Introduction
Chronic hepatitis B (CHB) is a major global health
concern. The main goal of CHB treatment is to reduce the risk of
chronic liver disease and related complications and improve the
quality of life and survival (1).
Guidelines for the management of hepatitis B virus (HBV) infection
indicate that hepatitis B surface antigen (HBsAg) loss is a key
endpoint of interest and is strongly recommended (2). Currently, there are two main
treatment options for CHB: treatment with a nucleos(t)ide analog
(NA) or pegylated interferon α-2a (Peg-IFN).
Antiviral therapy aimed at reducing HBV DNA levels
to the extent possible is important in the management of CHB, and
NA renders HBV DNA undetectable in most patients with CHB. However,
HBsAg clearance is unlikely to occur during a patient's lifetime,
even if HBV replication is well-controlled (3,4).
Peg-IFN is known to enhance innate immunity by preventing HBV
protein formation and depleting the covalently closed circular DNA
(cccDNA) pool in the liver (5-7).
Therefore, PEG-IFN therapy may result in greater HBsAg reduction
than NA therapy (8).
In previous studies, Peg-IFN add-on therapy to NA
was reported to be superior to monotherapy in terms of promoting
HBeAg loss and HBsAg reduction in patients with HBeAg-positive CHB
(9,10). Regarding the impact of HBsAg
levels, there are few studies with short-term follow-up after the
end of treatment in HBeAg-negative CHB patients who received
Peg-IFN in addition to NA; however, there are no reports of
long-term follow-up. Moreover, the conditions under which HBsAg
levels are further reduced in patients are not well understood.
The purpose of this study is to investigate the
conditions under which Peg-IFN is effective by observing changes in
HBsAg levels during and one year after Peg-IFN administration in
HBeAg negative patients who received NA treatment and remained HBV
DNA negative.
Patients and methods
Patients
This study included 17 patients with CHB who began
Peg-IFN therapy at the Department of Gastroenterology and
Neurology, Kagawa University Hospital, Japan, between September
2013 and April 2021. All patients were serum HBsAg-positive,
HBsAb-negative, HBeAg-negative and HBeAb-positive. They had
received NA therapy for more than 1 year, and their HBV DNA levels
were below detection sensitivity. The patients had no history of
interferon (IFN) treatment. All patients had hepatitis B and were
free of other viruses, such as the hepatitis C virus or autoimmune
hepatitis. None of the patients had a history of drug abuse or
alcoholic hepatitis. The exclusion criteria were neutrophil count
<1,500/mm3, platelet count <90,000/mm3,
hemoglobin concentration <10 g/dl, cirrhosis, history of organ
transplantation, and other known diseases for which Peg-IFN therapy
was not suitable. This study was approved by the Ethics Committee
of Kagawa University Hospital on June 20, 2019 (approval no.
2019032). An oral informed consent was obtained from all the
participants. Written informed consent was not required by the
ethics committee because the data involved routinely collected
medical data. All data were obtained from patient medical
records.
Peg-IFN therapy
Patients received subcutaneous injections of Peg-IFN
α-2a (PEGASYS: Hoffmann-La Roche Inc., Basel, Switzerland) at 180
µg per week for 48 weeks. The dose of Peg-IFN was modified for
adverse events according to the manufacturer's recommendations. The
patients were followed up monthly for 12 months after treatment
completion.
Laboratory tests
Virological and biochemical assessments were
performed monthly and follow-up data were collected. HBsAg levels
were measured using ARCHITECT i2000 (Abbott Laboratories, Chicago,
IL, USA). HBV genotypes were determined using enzyme immunoassays.
HBV DNA levels were measured using a Cobas®TaqMan®48 analyzer
(Roche Molecular Systems, Branchburg, NJ, USA). Blood HBV DNA
measurements were performed using the COBAS TaqMan HBV Test, v 2.0
(Roche Diagnostics K.K, Tokyo, Japan) (~2018/6) and the COBAS
6800/8800 HBV System (Roche Diagnostics K.K) (2018/7~) was used for
this study, which is based on a polymerase reaction assay. HBV DNA
values >1.3 log IU/ml were considered positive.
Assessment of effectiveness
In this study, responders were defined as those with
a 50% or greater decrease in HBsAg levels from baseline at week 96.
Conversely, non-responders were defined as those with a decrease in
HBsAg levels of less than 50% from baseline at week 96.
Statistical analysis
Data on baseline characteristics are presented as
median values. Patient characteristics were compared using unpaired
Student's t-test. Fisher's exact tests were used to analyze
categorical variables in both groups. Repeated measures ANOVA and
the Tukey's test was performed to analyze the changes in serum
HBsAg levels from baseline at weeks 0, 48 and 96. The mean value
indicates a change in the amount of HBsAg. The results were
considered significant at P<0.05. All statistical calculations
were performed using the JMP software (version 15, SAS Institute,
USA).
Ethical approval statement
This study was approved by the Ethics Committee of
Kagawa University Hospital (approval no. 2019032). Oral informed
consent was obtained from all participants. The ethics committee
waived written informed consent because the data were collected
routinely in medical practice. Information was removed from the
data prior to analysis, and all methods were performed in
accordance with relevant guidelines and regulations.
Results
Baseline characteristics
The patients' baseline characteristics are
summarized in Table I.
 | Table IClinical and demographic
characteristics of patients at the start of Peg-IFN treatment. |
Table I
Clinical and demographic
characteristics of patients at the start of Peg-IFN treatment.
| Characteristic | Value |
|---|
| Sex, male/female | 12/5 |
| Age, years | 55.5 (41.6-69.9) |
| Genotype,
B/C/unknown | 1/11/5 |
| HBsAg titer,
lU/ml | 475 (4-19,049) |
| AST, IU/l | 22 (18-43) |
| ALT, IU/l | 23 (11-62) |
| Albumin, g/dl | 4.4 (4.0-4.8) |
| Total bilirubin,
mg/dl | 0.8 (0.3-2.8) |
| Platelet,
x103/µl | 16.6 (8.4-45.7) |
| PT, % | 85 (70-129) |
| Fib-4 index | 0.8 (0.6-3.9) |
| NA use start age,
years | 51.2 (35.1-63.8) |
| NA use period until
Peg-IFN treatment starts, years | 4.8 (1.0-13.5) |
HBsAg response
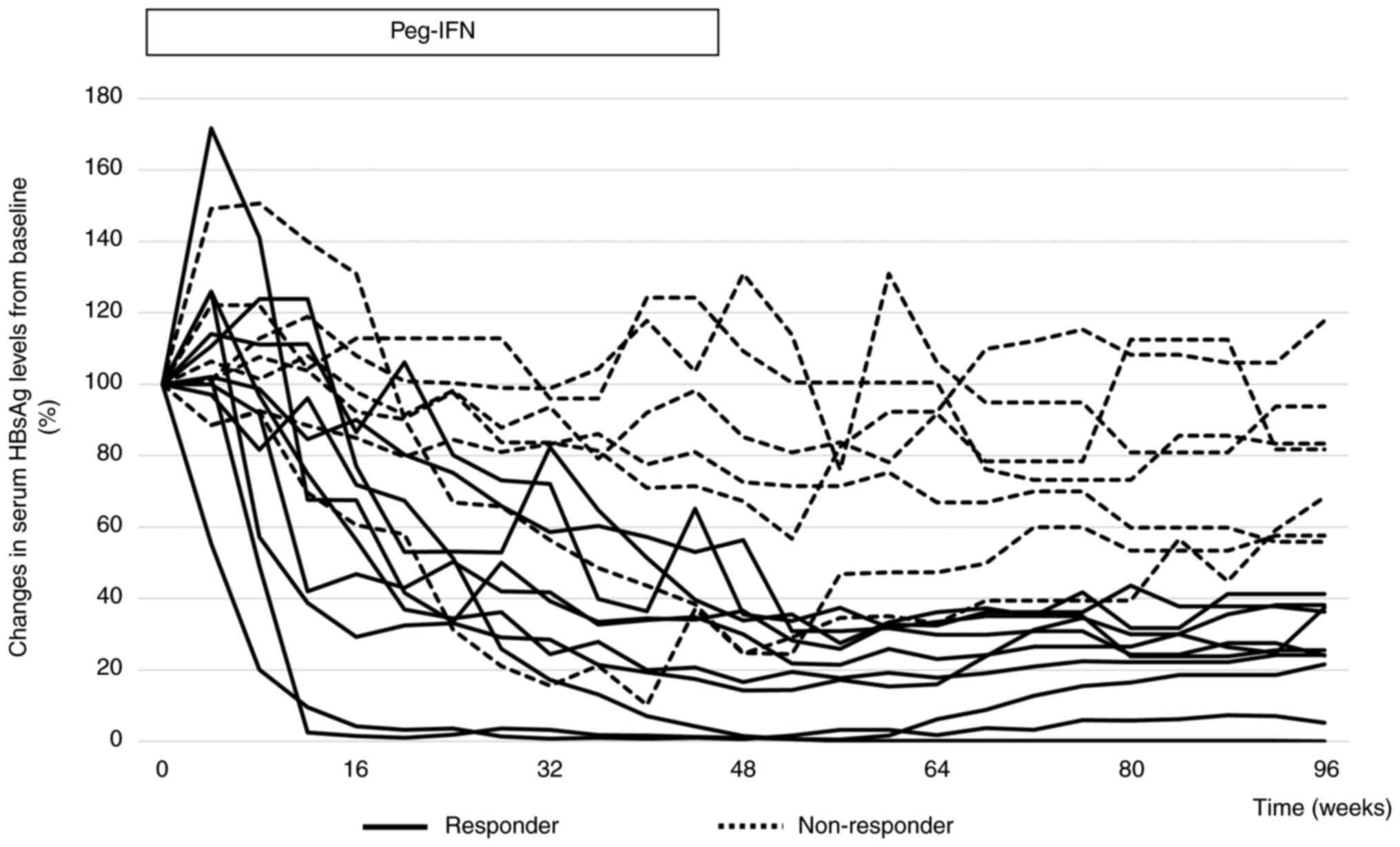
The clinical course of HBsAg in all patients is
shown in Fig. 1. In 10 of the 17
patients (58.8%), HBsAg levels decreased by more than 50% from
baseline at week 96. No patient achieved HBsAg clearance. In two
patients, HBsAg levels were higher at week 48 than at baseline.
However, in these two cases, HBsAg levels decreased from baseline
at week 96. In only one case, the HBsAg level increased at week 96
compared to that at baseline. In 9 of the 17 patients (52.9%),
HBsAg levels were higher at week 96 than at week 48.
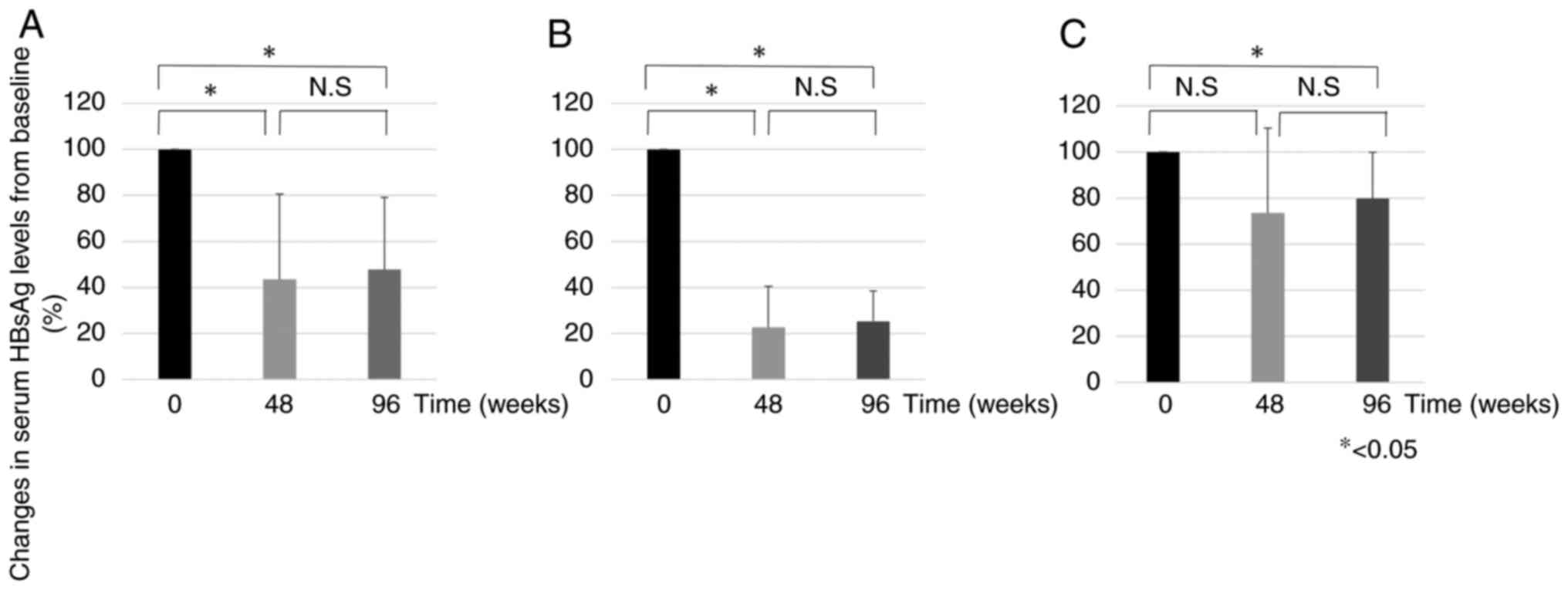
Fig. 2 shows the
mean HBsAg levels at weeks 48 and 96 compared to baseline. In all
groups, HBsAg levels were 43.5 and 47.7% at weeks 48 and 96,
respectively (Fig. 2A). In the
responder group, the percentages were 22.6 and 25.3% at weeks 48
and 96 weeks, respectively (Fig.
2B). In the non-responder group, the percentages were 73.5 and
79.7% at 48 and 96 weeks, respectively (Fig. 2C). In the responder group, there
was a significant decrease in HBsAg levels at week 48 compared with
baseline, but not in the non-responder group. All groups showed a
significant decrease in HBsAg levels at week 96 compared to
baseline. All groups showed an increase in HBsAg levels at week 96
compared to week 48, but the difference was not significant.
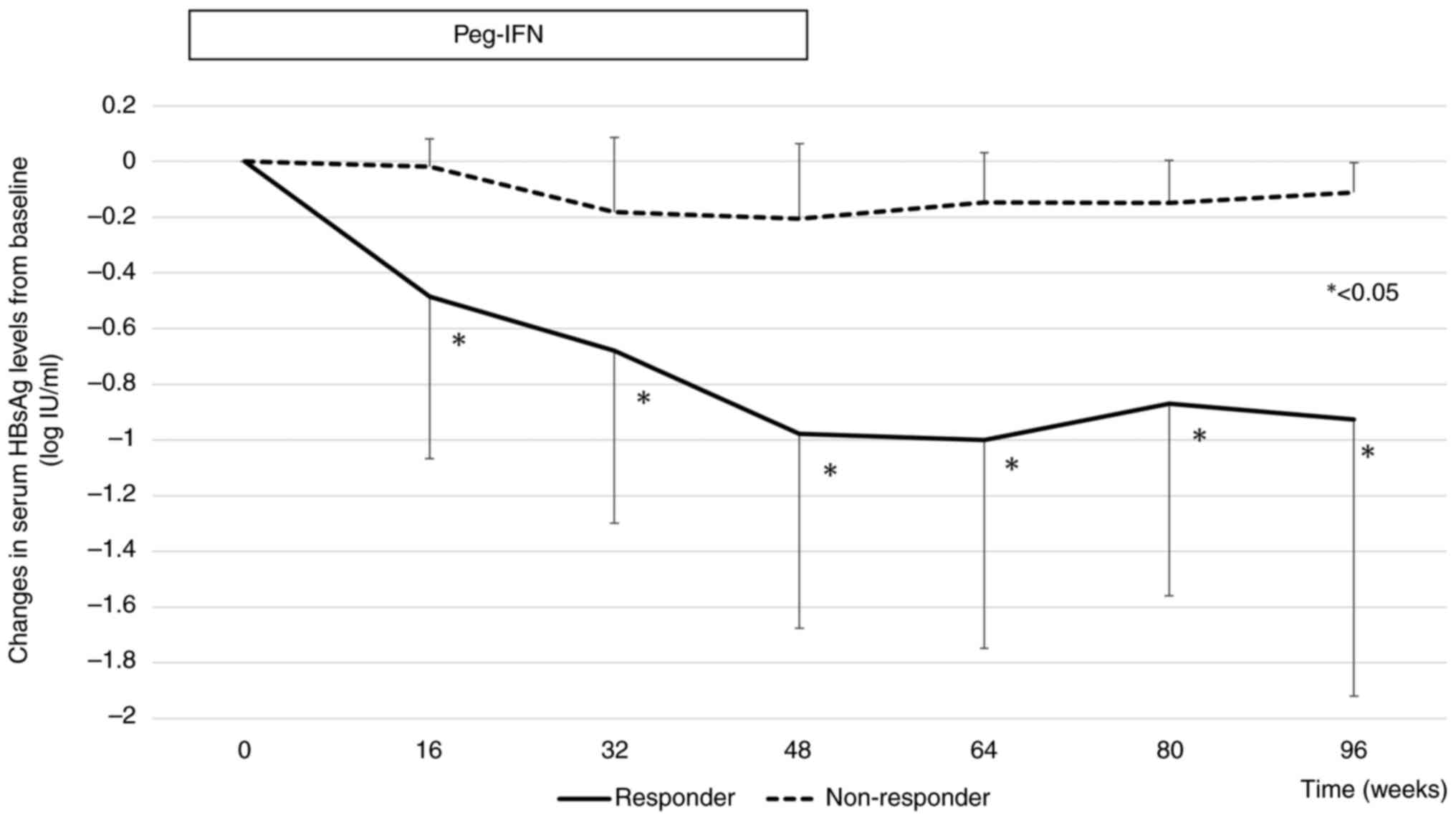
Changes in serum HBsAg levels from baseline in the
responder and non-responder groups are shown in Fig. 3. The mean declines in HBsAg levels
from baseline were -0.97, and -0.92 log IU/ml at weeks 48 and 96,
respectively, in the responder group, and -0.20, and -0.11 log
IU/ml at weeks 48 and 96, respectively, in the non-responder group.
From week 16 of Peg-IFN therapy, there was a significant difference
in the decrease in HBsAg levels from baseline between responders
and non-responders.
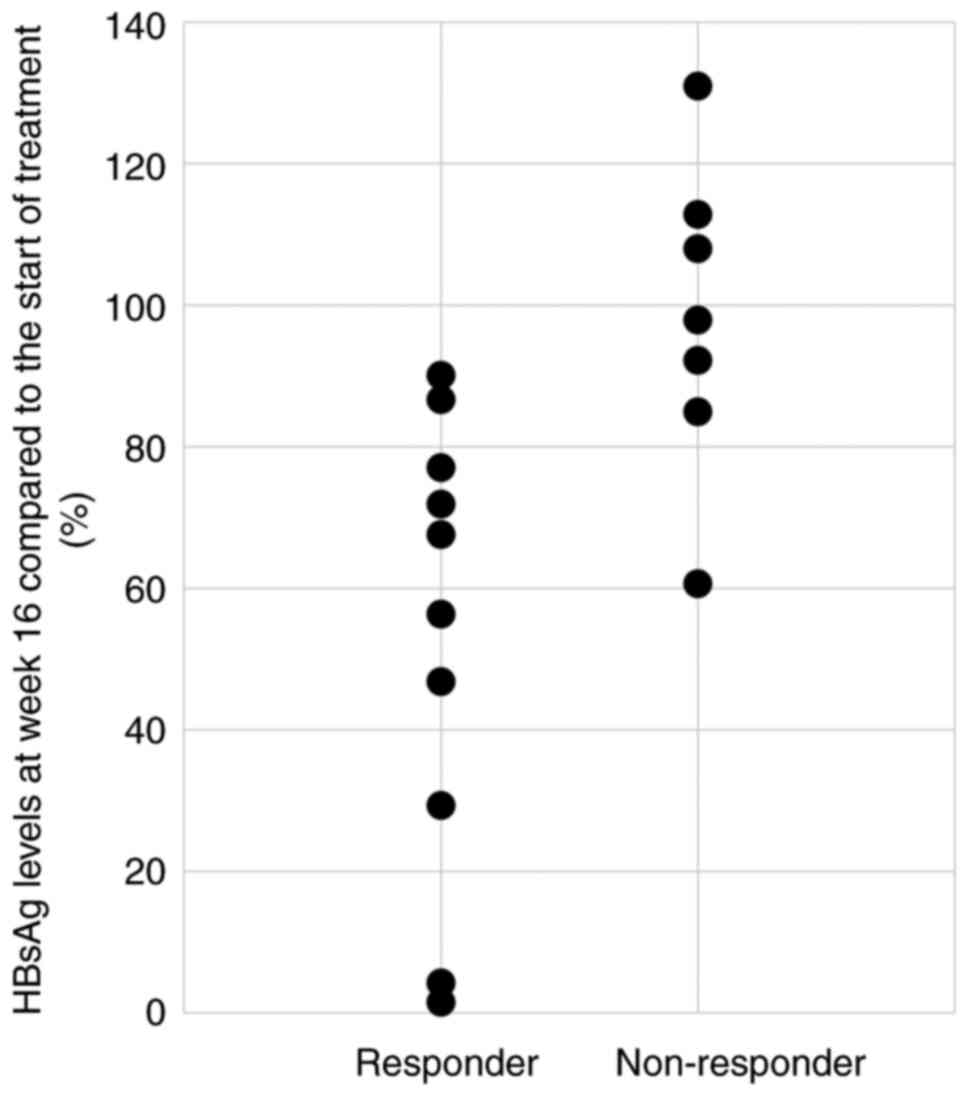
Fig. 4 shows the
HBsAg levels at week 16 compared to baseline for each case in the
responder and non-responder groups. In the responder group, 5
patients (50%) had a decrease in HBsAg level to 60% or less at week
16 compared to baseline. Conversely, there were no cases in the
non-responder group in which the HBsAg levels decreased below 60%.
The Fisher's exact test showed a significant difference between the
responding and non-responding groups in terms of whether HBsAg
levels reached 60% or less at week 16 compared to baseline
(Table II).
 | Table IIHBsAg levels from baseline. |
Table II
HBsAg levels from baseline.
| Group | HBsAg ≥60% | HBsAg <60% | P-value |
|---|
| Responder (n) | 5 | 5 | <0.05 |
| Non-responder
(n) | 7 | 0 | |
Contributing factors for prediction of
responders
A comparison of pretreatment factors between
responders and non-responders is presented in Table III. In the univariate analysis,
age at the start of NA use and the duration of NA use until the
start of treatment were significant pre-treatment factors
associated with HBsAg response.
 | Table IIIComparison of pre-treatment clinical
and demographic characteristics between the HBsAg responder and
non-responder groups. |
Table III
Comparison of pre-treatment clinical
and demographic characteristics between the HBsAg responder and
non-responder groups.
| Factors | Responder (n=10) | Non-responder
(n=7) | P-value |
|---|
| Sex, male/female | 8/2 | 4/3 | 0.314 |
| Age, years | 49.5 (41.6-69.9) | 57.0 (52.0-67.0) | 0.181 |
| Genotype,
B/C/unknown | 0/6/3 | 1/5/2 | 0.190 |
| HBsAg titer,
IU/ml | 433 (132-19,049) | 726 (4-3,473) | 0.575 |
| AST, IU/l | 21 (19-37) | 23 (18-43) | 0.428 |
| ALT, IU/l | 18 (13-62) | 23 (11-43) | 0.775 |
| Albumin, g/dl | 4.4 (4.0-4.8) | 4.4 (4.1-4.7) | 0.306 |
| Total bilirubin,
mg/dl | 0.9 (0.3-1.6) | 0.7 (0.4-2.8) | 0.916 |
| Platelet,
x103/µl | 18.0 (8.4-25.9) | 16.4 (10.9-45.7) | 0.414 |
| PT, % | 85 (70-98) | 96 (78-129) | 0.150 |
| Fib-4 index | 1.8 (0.8-2.8) | 1.5 (0.6-3.9) | 0.699 |
| NA use start age,
years | 45.4
(35.1-61.1) | 55.7
(47.2-63.8) | 0.025 |
| NA use period until
Peg-IFN treatment starts, years | 7.3 (3.0-13.5) | 1.5 (1.0-6.1) | 0.004 |
Discussion
In this study, we evaluated the effect of adding
Peg-IFN to NA on HBsAg reduction in HBeAg-negative patients whose
serum HBV DNA was undetectable after NA treatment. From a clinical
perspective, predicting whether a patient can achieve HBsAg
clearance before initiating Peg-IFN therapy is important. After 48
weeks of Peg-IFN therapy, the group whose HBsAg level was 50% of
the level before initiation and after 48 weeks of observation (week
96) was defined as the responder group and was compared with the
non-responder group.
Previous studies on a larger group of patients
reported that Peg-IFN treatment was effective as long as the
patient was in the immune reaction phase, regardless of whether the
patient was HBeAg positive or negative (11,12).
However, to our knowledge, the first study was on the
administration of Peg-IFN in HBeAg-positive cases (11). The second study had a design in
which patients who became HBeAg-negative with NAs were administered
Peg-IFN in combination for several weeks, and then Peg-IFN was
administered alone (12). Our
study differs from theirs in that we limited our subjects to
HBeAg-negative and HBeAb-positive cases and did not discontinue NA
even after starting Peg-IFN therapy. Our study is the first report
in this regard.
Although NAs strongly suppress HBV DNA, a large
multicenter long-term NAs therapy cohort study showed a 10-year
HBsAg loss rate of 2.1% and an annual incidence rate of only 0.22%
with little hope of HBsAg seroclearance (13). According to a study by
Papatheodoridis et al, HBsAg levels decreased by a median of
0.13 and 0.17 log IU/ml at 48 and 96 weeks, respectively, compared
to baseline, in HBeAg-negative CHB patients administered NA
monotherapy (14). The present
study did not directly compare the HBsAg reduction rate between NA
monotherapy and Peg-IFN and NA combination therapy. This is a
potential limitation of this study. However, it seems that the
addition of Peg-IFN to NA does not reduce HBsAg compared to NA
monotherapy because the rate of decrease in HBsAg levels is 0.20
log IU/ml and 0.11 log IU/ml at 48 and 96 weeks, respectively, in
non-responders. However, in the response group, the decrease in the
amount of HBsAg from the baseline was 0.97 log IU/ml at 48 weeks
and 0.92 log IU/ml at 96 weeks, which was remarkable. From this
perspective, it seems worthwhile to consider targeting and
administering Peg-IFN to patients who are expected to respond to
it.
NAs have been demonstrated to suppress liver
fibrosis and reduce the incidence of HCC (15-17).
To obtain such effects, it is necessary to use NAs continuously for
long periods, which may cause adherence and compliance problems.
Additionally, patients from resource-limited countries or regions
cannot afford the financial burden of long-term NAs therapy
(18). Our study showed that the
highest rate of HBsAg reduction was observed in patients who were
younger when NA therapy was initiated, and had a longer history of
NAs use. Based on this result, young patients taking NAs may also
consider Peg-IFN combination therapy with the goal of achieving
early seroclearance of HBsAg. Better use of different combinations
of antiviral drugs could make the treatment more cost-effective and
drug-free.
At week 16, a significant difference was observed
between the responding and non-responding groups in terms of the
rate of decrease in HBsAg levels. If the HBsAg level at week 16 was
<60% of that at the start of Peg-IFN therapy, the response to
Peg-IFN therapy was considered good. Based on these results, it may
be necessary to observe the rate of decrease in HBsAg levels at
week 16 and to consider continuing Peg-IFN as an add-on
therapy.
While some studies have shown that pretreatment
HBsAg titer is an important factor associated with the outcome of
HBsAg loss in Peg-IFN therapy (19,20),
long-term observational studies have reported that early serum
HBsAg decline is highly predictive of HBsAg serum clearance
(21-24).
In a previous study, we reported that HBsAg titers <120 IU/ml
may achieve HBsAg clearance with Peg-IFN monotherapy (25). In contrast, this study showed that
when Peg-IFN was added to NA, there was no significant difference
in the HBsAg levels between responders and non-responders at the
start of treatment. In the current study, we focused on
HBeAg-negative and HBeAb-positive patients; therefore, it is
possible that the baseline HBsAg level was not high, and the small
number of cases led to different results.
Although IFN-α therapy has a limited treatment
period, it has the advantage of lasting effects even after
treatment (26). In this study, we
defined the responder and non-responder groups based on the HBsAg
level at 1-year follow-up (week 96); however, we believe that
extending the observation period will deepen our understanding of
the therapeutic effects.
This study has certain limitations, the most
important of which is its retrospective design. This increases the
probability that the treatment assignment is subject to selection
bias. The study required weekly hospital visits for Peg-IFN
administration for 48 weeks and was limited to patients with time
and financial resources. It is also possible that a selection bias
may have been at work that excluded older individuals from the
study, as they may not have benefited sufficiently from
participation given their prognosis. In addition, the sample size
was relatively small. This raises questions regarding whether the
results can be generalized. Larger prospective studies are required
to confirm and extend the results of this study.
In conclusion, in HBeAg-negative patients with CHB,
the addition of Peg-IFN to NA therapy can further reduce the amount
of HBsAg in a population that is younger when NA is initiated and
undergoes NA therapy for a prolonged duration. In addition, a
decrease in HBsAg below 60% of the pre-start level at week 16 after
the start of Peg-IFN therapy could be a marker for predicting
efficacy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SM, MO, TH and TM contributed to the study
conception and design. Data collection and analysis were performed
by SM, KF, KT, MN, KO, TT, JT and AM. The first draft of the
manuscript was written by SM and TM. SM and KF confirm the
authenticity of all the raw data. All authors commented on previous
versions of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Kagawa University Hospital (approval no. 2019032). Oral informed
consent was obtained from all the participants. Written informed
consent was not required by the ethics committee because the data
involved routinely collected medical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wigfield P, Sbarigia U, Hashim M, Vincken
T and Heeg B: Are published health economic models for chronic
hepatitis B appropriately capturing the benefits of HBsAg Loss? A
systematic literature review. Pharmacoecon Open. 4:403–418.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sarin SK, Choudhury A, Sharma MK, Maiwall
R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H,
et al: Acute-on-chronic liver failure: Consensus recommendations of
the Asian Pacific association for the study of the liver (APASL):
An update. Hepatol Int. 13:353–390. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chevaliez S, Hézode C, Bahrami S, Grare M
and Pawlotsky JM: Long-term hepatitis B surface antigen (HBsAg)
kinetics during nucleoside/nucleotide analogue therapy: Finite
treatment duration unlikely. J Hepatol. 58:676–683. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zoutendijk R, Hansen BE, Van Vuuren AJ,
Boucher CA and Janssen HL: Serum HBsAg decline during long-term
potent nucleos(t)ide analogue therapy for chronic hepatitis B and
prediction of HBsAg loss. J Infect Dis. 204:415–418.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boni C, Penna A, Bertoletti A, Lamonaca V,
Rapti I, Missale G, Pilli M, Urbani S, Cavalli A, Cerioni S, et al:
Transient restoration of anti-viral T cell responses induced by
lamivudine therapy in chronic hepatitis B. J Hepatol. 39:595–605.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tjwa ET, Van Oord GW, Hegmans JP, Janssen
HL and Woltman AM: Viral load reduction improves activation and
function of natural killer cells in patients with chronic hepatitis
B. J Hepatol. 54:209–218. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Swiecki M and Colonna M: Type I
interferons: Diversity of sources, production pathways and effects
on immune responses. Curr Opin Virol. 1:463–475. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wursthorn K, Lutgehetmann M, Dandri M,
Volz T, Buggisch P, Zollner B, Longerich T, Schirmacher P, Metzler
F, Zankel M, et al: Peginterferon alpha-2b plus adefovir induce
strong cccDNA decline and HBsAg reduction in patients with chronic
hepatitis B. Hepatology. 44:675–684. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brouwer WP, Xie Q, Sonneveld MJ, Zhang N,
Zhang Q, Tabak F, Streinu-Cercel A, Wang JY, Idilman R, Reesink HW,
et al: Adding pegylated interferon to entecavir for hepatitis B e
antigen-positive chronic hepatitis B: A multicenter randomized
trial (ARES study). Hepatology. 61:1512–1522. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li GJ, Yu YQ, Chen SL, Fan P, Shao LY,
Chen JZ, Li CS, Yi B, Chen WC, Xie SY, et al: Sequential
combination therapy with pegylated interferon leads to loss of
hepatitis B surface antigen and Hepatitis B e Antigen (HBeAg)
seroconversion in HBeAg-positive chronic hepatitis B patients
receiving long-term entecavir treatment. Antimicrob Agents
Chemother. 59:4121–4128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brouwer WP, Chan HLY, Lampertico P, Hou J,
Tangkijvanich P, Reesink HW, Zhang W, Zhang W, Mangia A, Tanwandee
T, et al: Genome-wide association study identifies genetic variants
associated with early and sustained response to (Pegylated)
interferon in chronic hepatitis B patients: The GIANT-B study. Clin
Infect Dis. 69:1969–1979. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu P, Shang J, Zhang W, Gong G, Li Y, Chen
X, Jiang J, Xie Q, Dou X, Sun Y, et al: HBsAg Loss with
Peg-interferon Alfa-2a in Hepatitis B patients with partial
response to nucleos(t)ide Analog: New switch study. J Clin Transl
Hepatol. 6:25–34. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hsu YC, Yeh ML, Wong GL, Chen CH, Peng CY,
Buti M, Enomoto M, Xie Q, Trinh H, Preda C, et al: Incidences and
determinants of functional cure during entecavir or tenofovir
disoproxil fumarate for chronic hepatitis B. J Infect Dis.
224:1890–1899. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Papatheodoridis G, Goulis J,
Manolakopoulos S, Margariti A, Exarchos X, Kokkonis G, Hadziyiannis
E, Papaioannou C, Manesis E, Pectasides D and Akriviadis E: Changes
of HBsAg and interferon-inducible protein 10 serum levels in naive
HBeAg-negative chronic hepatitis B patients under 4-year entecavir
therapy. J Hepatol. 60:62–68. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tenney DJ, Rose RE, Baldick CJ,
Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J,
Wilber RB and Colonno RJ: Long-term monitoring shows hepatitis b
virus resistance to entecavir in nucleoside-naïve patients is rare
through 5 years of therapy. Hepatology. 49:1503–1514.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chang TT, Liaw YF, Wu SS, Schiff E, Han
KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, et al: Long-term
entecavir therapy results in the reversal of fibrosis/cirrhosis and
continued histological improvement in patients with chronic
hepatitis B. Hepatology. 52:886–893. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang SC, Lee CM, Hu TH, Wang JH, Lu SN,
Hung CH, Changchien CS and Chen CH: Virological response to
entecavir reduces the risk of liver disease progression in
nucleos(t)ide analogue-experienced HBV-infected patients with prior
resistant mutants. J Antimicrob Chemother. 68:2154–2163.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liaw YF: Antiviral therapy of chronic
hepatitis B: Opportunities and challenges in Asia. J Hepatol.
51:403–410. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wu FP, Yang Y, Li M, Liu YX, Li YP, Wang
WJ, Shi JJ, Zhang X, Jia XL and Dang SS: Add-on pegylated
interferon augments hepatitis B surface antigen clearance vs
continuous nucleos(t)ide analog monotherapy in Chinese patients
with chronic hepatitis B and hepatitis B surface antigen ≤ 1500
IU/mL: An observational study. World J Gastroenterol. 26:1525–1539.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yoshida K, Enomoto M, Tamori A, Nishiguchi
S and Kawada N: Combination of entecavir or tenofovir with
pegylated interferon-α for long-term reduction in hepatitis B
surface antigen levels: Simultaneous, sequential, or add-on
combination therapy. Int J Mol Sci. 2021; 22: 1456, 2021.
|
|
21
|
Brunetto MR, Moriconi F, Bonino F, Lau GK,
Farci P, Yurdaydin C, Piratvisuth T, Luo K, Wang Y, Hadziyannis S,
et al: Hepatitis B virus surface antigen levels: A guide to
sustained response to peginterferon alfa-2a in HBeAg-negative
chronic hepatitis B. Hepatology. 49:1141–1150. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Moucari R, Mackiewicz V, Lada O, Ripault
MP, Castelnau C, Martinot-Peignoux M, Dauvergne A, Asselah T, Boyer
N, Bedossa P, et al: Early serum HBsAg drop: A strong predictor of
sustained virological response to pegylated interferon alfa-2a in
HBeAg-negative patients. Hepatology. 49:1151–1157. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rijckborst V, Hansen BE, Cakaloglu Y,
Ferenci P, Tabak F, Akdogan M, Simon K, Akarca US, Flisiak R,
Verhey E, et al: Early on-treatment prediction of response to
peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using
HBsAg and HBV DNA levels. Hepatology. 52:454–461. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Boglione L, Cusato J, Cariti G, Di Perri G
and D'Avolio A: Role of HBsAg decline in patients with chronic
hepatitis B HBeAg-negative and E genotype treated with
pegylated-interferon. Antiviral Res. 136:32–36. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mimura S, Fujita K, Takuma K, Nakahara M,
Oura K, Tadokoro T, Kobara H, Tani J, Morishita A, Himoto T and
Masaki T: Effect of pegylated interferon alfa-2a in HBeAg-negative
chronic hepatitis B during and 48 weeks after off-treatment
follow-up: the limitation of pre-treatment HBsAg load for the
seroclearance of HBsAg. Intern Emerg Med. 16:1559–1565.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brunetto MR and Bonino F: Interferon
therapy of chronic hepatitis B. Intervirology. 57:163–170.
2014.PubMed/NCBI View Article : Google Scholar
|