Introduction
The ‘atopic march’, at times referred to as the
‘allergic march’, is the progression of allergic conditions as they
develop through infancy and childhood. These allergic conditions
have common genetic and environmental predisposing factors, share
immunologic features of one or more allergen-specific T helper type
2 responses and are characterized by a ‘type 2’ effector phase that
includes the generation of specific Immunoglobulin E (IgE),
granulocyte activation and other features, such as mucous
production and edema. A key trait of the atopic march is that the
presence of one allergic condition increases the risk for the
development of others; hence the term ‘march’ (1). The atopic march typically begins with
atopic dermatitis (AD) and then progresses to IgE-mediated food
allergy (FA), asthma and allergic rhinitis (AR) (2).
The positive rate of specific IgE antibodies to food
allergens, such as eggs, is high in infancy (3), and the positive rate of inhalant
allergens (e.g., mites and house dust) increases with age. In
addition, the prevalence of food allergies has increased in the
past 30 years, particularly in industrialized countries (3). Among specific IgE measuring reagents,
diagnostic reagents that may measure multiple items simultaneously
are used for screening for sensitized allergens. MAST III and MAST
IV (hereinafter referred to as ‘MAST’) are the most commonly
available in Japan and Korea. MAST tests >33 specific IgE items
simultaneously with a small amount of serum and is able to detect
changes in specific antibody titers of multiple allergens in the
atopic march during infancy. The results obtained may enable us to
understand the tendency of allergy onset and provide preventive
medical knowledge of future allergy risk.
The eosinophil is a major effector cell in allergic
disease (4). Therefore, direct
measurement of eosinophilic inflammation should aid in the
diagnosis, treatment and monitoring of allergic disease. However,
eosinophil counts/percentages provide only a limited understanding
of eosinophil activity. It has been suggested that the secretory
activity of eosinophils would be better measured by determining the
concentration of eosinophils and their tendency to release
mediators (5). In the past few
decades, eosinophil-derived neurotoxin (EDN) has emerged as a
promising biomarker for eosinophil activity and it has been studied
in a number of inflammatory diseases, including asthma (6-10).
EDN has been used successfully as a predictive marker for recurrent
wheezing after respiratory viral infection (11), but there are few reports of using
EDN levels as a predictor of airway allergic disease in preschool
children.
In the present study, it was investigated whether
there is an association between blood EDN levels and the onset of
allergic disease in preschool children.
Subjects and methods
Subjects
Children of <1 year of age with food allergy
disease and their controls who visited hospitals in Tochigi, Japan
affiliated with the Tochigi Pediatric Allergy Study Group were
enrolled. A total of 123 patients were initially enrolled in the
study from March 2018 to December 2019, comprising 88 males and 35
females, along with 37 Controls.
The inclusion criteria were as follows: i) Male and
female children <1 year of age at the time of enrolment; ii)
children diagnosed with FA or AD; iii) those for whom written
informed consent of their legal guardians was obtained. The
diagnostic criteria for AD were ‘chronic eczema lasting for >2
months, with other skin diseases excluded’. As controls, healthy
children and those with non-allergic disease (children without
allergic symptoms) were included (age- and sex-matched). These
children were visiting well-baby clinics for vaccination. Even if
all specific IgE levels were negative or there were no allergic
symptoms, it was possible for allergies to develop during the
clinical study period, in which case the final decision to
include/exclude the subject was made at the end of the study.
Individuals to whom any of the following applied
were excluded from the study: i) Patients with a history of
cardiac, hepatic or renal disease who were undergoing treatment
(excluding those receiving oral immunotherapy); ii) patients
suspected of having a serious infectious disease, such as human
immunodeficiency virus, hepatitis B virus (HBV) or HCV; iii) other
subjects deemed ineligible by the principal investigator or the
investigator in charge of the study. Written informed consent was
given by the legal guardians of all study subjects and this study
was approved by the Research Ethics Committee of Dokkyo Medical
University Hospital [Institutional Review Board (IRB) no.
26096].
Blood specimen collection
BD Vacutainer (Becton Dickinson and Company) serum
separation tubes were used to collect blood specimens, which were
collected once at the beginning of the study. The tourniquet was
removed from the arm as soon as blood flowed to prevent
hemoconcentration. Care was taken to perform venipuncture in a
manner to minimize any complication. The nurse performing the
venipuncture observed universal precautions for the prevention of
bloodborne pathogen transmission.
Serum EDN measurement
Serum specimens were prepared as described by
Peterson et al (12). In
brief, serum was prepared by allowing blood to clot at 25˚C for 1
h, then centrifuged at 1,350 x g for 10 min at 4˚C. Each serum
specimen was aliquoted into a new plastic tube and stored at -70˚C
until the assay.
The central laboratory at Inje University Sanggye
Paik Hospital in Seoul, Korea was used for serum EDN (sEDN)
measurements. sEDN concentrations were measured using the
K®EDN 'sandwich; enzyme-linked immunosorbent assay
(ELISA) kit (cat. no. SB-00029; SKIMS-BIO Co.) (13), with results expressed in ng/ml.
This ELISA detects human EDN with a minimum detection limit of 6.0
ng/ml and maximum detection limit of 400 ng/ml, and does not
cross-react with eosinophil cationic protein (ECP). The method
described by Morioka et al (14) was followed but modified slightly.
In brief, Nunc MaxiSorp 96-well plates were coated overnight at 4˚C
with 100 µl of mouse anti-human EDN monoclonal antibody (mAb) (cat.
no. KBT-K3231066P) diluted in PBS. The wells were blocked overnight
at 4˚C with 200 µl of blocking buffer [1X PBS, 1% bovine serum
albumin (BSA), 10% sucrose]. Standard EDN was diluted with 50 mM
tris pH 8.0 containing 0.05% Tween 20 buffer (Sigma-Aldrich; Merck
KGaA), 0.15 M NaCl and 0.5% BSA (termed assay diluent). The range
of measurements was 0.6-40 ng/ml, indicating assay sensitivity was
<0.6 ng/ml. Between each subsequent step, plates were washed
three times in PBS containing 0.05% Tween 20. Samples were then
diluted in 50 mM tris pH 8.0 containing 0.05% Tween 20 and 0.15 M
NaCl. Standards and diluted samples (100 µl) were applied to the
plates and incubated at room temperature for 1 h. After washing,
100 µl of horseradish-peroxidase-labeled mouse anti-human EDN mAb,
included in the ELISA kit, was added to the wells and incubated at
room temperature for 1 h. After another wash, the peroxidase
substrate tetramethylbenzidine (Sigma-Aldrich; Merck KGaA) was
added (100 µl/well) and incubated for 10 min at room temperature.
Enzyme reactions were stopped with 1N HCl (100 µl/well). Absorbance
was measured at 450 nm by a Micro Plate Reader Infinite 200 PRO
(TECAN Group). sEDN was determined from a dose-response curve by
multiplying the value read from the standard curve by the dilution
factor.
IgE measurement
The human IgE ELISA quantitation kit (cat. no.
CB-0035; CosmoBio) was used to measure total IgE levels. The
sensitivity limit for IgE was 15.6 ng/ml.
The subjects underwent a blood test for specific IgE
(sIgE) antibodies using a MAST at enrollment and every six months
thereafter at each center. Allergens tested using MAST
Immunosystems IV (Hitachi Chemical Co.) were classified as food
allergens (wheat, milk, egg white, ovomucoid, peanut, soybean,
rice, buckwheat, sesame, tomato, peach, kiwi, banana, tuna, salmon,
shrimp, crab, pork, beef and chicken); mite allergens (mason mite
and house dust), animal allergens (dog dander and cat dander); and
pollen allergens (timothy, Dactylis, ragweed, wormwood,
cedar, Cupressaceae, Alnus and white birch).
Candida, Alternaria, Aspergillus and latex
allergens were excluded from the study. If a subject was positive
for one allergen in a group, the subject was considered to be
positive for the group.
Statistical analysis
This study was powered with a two-sided test with
the significance level set at 0.05. For continuous variables, the
number of subjects observed, mean, standard deviation, median,
minimum and maximum values were presented, and the frequency and
percentage were presented for categorical data. For normally
distributed data, continuous data were tested using the two-samples
t-test. If data were not normally distributed, the Wilcoxon
rank-sum test (Mann-Whitney U-test) was used. All statistical
analyses were performed with IBM SPSS Statistics v.24.0 (IBM
Corp.).
Results
Patient characteristics
The study included 123 registered patients,
comprising 88 males and 35 females. During the study, a total of 37
subjects dropped out: 23 did not visit the clinic for examination,
12 withdrew consent and 2 deviated from the protocol. Finally, the
study analyzed 86 subjects and 37 Controls.
Baseline subject characteristics are displayed in
Tables I and II. The most common allergic disease was
atopic dermatitis [n=50 (58.1%)], followed by food allergy [n=48
(55.8%)] and asthma [n=9 (10.5%)].
 | Table ICharacteristics of the patients
(n=86). |
Table I
Characteristics of the patients
(n=86).
| Parameter | Value |
|---|
| Gender | |
|
Male | 61 |
|
Female | 25 |
| Age at first visit,
months | 8.7±3.4
(5.2-11.8) |
| Body
heighta, cm | 69.6±5.3 |
| Body
weightb, kg | 8.4±1.0 |
| Clinical symptoms of
pediatric asthma | 9 (10.5) |
| Atopic
dermatitis | 50 (58.1) |
| Allergic
rhinitis | 3 (3.5) |
| Food allergy | 48 (55.8) |
|
Eggs | 28 |
|
Milk | 19 |
|
Flour | 12 |
|
Other | 7 |
| Other clinical
symptoms | 5 (5.8) |
| Asthma | Mild, 8; moderate,
1 |
|
With
complications of asthma | 1 (1.2) |
|
With history
of asthma | 57 (66.3) |
| Family history of
allergic diseases | |
|
Sibling | 25 (29.1) |
|
Father | 42 (48.8) |
|
Mother | 47 (54.7) |
| Pets | 23 (26.7) |
| Passive
smoking | 15 (17.4) |
 | Table IITreatment details at patient
registration (n=86). |
Table II
Treatment details at patient
registration (n=86).
| Parameter | Value |
|---|
| Oral
immunotherapy | 2 (2.3) |
| Antihistamines | 21 (24.4) |
| Asthma
medication | 4 (4.7) |
| Inhaled steroid
medication | 3 |
| Leukotriene
antagonist | 2 |
| Long-acting
β2-stimulant | 1 |
| Intala
inhalation | 2 |
| Atopic dermatitis
coating | 41 (47.7) |
| Steroid
ointment | 41 |
| Protopic
ointment | 1 |
| Food removal | 41 (47.7) |
|
Eggs | 30 |
|
Milk | 21 |
|
Flour | 11 |
|
Soya | 1 |
|
Other | 3 |
| Skincare | 46 (53.5) |
|
Moisturizer | 4 (4.7) |
Cumulative sensitization rates to
allergens
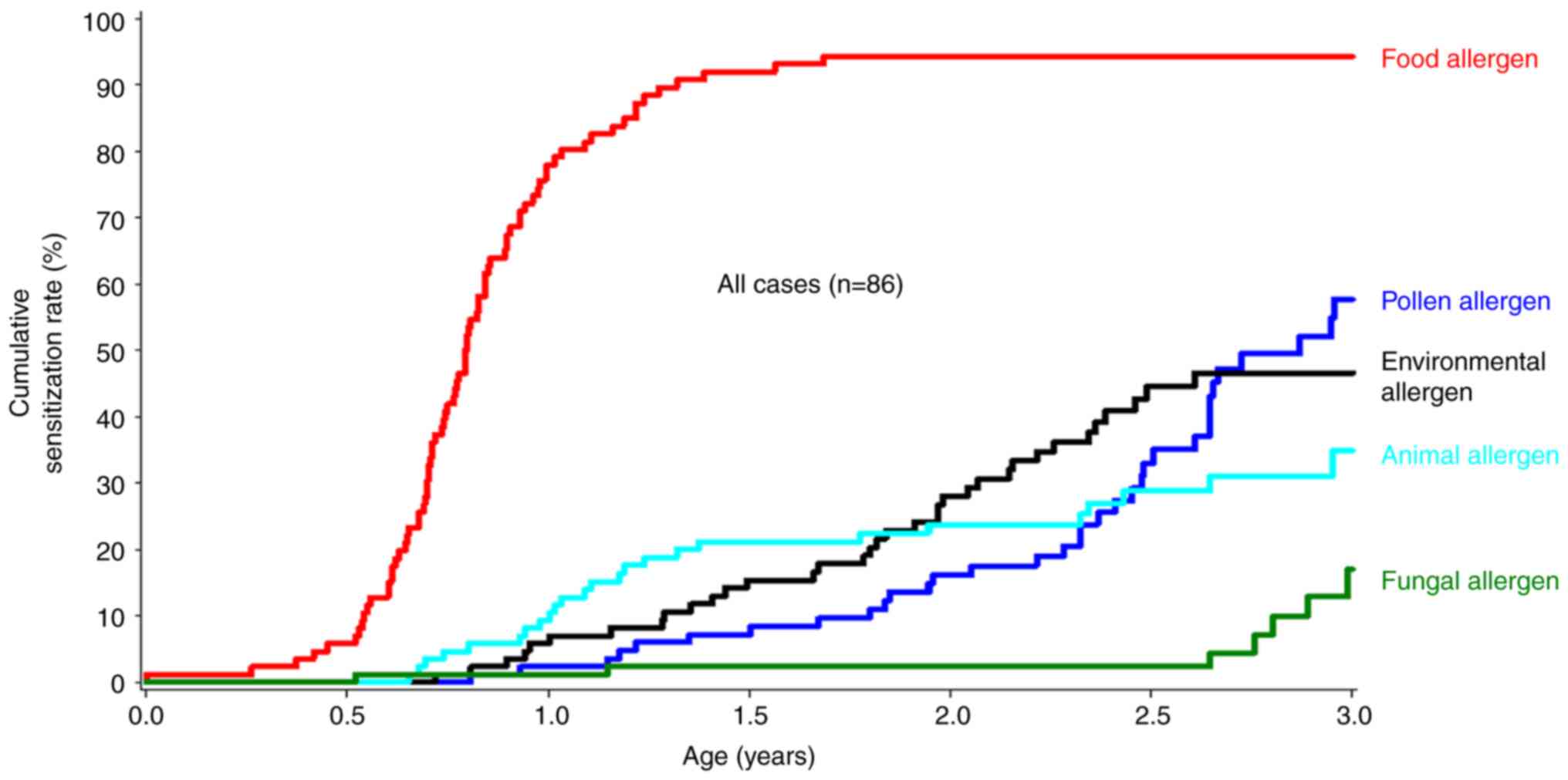
As indicated in Fig.
1, food allergens were the most common, followed by pollen
allergens, environmental allergens (Dermatophagoides
pteronyssinus, Dermatophagoides farinae), animal allergens and
fungal allergens. The cumulative sensitization rate of food
allergens reached a plateau at one-and-a-half years of age.
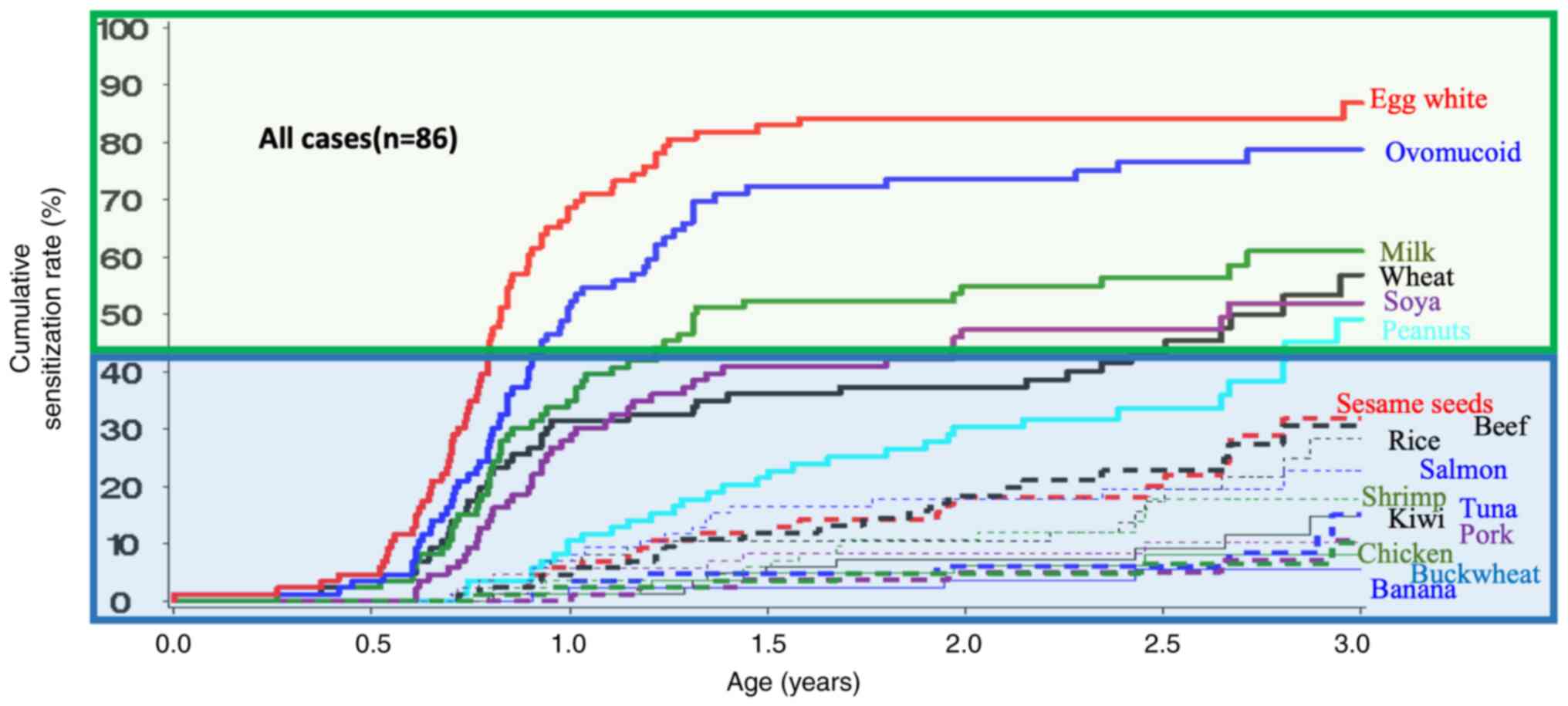
Fig. 2 presents the
cumulative sensitization rate of each food allergen for all
subjects (n=86). Egg white was the most common food allergen, with
a cumulative sensitization rate of >80%. By the end of the
3-year study period, food allergens with a cumulative sensitization
rate of >50% were as follows: Egg white, ovomucoid, milk, wheat,
soya and peanuts. All other food allergens had a cumulative
sensitization rate of <50%.
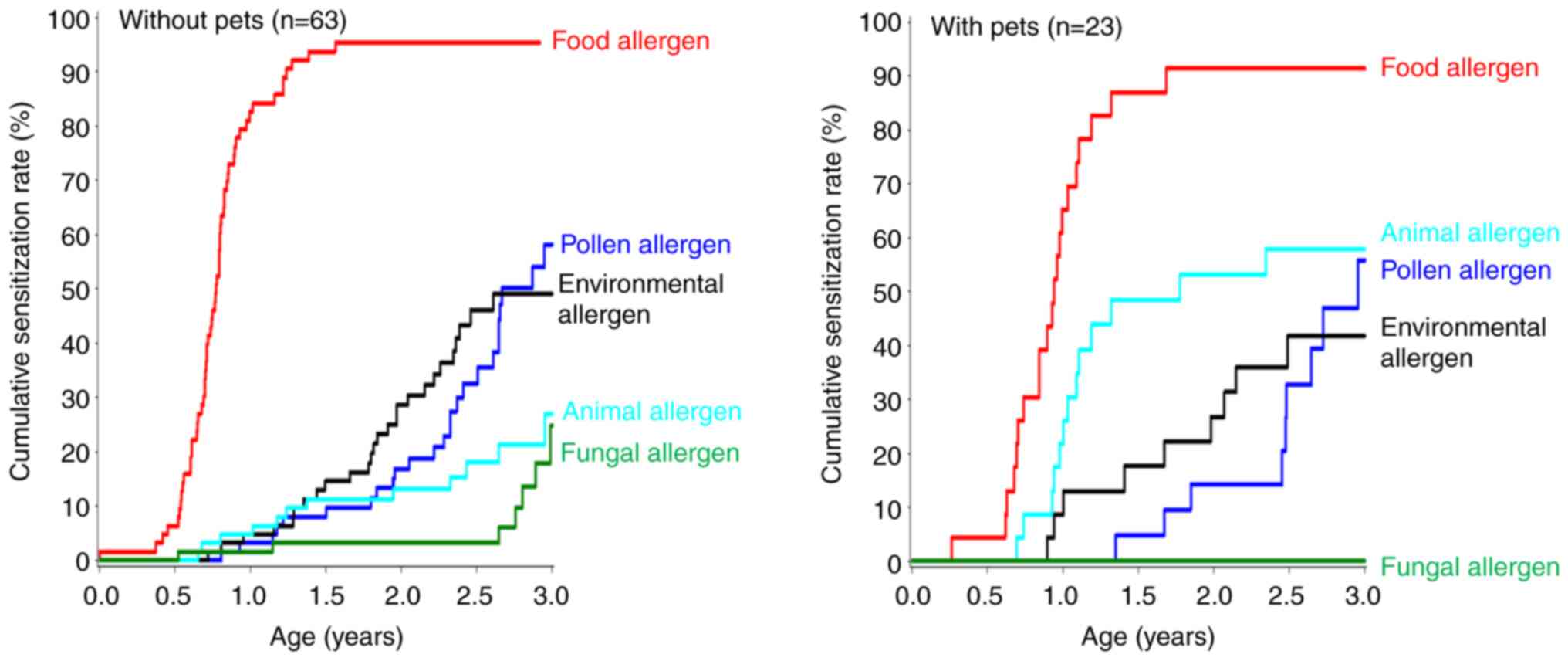
Fig. 3 displays the
effects of keeping pets on allergen development. Of note, the group
with pets (n=23) was much more likely to develop an animal allergen
by the age of 3 years than the group that did not have pets (n=63)
(60 vs. 30%). Because of this, animal allergens were the 2nd most
common type of allergen in the group with pets. Pollen and
environmental allergens were common (>50% cumulative
sensitization rate) in both groups.
Cumulative sensitization rates were similar between
males (n=61) and females (n=25), with food allergens being the most
common, followed by pollen, environmental, animal and fungal
allergens (data not shown).
Subjects were divided into those exposed to smoking
in the house (i.e., passive smoking) (n=15) and those that were not
(n=71). The ‘passive smoking group’ initially began to develop an
environmental allergy at an earlier age, but by the age of 3 years,
the ‘no passive smoking group’ had a much higher cumulative
sensitization rate than the passive smoking group (50% vs. 35%)
(data not shown).
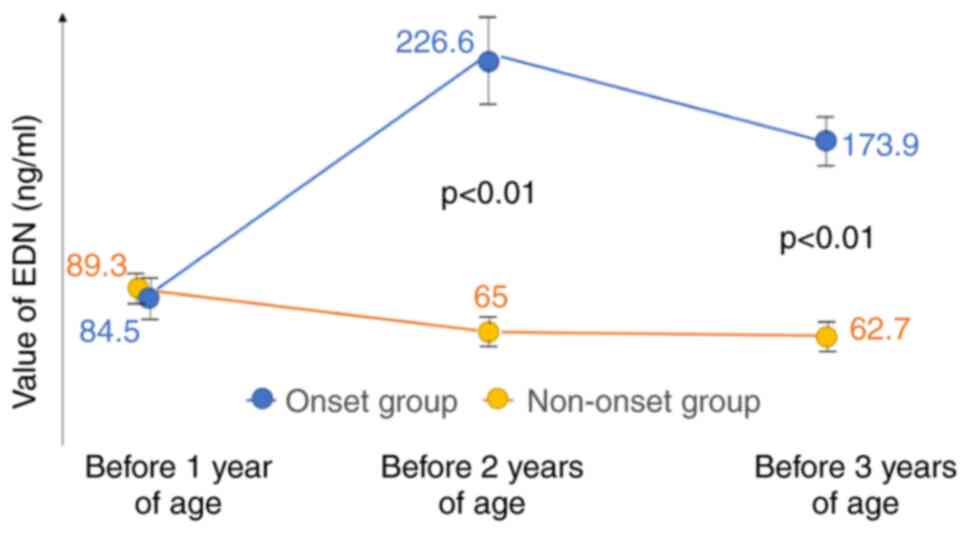
In Fig. 4, subjects
were divided into those who had developed an airway allergic
disease, asthma or allergic rhinitis (Onset Group) and those who
did not develop any airway allergic disease (Non-onset Group) by
the age of 3. The EDN value of each group was measured at 3
time-points: Before 1 year of age, before 2 years of age and before
3 years of age. Before 1 year of age, the EDN values in each group
were similar (84.5 vs. 89.3 ng/ml; Onset Group vs. Non-onset
Group). However, before 2 years of age, the EDN values exhibited a
marked divergence (226.6 vs. 65.0 ng/ml; P<0.01) and remained
divergent at the 3rd time-point (before 3 years of age) (173.9 vs.
62.7 ng/ml; P<0.01).
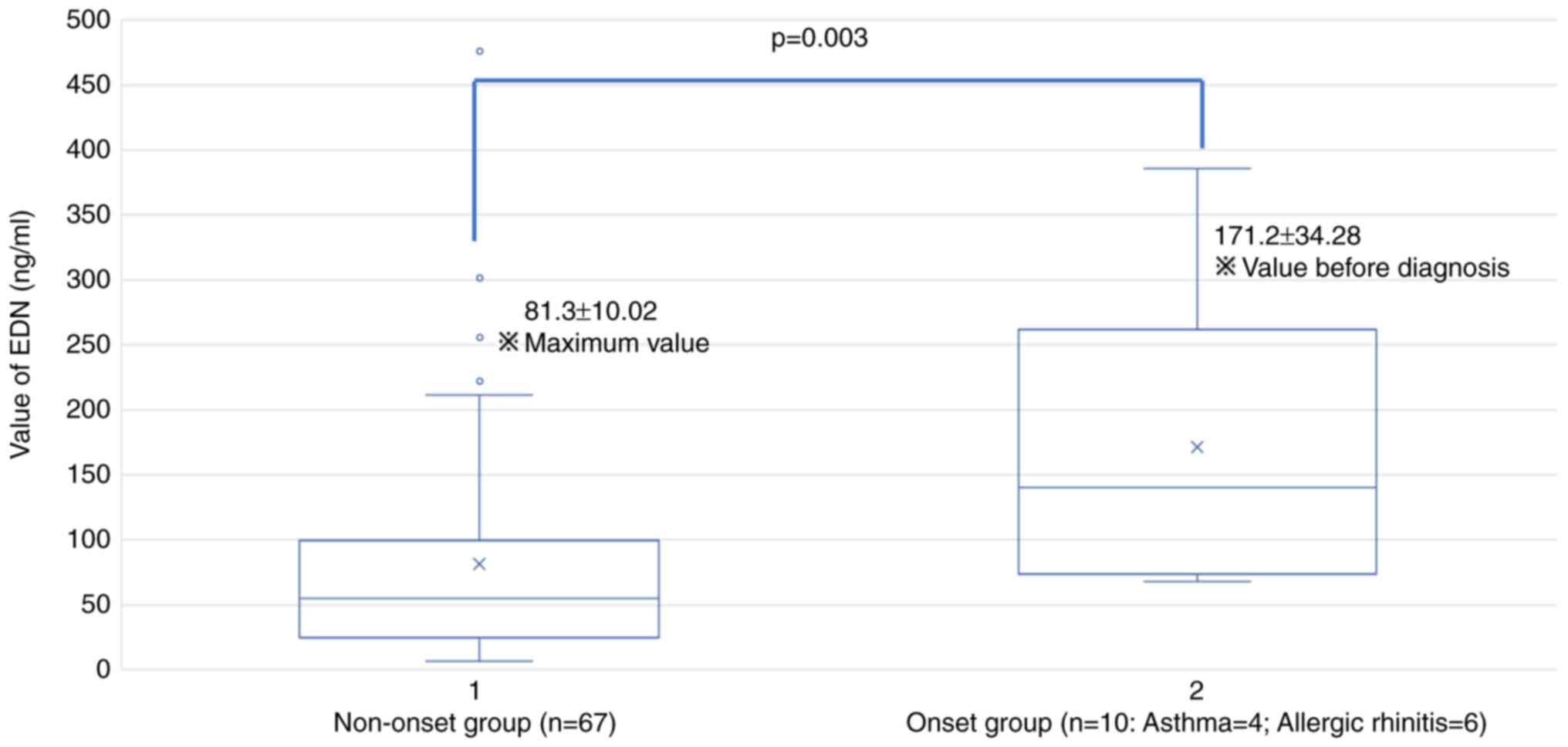
In Fig. 5, EDN
values before diagnosis were compared between the two groups: Onset
and Non-onset. The EDN levels in the Onset Group were much greater
than those in the Non-onset Group (171.2±34.28 vs. 81.3±10.02
ng/ml; P=0.003). The Onset Group consisted of 4 subjects with
asthma and 6 with AR.
Looking at the two groups [Onset (n=10) and
Non-onset (n=67)], EDN levels were compared twice: i) Before 1 and
2 years of age; and ii) before 1 and 3 years of age. There was no
significant difference in the first comparison but there was a
significant difference in the second (P=0.001) (data not
shown).
When EDN levels in subjects grouped according to
initial symptoms were compared [AD (n=22) vs. FA (n=15) vs. AD+FA
(n=37)], there were no significant differences (data not
shown).
Discussion
To date, only a small number of studies using EDN
levels as a predictor of airway allergic disease in preschool
children have been published (6,9,11).
In the present study, it was found that young children with
elevated EDN levels were more likely to develop allergic disease in
their first three years of life. Over the last decades, it has been
demonstrated that the presence of one allergic condition may
increase the risk for the development of others (1,2,15,16).
This ‘march’ typically begins with AD and then progresses to
IgE-mediated FA, asthma and AR. It is therefore highly beneficial
to determine factors that may increase the likelihood of allergic
disease development. One of these factors may be elevated
eosinophil activity.
Allergies may develop at a young age. In the present
study, an inclusion criterium was the presence of a food allergen,
so all subjects were sensitive to at least one type of food. The
other allergen groups (i.e., pollen, environmental, animal and
fungal) were much less common, with only pollen allergens exceeding
a 50% cumulative sensitization rate by the age of 3 years. There
may also be a difference between individuals sensitive to only one
food (termed ‘monosensitization’) and those sensitive to two or
more food types (termed ‘polysensitization’). A study of Korean
children found that risk factors for polysensitization included
parental history of allergic diseases (i.e., AD), birth season
(i.e., summer/fall) and exclusive breastfeeding in the first 6
months of life (17). Allergic
sensitization may have an important synergistic role in the atopic
march. A Canadian longitudinal study investigated whether allergic
sensitization enhances associations between atopic dermatitis in
infancy with subsequent allergic diseases, including asthma, FA and
AD (15). They found that AD
without concomitant allergic sensitization was not associated with
any increased risk of asthma at 3 years of age, but AD with
allergic sensitization increased the asthma risk >7-fold. They
also found that AD and allergic sensitization increased the risk of
food allergy development.
In the patients of the present study with household
pets, animal allergy was much more likely to develop than in
patients without pets. The role of early exposure to pets in the
development of atopy remains controversial, with studies producing
conflicting results (18-21).
The amount of allergen exposure may be a possible explanation for
this, as evidence exists that exposure to high levels of pet
allergens (i.e., more pets) may decrease a child's risk of
sensitization by inducing immune tolerance. Studies have
demonstrated an inverse dose-response association between
early-life pet exposure and risk of asthma and allergy (22,23).
Before 1 year of age, two groups of the present
study (Onset and Non-onset) exhibited statistically similar EDN
levels. However, as the patients aged, EDN levels exhibited a
marked divergence before 2 years of age and maintained this
difference up until 3 years of age, which was the endpoint of the
present study. Elevated EDN levels appear to be linked to onset of
airway allergic disease. It should also be noted that children
exhibited increased EDN levels before the onset of airway allergic
disease. Elevated EDN levels are a sign of eosinophil activity
(i.e., activation and degranulation).
Typically, patients under the age of 6 years cannot
fully participate in traditional measures of lung function,
therefore making airway disease diagnosis difficult. A delay
between elevation of immune cells, e.g. eosinophils, and onset of
lung dysfunction is frequently observed (24). If treatment decisions are based
solely on lung function, this may lead to a delay in care and
unnecessary morbidity in the patient. Biomarkers are measurable
indicators that link an underlying pathophysiological pathway to a
disease (25,26). Finding a reliable and accurate
biomarker for inflammatory disease, such as asthma, AD and FA, may
be an invaluable tool for diagnosis, treatment and monitoring. One
such possible tool is EDN, an eosinophil granule protein released
almost exclusively by eosinophils (4). Therefore, EDN levels would directly
correspond with eosinophil activity. Several studies have
demonstrated the efficacy of EDN levels as a biomarker for
eosinophilic inflammation (9,6-11,27-30),
highlighting its ability to represent the secretory activity of
eosinophils, a combination of the concentration of eosinophils and
their tendency to release degranulation products. Measuring EDN is
quick, simple and accurate with the recently developed K-EDN ELISA
kit. EDN is more easily recovered from serum specimens than other
eosinophil granule proteins, such as ECP, and may be recovered from
numerous different specimen types, including sputum, saliva, nasal
and bronchoalveolar lavage, serum, plasma and urine (5,29).
Putting into practice EDN as a diagnostic tool has
led to promising results. A study by Kim et al (8) found that EDN levels had predictive
value for asthma [sensitivity, 81.3%; specificity, 87.1%; positive
predictive value (PPV), 90.7%; negative predictive value (NPV),
75.0%]. A more recent study by Amer et al (31) also demonstrated the predictive
value of EDN for asthma [sensitivity, 100%; specificity, 64.7%;
PPV, 91.9%; NPV, 100%] and a strong correlation with severity.
Among the patients of the present study, 10 eventually developed
allergic disease (4 with asthma and 6 with allergic rhinitis). In
this group, EDN levels were significantly higher at ‘prior to 2
years of age’ and ‘prior to 3 years of age’ when compared to the
group with no onset of allergic disease. EDN has also been used as
a predictive biomarker for recurrent wheezing development after RSV
bronchiolitis, a common airway infection in young children. Using
53 ng/ml as a cutoff value, EDN levels at 3 months after RSV
infection had a PPV of 57%, NPV of 76%, sensitivity of 72% and
specificity of 62% for recurrent wheezing (11).
The present study highlights the utility of EDN as a
biomarker for allergic disease development, helping clinicians
diagnose, treat and monitor underlying eosinophilic inflammation.
EDN levels allow the clinician to stratify patients according to
treatable eosinophilic inflammation (32) and have the advantage of being
measurable in several biological fluids, even in young children.
More studies are needed looking at the potential link between
elevated EDN levels and airway allergic disease in young
children.
Acknowledgements
The abstract was presented at the 2023 AAAAI Annual
Meeting on 2/25/2023 in San Antonio, USA and published as abstract
no. 316691/DOI: https://doi.org/10.1016/j.jaci.2022.12.783 in The
Journal of Allergy and Clinical Immunology Volume 151, Issue 2,
Supplement, AB336.
Funding
Funding: Funding for MAST costs and data analysis were provided
by Showa Denko Materials Co., Ltd.
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YT, CKK and SY designed the study. YT, CKK and SY
checked and confirmed the authenticity of the raw data. YT, CKK,
ZC, JP, ShinY, MK and ShigY collected, analyzed and interpreted the
data. YT, CKK, ZC and SY wrote the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All legal guardians of patients provided written
informed consent before participating in this study and this study
was approved by the Research Ethics Committee of Dokkyo Medical
University Hospital (approval no. 26096).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hill DA and Spergel JM: The atopic march:
Critical evidence and clinical relevance. Ann Allergy Asthma
Immunol. 120:131–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hill DA, Grundmeier RW, Ram G and Spergel
JM: The epidemiologic characteristics of healthcare
provider-diagnosed eczema, asthma, allergic rhinitis, and food
allergy in children: A retrospective cohort study. BMC Pediatr.
16(133)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Renz H, Allen KJ, Sicherer SH, Sampson HA,
Lack G, Beyer K and Oettgen HC: Food allergy. Nat Rev Dis Primers.
4(17098)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hogan SP, Rosenberg HF, Moqbel R, Phipps
S, Foster PS, Lacy P, Kay AB and Rothenberg ME: Eosinophils:
Biological properties and role in health and disease. Clin Exp
Allergy. 38:709–750. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Venge P: Monitoring the allergic
inflammation. Allergy. 59:26–32. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim H, Kwon GE, Kim YH, Callaway Z, Han
YS, Seo JJ, Jiao F and Kim CK: Comparison of serum
eosinophil-derived neurotoxin levels between wheezing and
non-wheezing groups in children with respiratory tract infection. J
Asthma. 57:1211–1215. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim CK, Callaway Z, Park JS, Nishimori H,
Ogino T, Nagao M and Fujisawa T: Montelukast reduces serum levels
of eosinophil-derived neurotoxin in preschool asthma. Allergy
Asthma Immunol Res. 10:686–697. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim CK, Callaway Z, Park JS and Kwon E:
Utility of serum eosinophil-derived neurotoxin (EDN) measurement by
ELISA in young children with asthma. Allergol Int. 66:70–74.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim CK, Callaway Z, Kim DW and Kita H:
Eosinophil degranulation is more important than eosinophilia in
identifying asthma in chronic cough. J Asthma. 48:994–1000.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim CK, Callaway Z, Fletcher R and Koh YY:
Eosinophil-derived neurotoxin in childhood asthma: correlation with
disease severity. J Asthma. 47:568–573. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim CK, Seo JK, Ban SH, Fujisawa T, Kim DW
and Callaway Z: Eosinophil-derived neurotoxin levels at 3 months
post-respiratory syncytial virus bronchiolitis are a predictive
biomarker of recurrent wheezing. Biomarkers. 18:230–235.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Peterson CG, Enander I, Nystrand J,
Anderson AS, Nilsson L and Venge P: Radioimmunoassay of human
eosinophil cationic protein (ECP) by an improved method.
Establishment of normal levels in serum and turnover in vivo. Clin
Exp Allergy. 21:561–567. 1991.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Murphy KP: The detection, measurement, and
characterization of antibodies, and their use as research and
diagnostic tools. Janeway's immunobiology. 8th edition. Garland
Science, New York, NY, 2012.
|
|
14
|
Morioka J, Kurosawa M, Inamura H, Nakagami
R, Mizushima Y, Chihara J, Yokoseki T, Kitamura S, Omura Y and
Shibata M: Development of a novel enzyme-linked immunosorbent assay
for blood and urinary eosinophil-derived neurotoxin: A preliminary
study in patients with bronchial asthma. Int Arch Allergy Immunol.
122:49–57. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tran MM, Lefebvre DL, Dharma C, Dai D, Lou
WYW, Subbarao P, Becker AB, Mandhane PJ, Turvey SE and Sears MR:
Canadian Healthy Infant Longitudinal Development Study
Investigators. Predicting the atopic march: Results from the
Canadian health infant longitudinal development study. J Allergy
Clin Immunol. 141:601–607. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schenider L, Hanifin J, Boguniewicz M,
Eichenfield LF, Spergel JM, Dakovic R and Paller AS: Study of the
atopic march: development of atopic comorbidities. Pediatr
Dermatol. 33:388–398. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim NY, Kim GR, Kim JH, Baek JH, Yoon JW,
Jee HM, Baek HS, Jung YH, Choi SH, Kim KE, et al: Food allergen
sensitization in young children with typical signs and symptoms of
immediate-type food allergies: A comparison between monosensitized
and polysensitized children. Korean J Pediatr. 58:330–335.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
de Moira AP, Strandberg-Larsen K, Bishop
T, Pedersen M, Avraam D, Cadman T, Calas L, Casas M, Guillain BL,
et al: Associations of early-life pet ownership with asthma and
allergic sensitization: A meta-analysis of more than 77,000
children from the EU Chil Cohort Network. J Allergy Clin Immunol.
150:82–92. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Anyo G, Brunekreef de Meer G, Aarts F,
Janssen NA and van Vliet P: Early, current and past pet ownership:
Associations with sensitization, bronchial responsiveness and
allergic symtpoms in school children. Clin Exp Allergy. 32:361–366.
2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lødrop Carlsen KC, Roll S, Carlsen KH,
Mowinckel P, Wijga AH, Brunekreef B, Torrent M, Roberts G, Arshad
SH, Kull I, et al: Does pet ownership in infancy lead to asthma or
allergy at school age? Poole analysis of individual participant
data from 11 European birth cohorts. PLoS One.
7(e43214)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fall T, Lundholm C, Ortquvist AK, Fall K,
Fang F, Hedhammar A, Kämpe O, Ilgelsson E and Almqvist C: Early
exposure to dogs and farm animals and the risk of childhood asthma.
JAMA Pediatr. 169(e153219)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hesselmar B, Hicke-Roberts A, Lundell AC,
Adlerberth I, Rudin A, Saalman R, Wennergren G and Wold AE:
Pet-keeping in early life reduces the risk of allergy in a
dose-dependent fashion. PLoS One. 13(e0208472)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ownby DR, Johnson CC and Peterson EL:
Exposure to dogs and cats in the first year of life and risk of
allergic sensitization at 6 to 7 years of age. JAMA. 288:963–972.
2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jatakanon A, Lim S and Barnes PJ: Changes
in sputum eosinophils predict loss of asthma control. Am J Respir
Crit Care Med. 161:64–72. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Agache I and Rogozea L: Asthma biomarkers:
Do they bring precision medicine closer to the clinic? Allergy
Asthma Immunol Res. 9:466–476. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Diamant Z, Vijverberg S, Alving K,
Bakirtas A, Bjermer L, Custovic A, Dahlen SE, Gaga M, van Wijk RG,
Del Giacco S, et al: Toward clinically applicable biomarkers for
asthma: an EAACI position paper. Allergy. 74:1835–1851.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim KW, Lee KE, Kim ES, Song TW, Sohn MH
and Kim KE: Serum eosinophil-derived neurotoxin (EDN) in diagnosis
and evaluation of severity and bronchial hyperresponsiveness in
childhood asthma. Lung. 185:97–103. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Taniuchi S, Chihara J, Kojima T, Yamamoto
A, Sasai M and Kobayashi Y: Serum eosinohil derived neurotoxin may
reflect more strongly disease severity in childhood atopic
dermatitis than eosinophil cationic protein. J Dermatol Sci.
26:79–82. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Goto T, Morioka J, Inamura H, Yano M,
Kodaira K, Igarashi Y, Abe S and Kurosawa M: Urinary
eosinophil-derived neurotoxin concentrations in patients with
atopic dermatitis: A useful clinical marker for disease activity.
Allergol Int. 56:433–438. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim CK, Kita H, Callaway Z, Kim HB, Choi
J, Fujisawa T, Shin BM and Koh YY: The roles of a Th2 cytokine and
CC chemokine in children with stable asthma: Potential implication
in eosinphil degranulation. Pediatr Allergy Immunol. 21:e697–e704.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Amer OT, Naguib MS, Allam AA and Fouad
EMI: Evaluation of serum eosinophil-derived neurotoxin level in
children with bronchial asthma and its relation to disease
severity. Egypt J Hosp Med. 80:951–957. 2020.
|
|
32
|
Malinovschi A, Rydell N, Fujisawa T,
Borres MP and Kim CK: Clinical potential of eosinophil-derived
neurotoxin in asthma management. J Allergy Clin Immunol Pract.
11:750–761. 2023.PubMed/NCBI View Article : Google Scholar
|