Introduction
Type 2 diabetes mellitus (T2DM) is a severe public
health issue, and its prevalence is gradually increasing (1). Reports indicate that 629 million
individuals will have T2DM by 2045, up from the current predicted
425 million sufferers (2). As T2DM
and its complications are associated with very high rates of
disability and mortality (3),
researchers are committed to exploring safe and effective
therapeutics to prevent and treat it. Diabetic nephropathy (DN,
also known as diabetic kidney disease) is one of the most common
chronic and destructive complications of diabetes. Approximately
20-40% of T2DM patients will develop DN (4), and this complication is most
prevalent in developed countries (5). The pathological features of DN
include proteinuria, glomerular hypertrophy, basement membrane
thickening, podocytopenia, extracellular matrix protein deposition,
and renal fibrosis. In particular, renal fibrosis is the primary
pathological manifestation of renal injury (6,7). It
is hypothesized that DN is the primary cause of death in diabetes
patients as it can eventually progress to chronic renal failure
(8). Therefore, timely and
effective treatment for DN is of significant importance.
Currently, treatment goals for DN primarily include
blood glucose control, reduction of hypertension, lipid control,
and inhibition of renal fibrosis (9). First-line angiotensin-converting
enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs)
are drugs commonly used in clinical practice (10). However, the discontinuation of
ACEIs and ARBs in patients with advanced chronic kidney disease
(CKD) has been associated with a slowing or decreased estimated
glomerular filtration rate (eGFR) (11). Therefore, alternative drugs have
been successively developed in clinical practice, such as
empagliflozin [a sodium-glucose cotransporter 2 (SGLT2) inhibitor],
and fasudil [a Rho-associated coiled-coil-containing protein kinase
(ROCK) inhibitor]. However, they also have significant side
effects. For example, empagliflozin is unable to control blood
glucose by regulating insulin (12), and although Fasudil can protect the
kidneys from DN, it does not lower blood pressure (13). Poursharif et al (14) discovered that SGLT2 inhibitors
improved renal function by slowing eGFR in patients with early and
advanced DN. Several studies have shown that Dapagliflozin (a SGLT2
inhibitor) is a novel class of diabetes drugs and a clinically
recognized therapeutic agent for DN (15-17).
It can effectively reduce renal injury caused by DN and inhibit
renal fibrosis. According to the Dapagliflozin and Prevention of
Adverse Outcomes in CKD (DAPA-CKD) trial, Dapagliflozin lowered the
risk of hospitalization for any reason (including heart disease,
renal and urinary disorders, metabolic and nutritional disorders,
and neoplasms) in CKD patients with or without type 2 diabetes
(18,19). The Empagliflozin Cardiovascular
Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG
OUTCOME) trial results showed that Dapagliflozin significantly
reduced the risk of cardiovascular (CV)-associated death in adults
with T2DM and established CV disease compared with placebo
(20). Thus, Dapagliflozin has a
promising treatment option for DN. However, the mechanism of action
of Dapagliflozin in the treatment of DN is still not fully
elucidated, and additional scientific investigation is needed to
improve our understanding.
Epithelial/endothelial mesenchymal transition
(EMT/EndMT) is an important pathogenic mechanism that occurs during
renal fibrosis (21-23).
EndMT is considered to be a special type of EMT that occurs during
the transition of glomerular endothelial cells to mesenchymal cells
(24). It is involved in tissue
wound repair and remodeling under physiological conditions while
promoting renal fibrosis under pathological conditions (25,26).
In animal models of DN, multiple signaling pathways such as the
TGF-β signaling pathway, Wnt signaling pathway, Hedgehog signaling
pathway, Fibroblast growth factor receptors (FGFRs) 1 signaling
pathway, and Sirtuin3 (SIRT3) signaling pathway are implicated in
the regulation of EndMT during renal fibrosis (27). In addition, inhibiting the
expression of proinflammatory factors, including tumor necrosis
factor-α (TNF-α) and interleukin (IL)-6 and IL-1β, in DN rats can
slow down the progression of nephropathy (28). Among these numerous related
pathological mechanisms, the present study focuses attention on the
association between transforming growth factor-β1 (TGF-β1)/SMAD
homolog (SMAD)3 signaling pathway and EMT, and whether
Dapagliflozin plays a role in the treatment of DN through this
mechanism.
TGF-β1 is a multifunctional regulatory polypeptide
that can control several cell activities, including cell
proliferation, differentiation, and apoptosis (29). Additionally, it plays an important
regulatory role in the development of DN (30). Studies have shown that Smad7
inhibits the TGF-β/Smad-mediated renal fibrosis signaling pathway
by blocking the activation of Smad2/3. The TGF-β/Smad pathway
participates in the pathogenesis of renal fibrosis (31). The TGF-β/Smad3 signal pathway has
been shown to be highly activated in DN (32). Li et al (33) found that high concentrations of
glucose in cell culture media activated the TGF-β/Smad pathway.
Inhibition of the TGF-β1/Smad signaling pathway can reduce renal
fibrosis in diabetic rats (34).
Numerous studies have also demonstrated that reducing EMT by
inhibiting the TGF-1/Smad pathway can significantly reduce renal
fibrosis and treat DN (35-37).
However, there are no reports on the contribution of
the TGF-β/Smad signaling pathway in the involvement of
Dapagliflozin treatment of renal fibrosis in T2DM rats. In
addition, since the efficacy of metformin in the treatment of T2DM
is well established, several relevant studies have used it as a
positive control drug when empirically researching the efficacy of
drugs. Therefore, metformin is also used as the positive control
drug in the present study. Therefore, when designing the research
scheme of the present study, the effect of the combined use of
Dapagliflozin and metformin on renal fibrosis in T2DM rats was not
assessed. Streptozotocin (STZ) was used to induce T2DM in the rat
model, in order to explore the mechanism of Dapagliflozin on renal
fibrosis in T2DM rats and provide a potentially novel direction for
the development of therapeutics for the treatment of DN.
Materials and methods
Experimental animals
A total of 24 healthy SPF-grade male SD rats,
weighing 180-220 g, were provided by the Experimental Animal Center
of Shanghai Changzheng Hospital. They were reared in an environment
with a relative humidity of 60%, a temperature of 22˚C, and a 12 h
light/dark cycle, with ad libitum access to water and food.
Experiments were performed 1 week after acclimation. The present
study was approved by the Shanghai Changzheng Hospital Animal
Ethics Committee.
Grouping and drug intervention
A total of 24 rats were randomly divided into a
normal (Control) group, Model group (STZ-induced T2DM rats), a
Dapagliflozin (Dapa) group, and a metformin (Met) group, with 6
rats in each group. Rats in the control group were fed a normal
diet, while those in the other groups were fed a high-glucose,
high-fat diet for 4 weeks (28).
After 4 weeks, the rats in each group were fasted for 12-16 h, and
the Control group was intraperitoneally injected with the same
amount of sodium citrate buffer. The other groups of rats were
intraperitoneally injected with 35 mg/kg streptozotocin (STZ;
Dalian Meilun Biology Technology Co., Ltd.). A week after STZ
induction, the fasting blood glucose (FBG) of rats was >16.7
mmol/l, and the 24-h urine output was >150% of normal,
suggesting that T2DM rat models were successfully constructed
(19). After successful modeling,
the Control and Model groups were given an equal amount of normal
saline; the Dapa group was given 1 mg/kg Dapagliflozin
(Dapagliflozin, AstraZeneca) (38,39)
once a day for 4 weeks (40); the
Met group was administered 200 mg/kg metformin (Squibb) once a day
for 4 weeks (40). The FBG levels
of these rats were tested once every week. Rats were anesthetized
by intraperitoneal injection of sodium pentobarbital (35 mg/kg)
after 4 weeks and peripheral blood was collected. The rats were
subsequently sacrificed by cervical dislocation, and kidney tissues
were removed for subsequent analysis.
Biochemical detection
Rat peripheral blood was taken, and the supernatant
was collected after centrifugation at 1250 x g, at 4˚C for 10 min.
The blood urea nitrogen (BUN), serum creatinine (SCr), 24 h urine
protein level, and glycosylated hemoglobin (HbA1c) were detected
using corresponding assay kits (Dalian Meilun Biology Technology
Co., Ltd.). Total cholesterol (TC; cat. no. msw E2142),
triglyceride (TG; cat. no. mlsw E2170), high-density lipoprotein
cholesterol (HDL-c; cat. no. mlsw E2457) and low-density
lipoprotein cholesterol (LDL-c; cat. no. msw E2171) in serum were
measured to assess lipid levels in each group of rats according to
the manufacturer's instructions (Dalian Meilun Biology Technology
Co., Ltd.). Additionally, 24-h urinary albumin and creatinine
excretion levels were measured, and albumin-to-creatinine ratios
were calculated using rat urinary albumin (cat. no. QC12245;
Shanghai Qincheng Biotechnology Co., Ltd.) and urinary creatinine
(cat. no. BY-PD6160S; Shanghai Baiyi Biotechnology Co., Ltd.) ELISA
kits. In addition, the serum levels of insulin (RC-R17363T, DRG
International) and TGF-β1 (mlsw E2400, Dalian Meilun Biology
Technology Co., Ltd.) were measured using rat-specific ELISA
kits.
Renal index tests
After the rat kidneys were separated and the water
was wiped dry, the kidneys were weighed. The renal index was
calculated as follows: Renal index=kidney weight/body weight.
Hematoxylin and eosin (H&E)
staining and periodic acid-Schiff (PAS) staining
The renal tissue was fixed in 4% paraformaldehyde
solution overnight at room temperature, dehydrated with an
increasing gradient of alcohol solutions, and embedded in a
paraffin block. The embedded tissue was cut into sections with a
thickness of 3 µm using a microtome. The sections were
deparaffinized with xylene, stained with H&E (Mexin), then
mounted with neutral resin, observed, and imaged under an optical
microscope (x100 and x200 magnification; Olympus Coporation). As
for PAS staining, the sections were stained to assess glomerular
mesangial expansion. Briefly, the paraffin sections were incubated
with 0.5% periodic acid for 5 min, rinsed with distilled water,
incubated with Schiff reagent for 15 min at room temperature,
washed in tap water for 5 min, counterstained with hematoxylin at
room temperature for 1 min, mounted using neutral resin, observed,
and imaged under an optical microscope (x100 and x200
magnification; Olympus Corporation). Image Pro Plus version 6.0
(Media Cybernetics, Inc.) was used to quantify the positively
stained area of the glomerular.
Masson staining
Paraffin-coated renal tissue sections were
deparaffinized and rehydrated with 100, 95, and 70% ethanol
solutions, Next, they were stained with Regaud's hematoxylin at
room temperature for 1 min and rinsed thoroughly with distilled
water. Subsequently, the sections were stained in Masson's acid
solution at room temperature (Fuzhou Maixin Biotech Co., Ltd.) for
10 min, washed with 2% glacial acetic acid, differentiated with 1%
molybdenum phosphate at room temperature for 5 min, and then
directly transferred to aniline blue solution (Fuzhou Maixin
Biotech Co., Ltd.) at room temperature for 5 min. Subsequently, the
sections were briefly rinsed in distilled water and differentiated
in 0.2% glacial acetic acid. In the post-staining step, they were
dehydrated with 95 and 100% ethanol solutions, washed with xylene,
and fixed with neutral resin at room temperature for 5 min.
Finally, the sections were observed and imaged under an optical
microscope (x100 and x200 magnification; Olympus Corporation).
Western blotting
The rat renal tissues were lysed using RIPA Lysis
Solution (Beyotime Institute of Biotechnology), centrifuged at 4˚C
at 13,400 x g for 15 min, and the supernatant was collected. BCA
protein assay reagent (Thermo Fisher Scientific, Inc.) was used to
determine the total protein concentration. The proteins (30
µg/lane) were loaded on a 10% SDS gel, resolved by SDS-PAGE, and
transferred to PVDF membranes. Next, membranes were blocked in
skimmed milk for 1 h at room temperature, the membranes were
incubated overnight at 4˚C with the primary antibodies which
include: Anti-α smooth muscle Actin antibody (SMA; cat. no.
ab124964, 1:1,000; Abcam), anti-Vimentin antibody (cat. no.
ab92547; 1:1,000; Abcam), anti-E Cadherin antibody (cat. no.
ab231303; 1:1,000; Abcam), anti-TGF-β1 antibody (cat. no. ab215715;
1:1,000; Abcam), anti-MADH7/SMAD7 antibody (cat. no. ab216428;
1:1,000; Abcam), anti-phospho (p)-Smad3 antibody (cat. no. ab63403;
1:1,000; Abcam), and anti-β actin antibody (cat. no. ab115777;
1:5,000; Abcam). Subsequently, the membranes were washed three
times with TBST, and then incubated with the secondary antibodies:
Goat anti-mouse IgG H&L (HRP) (cat. no. ab205719; 1:5,000;
Abcam) or goat anti-rabbit IgG H&L (HRP) (cat. no. ab205718;
1:5,000; Abcam) at room temperature for 1 h. Signals were
visualized using a chemiluminescent solution, and the proteins were
imaged in the exposure apparatus (41).
Statistical analysis
Data are presented as the mean ± SD. SPSS version
21.0 (IBM Corp.) was used for data analysis. Differences between
multiple groups were analyzed using a one-way ANOVA followed by a
post hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Dapagliflozin improves renal
functional impairment in T2DM rats
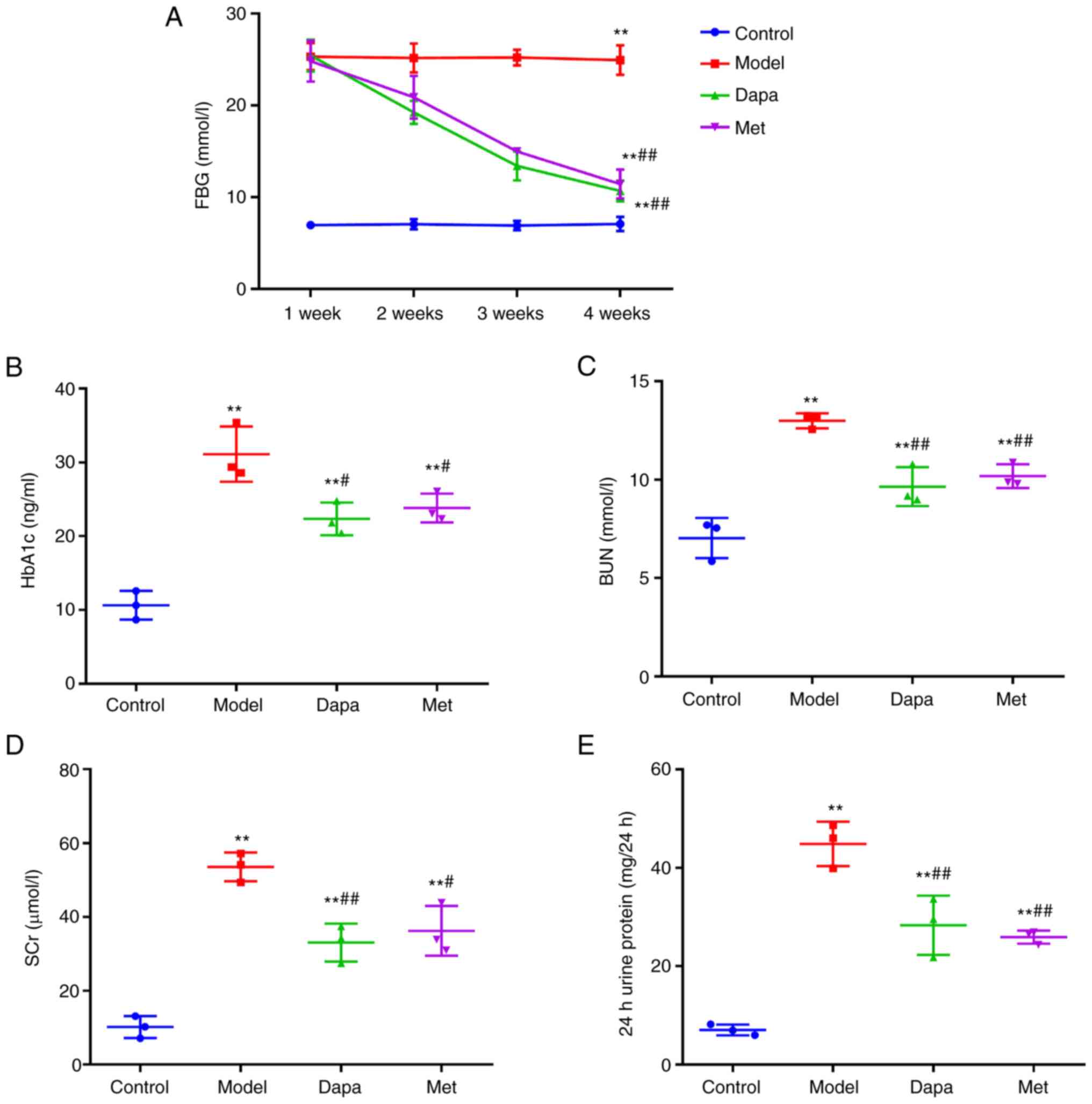
Firstly, the renal function indices of rats were
tested to determine the effect of Dapagliflozin on the kidneys of
T2DM rats. The results showed that during the 4 weeks of
intragastric administration, the FBG levels in the Control group of
rats were maintained at a normal level of ~7 mmol/l, while that in
the Model group was >16.7 mmol/l (remaining in the high glucose
range). Starting at 2 week, the FBG levels of the Dapa group and
the Met group gradually decreased with the duration of medication,
but the FBG levels of the Dapa group were lower than that of the
Met group (Fig. 1A). Following 4
weeks of intragastric administration, the levels of HbA1c, BUN,
SCr, and 24 h urine protein in the Model group were significantly
higher than those in the Control group; while Dapagliflozin and
metformin significantly reduced HbA1c, BUN, SCr, and 24 h urine
protein levels in rats after STZ induction (Fig. 1B-E). These results indicate that
Dapagliflozin has a protective effect on the kidneys of T2DM
rats.
Dapagliflozin decreases lipid levels
and increases insulin levels in T2DM rats
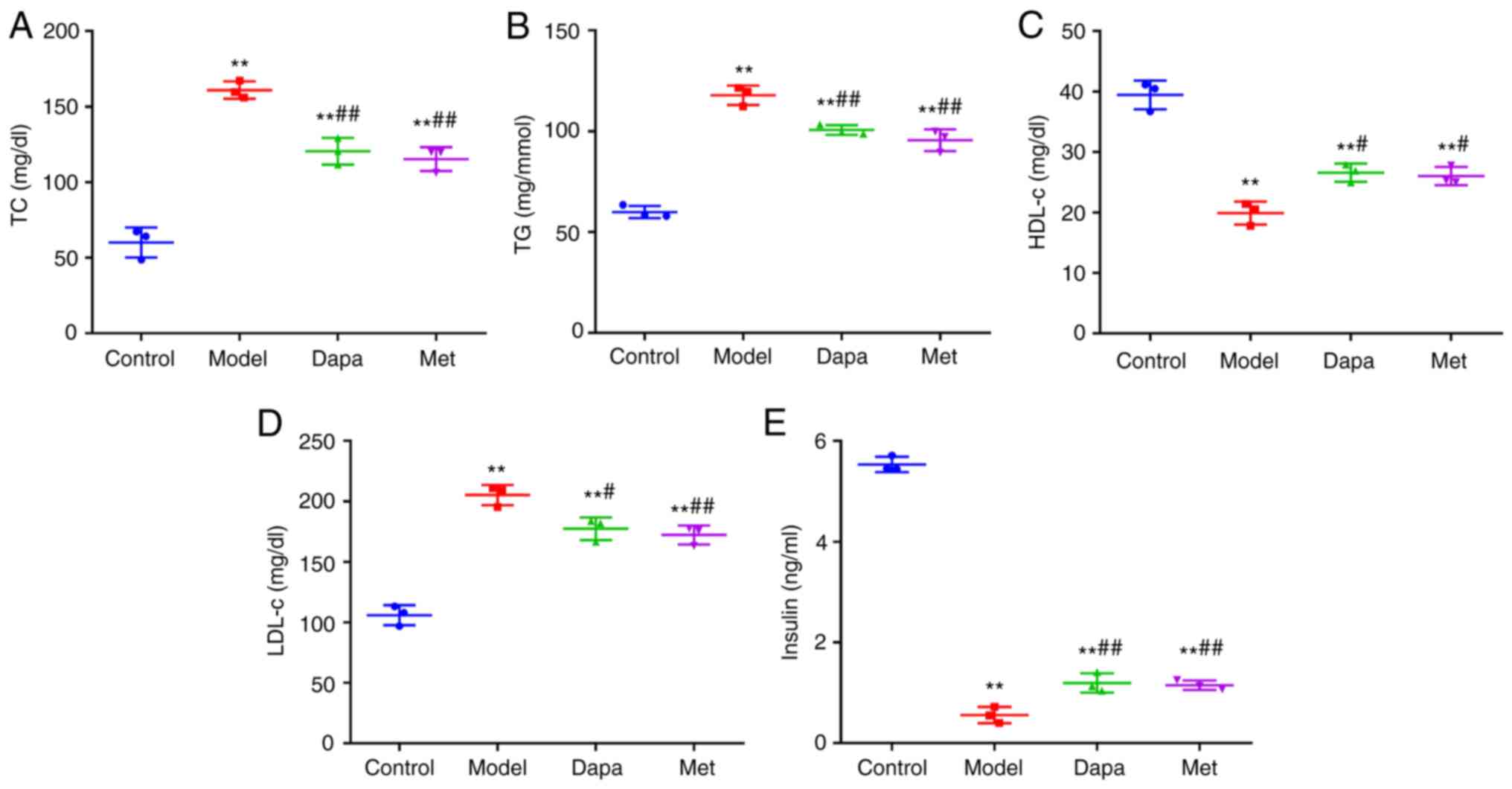
Subsequently, the effects of Dapagliflozin on lipid
and insulin levels in T2DM rats. The results showed that the serum
TC, TG, and LDL-c levels were significantly increased, and the
levels of HDL-c and insulin were markedly decreased in STZ-induced
T2DM rats compared with the Control group. Compared with the Model
group, treatment with Dapagliflozin and metformin significantly
lowered the serum levels of TC, TG, and LDL-c and elevated the
levels of HDL-c and insulin in T2DM rats (Fig. 2A-E). Accordingly, Dapagliflozin
decreased the lipid levels while increasing insulin levels in T2DM
rats.
Dapagliflozin improves renal tissue
damage in T2DM rats
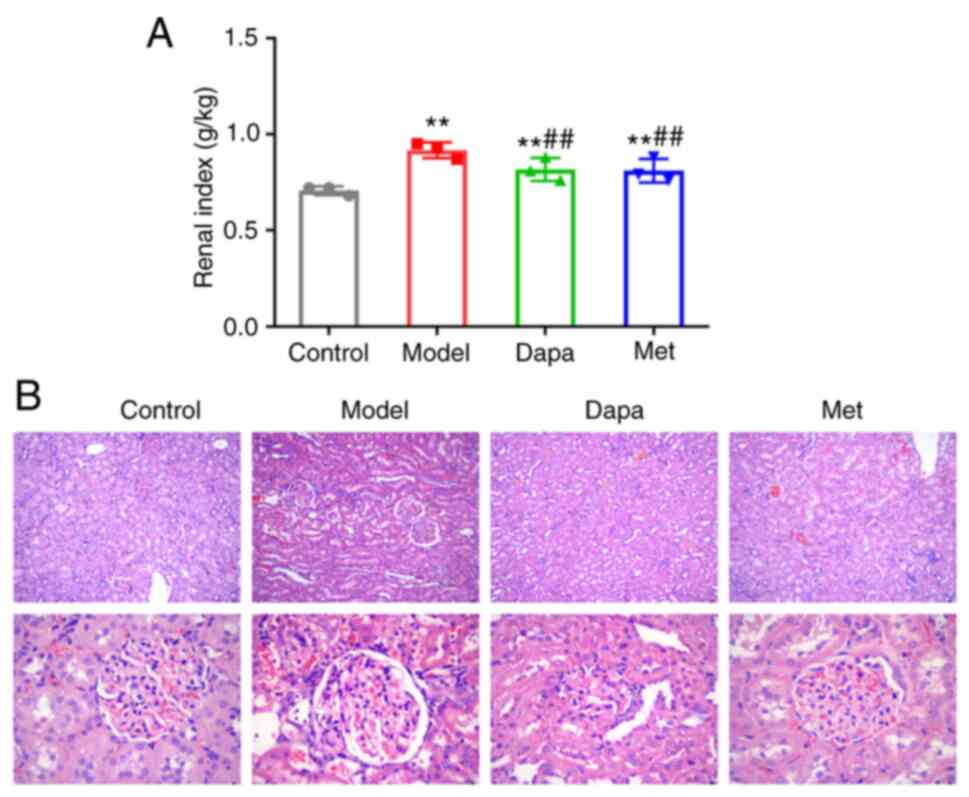
Next, the effect of Dapagliflozin on the morphology
and structure of the renal tissue in diabetic rats was observed.
The Model group showed a notably higher renal index of rats than
the Control group; the Dapa and Met groups displayed a much lower
renal index than the Model group (Fig.
3A). According to the H&E staining results, the renal
tissues of the Control group did not exhibit any significant
pathological changes, and the histology of the glomeruli and renal
tubules was normal. The renal tissues in the Model group showed
degeneration and fibrosis, detached renal tubular epithelial cells,
edema, glomerular mesangial cell proliferation, glomerular basement
membrane thickening, renal tubular dilatation, and atrophy. In the
Dapa and Met groups, the extent of renal disease was reduced, and
the proliferation of glomerular mesangial cells was reduced
(Fig. 3B). Thus, Dapagliflozin may
reduce renal tissue damage in diabetic rats.
Dapagliflozin reduces renal fibrosis
in T2DM rats
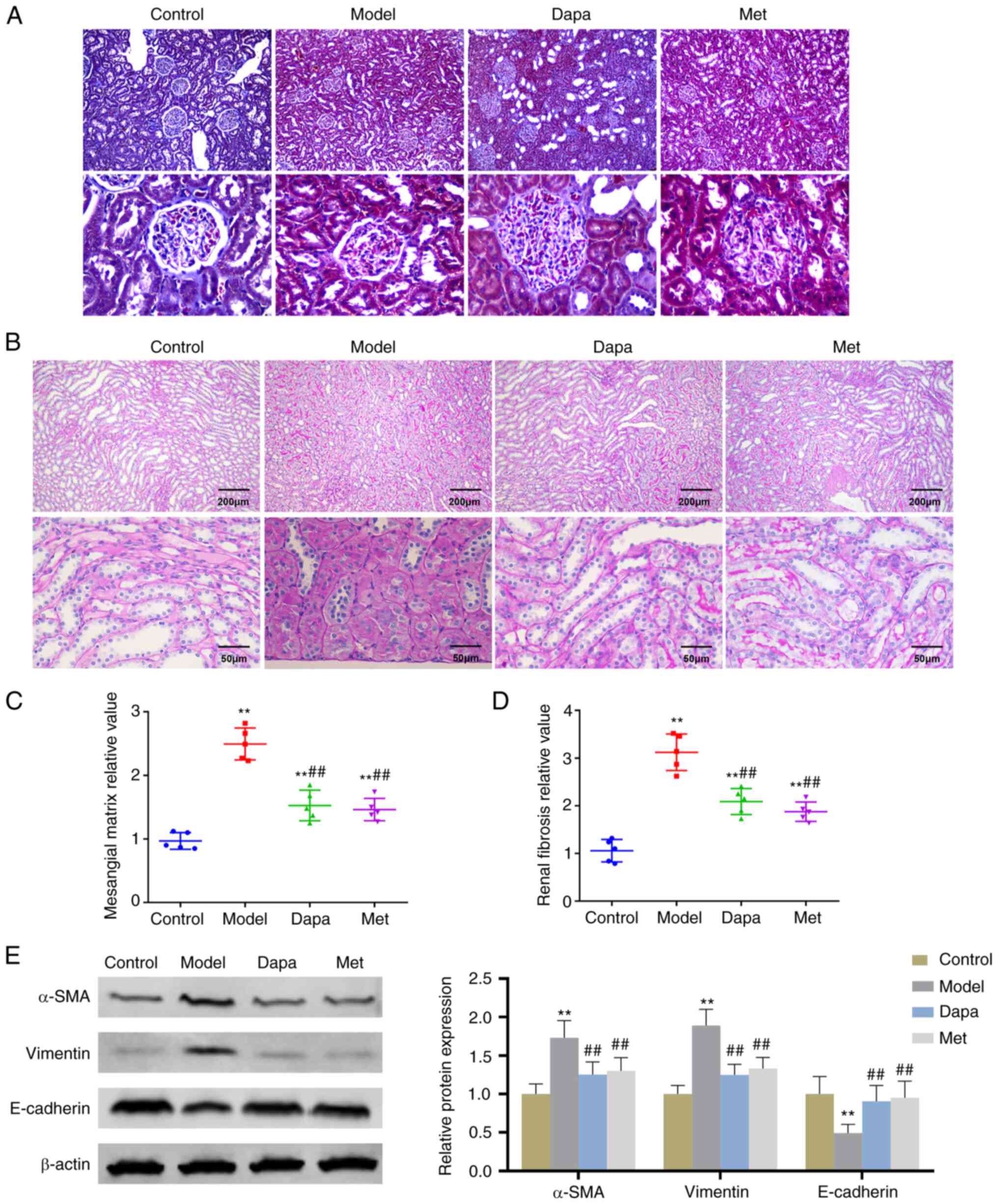
Moreover, the effect of Dapagliflozin on renal
fibrosis in diabetic rats was studied. Masson staining results
showed that the glomerulus, renal tubules, and interstitial
collagen deposition in the Control group were physiologically
normal. However, in the Model group, the glomerular basement
membrane was notably thicker, collagen deposition in the renal
interstitium increased, and the basement membrane of certain renal
tubules was also thicker, accompanied by a degree of dilation and
vacuole-like lesions. As for the Dapa and Met groups, the collagen
deposition in glomeruli and interstitial regions was significantly
lower compared with the Model group (Fig. 4A). With respect to PAS staining
results, glomerular mesangial matrix deposition and the degree of
renal fibrosis were notably increased in the Model group; after
treatment with Dapagliflozin and Metformin, glomerular mesangial
matrix deposition and the renal fibrosis in T2DM rats were
effectively improved (Fig. 4B-D).
In addition, compared with the Control group, the expression levels
of renal fibrosis-related proteins α-SMA and Vimentin in the renal
tissue of the Model group were significantly increased, while the
expression levels of E-cadherin were remarkedly reduced. As opposed
to the Model group, a notable drop in the expression levels of
α-SMA and Vimentin and a significant increase in the expression
levels of E-cadherin were observed in the kidney tissues of rats in
the Dapa group and Met groups (Fig.
4E). This shows that Dapagliflozin inhibited kidney fibrosis in
diabetic rats.
Dapagliflozin inhibits the activation
of the TGF-β1/Smad signaling pathway in T2DM rats
Finally, to explore the molecular mechanism by which
Dapagliflozin exerted its protective effects on the kidneys of
diabetic rats, the expression of TGF-β1/Smad signaling
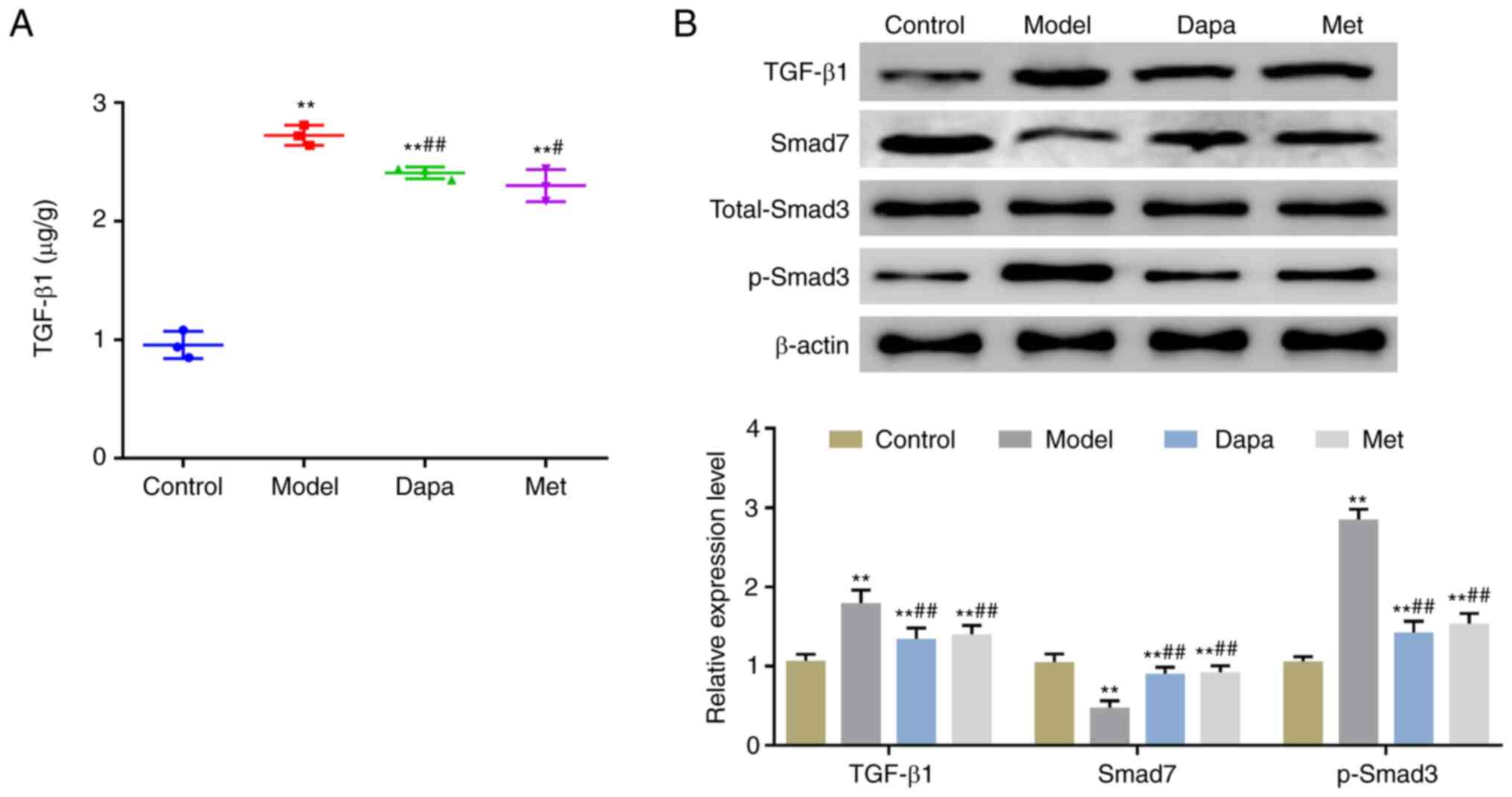
pathway-related proteins was determined. ELISA results revealed
that the serum levels of TGF-β1 were significantly higher in
STZ-induced T2DM rats compared with the Control rats, and were
significantly lower in the Dapa and Met groups compared with the
Model group (Fig. 5A). In terms of
western blotting results, STZ induced a significant increase in the
protein expression levels of TGF-β1 and p-Smad3 as well as a
significant decrease in the expression of Smad7 protein in rat
kidney tissues. Compared with the Model group, the Dapa and Met
groups exhibited significantly reduced expression levels of TGF-β1
and p-Smad3, and increased expression levels of Smad7 protein in
the kidney tissues. In addition, the protein expression levels of
total Smad3 were not significantly altered in the tissues of rats
in each group (Fig. 5B). Together,
these results showed that Dapagliflozin may protect the renal
function of diabetic rats by inhibiting the activity of the
TGF-β1/Smad signaling pathway.
Discussion
Diabetes mellitus, resulting from complete or
reduced insulin secretion and/or insufficient insulin action, is
characterized by chronic hyperglycemia and impaired metabolism of
carbohydrates, lipids, and proteins. T2DM is the most common form
of diabetes, accounting for 90-95% of all diabetic patients
(42). In China, diabetes and
prediabetes are more prevalent among individuals >20 years old,
with 15.5% of these instances being T2DM (43). The most common cause of CKD, DN,
can lead to end-stage renal disease and even patient death
(44,45). Therefore, it is crucial to treat DN
as soon as possible. Dapagliflozin, as an anti-diabetic drug, is
currently used clinically to treat DN. Studies have revealed
several effects of Dapagliflozin, such as reducing the risk of
renal endpoints by 47%, lowering the weight and blood pressure of
DN patients, and improving blood glucose and urine protein levels
(46). If abnormalities are seen
in serum BUN, SCr, and 24 h urine protein levels, which are
currently used to evaluate renal function, this indicates that
diabetes may cause nephropathic complications (47). In the present study, T2DM rat
models were established by intraperitoneal injection of 35 mg/kg
STZ. It was found that the levels of FBG, HbA1c, BUN, SCr, 24 h
urine protein, and lipids, as well as the renal index of T2DM rats
increased significantly, whereas insulin levels decreased
significantly. Additionally, the proliferation of glomerular
mesangial cells, the thickening of the glomerular basement
membrane, the dilatation and atrophy of renal tubules, accompanied
by a certain degree of dilatation, and vacuole-like lesions were
visible in the renal tissues. However, renal injury, lipid levels,
and insulin levels were effectively improved in T2DM rats after
Dapagliflozin treatment. It is evident that Dapagliflozin can
protect rats from STZ-induced T2DM.
The pathogenesis of DN is complicated. Renal
interstitial fibrosis is a common route from the development of
T2DM nephropathy to end-stage renal disease, which is primarily
caused by the excessive accumulation of extracellular matrix (ECM)
in the renal interstitium. This results in a decrease in the
expression of epithelial cell markers such as E-cadherin and an
increase in the expression of interstitial cell markers such as
α-SMA and Vimentin. Under normal circumstances, fibrocytes do not
express α-SMA, so α-SMA expression levels are often used as an
indicator of fibrosis (48).
In the present study, it was found that STZ induced
significant thickening of the glomerular basement membrane in the
renal tissue of T2DM rats, collagen deposition in the renal
interstitium, and thickening of the tubular basement membrane.
Additionally, the expression levels of α-SMA and Vimentin increased
significantly in the renal tissue and the E-cadherin expression
levels decreased significantly. It follows that STZ can induce
renal fibrosis in rats. After treatment with Dapagliflozin, the
pathological changes such as renal fibrosis and collagen deposition
in the renal interstitium of T2DM rats were alleviated; at the same
time, the protein expression levels of α-SMA and vimentin in rat
renal tissues were significantly reduced, whereas the protein
expression levels of E-cadherin increased significantly.
Collectively, Dapagliflozin can effectively alleviate renal tissue
fibrosis in T2DM rats.
Further, the molecular mechanism through which
Dapagliflozin improved renal fibrosis in T2DM rats was explored.
TGF-β1 is involved in the growth and differentiation of various
cells such as hepatocytes, renal cells, and cardiac cells, as well
as in the synthesis and accumulation of extracellular matrix, which
can lead to renal interstitial fibrosis and even glomerulosclerosis
(49). Moreover, TGF-β1 promotes
fibroblast transformation and ECM synthesis by regulating matrix
metalloproteinases and inhibits ECM decomposition. Subsequently, it
not only leads to podocyte apoptosis, basement membrane
exfoliation, and protein leakage, it also reduces the number of
nephrons and causes glomerulosclerosis (50). Hyperglycemia has been demonstrated
to induce an increase in the expression of TGF-β1 in mesangial
cells, thereby resulting in the accumulation of extracellular
matrix (51). Smad protein is the
transduction molecule of the TGF-β family signal from the receptor
to the nucleus, and at present, it is hypothesized that Smad is the
only substrate of TGF-β, and imbalances in the expression are the
molecular basis of renal fibrosis (52,53).
The Smad-dependent signaling pathway is a classical signaling
pathway by which TGF-β1 induces fibrosis (52,53).
Smad3 is an activated Smad protein and a downstream regulator of
TGF-β1. Increased expression of Smad in DN animal models indicates
that Smad3 is crucial to the development of DN fibrosis. The
concentration of Smad3 in the fibrotic kidney model is
significantly higher than in normal tissues (54,55).
Smad7, an inhibitory Smad protein, can compete with Smad2 or Smad3
to bind to the TGF-β receptor I to inhibit Smad2/Smad3
phosphorylation and its translocation to the nucleus, and increase
ubiquitin-mediated degradation of TGF-β receptor I. As a joint
result of these actions, it negatively regulates the TGF-β1/Smad
pathway. It has been recognized that Smad7, as an endogenous TGF-β1
antagonist, inhibits the TGF-β1/Smad signaling pathway (56) and reduces the progression of
experimental renal fibrosis (57).
In the present study, it was found that the expression levels of
TGF-β1 and p-Smad3 in the kidney tissue of T2DM rats were
significantly increased, and the expression levels of Smad7 were
considerably reduced. After treatment with Dapagliflozin, the
protein expression levels of TGF-β1 and p-Smad3, conversely,
decreased significantly while the protein expression levels of
Smad7 increased significantly in the kidney tissue of rats induced
with STZ. Briefly, the TGF-β1/Smad signaling pathway may be one of
the mechanisms by which Dapagliflozin reduces renal fibrosis.
The novelty of the present study is that it was
demonstrated for the first time that Dapagliflozin alleviates renal
fibrosis in T2DM rats by inhibiting the TGF-β1/Smad signaling
pathway and has a protective effect on STZ-induced T2DM rats.
However, this study lacked simultaneous in vitro
observations and validation through other phenotypic rescue
experiments, and only observed changes in indicators under the
actions of drugs. The present study is a preliminary observational
experiment. To confirm the findings of the results, further
mechanistic explanations are required in future studies. Secondly,
in the animal intervention experiments, the effect of the combined
use of Dapagliflozin and Metformin on DN was not explored, and a
non-disease group only treated with Dapagliflozin was not included
to exclude any potential effects of Dapagliflozin on the normal
health status. Additionally, whether Dapagliflozin exerts its
therapeutic effect in a dose-dependent manner has also not been
explored. STZ has significant defects in the construction of DN
models due to its islet destruction and nephrotoxic effects. In
view of this, there is an urgent need to identify more reliable
modeling methods to compensate for these deficiencies. Finally, the
present study did not fully detect all physiological indicators in
the rats. It is necessary to include indicators such as blood
glucose, blood lipids, blood uric acid, and urine protein (albumin
excretion rate and creatinine clearance rate) to better evaluate
changes in liver and kidney function.
In summary, Dapagliflozin can improve STZ-induced
renal fibrosis in T2DM rats, and its mechanism may be related to
the reduced activity of the TGF-β1/Smad signaling pathway. This
finding provides a theoretical basis for the medicinal use of
Dapagliflozin. However, further study is required to understand how
Dapagliflozin affects the TGF-1/Smad signaling pathway and treat
DN.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SX, YL, and WT conceived and designed the study, and
wrote the manuscript. YL and XL analyzed the data. SX and WT
collected and provided the samples for the study. SX and WT confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Shanghai
Changzheng Hospital Animal Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Carracher AM, Marathe PH and Close KL:
International diabetes federation 2017. J Diabetes. 10:353–356.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ji L, Hu D, Pan C, Weng J, Huo Y, Ma C, Mu
Y, Hao C, Ji Q, Ran X, et al: Primacy of the 3B approach to control
risk factors for cardiovascular disease in type 2 diabetes
patients. Am J Med. 126:925 e11–22. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sutariya B and Saraf M: Betanin, isolated
from fruits of Opuntia elatior Mill attenuates renal fibrosis in
diabetic rats through regulating oxidative stress and TGF-β
pathway. J Ethnopharmacol. 198:432–443. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhu PJ: Renal Fibrosis and Anti-fibrosis
Treatment Research. Chinese Journal of Integrated Traditional and
Western Nephrology 114-117, 2004.
|
|
6
|
Zhang X, Guo K, Xia F, Zhao X, Huang Z and
Niu J: FGF23C-tail improves diabetic nephropathy by
attenuating renal fibrosis and inflammation. BMC Biotechnol.
18(33)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Forbes JM and Cooper ME: Mechanisms of
diabetic complications. Physiol Rev. 93:137–188. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tuttle KR, Bakris GL, Bilous RW, Chiang
JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K,
Narva AS, Navaneethan SD, et al: Diabetic kidney disease: A report
from an ADA consensus conference. Am J Kidney Dis. 64:510–533.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dekkers CCJ, Gansevoort RT and Heerspink
HJL: New diabetes therapies and diabetic kidney disease
progression: The role of SGLT-2 inhibitors. Curr Diab Rep.
18(27)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Messerli FH, Bangalore S, Bavishi C and
Rimoldi SF: Angiotensin-Converting enzyme inhibitors in
hypertension: To use or not to use? J Am Coll Cardiol.
71:1474–1482. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bhandari S, Mehta S, Khwaja A, Cleland
JGF, Ives N, Brettell E, Chadburn M and Cockwell P: STOP ACEi Trial
Investigators. Renin-Angiotensin system inhibition in advanced
chronic kidney disease. N Engl J Med. 387:2021–2032.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li J, Liu H, Takagi S, Nitta K, Kitada M,
Srivastava SP, Takagaki Y, Kanasaki K and Koya D: Renal protective
effects of empagliflozin via inhibition of EMT and aberrant
glycolysis in proximal tubules. JCI Insight.
5(e129034)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Komers R, Oyama TT, Beard DR, Tikellis C,
Xu B, Lotspeich DF and Anderson S: Rho kinase inhibition protects
kidneys from diabetic nephropathy without reducing blood pressure.
Kidney Int. 79:432–442. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Poursharif S, Hamza S and Braam B: Changes
in Proximal tubular reabsorption modulate microvascular regulation
via the TGF system. Int J Mol Sci. 23(11203)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xue J, Wang L, Sun Z and Xing C: Basic
research in diabetic nephropathy health care: A study of the
renoprotective mechanism of metformin. J Med Syst.
43(266)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shen Y, Miao N, Xu J, Gan X, Xu D, Zhou L,
Xue H, Zhang W and Lu L: Metformin prevents renal fibrosis in mice
with unilateral ureteral obstruction and inhibits Ang II-Induced
ECM production in renal fibroblasts. Int J Mol Sci.
17(146)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rajasekeran H, Lytvyn Y and Cherney DZ:
Sodium-glucose cotransporter 2 inhibition and cardiovascular risk
reduction in patients with type 2 diabetes: The emerging role of
natriuresis. Kidney Int. 89:524–526. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schechter M, Jongs N, Chertow GM, Mosenzon
O, McMurray JJV, Correa-Rotter R, Rossing P, Langkilde AM, Sjostrom
CD, Toto RD, et al: Effects of dapagliflozin on hospitalizations in
patients with chronic kidney disease: A post Hoc analysis of
DAPA-CKD. Ann Intern Med. 176:59–66. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
McEwan P, Darlington O, Miller R, McMurray
JJV, Wheeler DC, Heerspink HJL, Briggs A, Bergenheim K and Garcia
Sanchez JJ: Cost-Effectiveness of dapagliflozin as a treatment for
chronic kidney disease: A health-economic analysis of DAPA-CKD.
Clin J Am Soc Nephrol. 17:1730–1741. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Reifsnider OS, Kansal AR, Gandhi PK,
Cragin L, Brand SB, Pfarr E, Fahrbach K and Ustyugova A:
Cost-effectiveness of empagliflozin versus canagliflozin,
dapagliflozin, or standard of care in patients with type 2 diabetes
and established cardiovascular disease. BMJ Open Diabetes Res Care.
9(e001313)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hutter S, van Haaften WT, Hunerwadel A,
Baebler K, Herfarth N, Raselli T, Mamie C, Misselwitz B, Rogler G,
Weder B, et al: Intestinal activation of pH-Sensing Receptor OGR1
[GPR68] contributes to fibrogenesis. J Crohns Colitis.
12:1348–1358. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lindquist JA and Mertens PR:
Myofibroblasts, regeneration or renal fibrosis-is there a decisive
hint? Nephrol Dial Transplant. 28:2678–2681. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zeisberg EM, Potenta SE, Sugimoto H,
Zeisberg M and Kalluri R: Fibroblasts in kidney fibrosis emerge via
endothelial-to-mesenchymal transition. J Am Soc Nephrol.
19:2282–2287. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cho JG, Lee A, Chang W, Lee MS and Kim J:
Endothelial to mesenchymal transition represents a key link in the
interaction between inflammation and endothelial dysfunction. Front
Immunol. 9(294)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Passerini AG, Milsted A and Rittgers SE:
Shear stress magnitude and directionality modulate growth factor
gene expression in preconditioned vascular endothelial cells. J
Vasc Surg. 37:182–190. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lu Y, Liu S, Zhang S, Cai G, Jiang H, Su
H, Li X, Hong Q, Zhang X and Chen X: Tissue inhibitor of
metalloproteinase-1 promotes NIH3T3 fibroblast proliferation by
activating p-Akt and cell cycle progression. Mol Cells. 31:225–230.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen Y, Zou H, Lu H, Xiang H and Chen S:
Research progress of endothelial-mesenchymal transition in diabetic
kidney disease. J Cell Mol Med. 26:3313–3322. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wahab NAA, Giribabu N, Kilari EK and
Salleh N: Abietic acid ameliorates nephropathy progression via
mitigating renal oxidative stress, inflammation, fibrosis and
apoptosis in high fat diet and low dose streptozotocin-induced
diabetic rats. Phytomedicine. 107(154464)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ilzecka J, Stelmasiak Z and Dobosz B:
Transforming growth factor-Beta 1 (tgf-Beta 1) in patients with
amyotrophic lateral sclerosis. Cytokine. 20:239–243.
2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu CL, Yan L, Cai KR, Sun K, Qi Y, Han
YL, Zhang XD and Sun XD: Effects of soybean isoflavones on
Wnt/β-catenin and the TGF-β1 signaling pathway in renal tissue of
type 2 diabetic rats. J Biol Regul Homeost Agents. 32:455–464.
2018.PubMed/NCBI
|
|
31
|
Sun M, Zhou W, Yao F, Song J, Xu Y, Deng
Z, Diao H and Li S: MicroRNA-302b mitigates renal fibrosis via
inhibiting TGF-β/Smad pathway activation. Braz J Med Biol Res.
54(e9206)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sun SF, Zhao TT, Zhang HJ, Huang XR, Zhang
WK, Zhang L, Yan MH, Dong X, Wang H, Wen YM, et al: Renoprotective
effect of berberine on type 2 diabetic nephropathy in rats. Clin
Exp Pharmacol Physiol. 42:662–670. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li Q, Ye F, Shi Y, Zhang L, Wang W, Tu Z,
Qiu J, Wang J, Li S, Bu H and Li Y: Nuclear translocation of SMAD3
may enhance the TGF-beta/SMADS pathway in high glucose
circumstances. Transplant Proc. 38:2158–2160. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu L, Wang Y, Yan R, Li S, Shi M, Xiao Y
and Guo B: Oxymatrine inhibits renal tubular EMT induced by high
glucose via upregulation of SnoN and inhibition of TGF-β1/smad
signaling pathway. PLoS One. 11(e0151986)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang Q, Liu X, Sullivan MA, Shi C and
Deng B: Protective Effect of Yi Shen Pai Du formula against
diabetic kidney injury via inhibition of oxidative stress,
inflammation, and epithelial-to-mesenchymal transition in db/db
mice. Oxid Med Cell Longev. 2021(7958021)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mou X, Zhou DY, Zhou D, Liu K, Chen LJ and
Liu WH: A bioinformatics and network pharmacology approach to the
mechanisms of action of Shenxiao decoction for the treatment of
diabetic nephropathy. Phytomedicine. 69(153192)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gan C, Zhang Q, Liu H, Wang G, Wang L, Li
Y, Tan Z, Yin W, Yao Y, Xie Y, et al: Nifuroxazide ameliorates
pulmonary fibrosis by blocking myofibroblast genesis: A drug
repurposing study. Respir Res. 23(32)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jaikumkao K, Pongchaidecha A, Chueakula N,
Thongnak LO, Wanchai K, Chatsudthipong V, Chattipakorn N and
Lungkaphin A: Dapagliflozin, a sodium-glucose co-transporter-2
inhibitor, slows the progression of renal complications through the
suppression of renal inflammation, endoplasmic reticulum stress and
apoptosis in prediabetic rats. Diabetes Obes Metab. 20:2617–2626.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Oraby MA, El-Yamany MF, Safar MM, Assaf N
and Ghoneim HA: Dapagliflozin attenuates early markers of diabetic
nephropathy in fructose-streptozotocin-induced diabetes in rats.
Biomed Pharmacother. 109:910–920. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jihua C, Cai C, Xubin B and Yue Y: Effects
of Dexmedetomidine on the RhoA/ROCK/Nox4 signaling pathway in renal
fibrosis of diabetic rats. Open Med (Wars). 14:890–898.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu WH, Liu SM, Lin SF and Huang HQ: Role
of berberine in fibronectin expression via S1P2-MAPK signaling
pathway in diabetic nephropathy. Chinese Pharmacological Bulletin.
29:723–728. 2013.
|
|
42
|
Tripathi BK and Srivastava AK: Diabetes
mellitus: Complications and therapeutics. Med Sci Monit.
12:RA130–RA147. 2006.PubMed/NCBI
|
|
43
|
Zhang PH, Chen ZW, Lv D, Xu YY, Gu WL,
Zhang XH, Le YL, Zhu HH and Zhu YM: Increased risk of cancer in
patients with type 2 diabetes mellitus: A retrospective cohort
study in China. BMC Public Health. 12(567)2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ou YL, Lee MY, Lin IT, Wen WL, Hsu WH and
Chen SC: Obesity-related indices are associated with albuminuria
and advanced kidney disease in type 2 diabetes mellitus. Ren Fail.
43:1250–1258. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tokunaga T, Fujiwara Y, Matsushita M,
Suzaki T and Suzaki E: Glomerular hypertrophy and hyperfiltration
in obesity-related diabetic (ob/ob) mouse. Analytical and
quantitative cytology and histology. 39:223–230. 2017.
|
|
46
|
Yuan XL and Wang SJ: Clinical efficacy of
dapagliflozin in patients with type 2 diabetic kidney disease.
Henan Medical Research. 29:1969–1971. 2020.
|
|
47
|
Zhang XR, Fu XJ, Zhu DS, Zhang CZ, Hou S,
Li M and Yang XH: Salidroside-regulated lipid metabolism with
down-regulation of miR-370 in type 2 diabetic mice. Eur J
Pharmacol. 779:46–52. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang CH, Punde TH, Huang CD, Chou PC,
Huang TT, Wu WH, Liu CH, Chung KF and Kuo HP: Fibrocyte trafficking
in patients with chronic obstructive asthma and during an acute
asthma exacerbation. J Allergy Clin Immunol. 135:1154–1162.e1-5.
2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tian C, Wang Y, Chang H, Li J and La X:
Spleen-Kidney supplementing formula alleviates renal fibrosis in
diabetic rats via TGF-β1-miR-21-PTEN signaling pathway. Evid Based
Complement Alternat Med. 2018(3824357)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Huang H, You Y, Lin X, Tang C, Gu X, Huang
M, Qin Y, Tan J and Huang F: Inhibition of TRPC6 signal pathway
alleviates podocyte injury induced by TGF-β1. Cell Physiol Biochem.
41:163–172. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Loeffler I, Hopfer U, Koczan D and Wolf G:
Type VIII collagen modulates TGF-β1-induced proliferation of
mesangial cells. J Am Soc Nephrol. 22:649–663. 2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Overstreet JM, Samarakoon R, Meldrum KK
and Higgins PJ: Redox control of p53 in the transcriptional
regulation of TGF-β1 target genes through SMAD cooperativity. Cell
Signal. 26:1427–1436. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kim D, Lee AS, Jung YJ, Yang KH, Lee S,
Park SK, Kim W and Kang KP: Tamoxifen ameliorates renal
tubulointerstitial fibrosis by modulation of estrogen receptor
α-mediated transforming growth factor-β1/Smad signaling pathway.
Nephrol Dial Transplant. 29:2043–2053. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Samarakoon R, Overstreet JM and Higgins
PJ: TGF-β signaling in tissue fibrosis: Redox controls, target
genes and therapeutic opportunities. Cell Signal. 25:264–268.
2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Liu S, Yu N, Zhang XL, Chen XQ and Tang
LQ: The regulation of berberine on the imbalance of TGF-β1/SnoN
expression in renal tissues of rats with early diabetic nephropathy
and the regulation of Smad signaling pathway. China Journal of
Chinese Material Madica. 37:3604–3610. 2012.
|
|
56
|
Ka SM, Yeh YC, Huang XR, Chao TK, Hung YJ,
Yu CP, Lin TJ, Wu CC, Lan HY and Chen A: Kidney-targeting Smad7
gene transfer inhibits renal TGF-β/MAD homologue (SMAD) and nuclear
factor kappaB (NF-κB) signalling pathways, and improves diabetic
nephropathy in mice. Diabetologia. 55:509–519. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Chatziantoniou C and Dussaule JC: Insights
into the mechanisms of renal fibrosis: Is it possible to achieve
regression? Am J Physiol Renal Physiol. 289:F227–F234.
2005.PubMed/NCBI View Article : Google Scholar
|