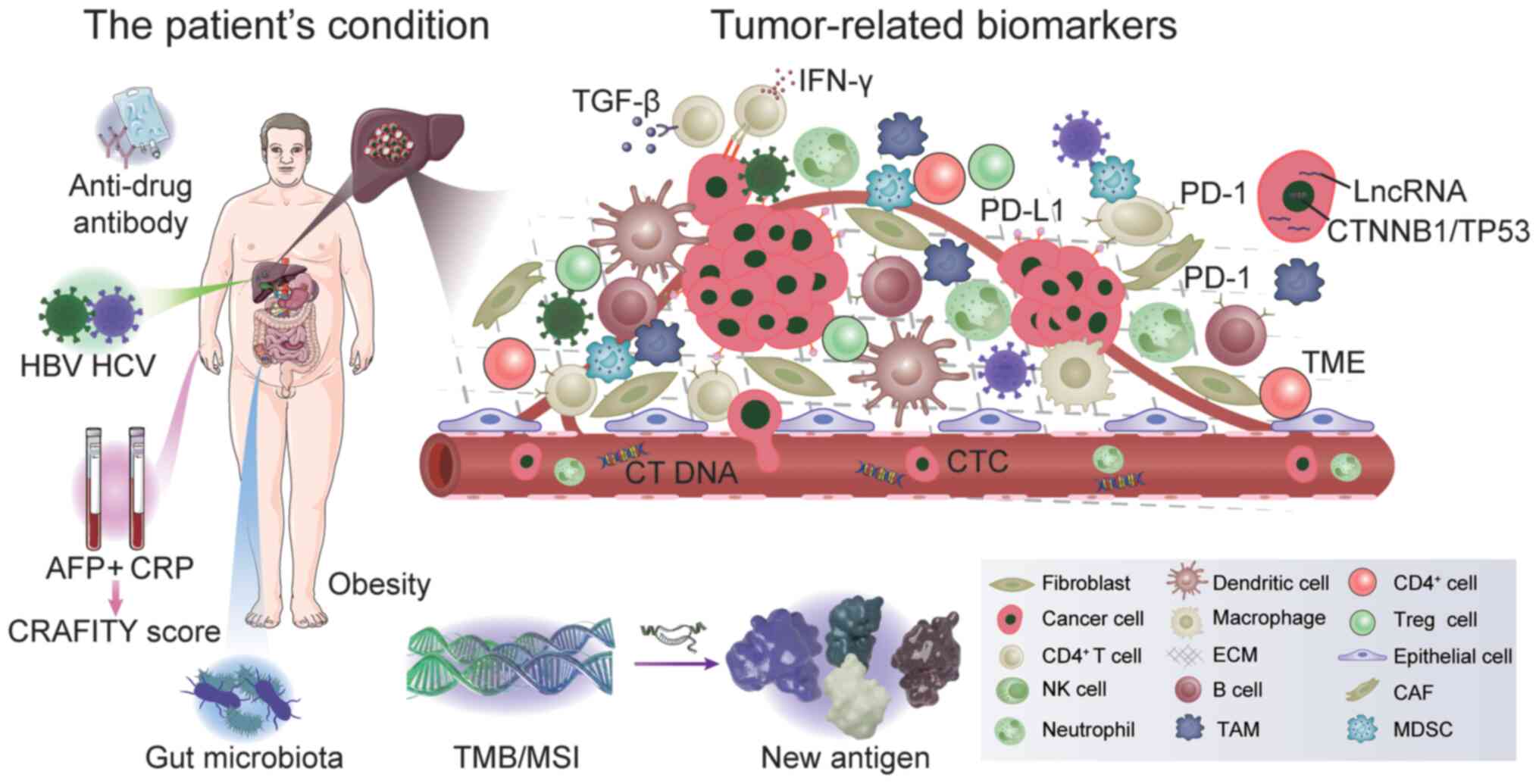
According to the 2020 global liver cancer
epidemiology, liver cancer is responsible for 4.69% of all cases of
cancer and 8.34% of all mortalities from cancer (1,2).
Hepatocellular carcinoma (HCC) pathogenesis has been associated
with infection by hepatitis B virus (HBV) and hepatitis C virus
(HCV), alcohol abuse, non-alcoholic steatohepatitis, cirrhosis, and
a family history of HCC, with cirrhosis caused by HBV being
important as it generates 60% of all cases in China (3). Although surgical resection,
radiofrequency ablation, transcatheter arterial chemoembolization
(TACE), radiotherapy and chemotherapy are used as potentially
curative treatments, the prognosis remains poor for patients with
advanced (stage 2-4) disease (3,4). The
emergence of cancer immunotherapies using immune checkpoint
inhibitors (ICIs) has begun a new era of anti-tumor therapy during
the past decade (5).
ICIs inhibit the activity of immune checkpoint
proteins, such as PD-1, PD-L1 and CTLA-4, which restrict the immune
response against tumors, thus reactivating antitumor activity
(6). This immunotherapy has
demonstrated promising results in patients with advanced,
inoperable liver cancer and those undergoing radiofrequency
ablation. For example, the IMbrve150 phase III trial demonstrated
reductions in both tumor progression and mortality with the
combined use of two ICIs, Atezolizumab and Bevacizumab, leading to
Food and Drug Administration (FDA) approval for this drug
combination as a first-line treatment for patients with
unresectable or metastatic HCC (7). Additionally, the CheckMate040 and
KEYNOTE-224 trials established Nivolumab and Pembrolizumab as
second-line immunotherapies for liver cancer, although subsequent
trials did not observe an improvement in overall survival (OS)
(8). Details of the current
immunotherapy clinical trials are provided in Table I. However, HCC is a heterogeneous
disease with multiple immunological features and thus, despite
encouraging results on specific forms of HCC, the use of
immunotherapy does not guarantee clinical benefit for all patients
with HCC (9). Data from randomized
controlled trials indicate that only 10-30% of patients with
advanced HCC who undergo immunotherapy achieve a complete response
(CR) or partial response (PR) (7,10-13).
A major contributing factor to this is the paucity of markers for
the early diagnosis and treatment of HCC. The identification and
application of predictive biomarkers that can accurately
distinguish patients that would benefit from immunotherapy could
enable the use of precision treatment in HCC immunotherapy,
allowing the proper allocation of medical resources and avoiding
the exposure of non-responsive patients to treatment toxicity.
Therefore, there is an urgent need for predictive markers, whether
positive or negative prognostic markers, to screen individuals for
immunotherapy suitability. The present review summarizes the
currently known biomarkers for immunotherapy, as presented in
Fig. 1.
HCC progression is known to be associated with HBV
or HCV infection and liver cirrhosis. However, evidence from the
CheckMate 040 and KEYNOTE-224 trials indicated that viral load or
immune responses to HBV/HCV may not necessarily influence T cell
activation and subsequent antitumor activity (14,15).
Furthermore, the results of a meta-analysis revealed that neither
HBV nor HCV affected the tumor immune microenvironment, and the
presence or absence of viral infection was not an effective
criterion for the selection of patients for programmed death 1
(PD-1)/programmed death-ligand 1 (PD-L1) immunotherapy (16).
Obesity and being overweight are considered to be
risk factors for numerous diseases, including cancer (17). Obesity caused by a high-fat diet
impairs CD8+ T cell infiltration and function, which
alters the immune microenvironment in mice and enhances tumor
growth (18). In contrast, another
study revealed that patients with advanced HCC that had higher body
mass indices (BMI; >25) appeared to have an improved prognosis
following immunotherapy (19).
In clinical practice, the serum AFP level represents
a primary indicator used in diagnosis and for monitoring the
effectiveness of liver cancer treatment (20). The AFP levels are increased in
~two-thirds of patients with HCC (21). The expression levels of certain
immune checkpoint proteins, such as SIGLEC15, CTLA4, CD274,
PDCD1LG2, PDCD1, TIGIT, LAG3 and HAVCR2, have been revealed to
differ with regards to the AFP level (22). It has been suggested that AFP could
be used as a prognostic biomarker for HCC immunotherapy. For
example, Spahn et al (23)
observed that baseline AFP concentrations <400 µg l-1
before the start of treatment were associated with increased rates
of PR or CR and reduced rates of progressive disease (PD). However,
in the CheckMate 459 trial, patients with high baseline AFP levels
(>400 ng/ml) had an increased overall survival (OS) (11). The objective response rate (ORR)
was revealed to be positively associated with the early stages of
AFP reduction therapy and PD-1 blockade, while progression-free
survival (PFS) and OS were also increased (2,24).
Therefore, the combination of AFP with other serum markers deserves
further investigation to improve diagnostic accuracy. For example,
previous studies have indicated that the C-reactive protein (CRP)
and AFP in immunotherapy (CRAFITY) score, which combines CRP with
AFP, can be used to predict treatment outcomes and
treatment-associated adverse events in patients with HCC undergoing
immunotherapy (25,26). However, there is still disagreement
over whether AFP can serve as a prognostic biomarker for
immunotherapy (25,27,28).
Blood inflammatory biomarkers are both affordable
and useful for the early identification of disease. It has been
suggested that a neutrophil-lymphocyte ratio (NLR) ≥5 and a
platelet-lymphocyte ratio (PLRs) ≥300 are independent prognostic
factors for OS, predicting reduced OS, PFS, ORR and an increased
risk of mortality in patients receiving immunotherapy (29,30).
Similarly, a multicenter study revealed that the NLR could predict
PFS in patients with unresectable HCC treated with Atezolizumab
plus Bevacizumab, particularly in patients with modified
albumin-bilirubin grade 1 or 2a (31).
According to recent studies, the gut microbiota
serves an important role in the development and occurrence of liver
cancer (36-38).
The underlying mechanism involves the gut-liver axis and is
associated with dysbiosis, intestinal permeability and bacterial
metabolites. Dysbiosis and intestinal permeability make it easier
for bacterial metabolites to reach the liver. Bacterial products
such as lipopolysaccharides (LPS) can cause inflammation and cancer
in the liver (39,40). In addition, Toll-like receptor 4
(TLR-4), which is widely distributed on the surfaces of various
liver cells and has been demonstrated to mediate hepatic
carcinogenesis, is the specific recognition receptor for LPS
(41). In a study by Chung et
al (36) the stools of eight
antibiotic-treated patients were collected for microbiota analysis.
Patients receiving Nivolumab demonstrated no alterations in the
diversity and composition of their gut microbiota. However, a
skewed Firmicutes/Bacteroidetes ratio and a low
Prevotella/Bacteroides ratio were revealed to predict a poor
immunotherapy response in patients with liver cancer, while the
presence of Akkermansia species suggested a positive prognosis
(36). Another study on 167
patients with hepatobiliary cancer treated with immunotherapy,
revealed that a number of bacteria, such as Lachnospiraceae
bacterium-GAM79, were associated with an improved OS and PFS after
treatment, while other bacteria, such as Veillonella, were
associated with an increased risk of immune-associated side effects
(37). There is also an
association between the diversity of the gut microbiota and the
levels of aspartate aminotransferase and alanine aminotransferase,
which reflect liver function (38). Stool samples from patients that
responded to anti-PD-1 therapy contained a greater taxonomic
abundance compared with those of non-responders. The
characterization of the dynamic changes in the gut microbiome can
be useful for an earlier prediction of anti-PD-1 treatment outcomes
in HCC. With the rapid development of microbial multi-omics,
analysis of the gut microbiota has potential as a predictive
biomarker for liver cancer immunotherapy. It has been reported that
fecal microbiota transplantation from donors that achieved CR/PR on
long-term anti-PD-1 therapy to patients failed to respond to
immunotherapy can increase the intra-tumoral lymphocyte
infiltration (42).
ICIs may be immunogenic and recognized by the human
immune system, which could lead to the induction of the humoral
immunity and subsequent adverse ADA responses (43). Different monoclonal antibodies are
associated with different rates of ADA development, with
Atezolizumab having the highest rate (~30%) compared with others
(5-10%) (44). ADAs may affect the
pharmacokinetics and pharmacodynamics of therapeutic antibodies,
and may even neutralize the therapeutic antibodies (45). A cohort study by Kim et al
(46) reported that increased ADA
levels at the second Atezolizumab injection (day 1 of chemotherapy
treatment cycle 2) may be associated with poor clinical outcomes.
Reducing Atezolizumab exposure in patients with advanced HCC,
Atezolizumab and Bevacizumab administration and an established ADA
level >1,000 ng/ml can accurately predict the curative effect
(46). Anti-Atezolizumab
antibody-positive patients did not demonstrate a reduction in the
frequency or severity of adverse events (44). However, a meta-analysis of 11
clinical trials, based on studies using Atezolizumab monotherapy or
combination therapy, demonstrated that unadjusted descriptive
analyses could not identify a clear association between the ADA
status and the frequency or severity of adverse events.
Furthermore, any ADA impact was not driven by neutralizing activity
(47). The most distinctive
feature of ADA assays is their lack of accurate quantification, as
there is no reliable calibration reference standard for ADAs
(48). Currently, there is no
effective method for predicting which drugs may cause ADAs.
Table II provides a brief
overview of the host-associated biomarkers that are used for HCC
immunotherapy.
PD-1 is an immunosuppressive transmembrane protein
that is expressed on the surface of cells such as T, B and myeloid
cells. By binding to PD-L1, it inhibits T cell activation and
proliferation, negatively stimulates T cells, blocks the T cell
receptor, and negatively impacts how the immune system combats
cancer (49,50). Inhibition of PD-1/PD-L1 prevents
the interaction between PD-1 on T cells and PD-L1 on tumor cells,
thus, restoring the T cell-mediated antitumor immune response
(51). However, anti-PD-1/PD-L1
therapies are only effective in 20-40% of patients (52). Numerous studies have demonstrated
that the expression level of PD-L1 on immune and tumor cells is
associated with the anti-PD-1 treatment response in HCC (14,15,53,54).
However, these studies varied in their detection techniques and
methods used to measure PD-L1, so there is no universal standard
for the detection and quantification of PD-L1(55). The most commonly used methods for
measuring PD-L1 are the tumor proportional score (TPS) and the
combined positive score (CPS) (56).
The KEYNOTE-244 study retrospectively analyzed the
association between PD-L1 expression levels and response to
Pembrolizumab treatment, finding a treatment response to
Pembrolizumab when PD-L1 was quantified using CPS but not when it
was quantified using TPS (15).
However, a study on the response to Nivolumab demonstrated
different outcomes. Patients that tested positive for PD-L1 (TPS
≥1%) had an increased median OS (28.1 months) compared with those
that tested negative for PD-L1 (median OS of 16.6 months) (13). Recently, a meta-analysis of nine
cohort studies (seven PD-L1 and three PD-1) demonstrated that
PD1/PDL-1 was a marker of poor survival rate regardless of OS, HR,
CI, disease-free survival (DFS) and other evaluation methods
(54). High PD-1/PD-L1 expression
levels were associated with aging, multiple tumors, high
α-fetoprotein levels and an advanced Barcelona Clinic liver cancer
stage (14,53). In addition, PD-L1, as measured by
CD274 (a PD-L1 messenger RNA) expression levels in the IMbrave150
trial, were revealed to be increased in patient with CR/PR compared
with that in patients with SD/PD. Patients with high CD274 levels
also demonstrated an increased PFS compared with those with low
expression levels (54). However,
PD-L1 expression levels are influenced by various factors. PD-L1
can be induced by IFN-γ, hypoxia or TLR-mediated pathways (57). Tumor heterogeneity and the tumor
interstitium were observed to be the primary causes of inconsistent
outcomes, followed by differences in detection methods (58). Thus, the value of the PD-L1
expression level as a predictive biomarker for immune checkpoint
blockade therapy in HCC has been reduced.
The CTNNB1 gene encodes the intracellular signaling
transducer β-catenin, which is essential for embryonic development,
cell fate determination, proliferation and migration (59). One of the key signaling pathways
that control liver regeneration, homeostasis and tumorigenesis is
the Wnt/β-catenin cascade (60,61).
In a mouse model of HCC, activation of this pathway promoted immune
evasion and conferred resistance to anti-PD-1 therapy (62,63).
Similar outcomes were observed in liver cancer. Harding et
al (64) reported that all 10
patients with mutations in components of the Wnt-β-catenin pathway
demonstrated PD and a reduced median survival rate compared with
patients without mutations. This implies that the Wnt-β-catenin
pathway is a marker of immunotherapy sensitivity (62). Additionally, patients with HCC with
mutations in CTNNB1 were revealed to have increased OS and PFS
compared with patients with no mutations in CTNNB1. Thus, CTNNB1
may serve as an independent prognostic factor in HCC following
immunotherapy (65,66).
Another dysregulated signaling pathway is the
transforming growth factor-β (TGF-β) pathway which is involved in
inflammation, fibrogenesis and immunomodulation in the HCC
microenvironment (67). Increased
TGF-β signaling may lead to T cell exhaustion through the
upregulation of PD-1 signaling, while inhibition of TGF-β signaling
may increase the anti-tumor immunity in HCC (68). Studies using mouse models have
indicated that a combination of blocking TGF-β signaling and
anti-PD-L1 antibodies could reduce TGF-β signaling, promote T cell
infiltration into the tumor environment, and reshape the immune
microenvironment, thus, stimulating effective anti-tumor immune
responses and tumor regression (69,70).
Numerous studies are investigating cancerous genes
in this era of precision medicine. Least absolute shrinkage and
selection operator regression analysis of data from The Cancer
Genome Atlas and International Cancer Genome Consortium dataset and
the International Cancer Genome Consortium database revealed nine
genes (ANP32B, BMI1, ASF1A, CDK5, BUB1, CBX3, CBX2, CDK1 and
BCORL1) to be independent predictors of HCC prognosis (71). Another study identified 11
immune-associated genes, NDRG1, MAPT, FABP6, CACYBP, HSP90AA1,
ISG20L2, NRAS, BRD8, OSGIN1, CD320 and PSMD14, that were used to
predict immune cell infiltration and construct a prognostic index
for the prediction of immunotherapy efficacy (72).
The number of somatic mutations per DNA megabase
(Mb), known as the TMB, is used to quantitatively evaluate the
mutations carried by tumor cells (73). Greater numbers of neo-antigens,
indicated by increased TMB, increases the likelihood that T cells
will be recognized, which is clinically associated with improved
ICI outcomes. Thus, the TMB is regarded as a reliable marker for
estimating the effectiveness of immunotherapy in HCC. Data on 17
types of cancer were collected in a study by Samstein et al
(74) confirming the initial
finding that a high TMB is associated to immunotherapy
effectiveness. Based in part on data from the KEYNOTE-158 study,
the FDA approved the use of Pembrolizumab for solid tumors with 10
or more mutations/Mb in June 2020. However, there is not a fixed
value of TMB for all types of cancer as the number of mutations
defining TMB-high status varies with the type of cancer (74,75).
Liver cancer has a median number of 4 mutations/Mb (n=755), with
only 0.8% of patients having TMB-high tumors.
Mismatch repair (MMR) in clinical practice is
assessed largely by the reactions of four representative
MMR-associated proteins (MLH1, MSH2, MSH6 and PMS2). One of the
missing proteins is called DNA mismatch repair deficiency (dMMR)
(78,79). MSI occurs during DNA replication,
leading to alterations in the length or base composition of the MS,
mainly as a result of dMMR. The MSI status of a tumor can be
categorized as stable (MSS), high instability (MSI-H) or low
instability (80). Perbolizumab
was given FDA approval in 2017 to treat MSI-H/dMMR solid tumors
that are unresectable or metastatic, have progressed after prior
therapy and for which there are no adequate alternative treatment
options. The first pan-cancer marker identified, MSI-H/dMMR, is now
being used to direct tumor immunotherapy, and has been demonstrated
to have clinical value for the treatment of tumors (81,82).
Even though the incidence of the MSI-H phenotype in HCC is low at
only ~2%, inflammation-mediated MMR pathway dysfunction may be to
blame for the accumulation of mutations observed during
hepatitis-associated tumorigenesis (78,83,84).
According to several reports, Pembrolizumab treatment completely
reverses MSI in patients with advanced HCC (84,85).
However, a study revealed that out of 50 patients, only one (2.0%)
was identified as MSI-H with high TMB, CD8+ lymphocyte
infiltration, and low VEGF expression levels, and that patient did
not experience as dramatic a response to Pembrolizumab treatment as
suggested by other reports (86).
MSI/dMMR is frequently used as a measure of the efficacy of
immunotherapy for colorectal cancer (87). The most recent clinical study on
neoadjuvant therapy for colorectal cancer included 12 patients with
MSI-H/dMMR, and it revealed that all patients that finished
treatment with checkpoint blockade had a clinically CR, without any
reported adverse events of grade 3 or higher (88). However, another study that compared
the OS of patients with resected colorectal cancer liver metastases
between patients with MSS and MSI revealed that patients with MSI
had a reduced OS, indicating a poor prognosis (89). The low proportion of patients with
high TMB or MSI in HCC compared with gastric and colon cancer, and
the sparse and contradictory information available, mainly from a
small number of case reports or case series, make it impossible to
determine predictive accuracy (78,86).
The TME describes the area surrounding the tumor,
containing various cell types, such as endothelial, immune cells
and fibroblasts. Extracellular components, such as cytokines, the
extracellular matrix, growth factors, hormones and peripheral blood
vessels, are associated to the development and metastasis of tumors
(90). In addition to these, the
TME in liver cancer also contains pit cells, Kupffer cells, hepatic
stellate cells, liver sinusoidal endothelial cells and
hematopoietic stem cells (91). As
CD8+ lymphocytes are the most common T cell subset, the
present review focuses on them. In several tumor types, high
expression levels of CD8+ tumor-infiltrating lymphocytes
(TILs) are associated with a favorable prognosis (92). High intra-tumoral CD8+
TIL levels have been associated with longer OS and DFS in a
meta-analysis involving a total of 3,509 patients (93). Nevertheless, according to the
experimental data from the CheckMate 040 trial, increased
CD3+ or CD8+ tumor-infiltrating T cells were
associated with improved survival rates and treatment responses,
although this association was not apparent (94). Additionally, prognosis was not
revealed to be associated to macrophage markers (14). Exhausted CD8+ T cells
also exhibit a lack of cytotoxicity, decreased release of
proinflammatory cytokines, such as IL-2, IL-12, IFN-γ and TNF-α,
increased expression levels of inhibitory receptors, such as PD-1
and CTLA-4, and transcriptional and epigenetic changes (95). Additionally, compared with other
types of cancer, HCC has an increased concentration of PD-1(Hi)
CD8+ T cells that express exhaustion-associated
inhibitory receptors, such as PD-1 and CTLA-4 on the surface of T
cells, which is indicative of a poor prognosis (96). Immunohistochemistry (IHC) has
demonstrated a strong association between an increased proportion
of CD38+ cells and an improved response to ICIs
(97). An unfavorable prognosis
was revealed to be predicted by the upregulation of the LDHA,
BFSP1, PPAT, NR0B1 and PFKFB4 genes, as demonstrated by a tissue
microarray analysis (98). Thus,
the TME can be used as a biomarker for the precise identification
of patients who are sensitive to immunotherapy. However, the
clinical use of TME components as biomarkers to predict the
response to immunotherapy in HCC appears challenging. There is a
need for the standardization and validation of test methods, test
timing and test interpretation.
Evaluation of the treatment of patients with liver
cancer should be performed throughout the treatment course, with
the need for convenient, rapid and reproducible methods. It is
evident that repeated multiple invasive biopsies of tumor tissue
are unacceptable to patients, and the detection of circulating
tumor cells (CTCs) in the blood via liquid biopsy would be more
convenient for clinical use. Peripheral blood can be used to detect
circulating biomarkers such as exosomes, circulating tumor DNA
(ctDNA), CTCs and metabolites (99). Single- or double-stranded DNA that
responds to tumor heterogeneity forms ctDNA, which is derived from
tumor cells (100). According to
a study by Cabel et al (101), synchronous changes in the ctDNA
levels and the tumor size at 8 weeks after immunotherapy were
predictors of DFP and OS in non-small cell lung cancer (NSCLC) and
colorectal cancer. However, the plasma contains only trace amounts
of ctDNA, which also fluctuates dynamically, resulting in a
fluctuating detection threshold and false negatives (102). Another possible circulating
biomarker is CTCs. The potential of HCC-CTCs expressing PD-L1 as
prognostic and predictive biomarkers was investigated in a study by
Winograd et al (103),
which revealed that PD-L1-positive CTCs were typical of advanced
HCC. Immunotherapy led to a good therapeutic response in patients
with PD-L1+ CTCs (103). According to a different study,
patients with 20% PD-L1-positive CTCs had an increased OS (median
not reached vs. 8.9 months) and PFS (median 6.1 vs. 2.9 months)
compared with patients with <20% PDL1-positive CTCs (76). These findings suggest that baseline
ctDNA and high CTC levels might be used as predictors to select
patients for immunotherapy and that dynamic changes in measured
CTCs might be used as an indicator of treatment response in liver
cancer, although this is still at an early stage. Further research
is required on other circulating biomarkers such as extracellular
vesicles and circulating RNA.
There are a number of immunotherapy drugs applied in
the treatment of liver cancer. Immunotherapy in combination with
other anti-tumor treatments, such as TKI, VEGFR, TACE or double
immunotherapy, is becoming more popular. It is challenging to
identify biomarkers for the assessment of immunotherapy efficacy. A
comprehensive treatment plan cannot be supported by a single
biomarker (104). It is important
to evaluate how different biomarkers interact, as is performed in
the CRAFITY score, which combines CRP and AFP as aforementioned
(105,106).
Analysis of the spatially distinct distribution of
different immune cell types in the TME and the dynamic interactions
between them has been demonstrated using multiplex
IHC/immunofluorescence, which allows the simultaneous analysis of
multiple immune parameters on the same paraffin-embedded tissue
section (107). In HCC, Ng et
al (97) revealed that the
total CD38+ cell ratio and
CD38+CD68+ macrophage density were indicators
of responsiveness to immune checkpoint blockade, and were an
improvement on the PD-L1 score or CD8+ T cell density.
Additionally, the combined use of two markers can improve the
prediction accuracy. In a recent study, the effects of TMB, gene
expression profiling and PD-1, combined and alone, on the prognosis
prediction in NSCLC were compared (108). It was revealed that the
combination of at least two biomarkers was more accurate compared
with the use of a single biomarker; however, combinations of three
biomarkers were not revealed to be predictive (108). In patients with NSCLC, Hurkmans
et al (109) investigated
the interaction of PD-L1, CD8+ T cell infiltrates and human
leukocyte antigens (HLA) class-I using IHC. The findings indicated
that patients with an increased PFS had high tumor mutation loads,
high infiltration of CD8+ T cells or no loss of HLA
class-I (109). In addition to
the combination of immune drugs, new anti-tumor therapies such as
photodynamic therapy and photothermal therapy can increase the
immune response of tumor cells by changing the TME, and demonstrate
synergistic effects (110).
Comprehensive ranking based on the fundamental molecular and
cellular pharmacological foundations and relevant mechanisms of
action to hit multiple targets, as well as further investigation of
the next-generation immunotherapies for patients with primary and
acquired drug resistance, may improve the prediction of the optimal
strategies (111,112). Currently, there are no data
available on the combined prediction of immunotherapy efficacy by
several indicators in liver cancer. Table III provides a brief summary of
tumor-associated biomarkers used in HCC immunotherapy.
In recent years, more immune-associated drugs,
including atezolizumab in combination with bevacizumab,
pembrolizumab and nivolumab, have been administered in clinical
settings. While progress has been made in the treatment of liver
cancer, not all patients respond effectively to immunotherapy. An
important problem that needs to be solved is how to identify
patients who would be sensitive to immunotherapy to avoid exposure
to drug toxicity and a waste of medical resources. Non-invasive
biomarkers are necessary. The collection and detection of NLR, PLR,
ctDNA, CTC and intestinal microorganisms is less traumatic to
patients, easier to collect and can achieve dynamic detection.
PD-1/PD-L1, genetic characteristics and the TME provide more
information on tumor heterogeneity. However, the treatment of liver
cancer is a combination of multiple treatment methods and various
treatment modes. In metastatic melanoma, Pires da Silva et
al (113) used conventional
clinical parameters, factors such as the Eastern Cooperative
Oncology Group Performance Status, presence/absence of liver and
lung metastases, amongst others, to establish a model for
predicting prognosis with validation in independent cohorts. The
model successfully predicted the responses and survival rate
outcomes of patients with metastatic melanoma after receiving
immunotherapy (109,113,114). Furthermore, given the presence of
tumor heterogeneity and the dynamic nature of the TME in liver
cancer, as well as the complex interactions and regulation between
the two, a single predictor is insufficient for the complexity of
treatment methods. A combinatorial, precise and diverse strategy is
thus necessary for immune biomarkers.
Not applicable.
Funding: This work was supported by the 1.3.5 Project for
Disciplines of Excellence, West China Hospital, Sichuan University
(grant no. ZYJC21043) and Sichuan Science and Technology Program
(grant no. 23ZDYF2874).
Not applicable.
MC conceived the topic for the present review and
wrote the manuscript. JW and XZ were responsible for reviewing and
editing the manuscript. ML revised the content of this review. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhu AX, Dayyani F, Yen CJ, Ren Z, Bai Y,
Meng Z, Pan H, Dillon P, Mhatre SK, Gaillard VE, et al:
Alpha-fetoprotein as a potential surrogate biomarker for
atezolizumab + bevacizumab treatment of hepatocellular carcinoma.
Clin Cancer Res. 28:3537–3545. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers.
7(6)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Anwanwan D, Singh SK, Singh S, Saikam V
and Singh R: Challenges in liver cancer and possible treatment
approaches. Biochim Biophys Acta Rev Cancer.
1873(188314)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y,
Guo X, Kang B, Hu R, Huang JY, Zhang Q, et al: Landscape of
infiltrating T cells in liver cancer revealed by single-cell
sequencing. Cell. 169:1342–1356.e16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wei SC, Duffy CR and Allison JP:
Fundamental mechanisms of immune checkpoint blockade therapy.
Cancer Discov. 8:1069–1086. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zayac A and Almhanna K: Hepatobiliary
cancers and immunotherapy: Where are we now and where are we
heading? Transl Gastroenterol Hepatol. 5(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ding X, He M, Chan A, Song QX, Sze SC,
Chen H, Man MKH, Man K, Chan SL, Lai PBS, et al: Genomic and
epigenomic features of primary and recurrent hepatocellular
carcinomas. Gastroenterology. 157:1630–1645.e6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Finn RS, Ryoo BY, Merle P, Kudo M,
Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et
al: Pembrolizumab as second-line therapy in patients with advanced
hepatocellular carcinoma in KEYNOTE-240: A randomized,
double-blind, phase III trial. J Clin Oncol. 38:193–202.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yau T, Park JW, Finn RS, Cheng AL,
Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, et
al: Nivolumab versus sorafenib in advanced hepatocellular carcinoma
(CheckMate 459): A randomised, multicentre, open-label, phase 3
trial. Lancet Oncol. 23:77–90. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li
Q, Lu Y, Chen Y, Guo Y, et al: Sintilimab plus a bevacizumab
biosimilar (IBI305) versus sorafenib in unresectable hepatocellular
carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study.
Lancet Oncol. 22:977–990. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kudo M: Durvalumab plus tremelimumab in
unresectable hepatocellular carcinoma. Hepatobiliary Surg Nutr.
11:592–596. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sangro B, Melero I, Wadhawan S, Finn RS,
Abou-Alfa GK, Cheng AL, Yau T, Furuse J, Park JW, Boyd Z, et al:
Association of inflammatory biomarkers with clinical outcomes in
nivolumab-treated patients with advanced hepatocellular carcinoma.
J Hepatol. 73:1460–1469. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ho WJ, Danilova L, Lim SJ, Verma R, Xavier
S, Leatherman JM, Sztein MB, Fertig EJ, Wang H, Jaffee E and
Yarchoan M: Viral status, immune microenvironment and immunological
response to checkpoint inhibitors in hepatocellular carcinoma. J
Immunother Cancer. 8(e000394)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Avgerinos KI, Spyrou N, Mantzoros CS and
Dalamaga M: Obesity and cancer risk: Emerging biological mechanisms
and perspectives. Metabolism. 92:121–135. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ringel AE, Drijvers JM, Baker GJ, Catozzi
A, García-Cañaveras JC, Gassaway BM, Miller BC, Juneja VR, Nguyen
TH, Joshi S, et al: Obesity shapes metabolism in the tumor
microenvironment to suppress anti-tumor immunity. Cell.
183:1848–1866.e26. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Akce M, Liu Y, Zakka K, Martini DJ, Draper
A, Alese OB, Shaib WL, Wu C, Wedd JP, Sellers MT, et al: Impact of
sarcopenia, BMI, and inflammatory biomarkers on survival in
advanced hepatocellular carcinoma treated with anti-PD-1 antibody.
Am J Clin Oncol. 44:74–81. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bellissimo F, Pinzone MR, Cacopardo B and
Nunnari G: Diagnostic and therapeutic management of hepatocellular
carcinoma. World J Gastroenterol. 21:12003–12021. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tsuchiya N, Sawada Y, Endo I, Saito K,
Uemura Y and Nakatsura T: Biomarkers for the early diagnosis of
hepatocellular carcinoma. World J Gastroenterol. 21:10573–10583.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cao W, Chen Y, Han W, Yuan J, Xie W, Liu
K, Qiu Y, Wang X and Li X: Potentiality of α-fetoprotein (AFP) and
soluble intercellular adhesion molecule-1 (sICAM-1) in prognosis
prediction and immunotherapy response for patients with
hepatocellular carcinoma. Bioengineered. 12:9435–9451.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Spahn S, Roessler D, Pompilia R, Gabernet
G, Gladstone BP, Horger M, Biskup S, Feldhahn M, Nahnsen S, Hilke
FJ, et al: Clinical and genetic tumor characteristics of responding
and non-responding patients to PD-1 inhibition in hepatocellular
carcinoma. Cancers (Basel). 12(3830)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sun X, Mei J, Lin W, Yang Z, Peng W, Chen
J, Zhang Y, Xu L and Chen M: Reductions in AFP and PIVKA-II can
predict the efficiency of anti-PD-1 immunotherapy in HCC patients.
BMC Cancer. 21(775)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hatanaka T, Kakizaki S, Hiraoka A, Tada T,
Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi
E, et al: Prognostic impact of C-reactive protein and
alpha-fetoprotein in immunotherapy score in hepatocellular
carcinoma patients treated with atezolizumab plus bevacizumab: A
multicenter retrospective study. Hepatol Int. 16:1150–1160.
2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Scheiner B, Pomej K, Kirstein MM, Hucke F,
Finkelmeier F, Waidmann O, Himmelsbach V, Schulze K, von Felden J,
Fründt TW, et al: Prognosis of patients with hepatocellular
carcinoma treated with immunotherapy-development and validation of
the CRAFITY score. J Hepatol. 76:353–363. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guan R, Mei J, Lin W, Deng M, Li S and Guo
R: Is the CRAFITY score a superior predictor of prognosis and
adverse events in hepatocellular carcinoma patients treated with
locoregional-immunotherapy? Hepatol Int. 17:1279–1288.
2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang M, Pan Y and Wang W: Prognostic
significance of the CRAFITY score in hepatocellular carcinoma
treated with immunotherapy: A systematic review and meta-analysis.
BMC Cancer. 23(236)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dharmapuri S, Özbek U, Lin JY, Sung M,
Schwartz M, Branch AD and Ang C: Predictive value of neutrophil to
lymphocyte ratio and platelet to lymphocyte ratio in advanced
hepatocellular carcinoma patients treated with anti-PD-1 therapy.
Cancer Med. 9:4962–4970. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Muhammed A, Fulgenzi CAM, Dharmapuri S,
Pinter M, Balcar L, Scheiner B, Marron TU, Jun T, Saeed A,
Hildebrand H, et al: The systemic inflammatory response identifies
patients with adverse clinical outcome from immunotherapy in
hepatocellular carcinoma. Cancers (Basel). 14(186)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ochi H, Kurosaki M, Joko K, Mashiba T,
Tamaki N, Tsuchiya K, Marusawa H, Tada T, Nakamura S, Narita R, et
al: Usefulness of neutrophil-to-lymphocyte ratio in predicting
progression and survival outcomes after atezolizumab-bevacizumab
treatment for hepatocellular carcinoma. Hepatol Res. 53:61–71.
2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jeon SH, Lee YJ, Kim HD, Nam H, Ryoo BY,
Park SH, Yoo C and Shin EC: Dynamic changes in peripheral blood
monocytes early after anti-PD-1 therapy predict clinical outcomes
in hepatocellular carcinoma. Cancer Immunol Immunother. 72:371–384.
2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Riedl JM, Barth DA, Brueckl WM, Zeitler G,
Foris V, Mollnar S, Stotz M, Rossmann CH, Terbuch A, Balic M, et
al: C-reactive protein (CRP) levels in immune checkpoint inhibitor
response and progression in advanced non-small cell lung cancer: A
Bi-center study. Cancers (Basel). 12(2319)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Klümper N, Saal J, Berner F,
Lichtensteiger C, Wyss N, Heine A, Bauernfeind FG, Ellinger J,
Brossart P, Diem S, et al: C reactive protein flare predicts
response to checkpoint inhibitor treatment in non-small cell lung
cancer. J Immunother Cancer. 10(e004024)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ramsey S: The role of the systemic
inflammatory response as a biomarker in immunotherapy for renal
cell cancer. Mol Diagn Ther. 13:277–281. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chung MW, Kim MJ, Won EJ, Lee YJ, Yun YW,
Cho SB, Joo YE, Hwang JE, Bae WK, Chung IJ, et al: Gut microbiome
composition can predict the response to nivolumab in advanced
hepatocellular carcinoma patients. World J Gastroenterol.
27:7340–7349. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mao J, Wang D, Long J, Yang X, Lin J, Song
Y, Xie F, Xun Z, Wang Y, Wang Y, et al: Gut microbiome is
associated with the clinical response to anti-PD-1 based
immunotherapy in hepatobiliary cancers. J Immunother Cancer.
9(e003334)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang L, Wu YN, Chen T, Ren CH, Li X and
Liu GX: Relationship between intestinal microbial dysbiosis and
primary liver cancer. Hepatobiliary Pancreat Dis Int. 18:149–157.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schwabe RF and Greten TF: Gut microbiome
in HCC-mechanisms, diagnosis and therapy. J Hepatol. 72:230–238.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Temraz S, Nassar F, Kreidieh F, Mukherji
D, Shamseddine A and Nasr R: Hepatocellular carcinoma immunotherapy
and the potential influence of gut microbiome. Int J Mol Sci.
22(7800)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dapito DH, Mencin A, Gwak GY, Pradere JP,
Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A,
Bataller R, et al: Promotion of hepatocellular carcinoma by the
intestinal microbiota and TLR4. Cancer Cell. 21:504–516.
2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Peng X, Gong C, Zhang W and Zhou A:
Advanced development of biomarkers for immunotherapy in
hepatocellular carcinoma. Front Oncol. 12(1091088)2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vaisman-Mentesh A, Gutierrez-Gonzalez M,
DeKosky BJ and Wine Y: The molecular mechanisms that underlie the
immune biology of anti-drug antibody formation following treatment
with monoclonal antibodies. Front Immunol. 11(1951)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Davda J, Declerck P, Hu-Lieskovan S,
Hickling TP, Jacobs IA, Chou J, Salek-Ardakani S and Kraynov E:
Immunogenicity of immunomodulatory, antibody-based, oncology
therapeutics. J Immunother Cancer. 7(105)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Enrico D, Paci A, Chaput N, Karamouza E
and Besse B: Antidrug antibodies against immune checkpoint
blockers: Impairment of drug efficacy or indication of immune
activation? Clin Cancer Res. 26:787–792. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kim C, Yang H, Kim I, Kang B, Kim H, Kim
H, Lee WS, Jung S, Lim HY, Cheon J and Chon HJ: Association of high
levels of antidrug antibodies against atezolizumab with clinical
outcomes and T-cell responses in patients with hepatocellular
carcinoma. JAMA Oncol. 8:1825–1829. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Peters S, Galle PR, Bernaards CA,
Ballinger M, Bruno R, Quarmby V, Ruppel J, Vilimovskij A, Wu B,
Sternheim N and Reck M: Evaluation of atezolizumab immunogenicity:
Efficacy and safety (Part 2). Clin Transl Sci. 15:141–157.
2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Myler H, Pedras-Vasconcelos J, Phillips K,
Hottenstein CS, Chamberlain P, Devanaryan V, Gleason C, Goodman J,
Manning MS, Purushothama S, et al: Anti-drug antibody validation
testing and reporting harmonization. AAPS J. 24(4)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sadreddini S, Baradaran B, Aghebati-Maleki
A, Sadreddini S, Shanehbandi D, Fotouhi A and Aghebati-Maleki L:
Immune checkpoint blockade opens a new way to cancer immunotherapy.
J Cell Physiol. 234:8541–8549. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kwok G, Yau TC, Chiu JW, Tse E and Kwong
YL: Pembrolizumab (Keytruda). Hum Vaccin Immunother. 12:2777–2789.
2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Beaver JA, Hazarika M, Mulkey F, Mushti S,
Chen H, He K, Sridhara R, Goldberg KB, Chuk MK, Chi DC, et al:
Patients with melanoma treated with an anti-PD-1 antibody beyond
RECIST progression: A US food and drug administration pooled
analysis. Lancet Oncol. 19:229–239. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ailia MJ, Heo J and Yoo SY: Navigating
through the PD-1/PDL-1 landscape: A systematic review and
meta-analysis of clinical outcomes in hepatocellular carcinoma and
their influence on immunotherapy and tumor microenvironment. Int J
Mol Sci. 24(6495)2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhu AX, Abbas AR, de Galarreta MR, Guan Y,
Lu S, Koeppen H, Zhang W, Hsu CH, He AR, Ryoo BY, et al: Molecular
correlates of clinical response and resistance to atezolizumab in
combination with bevacizumab in advanced hepatocellular carcinoma.
Nat Med. 28:1599–1611. 2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Paver EC, Cooper WA, Colebatch AJ,
Ferguson PM, Hill SK, Lum T, Shin JS, O'Toole S, Anderson L,
Scolyer RA and Gupta R: Programmed death ligand-1 (PD-L1) as a
predictive marker for immunotherapy in solid tumours: A guide to
immunohistochemistry implementation and interpretation. Pathology.
53:141–156. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Doroshow DB, Bhalla S, Beasley MB, Sholl
LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS and Hirsch FR:
PD-L1 as a biomarker of response to immune-checkpoint inhibitors.
Nat Rev Clin Oncol. 18:345–362. 2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Dong ZY, Wu SP, Liao RQ, Huang SM and Wu
YL: Potential biomarker for checkpoint blockade immunotherapy and
treatment strategy. Tumour Biol. 37:4251–4261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kim MS, Xu A, Haslam A and Prasad V:
Quality of biomarker defined subgroups in FDA approvals of
PD-1/PD-L1 inhibitors 2014 to 2020. Int J Cancer. 150:1905–1910.
2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
He S and Tang S: WNT/β-catenin signaling
in the development of liver cancers. Biomed Pharmacother.
132(110851)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Perugorria MJ, Olaizola P, Labiano I,
Esparza-Baquer A, Marzioni M, Marin JJG, Bujanda L and Banales JM:
Wnt-β-catenin signalling in liver development, health and disease.
Nat Rev Gastroenterol Hepatol. 16:121–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Llovet JM, Montal R, Sia D and Finn RS:
Molecular therapies and precision medicine for hepatocellular
carcinoma. Nat Rev Clin Oncol. 15:599–616. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Pinyol R, Sia D and Llovet JM: Immune
exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies
in HCC. Clin Cancer Res. 25:2021–2023. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ruiz de Galarreta M, Bresnahan E,
Molina-Sánchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela
V, Casanova-Acebes M, Dhainaut M, et al: β-catenin activation
promotes immune escape and resistance to anti-PD-1 therapy in
hepatocellular carcinoma. Cancer Discov. 9:1124–1141.
2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Harding JJ, Nandakumar S, Armenia J,
Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika
I, et al: Prospective genotyping of hepatocellular carcinoma:
Clinical implications of next-generation sequencing for matching
patients to targeted and immune therapies. Clin Cancer Res.
25:2116–2126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Wang Z, Sheng YY, Gao XM, Wang CQ, Wang
XY, Lu XU, Wei JW, Zhang KL, Dong QZ and Qin LX: β-catenin mutation
is correlated with a favorable prognosis in patients with
hepatocellular carcinoma. Mol Clin Oncol. 3:936–940.
2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Chen L, Zhou Q, Liu J and Zhang W: CTNNB1
alternation is a potential biomarker for immunotherapy prognosis in
patients with hepatocellular carcinoma. Front Immunol.
12(759565)2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Caja L, Dituri F, Mancarella S,
Caballero-Diaz D, Moustakas A, Giannelli G and Fabregat I: TGF-β
and the tissue microenvironment: Relevance in fibrosis and cancer.
Int J Mol Sci. 19(1294)2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Chen J, Gingold JA and Su X:
Immunomodulatory TGF-β signaling in hepatocellular carcinoma.
Trends Mol Med. 25:1010–1023. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Mariathasan S, Turley SJ, Nickles D,
Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita
JL, Cubas R, et al: TGFβ attenuates tumour response to PD-L1
blockade by contributing to exclusion of T cells. Nature.
554:544–548. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Horn LA, Chariou PL, Gameiro SR, Qin H,
Iida M, Fousek K, Meyer TJ, Cam M, Flies D, Langermann S, et al:
Remodeling the tumor microenvironment via blockade of LAIR-1 and
TGF-β signaling enables PD-L1-mediated tumor eradication. J Clin
Invest. 132(e155148)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wu ZH, Yang DL, Wang L and Liu J:
Epigenetic and immune-cell infiltration changes in the tumor
microenvironment in hepatocellular carcinoma. Front Immunol.
12(793343)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Dai Y, Qiang W, Lin K, Gui Y, Lan X and
Wang D: An immune-related gene signature for predicting survival
and immunotherapy efficacy in hepatocellular carcinoma. Cancer
Immunol Immunother. 70:967–979. 2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Klempner SJ, Fabrizio D, Bane S, Reinhart
M, Peoples T, Ali SM, Sokol ES, Frampton G, Schrock AB, Anhorn R
and Reddy P: Tumor mutational burden as a predictive biomarker for
response to immune checkpoint inhibitors: A review of current
evidence. Oncologist. 25:e147–e159. 2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Samstein RM, Lee CH, Shoushtari AN,
Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ,
Omuro A, et al: Tumor mutational load predicts survival after
immunotherapy across multiple cancer types. Nat Genet. 51:202–206.
2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Marabelle A, Fakih M, Lopez J, Shah M,
Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin
JA, Miller WH Jr, et al: Association of tumour mutational burden
with outcomes in patients with advanced solid tumours treated with
pembrolizumab: Prospective biomarker analysis of the multicohort,
open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21:1353–1365.
2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu
R, Zhang G, Zhao C, Zhang Y, Chen C, et al: Anti-PD-1 antibody
SHR-1210 combined with apatinib for advanced hepatocellular
carcinoma, gastric, or esophagogastric junction cancer: An
open-label, dose escalation and expansion study. Clin Cancer Res.
25:515–523. 2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ang C, Klempner SJ, Ali SM, Madison R,
Ross JS, Severson EA, Fabrizio D, Goodman A, Kurzrock R, Suh J and
Millis SZ: Prevalence of established and emerging biomarkers of
immune checkpoint inhibitor response in advanced hepatocellular
carcinoma. Oncotarget. 10:4018–4025. 2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Eso Y, Shimizu T, Takeda H, Takai A and
Marusawa H: Microsatellite instability and immune checkpoint
inhibitors: Toward precision medicine against gastrointestinal and
hepatobiliary cancers. J Gastroenterol. 55:15–26. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Malapelle U, Parente P, Pepe F, De Luca C,
Pisapia P, Sgariglia R, Nacchio M, Gragnano G, Russo G, Conticelli
F, et al: Evaluation of micro satellite instability and mismatch
repair status in different solid tumors: A multicenter analysis in
a real world setting. Cells. 10(1878)2021.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Cohen R, Hain E, Buhard O, Guilloux A,
Bardier A, Kaci R, Bertheau P, Renaud F, Bibeau F, Fléjou JF, et
al: Association of primary resistance to immune checkpoint
inhibitors in metastatic colorectal cancer with misdiagnosis of
microsatellite instability or mismatch repair deficiency status.
JAMA Oncol. 5:551–555. 2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Marcus L, Lemery SJ, Keegan P and Pazdur
R: FDA approval summary: Pembrolizumab for the treatment of
microsatellite instability-high solid tumors. Clin Cancer Res.
25:3753–3758. 2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Prasad V, Kaestner V and Mailankody S:
Cancer drugs approved based on biomarkers and not tumor Type-FDA
approval of pembrolizumab for mismatch repair-deficient solid
cancers. JAMA Oncol. 4:157–158. 2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Bonneville R, Krook MA, Kautto EA, Miya J,
Wing MR, Chen HZ, Reeser JW, Yu L and Roychowdhury S: Landscape of
microsatellite instability across 39 cancer types. JCO Precis
Oncol. 2017(PO.17.00073)2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Kawaoka T, Ando Y, Yamauchi M, Suehiro Y,
Yamaoka K, Kosaka Y, Fuji Y, Uchikawa S, Morio K, Fujino H, et al:
Incidence of microsatellite instability-high hepatocellular
carcinoma among Japanese patients and response to pembrolizumab.
Hepatol Res. 50:885–888. 2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Ando Y, Yamauchi M, Suehiro Y, Yamaoka K,
Kosaka Y, Fuji Y, Uchikawa S, Kodama K, Morio K, Fujino H, et al:
Complete response to pembrolizumab in advanced hepatocellular
carcinoma with microsatellite instability. Clin J Gastroenterol.
13:867–872. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Mukai S, Kanzaki H, Ogasawara S, Ishino T,
Ogawa K, Nakagawa M, Fujiwara K, Unozawa H, Iwanaga T, Sakuma T, et
al: Exploring microsatellite instability in patients with advanced
hepatocellular carcinoma and its tumor microenvironment. JGH Open.
5:1266–1274. 2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Buchler T: Microsatellite instability and
metastatic colorectal cancer-a clinical perspective. Front Oncol.
12(888181)2022.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Cercek A, Lumish M, Sinopoli J, Weiss J,
Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M,
Sugarman R, et al: PD-1 blockade in mismatch repair-deficient,
locally advanced rectal cancer. N Engl J Med. 386:2363–2376.
2022.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Turner KM, Delman AM, Wima K, Quillin RC,
Shah SA, Ahmad SA, Patel SH and Wilson GC: Microsatellite
instability is associated with worse overall survival in resectable
colorectal liver metastases. Am J Surg. 225:322–327.
2023.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Wu T and Dai Y: Tumor microenvironment and
therapeutic response. Cancer Lett. 387:61–68. 2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Oura K, Morishita A, Tani J and Masaki T:
Tumor immune microenvironment and immunosuppressive therapy in
hepatocellular carcinoma: A review. Int J Mol Sci.
22(5801)2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zheng X, Jin W, Wang S and Ding H:
Progression on the roles and mechanisms of tumor-infiltrating T
lymphocytes in patients with hepatocellular carcinoma. Front
Immunol. 12(729705)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Xu X, Tan Y, Qian Y, Xue W, Wang Y, Du J,
Jin L and Ding W: Clinicopathologic and prognostic significance of
tumor-infiltrating CD8+ T cells in patients with hepatocellular
carcinoma: A meta-analysis. Medicine (Baltimore).
98(e13923)2019.PubMed/NCBI View Article : Google Scholar
|
|
94
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502.
2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Wang C, Singer M and Anderson AC:
Molecular dissection of CD8(+) T-cell dysfunction. Trends Immunol.
38:567–576. 2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Ma J, Zheng B, Goswami S, Meng L, Zhang D,
Cao C, Li T, Zhu F, Ma L, Zhang Z, et al: PD1Hi
CD8+ T cells correlate with exhausted signature and poor
clinical outcome in hepatocellular carcinoma. J Immunother Cancer.
7(331)2019.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Ng HHM, Lee RY, Goh S, Tay ISY, Lim X, Lee
B, Chew V, Li H, Tan B, Lim S, et al: Immunohistochemical scoring
of CD38 in the tumor microenvironment predicts responsiveness to
anti-PD-1/PD-L1 immunotherapy in hepatocellular carcinoma. J
Immunother Cancer. 8(e000987)2020.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Gu X, Guan J, Xu J, Zheng Q, Chen C, Yang
Q, Huang C, Wang G, Zhou H, Chen Z and Zhu H: Model based on five
tumour immune microenvironment-related genes for predicting
hepatocellular carcinoma immunotherapy outcomes. J Transl Med.
19(26)2021.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Maravelia P, Silva DN, Rovesti G, Chrobok
M, Stål P, Lu YC and Pasetto A: Liquid biopsy in hepatocellular
carcinoma: Opportunities and challenges for immunotherapy. Cancers
(Basel). 13(4334)2021.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Cheng F, Su L and Qian C: Circulating
tumor DNA: A promising biomarker in the liquid biopsy of cancer.
Oncotarget. 7:48832–48841. 2016.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Cabel L, Riva F, Servois V, Livartowski A,
Daniel C, Rampanou A, Lantz O, Romano E, Milder M, Buecher B, et
al: Circulating tumor DNA changes for early monitoring of anti-PD1
immunotherapy: A proof-of-concept study. Ann Oncol. 28:1996–2001.
2017.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Peng Y, Mei W, Ma K and Zeng C:
Circulating tumor DNA and minimal residual disease (MRD) in solid
tumors: Current horizons and future perspectives. Front Oncol.
11(763790)2021.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Winograd P, Hou S, Court CM, Lee YT, Chen
PJ, Zhu Y, Sadeghi S, Finn RS, Teng PC, Wang JJ, et al:
Hepatocellular carcinoma-circulating tumor cells expressing PD-L1
are prognostic and potentially associated with response to
checkpoint inhibitors. Hepatol Commun. 4:1527–1540. 2020.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Cheng AL, Hsu C, Chan SL, Choo SP and Kudo
M: Challenges of combination therapy with immune checkpoint
inhibitors for hepatocellular carcinoma. J Hepatol. 72:307–319.
2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Lee JS and Ruppin E: Multiomics prediction
of response rates to therapies to inhibit programmed cell death 1
and programmed cell death 1 ligand 1. JAMA Oncol. 5:1614–1618.
2019.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Lu S, Stein JE, Rimm DL, Wang DW, Bell JM,
Johnson DB, Sosman JA, Schalper KA, Anders RA, Wang H, et al:
Comparison of biomarker modalities for predicting response to
PD-1/PD-L1 checkpoint blockade: A systematic review and
meta-analysis. JAMA Oncol. 5:1195–1204. 2019.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Giraldo NA, Nguyen P, Engle EL, Kaunitz
GJ, Cottrell TR, Berry S, Green B, Soni A, Cuda JD, Stein JE, et
al: Multidimensional, quantitative assessment of PD-1/PD-L1
expression in patients with Merkel cell carcinoma and association
with response to pembrolizumab. J Immunother Cancer.
6(99)2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Kim H, Kwon HJ, Kim ES, Kwon S, Suh KJ,
Kim SH, Kim YJ, Lee JS and Chung JH: Comparison of the predictive
power of a combination versus individual biomarker testing in
non-small cell lung cancer patients treated with immune checkpoint
inhibitors. Cancer Res Treat. 54:424–433. 2022.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Hurkmans DP, Kuipers ME, Smit J, van
Marion R, Mathijssen RHJ, Postmus PE, Hiemstra PS, Aerts JGJV, von
der Thüsen JH and van der Burg SH: Tumor mutational load,
CD8+ T cells, expression of PD-L1 and HLA class I to
guide immunotherapy decisions in NSCLC patients. Cancer Immunol
Immunother. 69:771–777. 2020.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Yin S, Chen Z, Chen D and Yan D:
Strategies targeting PD-L1 expression and associated opportunities
for cancer combination therapy. Theranostics. 13:1520–1544.
2023.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Lemaire V, Shemesh CS and Rotte A:
Pharmacology-based ranking of anti-cancer drugs to guide clinical
development of cancer immunotherapy combinations. J Exp Clin Cancer
Res. 40(311)2021.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Marei HE, Hasan A, Pozzoli G and
Cenciarelli C: Cancer immunotherapy with immune checkpoint
inhibitors (ICIs): Potential, mechanisms of resistance, and
strategies for reinvigorating T cell responsiveness when resistance
is acquired. Cancer Cell Int. 23(64)2023.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Pires da Silva I, Ahmed T, McQuade JL,
Nebhan CA, Park JJ, Versluis JM, Serra-Bellver P, Khan Y, Slattery
T, Oberoi HK, et al: Clinical models to define response and
survival with anti-PD-1 antibodies alone or combined with
ipilimumab in metastatic melanoma. J Clin Oncol. 40:1068–1080.
2022.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Rotte A: Predictive models for response
and survival in patients treated with anti-PD-1 monotherapy or with
anti-PD-1 and ipilimumab combination: Editorial commentary. Ann
Transl Med. 11(227)2023.PubMed/NCBI View Article : Google Scholar
|