Introduction
The coronavirus disease 2019 (COVID-19) mainly
affects the respiratory system, although it affects every organ
system (1). Extrapulmonary
involvement includes dysregulation of the immune system, metabolic
complications and adverse effects on various organs of the
cardiovascular, renal, nervous, endocrine, musculoskeletal and
other systems (1). The majority of
infections are self-limiting, with patients returning to their
usual state of health 12-14 days after receiving a positive test
result, but 20% of symptomatic, infected, unvaccinated adults need
hospitalization (2).
In the acute stage of COVID-19, kidney involvement
is very common among patients that are hospitalized, with acute
kidney injury (AKI) occurring in 15-28% of all intensive care unit
admissions (3,4). COVID-19 has been hypothesized to
affect the kidney by direct mechanisms, such as viral entry, local
inflammation/complement activation and glomerulopathy (5). The kidney can also be affected
indirectly, using nephrotoxic drugs, sepsis, systemic inflammation
and hypercoagulability with thromboembolic disease, among others
(4). After the acute phase, a
number of symptoms and effects on various organs prevail (such as
fatigue, shortness of breath, chest pain and digestive problems
among others), which is why the term ‘chronic COVID-19’ or ‘long
COVID-19’ was coined, which describes the chronic impact of
COVID-19 at all levels, although it is generally used to indicate
symptomatology persisting for >12 weeks after infection
(1). While AKI can lead to chronic
kidney disease (CKD), little is known about the long-term effects
of COVID-19 on kidney function (4).
The estimated glomerular filtration rate (eGFR) from
serum creatinine is a common clinical indicator that physicians use
as a pragmatic reference to kidney function (6). The longitudinal changes in eGFR after
suffering from COVID-19 has been a subject of study with
contradictory results, reporting that it did not cause changes
(7), caused slight reduction
(8), or that the changes were
heterogeneous between patients (9). The aim of the present cohort study
was to examine the changes in eGFR after one year post infection
(4-, 8- and 12-months after), compared with eGFR before infection
in a cohort of COVID-19 survivors (hospitalized or treated at home
during the acute phase), and whether these changes are associated
with any clinical characteristics of the patient.
Materials and methods
Study subjects
The study subjects consisted of patients with a mean
age of 50.0±14.3 years, comprising 56.3% females and 43.7% males.
Additional demographic data can be found in Table SI. A prospective cohort study was
conducted with patients that sought medical consultation at the
General Hospital [number 1 of the Mexican Institute of Social
Security Institute (IMSS), (Colima, Mexico)] between 28th September
2020 and 30th December 2020. Patients had COVID-19-associated
symptoms and had a positive diagnosis of severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2) from reverse transcription-PCR.
Patients included in the present cohort received at-home ambulatory
care or usual hospital treatment. Patients that were asymptomatic
and paucisymptomatic were not included in the present study as
previously defined (2). The
inclusion criteria for the present study were: i) Patients were
over the age of 18-years old; ii) patients were symptomatic during
the acute phase of COVID-19; iii) patients were suffering from
COVID-19 for the first time; and iv) patients were non-vaccinated
for COVID-19. In addition, patients were required to have routine
clinical laboratory tests to estimate the GFR from serum creatine
within 2-months before COVID-19 infection. These tests were part of
routine care unrelated to acute illness or pregnancy, ensuring a
more accurate assessment of kidney function changes associated with
COVID-19. The exclusion criteria of the present study were: i)
Patients that were pregnant; and ii) patients that were undergoing
renal replacement therapy treatment before infection with COVID-19.
The present cohort study only included patients that survived the
COVID-19 infection. Patients that did not survive during the acute
stage of the disease or within the first 4-months after COVID-19
infection were not considered in the analysis. In addition, patient
data were excluded after any of the following events: i) The
patient decided to withdraw from the study voluntarily; ii) the
patient was diagnosed with COVID-19 for the second time; or iii)
the patient had a positive pregnancy result. The study was approved
by the local health research committee of the IMSS-Colima, Mexico
(approval no. R-2020-601-041). Inclusion in the study was
voluntary, and each patient or their legal representative signed an
informed consent letter in cases where the patient could not
sign.
Measures and follow-up
Data were collected and entered into medical records
when patients sought medical care due to COVID-19 infection.
Universal variables (such as age, sex and education),
pathological/non-pathological personal history (such as blood type
and comorbidities) and signs and symptoms associated to COVID-19
were collected. Other collected data included treatment and
hospital admissions. The patients overall self-assessment or
symptom severity score was recorded for each patient routinely
(part of the standard care protocol during the patients'
interactions with the healthcare system) as previously described
[0-10-point Visual Analog Scale; from ‘very well’ (a score of 0) to
‘very poorly’ (a score of 10)] (1,9).
Patients were evaluated at 4-, 8-, and 12-months after COVID-19
(time periods following the initial COVID-19 infection/positive
result). The primary aim of the present study was to determine the
eGFR, according to the MDRD-4 formula or Levey equation (10), by establishing a baseline value for
eGFR before COVID-19 infection (within 2 months before the onset of
the illness). Subsequently, the study aimed to measure eGFR at 4, 8
and 12 months following COVID-19 infection. An eGFR of <90
ml/min/1.73 m2 was considered a low eGFR value. The
persistence of symptoms (‘long COVID’) was also evaluated according
to the previous definition (continuation of COVID-19-related
symptoms beyond the acute phase of the illness) (1).
Sample size
The sample size for the present study was calculated
based on a previous report that followed the eGFR for 6-months in
patients with a disease that affects renal function (diabetes),
finding that 71.7% of patients had a reduction in the eGFR while
the eGFR for 28.3% of patients remained unchanged (11). In total, 20 patients from each
group (with and without reduction) were needed to reach the
required power (0.8). Once the study was completed, the statistical
power of detecting an eGFR reduction at 12-months post-COVID-19
(≥10 ml/min/1.73 m2) in patients with normal vs. low
eGFR pre-COVID-19 was calculated, resulting in 85.4%.
Statistical analysis
Data are expressed as percentages or mean ± standard
deviation. The normal distribution of the data was verified using
the Kolmogorov-Smirnov test. Fisher's exact tests and
Cochran-Mantel-Haenszel chi-square tests were used to compare
qualitative variables across multiple periods of time. Fisher's
exact test was selected when the expected cell counts were
anticipated to be ≤5 in >20% of the cells within a category
(12). Two-way mixed ANOVAs,
followed by the Bonferroni post hoc test, were used to compare the
eGFRs across different evaluation periods and assess the
differences between the pre-COVID-19 and 12-months post-COVID-19
periods. Univariate and multivariate binary logistic regression
analyzes were used to determine the probability of developing low
eGFR at the pre-COVID-19 time (binomial outcome: Yes or no) with
the presence of different general or clinical characteristics of
the patients (transversal analyzes in pre-COVID-19). The data were
summarized as odds ratios (ORs) with 95% confidence intervals (CIs)
and P-values (cross-sectional analyzes in pre-COVID-19 data). For
longitudinal analysis, the change from the baseline eGFR
(pre-COVID-19) was used to examine the absolute differences between
the post-COVID-19 evaluation periods. Pearson correlations were
used for bivariate analyzes (eGFR of the baseline and the change in
eGFR) in various strata of the patients studied. The area under the
receiver operating characteristic (ROC) curve, CI, cut-off point,
sensitivity and specificity of the eGFR in the pre-COVID-19 period
was calculated to discriminate patients that would develop a
decrease in the eGFR during the post-COVID-19 period.
For association analyzes, multivariate generalized
linear mixed models (GENLINMIXED in SPSS (version 20; IBM Corp.)
with separate random intercepts and a binary logistic regression
link were used, as previously reported (13,14).
The longitudinal nature of the data was accounted for through two
random variables: i) The pandemic time points (pre-COVID-19 or
post-COVID-19); and ii) the month of survey (months 1-12, ordinal
scale), which is an indicator of time separation between the two
different time-points. The target variable was the dichotomic
reduction of eGFR (≥10 ml/min/1.73 m2; yes or no). The
fixed effects include continuous variables (age and body mass
index) and dichotomous variables divided as yes or no for various
pre-COVID-19 and COVID-19 clinical characteristics (high blood
pressure and B-positive blood type). Analysis involving a two-way
interaction (A x B, where A and B are factors) between the
principal risk factors for the reduction of the eGFR post-COVID-19
were made in separate models. The aim was to obtain the marginal
risk result from the aforementioned model, in which the binomial
regression parameters of multivariate analysis were summarized as
relative risk (RR) with a 95% CI and P-values. CinCalc version 1
(https://clincalc.com/stats/Power.aspx) (15) was used to calculate statistical
power and sample size. The rest of the analyzes were performed with
SPSS Statistics version 20 (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
In total, 311 patients were screened, of which 99
were included because the patients had an eGFR test 2-months before
experiencing COVID-19 for the first time. During post-COVID-19
infection follow-ups, all patients remained for the first 4-months;
at 8-months, 88 patients were analyzed; and 77 patients completed
12-months of follow-up. Not all patients completed the year of
follow-up because, after 4-months, the patient either decided to
voluntarily abandon the study and to not continue with the periodic
evaluations (n=14), or the patient suffered from COVID-19 for the
second time (n=8). As all patients had at least two measurements
(pre-COVID-19 and 4-months post-COVID-19), the 99 patients were
included in the analysis. Table
SI presents the main personal history, clinical characteristics
during and after COVID-19, and eGFR at different follow-up
periods.
Risk factors for a low eGFR
pre-COVID-19
In the population analyzed, having a low eGFR
(<90 ml/min/1.73 m2) pre-COVID-19 was associated with
age, arterial hypertension and smoking (Table SII). After multivariate analysis,
it was revealed that only hypertension (OR=2.91, 95% CI=1.02-8.32,
P=0.047) and smoking (OR=3.52, 95% CI=1.27-9.72, P=0.015) increased
the risk, by ~3x, of having a low eGFR (Table SII).
Risk factors for a reduction in the
eGFR post-COVID-19
Table I presents
the factors associated with a reduced eGFR of ≥10 ml/min/1.73
m2 during the first year after acute COVID-19. The
multivariate analysis indicated that being hospitalized (RR=2.90,
95% CI=1.10-7.68, P=0.032), being treated with Ivermectin
(RR=14.02, 95% CI=4.11-47.80, P<0.001) or anticoagulants
(RR=6.51, 95% CI=2.69-15.73, P<0.001) were factors that
increased the risk of a reduced eGFR during the first year
post-COVID-19. By contrast, a low eGFR (<90 ml/min/1.73
m2) before the disease (RR=0.09, 95% CI=0.03-0.25,
P<0.001), having a B-positive blood type (RR=0.29, 95%
CI=0.10-0.85, P=0.024), being diabetic (RR=0.06, 95% CI=0.02-0.17,
P<0.001), taking vitamin C during the acute phase of COVID-19
(RR=0.23, 95% CI=0.06-0.83, P=0.025) or suffering from chronic
symptoms of COVID-19 (RR=0.24, 95% CI=0.09-0.65, P=0.005), were
protective factors that reduced the probability of a reduced eGFR
post-COVID-19 (Table I).
 | Table IRelative risk from multivariate
generalized linear mixed model with binary logistic regression link
of various pre-COVID-19 and COVID-19 clinical characteristics to
have a reduction of eGFR (≥10 ml/min/1.73 m2) during the
first year after acute COVID-19. |
Table I
Relative risk from multivariate
generalized linear mixed model with binary logistic regression link
of various pre-COVID-19 and COVID-19 clinical characteristics to
have a reduction of eGFR (≥10 ml/min/1.73 m2) during the
first year after acute COVID-19.
| A, Pre-COVID-19
characteristics |
|---|
| | 95% CI | |
|---|
| Covariate | AdRR | Lower | Upper | P-value |
|---|
| Low
eGFRa | 0.09 | 0.03 | 0.25 | <0.001 |
| Age, years | 0.98 | 0.96 | 1.01 | 0.322 |
| Female | 0.59 | 0.27 | 1.27 | 0.178 |
| High
schoolb | 0.57 | 0.30 | 1.08 | 0.086 |
| B blood
typec | 0.29 | 0.10 | 0.85 | 0.024 |
| BMI | 0.96 | 0.91 | 1.02 | 0.191 |
| Diabetes | 0.06 | 0.02 | 0.17 | <0.001 |
| HBP | 1.88 | 0.78 | 4.54 | 0.158 |
| Smoking | 0.59 | 0.24 | 1.41 | 0.232 |
| Alcohol | 2.17 | 0.97 | 4.87 | 0.059 |
| B, COVID-19 disease
characteristics |
| | 95% CI | |
| Covariate | AdRR | Lower | Upper | P-value |
| During acute
phase | | | | |
|
High
symptomsd | 0.81 | 0.37 | 1.75 | 0.589 |
|
Hospitalized | 2.90 | 1.10 | 7.68 | 0.032 |
|
Para/NSAIDs | 0.58 | 0.01 | 23.82 | 0.774 |
|
Antivirals | 1.20 | 0.51 | 2.84 | 0.672 |
|
Antibiotics | 0.49 | 0.19 | 1.26 | 0.139 |
|
Ivermectin | 14.02 | 4.11 | 47.80 | <0.001 |
|
Steroids | 0.83 | 0.43 | 1.61 | 0.575 |
|
Anticoagulants | 6.51 | 2.69 | 15.73 | <0.001 |
|
Vitamins | | | | |
|
B
complex | 0.51 | 0.14 | 1.88 | 0.308 |
|
C | 0.23 | 0.06 | 0.83 | 0.025 |
|
D | 0.58 | 0.12 | 2.87 | 0.500 |
| Long
COVIDe | 0.24 | 0.09 | 0.65 | 0.005 |
The pre-COVID-19 eGFR (baseline eGFR value) was a
significant predictor of the loss of renal function post-COVID-19.
The area under the ROC curve for pre-COVID eGFR as a predictor of
the reduction in eGFR (≥10 ml/min/1.73 m2) one year
after COVID-19 was 0.81 (95% CI=0.66-0.96, P<0.001) with a
pre-COVID-19 eGFR cut-off of 89.9 ml/min/1.73 m2
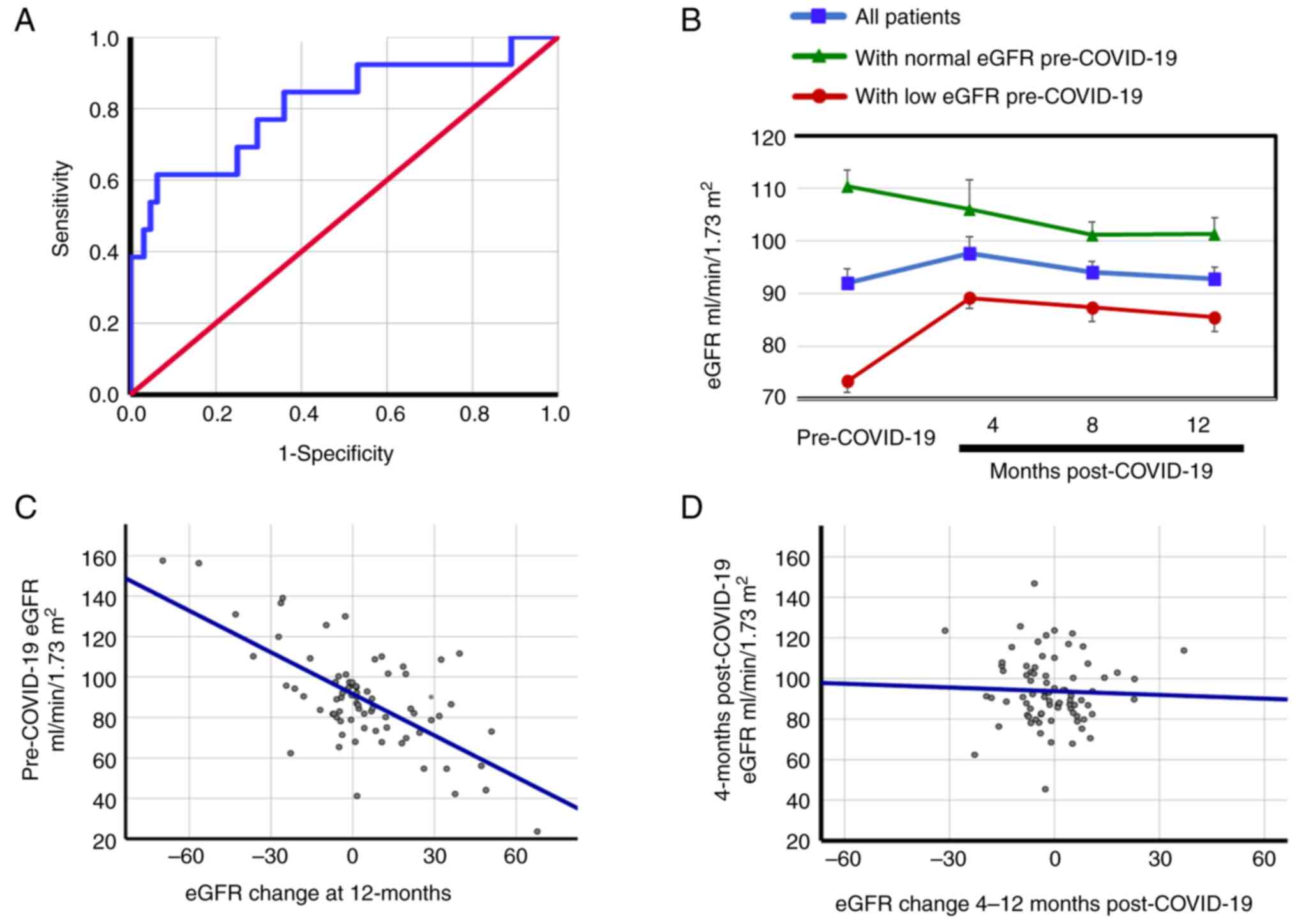
(Fig. 1A), and sensitivity of 0.85
and a specificity of 0.39. This suggests that a negative result (an
eGFR <89.9 ml/min/1.73 m2) can be an adequate
‘rule-out’ test for post-COVID-19 eGFR reduction (16).
In Table II the
proportion of patients with a reduced eGFR (≥10 ml/min/1.73
m2) across three follow-up periods within the initial
year post-acute COVID-19 infection, categorized by diverse clinical
characteristics, is presented. A comprehensive statistical analysis
was undertaken to understand these results, employing distinct
tests to reveal differences within table cells. For each category,
a careful selection of statistical tests was made to ensure the
accuracy and relevance of the analysis. It was observed at
12-months post-COVID-19 (Table
II), the largest difference between the proportion of patients
with reduced eGFR was between patients with normal vs. low
pre-COVID-19 eGFR (30.6 vs. 4.9%, respectively). Other variables
with apparent differences in the proportion of patients with a
reduced eGFR were: i) The presence or absence of a B positive blood
type; ii) diabetes; iii) hospitalization; and iv) the use of
anticoagulants during acute COVID-19 (Table II).
 | Table IINumber and proportion of patients
with reduced eGFR values (≥10 ml/min/1.73 m2), compared
with pre-COVID-19 values, in three periods during the first year
after acute COVID-19 according to diverse clinical
characteristics. |
Table II
Number and proportion of patients
with reduced eGFR values (≥10 ml/min/1.73 m2), compared
with pre-COVID-19 values, in three periods during the first year
after acute COVID-19 according to diverse clinical
characteristics.
| | Month after
COVID-19 | |
|---|
| | 4 (n=99) eGFR
reduction, n (%) | 8 (n=88) eGFR
reduction, n (%) | 12 (n=77) eGFR
reduction, n (%) | |
|---|
| Clinical
characteristics | No | Yes | No | Yes | No | Yes |
P-valuea |
|---|
| All | 82 (82.8) | 17 (17.2) | 68 (77.3) | 20 (22.7) | 64 (83.1) | 13 (16.9) | |
| Pre-COVID-19 low
eGFRb | | | | | | | |
|
No | 34 (68.0) | 16 (32.0) | 26 (60.5) | 17 (39.5) | 25 (69.4) | 11 (30.6) |
<0.001c |
|
Yes | 48 (98.0) | 1 (2.0) | 42 (93.3) | 3 (6.7) | 39 (95.1) | 2 (4.9) | |
| B blood
typed | | | | | | | |
|
No | 75 (81.5) | 17 (18.4) | 62 (75.6) | 20 (24.4) | 59 (81.9) | 13 (18.1) | 0.049c |
|
Yes | 7 (100.0) | 0 (0.0) | 6 (100.0) | 0 (0.0) | 5 (100.0) | 0 (0.0) | |
| Diabetes | | | | | | | |
|
No | 49 (77.8) | 14 (22.2) | 38 (69.1) | 17 (30.9) | 34 (75.6) | 11 (24.4) |
<0.001c |
|
Yes | 33 (91.7) | 3 (8.3) | 30 (90.9) | 3 (9.1) | 30 (93.8) | 2 (6.3) | |
| Hospitalized | | | | | | | |
|
No | 41 (85.4) | 7 (14.6) | 33 (82.5) | 7 (17.5) | 32 (94.1) | 2 (5.9) | 0.039e |
|
Yes | 41 (80.4) | 10 (19.6) | 35 (72.9) | 13 (27.1) | 32 (74.4) | 11 (25.6) | |
| Ivermectin | | | | | | | |
|
No | 70 (83.3) | 14 (16.7) | 60 (78.9) | 16 (17.2) | 54 (81.8) | 12 (18.2) | 0.824c |
|
Yes | 12 (80.0) | 3 (20.0) | 8 (66.7) | 4 (33.3) | 10 (90.9) | 1 (9.1) | |
| Anticoagulant | | | | | | | |
|
No | 50 (87.7) | 7 (12.3) | 40 (85.1) | 7 (14.9) | 37 (90.2) | 4 (9.8) | 0.005e |
|
Yes | 32 (76.2) | 10 (23.8) | 28 (68.3) | 13 (31.7) | 27 (75.0) | 9 (25.0) | |
| Vitamin C | | | | | | | |
|
No | 55 (84.6) | 10 (15.4) | 45 (77.6) | 13 (22.4) | 42 (80.8) | 10 (19.2) | 0.901e |
|
Yes | 27 (79.4) | 7 (20.6) | 23 (76.7) | 7 (23.3) | 22 (88.0) | 3 (12.0) | |
| Long
COVIDf | | | | | | | |
|
No | 24 (80.0) | 6 (20.0) | 19 (79.2) | 5 (20.8) | 18 (90.0) | 2 (10.0) | 0.899e |
|
Yes | 58 (84.0) | 11 (16.0) | 49 (76.6) | 15 (23.4) | 46 (80.7) | 11 (19.3) | |
In Table III
shows the differences between the pre-COVID-19 and 12-months
post-COVID-19 periods. Using the absolute values of the eGFRs
before and 12-months after COVID-19 infection, no changes were
observed when all patients were considered [92.1±26.7 vs. 92.9±19.3
ml/min/1.73 m2, respectively (P=1.000)] (Table III), which could suggest that
COVID-19 does not influence this variable. However, in patients
with a normal pre-COVID-19 eGFR (>90 ml/min/1.73 m2),
the eGFR was significantly reduced 12-months post-COVID-19 compared
with pre-COVID-19 [101.4±18.3 vs. 110.4±22.4 ml/min/1.73
m2, respectively (P<0.001)]. By contrast, in patients
with a low pre-COVID-19 eGFR, the eGFR increased from 73.4±15.5 to
85.5±17.2 ml/min/1.73 m2 pre-COVID-19 and 12-months
post-COVID-19, respectively (P<0.001). The presence or absence
of other variables did not seem to influence the eGFR levels
between the pre-COVID-19 and 12-months post-COVID-19 periods.
 | Table IIIeGFR (ml/min/1.73 m2)
before and 12-months after COVID-19 according to diverse clinical
characteristics. |
Table III
eGFR (ml/min/1.73 m2)
before and 12-months after COVID-19 according to diverse clinical
characteristics.
| Clinical
characteristic | Pre-COVID-19 | 12-months after
COVID-19 infection |
P-valuea |
|---|
| All | 92.1±26.7 | 92.9±19.3 | 1.000 |
| Pre COVID-19 low
eGFRb | | | |
|
No | 110.4±22.4 | 101.4±18.3 | <0.001 |
|
Yes | 73.4±15.5 | 85.5±17.2 | <0.001 |
| B blood
typec | | | |
|
No | 92.3±27.6 | 94.4±18.3 | 0.998 |
|
Yes | 87.1±23.2 | 83.2±10.6 | 1.000 |
| Diabetes | | | |
|
No | 97.7±28.0 | 93.9±18.1 | 1.000 |
|
Yes | 82.2±21.4 | 91.4±21.1 | 0.999 |
| Hospitalized | | | |
|
No | 95.2±17.1 | 95.6±18.3 | 0.998 |
|
Yes | 89.5±33.1 | 90.7±19.9 | 0.993 |
| Ivermectin | | | |
|
No | 92.9±28.2 | 94.0±19.5 | 1.000 |
|
Yes | 87.5±17.7 | 86.6±18.1 | 1.000 |
| Anticoagulants | | | |
|
No | 94.3±26.4 | 95.3±19.9 | 0.836 |
|
Yes | 89.1±27.5 | 90.3±18.7 | 0.897 |
| Vitamin C | | | |
|
No | 92.8±27.8 | 91.6±20.1 | 1.000 |
|
Yes | 90.7±25.4 | 95.8±17.8 | 0.998 |
| Long
COVIDd | | | |
|
No | 93.8±23.2 | 92.3±15.4 | 1.000 |
|
Yes | 90.9±28.4 | 92.1±20.1 | 1.000 |
Significance of considering the eGFR
value before COVID-19 as the baseline value
Fig. 1B indicates
that patients with low eGFRs pre-COVID-19 have increased eGFRs
12-months post-COVID-19 infection compared with pre-COVID-19
[85.5±17.2 vs. 73.4±15.5 ml/min/1.73 m2, respectively
(P<0.001)], while the eGFRs were reduced in patients with normal
eGFR pre-COVID-19 (110.4±22.4 ml/min/1.73 m2
pre-COVID-19 vs. 101.4±18.3 ml/min/1.73 m2 12-months
post-COVID-19, P<0.001). However, when the changes in the eGFRs
only in the post-COVID-19 stages were considered, taking the
4-months post-COVID-19 eGFRs as the baseline, the changes in eGFRs
were not significant in any of the subgroups of patients. For
example, the 4- and 12-months post-COVID-19 eGFRs in the patients
with normal eGFRs pre-COVID-19 were 106.1±16.2 vs. 101.4±18.3
ml/min/1.73 m2, respectively (P=0.207), and in the
patients with low eGFRs pre-COVID-19 the 4- and 12-months
post-COVID-19 eGFRs were 89.2±14.8 vs. 85.5±17.2 ml/min/1.73
m2, respectively (P=0.126). Additionally, Fig. 1C indicates that there is a high
correlation between pre-COVID-19 eGFR and the change in eGFR at
12-months post-COVID-19 (r=-0.664; P<0.001). This lost
significance when the eGFR at 4-months post-COVID-19 was correlated
with the change generated between 4- and 12-months post-COVID-19
(r=-0.039; P=0.369) (Fig. 1D). The
previous results indicated variations in the effects of the
COVID-19 infection on the eGFR value among survivors. The eGFR
changes depended on the value used as the baseline for the
comparison (such as the pre-COVID-19 or the 4-months post-COVID-19
eGFR) and subsequently, its clinical implications.
Interactions between variables that
affect changes in the eGFR
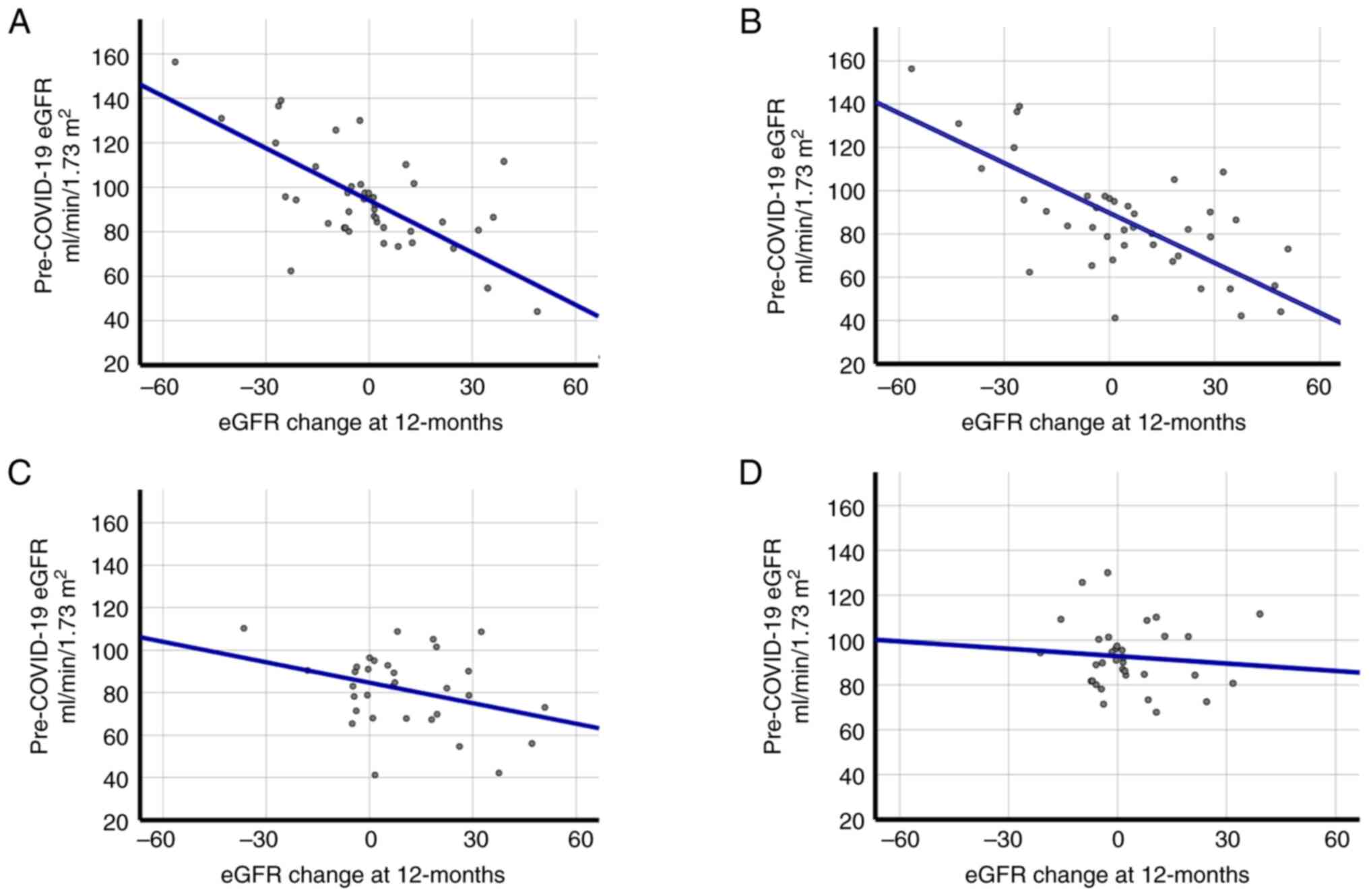
The high correlation between the pre-COVID-19 eGFR
and the change in the eGFR 12-months post-COVID-19 may be
influenced by diabetes or whether the patient was hospitalized
during COVID-19. Fig. 2 presents
that the correlations between the eGFR pre-COVID-19 with the eGFR
change at 12-months are maintained in patients without diabetes
(Fig. 2A; r=-0.754; P<0.001)
and in patients that were hospitalized (Fig. 2B; r=-0.764; P<0.001). This was
not observed in patients with diabetes (Fig. 2C; r=-0.330; P=0.065) and in
patients that were not hospitalized (Fig. 2D; r=-0.096; P=0.589).
Additionally, a multivariate generalized linear
mixed model with a binary logistic regression link was performed to
confirm the interactions between diverse characteristics and
reduced eGFR (≥10 ml/min/1.73 m2) during the first year
after acute COVID-19 (Table IV).
Analysis involving a two-way interaction (A x B, where A and B are
factors) indicated that the combination of patients with a normal
pre-COVID-19 eGFR without diabetes (RR=58.60, 95% CI=11.62-295.38,
P<0.001) or with being hospitalized for COVID-19 (RR=38.07, 95%
CI=8.68-167.00, P<0.001), or patients without diabetes combined
with being hospitalized (RR=11.17, 95% CI=1.95-64.05, P=0.007), had
a higher risk compared with the outcome of the variables
separately. Fig. 2 and Table IV suggested a strong interaction
between the principal risk factors (normal pre-COVID eGFR, without
diabetes and hospitalization in acute COVID-19).
 | Table IVRelative risk from multivariate
generalized linear mixed model with binary logistic regression link
(models without or involving a two-way interaction) of principal
risk factors to have a reduction of eGFR (≥10 ml/min/1.73
m2) during the first year after acute COVID-19. |
Table IV
Relative risk from multivariate
generalized linear mixed model with binary logistic regression link
(models without or involving a two-way interaction) of principal
risk factors to have a reduction of eGFR (≥10 ml/min/1.73
m2) during the first year after acute COVID-19.
| A, Principal risk
factors in a model without interactions |
|---|
| Risk factors to
reduction of eGFR | | 95% CI | |
|---|
| Pre-COVID-19
eGFR | Diabetes | Hospitalized | AdRR | Lower | Upper | P-value |
|---|
| Normala | - | - | 11.55 | 4.53 | 29.45 | <0.001 |
| - | No | - | 6.78 | 2.48 | 18.53 | <0.001 |
| - | - | Yes | 6.17 | 2.39 | 15.96 | <0.001 |
| B, Model involving
a two-way interaction (pre-COVID-19 eGFR and diabetes) |
| Risk factors to
reduction of eGFR | | 95% CI | |
| Pre-COVID-19
eGFR | Diabetes | Hospitalized | AdRR | Lower | Upper | P-value |
| Normala | No | - | 58.60 | 11.62 | 295.38 | <0.001 |
| Normala | Yes | - | 6.34 | 1.22 | 32.91 | 0.028 |
| Lowb | No | - | 3.93 | 0.78 | 19.67 | 0.097 |
| C, Model involving
a two-way interaction (pre-COVID-19 eGFR and hospitalization) |
| Risk factors to
reduction of eGFR | | 95% CI | |
| Pre-COVID-19
eGFR | Diabetes | Hospitalized | AdRR | Lower | Upper | P-value |
| Normala | - | Yes | 38.07 | 8.68 | 167.00 | <0.001 |
| Lowb | - | Yes | 1.70 | 0.32 | 9.16 | 0.533 |
| Normala | - | No | 4.14 | 1.06 | 16.31 | 0.042 |
| D, Model involving
a two-way interaction (diabetes and hospitalization) |
| Risk factors to
reduction of eGFR | | 95% CI | |
| Pre-COVID-19
eGFR | Diabetes | Hospitalized | AdRR | Lower | Upper | P-value |
| - | No | Yes | 11.17 | 1.95 | 64.05 | 0.007 |
| - | Yes | Yes | 1.03 | 0.17 | 6.34 | 0.968 |
| - | No | No | 1.22 | 0.21 | 6.83 | 0.818 |
Discussion
The present cohort study revealed that the changes
in the eGFR among unvaccinated COVID-19 patients 1 year after the
infection vary depending on the clinical characteristics of the
patient. The main risk factors associated with a decreased eGFR of
≥10 ml/min/1.73 m2 were: i) Having a normal eGFR value
before COVID-19; ii) not having diabetes; and iii) being
hospitalized. While an increased eGFR was observed in patients
with: i) Low pre-COVID-19 eGFR; and ii) diabetes and no
hospitalization. Due to the observed variation in the longitudinal
changes in the eGFR during the first year of COVID-19 infection and
its potential clinical implications for kidney health, changes in
the eGFR should probably be evaluated using a pre-COVID-19 eGFR
value instead of only using values post-infection which may falsely
indicate no changes or improvements in kidney function.
The present study revealed that, 1 year after the
initial COVID-19 infection, the majority of patients with a
significantly reduced eGFR had a normal eGFR pre-COVID-19 (30.6%),
compared with the 4.9% of patients with a low eGFR pre-COVID-19. By
contrast, patients with a low eGFR before COVID-19 infection
(73.4±15.5 ml/min/1.73 m2) had a significantly
(P<0.001) increased eGFR 12-months after COVID-19 infection
(85.5±17.2 ml/min/1.73 m2). Additionally, having
diabetes, was a protective factor against a reduced eGFR value
while not having diabetes was a risk factor.
These results, which initially seemed to contradict
what is currently known about traditional risk factors for changes
in eGFR and kidney disease, could be explained by variations in the
expression of angiotensin-converting enzyme 2 (ACE2) in patients
with diabetes and CKD. ACE2 is an enzyme attached to the membranes
of cells in the lungs, arteries, heart, kidney and intestines,
which serves as a cell-surface receptor and is the entry point into
cells for coronaviruses, including SARS-CoV-2(17). Higher expression levels of ACE2 in
patients with comorbidities such as cardiovascular disease, chronic
obstructive pulmonary disease and diabetic pancreatic islets
increase the susceptibility of contracting SARS-CoV-2 infection and
subsequent COVID-19 severity (18). However, it has been demonstrated
that the expression levels of ACE2 in the kidney is reduced in its
glomerular and tubular region in patients with diabetes, as well as
in various nephropathies (diabetes, hypertension and lupus), as
well as in chronic kidney disease (18). This could explain why patients
living with diabetes and CKD before COVID-19 infection have a
protective factor for reduced kidney function once they survived
COVID-19. This is due to the lower number of receptors for the
virus (ACE2) that they have in their kidneys. However, kidney
damage and diabetes are factors for greater disease severity and
mortality in COVID-19(2), which
was not analyzed in the present study.
The increased eGFR post-COVID-19 in the patients
with a low eGFR before COVID-19 should be taken with caution since
it may not necessarily reflect an improvement in renal function
since it is known that an increase in eGFR could be a mechanism for
kidney damage in several clinical conditions (2) (AKI, dehydration and hyperfiltration
in diabetes). Therefore, only evaluating eGFR after COVID-19
infection may not be the most appropriate way to assess kidney
function in surviving patients as it could increase after COVID-19
and be interpreted as a normal or improved function, instead the
pre-COVID-19 eGFR (baseline) should also be evaluated. The findings
of the present study demonstrated that patients that were
hospitalized had a significantly increased reduction of eGFR
compared with patients that were not hospitalized, which is
consistent with previous reports (19,20).
Furthermore, the present study revealed that the use of
anticoagulants and Ivermectin during the acute illness increased
the risk of reducing renal filtration during the first year after
suffering from COVID-19 (6 and 14 times, respectively) (Table I). Coagulopathy with COVID-19
disease is widely reported, and the use of anticoagulants has been
established to combat this disorder (17,21).
It is probable that in the present study, the use of anticoagulants
was associated with the reduction of post-COVID-19 eGFR, not
because of the drug itself, but because of the probable
hypercoagulability present in the patients that conditioned the use
of anticoagulants. Ivermectin has been proposed and used to treat
COVID-19 in different demographic groups. Clinical trials have not
demonstrated significant beneficial effects (22), although its usefulness is still
under discussion (23). However,
although Ivermectin has been postulated to be safe for COVID-19
treatment, there is debate since it is well-known that it can cause
adverse effects (24). Studies in
rats demonstrated that Ivermectin can compromise kidney and liver
integrity (25,26).
The present study demonstrated for the first time
that Ivermectin can cause affectation at the glomerular filtration
level in patients with COVID-19. However, studies with a larger
number of patients are needed to confirm this finding.
By contrast, the use of vitamin C was a protective
factor, which reduced the probability of lowering eGFR
post-COVID-19 by >4 times. Vitamin C, also known as ascorbic
acid, is a water-soluble vitamin. It is an antioxidant and acts as
a scavenger of free radicals, giving it anti-inflammatory
properties. Vitamin C serves a crucial role in modulating cellular
immunity and maintaining vascular integrity (27,28).
Animal trials have demonstrated that vitamin C can prevent kidney
damage caused by Ivermectin administration (27,29).
In experiments with rabbits treated with Ivermectin and vitamin C,
there was a decrease in serum urea, reducing a number of the
adverse effects of Ivermectin (30). As a result, a number of studies
strongly recommend coadministration of vitamin C when prescribing
Ivermectin (27,28).
Vitamin C has been recommended for patients with
COVID-19 as it may act as a protective factor against glomerular
filtration loss caused by COVID-19 or associated pathophysiological
processes. It could also reduce the toxicity of drugs such as
Ivermectin (31,32). Additionally, vitamin C stimulates
endothelial cell proliferation, prevents apoptosis and preserves
endothelial function while enhancing nitric oxide generation
(33).
In addition to other factors, blood type was also
analyzed in the present study. Recent research indicates that
individuals with blood type B may have different immune responses
and susceptibility patterns to viral infections compared with other
blood groups (34). In the present
study, it was observed that having blood type B (B and AB groups)
was correlated with being a protective factor against the loss of
eGFR. This is consistent with previous studies that demonstrate
that this blood group is protective for long-COVID-19 (1,35).
It has also been demonstrated that patients with blood type B/AB
exhibited a longer median time to end-stage renal disease compared
with patients with blood type O/A (36), which suggests that the B blood
group antigen may have a protective effect against the progression
of IgA nephropathy. This association could potentially be
associated with its influence on the inflammatory status of the
patient (36). However, further
research is necessary to fully understand the effects of blood type
B on kidney function.
A strength of the present study was that the
pre-COVID-19 data was considered as the baseline to analyze the
longitudinal changes of eGFR post-illness, which helped to assess
those changes with more certainty. As presented in Fig. 2, taking only post-COVID-19 data, as
has been performed, to the best of our knowledge, in the majority
of studies analyzing renal function (7,8),
could lead to incorrect interpretations. Failure to stratify the
population would also lead to inaccurate results. For example, if
the data of the entire population was used and compared before and
after a year of COVID-19, no changes would be observed (92.1±26.7
vs. 92.9±19.3 ml/min/1.73 m2, P=1.000) in the eGFR,
which is not correct for all subgroups of patients. The present
study also had limitations, mainly the number of patients, a lack
of urinary protein/albumin detection before and after COVID-19, and
other additional markers of inflammation or coagulopathy that would
have enriched the work. These aspects would be desirable to
consider in future research.
In summary, the changes in the eGFR associated to
COVID-19 infection in unvaccinated patients were highly variable
and depended on the characteristics of the patient. Considering an
eGFR value before COVID-19 as a baseline for the comparison
appeared to be crucial for the interpretation of the results. Other
factors were also identified as increasing the chance of reducing
the eGFR (such as the use of Ivermectin or anticoagulants), or as
protective factors (such as vitamin C treatment or B blood type).
These factors interact with each other to further increase risk.
Renal function in COVID-19 survivors is a relevant topic that
requires further investigation. Identifying characteristics of
those patients with changes in eGFR after COVID-19 may help
prioritize which patients need close outpatient follow-up
post-pandemic. An eGFR <90 ml/min/1.73 m2 cannot
diagnose CKD, especially for patients with an eGFR between 60 and
90 ml/min/1.73 m2 in the absence of albuminuria;
however, patients with the preclinical manifestation of kidney
damage should not be overlooked as albumin in urine was not
measured. Future studies are required to answer these
questions.
Supplementary Material
Main personal history and clinical
characteristics of the participants.
Univariate and multivariate logistic
regression analyses of factors associated with low eGFR (<90
ml/min/1.73m2) during the pre-coronavirus disease
2019period.
Acknowledgements
The authors would like to thank Dr Janet
Diaz-Martinez from Florida International University (Miami, USA)
and the contributions of Professor Julio V. Barrios Nuñez from
University of Colima (Colima, Mexico) for their assistance with
English language editing.
Funding
Funding: The present study was supported by the National Council
of Humanities, Sciences and Technologies (CONAHCYT) (grant no.
319282; Call for Frontier Science, Modality: Paradigms and
Controversies of Science, ).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JGE and IDE participated in the conception of the
study. KILA, JAGS and MICR participated in the acquisition of the
data for the work. JDM, JGOO, EMZ, VM, VGCM, HRTL JAGS, MROC, GAHF,
FRL, MICR and IDE participated in the design of the study, and
HRTL, JAGS, MROC, GAHF, KILA and IDE participated in the
analysis/interpretation of the data. JGE, JDM, KILA, MROC and IDE
drafted and revised the article. JGE, VGCM, KILA, JAGS, MICR and
IDE provided intellectual content of critical importance to the
work described. All authors read and approved the final version of
the manuscript and confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The study was approved by the local health research
committee of the IMSS-Colima, Mexico (approval no. R-2020-601-041)
on the 24th September 2020. Inclusion in the study was voluntary,
and each patient or their legal representative signed an informed
consent letter in cases where the patient could not sign.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID IDs: Jose Guzmán-Esquivel,
0000-0001-9678-8899; Janet Diaz-Martinez, 0000-0003-3295-4768; Jose
G. Ortega-Ortiz, 0009-0005-3920-8153; Efren Murillo-Zamora,
0000-0002-1118-498X; Valery Melnikov, 0000-0002-0869-6394; Héctor
R. Tejeda-Luna, 0009-0009-1573-4641; Vanessa G. Cosio-Medina,
0009-0002-7023-4710; Kara I. Llerenas-Aguirre, 0009-0002-1331-9136;
Jose A. Guzman-Solorzano, 0000-0003-2326-9822; Gustavo A.
Hernandez-Fuentes, 0000-0003-4685-3095; Maria R. Ochoa-Castro,
0000-0002-4347-4973; Martha I. Cardenas-Rojas, 0000-0001-7117-5922;
Fabian Rojas-Larios, 0000-0003-1744-9173; Ivan Delgado-Enciso,
0000-0001-9848-862X.
References
|
1
|
Guzman-Esquivel J, Mendoza-Hernandez MA,
Guzman-Solorzano HP, Sarmiento-Hernandez KA, Rodriguez-Sanchez IP,
Martinez-Fierro ML, Paz-Michel BA, Murillo-Zamora E, Rojas-Larios
F, Lugo-Trampe A, et al: Clinical characteristics in the acute
phase of COVID-19 that predict long COVID: Tachycardia, myalgias,
severity, and use of antibiotics as main risk factors, while
education and blood group B are protective. Healthcare (Basel).
11(197)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Delgado-Enciso I, Paz-Garcia J,
Barajas-Saucedo CE, Mokay-Ramírez KA, Meza-Robles C, Lopez-Flores
R, Delgado-Machuca M, Murillo-Zamora E, Toscano-Velazquez JA,
Delgado-Enciso J, et al: Safety and efficacy of a COVID-19
treatment with nebulized and/or intravenous neutral electrolyzed
saline combined with usual medical care vs usual medical care
alone: A randomized, open-label, controlled trial. Exp Ther Med.
22(915)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Diao B, Wang C, Wang R, Feng Z, Zhang J,
Yang H, Tan Y, Wang H, Wang C, Liu L, et al: Human kidney is a
target for novel severe acute respiratory syndrome coronavirus 2
infection. Nat Commun. 12(2506)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yende S and Parikh CR: Long COVID and
kidney disease. Nat Rev Nephrol. 17:792–793. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Faour WH, Choaib A, Issa E, Choueiry FE,
Shbaklo K, Alhajj M, Sawaya RT, Harhous Z, Alefishat E and Nader M:
Mechanisms of COVID-19-induced kidney injury and current
pharmacotherapies. Inflamm Res. 71:39–56. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Spruill WJ, Wade WE and Cobb HH III:
Comparison of estimated glomerular filtration rate with estimated
creatinine clearance in the dosing of drugs requiring adjustments
in elderly patients with declining renal function. Am J Geriatr
Pharmacother. 6:153–160. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tong M, Yan X, Jiang Y, Jin Z, Zhu S, Zou
L, Liu Y, Zheng Q, Chen G, Gu R, et al: Endothelial biomarkers in
patients recovered from COVID-19 one year after hospital discharge:
A cross-sectional study. Mediterr J Hematol Infect Dis.
14(e2022033)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nugent J, Aklilu A, Yamamoto Y, Simonov M,
Li F, Biswas A, Ghazi L, Greenberg H, Mansour G, Moledina G and
Wilson FP: Assessment of acute kidney injury and longitudinal
kidney function after hospital discharge among patients with and
without COVID-19. JAMA Netw Open. 4(e211095)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou M, Tan X, Luo P, Xu J, Yin Z, Liao T,
Wang S, Wang Z and Jin Y: Changes in glomerular filtration rate and
metabolomic differences in severely ill coronavirus disease
survivors 3 months after discharge. Biochim Biophys Acta Mol Basis
Dis. 1868(166289)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Levey AS, Bosch JP, Lewis JB, Greene T,
Rogers N and Roth D: A more accurate method to estimate glomerular
filtration rate from serum creatinine: A new prediction equation.
Modification of diet in renal disease study group. Ann Intern Med.
130:461–470. 1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ruggenenti P, Cortinovis M, Trillini M,
Parvanova A, Abbate M, Satriano C, Salvetti F, Bossi AC, Trevisan
R, Perna A, et al: Long-term kidney and systemic effects of calorie
restriction in overweight or obese type 2 diabetic patients
(C.Re.S.O. 2 randomized controlled trial). Diabetes Res Clin Pract.
185(109804)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
McDonald JH: Biological statistics. 1st
edition. California State, 2023.
|
|
13
|
Jaffa MA, Gebregziabher M, Luttrell DK,
Luttrell LM and Jaffa AA: Multivariate generalized linear mixed
models with random intercepts to analyze cardiovascular risk
markers in type-1 diabetic patients. J Appl Stat. 43:1447–1464.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Baum MK, Tamargo JA, Diaz-Martinez J,
Delgado-Enciso I, Meade CS, Kirk GD, Mehta SH, Moore R, Kipke MD,
Shoptaw SJ, et al: HIV, psychological resilience, and substance
misuse during the COVID-19 pandemic: A multi-cohort study. Drug
Alcohol Depend. 231(109230)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rosner B: Fundamentals of biostatistics.
3rd edition. PWS-Kent Pub. Co, Boston, Mass, 1990.
|
|
16
|
Pewsner D, Battaglia M, Minder C, Marx A,
Bucher HC and Egger M: Ruling a diagnosis in or out with ‘SpPIn’
and ‘SnNOut’: A note of caution. BMJ. 329:209–213. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hardenberg JHB and Luft FC: Covid-19, ACE2
and the kidney. Acta Physiol (Oxf). 230(e13539)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rodrigues R and Costa De Oliveira S: The
impact of angiotensin-converting enzyme 2 (ACE2) expression levels
in patients with comorbidities on COVID-19 severity: A
comprehensive review. Microorganisms. 9(1692)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zavori L, Molnar T, Varnai R, Kanizsai A,
Nagy L, Vadkerti B, Szirmay B, Schwarcz A and Csecsei P: Cystatin-c
may indicate subclinical renal involvement, while orosomucoid is
associated with fatigue in patients with long-COVID syndrome. J
Pers Med. 13(371)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Torjesen I: Covid-19: Infection increases
the risk of kidney disease even in mild cases, finds study. BMJ.
374(n2189)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Reis S, Popp M, Schießer S, Metzendorf MI,
Kranke P, Meybohm P and Weibel S: Anticoagulation in COVID-19
patients-an updated systematic review and meta-analysis. Thromb
Res. 219:40–48. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Reis G, Silva EASM, Silva DCM, Thabane L,
Milagres AC, Ferreira TS, Dos Santos CVQ, Campos VHS, Nogueira AMR,
de Almeida APFG, et al: Effect of early treatment with Ivermectin
among patients with COVID-19. N Engl J Med. 386:1721–1731.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Furlan L: Ivermectin treatment for
COVID-19. N Engl J Med. 387(e66)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Barus R, Gautier S and Wabont G:
Ivermectin treatment for COVID-19. N Engl J Med. 387(e66)2022.
|
|
25
|
Arise RO and Malomo SO: Effects of
ivermectin and albendazole on some liver and kidney function
indices in rats. Afr J Biochem Res. 3:190–197. 2009.
|
|
26
|
Nunes LLA and Lima TDM: Use of medicines
for COVID-19 treatment in patients with loss of kidney function: A
narrative review. J Bras Nefrol. 43:254–262. 2021.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
27
|
Tawfeek SE, Domouky AM and Abdel-Kareem
RH: Protective effect of vitamin C against ivermectin induced
nephrotoxicity in different age groups of male wistar rats:
Bio-histopathological study. Anat Cell Biol. 54:501–517.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Niki E: Action of ascorbic acid as a
scavenger of active and stable oxygen radicals. Am J Clin Nutr. 54
(6 Suppl):1119S–1124S. 1991.PubMed/NCBI View Article : Google Scholar
|
|
29
|
GabAllh MS, El-mashad ABE, Amin AA and
Darweish MM: Pathological studies on effects of ivermectin on male
and female rabbits. Benha Vet Med J. 32:104–112. 2017.
|
|
30
|
Khawla B, Al-Deen A and Al-Masoudi EA,
Majeed SK and Al-Masoudi EA: Histopathological and biochemical
effects of ivermectin on kidney functions, lung, and the
ameliorative effects of vitamin C in rabbits (lupus cuniculus).
Basrah J Vet Res. 14:110–124. 2016.
|
|
31
|
Xu F, Wen Y, Hu X, Wang T and Chen G: The
potential use of vitamin C to prevent kidney injury in patients
with COVID-19. Diseases. 9(46)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hazan S, Dave S, Gunaratne AW, Dolai S,
Clancy RL, McCullough PA and Borody TJ: Effectiveness of
ivermectin-based multidrug therapy in severely hypoxic, ambulatory
COVID-19 patients. Future Microbiol. 17:339–350. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
May JM and Harrison FE: Role of vitamin C
in the function of the vascular endothelium. Antioxid Redox Signal.
19:2068–2083. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rana R, Ranjan V and Kumar N: Association
of ABO and Rh blood group in susceptibility, severity, and
mortality of coronavirus disease 2019: A hospital-based study from
Delhi, India. Front Cell Infect Microbiol.
11(767771)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim Y, Latz CA, DeCarlo CS, Lee S, Png
CYM, Kibrik P, Sung E, Alabi O and Dua A: Relationship between
blood type and outcomes following COVID-19 infection. Semin Vasc
Surg. 34:125–131. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang M, Xie J, Ouyang Y, Zhang X, Shi M,
Li X, Wang Z, Shen P, Ren H, Zhang W, et al: ABO blood type is
associated with renal outcomes in patients with IgA nephropathy.
Oncotarget. 8:73603–73612. 2017.PubMed/NCBI View Article : Google Scholar
|