Introduction
Several essential trace elements are required for
the maintenance of numerous physiological functions, since the
elements act as important components of metalloproteins and
metalloenzymes (1). It is well
established that deficient or excessive levels of essential trace
elements can result in the development of chronic liver diseases
(CLDs), such as viral chronic hepatitis/cirrhosis, alcoholic liver
disease, nonalcoholic fatty liver disease (NAFLD) and autoimmune
liver diseases (2,3). Metabolic abnormalities, including
insulin resistance and dyslipidemia, are closely associated with
the development of hepatic steatosis and hepatic fibrosis (4). Such metabolic abnormalities are also
related to disorders of the metabolism of some trace elements,
including zinc (Zn), selenium (Se) and iron (Fe) (5).
Strong correlations between impaired trace element
metabolism and insulin resistance, hepatic steatosis or hepatic
fibrosis have been observed in patients with hepatitis C virus
(HCV)-related CLD (CLD-C) (6-9).
Recent studies have also revealed that serum Zn and Se levels are
inversely associated with the severity of hepatic fibrosis in
patients with NAFLD or nonalcoholic steatohepatitis (NASH)
(10,11), whereas serum ferritin levels have
been shown to be increased in parallel with the degree of hepatic
fibrosis in such patients (12).
Furthermore, it has been demonstrated that serum copper (Cu) levels
are decreased as disease activity becomes more severe in patients
with NASH (13,14).
Patients with NASH have some similar common clinical
features as patients with CLD-C, including insulin resistance,
hepatic steatosis and iron overload (15,16).
However, to the best of our knowledge, it is not yet known as to
whether the involvement of essential trace elements in the
pathogenesis of NASH is identical to that in CLD-C.
The present study aimed to verify the involvement of
four essential trace elements (Fe, Zn, Se and Cu) in the
pathogeneses of CLD-C and NASH, and to compare the involvement of
these trace elements between groups of patients with CLD-C and
NASH.
Materials and methods
Study population
The present study was conducted retrospectively. A
total of 66 patients with CLD-C and 26 patients with NASH were
randomly selected from patients admitted to the Hospital of Kagawa
University Faculty of Medicine (Miki, Japan) between January 2015
and December 2019. The pathological diagnosis of NASH was
determined on the basis of Matteoni's classification (17).
All of the selected patients with CLD-C had
detectable serum HCV-RNA as determined by PCR, and exhibited
histological findings compatible with chronic hepatitis or liver
cirrhosis.
The study protocol complied with all of the
provisions of The Declaration of Helsinki. The design of the study
was approved by the Ethical Committees of both the Kagawa
Prefectural University of Health Sciences (approval no. 305;
Takamatsu, Japan) and Kagawa University Faculty of Medicine
(approval no. 2020-045). Written informed consent was obtained from
each individual.
Laboratory assessments
HCV-RNA was quantitatively detected with the COBAS
TaqMan HCV assay (Roche Molecular Diagnostics) as previously
described (18). Serum albumin
(Alb) and alanine aminotransferase (ALT), ferritin, glucose and
insulin levels were measured using standard laboratory techniques.
Insulin resistance was estimated based on the homeostasis model for
the assessment of insulin resistance (HOMA-IR) value using the
following equation: HOMA-IR value=fasting insulin (µU/m) x fasting
glucose (mg/dl)/405. The normal ranges of serum Alb level, ALT
level and HOMA-IR value were 3.5-5.5 g/dl, <35 IU/l and <1.6,
respectively. Serum Zn, Se and Cu levels were determined by atomic
absorption spectrometry, as previously described (19). The serum Zn levels were measured in
the morning after the patients underwent an overnight fast due to
its circadian rhythm.
Zn deficiency was defined as a serum Zn level <60
µg/dl (20) and Se deficiency was
defined as a serum Se level <10 µg/dl (21). The serum ferritin level in each
patient was also measured as a serological hallmark of iron storage
in the liver. Iron overload was defined as a serum ferritin level
exceeding 1.5 times the upper normal range (>300 ng/ml in women
and >450 ng/ml in men) (22).
The normal range of serum Cu levels was 70-132 µg/dl.
Histological assessments
Each liver biopsy was conducted under the guidance
of ultrasound prior to treatment, using 16-gauge needles to obtain
the liver specimen. The tissue samples were fixed in 10% formalin
and embedded in paraffin. The tissue sections were stained with
hematoxylin and eosin for pathological evaluation, as previously
described (23). The stage of
hepatic fibrosis was based on the New Inuyama Classification system
(24), which provides the standard
criteria for the histological assessment of chronic hepatitis in
Japan. Briefly, the staging of hepatic fibrosis was scored as
follows from F0 to F4.: F0, no
fibrosis in the liver specimen; F1, portal expansion;
F2, bridging fibrosis; F3, bridging fibrosis
with lobular distortion; and F4, liver cirrhosis. The
severity of hepatic steatosis was classified into grade 0 to 3,
based on the classification proposed by Brunt et al
(25). Hepatic steatosis in 0,
<33, 33-66 and >66% of hepatocytes was defined as grade 0, 1,
2 and 3, respectively.
Statistical analysis
Statistical analyses were conducted using JMP 14
(SAS Institute, Inc.). Data are presented as the mean ± standard
deviation. The Mann-Whitney U-test and the Kruskal-Wallis test were
used to compare variables between two groups and more than three
groups, respectively. When significant differences were identified
by the Kruskal-Wallis test, the Dunn-Bonferroni test was used for
pairwise comparisons. The correlation between quantitative
variables was analyzed by Pearson's test. Fisher's exact
probability test was used to compare differences in frequencies.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics
Table I summarizes
the clinical characteristics of the patients. No significant
differences in age or sex were identified between the CLD-C and
NASH groups; however, patients with NASH had significantly higher
body mass index (BMI) values compared with those of patients with
CLD-C. The serum ALT levels and HOMA-IR values were also
significantly higher in the NASH group compared with those in the
CLD-C group. The serum Alb levels were similar between the two
groups, indicating that hepatic reserve in patients with NASH was
similar to that in patients with CLD-C. Histologically, patients
with NASH had significantly more severe hepatic steatosis than
patients with CLD-C, whereas the severity of hepatic fibrosis was
nearly identical between the two groups.
 | Table ICharacteristics of patients with
CLD-C and NASH. |
Table I
Characteristics of patients with
CLD-C and NASH.
| Characteristic | CLD-C (n=66) | NASH (n=26) | P-value |
|---|
| Age,
yearsa | 59.3±8.5
(35-81) | 60.8±11.6
(36-81) | 0.4483 |
| Sex (male/female),
n | 38/28 | 10/16 | 0.0776 |
| BMI,
kg/m2a | 23.9±3.4
(16.6-34.1) | 27.9±3.8
(20.7-35.3) | 0.0001 |
| ALT,
IU/la | 70.8±55.3
(15-287) | 102.8±65.0
(23-301) | 0.0057 |
| Alb,
g/dla | 4.0±0.5
(2.7-5.1) | 4.2±0.4
(3.6-5.1) | 0.1792 |
|
HOMA-IRa | 2.06±1.22
(0.52-5.74) | 4.49±2.90
(0.89-13.0) | <0.0001 |
| Hepatic steatosis
(Gr 0/1/2/3), n | 32/24/10/0 | 0/10/10/6 | <0.0001 |
| Hepatic fibrosis
(F1/2/3/4), n | 19/22/15/10 | 13/2/11/0 | 0.1637 |
Distribution of circulating trace
elements
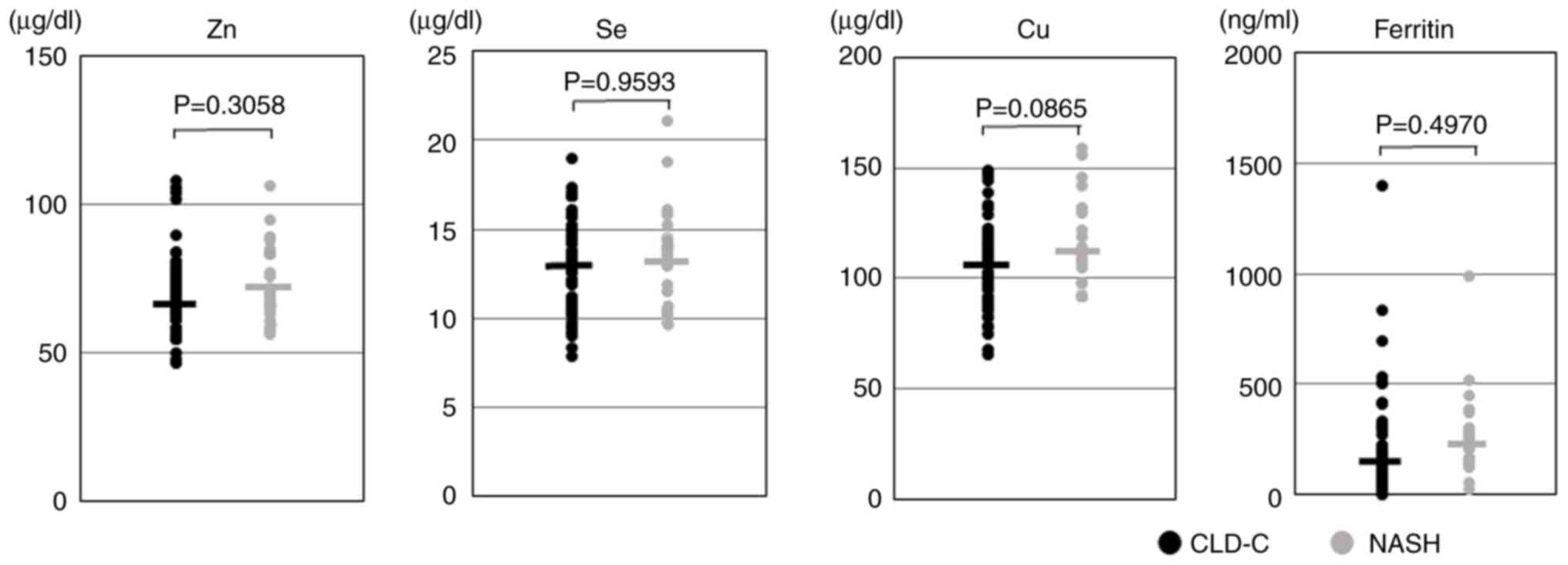
The present study investigated the distribution of
serum Zn, Se, Cu and ferritin levels in the CLD-C and NASH groups,
and compared them (Fig. 1). Eight
of the 66 (12.1%) patients with CLD-C and three of the 26 (11.5%)
patients with NASH were diagnosed as having Zn deficiency. The mean
serum Zn levels in the CLD-C group were similar to those in the
NASH group (70.8±12.3 vs. 73.9±12.3 µg/dl; P=0.3058). Similarly,
seven of the 66 (10.6%) patients with CLD-C and two of the 26
(7.7%) patients with NASH were diagnosed with Se deficiency, and
mean serum Se levels were almost identical between the groups
(13.1±2.5 vs. 13.3±2.8 µg/dl; P=0.9593). These Zn- and Se-deficient
patients were free from symptoms. The mean serum Cu levels in the
NASH group tended to be higher than those in the CLD-C group
(115.9±20.6 vs. 106.3±19.0 µg/dl; P=0.0865). The frequencies of
hyperferritinemia (>300 ng/ml in women and >450 ng/ml in men)
in the CLD-C and NASH groups were 10.6 and 11.5%, respectively. The
mean serum ferritin levels in the CLD-C group was almost identical
to those in the NASH group (219.0±241.5 vs. 255.9±187.0 ng/ml;
P=0.4970).
The present study also examined the differences in
the trace elements between male and female patients. There were no
significant differences in serum Zn or Se levels between male and
female patients in both the CLD-C and NASH groups. However, the
serum Cu levels were significantly higher in the female patients
with CLD-C compared with those in the male patients with CLD-C
(113.0±16.5 vs. 101.4±19.4 mg/dl; P=0.0100), whereas the serum
ferritin levels were significantly lower in the female patients
with NASH compared with those in the male patients (186.4±92.6 vs.
367.1±246.0 ng/ml; P=0.0132) (data not shown).
Biochemical and nutritional factors
associated with serum trace elements
The present study analyzed the laboratory factors
associated with circulating trace element levels in the patients.
As shown in Table II, the serum
ALT levels were significantly correlated with the serum ferritin
levels in both the CLD-C (r=0.667, P<0.0001) and NASH (r=0.523,
P=0.0061) groups. The serum Alb levels were significantly
correlated with the serum Zn levels (CLD-C: r=0.475, P<0.0001;
NASH: r=0.669, P=0.0002) and serum Se levels (CLD-C: r=0.516,
P<0.0001; NASH: r=0.592, P=0.0015) in both groups. A positive
correlation was also observed between serum Alb and Cu levels in
patients with NASH (r=0.446, P=0.0330).
 | Table IICorrelations between serum trace
element levels and biochemical or nutritional factors in patients
with CLD-C and NASH. |
Table II
Correlations between serum trace
element levels and biochemical or nutritional factors in patients
with CLD-C and NASH.
| | CLD-C (n=66) | NASH (n=26) |
|---|
| Factor | Element | r-value | P-value | r-value | P-value |
|---|
| ALT | Zn | 0.122 | 0.3305 | 0.110 | 0.5911 |
| | Se | 0.101 | 0.4388 | -0.116 | 0.5710 |
| | Cu | 0.022 | 0.8642 | 0.259 | 0.2331 |
| | Ferritin | 0.667 | <0.0001 | 0.523 | 0.0061 |
| Alb | Zn | 0.475 | <0.0001 | 0.669 | 0.0002 |
| | Se | 0.516 | <0.0001 | 0.592 | 0.0015 |
| | Cu | 0.208 | 0.1131 | 0.446 | 0.0330 |
| | Ferritin | 0.163 | 0.2635 | -0.090 | 0.6631 |
| HOMA-IR | Zn | -0.290 | 0.0192 | -0.451 | 0.0309 |
| | Se | -0.275 | 0.0332 | -0.061 | 0.7823 |
| | Cu | 0.032 | 0.8034 | -0.080 | 0.7379 |
| | Ferritin | 0.419 | 0.0022 | 0.411 | 0.0423 |
| BMI | Zn | -0.078 | 0.5677 | -0.194 | 0.3419 |
| | Se | 0.012 | 0.9334 | -0.182 | 0.3732 |
| | Cu | 0.094 | 0.4980 | 0.185 | 0.3993 |
| | Ferritin | 0.144 | 0.3383 | -0.064 | 0.7565 |
The HOMA-IR values were positively correlated with
serum ferritin levels in both the CLD-C (r=0.419, P=0.0022) and
NASH (r=0.411, P=0.0423) groups. There was an inverse association
between the HOMA-IR values and serum Zn levels in the CLD-C group
(r=-0.290, P=0.0192) and am inverse correlation in the NASH group
(r=-0.451, P=0.0309), whereas an inverse association was observed
between the HOMA-IR values and serum Se levels in patients with
CLD-C only (r=-0.275, P=0.0332). BMI values were not correlated
with the levels of any of the four essential trace elements in
either patients with CLD-C or NASH.
Histological factors associated with
serum trace element levels
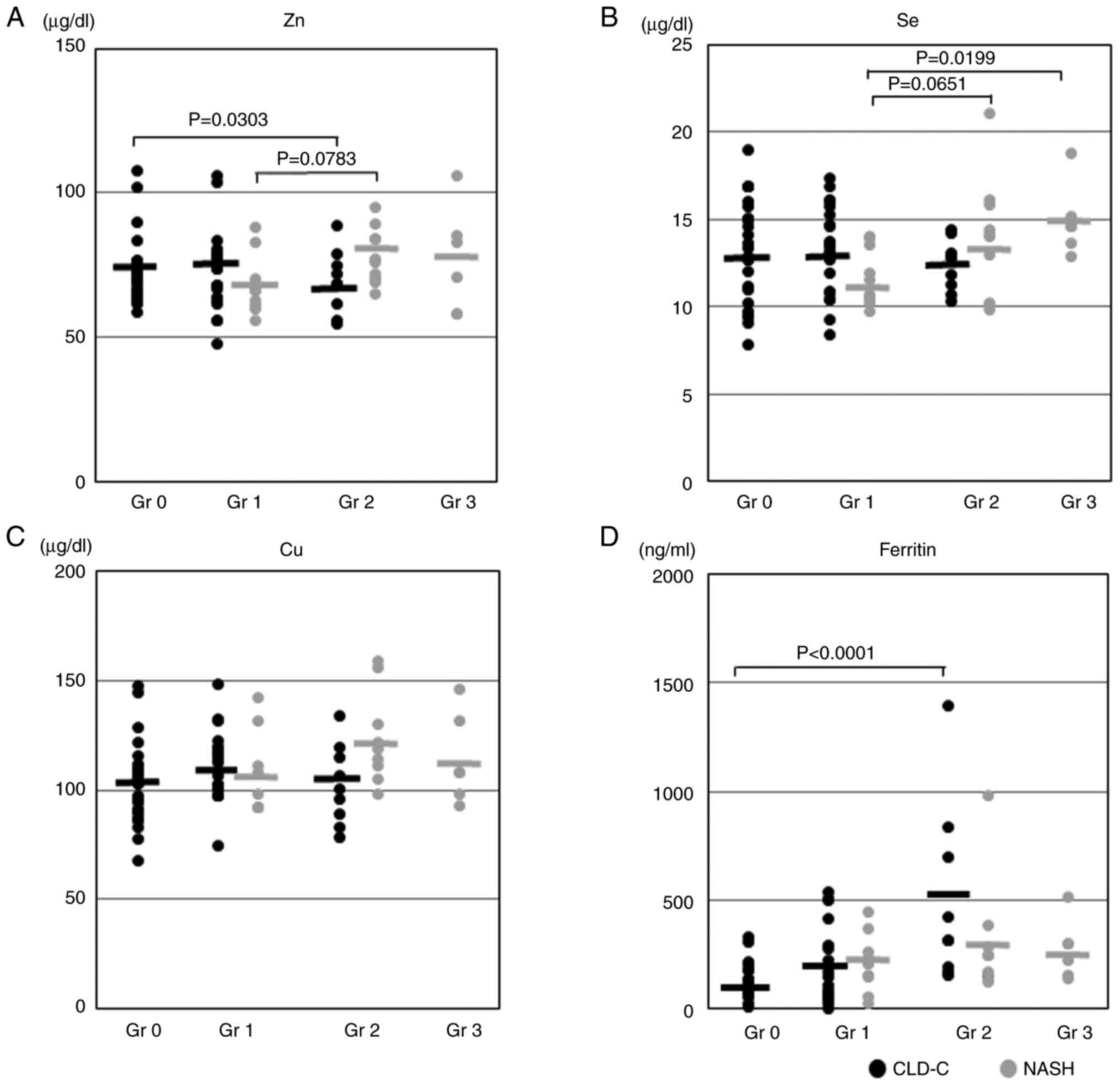
Subsequently, the present study investigated which
trace element affected hepatic steatosis in CLD-C and NASH
patients. As shown in Fig. 2A, the
serum Zn levels were significantly decreased as the grade of
hepatic steatosis became more severe in patients with CLD-C. The
serum Zn levels in patients with NASH and grade 2 steatosis tended
to be higher than those in patients with NASH and grade 1 steatosis
(78.1±9.6 vs. 68.1±10.1 µg/dl; P=0.0783). The serum Se levels in
patients with NASH and grade 3 steatosis were significantly higher
than those in patients with NASH and grade 1 steatosis (15.0±2.1
vs. 11.6±1.6 µg/dl; P=0.0199; Fig.
2B). The serum ferritin levels in patients with CLD-C were
significantly increased in proportion to the grade of hepatic
steatosis (Fig. 2D). However, no
significant association was identified between serum Cu levels and
the severity of hepatic steatosis in the NASH or CLD-C groups
(Fig. 2C).
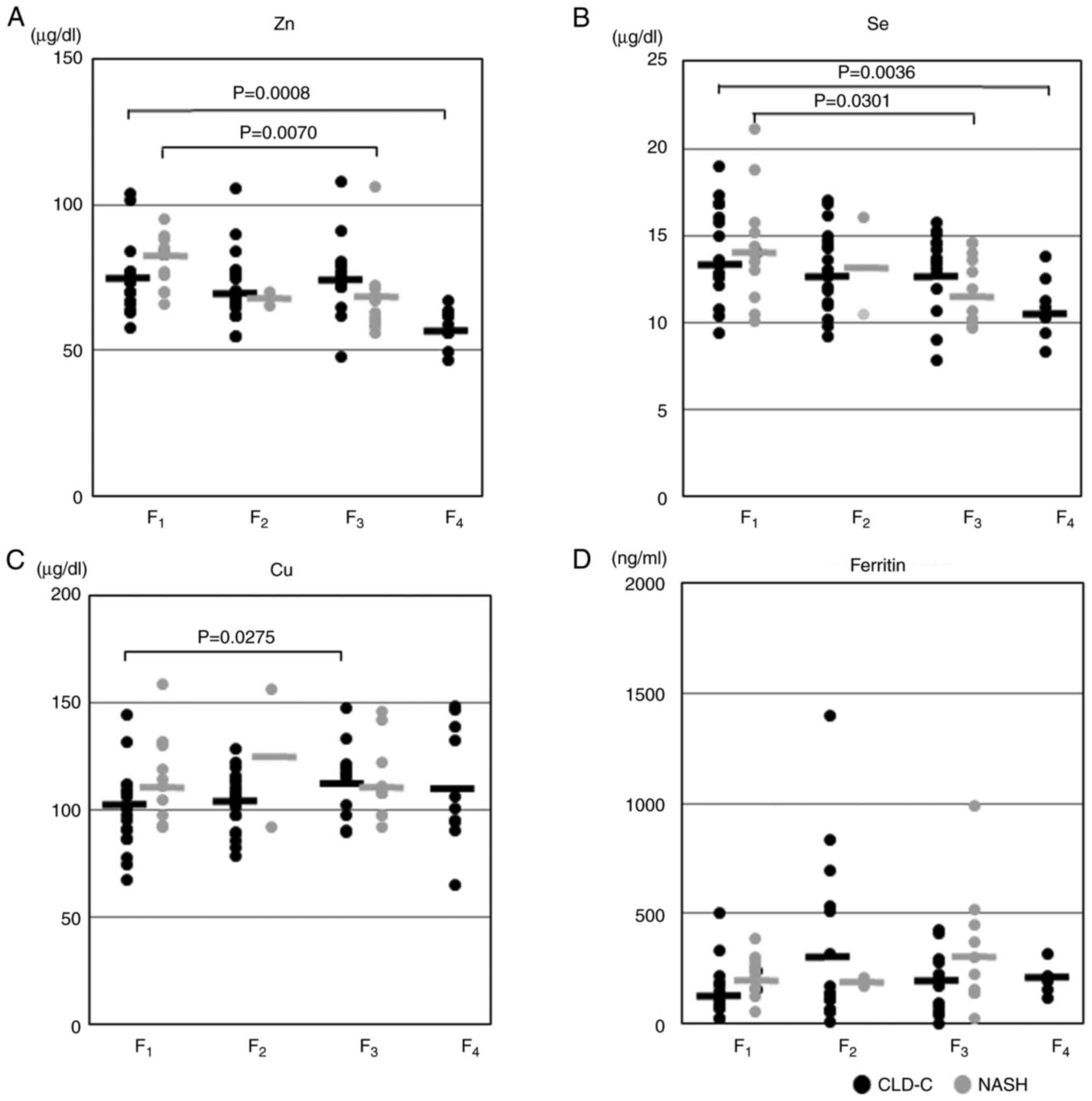
The association between serum trace element levels
and the stages of hepatic fibrosis were also assessed in patients
with CLD-C and NASH. In both groups, serum Zn levels were
significantly reduced as the stage of hepatic fibrosis progressed
(Fig. 3A). Similarly, serum Se
levels were decreased in proportion to the severity of hepatic
fibrosis in both groups (Fig. 3B).
Serum Cu levels in patients with CLD-C and F3 hepatic
fibrosis were significantly higher than those in patients with
CLD-C and F1 hepatic fibrosis (114.8±15.1 vs. 100.1±18.5
µg/dl; P=0.0275; Fig. 3C). By
contrast, no significant associations were observed between serum
ferritin levels and the severity of hepatic fibrosis in either
CLD-C or NASH groups (Fig.
3D).
Discussion
The results of the present study indicated that some
essential trace elements that contribute to hepatic inflammation,
hepatic fibrosis and hepatic reserve were common between patients
with CLD-C and patients with NASH. However, other essential trace
elements involved in hepatic steatosis and insulin resistance
differed between the CLD-C and NASH groups, although their average
levels were approximately equivalent in the two groups. To the best
of our knowledge, the present study is the first to compare the
involvement of these essential trace elements in the pathogenesis
of CLD-C with the pathogenesis of NASH.
It is well established that iron deposition in the
liver can initiate reactive oxygen species and subsequently lead to
hepatic inflammation in patients with CLD-C or NASH (26). The attenuation of iron overload by
phlebotomy thus results in improved serum transaminase levels in
such patients (27). The present
findings confirmed the strong correlation between serum ALT and
ferritin levels in patients with CLD-C and those with NASH.
Hypoalbuminemia derived from an unfavorable hepatic
reserve results in a relative increase in α2 macroglobulin, which
more strongly binds to Zn, eventually causing a substantial
increase in the urinary excretion of Zn (28). Thus, the present study suggests
that serum Zn levels are associated with serum Alb levels in
patients with CLD-C and NASH.
A decline in serum Se levels is frequently observed
in patients with decompensated liver cirrhosis (29). However, lower serum Se levels do
not indicate Se deficiency; rather, they imply an unfavorable
hepatic reserve in these patients (29). The present study revealed a
positive correlation between serum Se and Alb levels in both the
CLD-C and NASH groups, suggesting that lower serum Se levels
reflect an unfavorable hepatic reserve in these patients.
Our previous study revealed a close correlation
between serum ferritin levels and HOMA-IR values in patients with
CLD-C (9). The present study
demonstrated a positive correlation between serum ferritin levels
and HOMA-IR values in patients with NASH patients as well as those
with CLD-C. Iron is likely to affect hepatic insulin sensitivity.
It was thus hypothesized that the hepatic extraction and metabolism
of insulin may be attenuated as the deposition of iron in the liver
becomes more severe, leading to hyperinsulinemia in CLD-C and NASH
(30,31).
The present study revealed that serum Zn levels were
inversely correlated with HOMA-IR values in patients with CLD-C
(9). Zn has been demonstrated to
serve a crucial role in the stabilization of the insulin-like
growth factor-1 (IGF-1) transcript (32). Zn deficiency can lead to an
impairment of IGF-1 synthesis and subsequently an increase in
insulin release from β cells in patients with CLD-C (33). The present analyses also elucidated
an inverse correlation between serum Zn levels and HOMA-IR values
in patients with NASH, which supports the inverse correlation
between serum Zn levels and HOMA-IR values (10). The present study hypothesized that
the putative mechanism by which Zn deficiency causes insulin
resistance in patients with NASH is probably equivalent to that in
patients with CLD-C. Notably, several studies have revealed a
decrease in IGF-1 release in patients with NASH (34). The present study also confirmed
that a significant correlation existed between serum Zn and IGF-1
levels in patients with NASH (data not shown).
Our previous study revealed an inverse correlation
between serum Se concentrations and HOMA-IR values in patients with
CLD-C (8). Lower serum Se levels
may impair the activation of mitogen-activated protein kinase,
leading to insulin resistance in such patients (35). Unexpectedly, the present study did
not show a significant correlation between serum Se levels and
HOMA-IR values in patients with NASH. It is of interest that a high
prevalence of type 2 diabetes mellitus (T2DM) is observed in
individuals with relatively high levels of serum Se or relatively
low Se levels (36). This result
may explain the reason why we did not observe an inverse
correlation between serum Se concentrations and HOMA-IR values in
patients with NASH.
Selenoprotein P (SeP) has been suggested to serve an
important role in insulin resistance (37). However, there are conflicting
findings regarding circulating SeP levels in patients with NASH. A
previous study detected higher serum SeP levels in patients with
NASH compared with those in healthy controls or patients with NAFLD
(38), whereas another study
documented lower SeP levels in patients with NASH (39).
The grades of hepatic steatosis have been reported
to be increased as serum Zn levels decrease in patients with CLD-C
(9). Lower serum Zn levels may
cause the inactivation of peroxisome proliferator-activated
receptor-α, and a subsequent facilitation of lipid peroxidation in
such patients (40). Nevertheless,
the reason why no negative association was detected between the
grade of hepatic steatosis and serum Zn levels in the patients with
NASH in the present study remains uncertain. It is of interest that
in a previous study the administration of zinc gluconate did not
result in the improvement of hepatic steatosis in patients with
NAFLD (41).
The present findings also demonstrated that the
severity of hepatic steatosis was increased in proportion to serum
Se levels in patients with NASH. Spaur et al (42) reported that higher serum Se levels
were associated with the severity of hepatic steatosis in
participants of the National Health and Nutrition Examination
Survey. In db/db rats (an animal model of T2DM), long-term
supplementation with selenite, which is one of the inorganic
selenium compounds, has been shown to result in the exacerbation of
hepatic steatosis by reducing the antioxidant defense capacity,
despite the improvement in hyperglycemia (43). By contrast, Bonnefont-Rousselot
et al (44) documented that
serum Se levels were independent of the grade of hepatic steatosis
in patients with NAFLD. In an experimental animal model of NASH,
the administration of selenoneine, an organoselenium compound, has
been shown to alleviate the degree of hepatic steatosis (45). As aforementioned, the relationship
between serum Se levels and the degree of hepatic steatosis remains
controversial.
It is well established that a decrease in the
activity of collagenase derived from Zn deficiency results in the
progression to more advanced hepatic fibrosis (46). This explains why the stage of
hepatic fibrosis became more severe as serum Zn levels gradually
decreased in patients with CLD-C and NASH in the present study.
A previous report revealed that supplementation with
Se can inhibit the procollagen synthesis in an animal model of
hepatic fibrosis via a decrease in oxidative stress, and the
subsequent reduced collagen formation and enhanced collagen
degradation (47). These results
may indicate that a decline in serum Se levels can lead to the
development of hepatic fibrosis in patients with CLD-C (8) or NASH (11). Concerning the serum Se
concentrations in the patients with NASH in the present study, it
was observed that the serum Se levels were increased in proportion
to the degree of hepatic steatosis, and that the serum Se levels
were decreased in parallel with the degree of hepatic fibrosis.
In the present study, serum Cu levels were increased
as the stage of hepatic fibrosis was enhanced in patients with
CLD-C, whereas these levels did not affect the stage of hepatic
fibrosis in patients with NASH. Cu is likely to serve a pivotal
role in hepatic fibrosis as a cofactor (48). Nobil et al (14) revealed that serum Cu levels in
patients with NAFLD with more severe activity were significantly
lower compared with those in patients with NAFLD and no activity
(14). By contrast, a recent study
reported a strong correlation between higher serum Cu levels and
the risk of NAFLD in women (49),
although no significant difference in serum Cu concentrations was
found in the present study between male and female patients with
NASH. Lower serum ceruloplasmin levels may cause a high
susceptibility to oxidative stress in hepatocytes, eventually
leading to lower circulating Cu concentrations in patients with
NAFLD with severe activity. Aigner et al (13) also observed that hepatic Cu content
was lower in patients with NASH than in those with NAFLD because of
lower Cu availability in the patients with NASH.
Serum ferritin levels may predict the severity of
hepatic fibrosis in patients with NASH (12). Excessive iron activates hepatic
stellate cells by increasing α-smooth muscle actin, collagen and
transforming growth factor-β (22). However, this was not confirmed in
patients with CLD-C or NASH in the present study.
There are several study limitations to consider.
First, some of the results obtained did not reach statistical
significance due to the small sample size. Therefore, it was not
possible to analyze the data in the present study separately for
male and female patients. A large-scale study is thus required to
verify the present findings. Second, the present study could not
confirm whether the female patients were in menopause, thus bias
may have occurred with respect to serum ferritin levels. Third, the
correlation between dietary intake and the circulating levels of
each trace element was not investigated, and the results may be
biased because of imbalanced dietary intake of these trace
elements. Moreover, the correlations between serum Zn, Se, Cu or
ferritin levels and HCV-RNA loads were not assessed. However, our
previous study explored the correlation between serum Se levels and
HCV-RNA load and no significant correlation was found (8).
In conclusion, the involvement of iron in the
pathogenesis of hepatic inflammation was common to both patients
with CLD-C and those with NASH. In addition, lower serum Zn and Se
levels indicated an unfavorable hepatic reserve and advanced
hepatic fibrosis in both of these groups. However, the roles of Zn
and Se in the pathogenesis of hepatic steatosis and insulin
resistance were distinct between the CLD-C and NASH groups. Further
studies are required to clarify the mechanisms by which disordered
essential trace element metabolism may evoke hepatic steatosis and
insulin resistance in patients with NASH.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH and SK designed the study. KF, SM, JT, AM and TM
collected the samples and supported the study techniques. TH
performed data analyses and wrote the original draft. TM edited the
original draft. TM and AM confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committees of both the Kagawa Prefectural University of Health
Sciences (approval no. 305) and Kagawa University Faculty of
Medicine (approval no. 2020-045). Written informed consent was
obtained from each individual.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nordberg M and Nordberg GF: Trace element
research-historical and future aspects. J Trace Elem Med Biol.
38:46–52. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kozeniecki M, Ludke R, Kerner J and
Patterson B: Micronutrients in liver disease: Roles, risk factors
for deficiency and recommendation for supplementation. Nutr Clin
Pract. 35:50–62. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Himoto T and Masaki T: Current trends of
essential trace elements in patients with chronic liver diseases.
Nutrients. 12(2084)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Khan RS, Bril F, Cusi K and Newsome PN:
Modulation of insulin resistance in nonalcoholic fatty liver
disease. Hepatology. 70:711–724. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dubey P, Thakur V and Chattopadhyay M:
Role of minerals and trace elements in diabetes and insulin
resistance. Nutrients. 12(1864)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Moriyama M, Matsumura H, Fukushima A,
Ohkido K and Arakawa Y, Nirei K, Yamagami H, Kaneko M, Tanaka N and
Arakawa Y: Clinical significance of evaluation of serum zinc
concentrations in C-viral chronic liver disease. Dig Dis Sci.
51:1967–1977. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Himoto T, Hosomi N, Nakai S, Deguchi A,
Kinekawa F, Matsuki M, Yachida M, Masaki T, Kurokochi K, Watanabe
S, et al: Efficacy of zinc administration in patients with
hepatitis C virus-related chronic liver disease. Scand J
Gastroenterol. 42:1078–1087. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Himoto T, Yoneyama H, Kurokohchi K, Inukai
M, Masugata H, Goda F, Haba R, Watanabe S, Kubota S, Senda S and
Masaki T: Selenium deficiency is associated with insulin resistance
in patients with hepatitis C virus-related chronic liver disease.
Nutr Res. 31:829–835. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Himoto T, Nomura T, Tani J, Miyoshi H,
Morishita A, Yoneyama H, Haba R, Masugata H and Masaki T:
Exacerbation of insulin resistance and hepatic steatosis deriving
from zinc deficiency in patients with HCV-related chronic liver
disease. Biol Trace Elem Res. 163:81–88. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ito T, Ishigami M, Ishizu Y, Kuzuya T,
Honda T, Ishikawa T, Toyoda H, Kumada T and Fujishiro M:
Correlation of serum zinc levels with pathological and laboratory
findings in patients with nonalcoholic fatty liver disease. Eur J
Gastroenterol Hepatol. 32:748–753. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Reja M, Makar M, Visaria A, Marino D and
Rustgi V: Increased serum selenium levels are associated with
reduced risk of advanced liver fibrosis and all-cause mortality in
NAFLD patients: National Health and Nutrition Examination Survey
(NHANES) III. Ann Hepatol. 19:635–640. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Buzzetti E, Petta S, Manuguerra R, Luong
TV, Cabibi D, Corradini E, Craxi A, Pinzani M, Tsochatzis E and
Pietrangelo A: Evaluating the association of serum ferritin and
hepatic iron with disease severity in non-alcoholic fatty liver
disease. Liver Int. 39:1325–1334. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aigner E, Strasser M, Haufe H, Sonnweber
T, Hohia F, Stadimayr A, Solioz M, Tilg H, Patsch W, Weiss G, et
al: A role for low hepatic copper concentrations in nonalcoholic
fatty liver disease. Am J Gastroenterol. 105:1978–1985.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nobili V, Siotto M, Bedogni G, Rava L,
Pietrobattista A, Panera N, Alisi A and Squitti R: Levels of serum
seruloplasmin associated with pediatric nonalcoholic fatty liver
disease. J Pediatr Gastroenterol Nutr. 56:370–375. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
García-Monzón C, Lo lacono O, Mayoral R,
González-Rodrínguez A, Miqulena-Colina ME, Lozano-Rodríguez T,
García-Pozo L, Vargas-Castrillón J, Casado M, Boscá L, et al:
Hepatic insulin resistance is associated with increased apoptosis
and fibrogenesis in nonalcoholic steatohepatitis and chronic
hepatitis C. J Hepatol. 54:142–152. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Milic S, Mikolasevic I, Orlic L, Devcic E,
Starcevic-Cizmarevic N, Stimac D, Kapovic M and Ristic S: The role
of iron and iron overload in chronic liver disease. Med Sci Monit.
22:2144–2151. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Matteoni CA, Younossi ZM, Gramlich T,
Boparai N, Liu YC and McCullough AJ: Nonalcoholic fatty liver
disease: A spectrum of clinical and pathological severity.
Gastroenterology. 116:1413–1419. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sandres-Sauné K, Abravanel F, Nicot F,
Peron JM, Alric L, Boineau J, Pasquier C and Izopet J: Detection
and quantitationof HCV RNA using real-time PCV after automated
sample processing. J Med Virol. 79:1821–1826. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sunderman FW Jr: Atomic absorption
spectrometry of trace metals in clinical pathology. Hum Pathol.
4:549–582. 1973.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kodama H, Tanaka M, Naito Y, Katayama K
and Moriyama M: Japan's practical guidelines for zinc deficiency
with a particular focus on taste disorders, inflammatory bowel
disease, and liver cirrhosis. Int J Mol Sci.
21(2941)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kodama H, Asagiri K, Ida S, Etani Y,
Koyama H, Soh H, Tanaka Y, Takayanagi M, Funakoshi M and Yoshida M:
Diagnosis and treatment of selenium deficiency. J Jpn Sci Clin
Nutr. 40:239–283. 2015.
|
|
22
|
Mehta KJ, Farnaud SJ and Sharp PA: Iron
and liver fibrosis: Mechanistic and clinical aspects World J.
Gastroenterol. 25:521–538. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cardiff RD, Miller CH and Munn RJ: Manual
hematoxylin and eosin staining of mouse tissue sections. Cold
Spring Harb Protc. 2014:655–658. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ichida F, Tsuji T, Omata M, Ichida T,
Inoue K, Kamimura T, Yamada G, Hino K, Yokosuka O and Suzuki H: New
Inuyama classification: New criteria for histological assessment of
chronic hepatitis. Int Hepatol Commun. 6:112–119. 1996.
|
|
25
|
Brunt EM, Janey CG, Di Bisceglie AM,
Neuschwander-Tetri BA and Bacon BR: Nonalcoholic steatohepatitis: A
proposal for grading and staging the histological lesions. Am J
Gastroenterol. 94:2467–2474. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kohgo Y, Ikuta K, Ohtake T, Torimoto Y and
Kato J: Iron overload and cofactors with special reference to
alcohol, hepatitis C virus infection and steatosis/insulin
resistance. World J Gastroenterol. 13:4699–4706. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Valenti L, Fracanzari AL, Dongiovanni P,
Rovida S, Rametta R, Fatta E, Pulixi EA, Maggioni M and Fargion S:
A randomized trial of iron depletion in patients with nonalcoholic
fatty liver disease and hyperferritinemia. World J Gastroenterol.
20:3002–3010. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Himoto T and Masaki T: Associations
between zinc deficiency and metabolic abnormalities in patients
with chronic liver disease. Nutrients. 10(88)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Burk RF, Early DS, Hill KE, Palmer IS and
Boeglin ME: Plasma selenium in patients with cirrhosis. Hepatology.
27:794–798. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Frenández-Real JM, López-Bermejo A and
Ricart W: Cross-talk between iron metabolism and diabetes.
Diabetes. 51:2348–2354. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fernandez M, Lokan J, Leung C and Grigg A:
A critical evaluation of the roles of iron overload in fatty liver
disease. J Gastroenterol Hepatol. 37:1873–1883. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
McNall AD, Etherton TD and Fosmire GJ: The
impaired growth induced by zinc deficiency in rats is associated
with decreased expression of hepatic insulin-like growth factor I
and growth hormone receptor genes. J Nutr. 125:874–879.
1995.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Himoto T, Tani J, Miyoshi H, Yoneyama H,
Mori H, Inukai M, Masugata H, Goda F, Senda S, Haba R and Masaki T:
The ratio of insulin-like growth factor/insulin-like growth
factor-binding protein-3 in sera of patients with hepatitis C
virus-related chronic liver disease as a predictive marker of
insulin resistance. Nutr Res. 33:27–33. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yao Y, Miao X, Zhu D, Li D, Zhang Y, Song
C and Liu K: Insulin-like growth factor-1 and non-alcoholic fatty
liver disease: A systemic review and-meta-analysis. Endocrine.
65:227–237. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Steinbrenner H: Interference of selenium
and selenoproteins with insulin-regulated carbohydrate and lipid
metabolism. Free Radic Biol Med. 65:1538–1547. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang XL, Yang TB, Wei J, Lei GH and Zeng
C: Association between serum selenium level and type 2 diabetes
mellitus: A non-linear dose-response meta-analysis of observational
studies. Nutr J. 15(48)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Polyzos SA, Kountouras J, Goulas A and
Duntas L: Selenium and selenoprotein P in nonalcoholic fatty liver
disease. Hormone (Athens). 19:61–72. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cetindagli I, Kara M, Tanoglu A, Ozalper
V, Aribal S, Hancerli Y, Unal M, Ozarı O, Hira S, Kaplan M and
Yazgan Y: Evaluation of endothelial dysfunction in patients with
nonalcoholic fatty liver disease: Association of selenoprotein P
with carotid intima-media thickness and endothelium-dependent
vasodilation. Clin Res Hepatol Gastroenterol. 41:516–524.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Polyzos SA, Kountouras J, Mavrouli M,
Katsinelos P, Doulberis M, Gavana E and Duntas L: Selenoprotein P
in patients with nonalcoholic fatty liver disease. Exp Clin
Endocrinol Diabetes. 127:598–602. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bao B, Prasad AS, Beck FW, Fitzgerald JT,
Snell D, Bao GW, Singh T and Cardozo LJ: Zinc decreases C-reactive
protein, lipid peroxidation, and inflammatory cytokines in elderly
subjects: A potential implication of zinc as an atheroprotective
agent. Am J Clin Nutr. 91:1634–1641. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fathi M, Alavinejad P, Haidari Z and Amani
R: The effect of zinc supplementation on steatosis severity and
liver function enzymes in overweight/obese patients with mild to
moderate non-alcoholic fatty liver following calorie-restricted
diet: A double-blind, randomized placebo-controlled trial. Biol
Trace Elem Res. 197:394–404. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Spaur M, Nigra AE, Sanchez TR, Navas-Acien
A, Lazo M and Wu HC: Association of blood manganese, selenium with
steatosis, fibrosis in the National Health and Nutrition
Examination Survey, 2017-2018. Environ Res.
213(113647)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang C, Yang S, Zhang N, Mu Y, Ren H, Wang
Y and Li K: Long-term supranutritional supplementation with
selenate decreases hyperglycemia and promotes fatty liver
degeneration by inducing hyperinsulinemia in diabetic db/db mice.
PLoS One. 9(e101315)2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bonnefont-Rousselot D, Ratziu V, Giral P,
Charlotte F, Beuclear I and Poynard T: Lido Study Group. Blood
oxidative stress markers are unreliable markers of hepatic
steatosis. Aliment Pharmacol Ther. 23:91–98. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Miyata M, Matsushita K, Shindo R,
Shimokawa Y, Sugiura Y and Yamashita M: Selenoneine ameliorates
hepatocellular injury and hepatic steatosis in a mouse model of
NAFLD. Nutrients. 12(1898)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Consolo M, Amoroso A, Spandidos DA and
Mazzarino MC: Matrix metalloproteinases and their inhibitors as
markers of inflammation and fibrosis in chronic liver disease
(Review). Int J Mol Med. 24:143–152. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ding M, Potter JJ, Liu X, Torbenson MS and
Mezey E: Selenium supplementation decreases hepatic fibrosis in
mice after chronic carbon tetrachloride administration. Biol Trace
Elem Res. 133:83–97. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Arain SA, Kazi TG, Afridi HI, Talpur FN,
Mughal MA, Shah F, Arian SS and Panhwar AH: Estimation of copper
and iron burden in biological samples of various stages of
hepatitis C and liver cirrhosis patients. Biol Trace Elem Res.
160:197–205. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen C, Zhou Q, Yang R, Wu Z, Yuan H,
Zhang N, Zhi M, Zhang Y, Ni X, Wang Z, et al: Copper exposure
association with prevalence of non-alcoholic fatty liver disease
and insulin resistance among US adults (NHANES 2011-2014).
Ecotoxicol Environ Saf. 218(112295)2021.PubMed/NCBI View Article : Google Scholar
|