
levels in the thrombus, and the infarct related arteries tend to be
completely occluded (29).
Therefore, thrombus, plaque rupture and lipid-rich plaque are
considered to indicate microcirculation dysfunction during
reperfusion therapy (30).
Furthermore, some findings from OCT studies show
that TCFA is one of the characteristics of vulnerable plaques which
are prone to rupture in coronary artery disease (31). Recently, TCFA was revealed to be
associated with greater plaque burden and plaque volume (32). A study by Araki et al
(33) also demonstrated that TCFA
is a predictor of subsequent rapid plaque progression (OR=5.85; 95%
CI, 2.01-17.03). In the present study, patients in the TIMI 0/1
group had a significantly higher prevalence of TCFA compared with
the TIMI 2/3 group (72.0 vs. 41.4%), and TCFA was independently
associated with poor initial TIMI blood flow. In addition, the
present study revealed that the TIMI 0/1 group had a larger CSA of
plaque rupture. This may be proportional to the content of the
lipid core flowing into the vascular lumen; that is, with the
larger CSA, the more lipid and thrombogenic components flow into
the lumen, thereby increasing the risk of blocking the lumen.
On the other hand, healed plaques are considered to
be a signature of prior plaque destabilization (13). Previous studies have demonstrated
that healed plaques in patients with STEMI are associated with a
high level of plaque vulnerability and inflammation (34,35).
Cao et al (36) also
reported that healed plaques are an independent predictor of side
branch occlusion (OR=18.8; 95% CI, 5.1-68.8). In the present study,
the TIMI 0/1 group had a higher proportion of healed structures
(47.0 vs. 29.3%), indicating that the plaques in the TIMI 0/1 group
were more vulnerable. The reason for this was that the combination
of plaque vulnerability, local inflammation and greater plaque
burden in addition to systemic inflammation may outweigh the
protective mechanism of plaque healing and predispose those plaques
to develop into an occlusive thrombus (13).
The present study has several limitations. First,
this is a single-center retrospective analysis with a relatively
small sample. Second, a small fraction of patients with STEMI
enrolled during the study were excluded, and selection bias may
have affected the results. Third, for patients with STEMI with a
TIMI grade 0, thrombus aspiration must be performed to achieve
blood perfusion, and the mechanical damage may alter the
morphological characteristics of the underlying plaque in these
patients. Fourth, coronary thrombus burden may affect OCT
assessment of vulnerable plaque characteristics; the present study
therefore excluded patients with a large thrombus burden. Fifth,
due to the physical characteristics of near-infrared light in OCT
technology, its relatively shallow penetration depth and fast
attenuation limits the ability to detect the fine structure of
plaque. Therefore, the results of this study still need to be
confirmed by large-scale multicenter studies.
In conclusion, the present study revealed that the
morphological characteristics of culprit coronary plaques (lipid
plaque, lipid length, maximum lipid arc, lipid index, TCFA, maximum
CSA of plaque rupture, healed plaque) are significantly associated
with poor initial TIMI blood flow before PCI in patients with
STEMI. Preoperative TIMI blood flow is important for patients with
STEMI, therefore, systematic evaluation of the plaque morphological
characteristics in patients with STEMI may contribute to early
diagnosis and effective intervention, and subsequently reduce the
occurrence of adverse cardiovascular events.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Key Science and
Technology Program of Henan Province (grant no. 122102310068).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS and YC conceived and designed the study. SD, HY
and SC collected data. HS, SD, HY and SC analyzed and interpreted
the data. HS and SD drafted the manuscript. YC, HY and SC reviewed
the manuscript. HS and YC confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Henan Provincial People's Hospital (approval no.
HNSRMYY-2017-47), and written informed consent for research
purposes was obtained from all patients at admission.
Patient consent for publication
Not applicable.
Competing interest.
The authors declare that they have no competing
interests.
References
|
1
|
Kuhn J, Olié V, Grave C, Le Strat Y,
Bonaldi C and Joly P: Estimating the future burden of myocardial
infarction in france until 2035: An illness-death model-based
approach. Clin Epidemiol. 14:255–264. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fang C, Yin Y, Jiang S, Zhang S, Wang J,
Wang Y, Li L, Wang Y, Guo J, Yu H, et al: Increased vulnerability
and distinct layered phenotype at culprit and nonculprit lesions in
STEMI versus NSTEMI. JACC Cardiovasc Imaging. 15:672–681.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sun R, Hu S, Guagliumi G, Jia H, Tian J,
Li L, Zhang S, Wang Y, Zhang S, Hou J and Yu B: Pre-infarction
angina and culprit lesion morphologies in patients with a first
ST-segment elevation acute myocardial infarction: Insights from in
vivo optical coherence tomography. EuroIntervention. 14:1768–1775.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bauer T, Zeymer U, Diallo A, Vicaut E,
Bolognese L, Cequier A, Huber K, Montalescot G, Hamm CW and Van't
Hof AW: ATLANTIC Investigators. Impact of preprocedural TIMI flow
on clinical outcome in low-risk patients with ST-elevation
myocardial infarction: Results from the ATLANTIC study. Catheter
Cardiovasc Interv. 95:494–500. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bouisset F, Deney A, Ferrières J,
Panagides V, Becker M, Riviere N, Yvorel C, Commeau P, Adjedj J,
Benamer H, et al: Mechanical complications in ST-elevation
myocardial infarction: The impact of pre-hospital delay. Int J
Cardiol. 345:14–19. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kalinskaya A, Dukhin O, Lebedeva A,
Maryukhnich E, Rusakovich G, Vorobyeva D, Shpektor A, Margolis L
and Vasilieva E: Circulating cytokines in myocardial infarction are
associated with coronary blood flow. Front Immunol.
13(837642)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Somuncu MU, Akgun T, Cakır MO, Akgul F,
Serbest NG, Karakurt H, Can M and Demir AR: The elevated soluble
ST2 predicts no-reflow phenomenon in ST-elevation myocardial
infarction undergoing primary percutaneous coronary intervention. J
Atheroscler Thromb. 26:970–978. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mirbolouk F, Gholipour M, Salari A,
Shakiba M, Kheyrkhah J, Nikseresht V, Sotoudeh N, Moghadam N,
Mirbolouk MJ and Far MM: CHA2DS2-VASc score predict no-reflow
phenomenon in primary percutaneous coronary intervention in primary
percutaneous coronary intervention. J Cardiovasc Thorac Res.
10:46–52. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Søndergaard FT, Beske RP, Frydland M,
Møller JE, Helgestad OKL, Jensen LO, Holmvang L, Goetze JP,
Engstrøm T and Hassager C: Soluble ST2 in plasma is associated with
post-procedural no-or-slow reflow after primary percutaneous
coronary intervention in ST-elevation myocardial infarction. Eur
Heart J Acute Cardiovasc Care. 12:48–52. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Stalikas N, Papazoglou AS, Karagiannidis
E, Panteris E, Moysidis D, Daios S, Anastasiou V, Patsiou V,
Koletsa T, Sofidis G, et al: Association of stress induced
hyperglycemia with angiographic findings and clinical outcomes in
patients with ST-elevation myocardial infarction. Cardiovasc
Diabetol. 21(140)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Araki M, Park SJ, Dauerman HL, Uemura S,
Kim JS, Di Mario C, Johnson TW, Guagliumi G, Kastrati A, Joner M,
et al: Optical coherence tomography in coronary atherosclerosis
assessment and intervention. Nat Rev Cardiol. 19:684–703.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Weng Z, Zhao C, Qin Y, Liu C, Pan W, Hu S,
He L, Xu Y, Zeng M, Feng X, et al: Peripheral atherosclerosis in
acute coronary syndrome patients with plaque rupture vs plaque
erosion: A prospective coronary optical coherence tomography and
peripheral ultrasound study. Am Heart J. 263:159–168.
2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fracassi F, Crea F, Sugiyama T, Yamamoto
E, Uemura S, Vergallo R, Porto I, Lee H, Fujimoto J, Fuster V and
Jang IK: Healed culprit plaques in patients with acute coronary
syndromes. J Am Coll Cardiol. 73:2253–2263. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shimokado A, Matsuo Y, Kubo T, Nishiguchi
T, Taruya A, Teraguchi I, Shiono Y, Orii M, Tanimoto T, Yamano T,
et al: In vivo optical coherence tomography imaging and
histopathology of healed coronary plaques. Atherosclerosis.
275:35–42. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Reindl M, Tiller C, Holzknecht M, Lechner
I, Henninger B, Mayr A, Brenner C, Klug G, Bauer A, Metzler B and
Reinstadler SJ: Association of myocardial injury with serum
procalcitonin levels in patients with ST-elevation myocardial
infarction. JAMA Netw Open. 3(e207030)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gibson CM, Cannon CP, Daley WL, Dodge JT
Jr, Alexander B Jr, Marble SJ, McCabe CH, Raymond L, Fortin T,
Poole WK and Braunwald E: TIMI frame count: A quantitative method
of assessing coronary artery flow. Circulation. 93:879–888.
1996.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jiao Y, Su Y, Shen J, Hou X, Li Y, Wang J,
Liu B, Qiu D, Sun Z, Chen Y, et al: Evaluation of the long-term
prognostic ability of triglyceride-glucose index for elderly acute
coronary syndrome patients: A cohort study. Cardiovasc Diabetol.
21(3)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li Z, Tang Z, Wang Y, Liu Z, Wang G, Zhang
L, Wu Y and Guo J: Assessment of radial artery atherosclerosis in
acute coronary syndrome patients: An in vivo study using optical
coherence tomography. BMC Cardiovasc Disord. 22(120)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yamanaka Y, Shimada Y, Tonomura D,
Terashita K, Suzuki T, Yano K, Nishiura S, Yoshida M, Tsuchida T
and Fukumoto H: Laser vaporization of intracoronary thrombus and
identifying plaque morphology in ST-segment elevation myocardial
infarction as assessed by optical coherence tomography. J Interv
Cardiol. 2021(5590109)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kanaya T, Noguchi T, Otsuka F, Asaumi Y,
Kataoka Y, Morita Y, Miura H, Nakao K, Fujino M, Kawasaki T, et al:
Optical coherence tomography-verified morphological correlates of
high-intensity coronary plaques on non-contrast T1-weighted
magnetic resonance imaging in patients with stable coronary artery
disease. Eur Heart J Cardiovasc Imaging. 20:75–83. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Russo M, Fracassi F, Kurihara O, Kim HO,
Thondapu V, Araki M, Shinohara H, Sugiyama T, Yamamoto E, Lee H, et
al: Healed plaques in patients with stable angina pectoris.
Arterioscler Thromb Vasc Biol. 40:1587–1597. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Higuma T, Soeda T, Yamada M, Yokota T,
Yokoyama H, Nishizaki F, Xing L, Yamamoto E, Bryniarski K, Dai J,
et al: Coronary plaque characteristics associated with reduced TIMI
(Thrombolysis in Myocardial Infarction) flow grade in patients with
st-segment-elevation myocardial infarction: A combined optical
coherence tomography and intravascular ultrasound study. Circ
Cardiovasc Interv. 9(e003913)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang J, Fang C, Zhang S, Li L, Lu J, Wang
Y, Wang Y, Yu H, Wei G, Yin Y, et al: Systemic and local factors
associated with reduced thrombolysis in myocardial infarction flow
in ST-segment elevation myocardial infarction patients with plaque
erosion detected by intravascular optical coherence tomography. Int
J Cardiovasc Imaging. 37:399–409. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu H, Dai J, Fang C, Jiang S, Mintz GS and
Yu B: Prevalence, morphology, and predictors of intra-stent plaque
rupture in patients with acute coronary syndrome: An optical
coherence tomography study. Clin Appl Thromb Hemost.
28(10760296221146742)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Majeed K, Hartman E, Mori TA, Alcock R,
Spiro J, Ligthart J, Witberg K, Hillis G, van Soest G and Schultz
C: The effect of stent artefact on quantification of plaque
features using optical coherence tomography (OCT): A feasibility
and clinical utility study. Heart Lung Circ. 29:874–882.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ando H, Amano T, Matsubara T, Uetani T,
Nanki M, Marui N, Kato M, Yoshida T, Yokoi K, Kumagai S, et al:
Comparison of tissue characteristics between acute coronary
syndrome and stable angina pectoris. An integrated backscatter
intravascular ultrasound analysis of culprit and non-culprit
lesions. Circ J. 75:383–390. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Soeda T, Higuma T, Abe N, Yamada M,
Yokoyama H, Shibutani S, Ong DS, Vergallo R, Minami Y, Lee H, et
al: Morphological predictors for no reflow phenomenon after primary
percutaneous coronary intervention in patients with ST-segment
elevation myocardial infarction caused by plaque rupture. Eur Heart
J Cardiovasc Imaging. 18:103–110. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Falk E, Nakano M, Bentzon JF, Finn AV and
Virmani R: Update on acute coronary syndromes: The pathologists'
view. Eur Heart J. 34:719–728. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Grover SP and Mackman N: Tissue factor in
atherosclerosis and atherothrombosis. Atherosclerosis. 307:80–86.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Feng X, Xu Y, Zeng M, Qin Y, Weng Z, Sun
Y, Gao Z, He L, Zhao C, Wang N, et al: Optical coherence tomography
assessment of coronary lesions associated with microvascular
dysfunction in ST-segment elevation myocardial infarction. Circ J.
4(CJ-23-0200)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tomaniak M, Katagiri Y, Modolo R, de Silva
R, Khamis RY, Bourantas CV, Torii R, Wentzel JJ, Gijsen FJH, van
Soest G, et al: Vulnerable plaques and patients: State-of-the-art.
Eur Heart J. 41:2997–3004. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dobrolińska MM, Gąsior P, Wańha W,
Pietraszewski P, Pociask E, Smolka G, Wojakowski W and Roleder T:
The influence of high-density lipoprotein cholesterol on maximal
lipid core burden indexing thin cap fibrous atheroma lesions as
assessed by near infrared spectroscopy. Cardiol J. 28:887–895.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Araki M, Yonetsu T, Kurihara O, Nakajima
A, Lee H, Soeda T, Minami Y, McNulty I, Uemura S, Kakuta T and Jang
IK: Predictors of rapid plaque progression: An optical coherence
tomography study. JACC Cardiovasc Imaging. 14:1628–1638.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li J, Sheng Z, Tan Y, Zhou P, Liu C, Zhao
H, Song L, Zhou J, Chen R, Chen Y and Yan H: Association of plasma
trimethylamine N-Oxide level with healed culprit plaques examined
by optical coherence tomography in patients with ST-Segment
elevation myocardial infarction. Nutr Metab Cardiovasc Dis.
31:145–152. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yin WJ, Jing J, Zhang YQ, Tian F, Zhang T,
Zhou SS and Chen YD: Association between non-culprit healed plaque
and plaque progression in acute coronary syndrome patients: An
optical coherence tomography study. J Geriatr Cardiol. 18:631–644.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cao Y, Mintz GS, Matsumura M, Zhang W, Lin
Y, Wang X, Fujino A, Lee T, Murai T, Hoshino M, et al: The relation
between optical coherence tomography-detected layered pattern and
acute side branch occlusion after provisional stenting of coronary
bifurcation lesions. Cardiovasc Revasc Med. 20:1007–1013.
2019.PubMed/NCBI View Article : Google Scholar
|