Introduction
Severe acute respiratory syndrome 2 (SARS-CoV-2) is
the causal agent of the COVID-19 outbreak (1). At the global level, according to the
National Center for Surveillance and Control of Communicable
Diseases, a total of 244,089,628 cases of infection and 4,958,793
COVID-19-associated deaths had been confirmed (as of October 2021)
(2).
Being primarily a respiratory disease with major
pulmonary complications, it is characterized by fever, a
non-productive cough, respiratory difficulties, dyspnea, and
hypoxia in most patients, and signs of interstitial pneumonia on
X-rays or CT scans; however, COVID-19 may also affect other organs,
including the brain (3).
Thromboembolic events have been reported after infection with
SARS-CoV-2, primarily in the pulmonary vasculature, as well as an
increased rate of thromboembolic complications of the nervous
system with subsequent cerebrovascular accidents (4). The most common complication after
infection with SARS-CoV-2 remains ischemic stroke, but cases of
cerebral venous sinus thrombosis have been confirmed (4).
This infectious disease, caused by the most recently
discovered coronavirus, COVID-19, can induce hypercoagulability
associated with an increased prevalence of venous thromboembolism
grouped with thrombotic complications, primarily connected to
pulmonary vascularization, but these thrombotic complications may
also involve the vasculature of the brain (for 2% of the patients
with confirmed SARS-CoV-2 infection). Currently, the mechanism of
thrombophilia associated with COVID-19 infection is not completely
understood. However, it is known that the severe inflammatory
response after the infection with SARS-CoV-2 virus leads to an
outburst of cytokines which induces a pro-coagulable state. The
virus has a specific pro-coagulative effect, and infection with
this virus produces large-scale endothelial dysfunction and
inflammation. Both Von Willebrand factor (VWF) and factor VIII
(FVIII) are known to be highly rated for patients with COVID-19.
These factors are not only connected to the risk of inflammation
and thrombosis but are strongly connected to endothelial lesions. A
decrease in the FVIII/VWF ratio in patients infected with the
SARS-CoV-2 virus is associated with a higher risk of aggravation of
the respiratory state followed by an increased need for oxygen
(4).
A well-known characteristic of infection with
SARS-CoV-2 is coagulopathy and the fact that hypercoagulability may
lead to other complications associated with COVID-19, in
particular, venous thromboembolic events and stroke. Disseminated
intravascular coagulation, increased D-dimer levels, modified
fibrinogen levels, fibrin or fibrinogen degradation products,
thrombocytopenia, and the presence of antiphospholipid antibodies
are all linked to SARS-CoV-2 infection and severity. However, there
are a few case reports that have described cerebral venous sinus
thrombosis (CVST) associated with SARS-CoV-2 infection (5).
Cerebral venous thrombosis (CVT) is caused by
complete or partial occlusion of the major cerebral venous sinuses
(thrombosis of the major cerebral venous sinuses) or smaller
cortical supply veins and represents a common cause of stroke
(6). It is a rare disease,
accounting for ~0.5% of all cerebrovascular diseases worldwide
(7). Prothrombotic hematological
conditions, hormonal (oral contraceptive pill, pregnancy,
puerperium, steroids), local factors (skull abnormalities/trauma,
compressing mass or infection), systemic illness (dehydration,
sepsis, malignancy or connective tissue disorders), or idiopathic
causes (7) are all risk
factors.
CVST is known to be a multifactorial disease, with
at least one risk factor for 85% of the affected adult patients.
Risk factors are associated with the Virchow triad of
thrombogenesis (lesions of the vessel wall, hypercoagulability, and
blood stasis). The most frequent risk factors associated with CVT
are prothrombotic conditions. Patients known to have hereditary
thrombophilia present with an increased susceptibility to
developing any form of thrombosis, CVST included; the most frequent
causes are V Leiden factor, polymorphism of G20210A prothrombin and
antiphospholipid syndrome, and the rarest risk factors are
antithrombin III deficiency, C protein deficiency and S protein
deficiency (8).
Previously, a prognosis of CVT was fatal, especially
due to tardive or post-mortem identification of the diagnosis. Most
patients diagnosed with CVT today have a good prognosis, largely
due to improvements in high-performance imaging methods. However,
CVT management is notably complicated by SARS-CoV-2 infection.
SARS-CoV-2 increases systemic hypercoagulability and
thromboembolism and is associated with cerebrovascular diseases,
particularly CVT (9).
For confirmation of neurological pathologies,
diagnosis is established according to clinical manifestations,
objective neurological examinations, paraclinical investigations
(biological data analyzed during a patient's stay, and biological
material collected to establish hereditary factors for thrombosis),
and based on cerebral imaging. The most commonly used imaging
methods are CT, native or with angiography, and magnetic resonance
imaging (MRI), native or with angiography. Furthermore, the device
used was a SIGNA Explorer 1.5 T (GE Healthcare). In order to
determine the hereditary thrombosis profile, the test for the
identification of mutations associated with thrombophilia,
biological material/blood was collected and the equipment used was
as follows: For acid DNA isolation a vortex combispin FVL-2400N
(Biosan) and a Thermo-Shaker TS-100 centrifuge Hettich Universal
320R (Biosan); for DNA amplification, a Real-Time thermal cycler
qTower 2.2 (Analytic Jena); and for hybridization and detection, a
Thermo-Shaker PST-60HL (Biosan). The presence of infection with
SARS-CoV-2 virus was detected using ARN SARS-CoV-2 testing by
reverse transcription-quantitative PCR.
The purpose of neurological treatment is to
eliminate the obstacle at the level of the sinus lumen or vessels
affected and to stop the progression of a thrombus, to cure the
prothrombotic status in order to prevent both venous thrombotic
events and relapse (10).
Materials and methods
The statistical data collected from patients with
neurological disorders and SARS-CoV-2 infection, who were admitted
to the Neurology Section of ‘Sf. Apostol Andrei’ Emergency Clinical
Hospital in Constanta, provide confirmation of the observed
pathological findings. The Ethics Committee for Clinical Studies at
the Constanta County Emergency Clinical Hospital approved the study
(approval no. 31/03.11.2021), which was performed in compliance
with the Declaration of Helsinki. All subjects provided written
informed consent prior to enrolment. In the present report, the
cases of three patients affected by a neurological disease (patient
no. 1: Left subacute venous transversal-sigmoid-jugular thrombosis;
patient no. 2: Right superior frontal cortical vein thrombosis,
superior sagittal sinus, sinuses' confluent, sigmoid sinuses and
transverse on the right side, and partially the right internal
jugular vein; and patient no. 3: Thrombosis of transverse and
sagittal sinuses) and diagnosed with COVID-19. Paraclinical
investigations included CT scan, MRI scan and electromyography.
Results
Patient no. 1
The first case presented is of a patient aged 66
years, known to be at high risk of hypertension, who was
hospitalized in November 2020 in the Neurology Clinic for a
language disorder that started 15 days before presentation.
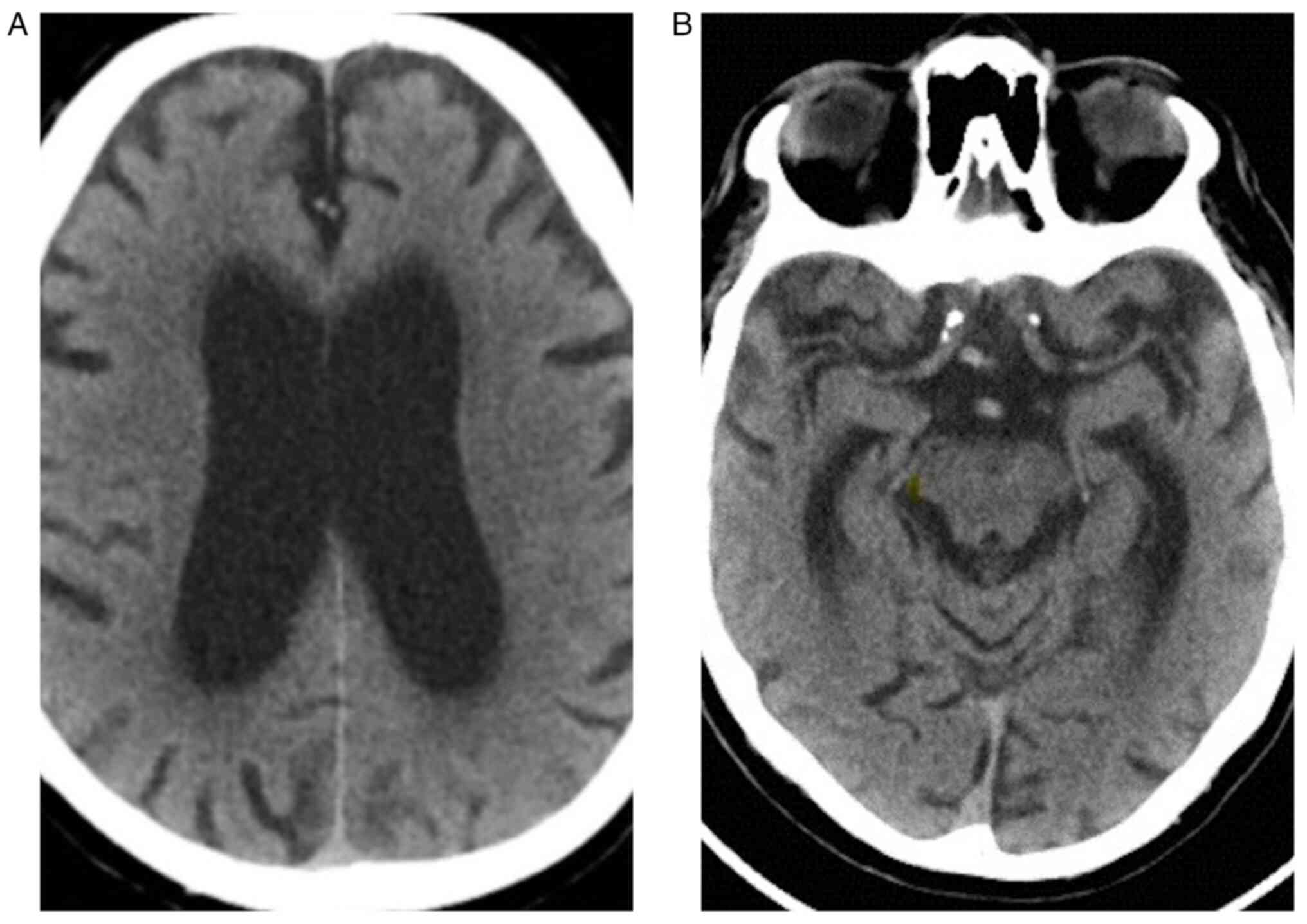
When the patient came to the Emergency Unit, he
underwent native cranial CT (Fig.
1). When hospitalized, the ARN SARS-CoV-2 test was negative.
During hospitalization, the patient presented dynamically increased
blood glycemia and increased glycated hemoglobin (glycated
hemoglobin, 11.2%; normal range, 4.8-5.6%), and thus a diabetes
consultation was requested. The patient was diagnosed with newly
discovered type II diabetes mellitus and insulin therapy was
started.
The neurological objective examination when the
patient was hospitalized was conscious and cooperative, prone to
roughness in his right superior member, with no motor deficit at
the level of inferior members, with no coordination or sensory
disorders, and no cutaneous plantar reflex indifferently
bilateral.
On the 4th day the patient submitted a neurological
examination, he was conscious, cooperative, and oriented in time
and space, with no movement deficits, no sensory disorders,
presented ataxia at the level of the bilateral inferior members,
orthostatic intolerance, and sustained walking. On the 8th day, the
objective of the neurological examination differed; the patient was
conscious, cooperative, and oriented in time and space, he
presented with normal ocular ability, no nystagmus, movement
deficits at the level of inferior members bilaterally proximal
right 2-3/5 and left 3-4/5, and bilateral brachia proximal right
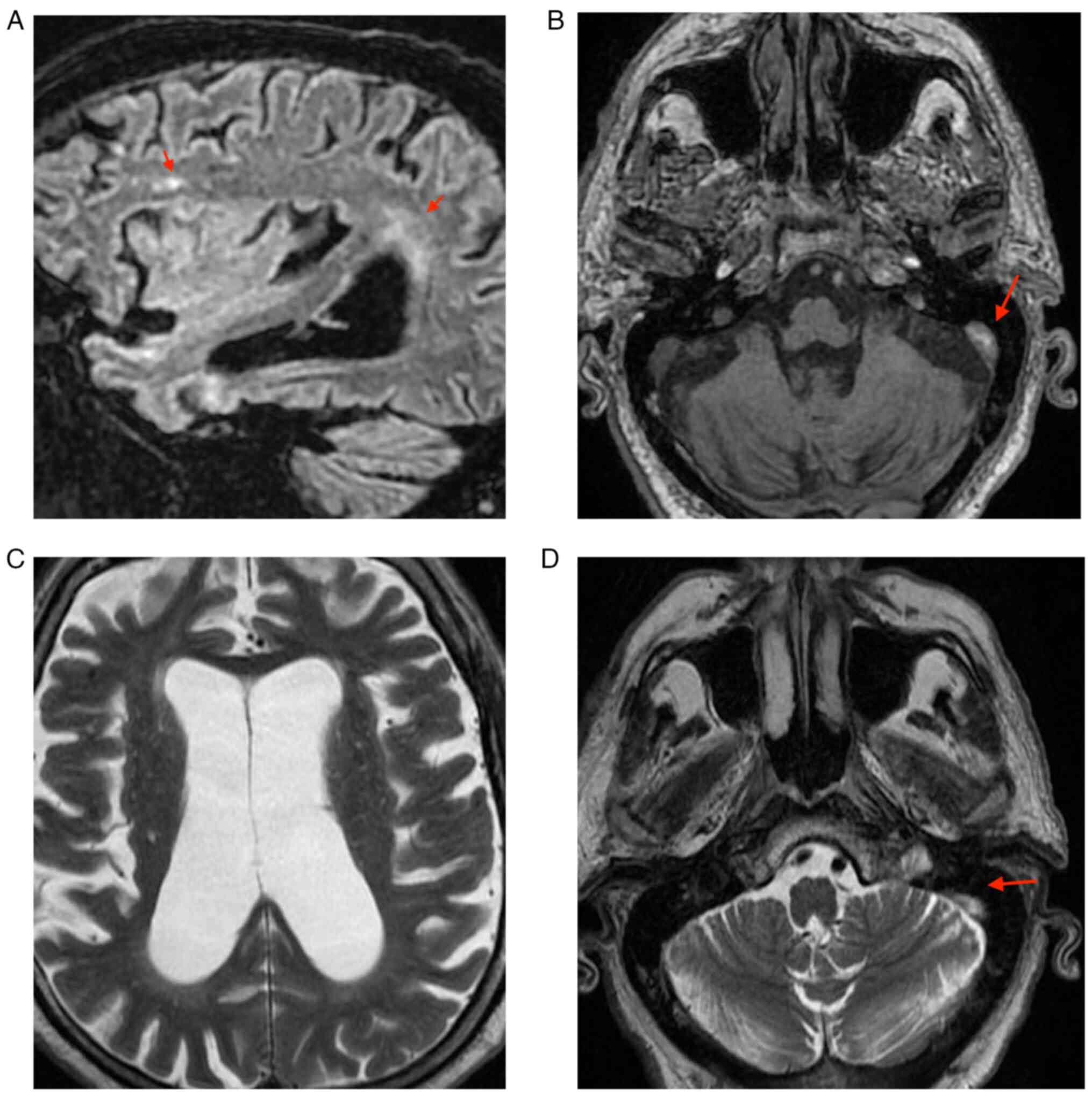
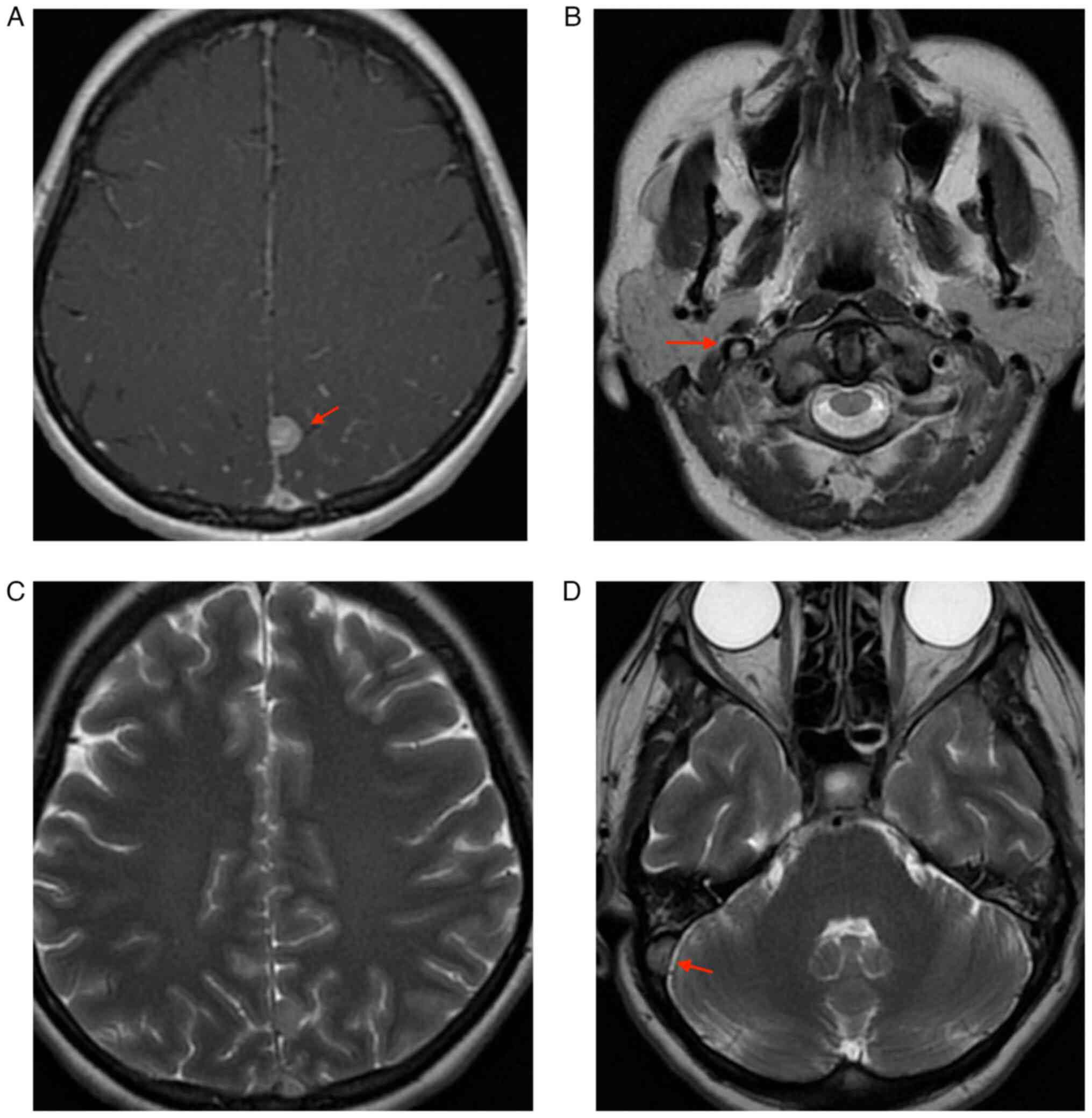
3/5 and left 3-4/5. The native cranial and brain MRI examination
which was performed on the 9th day of hospitalization highlighted
left subacute venous transversal-sigmoid-jugular thrombosis (as
depicted by the arrows in Fig.
2D); supratentorial demyelinating lesions (as depicted by the
arrows in Fig. 2A and B), most probably with ischemic vascular
sublayer; and cerebral abiotrophy which surpassed the age limit
(Figs. 1 and 2). During the 9th day of hospitalization,
the patient underwent electromyography, and was diagnosed with
predominantly sensitive axonal polyneuropathy, and on the 14th day
of hospitalization, the Echo Doppler of cervical vessels
highlighted bilateral carotid atheromatosis. In addition, the
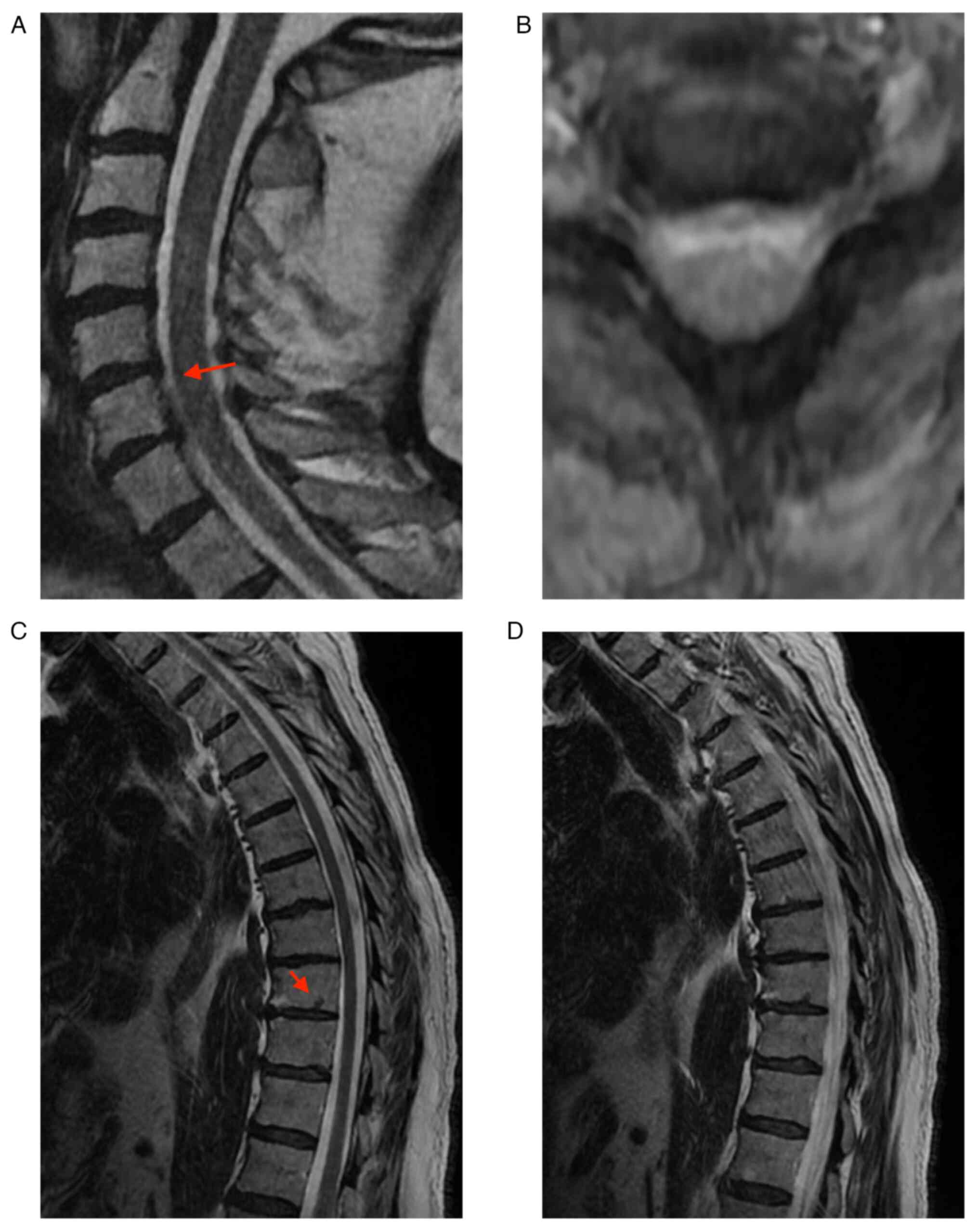
patient underwent native Nuclear Magnetic Resonance (NMR)
examination of the cervical and thoracic spine and under the
reserve of movement artifacts, and the results were: T7, T9, and
T10 intraspongious herniation (as depicted by the arrows in
Fig. 3C); C5-C6 herniated disk (as
depicted by the arrows in Fig.
3A), compression over the anterior spine of LCR; C6-C7 left
median and paramedian protrusion, with C7 left intraforaminal
radicular conflict (Fig. 3).
On the 14th day of hospitalization, the patient's
health status had changed, as he exhibited desaturation up to 89%
and was feverish (body temperature, 37.3˚C). RT-PCR was performed
to determine whether a SARS-CoV-2 infection was present; the
results were positive, and the patient was isolated on the 16th day
and transferred to the Municipal Hospital in Medgidia (support
hospital for COVID-infected patients) in order to receive special
treatment. When transferred, the patient was submitted to a
neurological examination, he was conscious, hardly cooperative,
with a symmetric face, brachial distal movement deficit 3-4/5,
crural 3/5, plantary cutanate reflex in bilateral flexion, and did
not cooperate for sensory stimuli and coordination tests. The
results of the patient examination are presented in Table I.
 | Table IBiological parameters of patient no.
1. |
Table I
Biological parameters of patient no.
1.
| Parameter (normal
range) | Day 1 | Day 3 | Day 8 | Day 12 | Day 14 |
|---|
| White blood cells
(4,000-10,000/µl) | 6.31 | 6.4 | - | 6.69 | - |
| Hemoglobin (12.6-17.4
g/dl) | 16.90 | 15.8 | - | 16 | - |
| Hematocrit
(37-51%) | 46.20 | 46.1 | - | 44.2 | - |
| Platelets
(150,000-450,000/µl) | 212.00 | 246 | - | 185 | - |
| Erythrocyte
sedimentation rate (<20 mm/h) | - | - | - | 26 | - |
| Aspartate
amino-transferase (0-37 U/l) | 22.92 | - | - | - | - |
| Alanine
amino-transferase (0-40 U/l) | 20.26 | - | - | - | - |
| Urea (<49
mg/dl) | 79.81 | 100 | - | 67 | - |
| Creatinine (<1.2
mg/dl) | 1.01 | 1.72 | 1.42 | 0.82 | - |
| Glycemia (100-125
mg/dl) | 670.24 | 449 | 154 | - | - |
| Vitamin B12
(191-663 pg/ml) | - | - | - | - | 892 |
| Potassium (3.5-5.1
mmol/l) | 4 | 4.6 | 4.1 | 3.3 | - |
| Sodium (136-145
mmol/l) | 127 | 136 | 134 | 141 | - |
During the 15 days of hospitalization in the
Neurology Department, the patient underwent treatment with cerebral
depletion (Manitol 20% 100 ml every 8 h-only on the first 4 days of
hospitalization), platelet antiaggregatory agent (Aspenter 75 mg
for the first 3 days; 3 tablets every day at lunchtime, followed by
2 tablets at lunchtime on day 4, and 1 tablet at lunchtime on days
5 and 6), therapy with vitamins (vitamin B1 100 mg 1 ampoule x2
every day, vitamin B6 250 mg ampoule x2 every day),
hydro-electrolytic rebalancing (normal saline solution 500 ml every
day, slowly endovenous drip), statin (Atorvastatine 20 mg 1 tablet
every day in the evening), gastric protector (Zencopan 40 mg 1
tablet every day in the morning), antihypertensive (Tertensif 1.5
mg 1 tablet every day in the morning), Atacand (8 mg 1 tablet every
day in the evening), rapid insulin (10 units at 08:00, 8 units at
13:00, 8 units at 19:00, and 6 units at 24:00),
low-molecular-weight heparin (Clexane 0.7 ml every 12 h,
subcutaneously from the 7 to 11th day) and oral anticoagulant
[Sintrom 4 mg 1/2 tablet from the 7th day, according to
International Normalized Ratio (INR)].
During hospitalization, the patient was
paraclinically monitored, the biological data obtained are
summarized in Tables II and
III.
 | Table IIResults of the coagulation tests in
patient no. 1. |
Table II
Results of the coagulation tests in
patient no. 1.
| Parameter (normal
range) | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 | Day 14 | Day 16 |
|---|
| INR (2.0-3.0) | 1.1 | 1.06 | 1.5 | 3.51 | 4.58 | 2.18 | 2.9 | 5.2 | 4.6 |
| Prothrombin time
(70-100%) | 87 | 91 | 57 | 21 | 16 | 36 | 26 | 14 | 16 |
| Clotting time
(11.7-15.3 sec) | 14.6 | 14.2 | 19.7 | 43.7 | 56.3 | 27.9 | 36.5 | 63.5 | 56.5 |
| Partial
thromboplastin time (<40 sec) | 32.4 | 34.9 | 35.4 | 47.8 | - | 52 | 51.8 | 64.6 | - |
 | Table IIIHereditary thrombophilia profile in
patient no. 1. |
Table III
Hereditary thrombophilia profile in
patient no. 1.
| Mutation | Wild type | Mutation
status | Genotype |
|---|
| FV G1691A
(Leiden) | Positive | Negative | Normal |
| FV H1299R (R2) | Positive | Negative | Normal |
| Prothrombin
G20210A | Positive | Negative | Normal |
| MTHFR C677T | Positive | Negative | Normal |
| MTHFR A1298C | Positive | Negative | Normal |
| Factor XIII
V34L | Positive | Negative | Normal |
| PAI-1 4G/5G | Positive 5G | Negative 4G | Homozygous
5G/5G |
| EPCR A4600G | Positive A | / | A2/A2 |
| EPCR G4678C | Positive G | / | A2/A2 |
On the 15th day of hospitalization, biological
material was collected to determine the hereditary thrombosis
profile which was transmitted to the Clinical Service of
Pathological Anatomy, a test for identifying the mutations
associated with cardiovascular disease and thrombophilia. The test
identified 9 mutations: FV G1691A (Leiden), FV H1299R (R2),
Prothrombin G20210A, MTHFR C677T, MTHFR A1298C, Factor XIII V34L,
PAI-1 4G/5G, EPCR A4600G, and EPCR G4678C.
The test identified the genotype 5G/5G of PAI-1 and
haplotype A2/A2 of EPCR.
Patient no. 2
The second case was a patient aged 41 years, with a
personal pathological history consisting of uterine fibrosis,
following treatment with oral contraceptives and a smoker, who was
transferred from the Section of Gynecology of the Emergency
Hospital in Tulcea to the Neurology Section of ‘Sf. Apostol Andrei’
Emergency Hospital in Constanta in January 2021, where she was
hospitalized for a period of 13 days. In the Gynecology Clinical
Section, a day before being transferred, the patient underwent a
native cerebral CT, which highlighted an occipital epidural
hematoma, for which she was transferred for additional
investigation.
The results of the neurological examination
performed in the Section of Neurology were: The patient was
conscious, cooperative, partially oriented in time and space, with
no cervix stiffness, normal ocular ability, left hemiparesis 4/5
easily ataxic, and Babinski present on the left side. The RT-PCR
SARS-CoV-2 test, which was performed when the patient was
hospitalized, was negative.
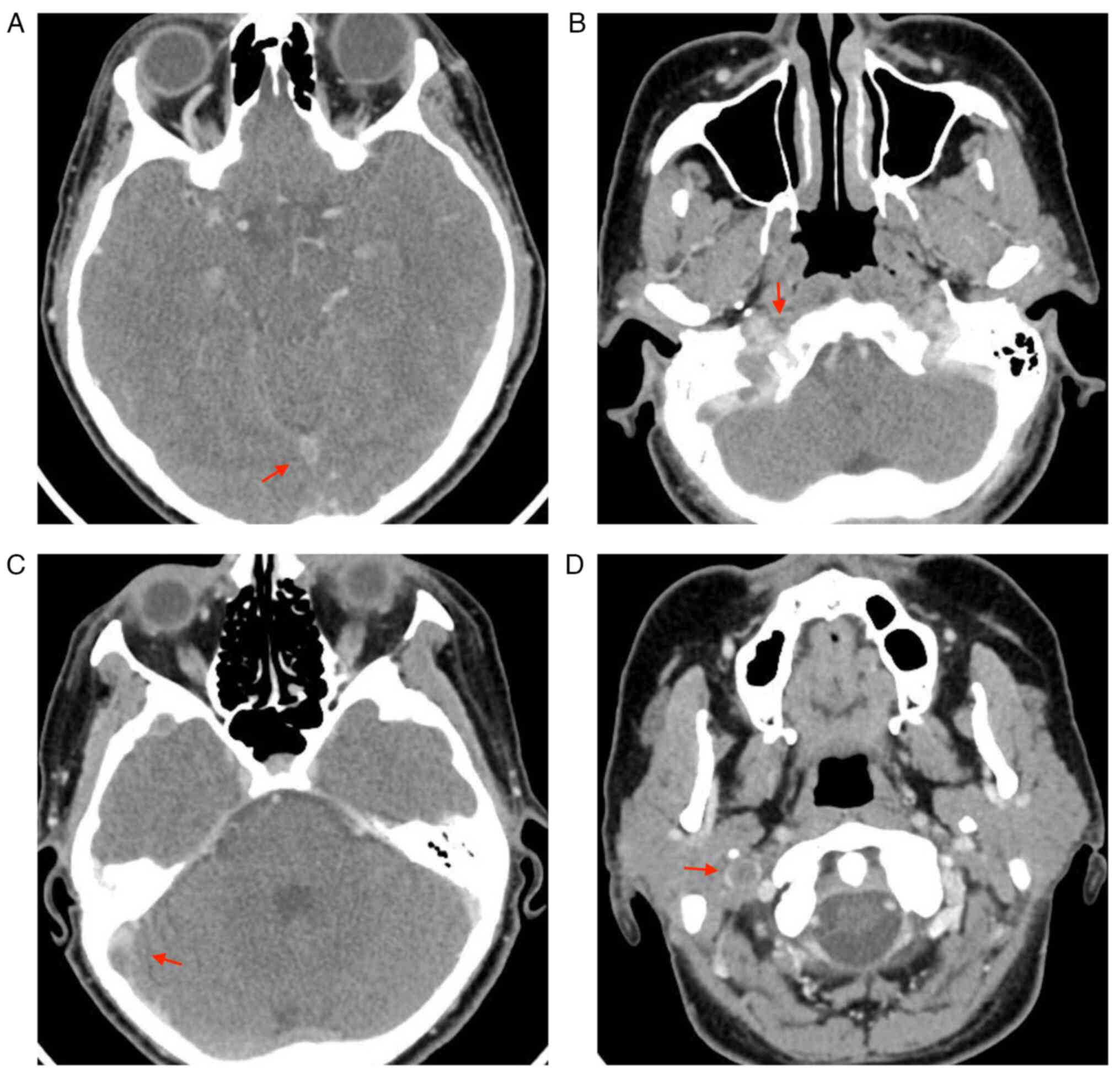
On the first day of hospitalization in the Clinical
Section of Neurology, the patient underwent a cranial and
encephalic CT and cranial CT angiogram, an examination which evoked
thrombosis of the right internal jugular vein (as depicted by the
arrows in Fig. 4D), sigmoid
sinuses (as depicted by the arrows in Fig. 4B), right transverse (as depicted by
the arrows in Fig. 4C) and
superior sagittal (as depicted by the arrows in Fig. 4A). On the first day of
hospitalization the patient underwent a cardiac assessment, the
results of which indicated that a thrombosis profile should be
performed, an X-ray of the heart and exploration according to the
affected protocol, and to follow a treatment with Heparin 25 000 UI
with a syringe pump at a rate of 2 ml/h with a Partial
Thromboplastin Time target of 50-70. On the 7th day of
hospitalization, the patient underwent a cardiac X-ray, which
indicated cavities of normal dimensions, without any kinetic
modifications on the left ventricle, an ejection fraction of 60%,
no abnormalities in the valves, no pulmonary hypertension, and
obstruction free cavities.
On the 4th day of hospitalization, a
dermato-venereological assessment was requested, which indicated
the fact that the patient was from another city, the reason for
which it was not known whether she was on the list of the people
affected by Lues. The following investigations were performed:
Tests highlighting non-specific antibodies by the Venereal Disease
Research Laboratory (VDRL) and Rapid Plasma Reagin test (RPR),
Treponema pallidum Hemagglutination Assay (TPHA) and treatment with
antibiotic injection (Moldamin 1,200,000 UI fl. X, 2 bottles every
5 days, 5 doses), but considering the anticoagulant medication, the
patient received oral antibiotic (Doxycycline 100 mg 1 tablet every
12 h for 4 weeks), probiotic (Eubiotic forte 1 tablet every day,
after meals, 2 h between antibiotic treatment), and proton pump
inhibitor (Nexium 20 mg 1 tablet every day, 20 min before meals). A
gynecological assessment was performed, the results of which showed
uterine fibrosis, metrorrhagia, and secondary anemia. On the 4th
day of hospitalization, the patient underwent a native cranial
cerebral NMR and a venous/segment angiography, which indicated
thrombosis in the right superior frontal subacute cortical
tardive-vein, superior sagittal sinus, sinuses' confluent, sigmoid
sinuses, and transverse on the right side (as depicted by the
arrows in Fig. 5), partially the
right internal jugular vein (as depicted by the arrows in Fig. 5B); and small left parasagittal
superior parietal meningioma (as depicted by the arrows in Fig. 5A).
On the 6th day of hospitalization, the patient
underwent a hematological assessment for thrombocytosis, which
indicated an iron deficit (severe posthemorrhagic hypochromic
microcitary anemia). As the patient was young, even if she was
following an anticoagulant treatment, a subsequent practice was
recommended: C protein, S protein, antithrombin III, factor V
Leiden mutation, mutation of prothrombin/factor II gene, lupus
anticoagulant, dosing serum homocysteine, iron supplementation, and
ferritin.
On the 9th day of hospitalization, biological
material was collected to determine the hereditary thrombosis
profile which was analyzed by the Clinical Service of Pathological
Anatomy, where assays for identifying the mutations associated with
the cardio-vascular disease and thrombophilia were performed. The
test identified 9 mutations: FV G1691A (Leiden), FV H1299R (R2),
Prothrombin G20210A, MTHFR C677T, MTHFR A1298C, Factor XIII V34L,
PAI-1 4G/5G, EPCR A4600G, and EPCR G4678C. The results of the
hereditary thrombophilia profile in patient no. 2 are shown in
Table IV.
 | Table IVHereditary thrombophilia profile in
patient no. 2. |
Table IV
Hereditary thrombophilia profile in
patient no. 2.
| Mutations | Wild type | Mutation | Genotype |
|---|
| FV G1691A
(Leiden) | Positive | Negative | Normal |
| FV H1299R (R2) | Positive | Negative | Normal |
| Prothrombin
G20210A | Positive | Negative | Normal |
| MTHFR C677T | Positive | Positive | Heterozygous |
| MTHFR A1298C | Positive | Positive | Heterozygous |
| Factor XIII
V34L | Positive | Negative | Normal |
| PAI-1 4G/5G | Negative 5G | Positive 4G | Homozygous
4G/4G |
| EPCR A4600G | Positive A | / | A1/A1 (H1/H1) |
| EPCR G4678C | Positive C | / | A1/A1 (H1/H1) |
The following genotypes were identified: Composed
heterozygote (double heterozygote) for mutations C677T and A1298C
of MTHFR and homozygote for mutation 4G of PAI-1. Moreover,
haplotype A1/A1 (H1/H1) of EPCR was also identified.
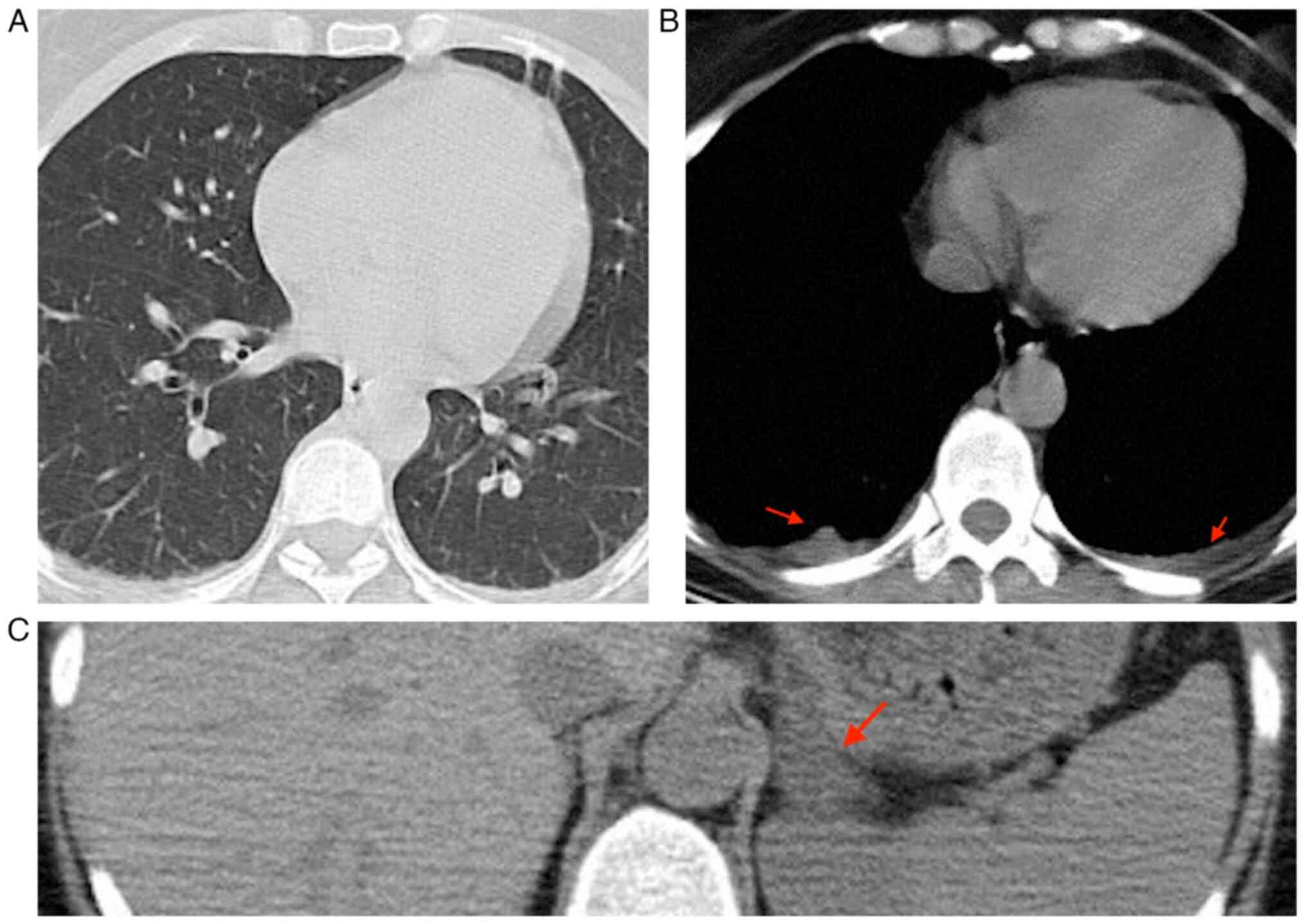
On the 10th day of hospitalization, the patient
reported experiencing headache and had a fever. As a result, a
native thorax CT scan was performed, revealing several findings.
The scan showed bilateral pleurisy in a small quantity (indicated
by the arrows in Fig. 6B), as well
as opacities in the right inferior lobe and alveoli. Additionally,
there were opacities in the left superior lobe in the form of
opaque glass. It was recommended to correlate these findings with
the PCR test. Furthermore, the CT scan also revealed a left
suprarenal adenoma (indicated by the arrows in Fig. 6C). It is a recommendation following
the patient's symptomatology and the immastigmatic investigation
carried out, because SARS-CoV-2 infection must always be suspected
even if it is not clear from the beginning, which is why studies
and articles (1-4)
have been published precisely to help doctors prevent further
complications and catch the infection early. The patient was tested
again on the 10th day of hospitalization, the results of the PCR
test for SARS-CoV-2 was positive. In the neurologic examination,
the patient was conscious, cooperative, and oriented in time and
space, with no cervical stiffness, normal ocular ability, no
nystagmus, no movement deficit, no sensory disorders, no
coordination disorders and plantary cutanate reflex in bilateral
flexion.
During the patient's hospitalization, various
treatments were administered. These included cerebral depletion
with mannitol (20% mannitol, 100 ml every 6 h for the first 5
days), hydro-electrolytic rebalancing with normal saline solution
(250 ml every 12 h), anti-inflammatory medication (Algocalmin, 1
g/2 ml, 1 bottle every 12 h), antiemetic medication
(Metoclopramide, 5 mg/ml, 1 bottle as needed), continuous infusion
of Heparin (25,000 units in 50 ml normal saline solution, with a
rate of 2 ml per hour) to maintain a target partial thromboplastin
time of 50-70 (for 11 days).
On the 8th day of hospitalization, additional
treatments were added to the regimen. These included oral
anticoagulant (Sintrom, 4 mg, 1 tablet per day according to INR),
oral antibiotic (Doxycycline, 100 mg, 1 tablet every 12 h for 4
weeks), probiotic supplement (Eubiotic forte, 1 tablet per day,
taken after meals, at least 2 h apart from the antibiotic), and a
proton pump inhibitor (Nexium, 20 mg, 1 tablet per day, taken 20
min before a meal). The patient's general condition was good during
hospitalization, she was discharged conscious, cooperative, and
oriented in time and space, with no sign of neurological focal
point, on the 14th day, with self-isolation according to the
indication of the Directorate for Public Health in Constanta and
continued the treatment indicated by the dermatologist for up to 4
weeks, and for the neurological condition, she continued the
treatment with oral anticoagulant (Sintrom 4 mg 1/4 tablet every
day according to INR target 2-3 and repeats INR on the 7th, 14th
day, then on a monthly basis).
The biological data dynamically obtained during the
period of hospitalization are summarized in Table V. The results of the coagulation
tests are summarized in Table
VI.
 | Table VBiological data of patient no. 2. |
Table V
Biological data of patient no. 2.
| Parameter (normal
range) | Day 1 | Day 2 | Day 4 | Day 5 | Day 10 | Day 12 |
|---|
| White blood cells
(4,000-10,000/µl) | 8.41 | 9.37 | 6.56 | 4.96 | 4.63 | 6.99 |
| Hemoglobin
(12.6-17.4 g/dl) | 8.4 | 8.6 | 8 | 7.9 | 8.1 | 8 |
| Hematocrit
(37-51%) | 28.7 | 29.9 | 28.2 | 27.1 | 27.9 | 27.4 |
| Platelets
(150,000-450,000/µl) | 573 | 625 | 607 | 437 | 413 | 376 |
| Erythrocyte
sedimentation rate (<20 mm/h) | 61 | - | - | - | 66 | - |
| Fibrinogen (200-400
mg/dl) | 360 | - | - | - | 405 | - |
| C-reactive protein
(0-5 mg/l) | 1.4 | - | 0.95 | - | - | - |
| Aspartate
amino-transferase (0-37 U/l) | 29 | - | - | - | 17 | - |
| Alanine
amino-transferase (0-40 U/l) | 22 | - | - | - | 26 | - |
| Cholesterol
(<200 mg/dl) | 240 | - | - | - | - | - |
| Cholesterol
low-density lipoprotein (<100 mg/dl) | 163 | - | - | - | - | - |
| Lactate
dehydrogenase (135-214 U/l) | - | - | - | - | 221 | - |
| Triglycerides
(<150 mg/dl) | 249 | - | - | - | - | - |
| Urea (<49
mg/dl) | 14 | 26 | 15 | - | 16 | 15 |
| Creatinine (<1.2
mg/dl) | 0.49 | 0.6 | 0.56 | - | 0.41 | 0.56 |
| Glycemia 100-125
mg/dl) | 110 | 107 | 101 | - | - | - |
| Potassium (3.5-5.1
mmol/l) | 3.5 | - | - | - | - | - |
| Sodium (136-145
mmol/l) | 137 | - | - | - | - | - |
| Rapid plasma reagin
test (<1, negative; >1 positive) | 1.61 | - | - | - | - | - |
| Anti-human
immunodeficiency virus (1+2) | Negative | - | - | - | - | - |
| Free thyroxine 4
(12-22 pmol/l) | 15.6 | - | - | - | - | - |
| Thyroid stimulating
hormone (0.27-4.2 µUl/ml) | 1.37 | - | - | - | - | - |
| Homocysteine(≤12
µmol/l) | 10.3 | - | - | - | - | - |
| D-dimer (0-0.5 µg
FEU/ml) | - | - | - | - | 1.17 | - |
 | Table VIResults of the coagulation tests in
patient no. 2. |
Table VI
Results of the coagulation tests in
patient no. 2.
| Parameter (normal
value) | Day 21 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 |
|---|
| INR (2.0-3.0) | 0.99 | - | - | - | - | - | 1.09 | - | 1.06 | 1.32 | 2.29 | 2.33 |
| Prothrombin time
(70-100%) | 101 | - | - | - | - | - | 89 | 92 | - | 68 | 35 | 34 |
| Clotting time
(11.7-15.3 sec) | 13.2 | - | - | - | - | - | 14.4 | 14 | - | 17.3 | 29.2 | 29.6 |
| Partial
thromboplastin time (<40 sec) | 37 | 35.1 | 47.5 | 69.2 | 87.9 | 62.2 | 64.5 | 75.7 | - | 58.8 | 155.4 | 75.4 |
Patient no. 3
The third case was of a patient aged 46, with
asthma, who came to the Emergency Unit in April 2021 for repeated
hematemesis in the morning, and was thus hospitalized in the
Gastroenterology Section for additional investigation and
etiological treatment. When admitted to the hospital, the patient
performed a SARS-CoV-2 rapid antigen test and PCR test for SARS
CoV-2 test which were both negative. When admitted to the clinical
section, the patient underwent an abdominal and pelvic X-ray which
showed the following: Absence of liquid in the peritoneal cavity,
liver steatosis, a hyperecogenous pancreas, apparently homogeneous;
gallbladder, and no modifications in the liver and kidneys.
Biologically during the admission to the Gastroenterology section:
Leukocytosis with neutrophilia, slight increase of amylase,
modified basal glycemia, hepatic cytolysis, thrombocytopenia,
nitrogen retention, and hepatic cholestasis syndrome.
On the 2nd day of hospitalization, the patient's
condition was aggravated, thus a neurological assessment was
requested for the deviation of eyeballs and force deficit at the
level of the right hemibody. During the neurological assessment,
the patient was preferentially looking towards the left-side and
presented right lateral homonymous hemianopsia, movement deficit at
the level of the right superior member 0/5 and right inferior
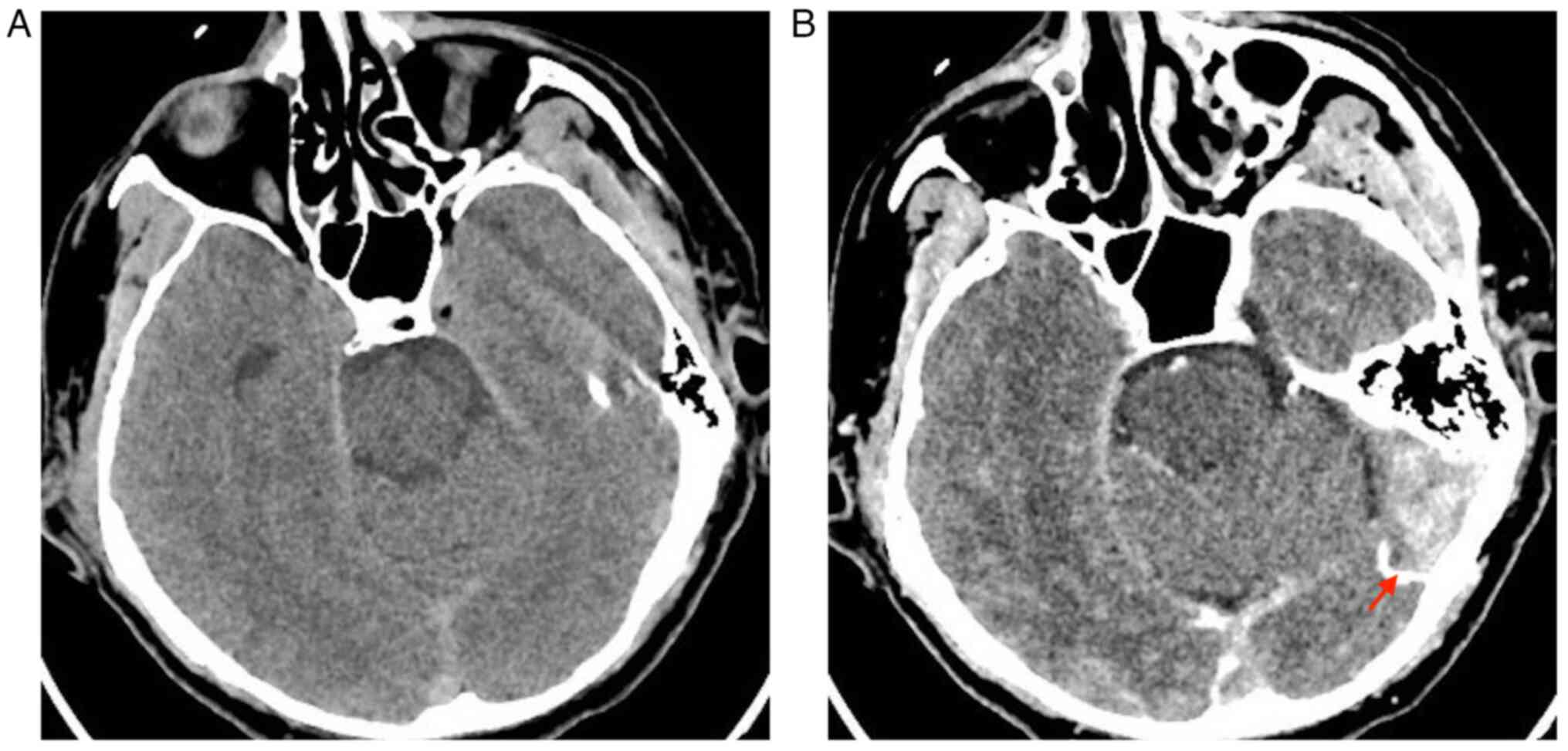
member 1/5. Cerebral CT was requested immediately. At the cranial
and encephalic CT performed before and after administering the
contrast substance showed the following: Thrombosis of the
transverse (as depicted by the arrows in Fig. 7B) and sagittal sinuses and some
bilateral frontal and parietal superior cortical veins (Fig. 7). At the neurological re-evaluation
and a cerebral CT performed on the same day, the neurological
assessment indicated a severe general condition, no cervical
stiffness, head and eyeballs deviated towards the left side,
reactive intermediary pupils, right hemiplegia, plantary cutanate
reflex in bilateral plantary indifference, and the patient
mobilized the left members at nociception. The patient was
transferred to the intensive care section-neurology and was
submitted for a cranial and encephalic IRM with angiography
sequence when the condition allowed.
On the 9th day of hospital admission, the PCR test
was positive. The patient's condition continued to be severe, and
on the 10th day, the patient exhibited a cardiac and respiratory
arrest, did not respond to cardiopulmonary resuscitation, and
therefore was declared exitus.
During the hospitalization, the patient received
treatment with cerebral depletive (20% mannitol),
hydro-electrolytic rebalancing solution (normal saline solution, 5%
glucose), proton pump inhibitor (Pantoprazole 40 mg, 1 bottle
intravenously every 12 h), antiemetic (Metoclopramide 5 mg/ml, 1
bottle when needed), therapeutic vitamins (vitamin B 1,100 mg 1
bottle x2 every day, vitamin B2 250 mg 1 bottle x2 every day,
vitamin C 750 mg 1 bottle x2 every day), cerebral trophic medicine
(Cerebrolysin 1 bottle x2 every day), anti-inflammatory
(Algocalmine 1 g/2 ml 1 bottle intravenous, Paracetamol 10 mg/ml 1
bottle every day), low molecular weight heparin (Clexane 0.6 ml 1
bottle subcutaneously every day), antihypertensive medication (Enap
1.25 mg/ml 1 bottle, Furosemide 20 mg/2 ml 1 bottle, when needed),
bronchodilator (Miofilin 24 mg/ml 1 bottle every 12 h), injectable
antibiotic (Ceftamil 1 g every 8 h).
During hospitalization, the patient was
paraclinically monitored and the biological data obtained are
summarized in the Table VII.
 | Table VIIBiological data of patient no. 3. |
Table VII
Biological data of patient no. 3.
| Parameter (normal
range) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 |
|---|
| White blood cells
(4,000-10,000/µl) | 13.74 | 16.04 | 21.59 | 9.66 | 12.61 | - | 12.12 | 8.65 |
| Hemoglobin
(12.6-17.4 g/dl) | 14.1 | 14 | 14.6 | 12.3 | 12 | - | 10.9 | 10.2 |
| Hematocrit
(37-51%) | 41.9 | 40.2 | 42.5 | 37.2 | 37 | - | 32.8 | 31 |
| Platelets
(150,000-450,000/µl) | 472 | 149 | 460 | 197 | 183 | - | 96 | 109 |
| Fibrinogen (200-400
mg/dl) | - | - | 879 | 774 | 706 | 686 | 686 | 593 |
| C-reactive protein
(0-5 mg/l) | - | - | - | - | - | - | 24.47 | 28.53 |
| Aspartate
amino-transferase (0-37 U/l) | 35.98 | - | 31 | - | 89 | - | 148 | 102 |
| Alanine
amino-transferase (0-40 U/l) | 89.61 | - | 59 | - | 86 | - | 109 | 81 |
| Urea (<49
mg/dl) | 33 | - | 78 | - | 66 | 92 | 151 | - |
| Creatinine (<1.2
mg/dl) | 0.83 | - | 1.76 | 1.46 | 1.51 | 2.84 | 4.93 | 7.67 |
| Glycemia (100-125
mg/dl) | 129 | - | - | - | - | - | - | - |
| Potassium (3.5-5.1
mmol/l) | - | 4.1 | - | - | - | - | - | - |
| Sodium (136-145
mmol/l) | - | 140 | - | - | - | - | - | - |
| D-Dimer (0-0.5 µg
FEU/ml) | 1.6 | - | - | - | - | - | - | - |
| Amylase (20-100
U/l) | 114.62 | - | - | - | - | - | - | - |
| Lipase (13-60
U/l) | 47.92 | - | - | - | - | - | - | - |
| Direct bilirubin
(<0.2 mg/dl) | - | - | 0.33 | - | - | - | 3.36 | 5.08 |
| Indirect bilirubin
(≤1 mg/dl) | - | - | - | - | - | - | 2.37 | 0.066 |
| Total bilirubin (≤1
mg/dl) | - | - | 0.55 | - | - | - | 5.74 | 5.14 |
Discussion
These case presentations describe cases of
neurological disease in patients associated with SARS-CoV-2
infection hospitalized in the Neurology Section of Constanta County
Hospital (Fig. 8). Although the
data is limited, observational studies such as these improve our
understanding of the evolution of a disease in patients affected by
CVT and similar diseases, and its evolution in association with
SARS-CoV-2 infection.
In the present report, the cases of two male
patients aged 46 and 66, and a female patient aged 41 are
presented. When hospitalized, each of these patients presented
negative on a PCR test for infection with SARS-CoV-2, but the
results of the test changed during hospitalization, along with
various localizations of CVT, different risk factors, and
distinctly associated pathologies. PCR may miss detection of
individuals with SARS-CoV-2 infection, and early sampling minimizes
false-negative diagnoses (11). It
may be the case that the PCR test was taken too early or taken
incorrectly, or the symptoms appeared a period of time after
infection, which is why they were diagnosed first with CVST and
then with COVID-19(5).
The patients had no direct contact with patients
infected with SARS-CoV-2. There was no data on the vaccination
status of patients, although this may not be as relevant given
numerous reports of individuals infected with COVID-19 following
vaccination or prior infection.
The first case described here was that of a male
patient, aged 66, with left subacute venous
transversal-sigmoid-jugular thrombosis indicated by the native
cerebral NMR. The patient's associated risk factors, such as high
blood pressure, newly discovered type II diabetes mellitus, and
bilateral carotid atheromatosis, appeared in the hereditary
thrombosis profile (genotype 5G/5G PAI-1 and haplotype A2/A2 of
EPCR) and on the 14th day of hospitalization, the patient tested
positive for COVID-19 following a PCR test.
The second case described a female patient, aged 41,
with thrombosis in the right superior frontal subacute cortical
tardive-vein, superior sagittal sinus, sinuses' confluent, sigmoid
sinuses, and transverse on the right side; partially the right
internal jugular vein was indicated on native NMR and angiography.
The patient's associated risk factors such as uterine fibrosis,
treatment with oral contraceptives, chronic tabagism, acute
syphilis, severe posthemorrhagic hypochromic microcitary anemia,
dyslipidemia and presented modifications at the hereditary
thromboliphic profile [composed heterozygote for C677T and A1298C
of MTHFR mutations, homozygote for 4G of PAI-1 mutation and
haplotype A1/A1 (H1/H1) of EPCR]. On the 10th day of
hospitalization, the patient was positive for COVID-19 infection
following a PCR test.
The third case was a male patient, aged 46, who had
thrombosis of the transverse and sagittal sinuses and in some
bilateral frontal and parietal superior cortical veins in the CT
native and contrast substance CT. The patient presented with
SARS-CoV-2 infection on the 9th day of hospitalization. He
presented with risk factors, such as high blood pressure, asthma,
acute renal failure, and syndrome of hepatic cytolysis. The
evolution of this patient's disease was not favorable, as he
developed a vascular coma, and cardiac and respiratory arrest
during hospitalization.
CVST presents as one of three clinical syndromes:
Isolated intracranial high blood pressure (characterized by
headaches, papillary edema, and visual problems), focal syndrome
(accompanied by convulsions reported in 39.3%, paresis in 37.2%,
and aphasia in 19.1% of cases), and encephalopathy (characterized
by the alteration of the mental state, extended neurological signs,
and coma). Risk factors include a genetic predisposition to
thrombophilia, which can be determined in patients with CVST by
assessing C protein deficit and S protein deficit (12), antithrombin III and factor V Leiden
levels, and mutations of the G20210A prothrombin gene. Other risk
factors, which may explain an increased predisposition to CVST in
women more than in men, may be due to use of oral contraceptives,
pregnancy, and puerperium. Among the risk factors, there are also
focal infections at the level of the head, neck, and sinuses, or
malign infections, particularly in older patients (12). However, a previous study indicated
that up to 12.5% of cases did not present with any associated
risks. Therefore, the lack of an associated risk suggests that the
infection with SARS-CoV-2 virus acted as a potential etiological
factor in the development of CVST (12).
In the study by Cavalcanti et al (13), three patients (<41 years old)
were infected with SARS-CoV-2 virus and cerebral vein sinus
thrombosis. One patient in the study presented with thrombosis in
both the superficial and profound venous systems. Another patient
showed involvement of the right sinus, Galen vein and internal
cerebral veins. The third patient had thrombosis in the profound
spinal veins. Additionally, two of these patients experienced
hemorrhagic venous heart attacks. On average, the time from the
onset of symptoms indicating SARS-CoV-2 infection to a thrombotic
event was 7 days, with a range of 2 to 7 days. It is worth noting
that one of the patients had recently been diagnosed with diabetic
ketoacidosis, while another patient was using oral contraceptives.
All three patients had an unfavorable evolution and eventually
succumbed to their conditions. Even though COVID-19 is severe and
its primary effect is acute respiratory distress, cardiac
disorders, acute renal disorders and thromboembolic events are
increasingly being reported (13).
The association between profound cerebral thrombosis and
potentially fatal complications may complicate the initial clinical
presentation of a patient infected with COVID-19. The cases
presented herein offer us a new perspective on the fact that such
cases display insights regarding the proofs accumulated according
to which COVID-19 contributes to hypercoagulation and therefore
increases the risk of mortality (13).
In the present study, the third case was an uncommon
manifestation of catastrophic CVT in a relatively young patient who
had previously only presented with asthma and was infected with
SARS-CoV-2, with an unfavorable outcome, leading to exitus. In the
study by Cavalcanti et al (13), in the case of symptomatic CVT with
a recent COVID-19 infection, one patient presented the same risk
factor as case 2 in the present study, the use of oral
contraceptives; COVID-19 infection was almost certainly a risk
factor synergistic in both studies. Based on the given statement,
it can be understood that in the present study, the third case
described an uncommon manifestation of catastrophic cerebral venous
thrombosis (CVT) in a relatively young patient. This patient had
previously only been diagnosed with asthma and was also infected
with SARS-CoV-2. Unfortunately, the outcome for this patient was
unfavorable, leading to death (exitus).
The statement then refers to a study conducted by
Cavalcanti et al (13),
where a similar risk factor was observed in a patient with
symptomatic CVT and recent COVID-19 infection. Specifically, in
both the present study (case 2) and the study by Cavalcanti et
al, the use of oral contraceptives was identified as a shared
risk factor. Additionally, it suggests that COVID-19 infection
likely acted as a synergistic risk factor in both cases, indicating
that the combination of COVID-19 and oral contraceptive use may
have contributed to the development of CVT.
Additionally, the study by Hameed et al
(9) also included one patient who
had used oral contraceptives. Another similarity of our study and
the case report by Cavalcanti et al (13) is the fact that the patients from
both studies received antibiotic treatments. A similarity between
our study and the studies by Cavalcanti et al (13) and Hameed et al (9) consisted of the fact that in the
present study, at least one patient exhibited dehydration, which is
known to be a contributor to the pathology of COVID-19.
Mowla et al (5) presented a case series of 13
individuals with symptomatic CVT and concurrent COVID-19 infection
from Iran, Singapore, and the United States. The mean age of the
SARS-CoV-2 positive patients was substantially greater than that of
the CVST-positive patients in the control sample. Their patients
exhibited a much lower prevalence of recognized CVST risk factors
than the general population. That is, the older age and
considerably fewer risk factors for CVST in comparison to the
non-SARS-CoV-2 infected comparison group suggested that SARS-CoV-2
infection may have served as a precursor for CVST. The statement
suggests that in the comparison group, the patients infected with
SARS-CoV-2 were older and had fewer risk factors for CVST compared
to the non-SARS-CoV-2 infected group. This observation indicates
that SARS-CoV-2 infection may have acted as a potential precursor
or trigger for the development of CVST, considering that the
infected group had an older age and fewer established risk factors
for the condition.
Hameed et al (9) studied 20 cases with symptomatic CVT
and recent COVID-19 infection from Pakistan, Egypt, Singapore, and
the United Arab Emirates. In the same study, the most frequent
neurological manifestations were headaches and seizures. Although
mortality was high, survivors had a favorable functional
neurological result.
The novelty of the present study compared with
previous studies, comes from the fact that our patients were also
tested for their hereditary thrombosis profile and the test
revealed gene mutations. Furthermore, the patients were previously
relatively healthy (one patient had asthma and the other was
diagnosed at admission with diabetes type 2) and did not have any
pathological antecedents in this area; thus, the COVID-19 infection
was almost certainly an additive risk factor. It is also noteworthy
as, in contrast to previous studies, in the present study, the
patients were admitted due to their neurological symptoms and the
COVID-19 infection was detected later. In the study of Hameed et
al (9), CVT was a presenting
characteristic in 65% of patients, whereas 35% of patients
developed CVT when receiving treatment for COVID-19 infection.
Additionally, in the study of Mowla et al (5), only one patient was asymptomatic at
presentation; thus, another novelty of the present study is that
the patients were asymptomatic at presentation, but CVST was
present and apparent.
Additionally, in patients with severe COVID-19,
rapid clinical deterioration or exacerbation could be associated
with a neurological event, possibly CVST, adding to the disease's
high fatality rate. Furthermore, physicians may consider SARS-CoV-2
infection as a differential diagnosis when dealing with patients
who exhibited these neurological symptoms simultaneously during the
COVID-19 pandemic in order to avoid a late diagnosis or
misdiagnosis. Furthermore, accurate epidemiological data and
pathophysiological results are necessary to aid future therapeutic
management.
In summary, the present case series provides cases
to exemplify that COVID-19 may serve as a significant contributor
to hypercoagulation, thus increasing the potential lethality of the
disease. Increased recognition of this uncommon but possibly
curable consequence of COVID-19 infection is thus recommended,
particularly given that the present is the only such study
assessing COVID-19 infection in patients with CVT from Romania and
South-East Europe, to the best of our knowledge.
In conclusion, patients with COVID-19 are at an
increased risk of stroke, especially in the first 10 days after
infection. A particular form of stroke is presented by CVT; the
pathophysiological mechanisms presented are immune systemic
processes, a cytokine storm leading to increased blood viscosity,
and thrombogenesis within the state of hypercoagulability, at the
same time producing inflammation marked by the increase of
prothrombotic factors.
Recent data collected from research and studies
supports that there is an increase in neurological pathologies,
such as thrombotic complications of the sinuses and cerebral
vessels for patients confirmed to be infected with SARS-CoV-2. Risk
factors for CVT include infections, oral contraceptives, pregnancy,
hematological disorders, mechanical or traumatic factors,
autoimmune inflammatory disorders or even malign pathologies.
Coexistent risk factors and age are well-defined risk factors for
the development of CVT. Moreover, an association of the infection
with SARS-CoV-2 virus for these patients may involve the onset of a
procoagulant cascade and the evolution of the clinical status of
the patient.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AA, LFM, AEG, CMM, SGP and SCC conceived the study.
AA, CMM, LFM, AEG, CMM and SCC collected the data. AA, LFM, AEG and
CMM analyzed the data. AA, CMM and LFM performed the investigation.
AA, CMM, LFM, DCJ, SDA and CEF were involved in study design. AA,
LFM, CMM, RAB and CAS: Software. CAS, FIR, AA, LFM, CMM and FIR:
Validation. AA, LFM, CMM, CEF, AZS and FIR: Visualization. CAS,
FIR, AA, LFM, CMM, AZS, SDA and SGP wrote the manuscript. AA, LFM,
CMM, AZS, SDA, SGP and RAB reviewed and edited the manuscript. AA,
AZS, CMM and LFM collected the data. AZS, SDA and SGP performed the
analysis and interpretation of data. All authors confirm the
authenticity of the raw data generated during the study.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the Constanta Clinical Hospital, (Constanta, Romania;
approval no. 31/03.11.2021).
Patient consent for publication
All patients consented in written form to the
publication of the findings and images based on their
examinations.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO):
Coronavirus disease (COVID-19). WHO, Geneva. Accessed December 30,
2021.
|
|
2
|
Centrul național de supraveghere şi
control al bolilor transmisibile-redheader. Cnscbt.ro.
|
|
3
|
Nannoni S, de Groot R, Bell S and Markus
HS: Stroke in COVID-19: A systematic review and meta-analysis. Int
J Stroke. 16:137–149. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abouhashem S, Eldawoody H and Taha MM:
Cerebral venous sinus thrombosis in patients with COVID-19
infection. Interdiscip Neurosurg. 24(101091)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mowla A, Shakibajahromi B, Shahjouei S,
Borhani-Haghighi A, Rahimian N, Baharvahdat H, Naderi S, Khorvash
F, Altafi D, Ebrahimzadeh SA, et al: Cerebral venous sinus
thrombosis associated with SARS-CoV-2; a multinational case series.
J Neurol Sci. 419(117183)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ulivi L, Squitieri M, Cohen H, Cowley P
and Werring DJ: Cerebral venous thrombosis: A practical guide.
Pract Neurol. 20:356–367. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gaillard F, Ranchod A, Alhusseiny K, et
al: Cerebral venous thrombosis. Radiopaedia. https://doi.org/10.53347/rID-4449. Accessed September
25, 2023.
|
|
8
|
Idiculla PS, Gurala D, Palanisamy M,
Vijayakumar R, Dhandapani S and Nagarajan E: Cerebral venous
thrombosis: A comprehensive review. Eur Neurol. 83:369–379.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hameed S, Wasay M, Soomro BA, Mansour O,
Abd-Allah F, Tu T, Farhat R, Shahbaz N, Hashim H, Alamgir W, et al:
Cerebral venous thrombosis associated with COVID-19 infection: An
observational, multicenter study. Cerebrovasc Dis Extra. 11:55–60.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Johansson A, Mohamed MS, Moulin TC and
Schiöth HB: Neurological manifestations of COVID-19: A
comprehensive literature review and discussion of mechanisms. J
Neuroimmunol. 358(577658)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mallett S, Allen AJ, Graziadio S, Taylor
SA, Sakai NS, Green K, Suklan J, Hyde C, Shinkins B, Zhelev Z, et
al: At what times during infection is SARS-CoV-2 detectable and no
longer detectable using RT-PCR-based tests? A systematic review of
individual participant data. BMC Med. 18(346)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bolaji P, Kukoyi B, Ahmad N and Wharton C:
Extensive cerebral venous sinus thrombosis: A potential
complication in a patient with COVID-19 disease. BMJ Case Rep.
13(e236820)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cavalcanti DD, Raz E, Shapiro M,
Dehkharghani S, Yaghi S, Lillemoe K, Nossek E, Torres J, Jain R,
Riina HA, et al: Cerebral venous thrombosis associated with
COVID-19. AJNR Am J Neuroradiol. 41:1370–1376. 2020.PubMed/NCBI View Article : Google Scholar
|