Introduction
Butorphanol is an opioid agonist-antagonist that
exhibits protective effects on specific organs, such as the brain,
the lungs and the heart (1). The
potential mechanism of action of butorphanol depends on the dual
pharmacological functions of κ-opioid and µ-receptors activated by
specific metabolites (2). It has
been acknowledged that butorphanol can be administered via
different routes, such as intravenous administration, intramuscular
injection and nasal spray (3).
Previous studies have shown that butorphanol is widely applied in
clinical practice. For example, as a synthetic opioid, butorphanol
has been reported to inhibit tumor growth and metastasis as well as
inflammation, oxidative stress and apoptosis in cerebrovascular
diseases (3,4). However, the neuroprotective effects
of butorphanol on neurodegenerative diseases have not yet been
determined.
Neurodegenerative diseases comprise several
biological events, including oxidative stress and programmed cell
death (5). Ferroptosis is a novel
iron-dependent process of programmed cell death featured by
excessive accumulation of lipid peroxides and reactive oxygen
species (6). Emerging evidence has
supported that ferroptosis is closely implicated in the process of
neurodegenerative diseases (7).
Hydrogen peroxide (H2O2) is often used to
establish the model of oxidative damage due to its strong oxidant
properties (8,9). In brain cells,
H2O2 acts as an intracellular regulator of
neuronal activity, growth and organelle function as well as a
diffusible messenger of neuron-glia signaling and interneuronal
communication, including the regulation of synaptic transmission
and plasticity (10). To
effectively clarify the effects of butorphanol on neurodegenerative
diseases, H2O2 was used to treat PC12 cells
so as to establish an in vitro model of oxidative
damage.
Among the kinases, mitogen-activated protein kinase
(MAPK) plays an indispensable role in neurodegeneration (11). The c-Jun NH2-terminal kinase (JNK)
and the p38 MAP kinase, belong to the MAPK family of enzymes and
are responsible for stress signaling and pro-inflammatory cytokine
stimulation (12). The transit of
upstream signals to downstream factors during the activation of the
JNK/p38 MAPK may mediate apoptosis, differentiation, growth or
immune responses (13-15).
Furthermore, the tight correlation between ferroptosis and JNK
signaling has also been highlighted (16,17).
In addition, the inhibition of JNK and MAPK enzymes can prevent
neural damage (18).
The purpose of the present study was to identify the
role of butorphanol in the prevention of neurodegenerative disease
cell model, and the mechanism of action of the association between
butorphanol and the JNK/p38 signaling pathway. The present study
may offer a novel strategy for the study of neurodegenerative
diseases.
Materials and methods
Cell culture and treatment
PC12 cells were obtained from BeNa Culture
Collection. The cells were incubated with DMEM supplemented with
10% FBS (Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, and
100 µg/ml streptomycin in a humid environment at 37˚C with 5%
CO2. PC12 cells were incubated in 96-well plates for 24
h. Subsequently, 400 µM H2O2 was incubated
with the cells for 1 h at room temperature so as to establish an
in vitro cell injury model. Following the indicated
treatment, PC12 cells were incubated with butorphanol at different
doses (1, 2 and 4 µM) for 24 h and a series of cellular experiments
were conducted to investigate the effects of butorphanol on
H2O2-induced PC12 cell injury.
Cell Counting Kit-8 (CCK-8)
PC12 cells were incubated in 96-well plates, at a
density of 5x103, for 24 h. Subsequently, 10 µl CCK-8
reagent (Beyotime Institute of Biotechnology) was added to each
well and the plate was incubated for an additional 2 h. The
absorbance was detected at a wavelength of λ=450 nm using a
microplate reader (Molecular Devices, LLC), and cell viability was
expressed as the ratio of the measured wavelength to the control
wavelength.
Detection of oxidative stress
The lysis of PC12 cells was performed by cell lysis
buffer (Beyotime Institute of Biotechnology; cat. no. P0013).
Subsequently, the cells were centrifuged at 10,000 x g for 10 min
at 4˚C and 200 µl supernatant was collected. Malondialdehyde (MDA;
cat. no. S0131S), superoxide dismutase (SOD; cat. no. S0088),
glutathione peroxidase (GSH-Px; cat. no. S0056) and catalase (CAT;
cat. no. S0082) (Beyotime Institute of Biotechnology) assay kits
were used to determine the contents of MDA, SOD, GSH-Px, and CAT,
separately.
Western blotting
PC12 cells were isolated using RIPA lysis buffer
(Beyotime Institute of Biotechnology; cat. no. P0013B) and
quantified by the bicinchoninic acid protein kit (Beyotime
Institute of Biotechnology; cat. no. P0011). Proteins (30 µg/lane)
were separated using 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes. The membranes were incubated for 2 h with 5%
skimmed milk or 5% BSA at room temperature and overnight at 4˚C
with primary antibodies against NADPH oxidase (Nox) 2 (cat. no.
ab129068; 1:5,000; Abcam), Nox4 (cat. no. ab154244; 1:500; Abcam),
Bcl-2 (cat. no. ab194583; 1:500; Abcam), Bax (cat. no. ab182733;
1:2,000; Abcam), cleaved-caspase 3 (cat. no. 9661; 1:500; Cell
Signaling Technology, Inc.), caspase 3 (cat. no. ab184787; 1:2,000;
Abcam), phosphorylated (p)-JNK (cat. no. ab76572; 1:5,000; Abcam),
p-p38 (cat. no. ab4822; 1:1,000; Abcam), JNK (cat. no. ab199380;
1:2,500; Abcam), p38 (cat. no. ab170099; 1:1,000; Abcam), GPX4
(cat. no. ab125066; 1:2,000; Abcam), ferritin heavy chain (FTH1;
cat. no. ab183781; 1:1,000; Abcam), long-chain-fatty-acid-CoA
ligase 4 (ACSL4; cat. no. ab155282; 1:10,000; Abcam) or GAPDH (cat.
no. ab181602; 1:10,000; Abcam). Subsequently, horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no.
ab97080; 1:5,000; Abcam) was applied to incubate the membranes for
an additional 2 h at room temperature. Finally, the visualization
of the protein bands was performed by an enhanced chemiluminescence
reagent (Thermo Fisher Scientific, Inc.). ImageJ software (version
1.52v; National Institutes of Health) was used for densitometric
analysis.
Terminal
deoxynucleoitidyl-transferase-mediated dUTP nick end labeling
(TUNEL)
With the application of a TUNEL kit (cat. no. C1091;
Beyotime Institute of Biotechnology), the effect of butorphanol on
H2O2-induced PC12 apoptosis was assessed. In
short, the fixation and permeabilization of PC12 cells on glass
slips were carried out with 4% paraformaldehyde at room temperature
for 30 min and 0.25% Triton-X 100 at room temperature for 5 min,
separately. Subsequently, the cells were labeled with TUNEL reagent
at 37˚C in the dark for 60 min. Finally, nuclear labeling was
performed using Vectashield mounting medium containing 50 µg/ml
DAPI for nuclear labeling (Vector Laboratories, Inc.) at room
temperature for 5 min. The luminescence intensity was controlled in
a fluorescent microscope with a set of fluorescein isothiocyanate
filters (green spectrum). Lastly, the images of positive apoptotic
cells in three random fields per coverslip were captured by a
fluorescent microscope (magnification, x200; Olympus Corporation).
The number of TUNEL-positive PC12 cells and the total number of
cells were counted using Image-Pro Plus 6.0 software (Media
Cybernetics). The percentage of TUNEL-positive PC12 cells in the
total cells were counted and the average value was calculated.
Lipid peroxidation
The detection of the oxidation of polyunsaturated
fatty acids in cell samples was performed by assessing
thiobarbituric acid-reactive substances (TBARS) produced during the
process of lipid peroxidation, which included lipid peroxides and
MDA. The determination of TBARS was performed by fluorimetry which
used 1,1,3,3-tetramethoxypropane as the standard.
Measurement of Fe2+
The collected cells were homogenized with
phosphate-buffered saline and centrifuged at 13,000 x g for 10 min
at 4˚C for the detection of iron concentration. The latter was
quantified by an iron colorimetric assay kit (cat. no. K390;
BioVision, Inc.).
Statistical analysis
All data are demonstrated as mean ± standard
deviation utilizing GraphPad Prism 8.0 software (GraphPad Software,
Inc.). Each experiment was repeated at least three times. Tukey's
post-hoc test and one-way analysis of variance were employed to
conduct statistical comparisons. P<0.05 was considered to
indicate a statistical significance.
Results
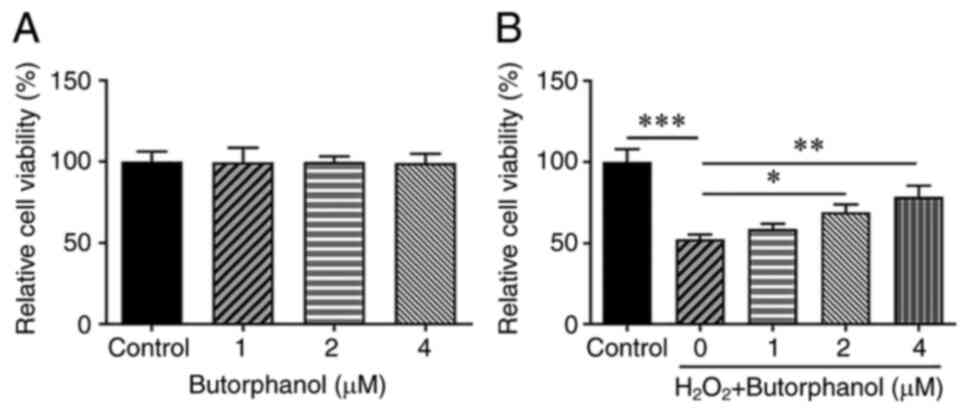
Effects of butorphanol treatment on
the viability of PC12 cells
To clarify the role of butorphanol in
neurodegenerative diseases, the viability of
H2O2-treated or untreated PC12 cells exposed
to butorphanol was measured to verify the hypothesis that
butorphanol protects against H2O2-induced
viability injury in PC12 cells. CCK-8 was used to investigate the
effects of butorphanol on the viability of PC12 cells as well as on
the viability of H2O2-treated PC12 cells. The
viability of PC12 cells remained unchanged following butorphanol
treatment (Fig. 1A). However, it
was noted that H2O2 treatment greatly reduced
the viability of PC12 cells in comparison with that of the control
group (Fig. 1B). Notably,
H2O2-treated PC12 cell viability was
gradually reversed with the increasing concentrations of
butorphanol, revealing that this compound promoted the viability of
H2O2-exposed PC12 cells in a
concentration-dependent manner.
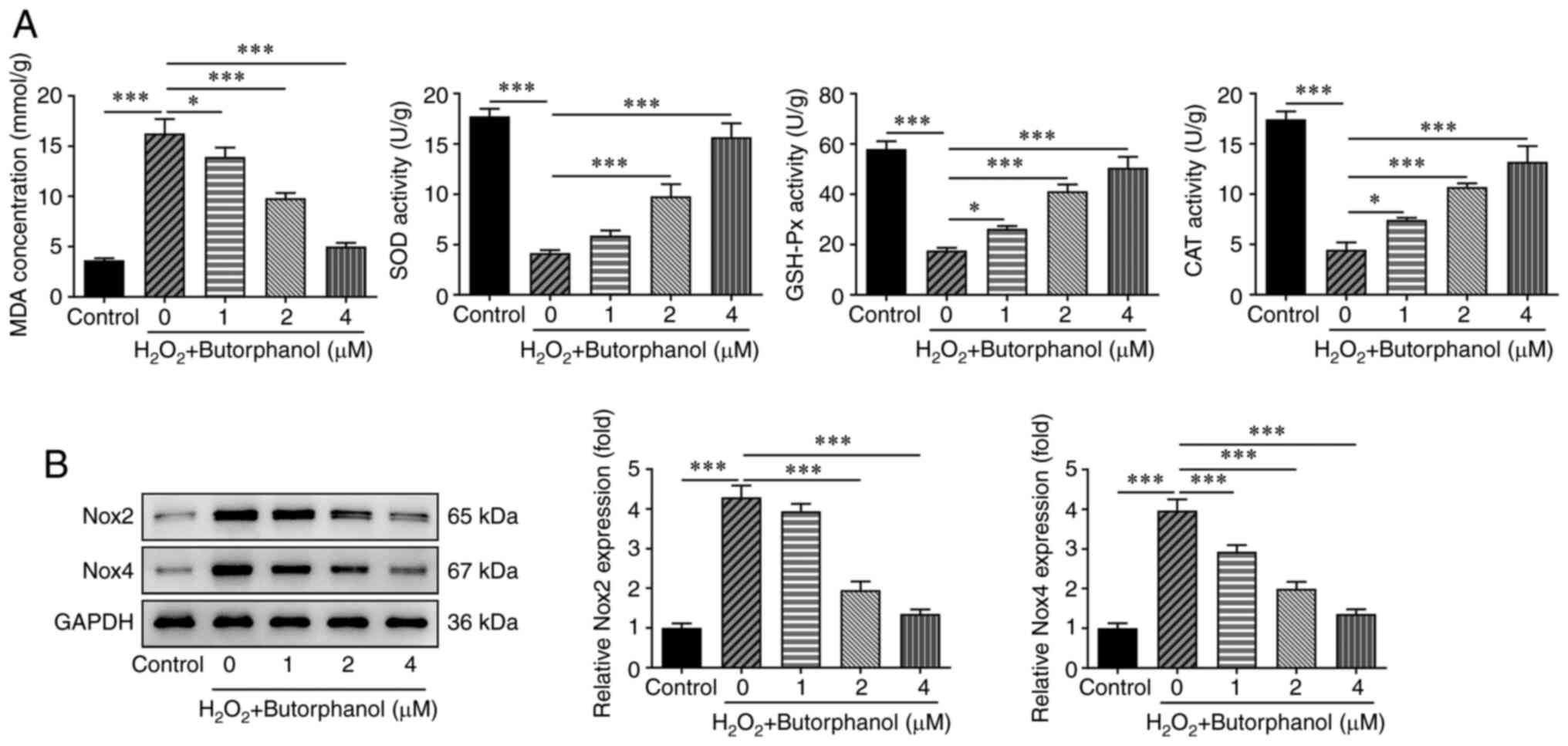
Butorphanol inhibits oxidative stress
in H2O2-treated PC12 cells
To explore the role of butorphanol in oxidative
stress of H2O2-treated PC12 cells, the
expression levels of oxidative stress factors including MDA, SOD,
GSH-Px and CAT were evaluated with the corresponding assay kits to
validate the hypothesis that butorphanol may also play a protective
role in H2O2-evoked oxidative stress in PC12
cells. In contrast to the control group, treatment of the cells
with H2O2 significantly increased MDA
concentration and significantly decreased SOD, GSH-Px and CAT
expression levels. Nevertheless, butorphanol reversed the effects
of H2O2 treatment on the expression of theses
markers in a dose-dependent manner, as demonstrated by the
decreased MDA concentration as well as the upregulated SOD, GSH-Px
and CAT expression levels in comparison with those of the
H2O2 group (Fig.
2A). In addition, the expression levels of oxidative
stress-related proteins, including Nox2 and Nox4 were significantly
increased in H2O2-treated PC12 cells, while
butorphanol treatment exhibited the opposite effects on these
proteins, suggesting that butorphanol imparted suppressive effects
on the induction of oxidative stress in
H2O2-treated PC12 cells (Fig. 2B).
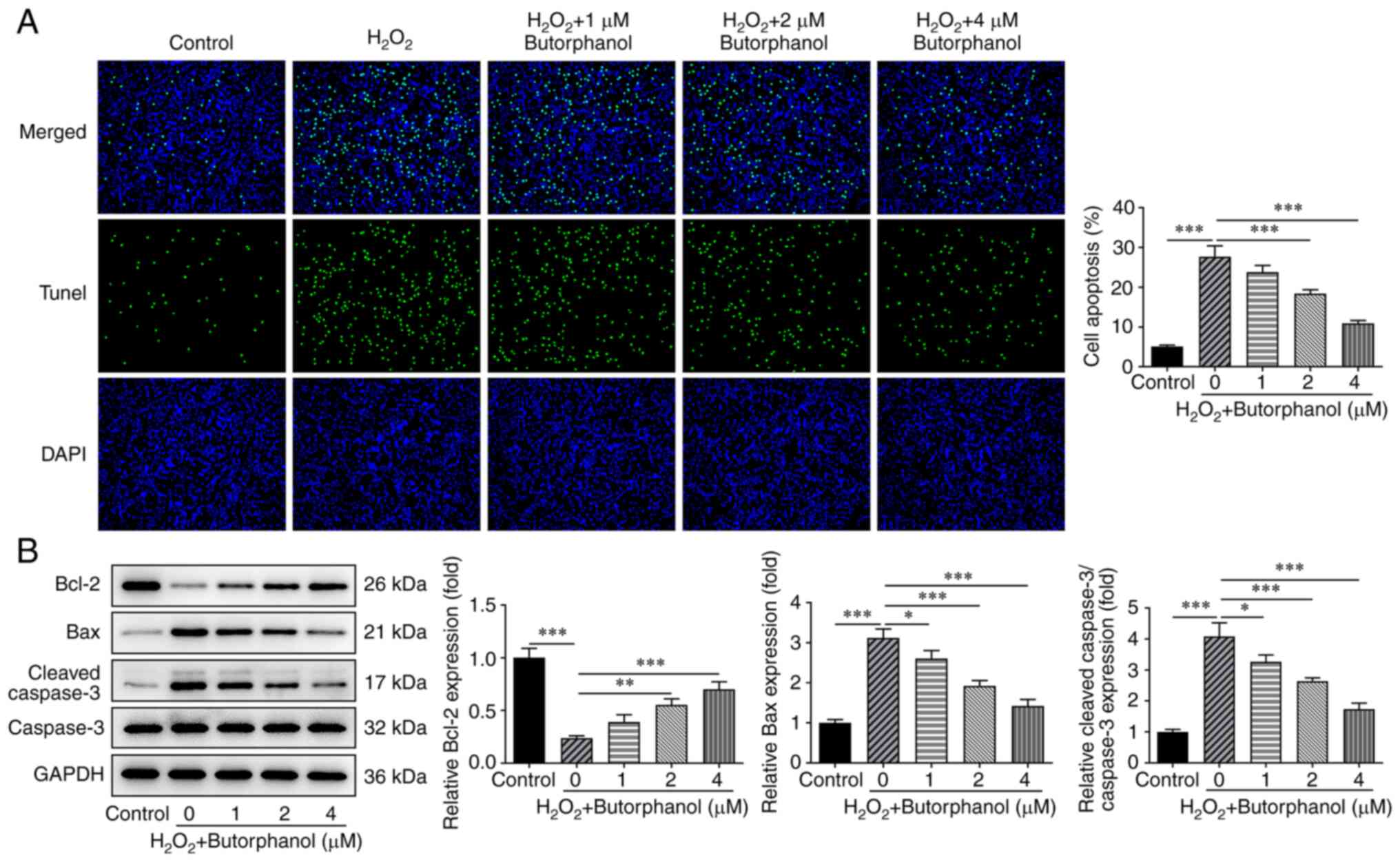
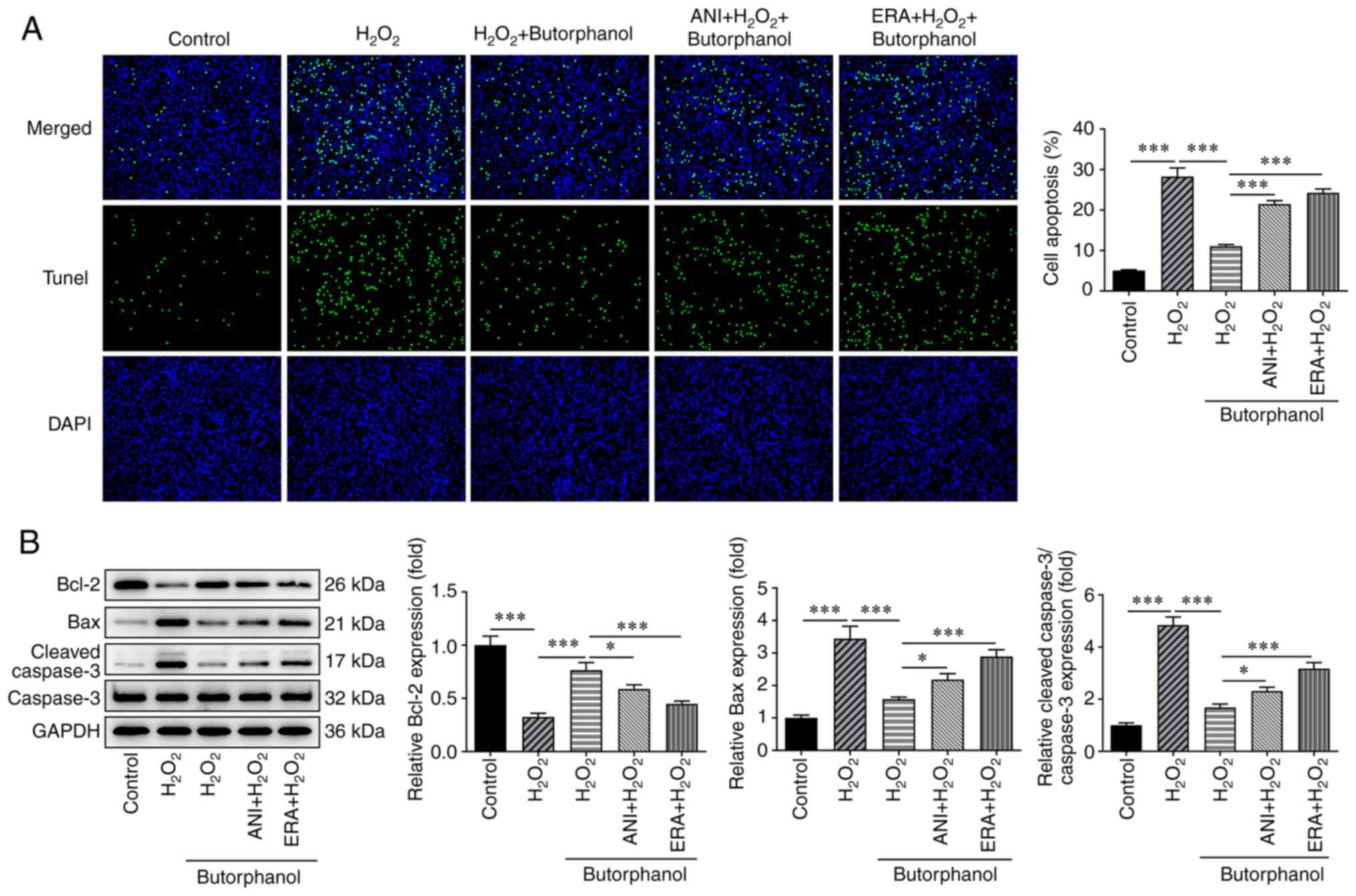
Butorphanol inhibits apoptosis in
H2O2-treated PC12 cells
The TUNEL assay was used explore the role of
butorphanol in H2O2-induced PC12 apoptosis,
and the expression levels of the apoptotic factors were assessed
using western blotting to validate the hypothesis that butorphanol
may hamper the apoptosis of H2O2-treated PC12
cells. The significantly increased levels of
H2O2-induced PC12 apoptosis were gradually
decreased by butorphanol treatment (Fig. 3A). Notably, butorphanol exhibited
inhibitory effects on H2O2-induced PC12
apoptosis in a dose-dependent manner. In addition,
H2O2 treatment significantly diminished Bcl-2
expression and markedly increased Bax and cleaved-caspase 3
expression, which were subsequently partially reversed by
butorphanol treatment, as determined by the upregulated Bcl-2
expression as well as the downregulated Bax and cleaved-caspase 3
expression in H2O2-treated PC12 cells
additionally treated with increasing doses of butorphanol (Fig. 3B). The aforementioned results
indicated that butorphanol repressed the apoptosis of
H2O2-treated PC12 cells.
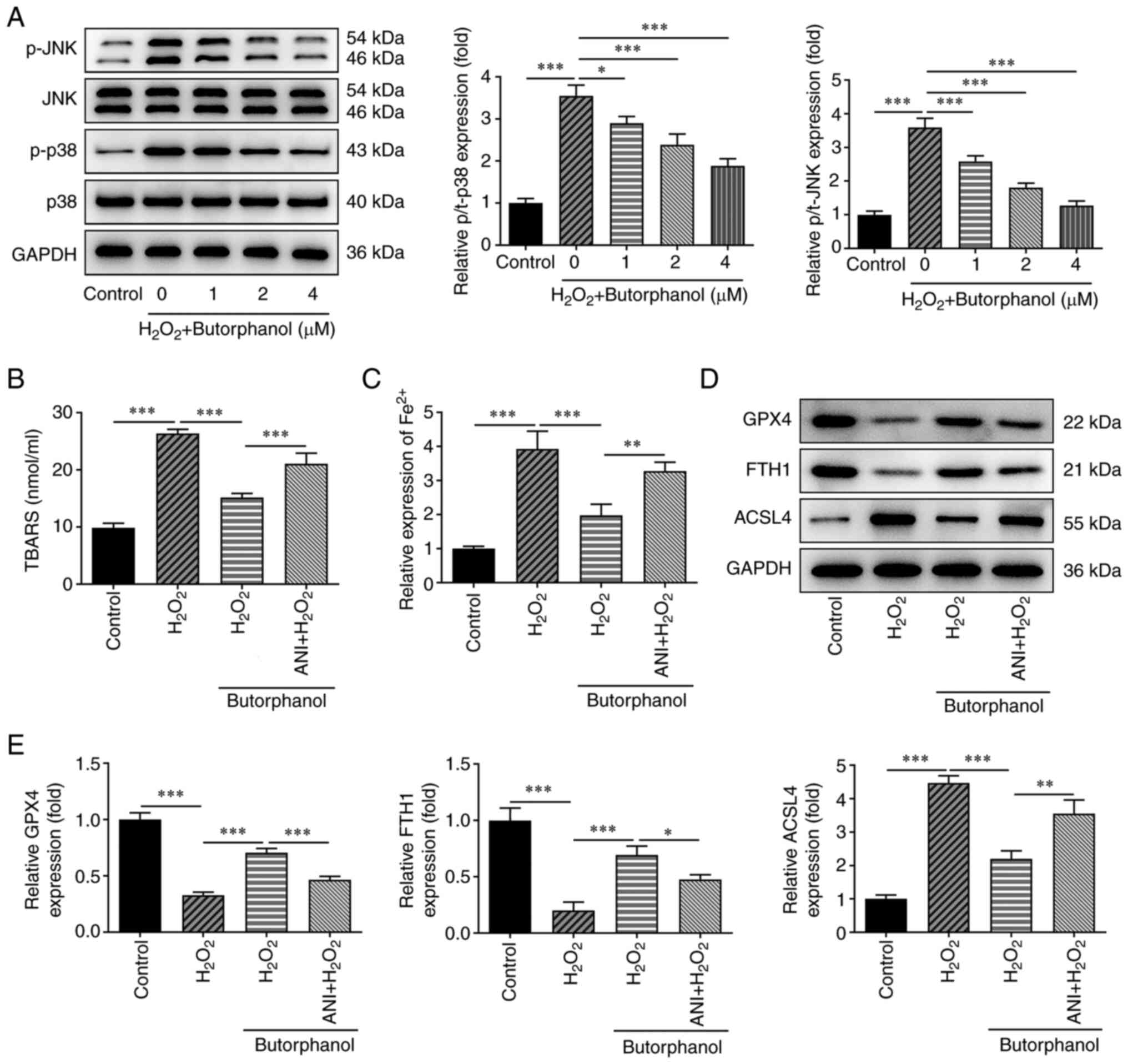
Butorphanol inhibits ferroptosis by
blocking the JNK/p38 signaling pathway
To examine the latent regulatory mechanism of
butorphanol in H2O2-treated PC12 cells, the
results obtained from western blotting indicated that the
significantly increased p-JNK and p-p38 expression levels in PC12
cells caused by H2O2 treatment were gradually
decreased following butorphanol administration compared with those
of the H2O2 group (Fig. 4A). Butorphanol (4 µM) was selected
for the remaining experiments. To further identify the mechanism of
the JNK/p38 signaling pathway, the JNK activator anisomycin (ANI)
was utilized to pre-treat the cells. Increased TBAR levels in
H2O2-treated PC12 cells were subsequently
inhibited by butorphanol administration, which was restored by ANI
(Fig. 4B).
In addition, butorphanol diminished the increased
levels of Fe2+ in H2O2-treated
PC12 cells, which were subsequently partially countervailed by ANI
administration in comparison with that of the
H2O2 + butorphanol group (Fig. 4C). Moreover, butorphanol treatment
downregulated ACSL4 expression and upregulated GPX4 and FTH1
expression levels in H2O2-treated PC12 cells
compared with those noted in the H2O2 group
(Fig. 4D and E). Nevertheless, ANI partially abolished
the effects of butorphanol on the expression levels of these
ferroptosis-related proteins, as determined by the increased ACSL4
expression and the decreased GPX4 and FTH1 expression in the ANI +
H2O2 + butorphanol group, suggesting that
butorphanol inhibited ferroptosis by blocking the JNK/p38 signaling
pathway.
Butorphanol inhibits ferroptosis by
blocking the JNK/p38 signaling pathway to suppress oxidative stress
in H2O2-treated PC12 cells
To further determine whether butorphanol
participates in H2O2-induced PC12 cell
oxidative stress by blocking the JNK/p38 signaling pathway and
suppressing ferroptosis, the ferroptosis inducer erastin (ERA) was
used at a concentration of 30 mM and the JNK activator ANI at a
concentration of 10 mM to treat the cells. Following treatment, the
oxidative stress levels were detected again. Compared with the
H2O2 + butorphanol group, ANI or ERA
treatment significantly enhanced the MDA levels and significantly
reduced the levels of SOD, GSH-Px and CAT (Fig. 5A). Furthermore, ANI or ERA
administration significantly improved the decreased Nox2 and Nox4
expression levels compared with those noted in the
H2O2 + butorphanol group, indicating that
butorphanol inhibited ferroptosis by blocking the JNK/p38 signaling
pathway to suppress oxidative stress in
H2O2-treated PC12 cells (Fig. 5B).
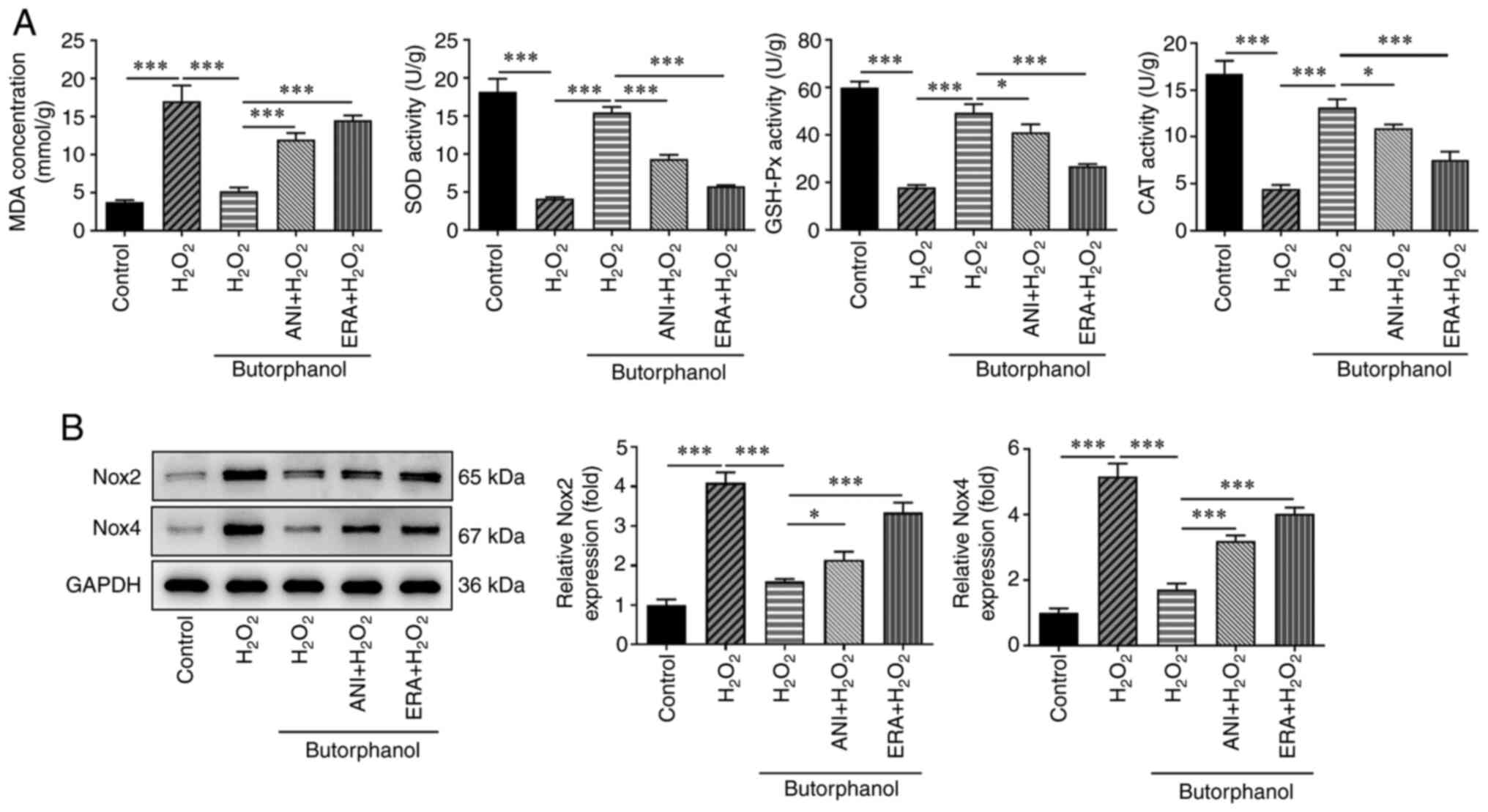 | Figure 5Butorphanol inhibits ferroptosis by
blocking the JNK/p38 signaling pathway to suppress oxidative stress
in H2O2-treated PC12 cells. (A) Levels of
MDA, SOD, GSH-Px and CAT were detected using the corresponding
assay kits. (B) Expression levels of the oxidative stress-related
proteins were detected using western blotting.
*P<0.05 and ***P<0.001. JNK, c-Jun
NH2-terminal kinase; H2O2, hydrogen peroxide;
MDA, malondialhedyde; SOD, superoxide dismutase; GSH-Px,
glutathione peroxidase; CAT, catalase; Nox, NADPH oxidase; ANI,
anisomycin; ERA, erastin. |
Butorphanol inhibits ferroptosis by
blocking the JNK/p38 signaling pathway to suppress
H2O2-induced PC12 apoptosis
To further determine whether butorphanol
participates in H2O2-induced PC12 apoptosis
by blocking the JNK/p38 signaling pathway and suppressing
ferroptosis, the apoptosis levels were detected again following the
addition of ERA and ANI. The results indicated that
H2O2 treatment increased the induction of
apoptosis of PC12 cells compared with the control group, which was
significantly reduced by butorphanol treatment (Fig. 6A). Evidently, ANI or ERA markedly
enhanced the apoptosis of butorphanol-treated PC12 cells exposed to
H2O2 compared with the
H2O2 + butorphanol group. Moreover, treatment
of the cells with H2O2 decreased Bcl-2 levels
and increased the levels of Bax and cleaved-caspase 3, which were
subsequently reversed by butorphanol, as determined by the enhanced
Bcl-2 expression as well as the reduced Bax and cleaved-caspase 3
expression levels in the H2O2 + butorphanol
group (Fig. 6B). Nevertheless, it
was noted that the simultaneous addition of ANI and ERA inhibited
Bcl-2 levels and promoted the levels of Bax and cleaved-caspase 3
compared with those noted in the H2O2 +
butorphanol group, implying that butorphanol inhibited ferroptosis
by blocking the JNK/p38 signaling pathway to suppress
H2O2-induced PC12 apoptosis.
Discussion
In the present study, H2O2 was
incubated with PC12 cells so as to establish a model of
neurodegenerative damage in vitro. Through a series of
functional experiments, it was revealed that butorphanol suppressed
the induction of oxidative stress, apoptosis and ferroptosis of
H2O2-treated PC12 cells. In addition,
subsequent experiments demonstrated that butorphanol inhibited
ferroptosis by blocking the JNK/p38 signaling pathway to suppress
oxidative stress and apoptosis in
H2O2-treated PC12 cells. To the best of our
knowledge, this is the first study to preliminarily clarify the
possible suppressive role of butorphanol in neurodegenerative
diseases as well as its close relation with the JNK/p38 signaling
pathway in neurodegenerative diseases.
Neurodegenerative diseases are sporadic and rare
hereditary disorders of the central nervous system, which can
contribute to the slow progressive loss of the functions of
specific neuron populations and their connections (19). Apoptosis is one of the mechanisms
triggering neurodegenerative diseases, during the process of which
the apoptotic rate is enhanced and the neural damage may be evident
(20). A previous study has
demonstrated that anti-apoptotic drugs can alter the apoptotic
pathway in neurodegenerative diseases as well as delay the disease
progression (21). In addition, it
is commonly acknowledged that oxidative stress is a major
regulatory element in neurodegenerative diseases (22). Furthermore, ferroptosis has been
reported to participate in the apoptosis and neuronal cell damage
in PC12 cells (23). Furthermore,
butorphanol, a synthetic selective opioid receptor antagonist, has
been shown to attenuate apoptosis and oxidative damage in neuronal
PC12 cells in recent studies (3,24).
In the present study, butorphanol had no influence on the viability
of PC12 cells, whereas it markedly decreased
H2O2-induced PC12 cell viability loss,
indicating that it could inhibit neuron cell viability loss in a
neurodegenerative disease cell model. Moreover, it was also found
that the increased Nox2 and Nox4 expressions caused by
H2O2 treatment were markedly reduced by
butorphanol treatment, which revealed the inhibitory effects of
butorphanol on oxidative stress in neurodegenerative diseases.
Furthermore, butorphanol suppressed
H2O2-induced apoptosis in PC12 cells and
downregulated the expression levels of the pro-apoptotic proteins
Bax and cleaved-caspase 3, suggesting its suppressive effects on
the induction of neuronal apoptosis in neurons.
The JNK/p38 signaling pathway is a critical signal
transduction pathway that participates in several physiological and
pathological responses, such as apoptosis, proliferation and
oxidative stress (25,26). The accumulation of
H2O2 has been shown to activate JNK/p38 MAPK
signaling in neuronal cells (27).
It has also been shown that butorphanol can block p38 and JNK
phosphorylation (28). Ferroptosis
plays a critical role in neuronal death and neurological disorders.
Concomitantly, iron depositions can be detected in specific brain
regions of cases with neurodegenerative diseases (7). A previous study suggested that
ferroptosis could be regulated by the Ras-JNK/p38 signaling pathway
(16). In the present study,
butorphanol markedly reduced the expression levels of p-JNK and
p-p38 in H2O2-treated PC12 cells, indicating
that it could block the JNK/p38 signaling pathway, which was
consistent with the results mentioned in a previous study (28). Moreover, butorphanol diminished the
levels of Fe2+ and ACSL4 in
H2O2-treated PC12 cells, which were
subsequently reversed by treatment with the JNK activator ANI,
suggesting that it could suppress ferroptosis by blocking the
JNK/p38 signaling pathway.
In order to further assess the relationship among
ferroptosis, oxidative stress, apoptosis and the JNK/p38 signaling
pathway, the ferroptosis inducer ERA was used to track the effects
of the inhibition of ferroptosis caused by the butorphanol-induced
blocking of the JNK/p38 signaling pathway on the induction of
apoptosis and oxidative stress in
H2O2-treated PC12 cells. In the present
study, it was shown that the decreased levels of MDA, Nox2 and Nox4
in H2O2-treated PC12 cells caused by
butorphanol were increased by ANI or ERA administration, indicating
that butorphanol inhibited ferroptosis by blocking the JNK/p38
signaling pathway to suppress oxidative stress in neurons.
Furthermore, the increased apoptosis levels and the upregulated Bax
and cleaved-caspase 3 expressions caused by ANI or ERA
administration could be observed in butorphanol-treated PC12 cells
exposed to H2O2. The aforementioned results
implied that butorphanol inhibited ferroptosis by blocking the
JNK/p38 signaling pathway, thus repressing oxidative stress and
apoptosis in a neurodegenerative disease model.
In conclusion, the present study investigated the
role of butorphanol and uncovered the relationship among
ferroptosis, oxidative stress, apoptosis and the JNK/p38 signaling
pathway in a neurodegenerative disease cell model. The findings may
provide new insights for the treatment of neurodegenerative
diseases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ, QS, PZ and YQ conceived and designed the study,
and acquired and interpreted the data. LJ, QS, PZ and YQ performed
the experiments. LJ, QS, PZ and YQ wrote the manuscript. LJ, QS, PZ
and YQ confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Luan G, Pan F, Bu L, Wu K, Wang A and Xu
X: Butorphanol promotes macrophage phenotypic transition to inhibit
inflammatory lung injury via κ receptors. Front Immunol.
12(692286)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jose DE, Ganapathi P, Anish Sharma NG,
Shankaranarayana P, Aiyappa DS and Nazim M: Postoperative pain
relief with epidural buprenorphine versus epidural butorphanol in
laparoscopic hysterectomies: A comparative study. Anesth Essays
Res. 10:82–87. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang Z, Wang L, Hu Y and Wang F:
Butorphanol protects PC12 cells against OGD/R-induced inflammation
and apoptosis. Mol Med Rep. 22:1969–1975. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang B, Li Y, Shen Y, Xu Y and Zhang C:
Butorphanol inhibits the malignant biological behaviors of ovarian
cancer cells via down-regulating the expression of TMEFF1. Onco
Targets Ther. 13:10973–10981. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dugger BN and Dickson DW: Pathology of
neurodegenerative diseases. Cold Spring Harb Perspect Biol.
9(a028035)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11(88)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Reichert CO, de Freitas FA, Sampaio-Silva
J, Rokita-Rosa L, Barros PL, Levy D and Bydlowski SP: Ferroptosis
mechanisms involved in neurodegenerative diseases. Int J Mol Sci.
21(8765)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang X, Zhao J, Han Z and Tang F:
Protective effects of semen crotonis pulveratum on trinitrobenzene
sulphonic acid-induced colitis in rats and
H2O2-induced intestinal cell apoptosis in
vitro. Int J Mol Med. 35:1699–1707. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cai X, Zhu L, Chen X, Sheng Y, Guo Q, Bao
J and Xu J: X/XO or H2O2 induced IPEC-J2 cell as a new in vitro
model for studying apoptosis in post-weaning piglets.
Cytotechnology. 68:713–724. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rice ME: H2O2: A dynamic neuromodulator.
Neuroscientist. 17:389–406. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Falcicchia C, Tozzi F, Arancio O,
Watterson DM and Origlia N: Involvement of p38 MAPK in synaptic
function and dysfunction. Int J Mol Sci. 21(5624)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Su AR, Qiu M, Li YL, Xu WT, Song SW, Wang
XH, Song HY, Zheng N and Wu ZW: BX-795 inhibits HSV-1 and HSV-2
replication by blocking the JNK/p38 pathways without interfering
with PDK1 activity in host cells. Acta Pharmacol Sin. 38:402–414.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lanna A, Gomes DC, Muller-Durovic B,
McDonnell T, Escors D, Gilroy DW, Lee JH, Karin M and Akbar AN: A
sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits
immunity during aging. Nat Immunol. 18:354–363. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li J, Xu B, Chen Z, Zhou C, Liao L, Qin Y,
Yang C, Zhang X, Hu Z, Sun L, et al: PI3K/AKT/JNK/p38 signalling
pathway-mediated neural apoptosis in the prefrontal cortex of mice
is involved in the antidepressant-like effect of pioglitazone. Clin
Exp Pharmacol Physiol. 45:525–535. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu K, Hu L and Hou J: Selective
suppression of Notch1 inhibits proliferation of renal cell
carcinoma cells through JNK/p38 pathway. Oncol Rep. 35:2795–2800.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ye F, Chai W, Xie M, Yang M, Yu Y, Cao L
and Yang L: HMGB1 regulates erastin-induced ferroptosis via
RAS-JNK/p38 signaling in HL-60/NRASQ61L cells. Am J
Cancer Res. 9:730–739. 2019.PubMed/NCBI
|
|
17
|
Zhang H, Jiao W, Cui H, Sun Q and Fan H:
Combined exposure of alumina nanoparticles and chronic stress
exacerbates hippocampal neuronal ferroptosis via activating
IFN-γ/ASK1/JNK signaling pathway in rats. J Hazard Mater.
411(125179)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aminzadeh A, Dehpour AR, Safa M,
Mirzamohammadi S and Sharifi AM: Investigating the protective
effect of lithium against high glucose-induced neurotoxicity in
PC12 cells: Involvements of ROS, JNK and P38 MAPKs, and apoptotic
mitochondria pathway. Cell Mol Neurobiol. 34:1143–1150.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Reith W: Neurodegenerative diseases.
Radiologe. 58:241–258. 2018.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
20
|
Radi E, Formichi P, Battisti C and
Federico A: Apoptosis and oxidative stress in neurodegenerative
diseases. J Alzheimers Dis. 42 (Suppl 3):S125–S152. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Erekat NS: Apoptosis and its therapeutic
implications in neurodegenerative diseases. Clin Anat. 35:65–78.
2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Singh A, Kukreti R, Saso L and Kukreti S:
Oxidative stress: A key modulator in neurodegenerative diseases.
Molecules. 24(1583)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wu C, Zhao W, Yu J, Li S, Lin L and Chen
X: Induction of ferroptosis and mitochondrial dysfunction by
oxidative stress in PC12 cells. Sci Rep. 8(574)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tsukamoto A, Iimuro M, Sato R, Yamazaki J
and Inomata T: Effect of midazolam and butorphanol premedication on
inhalant isoflurane anesthesia in mice. Exp Anim. 64:139–145.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bu X, Xia W, Wang X, Lu S and Gao Y:
Butylphthalide inhibits nerve cell apoptosis in cerebral infarction
rats via the JNK/p38 MAPK signaling pathway. Exp Ther Med.
21(565)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lv WP, Li MX and Wang L: Peroxiredoxin 1
inhibits lipopolysaccharide-induced oxidative stress in lung tissue
by regulating P38/JNK signaling pathway. Eur Rev Med Pharmacol Sci.
21:1876–1883. 2017.PubMed/NCBI
|
|
27
|
Li WW, Gao XM, Wang XM, Guo H and Zhang
BL: Icariin inhibits hydrogen peroxide-induced toxicity through
inhibition of phosphorylation of JNK/p38 MAPK and p53 activity.
Mutat Res. 708:1–10. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Huang LH, Li J, Gu JP, Qu MX, Yu J and
Wang ZY: Butorphanol attenuates myocardial ischemia reperfusion
injury through inhibiting mitochondria-mediated apoptosis in mice.
Eur Rev Med Pharmacol Sci. 22:1819–1824. 2018.PubMed/NCBI View Article : Google Scholar
|