Introduction
Toxic epidermal necrolysis (TEN) is one of the few
acute and severe allergic diseases in dermatology. The incidence
rate is low (1.58-2.26 cases/million individuals), but the
mortality rate can reach ~61% (1).
Drugs are the most common pathogenic factors for this condition.
Certain antibiotics, such as sulfonamide and penicillin, as well as
some psychotropic drugs, such as barbiturates and phenytoin, have
been occasionally reported to cause TEN (2). However, the pathogenic factors of TEN
do not only include drugs, but also other factors. For example,
ultraviolet radiation (UVR), which everyone is exposed to in daily
life, but which can also trigger TEN in the form of
photodistributed TEN (3).
The onset of TEN exhibits certain typical features,
with local erythema of the body at the initial stage, accompanied
by pain. After 1 to 2 weeks, the disease can rapidly develop from
erythema to bullous skin lesions, after which peeling skin tissues
can appear. During patient care, external forces (such as rubbing
or squeezing) can cause patches of bullous skin to peel off (which
is known as the Nikolsky sign), and the peeled wound is similar to
a deep second-degree scald. The skin-stripping area is an important
index for evaluating TEN. According to the percentage of
skin-stripping area compared with the whole-body surface area, the
disease is referred to by different names (4). A ratio <10% is referred to as
Stevens-Johnson syndrome (SJS), a ratio >30% is referred to as
TEN and a ratio >10% but <30% is referred to as overlapping
SJS and TEN (5). In addition to
skin symptoms, there can also be systemic symptoms, such as fever,
fatigue, chills and muscle soreness. Exfoliation can also occur in
other parts of the mucosa, such as the eyes, lips and external
genitalia. If the disorder is combined with the exfoliation of
other parts of the mucosa, it indicates that the condition has
become notably serious. If the disorder is not properly treated,
patients are prone to electrolyte disturbances and can even die due
to the dysfunction of multiple organs (6). The aim of the present study was to
highlight the fact that physicians should be alert to the
possibility of the development of erythema multiforme into TEN when
patients are treated with multiple drugs. The study summarizes the
treatment methods and experiences of patients with TEN both
domestically (China) and internationally, providing reference for
the diagnosis and treatment of TEN in the future.
Case report
A 58-year-old male patient was hospitalized in
Shaoxing People's Hospital (Shaoxing, China) in April 2022 for
treatment due to haematemesis and tarry stool for 1 day.
Preliminary diagnoses included the following: i) Oesophageal and
gastric variceal bleeding; ii) chronic hepatitis B; iii) malignant
liver tumour; iv) portal hypertension; v) portal vein embolism; vi)
anaemia; vii) ascites; and viii) spleen enlargement. The basis for
this diagnosis was a previous history of hepatitis B, cirrhosis and
gastrointestinal bleeding, and end-stage chronic liver disease. A
physical examination revealed an anemic facial appearance and
abdominal wall varicose veins. Palpation revealed splenomegaly with
positive results for ascites. The main laboratory tests revealed
the following results: Red blood cell count, 2.67x1012/l
(normal, 4.5-5.5x1012/l); hemoglobin level, 82 g/l
(normal, 120-160 g/l); hepatitis B virus surface antigen,
>250.00 IU/ml (normal, <0.05 IU/ml); hepatitis B virus e
antibody, 0.89 PEIU/ml (normal, 0-0.2 PEIU/ml); hepatitis B virus
core antibody, 12.84 PEIU/ml (normal, <0.9 PEIU/ml); total
protein, 58 g/l (normal, 60-80 g/l); and albumin, 29.6 g/l (normal,
35-50 g/l). A routine ultrasound examination revealed liver
cirrhosis and a liver mass, portal vein widening with thrombosis,
liver and spleen enlargement, gallbladder wall oedema and a large
amount of ascites. After admission, the patient was treated with
Losec® (40 mg, twice a day, intravenous) for gastric
acid inhibition, Stilamin® (3 mg, every 8 h,
intravenous) and terlipressin (1 mg, every 6 h intravenous) for
increased blood pressure, furosemide (20 mg, once a day at 10 am,
intravenous) for diuresis and polyene phosphatidylcholine (456 mg,
once a day at 10 am, intravenous) for liver protection and
rehydration.
Diagnosis and treatment
In April 2022, on the 6th day after hospitalization,
the patient underwent endoscopic surgery with the use of a venous
ligation ring, sclerosing agent (10 ml lauromacrogol injection; the
main ingredient is 100 mg lauromacrogol, while other ingredients
include ethanol and sterile water for injection) and medical
adhesive (2 ml COMPONT, Beijing COMPONT Medical Equipment Co., Ltd;
the main component is α-N-butyl cyanoac rylate, and other
components include small amounts of stabilizers, such as
hydroquinone, toluene yellow acid and sulfur dioxide). On the
second day after endoscopic surgery, the patient developed a
scattered rash with itching; therefore, the patient was advised to
stop using Stilamin and instead use octreotide injections.
Dexamethasone (10 mg, once a day at 8 am, intravenous) was used for
relieve the skin allergy symptoms.
After 3 days of treatment with dexamethasone (10 mg,
once a day at 8 am, intravenous), the number of rashes increased.
The patient experienced a burning and pain sensation on the lips
and conjunctiva, and in addition, oedematous skin erythema, loose
epidermis and a bullous appearance was observed. Increased
secretion was observed in the corners of the eyes. Dermatologists
diagnosed erythema multiforme based on the aforementioned symptoms.
The possibility of drug allergies was considered, but the patient
denied a history of antipyretic and analgesic drugs,
anti-inflammatory drugs, new drugs or sensitizing drugs within 1
month before admission. Subsequently, it was found that
dexamethasone had a poor therapeutic effect on the skin symptoms.
The rash symptoms were not effectively treated and gradually
worsened. The doctor's assessment indicated that the patient needed
to use steroids for a longer period, and the dosage of steroids
used was high. Therefore, dexamethasone treatment was stopped and
changed to treatment with methylprednisolone (60 mg, once a day at
8 am, intravenous). As methylprednisolone has minimal side effects
on the digestive system and liver function (7), it was hypothesised that using
methylprednisolone would be more effective for treating the
patient.
On the thirteenth day after endoscopic surgery, a
large number of blisters appeared on the patient's back, and some
of the blisters fused. The overall area of skin lesions had
increased compared with previously. Furthermore, part of the skin
became exfoliated, the wound was eroded (Fig. 1A-C), and Nissl's sign was positive.
The laboratory examination results were as follows: IgG, 24.85 g/l
(normal, 7-16.6 g/l); and IgA, 0.3 g/l (normal, 0.7-4 g/l). Other
immunoglobulin levels were generally normal. In addition, the
blisters on the lips of the patient partially ruptured and expanded
to the surrounding area, with epidermal erosion and bloody
exudation. The pain was more severe compared with before, and some
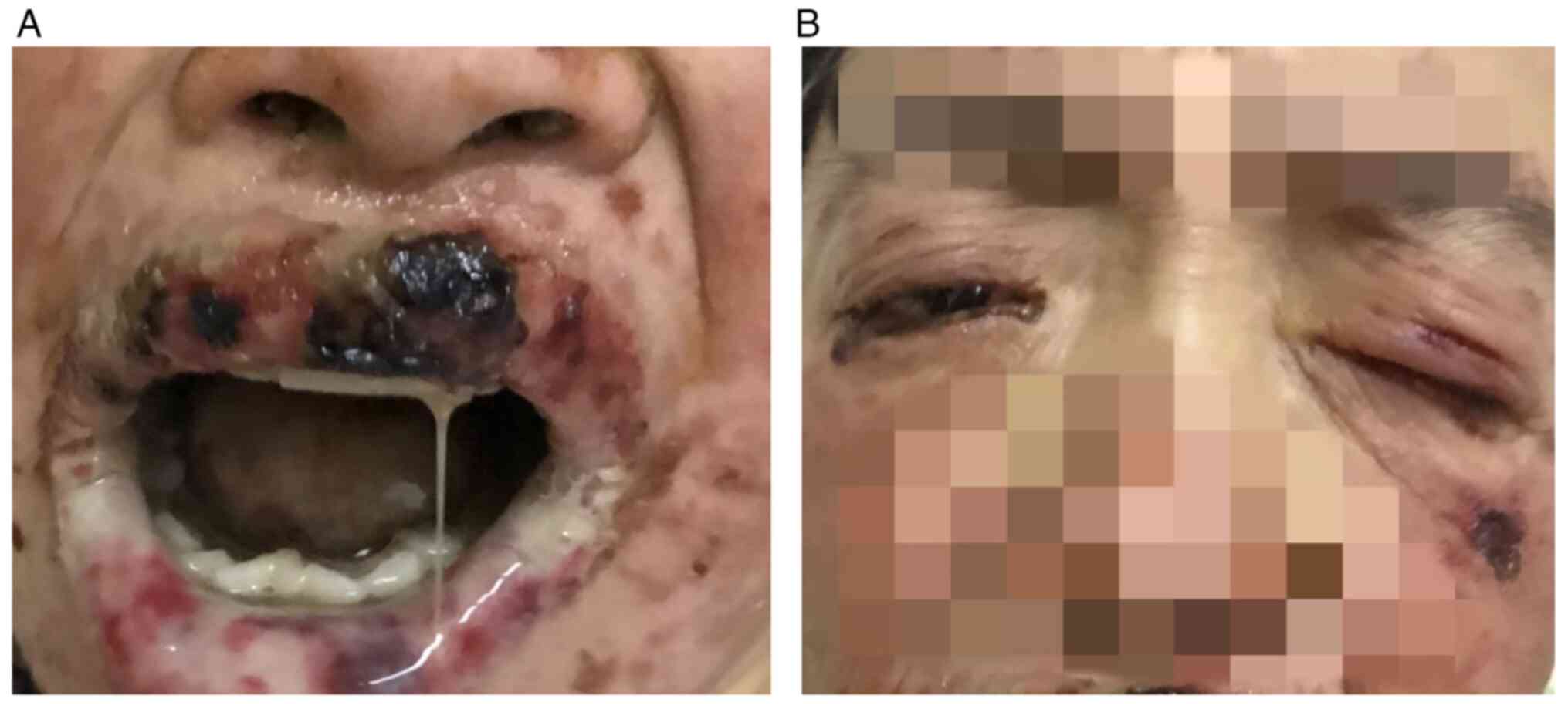
skin lesions were covered with a grey-white pseudomembrane
(Fig. 2A). Pain and swelling in
both eyes, conjunctival congestion, increased discharge from the
corners of the eyes and difficulty opening the eyes were observed
(Fig. 2B). Combined with the
aforementioned symptoms, the revised diagnosis was TEN. When
considering that patients with upper gastrointestinal bleeding
should not use an excessive dose of corticosteroids, according to
the recommendations of the Guideline for Primary Care of Drug
Eruption (2022) (8), γ globulin
(20 g, qd8, intravenous) and methylprednisolone (100 mg, qd8,
intravenous) were administered. The gastrointestinal bleeding of
the patient improved, and the patient could tolerate oral
medication. Therefore, a new generation proton pump inhibitor,
rabeprazole sodium enteric-coated tablets (20 mg, once a day in the
morning, oral), with greater efficacy, was used instead. The
patient's disease had now progressed to TEN, so the risk factors of
TEN should be reduced as much as possible. Therefore, the use of
omeprazole sodium (Losec®) and octreotide injection for
treatment was discontinued.
After 4 days, the rash had still not obviously
improved, and the erosion and exfoliation of the trunk skin were
worse compared with before (with obvious pain). Adalimumab (40 mg,
once, subcutaneous) was used for treatment, and Kangfu New
Liquid-soaked gauze (Innermongolia Jingxin Pharmaceutical Co., Ltd)
was applied on the wound (Fig.
3A), and erythromycin ointment to resist mucosal infections.
After 2 days, the rash had improved. On the 4th day after treatment
with adalimumab, the wound was dry, and part of the scab skin fell
off (Fig. 3B). After 10 days, the
rash had generally recovered (Fig.
3C). At the time of writing this case report, there have been
no signs of recurrence of the patient's rash.
Discussion
TEN is a type of acute and severe allergic
autoimmune skin reaction. It can also be caused by a number of
other factors, but the majority of them involve drugs. The research
on its pathogenesis has not reached a unified consensus (9). Based on current international medical
research, HLA genes can be closely related to drug-induced TEN
(10). Mutation of the cytochrome
P450 gene can lead to a slower metabolism of related drugs (such as
diazepam, warfarin and phenytoin), thereby increasing the risk of
TEN (11). Certain infectious
factors, such as human herpesvirus, Epstein-Barr virus and
Mycoplasma pneumoniae, are also associated with the onset of
TEN. Autoimmune diseases and active malignant tumours are also
potential factors for the onset of TEN (12).
The treatment of TEN should be considered from
multiple aspects, and multiple methods of combined treatment should
be started as soon as possible (12). It is necessary to avoid the
presence of pathogenic factors, reduce the clinical symptoms of
patients and provide supportive treatment for preventing secondary
internal organ failure (13).
Discontinuation is required of the drugs that the patient is
currently using that may cause TEN. Use of medications with adverse
reactions, such as rashes or skin allergies, should be avoided as
much as possible. Garcia-Doval et al (14) reviewed the clinical data of 113
patients with SJS or TEN and revealed that early cessation of
allergenic factors (drugs) can significantly reduce patient
mortality. In addition, supportive treatment for TEN should be
provided (15), including
maintaining the fluid and electrolyte balance of the patient
(16), symptomatic management of
skin lesions and mucous membranes and prevention and control of
infection (17). Appropriate
medication is actively used for treatment, including
glucocorticoids (18),
immunosuppressants (19,20), traditional Chinese medicine
(21,22), human immunoglobulins (23-25),
cyclosporine (26),
cyclophosphamide and thalidomide, among others. The main function
of these treatment plans is to maintain the balance of vital signs
in the body, improve the body's own immunity, reduce other harmful
factors and accelerate the skin healing ability. By using these
comprehensive treatments, the patient's disease can be effectively
alleviated. Han et al (27)
reported the use of plasma exchange to treat patients with TEN, but
Clark and Huang (28) suggested
that using plasma exchange alone to treat TEN still has certain
shortcomings. Although plasma exchange can remove some toxins from
the blood, it may also consume coagulation factors, increase the
risk of bleeding and lead to electrolyte deficiency (such as
hypocalcemia). After plasma exchange, the body's immune system is
still very poor, which is not conducive to recovery from the
disease (28).
The patient in the present case suffered from both
acute gastrointestinal bleeding and end-stage chronic liver
disease. After hospitalization, a number of types of drugs were
used, so the root cause of TEN is relatively complex. The patient
developed a rash on the second day after endoscopic surgery (and
also the second day after the use of sclerosing agent and medical
adhesive), therefore the timing of the occurrence was quite
important. According to the relevant literature (29,30)
and combined with the characteristics of the present case, the time
of the skin allergic reaction after using omeprazole sodium for
injection (Losec), somatostatin for injection (Stilamin) or
terlipressin is inconsistent with the actual time the skin rash
developed in the present case. Therefore, the present study focused
on the hypothesis that the predisposing factors of TEN were related
to the use of medical adhesives or the sclerosing agent.
The rash of the present patient was initially
considered to be erythema multiforme, which is usually associated
with infection (31). The typical
skin pathology in the initial stage mainly involves erythema with
different degrees of target lesions (or iris lesions), which is a
skin disease related to acute onset and inflammation that is
usually accompanied by mucosal damage and can heal itself; however,
it can easily recur, and its mortality rate is low (32,33).
As demonstrated by the experience of diagnosis and treatment in the
present case, if erythema multiforme appears in patients with
complicated medication usage, it is necessary to examine whether
this may constitute an important precursor of SJS or TEN.
The current patient developed a rash after 2 days of
digestive endoscopy and rapidly developed TEN, which, to the best
of our knowledge, is rarely reported. In addition, life support
treatments (such as supplement of effective circulating blood
volume, desensitization with glucocorticoids, wet compresses with
Kangfu New Liquid-soaked gauze, erythromycin ointment to resist
mucosal infections, intravenous injections of human γ globulin to
enhance the disease resistance of the body and treatment with
adalimumab, which is a TNF antagonist) can have beneficial effects.
Adalimumab can be used to treat rheumatoid arthritis and ankylosing
spondylitis and improve symptoms, such as joint stiffness, pain,
redness, swelling and deformation. Considering that adalimumab can
exert an antagonistic effect on TNF, it was also suitable for the
present patient (34). Compared
with the anti-inflammatory effects of glucocorticoids such as
dexamethasone and methylprednisolone, adalimumab plays a regulatory
role in the biological response induced or regulated by TNF,
thereby regulating immune suppression (35). Therefore, on the basis of
comprehensive treatment, adalimumab exerts specific advantages,
which results in the skin symptoms of patients becoming benign. In
addition, if patients are initially treated with adalimumab when
their skin exhibits erythema multiforme, it is unclear whether the
development of TEN can be controlled. In future clinical practice,
the identification of valuable cases for in-depth study is
necessary to obtain new insights into the development of TEN.
Acknowledgements
Not applicable.
Funding
Funding: This study was financially supported by grants from the
China Postdoctoral Science Foundation (grant no. 2023M733164) and
the Medicine and Health Science and Technology Plan of Zhejiang
Provincial Health Commission (grant no. 2023RC306).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LH and WC wrote the draft of the manuscript. WC and
ZZ obtained the clinical data and conceived the methodology. XG, QL
and HC performed the research and analyzed the data. LH and ZZ made
substantial contributions to conception and design, acquisition,
analysis and interpretation of data, and writing/editing/revising
the manuscript. YZ wrote and reviewed the manuscript. YZ and JD
contributed to the conception and design of the study and confirmed
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient who participated in the study provided
written informed consent for the publication of any associated
data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kinoshita Y and Saeki H: A review of toxic
epidermal necrolysis management in Japan. Allergol Int. 66:36–41.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ordoñez L, Salgueiro E, Jimeno FJ and
Manso G: Spontaneous reporting of Stevens-Johnson syndrome and
toxic epidermal necrolysis associated with antiepileptic drugs. Eur
Rev Med Pharmacol. 19:2732–2737. 2015.PubMed/NCBI
|
|
3
|
Gaghan LJ, Coates MM, Crouse LN, Miedema
J, Mervak JE and Ziemer CM: Photodistributed toxic epidermal
necrolysis: Case report and review of current literature. JAMA
Dermatol. 158:787–790. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Maity S, Banerjee I, Sinha R, Jha H, Ghosh
P and Mustafi S: Nikolsky's sign: A pathognomic boon. J Family Med
Prim Care. 9:526–530. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lerch M, Mainetti C, Terziroli
Beretta-Piccoli B and Harr T: Current perspectives on
stevens-johnson syndrome and toxic epidermal necrolysis. Clin Rev
Allergy Immunol. 54:147–176. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen CB, Abe R, Pan RY, Wang CW, Hung SI,
Tsai YG and Chung WH: An updated review of the molecular mechanisms
in drug hypersensitivity. J Immunol Res.
2018(6431694)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Torrelo A: Methylprednisolone aceponate
for atopic dermatitis. Int J Dermatol. 56:691–697. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chinese Medical Association, Chinese
Medical Journals Publishing House, Chinese Society of Dermatology,
Chinese Society of General Practice, Editorial Board of Chinese
Journal of General Practitioners of Chinese Medical Association,
Expert Group of Guidelines for Primary Care of Skin and Venereal
Disease. Guideline for primary care of drug eruption (2022). Chin J
Gen Pract. 21:804–813. 2022.(In Chinese).
|
|
9
|
Wilkerson RG: Drug hypersensitivity
reactions. Emerg Med Clin North Am. 40:39–55. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ueta M: Susceptibility genes and HLA for
cold medicine-related SJS/TEN with SOC. Front Genet.
13(912478)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tassaneeyakul W, Prabmeechai N, Sukasem C,
Kongpan T, Konyoung P, Chumworathayi P, Tiamkao S, Khunarkornsiri
U, Kulkantrakorn K, Saksit N, et al: Associations between HLA class
I and cytochrome P450 2C9 genetic polymorphisms and
phenytoin-related severe cutaneous adverse reactions in a Thai
population. Pharmacogenet Genomics. 26:225–234. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Grünwald P, Mockenhaupt M, Panzer R and
Emmert S: Erythema multiforme, Stevens-Johnson syndrome/toxic
epidermal necrolysis-diagnosis and treatment. J Dtsch Dermatol Ges.
18:547–553. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schneider JA and Cohen PR: Stevens-Johnson
Syndrome and toxic epidermal necrolysis: A concise review with a
comprehensive summary of therapeutic interventions emphasizing
supportive measures. Adv Ther. 34:1235–1244. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Garcia-Doval I, LeCleach L, Bocquet H,
Otero XL and Roujeau JC: Toxic epidermal necrolysis and
Stevens-Johnson syndrome: Does early withdrawal of causative drugs
decrease the risk of death? Arch Dermatol. 136:323–327.
2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rizzo JA, Johnson R and Cartie RJ:
Pediatric toxic epidermal necrolysis: Experience of a Tertiary Burn
Center. Pediatr Dermatol. 32:704–709. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Noe MH and Micheletti RG: Diagnosis and
management of Stevens-Johnson syndrome/toxic epidermal necrolysis.
Clin Dermatol. 38:607–612. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jacobsen A, Olabi B, Langley A, Beecker J,
Mutter E, Shelley A, Worley B, Ramsay T, Saavedra A, Parker R, et
al: Systemic interventions for treatment of Stevens-Johnson
syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN
overlap syndrome. Cochrane Database Syst Rev.
3(CD013130)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zimmermann S, Sekula P, Venhoff M,
Motschall E, Knaus J, Schumacher M and Mockenhaupt M: Systemic
immunomodulating therapies for stevens-johnson syndrome and toxic
epidermal necrolysis: A systematic review and meta-analysis. JAMA
Dermatol. 153:514–522. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chafranska L, Saunte DM, Behrendt N,
Nygaard U, Christensen RJ, Sand C and Jemec GB: Pediatric toxic
epidermal necrolysis treated successfully with infliximab. Pediatr
Dermatol. 36:342–345. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Da W, Zhu J, Wang L and Lu Y: Adalimumab
for Crohn's disease after infliximab treatment failure: A
systematic review. Eur J Gastroenterol Hepatol. 25:885–891.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zeng C, Liao Q, Hu Y, Shen Y, Geng F and
Chen L: The role of periplaneta americana (Blattodea: Blattidae) in
modern versus traditional Chinese medicine. J Med Entomol.
56:1522–1526. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fu S, Chen J, Zhang C, Shi J, Nie X, Hu Y,
Fu C, Li X and Zhang J: Gastroprotective effects of periplaneta
Americana L. extract against ethanol-induced gastric ulcer in mice
by suppressing apoptosis-related pathways. Front Pharmacol.
12(798421)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Barron SJ, Del Vecchio MT and Aronoff SC:
Intravenous immunoglobulin in the treatment of Stevens-Johnson
syndrome and toxic epidermal necrolysis: A meta-analysis with
meta-regression of observational studies. Int J Dermatol.
54:108–115. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huang YC, Chien YN, Chen YT, Li YC and
Chen TJ: Intravenous immunoglobulin for the treatment of toxic
epidermal necrolysis: A systematic review and meta-analysis. G Ital
Dermatol Venereol. 151:515–524. 2016.PubMed/NCBI
|
|
25
|
Yang Y, Xu J, Li F and Zhu X: Combination
therapy of intravenous immunoglobulin and corticosteroid in the
treatment of toxic epidermal necrolysis and Stevens-Johnson
syndrome: A retrospective comparative study in China. Int J
Dermatol. 48:1122–1128. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Al Rajaibi R, Al Rumhi T and Al Abri AM:
Carbamazepine-Induced stevens-johnson syndrome/toxic epidermal
necrolysis overlap treated successfully with oral cyclosporin: Case
report and literature review. Sultan Qaboos Univ Med J. 21:491–494.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Han F, Zhang J, Guo Q, Feng Y, Gao Y, Guo
L, Hou Y, An J, Wang X, Yan B, et al: Successful treatment of toxic
epidermal necrolysis using plasmapheresis: A prospective
observational study. J Crit Care. 42:65–68. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Clark WF and Huang SS: Introduction to
therapeutic plasma exchange. Transfus Apher Sci. 58:228–229.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Thakor AS, Burke A, Handfield-Jones S,
Sinha A, Palmer M and Burns A: Toxic epidermal necrolysis and
neutropaenia: Complications of omeprazole. Australas J Dermatol.
50:207–210. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin CY, Wang CW, Hui CR, Chang YC, Yang
CH, Cheng CY, Chen WW, Ke WM and Chung WH: Delayed-type
hypersensitivity reactions induced by proton pump inhibitors: A
clinical and in vitro T-cell reactivity study. Allergy. 73:221–229.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Trayes KP, Love G and Studdiford JS:
Erythema multiforme: Recognition and management. Am Fam Physician.
100:82–88. 2019.PubMed/NCBI
|
|
32
|
Soares A and Sokumbi O: Recent updates in
the treatment of erythema multiforme. Medicina (Kaunas).
57(921)2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rykiel K, Melchor J, Motie I, Mulles K and
Farhangi V: Recurrent erythema multiforme major following COVID-19
infection. Cureus. 15(e42646)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu X, Hu R, Peng L, Liu M and Sun Z:
Efficacy and safety of adalimumab biosimilars: Current critical
clinical data in rheumatoid arthritis. Front Immunol.
12(638444)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yamamoto T: An update on adalimumab for
pyoderma gangrenosum. Drugs Today (Barc). 57:535–542.
2021.PubMed/NCBI View Article : Google Scholar
|