1. Introduction
Extracellular vesicles (EVs) are lipid bilayer
membrane vesicles released by varying types of activated and
apoptotic cells through different mechanisms. They are mainly
divided into three categories: Exosomes, microvesicles and
apoptotic bodies according to different sizes, sources and markers.
Among them, exosomes and smaller microvesicles can be considered as
small EVs (sEVs) (1). EVs can be
used as intercellular communication media to carry proteins, RNA,
free fatty acids, metabolites, and other substances to the distal
or adjacent cells, which play a biological function of maintaining
cell and tissue homeostasis (2,3). EVs
are extracellular matrix (ECM)-specific regulators that carry
proteases and signaling molecules that can alter the composition of
the ECM by modulating target cells and are thus an integral part of
the extracellular environment (4).
In previous years, EVs have received extensive attention, having
been recognized as potential biomarkers and drug delivery systems
that play important role in the diagnosis and treatment of diseases
(5).
Ovarian disease is a common gynecological and
obstetric disease, which can be divided into malignant ovarian
cancer, premature ovarian insufficiency (POI), polycystic ovary
syndrome (PCOS), and other non-malignant diseases. Ovarian cancer
is a gynecological disease entity that is mostly diagnosed at late
stage and has a high recurrence rate. More than 95% of ovarian
cancer occurs in middle-aged women over 45 years-old and most of
them are epithelial ovarian cancer (EOC) of various histological
types. EOC can be stratified into two categories: slow-growing type
I ovarian cancer and inert tumors and type II ovarian cancer with
clinically invasive tumors (6-8).
EOC can be classified into four histological subtypes: serous
carcinomas, endometrioid carcinomas, mucinous carcinomas and clear
cell carcinomas (9). Among the
aforementioned subdivisions, serous carcinomas are the most common,
accounting for ~45%. High-grade serous ovarian cancer (HGSOC) is
derived from serous tubal intraepithelial carcinoma and is often
characterized by mutations in TP53, distinct from specific
mutations in genes such as KRAS, BRAF, or ERBB2 that are common in
low-grade serous ovarian cancer (LGSOC). These two types of HGSOC
and LGSOC are considered to develop through two independent
pathways (10,11). For HGSOCs, prevention through
resection of ovarian cancer or use of oral contraceptive pills to
prevent oviposition may reduce its mortality (12). Endometrioid ovarian cancer accounts
for ~13% of EOC, and its incidence varies by region. According to
statistics, the incidence of endometrioid ovarian cancer in Asia is
higher than that in other regions of the world (9). Mucinous ovarian cancer is a rare but
important subtype of ovarian cancer, accounting for ~13% of EOC
(9). This subtype of ovarian
cancer is usually associated with mutations in genes such as KRAS
and HER2, and its development is mainly confined to the pelvic
cavity (13). The incidence of
clear cell carcinoma accounts for ~3% of EOC. And the pathogenesis
of this tumor is associated with frequent mutations in AT-rich
interaction domain 1A (ARID1A) and PIK3CA (9,13).
Compared with other ovarian cancers, there are significant
geographical and ethnic differences in its incidence (14).
Ovarian cancer metastasizes in two ways: Passive
dissemination and hematogenous metastasis. Passive dissemination of
ovarian cancer cells is the most common, where the cancer cells
dislodge from the primary tumor and spread to the greater omentum
of the peritoneum and adjacent organs with peritoneal fluid,
whereas hematogenous metastasis of ovarian cancer cells spreads
through two links of introgression and extravasation to surrounding
tissues (15). Currently,
effective early screening methods for ovarian cancer are lacking,
and the survival rate of patients after a late diagnosis is low.
The disease is mainly treated with traditional treatment
modalities, including chemotherapy and surgery, whereas new
treatment methods, such as targeted therapy and immunomodulation
therapy, are constantly improving and are gradually being applied
in the clinic. However, effective treatment of ovarian cancer
remains a difficult problem, and seeking effective treatment is the
key to curing ovarian cancer (16,17).
POI is an ovarian disease characterized by
insufficient estrogen secretion, increased gonadotropin secretion,
and premature menopause in women under the age of 40 years
(18). As a common gynecological
disease with an incidence rate of ~1% in women before the age of 40
years, POI has various causes, including genetic factors such as
chromosomal abnormalities (X-chromosome terminal deletion and
reduced number) and gene mutations [mutations in forkhead box L2
(FOXL2), basonuclin zinc finger protein 1 (BNC1)], spontaneous
factors such as autoimmune diseases (primary adrenal insufficiency,
hypothyroidism), and iatrogenic factors such as radiotherapy and
chemotherapy (19-22).
POI is mainly caused by genetic mechanisms leading to the reduction
of gene dosage and the destruction of meiosis, but also includes
autoimmune attacks on the ovary and some rare causes, such as
galactosemia (23-25).
Patients with POI present with a pathological state of estrogen
insufficiency; therefore, hormone replacement therapy can be used
to relieve various adverse symptoms. However, this therapy does not
fundamentally solve the problems of impaired ovarian function and
low fertility, and exosomal therapy may be an effective treatment
option (25,26).
PCOS is a disease characterized by increased
androgen secretion (acne, hirsutism, alopecia, and other symptoms),
polycystic ovary, and menstrual disorders (>35 days), which
greatly affect a woman's quality of life (27,28).
As a common heritable endocrine disease with a prevalence of 3-10%
at a certain geographical location and race, its genetic,
environmental, behavioral, and endocrine factors greatly affect a
woman's life. PCOS will transform from a reproductive disease to a
metabolic disorder with age, which will also predispose women to
cardiovascular disease and type II diabetes in later life (29,30).
Treatment of PCOS includes both medical and surgical treatments.
Among these, inositol and insulin have been confirmed as
therapeutic agents for PCOS (31,32).
In severe cases, laparotomy can be performed and laparoscopic
ovarian drilling is an effective method (33).
EVs have been shown to be extensively involved in
ovarian cancer drug resistance and promote cancer cell metastasis
in vivo through molecules such as mixed cargoes carrying
biological effectors and microRNAs (miRNAs or miRs) (34). They can also regulate the function
of immune cells and promote cellular immune evasion by inducing
macrophage polarization, inhibiting dendritic cell (DC) activation,
suppressing natural killer (NK) cell cytotoxicity, and regulating T
cell function, among other key processes in ovarian carcinogenesis
(35). Simultaneously, EVs in the
ovarian follicular fluid of female patients with PCOS contain a
variety of differentially expressed miRNAs and proteins, which are
widely involved in the transfer of genetic information between
somatic cells and oocytes, as well as in the hormone metabolism
pathway of oocytes (36).
Moreover, exosomes derived from mesenchymal stem cell (MSC) species
regulate androgen production in vitro by virtue of their
cargo carrier function as the main molecule for reversing PCOS,
thereby restoring fertility in PCOS mice (37). In the development of POI, EVs
derived from a variety of stem cells such as embryonic stem cells
and human umbilical cord MSCs (HUCMSCs) play an important role in
intercellular communication by transporting several molecules such
as miRNAs from donors to recipients through their own transport
function leading to the occurrence of increased follicular growth,
decreased follicular atresia and restoration of hormone levels,
thus playing an important role (38,39).
EVs are closely associated with the development of ovarian cancer,
POI and PCOS. Therefore, in the present review, the role of the
biological characteristics of EVs in ovarian cancer, POI and PCOS
was mainly highlighted.
2. EVs
As a small vesicle that can be separated from cells
and biological fluids, EVs can be divided into exosomes,
microvesicles and apoptotic bodies according to different sizes and
sources (2). An exosome is a small
vesicle with a diameter of ~20-100 nm. As a carrier that shuttles
microRNA, drug molecules, and other substances, exosome proteins
can mediate the exchange of information between cells (40). Exosomes are initially
multivesicular bodies (MVBs) produced by the inward budding of
cells. After fusing with the plasma membrane, the contents are
secreted into the extracellular environment. Regulated by proteins,
ceramides and other substances, its biosynthesis and secretion are
complex. For example, ISGy modification, which belongs to
ubiquitination modification, can induce the aggregation and
degradation of MVB protein TSG101, reduce the number of MVB, and
thus inhibit the secretion of exosomes (41-43).
Various bioactive molecules including DNA, proteins, mRNA, and
non-coding RNA are released by exosomes, and its lipid bilayer
structure can effectively protect its contents from the
decomposition of extracellular nuclease and proteinase; hence, it
can stably exist in body fluids. The biological characteristics of
exosomes include stability and low toxicity. Furthermore, they play
an important role in targeted drug therapy of ovarian diseases
through binding with targeted ligand/homing peptide and exosome
transmembrane structure (44,45).
Among the contents of exosomes, proteins such as tetraspanins (CD9,
CD63, CD81 and CD151), Rab protein, connexin, and exosomal nucleic
acid molecules, such as miR-214 and miR-10b can be used as
biomarkers for cancer treatment. Moreover, compared with normal
cells, tumor cells release more exosomes; thus, their biomarker
characteristics for diagnosis and treatment have become a major
focus; however, exosome separation and purification are
time-consuming and costly. Therefore, at present, medical
researchers are exploring electrochemical sensing and other
technologies to manufacture various devices to address this problem
so that this exosome feature can be applied to the clinical
treatment of cancer (46,47). Microvesicles, as vesicles with a
diameter of ~200-1,000 nm formed through an outward extrusion of
the plasma membrane, can affect disease occurrence by regulating
cell processes including promoting cell growth and invasion,
angiogenesis, and cell type changes, among others, and play an
important role in the research and treatment of ovarian diseases
(48). Apoptotic bodies are small
vesicles formed by the decomposition of cells after apoptosis,
which can carry substances that can be used in apoptotic cells to
normal cells. Concurrently, studies have shown that apoptotic
bodies can transfer chemotherapeutic drugs with proximity effect to
tumor cells to play a therapeutic role. Hence, drugs can penetrate
into the tumor and maximize its destructive effect (49,50).
The biological characteristics of the three types of EVs allow us
to discuss their role in ovarian diseases.
3. Exosomes and the treatment of ovarian
cancer
Contents of exosomes and ovarian
cancer metastasis
Exosomal content miRNAs are a group of endogenous,
22 nt-long non-coding regulatory genes that function as gene
regulatory molecules in the control of life activities in
multicellular plants and animals (51). Chen et al (52) discovered that the conserved gene
sequence can preserve the genome structure by comparing the
human-mouse genome and examining the gene order stability of nearby
miRNA regions. In order to cleave or suppress translational
activity, miRNAs may interact with their target mRNAs (51). In a study by John et al
(53) employed three validation
techniques-retrospective, statistical and indirect experimental-to
demonstrate that the majority of miRNAs are selective in the mRNAs
they interact with and cleave. They are both multiplexed (one miRNA
may target several genes) and synergistic (several miRNAs can
regulate one gene) (53). MiRNAs
are now considered to have a role in the development of ovarian
cancer. In consequence, because of their affinity for the raft-like
outer part of the MVB membrane, miRNAs can be specifically mediated
into exosomes (54). Exosomes are
vesicle carriers that facilitate cellular information transfer and
may carry different miRNAs that affect ovarian cancer cells. Rashed
et al (55) identified that
miR-940 targets the proto-oncogene tyrosine protein kinase and
inhibits the expression of its downstream protein, preventing the
invasive metastasis of ovarian cancer cells. MiR-940 is enriched in
exosomes derived from ovarian cancer SKOV3-IP1, HeyA8 and HeyA8-MDR
cells. MiR-124, another exosomal component produced from ovarian
cancer cells, similarly prevents cell metastasis. By inhibiting the
production of the protein sphingosine kinase 1, which has a
pro-carcinogenic impact, this miRNA achieves its oncogenic
mechanism (56). Moreover, ovarian
cancer-derived exosomal miR-205 and ascites-derived exosomal
miR-6780b-5p enhance ovarian cancer cell metastasis by promoting
angiogenesis and epithelial-mesenchymal transition (EMT) of cells,
respectively (57,58). In addition, exosomal miR-323-3p
derived from adipose MSCs (AMSCs) can reduce the apoptosis of
cumulus cells and promote their proliferation by acting on PDCD4 in
patients with PCOS. Additionally, miR-199a-5p, an ovarian cancer
cell-derived exosome, has the ability to control hypoxia-inducible
factor-2, which enhances tight junctions in vascular endothelial
cells and prevents the spread of cancer cells (59). It is clear that the overexpression
of these tumor suppressor exosomal miRNAs can prevent cancer cell
metastasis, obstruct ovarian carcinogenesis, and potentially
function as biomarkers and therapeutic targets in the treatment of
ovarian cancer. Exosomal miRNAs can also encourage the spread of
ovarian cancer cells. In a recent study, Cao et al (60) revealed that miR-21-5P, which is
highly expressed in the exosomes of patients with ovarian cancer,
promotes the expression of cyclin synthesis-dependent kinase 6 and
anti-apoptotic proteins in cancer cells while inhibiting the
expression of pro-apoptotic proteins to facilitate the migration of
ovarian cancer cells. Exosomes from ovarian cancer and ascites,
respectively, can promote angiogenesis and EMT, which can both
increase ovarian cancer cell metastasis (57,58).
In addition, EOC exosomes have the ability to carry miR-125b-5p,
miR-181d-5p and miR-21-3p to M2 macrophages, where they can
increase their polarization and aid in the metastasis of EOC cells
(61). These exosomal miRNAs that
encourage tumor cell metastasis may serve as targets in the therapy
of ovarian cancer. In conclusion, both strategies-blocking the
release of exosomes that restrict cancer cell metastasis from
ovarian cancer tissues and focusing on exosomes that promote it-can
decrease tumor cell metastasis and may prove to be useful
modalities for the treatment of ovarian cancer.
In addition to exocytotic miRNAs, non-miRNA
substances such as plasmids and proteins can also be used as
information carriers to influence the metastasis of ovarian cancer.
Transforming growth factor 1 (TGF-1) may be carried by
cancer-associated fibroblast-derived exosomes, according to a
research by Li et al (62).
This TGF-1-carrying exosome, when ingested by ovarian cancer,
initiates the disease's EMT process and encourages the spread of
ovarian cancer cells. Meanwhile, studies have shown that exosomes
derived from DC carrying Killer Cell Lectin Like Receptor K1
(NKG2D) ligands can bind to the NKG2D receptor on the surface of NK
cells and activate NK cells, thus promoting the immune rejection of
tumors and inhibiting tumor growth. In addition, Viaud et al
(63) have injected dexamethasone
vaccine into 15 patients with melanoma in Phase I clinical trials
and found an increase in the number of NK cells in some patients.
In two of these patients, the tumor decreased. It has also been
demonstrated that exosomes released by ovarian cancer cells allow
subpopulations of cells with high invasive metastatic properties to
transmit those qualities to cells with lower metastatic capacities.
By way of their endoplasmic CD44 transfer, for instance, exosomes
released by highly metastatic H08910PM cells might encourage the
metastasis of low metastatic HO8910 cells (64). Pro-metastatic action of exosomes,
in turn, is associated with the ability of the cells from whence
they come to invade. Exosomes produced from highly metastatic
ovarian cancer cell lines include specific, highly expressed
pro-metastatic proteins that can control the Wnt/β-catenin
signaling pathway and encourage the spread of tumor cells.
Additionally, these exosomes are more likely to promote metastasis
than exosomes from ovarian cancer cells with little metastatic
potential (65). Other RNA
molecules in the exosome also influence the spread of ovarian
cancer. Exosomes containing the cyclic RNAs CircPUM1 and CircWHSC1,
for instance, operate on peritoneal mesothelial cells and promote
peritoneal metastasis of ovarian cancer by taking up and releasing
miRNAs in the form of sponges to maintain high expression levels
(66,67). Therefore, by blocking metastasis as
therapeutic targets, such exosomes carrying non-miRNA components
may potentially be used in the treatment of ovarian cancer. The
part exosomes play in the spread of ovarian cancer was reviewed by
the authors and the remarkable therapeutic potential of exosomes
was identified.
Targeted therapy of ovarian cancer
with exosome
At present, no optimal treatment for ovarian cancer,
as a gynecological disease with high incidence, has been found,
however studies have revealed that targeted treatment of ovarian
cancer may address this issue. Therapeutic exosome targeting to
treat ovarian cancer may include passive targeting of exosomes
utilizing the tropism of natural exosomal cells and active
targeting of exosomes utilizing tissue exosome surface engineering
(68). Exosomes are tiny,
extensively dispersed in bodily fluids, and readily pass through
blood vessel walls. At the same time, compared with in vitro
manufactured carriers, human-derived exosomes are less immunogenic
and more biocompatible (69). The
structure can easily escape pursuit by the immune system and can
carry nano-molecules for targeted transport to specific tissues
(70). Additionally, it has been
demonstrated that hybrid exosomes, which combine the benefits of
exosome and liposome delivery methods, have great stability and
high drug release rates, and may differently target medications to
normal and tumor cells, making the targeting of tumor therapy
easier (71). Because of the
characteristics summarized in the previous section, exosomes may be
useful as delivery vehicles for drugs used to treat ovarian cancer.
For example, bone marrow-derived MSC exosomes have specific
targeting functions and can activate signaling pathways of ovarian
cancer cell proliferation and metastasis, thus promoting tumor
formation (72). It is possible to
treat ovarian cancer using a strategy that specifically inhibits
MSC exosomes. Meanwhile, Hadla et al (73) found that exosomes increase the
therapeutic index of doxorubicin (DOX) in a mouse model of ovarian
cancer. In that study a human HGSOC mouse model of ovarian cancer
was constructed, when 5x106 spontaneously transformed
mouse ovarian surface epithelial cells mixed with 30% stromal gel
was subcutaneously implanted into the lateral abdomen of FVB/N
mice. A total of 3 mg/kg of DOX and 3 or 6 mg/kg of exoDOX were
injected intraperitoneally bi-weekly after tumors had reached a
size of >50 mm3. It was found that at high
concentrations, exoDOX was more effective than free DOX alone,
while having lower toxicity, thereby increasing the potential of
DOX in the treatment of ovarian cancer. Triptolide (TP) is an
herbal ingredient with anti-tumor effects. In 2019, Liu et
al (74) found that the
transportation of TP through exosomes, a carrier, significantly
inhibited the proliferation of ovarian cancer cells and attenuated
or delayed drug toxicity. A total of 2x106 SKOV3 cells
were injected subcutaneously into Balb/c nude mice to perform mouse
ovarian cancer modeling. When the tumor volume reached ~100
mm3, TP content of 0.2 mg/kg was injected
intraperitoneally into mice twice a week for 4 weeks. This may be a
promising strategy for TP treatment of ovarian cancer. In
conclusion, exosomes play an important role in the treatment of
ovarian cancer and can be used as the main safety delivery vehicle
for future ovarian cancer drugs. In addition, Huang et al
(75) prepared engineered exosomes
(cRGD-Exo-MEG3) modified with c(RGDyK) and carrying the long
non-coding RNA maternally expressed gene 3 (lncRNA MEG3) by
engineering technology. Both in vivo and in vitro,
this exosome can target the lncRNA MEG3, which exerts
anti-osteosarcoma (OS) effects, to tumors more efficiently,
enhancing the anti-OS effects of MEG3 and thus inhibiting tumor
growth. Moreover, Liang et al (76) combined 10 µg 5-fluorouracil (5-FU)
and 400 nm miR-21 inhibitor oligonucleotide (miR-21i) with exosomes
at a protein concentration of 10 µg. They obtained engineered
exosomes loaded with 5-FU and miR-21i and injected them into a
human colon cancer cell line (HCT-1165FR) using electroporation.
The results revealed that the combination of 5-FU and miR-21i
significantly inhibited cancer cell proliferation. Moreover,
injection of 5-FU and miR-21i exosomes into nude mice inhibited
tumor growth. This further demonstrated that miR-21i and 5-FU
exosomes can target cancer cell delivery and improve the efficacy
of CRC treatment. Exosomes play an important role in the targeted
treatment of cancer, so that the targeted treatment of exosomes may
have potential therapeutic value in ovarian cancer.
Immunotherapy of ovarian cancer with
exosome
Spontaneous immune responses and immune evasion
mechanisms are present in ascites, peripheral blood, and tumors of
patients with multiple ovarian cancers, and unlike conventional
surgical and platinum-based treatments, immunotherapy for ovarian
cancer is emerging (77). At
present, the main direction of ovarian cancer immunotherapy is the
development of biomarkers and targeted therapy. Some treatment
pathways based on immunotherapy have proven pre-clinical success
and entered clinical trials, such as immune checkpoint blockade and
cancer vaccines (78,79). For example, the intravenous
anti-programmed cell death protein 1 (PD-1) antibody nivolumab to
block PD-1 signaling in 20 patients with platinum-resistant,
recurrent, or advanced ovarian cancer has been tested in a phase II
clinical trial. The results demonstrated a disease control rate of
45% (78). Nivolumab is currently
approved by the Food and Drug Administration for the treatment of
ovarian cancer (80). At the same
time, another study has injected oxidized whole-tumor lysate DC
vaccine into the nodules of patients with recurrent ovarian cancer
and found that the injection of the vaccine was associated with
prolonged survival (79). As the
vaccine in the present study was only in the pilot clinical stage,
and due to certain limitations, such as production difficulties and
lack of immunogenicity of lysates, it is not currently approved for
human treatment. In addition, several studies have shown that
lymphocyte infiltration is the manifestation of tumor immune
response. Tumor-infiltrating T cells are closely associated with
improved clinical outcomes and prolonged survival in advanced
ovarian cancer and are considered to have clinical significance as
an independent prognostic marker for ovarian cancer (81,82).
Zhang et al (81) analyzed
the distribution of tumor-infiltrating T cells in 186 frozen
specimens of stage III or IV ovarian cancer and found that the
5-year overall survival rate of patients with tumors containing
T-cell infiltration (38%) was higher than that of patients without
T-cell infiltration (4.5%). Meanwhile, Han et al (82) conducted T cell infiltration
analysis on tumor samples of 150 EOC patients and also found that T
cell tumor infiltration was significantly associated with improved
survival rate of patients. Despite numerous advances in ovarian
cancer immunotherapy strategies, new strategies for immunotherapy
still need to be explored to improve the survival of ovarian cancer
patients. Exosomes, which can act as carriers to mediate cell
communication and evade immune rejection by the human body, have
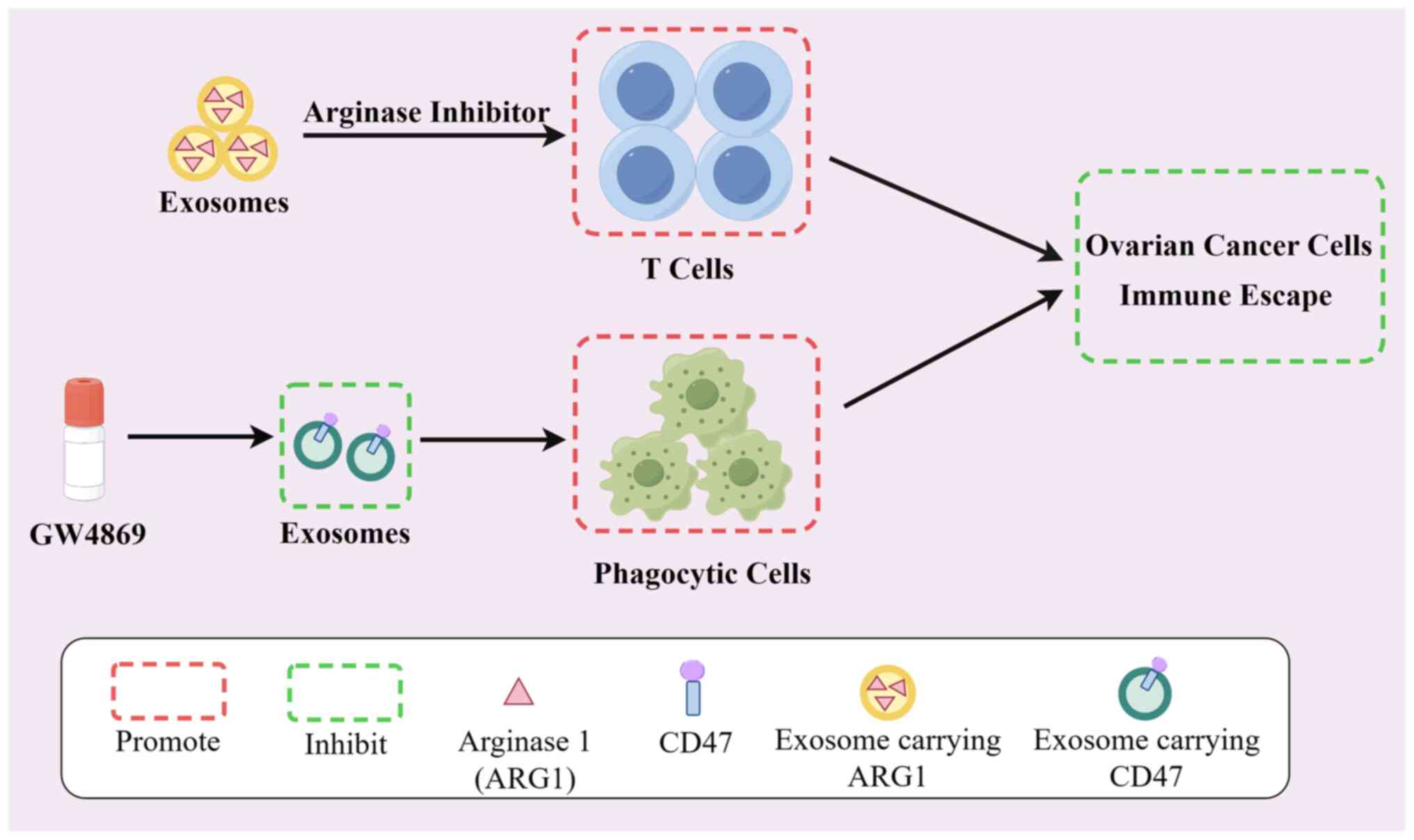
the potential for immune modulation. It has been identified that
ascites and plasma of patients with ovarian cancer contain tiny EVs
with internal Arginase 1 that may infiltrate antigen-presenting
cells, lowering T-cell activity and assisting the tumor in evading
immune system surveillance. However, the arginase inhibitor (OAT-1)
can block the immune escape process of cancer cells and reverse the
tumor growth-promoting effects of EV (83). Labani-Motlagh et al
(84) also demonstrated that
exosome-derived NKG2D from patient ovarian cancer cells or ascites
promoted cytotoxicity of NK cells, leading to immunological escape.
CD47, a protein expressed on the surface of most tumours, interacts
with signal-regulated protein alpha (SIRPα) on the surface of
phagocytes, thereby inhibiting the phagocytic ability of phagocytes
to engulf tumour cells (85).
Overexpression of CD47 in tumor cell-derived exosomes facilitates
an immune evasion response in ovarian cancer cells. Exosomes can be
used as a novel immune checkpoint, its biogenesis/release inhibitor
GW4869 can inhibit the spread of ovarian cancer cells by indirectly
reducing the expression of CD47 through the inhibition of exosome
secretion for the treatment of ovarian cancer (86). Meanwhile, it was found that
exosomes carrying SIRPα variants could block the normal interaction
between CD47 and SIRPα, enhancing phagocytosis in the T-cell
response and facilitating tumor clearance (85). Furthermore, PD-1, secreted by
exosomes derived from activated T cells, can interact with
programmed death-ligand 1 to impede the inhibitory effect of PD-1
on the activity of toxic T cells, with implications for tumour
clearance (87). It is clear that
exosomes may have some effect on tumours by altering the immune
response of the organism. For the treatment of ovarian cancer, this
immunotherapy of ovarian cancer by exosomes may become an effective
modality (Fig. 1).
Implications of exosomes for the
treatment of ovarian cancer
Numerous studies have shown that the interaction
between the tumor and its microenvironment is the key for tumor
therapy, and exosomes, as the mediators between tumor cells and the
tumor microenvironment, are of great significance for the treatment
of ovarian cancer (88). In
targeting and in immunotherapy of ovarian cancer, exosomes
undoubtedly play an irreplaceable role. the possible role of
exosomal targeted therapies and immunotherapy in ovarian cancer as
well as in numerous other cancers has been already summarized in
the aforementioned paragraphs, showing the potential of exosomes in
cancer therapy. In ovarian cancer treatment, exosomes have shown
therapeutic potential in addressing the issue of drug resistance in
ovarian cancer cells. For example, the downregulation of circular
RNA, cerebellar degeneration related 1 as in the exosomes of
cisplatin in patient cells can sensitize ovarian cancer to
cisplatin and reduce ovarian cancer cell resistance by promoting
miR-1270 expression, which regulates the suppressor of cancer cell
invasion and favors cisplatin treatment of ovarian cancer (89). Meanwhile, exosomes widely exist in
body fluids such as peripheral blood and are widely distributed and
highly stable. Studies have proved that exosomal microRNAs in
patients with ovarian cancer exhibit characteristics different from
those in healthy women and can be used as a biomarker to aid in the
treatment of ovarian cancer (90,91).
At present, the screening and diagnostic methods of ovarian cancer
are not advanced. Coupled with the asymptomatic growth of ovarian
cancer, most ovarian cancers are diagnosed late, resulting in poor
survival. Moreover, the current traditional treatment technologies
such as combined platinum compounds and taxane chemotherapy failed
to achieve favorable treatment results, and a high recurrence rate
remains present. Currently, different targeted therapies and
biological drug therapies that are expected to transform ovarian
cancer into a chronic disease have not shown to be efficacious in
terms of cure (92,93). The exosome is an important
participant in ovarian cancer therapies such as targeted therapy
and immunotherapy, and its presence is undoubtedly of great value
in the treatment of ovarian cancer.
4. Exosomes and non-ovarian cancer
disease
Exosomes and the treatment of POI
As transferable vesicles, the miRNA in exosomes
affects POI treatment. Chemotherapeutic agents have been widely
used in the construction of POI animal models. A previous study has
reported that for the construction of POI mouse models,
cyclophosphamide (CTX) (120 mg/kg)/busulfan (12 mg/kg) treatment
for at least 2 weeks or cisplatin (2 mg/kg) treatment for more than
10 days was found to be the most effective way to construct POI
mice (94). For the induction of
POI rats, a loading dose of 200 mg/kg CTX and a maintenance dose of
8 mg/kg CTX for 14 days is the most effective method (95). A study has shown that exosomes
derived from bone marrow mesenchymal cells (BMSCs) can carry
miR-144-5p that can mediate the activation of pI3K/AKT pathway and
target ovarian granulosa cells (GCs) damaged by CTX, so as to
inhibit GC apoptosis in rats with POI caused by CTX and improve
ovarian function (96).
Concurrently, the exocrine miR-644-5p from this cell can regulate
the expression of p53 ovarian GCs in POI mice model induced by
cisplatin and then inhibit apoptosis (97). This shows that miR-144-5p and
miR-644-5p in BMSCs play an important role in the treatment of POI
caused by CTX and cisplatin, respectively. Other studies have
revealed that miRNA-1246 and miRNA-21-5p carried by human amniotic
epithelial cell-derived exosomes can be transferred to ovarian GCs,
reducing chemotherapy-induced GC apoptosis in POI mice model by
inhibiting the expression of cleaved caspase 3 protein (98); exosome miR-10a derived from
amniotic fluid stem cells can also inhibit the apoptosis of ovarian
GCs in POI mice model induced by chemotherapy and is conducive to
ovarian growth and development. They can all be used as therapeutic
targets for chemotherapy-induced POI (99). In addition to treating
chemotherapy-induced POI mouse models, some studies have found that
exosomes derived from HUCMSCs can carry miR-146a-5p or miR-21-5p to
regulate the activation of primitive follicles and improve
fertility in natural aging mice model with low ovarian reserve
(100). This exocrine, highly
expressed miR-21 from human MSCs can reduce the expression of
phosphorylated lysine oxidase like 2 and Yes-associated protein by
reducing the expression of its target large tumor suppressor 1,
thereby increasing the secretion of estrogen from ovarian GCs (KGN
and SVOG) and repairing ovarian function, which affects the
treatment of POI (101). These
studies indicated that various cell-derived exocrine miRNAs play an
important role in the treatment of POI by CTX, cisplatin,
chemotherapy and other injuries.
Exosomes and the treatment of
PCOS
In the current treatment of PCOS, the use of
exosomes may be considered effective. A study has demonstrated that
S100 calcium-binding protein A9 (S100-A9) contained within the
follicular fluid-derived exosomes of the ovary of PCOS patients can
activate nuclear factor kappa B of steroid human granulosa tumor
cell signaling pathways, which in turn promote the occurrence of
inflammatory reactions and reduce steroid production (102). Simultaneously, miR-143-3p
exosomes from the same source can act on the target bone
morphogenetic protein receptor type 1A, inhibit the activity of KGN
Smad 1/5/8 signaling pathway, and promote the increase of apoptotic
factors in GCs (Primary GCs and KGN) (103). Additionally, circLDLR in exosomes
can inhibit miR-1294 expression that can directly bind to
Cytochrome P450 Family 19 Subfamily A Member 1 (CYP19A1) gene in
KGN cells and promote the expression of CYP19A1 gene. Patients with
PCOS with a decreased CYP19A1 gene expression in GCs can be treated
by increasing the secretion of estrogen E2, which can be used as a
treatment tool by regulating the function of PCOS GCs (104). There are also numerous miRNAs in
exosomes that have therapeutic targets for PCOS. For example,
exosome miR-424-5p in PCOS follicular fluid can target GC cell
division cycle associated 4 gene, block the
Rb/E2F1 pathway mediated by this gene, and
promote apoptosis and aging of GCs in patients with PCOS (105). In addition, exosomal miR-323-3p
derived from AMSCs can reduce the apoptosis of cumulus cells and
promote their proliferation by acting on PDCD4 in mice with PCOS
(106). In conclusion, exosomes
and their contents have great potential and may help in the
treatment of PCOS.
5. Microvesicles and ovarian disease
Microvesicles and the treatment of
ovarian cancer
As a therapeutic carrier, microvesicles have great
potential in the treatment of ovarian cancer. It was demonstrated
that microvesicles derived from adipose tissue-derived human
immortalized MSCs could carry proapoptotic and growth inhibitory
factors to inhibit the proliferation of ovarian cancer by acting on
ovarian cancer target cells through different pathways (107). Microvesicles derived from M1
macrophages simultaneously carrying DOX can specifically recognize
tumor cells and transport DOX to the nucleus, which can induce
apoptosis and significantly inhibit metastasis of advanced ovarian
cancer cells (108).
Microvesicles can not only act as a substance inhibiting ovarian
cancer metastasis but also serve as a promotive substance for
ovarian cancer development, thus inhibiting the release of
microvesicles from tumor cells can be a therapeutic modality. A
study has shown that simvastatin can effectively inhibit the
production of HGSOC cell-derived microvesicles, hinder the
secretion of microvesicles, and inhibit cell spreading and
metastasis by regulating the content and action of microvesicles in
ovarian cancer cells (109).
Additionally, O2-3-aminopropyldiazeniumdiolate (3f) can
prevent the generation of triple-negative breast cancer-derived
microvesicles by epigenetic modification, thereby attenuating the
microvesicle pro-metastatic function and inhibiting the metastasis
of cancer cells, which suggests that 3f plays a role in hindering
microvesicle secretion from cancer cells. This therapeutic
potential requires further research (110). Moreover, it has been investigated
that combined microbubble and ultrasound irradiation may be
effective as an ovarian cancer gene therapy to increase the
metastatic efficiency of siRNA-TPX2 plasmids that can inhibit the
phosphorylation of p38 and Mitogen-Activated Protein Kinase 8,
preventing the metastasis and invasion of ovarian cancer cells
(111). In summary, microvesicles
play an important role in the treatment of ovarian cancer.
Microvesicles and the treatment of POI
and PCOS
POI and PCOS are ovarian disorders that are
considered distressing for women. Microvesicles have shown
therapeutic potential in addition to the common treatments such as
hormone therapy and pharmacotherapy. In the treatment of POI,
studies have shown that transplantation of HUCMSC-derived
microvesicles can favorably activate the
phosphatidylinositol-3-kinase-serine/threonine kinase signaling
pathway and promote the production of proangiogenic factors,
restoring the angiogenic function in the ovaries of mice with PCOS
(112). In the treatment of PCOS,
MSCs-MVs have been reported to be able to rescue ovarian function
by regulating hormone levels, inhibiting follicular atresia and
promoting follicle development (113). The current understanding of the
role that microvesicles play in the treatment of POI and PCOS
remains unclear, and its research remains in its infancy. However,
existing studies have demonstrated that microvesicles may be
promising in the treatment of POI and PCOS, and exploring the
therapeutic relationship between microvesicles and both disease
entities is warranted.
6. Apoptotic bodies and the treatment of
ovarian disease
Recently, studies of apoptotic bodies in the
treatment of ovarian diseases mainly focus on the relationship
between apoptotic bodies and tumor treatment; however, there are
very few studies on its role in the treatment of POI and PCOS.
Therefore, the potential role of apoptotic bodies in the treatment
of ovarian cancer was mainly summarized in the present review.
First, apoptotic bodies can carry the remaining proapoptotic drugs
deep into the tumor interior through a proximity effect, improving
the efficiency of drug-induced apoptosis, treating tumors, and
inhibiting tumor growth (50).
Second, studies have shown that tumor cell-derived apoptotic bodies
contribute to tumorigenesis. For example, cancer cell-produced
apoptotic bodies with growth arrest specific 6 (Gas6) ligand of
receptor tyrosine kinase (AxL) as well as phosphatidylserine (PS)
are able to promote the spread of tumor cells through the
PS-Gas6-AxL signaling pathway (114). Μeanwhile, fibroblasts that can
take up cellular chromosomal DNA tumor cell-derived apoptotic
bodies favor the horizontal transfer of oncogenes between cells and
promote tumorigenesis (115). The
highly potent procoagulant effect exerted by the apoptotic bodies
produced by tumor cells induced by the chemotherapeutic treatment
of additional tumors invite the formation of tumor thrombi
(116). These studies suggested
that apoptotic bodies may serve as therapeutic targets in cancer.
As there are few related literatures regarding the relationship
between current apoptotic bodies and ovarian cancer treatment, the
potential of apoptotic bodies in the treatment of ovarian cancer
through the role it plays in tumor therapy should be further
investigated.
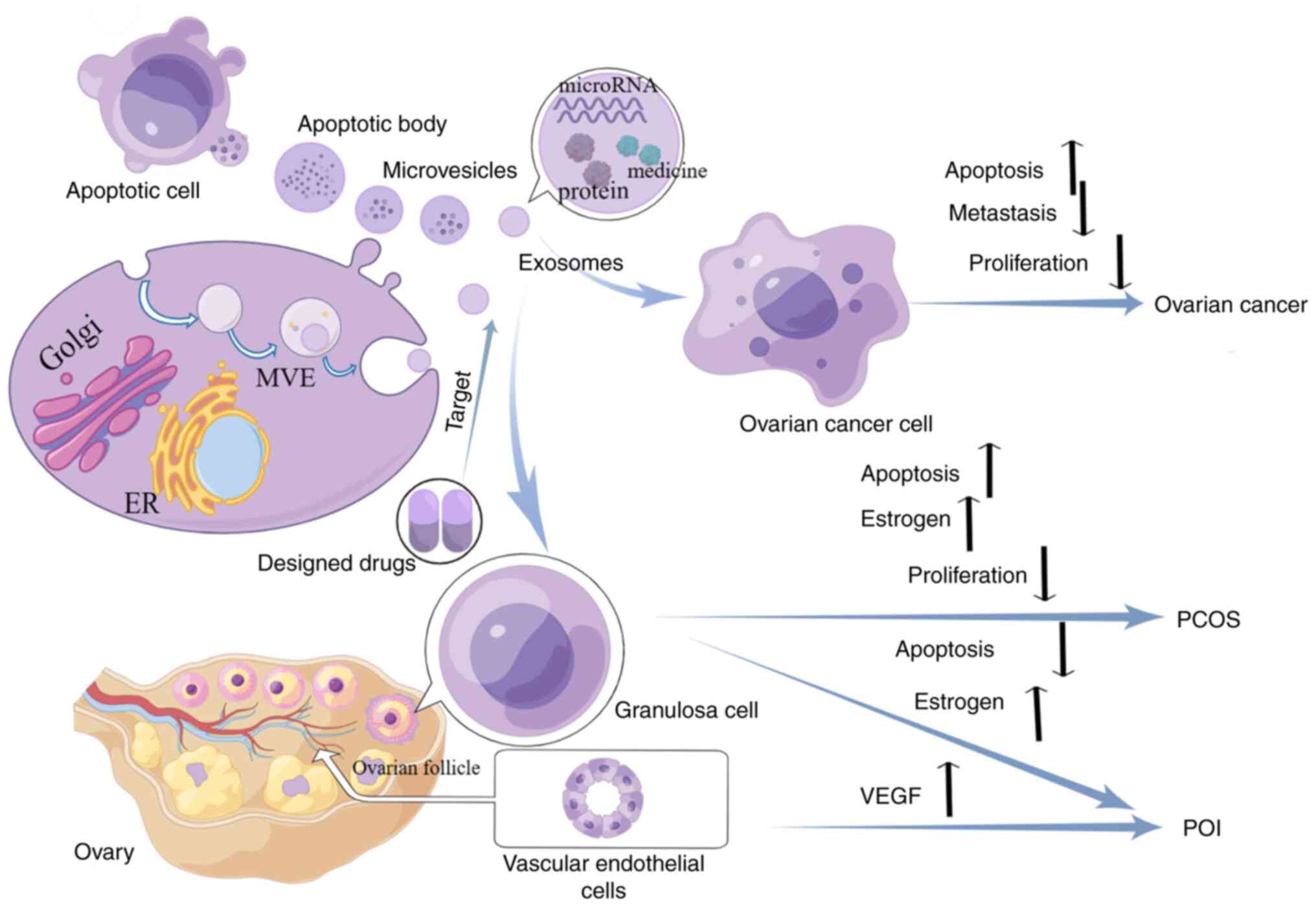
7. Conclusion and outlook
In the present review, the role and therapeutic
potential of EVs in the management of three ovarian diseases,
namely, ovarian cancer, POI and PCOS were mainly summarized
(Fig. 2). These three types of EVs
are able to participate in ovarian disease development by
transporting miRNAs (Table I) or
non-miRNAs (Table II). Currently,
ovarian disease is considered a common disease of women. However,
until present, no suitable treatment has been identified. The
application of EV therapy may have higher therapeutic benefits
relative to conventional therapies. There are currently three
reasons why exosomes for the treatment of human ovarian disease are
not approved by the Food and Drug Administration or other
regulatory agencies. First, the absolute isolation and definition
of the size or biogenesis of various EVs (including exosomes) has
not yet been determined. Secondly, the mechanism of action of
exosome therapy is also lacking in-depth and detailed exploration.
Finally, exosomes change their properties after manipulation in
vitro, which can have adverse effects on patients. Therefore,
investment in these novel therapeutic therapies should be applied
to realize the potential of EVs in the treatment of ovarian
diseases. Hopefully, in the near future, the most suitable therapy
for ovarian disease will be discovered to successfully solve this
medical dilemma.
 | Table IRole of exosomal miRNA in the
occurrence and development of ovarian diseases. |
Table I
Role of exosomal miRNA in the
occurrence and development of ovarian diseases.
| miRNA | Source of
exosomes | Model | In vitro or
in vivo study | Function | (Refs.) |
|---|
| Ovarian cancer | | | | | |
|
miR-940 | Ovarian cancer
cells | HeyA8, SKOV3IP1,
A2780-PAR, A2780-CP20, HIO-180 | In
vitro | Target the
proto-oncogene SRC and inhibits the expression of its downstream
protein, preventing the invasive metastasis of ovarian cancer
cells | (55) |
|
miR-124 | Ovarian cancer
cells | SKOV3, HO8910pm
cell lines | In
vitro | Inhibit the
production of the protein SphK1, which has a pro-carcinogenic
impact, to achieve its oncogenic mechanism | (56) |
|
miR-199a-5p | Ovarian cancer
cells | A2780, UWB, and
Hey, Anglne cell line | In
vitro | Control
hypoxia-inducible factor-2 to enhance tight junctions in vascular
endothelial cells and prevent the spread of cancer cells | (59) |
|
miR-21-5P | Ovarian cancer
cells | A2780, SKOV3,
BALB/C nude mice | In vitro and
in vivo | Promote the
expression of CDK6 and anti-apoptotic proteins in cancer cells
while inhibiting the expression of pro-apoptotic proteins to
facilitate the migration of ovarian cancer cells | (60) |
|
miR-205 | Ovarian cancer
cells | HUVECs, BALB/c nude
mice | In vitro and
in vivo | Enhance ovarian
cancer cell metastasis by promoting angiogenesis and EMT of
cells | (57) |
|
miR-6780b-5p |
Hydroperitoneum | A2780, SKOV3,
CAOV3, ES2, orthotopic xenograft mouse model of ovarian cancer | In vitro and
in vivo | Enhance ovarian
cancer cell metastasis by promoting angiogenesis and EMT of
cells | (58) |
|
miR-125b-5p,
miR-181d-5p, miR-21-3p | EOC | SKOV3, HO-8910, the
monocyte cell line U937 | In
vitro | Delivered to M2
macrophages and induce their polarization and aid in the metastasis
of EOC cells | (61) |
| POI | | | | | |
|
miR-144-5p | BMSCs | CTX-damaged GCs,
CTX-induced rats | In vitro and
in vivo | Mediate the
activation of pI3K/AKT pathway and target ovarian GCs damaged by
CTX, so as to inhibit GC apoptosis in rats with POI caused by CTX
and improve ovarian function | (96) |
|
miR-644-5p | BMSCs | Cisplatin-induced
primary GCs, Cisplatin-induced POI mouse | In vitro and
in vivo | Regulate the
expression of p53 ovarian GCs in POI mice model induced by
cisplatin and then inhibit apoptosis | (97) |
|
miR-21-5P,
miRNA-1246 | hAEC |
Chemotherapy-induced GC,
chemotherapy-induced POI mice | In vitro and
in vivo | Delivered to
ovarian GCs and reduce chemotherapy-induced GC apoptosis in POI
mice model by inhibiting the expression of cleaved caspase 3
protein | (98) |
|
miR-10a | AFSC | CTx-damaged GCs,
CTx-induced POI mice | In vitro and
in vivo | Inhibit the
apoptosis of ovarian GCs in POI mice model induced by chemotherapy
and is conducive to ovarian growth and development | (99) |
|
miR-21 | hMSCs | KGN, SVOG | In
vitro | Reduce the
expression of LOXL2 and YAP by reducing the expression of its
target LATS1, thereby increasing the secretion of estrogen from
ovarian GCs (KGN and SVOG) and repairing ovarian function | (101) |
|
miR-146a-5p,
miR-21-5p | HucMSC | Primordial oocytes,
aged female mice | In vitro and
in vivo | Regulate the
activation of primitive follicles and improve fertility in female
mice with low ovarian reserve | (100) |
| PCOS | | | | | |
|
miR-323-3p | AMSCs | GCs,
letrozole-induced PCOS mouse | In vitro and
in vivo | Reduce the
apoptosis of cumulus cells and promote their proliferation by
acting on PDCD4 in mice with PCOS | (106) |
|
miR-143-3p | Follicular
fluid | KGN | In
vitro | Act on the target
BMPR1A, inhibit the activity of KGN Smad1/5/8 signaling pathway,
and promote the increase of apoptotic factors in GCs (Primary GCs
and KGN) | (103) |
 | Table IIRole of non-miRNA in EVs in the
occurrence and development of ovarian diseases. |
Table II
Role of non-miRNA in EVs in the
occurrence and development of ovarian diseases.
| Non-microRNA | Types of EVs | Source of EVs | Model | In vitro or
in vivo study | Function | (Refs.) |
|---|
| Ovarian cancer | | | | | | |
|
TGF-1 | Exosome | Cancer-associated
fibroblast | SKOV-3 and CAOV-3
cell lines | In
vitro | Initiates the
epithelial-mesenchymal transition process of the disease and
encourages the spread of ovarian cancer cells | (62) |
|
CD44 | Exosome | H08910PM cells | HO8910 cells | In
vitro | Metastasis of low
metastatic HO8910 cells may be encouraged | (64) |
|
CircPUM1,
CircWHSC1 | Exosome | Ovarian cancer
cells | A2780, CAOV3,
OVCAR3, BALB/c nude mice | In vitro and
in vivo | Operates on
peritoneal mesothelial cells and promotes peritoneal metastasis of
ovarian cancer | (66,67) |
|
ARG1 | Exosome | Ascites,
plasma | Murine ovarian
cancer cell line ID8, C57BL/6 mice | In vitro and
in vivo | Enters
antigen-presenting cells to reduce T-cell activity and help tumors
escape surveillance by the immune system | (83) |
|
NKG2D | Exosome | Ovarian cancer
cells, ascites | OVCAR-3, K562
cells | In
vitro | Promotes the
cytotoxicity of NK cells and causes immune escape | (84) |
|
CD47 | Exosome | Ovarian cancer
cells | BALB/c nude
mice | In vivo | Facilitates an
immune evasion response in ovarian cancer cells | (86) |
|
PD-1 | Exosome | T cells | PY8119, C57BL/6
mice | In vitro and
in vivo | Interacts with
programmed death-ligand 1 to impede the inhibitory effect of
programmed death-1 on the activity of toxic T cells | (87) |
|
Cdr1as | Exosome | Ovarian cancer
cells | A2780, SKOV-3,
BALB/c athymic mice | In vitro and
in vivo | Downregulation of
cdr1as can promote the expression of miR-1270, which regulates
cancer cell invasion inhibitor, sensitizing ovarian cancer to
cisplatin and reducing the drug resistance of ovarian cancer
cells | (89) |
|
Proapoptotic
and growth inhibitory factors | Microvesicle | Human immortalized
mscs | ES-2, OAW-42 | In
vitro | It can inhibit the
proliferation of ovarian cancer by acting on ovarian cancer target
cells through different pathways | (107) |
|
Dox | Microvesicle | M1 macrophages | SKOV3, CHO BALB/c
nude mice | In vitro and
in vivo | Specifically
recognizes tumor cells and transports Dox to the nucleus, which can
induce apoptosis and significantly inhibit metastasis of advanced
ovarian cancer cells | (108) |
| PCOS | | | | | | |
|
S100-A9 | Exosome | Follicle fluid of
the ovary of PCOS patients | KGN | In
vitro | Activates nuclear
factor kappa B of steroid human granulosa tumor cell signaling
pathways, which in turn promote the occurrence of inflammatory
reactions and reduce steroid production | (102) |
|
CircLDLR | Exosome | Follicle fluid | KGN | In
vitro | Inhibits miR-1294
expression that can directly bind to CYP19A1 gene in KGN cells and
promotes the expression of CYP19A1 gene | (104) |
|
Gas6 ligand,
PS | Apoptotic body | Cancer cells | MDA-MB-231, HCC827,
SK-MES-1 | In
vitro | Promotes the spread
of tumor cells through the PS-Gas6-AXL signaling pathway | (114) |
Acknowledgements
All the figures in this article are made using
Figdraw (www.figdraw.com).
Funding
Funding: The present study was supported by the Research Fund
for Lin He's Academician Workstation of New Medicine and Clinical
Translation in Jining Medical University (grant nos. JYHL2021MS13
and JYHL2021MS10) and College Students' Innovation Training Program
of Jining Medical University (grant nos. S202310443062 and
cx2023062z).
Availability of data and materials
Not applicable.
Authors' contributions
KM and XW contributed to the study conception and
design. YZ and JZ performed the research and were major
contributors in writing the manuscript. LH, ZZ, CW and WL
contributed to the acquisition, analysis and systematization of
data. All authors read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walbrecq G, Margue C, Behrmann I and Kreis
S: Distinct cargos of small extracellular vesicles derived from
hypoxic cells and their effect on cancer cells. Int J Mol Sci.
21(5071)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zarà M, Guidetti GF, Camera M, Canobbio I,
Amadio P, Torti M, Tremoli E and Barbieri SS: Biology and role of
extracellular vesicles (EVs) in the pathogenesis of thrombosis. Int
J Mol Sci. 20(2840)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Skotland T, Sagini K, Sandvig K and
Llorente A: An emerging focus on lipids in extracellular vesicles.
Adv Drug Deliv Rev. 159:308–321. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang W, Jo H, Park S, Kim H, Kim SI, Han
Y, Lee J, Seol A, Kim J, Lee M, et al: Integrated analysis of
ascites and plasma extracellular vesicles identifies a miRNA-based
diagnostic signature in ovarian cancer. Cancer Lett.
542(215735)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kuhlmann JD, Chebouti I, Kimmig R,
Buderath P, Reuter M, Puppel SH, Wimberger P and Kasimir-Bauer S:
Extracellular vesicle-associated miRNAs in ovarian cancer-design of
an integrated NGS-based workflow for the identification of
blood-based biomarkers for platinum-resistance. Clin Chem Lab Med.
57:1053–1062. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Koshiyama M, Matsumura N and Konishi I:
Subtypes of ovarian cancer and ovarian cancer screening.
Diagnostics (Basel). 7(12)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
US Preventive Services Task Force.
Grossman DC, Curry SJ, Owens DK, Barry MJ, Davidson KW, Doubeni CA,
Epling JW Jr, Kemper AR, Krist AH, et al: Screening for ovarian
cancer: US preventive services task force recommendation statement.
JAMA. 319:588–594. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stewart C, Ralyea C and Lockwood S:
Ovarian cancer: An integrated review. Semin Oncol Nurs. 35:151–156.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Coburn SB, Bray F, Sherman ME and Trabert
B: International patterns and trends in ovarian cancer incidence,
overall and by histologic subtype. Int J Cancer. 140:2451–2460.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kurman RJ: Origin and molecular
pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol. 24
(Suppl 10):x16–x21. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Singer G, Oldt R III, Cohen Y, Wang BG,
Sidransky D, Kurman RJ and Shih IeM: Mutations in BRAF and KRAS
characterize the development of low-grade ovarian serous carcinoma.
J Natl Cancer Inst. 95:484–486. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Karnezis AN, Cho KR, Gilks CB, Pearce CL
and Huntsman DG: The disparate origins of ovarian cancers:
Pathogenesis and prevention strategies. Nat Rev Cancer. 17:65–74.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Prat J: New insights into ovarian cancer
pathology. Ann Oncol. 23 (Suppl 10):x111–x117. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chan JK, Teoh D, Hu JM, Shin JY, Osann K
and Kapp DS: Do clear cell ovarian carcinomas have poorer prognosis
compared to other epithelial cell types? A study of 1411 clear cell
ovarian cancers. Gynecol Oncol. 109:370–376. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yeung TL, Leung CS, Yip KP, Au Yeung CL,
Wong ST and Mok SC: Cellular and molecular processes in ovarian
cancer metastasis. A review in the theme: Cell and molecular
processes in cancer metastasis. Am J Physiol Cell Physiol.
309:C444–C456. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chandra A, Pius C, Nabeel M, Nair M,
Vishwanatha JK, Ahmad S and Basha R: Ovarian cancer: Current status
and strategies for improving therapeutic outcomes. Cancer Med.
8:7018–7031. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kurnit KC, Fleming GF and Lengyel E:
Updates and new options in advanced epithelial ovarian cancer
treatment. Obstet Gynecol. 137:108–121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Woad KJ, Watkins WJ, Prendergast D and
Shelling AN: The genetic basis of premature ovarian failure. Aust N
Z J Obstet Gynaecol. 46:242–244. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Howell S and Shalet S: Gonadal damage from
chemotherapy and radiotherapy. Endocrinol Metab Clin North Am.
27:927–943. 1998.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ishizuka B: Current understanding of the
etiology, symptomatology, and treatment options in premature
ovarian insufficiency (POI). Front Endocrinol (Lausanne).
12(626924)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang F, Liu Y, Ni F, Jin J, Wu Y, Huang Y,
Ye X, Shen X, Ying Y, Chen J, et al: BNC1 deficiency-triggered
ferroptosis through the NF2-YAP pathway induces primary ovarian
insufficiency. Nat Commun. 13(5871)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Domniz N and Meirow D: Premature ovarian
insufficiency and autoimmune diseases. Best Pract Res Clin Obstet
Gynaecol. 60:42–55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Goswami D and Conway GS: Premature ovarian
failure. Hum Reprod Update. 11:391–410. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Szeliga A, Calik-Ksepka A,
Maciejewska-Jeske M, Grymowicz M, Smolarczyk K, Kostrzak A,
Smolarczyk R, Rudnicka E and Meczekalski B: Autoimmune diseases in
patients with premature ovarian insufficiency-our current state of
knowledge. Int J Mol Sci. 22(2594)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sullivan SD, Sarrel PM and Nelson LM:
Hormone replacement therapy in young women with primary ovarian
insufficiency and early menopause. Fertil Steril. 106:1588–1599.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang S, Zhu D, Mei X, Li Z, Li J, Xie M,
Xie HJW, Wang S and Cheng K: Advances in biomaterials and
regenerative medicine for primary ovarian insufficiency therapy.
Bioact Mater. 6:1957–1972. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sirmans SM and Pate KA: Epidemiology,
diagnosis, and management of polycystic ovary syndrome. Clin
Epidemiol. 6:1–13. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Meier RK: Polycystic ovary syndrome. Nurs
Clin North Am. 53:407–420. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wolf WM, Wattick RA, Kinkade ON and Olfert
MD: Geographical prevalence of polycystic ovary syndrome as
determined by region and race/ethnicity. Int J Environ Res Public
Health. 15(2589)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Louwers YV and Laven JSE: Characteristics
of polycystic ovary syndrome throughout life. Ther Adv Reprod
Health. 14(2633494120911038)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Patel S: Polycystic ovary syndrome (PCOS),
an inflammatory, systemic, lifestyle endocrinopathy. J Steroid
Biochem Mol Biol. 182:27–36. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pauli JM, Raja-Khan N, Wu X and Legro RS:
Current perspectives of insulin resistance and polycystic ovary
syndrome. Diabet Med. 28:1445–1454. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mitra S, Nayak PK and Agrawal S:
Laparoscopic ovarian drilling: An alternative but not the ultimate
in the management of polycystic ovary syndrome. J Nat Sci Biol Med.
6:40–48. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tian W, Lei N, Zhou J, Chen M, Guo R, Qin
B, Li Y and Chang L: Extracellular vesicles in ovarian cancer
chemoresistance, metastasis, and immune evasion. Cell Death Dis.
13(64)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ingenito F, Roscigno G, Affinito A, Nuzzo
S, Scognamiglio I, Quintavalle C and Condorelli G: The Role of
Exo-miRNAs in cancer: A focus on therapeutic and diagnostic
applications. Int J Mol Sci. 20(4687)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang Y, Lang P, Zhang X, Wu X, Cao S, Zhao
C, Shen R, Ling X, Yang Y and Zhang J: Molecular characterization
of extracellular vesicles derived from follicular fluid of women
with and without PCOS: Integrating analysis of differential miRNAs
and proteins reveals vital molecules involving in PCOS. J Assist
Reprod Genet. 40:537–552. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Park HS, Cetin E, Siblini H, Seok J,
Alkelani H, Alkhrait S, Liakath Ali F, Mousaei Ghasroldasht M,
Beckman A and Al-Hendy A: Therapeutic potential of mesenchymal stem
cell-derived extracellular vesicles to treat PCOS. Int J Mol Sci.
24(11151)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Geng Z, Guo H, Li Y, Liu Y and Zhao Y:
Stem cell-derived extracellular vesicles: A novel and potential
remedy for primary ovarian insufficiency. Front Cell Dev Biol.
11(1090997)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fu YX, Ji J, Shan F, Li J and Hu R: Human
mesenchymal stem cell treatment of premature ovarian failure: New
challenges and opportunities. Stem Cell Res Ther.
12(161)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dorayappan KDP, Wallbillich JJ, Cohn DE
and Selvendiran K: The biological significance and clinical
applications of exosomes in ovarian cancer. Gynecol Oncol.
142:199–205. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li SP, Lin ZX, Jiang XY and Yu XY:
Exosomal cargo-loading and synthetic exosome-mimics as potential
therapeutic tools. Acta Pharmacol Sin. 39:542–551. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Savina A, Furlán M, Vidal M and Colombo
MI: Exosome release is regulated by a calcium-dependent mechanism
in K562 cells. J Biol Chem. 278:20083–20090. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Villarroya-Beltri C, Baixauli F,
Mittelbrunn M, Fernández-Delgado I, Torralba D, Moreno-Gonzalo O,
Baldanta S, Enrich C, Guerra S and Sánchez-Madrid F: ISGylation
controls exosome secretion by promoting lysosomal degradation of
MVB proteins. Nat Commun. 7(13588)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yang H, Fu H, Xu W and Zhang X: Exosomal
non-coding RNAs: A promising cancer biomarker. Clin Chem Lab Med.
54:1871–1879. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lin Y, Lu Y and Li X: Biological
characteristics of exosomes and genetically engineered exosomes for
the targeted delivery of therapeutic agents. J Drug Target.
28:129–141. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jalalian SH, Ramezani M, Jalalian SA,
Abnous K and Taghdisi SM: Exosomes, new biomarkers in early cancer
detection. Anal Biochem. 571:1–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Makler A and Asghar W: Exosomal biomarkers
for cancer diagnosis and patient monitoring. Expert Rev Mol Diagn.
20:387–400. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sedgwick AE and D'Souza-Schorey C: The
biology of extracellular microvesicles. Traffic. 19:319–327.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xu X, Lai Y and Hua ZC: Apoptosis and
apoptotic body: Disease message and therapeutic target potentials.
Bioscience reports. 39(BSR20180992)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhao D, Tao W, Li S, Chen Y, Sun Y, He Z,
Sun B and Sun J: Apoptotic body-mediated intercellular delivery for
enhanced drug penetration and whole tumor destruction. Sci Adv.
7(eabg0880)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen J, Chen W and Li Y: Conservation of
gene order in human microRNA-neighboring regions. Genome.
55:701–704. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human microRNA targets. PLoS Biol.
2(e363)2004.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Janas T, Janas MM, Sapoń K and Janas T:
Mechanisms of RNA loading into exosomes. FEBS Lett. 589:1391–1398.
2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Rashed MH, Kanlikilicer P,
Rodriguez-Aguayo C, Pichler M, Bayraktar R, Bayraktar E, Ivan C,
Filant J, Silva A, Aslan B, et al: Exosomal miR-940 maintains
SRC-mediated oncogenic activity in cancer cells: A possible role
for exosomal disposal of tumor suppressor miRNAs. Oncotarget.
8:20145–20164. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang H, Wang Q, Zhao Q and Di W: MiR-124
inhibits the migration and invasion of ovarian cancer cells by
targeting SphK1. J Ovarian Res. 6(84)2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang
J and Wu X: Ovarian cancer cell-secreted exosomal miR-205 promotes
metastasis by inducing angiogenesis. Theranostics. 9:8206–8220.
2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Cai J, Gong L, Li G, Guo J, Yi X and Wang
Z: Exosomes in ovarian cancer ascites promote
epithelial-mesenchymal transition of ovarian cancer cells by
delivery of miR-6780b-5p. Cell Death Dis. 12(210)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lian XY, Zhang H, Liu Q, Lu X, Zhou P, He
SQ, Tang RX and Cui J: Ovarian cancer-excreted exosomal miR-199a-5p
suppresses tumor metastasis by targeting hypoxia-inducible
factor-2α in hypoxia microenvironment. Cancer Commun (Lond).
40:380–385. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Cao J, Zhang Y, Mu J, Yang D, Gu X and
Zhang J: Exosomal miR-21-5p contributes to ovarian cancer
progression by regulating CDK6. Human Cell. 34:1185–1196.
2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chen X, Zhou J, Li X and Wang X, Lin Y and
Wang X: Exosomes derived from hypoxic epithelial ovarian cancer
cells deliver microRNAs to macrophages and elicit a tumor-promoted
phenotype. Cancer Lett. 435:80–91. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Li W, Zhang X, Wang J, Li M, Cao C, Tan J,
Ma D and Gao Q: TGFβ1 in fibroblasts-derived exosomes promotes
epithelial-mesenchymal transition of ovarian cancer cells.
Oncotarget. 8:96035–96047. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Viaud S, Terme M, Flament C, Taieb J,
André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz
T, et al: Dendritic cell-derived exosomes promote natural killer
cell activation and proliferation: A role for NKG2D ligands and
IL-15Ralpha. PLoS One. 4(e4942)2009.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Shen X, Wang C, Zhu H, Wang Y, Wang X,
Cheng X, Ge W and Lu W: Exosome-mediated transfer of CD44 from
high-metastatic ovarian cancer cells promotes migration and
invasion of low-metastatic ovarian cancer cells. J Ovarian Res.
14(38)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Alharbi M, Lai A, Guanzon D, Palma C,
Zuñiga F, Perrin L, He Y, Hooper JD and Salomon C: Ovarian
cancer-derived exosomes promote tumour metastasis in vivo: An
effect modulated by the invasiveness capacity of their originating
cells. Clin Sci (Lond). 133:1401–1419. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Guan X, Zong ZH, Liu Y, Chen S, Wang LL
and Zhao Y: circPUM1 promotes tumorigenesis and progression of
ovarian cancer by sponging miR-615-5p and miR-6753-5p. Mol Ther
Nucleic Acids. 18:882–892. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Zong ZH, Du YP, Guan X, Chen S and Zhao Y:
CircWHSC1 promotes ovarian cancer progression by regulating MUC1
and hTERT through sponging miR-145 and miR-1182. J Exp Clin Cancer
Res. 38(437)2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo
JK and Choi C: Biodistribution of exosomes and engineering
strategies for targeted delivery of therapeutic exosomes. Tissue
Eng Regen Med. 18:499–511. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Zhang Y, Bi J, Huang J, Tang Y, Du S and
Li P: Exosome: A review of its classification, isolation
techniques, storage, diagnostic and targeted therapy applications.
Int J Nanomedicine. 15:6917–6934. 2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Sharma S, Zuñiga F, Rice GE, Perrin LC,
Hooper JD and Salomon C: Tumor-derived exosomes in ovarian
cancer-liquid biopsies for early detection and real-time monitoring
of cancer progression. Oncotarget. 8:104687–104703. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rayamajhi S, Nguyen TDT, Marasini R and
Aryal S: Macrophage-derived exosome-mimetic hybrid vesicles for
tumor targeted drug delivery. Acta Biomater. 94:482–494.
2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Xie X, Wu H, Li M, Chen X, Xu X, Ni W, Lu
C, Ni R, Bao B and Xiao M: Progress in the application of exosomes
as therapeutic vectors in tumor-targeted therapy. Cytotherapy.
21:509–524. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hadla M, Palazzolo S, Corona G, Caligiuri
I, Canzonieri V, Toffoli G and Rizzolio F: Exosomes increase the
therapeutic index of doxorubicin in breast and ovarian cancer mouse
models. Nanomedicine (Lond). 11:2431–2441. 2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Liu H, Shen M, Zhao D, Ru D, Duan Y, Ding
C and Li H: The effect of triptolide-loaded exosomes on the
proliferation and apoptosis of human ovarian cancer SKOV3 cells.
Biomed Res Int. 2019(2595801)2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Huang X, Wu W, Jing D, Yang L, Guo H, Wang
L, Zhang W, Pu F and Shao Z: Engineered exosome as targeted lncRNA
MEG3 delivery vehicles for osteosarcoma therapy. J Control Release.
343:107–117. 2022.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si
K, Sun B, Chen B and Xiao Z: Engineered exosomes for targeted
co-delivery of miR-21 inhibitor and chemotherapeutics to reverse
drug resistance in colon cancer. J Nanobiotechnology.
18(10)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Coukos G, Tanyi J and Kandalaft LE:
Opportunities in immunotherapy of ovarian cancer. Ann Oncol. 27
(Suppl 1):i11–i15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hamanishi J, Mandai M, Ikeda T, Minami M,
Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S,
et al: Safety and antitumor activity of Anti-PD-1 antibody,
nivolumab, in patients with platinum-resistant ovarian cancer. J
Clin Oncol. 33:4015–4022. 2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Tanyi JL, Bobisse S, Ophir E, Tuyaerts S,
Roberti A, Genolet R, Baumgartner P, Stevenson BJ, Iseli C, Dangaj
D, et al: Personalized cancer vaccine effectively mobilizes
antitumor T cell immunity in ovarian cancer. Sci Transl Med.
10(eaao593)2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Sangwan K, Sharma V and Goyal PK:
Pharmacological profile of novel anti-cancer drugs approved by
USFDA in 2022: A review. Curr Mol Med: Jun 22, 2023 (Epub ahead of
print).
|
|
81
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence,
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Han LY, Fletcher MS, Urbauer DL, Mueller
P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers
MT, et al: HLA class I antigen processing machinery component
expression and intratumoral T-Cell infiltrate as independent
prognostic markers in ovarian carcinoma. Clin Cancer Res.
14:3372–3379. 2008.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Czystowska-Kuzmicz M, Sosnowska A, Nowis
D, Ramji K, Szajnik M, Chlebowska-Tuz J, Wolinska E, Gaj P, Grazul
M, Pilch Z, et al: Small extracellular vesicles containing
arginase-1 suppress T-cell responses and promote tumor growth in
ovarian carcinoma. Nat Commun. 10(3000)2019.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Labani-Motlagh A, Israelsson P, Ottander
U, Lundin E, Nagaev I, Nagaeva O, Dehlin E, Baranov V and
Mincheva-Nilsson L: Differential expression of ligands for NKG2D
and DNAM-1 receptors by epithelial ovarian cancer-derived exosomes
and its influence on NK cell cytotoxicity. Tumour Biol.
37:5455–5466. 2016.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Koh E, Lee EJ, Nam GH, Hong Y, Cho E, Yang
Y and Kim IS: Exosome-SIRPα, a CD47 blockade increases cancer cell
phagocytosis. Biomaterials. 121:121–129. 2017.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Shimizu A, Sawada K, Kobayashi M, Yamamoto
M, Yagi T, Kinose Y, Kodama M, Hashimoto K and Kimura T: Exosomal
CD47 plays an essential role in immune evasion in ovarian
cancerexosomal CD47 regulates immune evasion in ovarian cancer. Mol
Cancer Res. 19:1583–1595. 2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Qiu Y, Yang Y, Yang R, Liu C, Hsu JM,
Jiang Z, Sun L, Wei Y, Li CW, Yu D, et al: Activated T cell-derived
exosomal PD-1 attenuates PD-L1-induced immune dysfunction in
triple-negative breast cancer. Oncogene. 40:4992–5001.
2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Tang MK and Wong AS: Exosomes: Emerging
biomarkers and targets for ovarian cancer. Cancer Lett. 367:26–33.
2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Zhao Z, Ji M, Wang Q, He N and Li Y:
Circular RNA Cdr1as upregulates SCAI to suppress cisplatin
resistance in ovarian cancer via miR-1270 suppression. Mol Ther
Nucleic Acids. 18:24–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Cheng L, Wu S, Zhang K, Qing Y and Xu T: A
comprehensive overview of exosomes in ovarian cancer: Emerging
biomarkers and therapeutic strategies. J Ovarian Res.
10(73)2017.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Ryu J and Thomas SN: Quantitative mass
spectrometry-based proteomics for biomarker development in ovarian
cancer. Molecules. 26(2674)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Cortez AJ, Tudrej P, Kujawa KA and
Lisowska KM: Advances in ovarian cancer therapy. Cancer Chemother
Pharmacol. 81:17–38. 2018.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Lee EH, Han SE, Park MJ, Kim HJ, Kim HG,
Kim CW, Joo BS and Lee KS: Establishment of effective mouse model
of premature ovarian failure considering treatment duration of
anticancer drugs and natural recovery time. J Menopausal Med.
24:196–203. 2018.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Qi Y, Zhu YM and Li B: Comparison of
Animal Models for Premature Ovarian Insufficiency Induced by
Different Doses of Cyclophosphamide: A Network Meta-analysis,
2022.
|
|
96
|
Yang M, Lin L, Sha C, Li T, Zhao D, Wei H,
Chen Q, Liu Y, Chen X, Xu W, et al: Bone marrow mesenchymal stem
cell-derived exosomal miR-144-5p improves rat ovarian function
after chemotherapy-induced ovarian failure by targeting PTEN. Lab
Invest. 100:342–352. 2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Sun B, Ma Y, Wang F, Hu L and Sun Y:
miR-644-5p carried by bone mesenchymal stem cell-derived exosomes
targets regulation of p53 to inhibit ovarian granulosa cell
apoptosis. Stem Cell Res Ther. 10(360)2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Zhang Q, Sun J, Huang Y, Bu S, Guo Y, Gu
T, Li B, Wang C and Lai D: Human amniotic epithelial cell-derived
exosomes restore ovarian function by transferring microRNAs against
apoptosis. Mol Ther Nucleic Acids. 16:407–418. 2019.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Xiao GY, Cheng CC, Chiang YS, Cheng WT,
Liu IH and Wu SC: Exosomal miR-10a derived from amniotic fluid stem
cells preserves ovarian follicles after chemotherapy. Sci Rep.
6(23120)2016.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Yang W, Zhang J, Xu B, He Y, Liu W and Li
J, Zhang S, Lin X, Su D, Wu T and Li J: HucMSC-Derived exosomes
mitigate the age-related retardation of fertility in female mice.
Mol Ther. 28:1200–1213. 2020.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Cai JH, Sun YT and Bao S: HucMSCs-exosomes
containing miR-21 promoted estrogen production in ovarian granulosa
cells via LATS1-mediated phosphorylation of LOXL2 and YAP. Gen Comp
Endocrinol. 321(114015)2022.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Li H, Huang X, Chang X, Yao J, He Q, Shen
Z, Ji Y and Wang K: S100-A9 protein in exosomes derived from
follicular fluid promotes inflammation via activation of NF-κB
pathway in polycystic ovary syndrome. J Cell Mol Med. 24:114–125.
2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Zhao Y, Pan S, Li Y and Wu X: Exosomal
miR-143-3p derived from follicular fluid promotes granulosa cell
apoptosis by targeting BMPR1A in polycystic ovary syndrome. Sci
Rep. 12(4359)2022.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Huang X, Wu B, Chen M, Hong L, Kong P, Wei
Z and Teng X: Depletion of exosomal circLDLR in follicle fluid
derepresses miR-1294 function and inhibits estradiol production via
CYP19A1 in polycystic ovary syndrome. Aging (Albany NY).
12:15414–15435. 2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Yuan D, Luo J, Sun Y, Hao L, Zheng J and
Yang Z: PCOS follicular fluid derived exosomal miR-424-5p induces
granulosa cells senescence by targeting CDCA4 expression. Cell
Signal. 85(110030)2021.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Zhao Y, Tao M, Wei M, Du S, Wang H and
Wang X: Mesenchymal stem cells derived exosomal miR-323-3p promotes
proliferation and inhibits apoptosis of cumulus cells in polycystic
ovary syndrome (PCOS). Artif Cells Nanomed Biotechnol.
47:3804–3813. 2019.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Szyposzynska A, Bielawska-Pohl A,
Krawczenko A, Doszyn O, Paprocka M and Klimczak A: Suppression of
ovarian cancer cell growth by AT-MSC microvesicles. Int J Mol Sci.
21(9143)2020.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Guo L, Zhang Y, Wei R, Zhang X, Wang C and
Feng M: Proinflammatory macrophage-derived microvesicles exhibit
tumor tropism dependent on CCL2/CCR2 signaling axis and promote
drug delivery via SNARE-mediated membrane fusion. Theranostics.
10:6581–6598. 2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Mancilla P, Liberona M, Kato S, Barra J,
Gonzalez A and Cuello M: Simvastatin modifies the internalization,
endocytic trafficking, and the content of ovarian cancer
cellderived extracellular microvesicles which are responsible of
inducing migration and invasion in vitro. Int J Gynecol Cancer. 30
(Suppl 3):A192–A193. 2020.
|
|
110
|
Kang F, Zhu J, Wu J, Lv T, Xiang H, Tian
J, Zhang Y and Huang Z: O2-3-Aminopropyl
diazeniumdiolates suppress the progression of highly metastatic
triple-negative breast cancer by inhibition of microvesicle
formation via nitric oxide-based epigenetic regulation. Chem Sci.
9:6893–6898. 2018.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Huang D, Chen J, Yang C and Wang M: TPX2
silencing mediated by joint action of microvesicles and ultrasonic
radiation inhibits the migration and invasion of SKOV3 cells. Mol
Med Rep. 17:7627–7635. 2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Yang Z, Du X, Wang C, Zhang J, Liu C, Li Y
and Jiang H: Therapeutic effects of human umbilical cord
mesenchymal stem cell-derived microvesicles on premature ovarian
insufficiency in mice. Stem Cell Res Ther. 10(250)2019.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Faruk EM: El desoky RE, El-Shazly AM and
Taha NM: Does exosomes derived bone marrow mesenchymal stem cells
restore ovarian function by promoting stem cell survival on
experimentally induced polycystic ovary in adult female albino
rats?(Histological and Immunohistochemical Study). J Stem Cell Res
Ther. 8(1000442)2018.
|
|
114
|
Zweemer AJM, French CB, Mesfin J, Gordonov
S, Meyer AS and Lauffenburger DA: Apoptotic bodies Elicit
Gas6-mediated migration of AXL-Expressing tumor cell. Mol Cancer
Res. 15:1656–1666. 2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Bergsmedh A, Szeles A, Henriksson M, Bratt
A, Folkman MJ, Spetz AL and Holmgren L: Horizontal transfer of
oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci USA.
98:6407–6411. 2001.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Muhsin-Sharafaldine MR, Kennedy BR,
Saunderson SC, Buchanan CR, Dunn AC, Faed JM and McLellan AD:
Mechanistic insight into the procoagulant activity of tumor-derived
apoptotic vesicles. Biochim Biophys Acta Gen Subj. 1861:286–295.
2017.PubMed/NCBI View Article : Google Scholar
|