Introduction
Liver diseases have become a serious public health
concern worldwide. Inflammation is involved in the pathological
process underlying a number of hepatic disorders, such as sepsis,
hepatitis, alcoholic and autoimmune liver diseases (1). Hepatic inflammation can induce
structural and functional liver damage, ultimately resulting in
liver failure or even cancer (2).
Anti-inflammatory treatment has been considered as an important
therapeutic strategy in the management of several hepatic disorders
(3,4).
Hepatic inflammation is characterized by the
excessive release of inflammatory cytokines (5). Hepatic cells are the targets of
inflammatory injury; however, following specific stimulation they
may also contribute to the production of cytokines (5,6).
Hepatic inflammation may be triggered by a variety of stimuli, such
as viral infection, alcohol and toxins (7,8).
Lipopolysaccharide (LPS) can induce the production of inflammatory
mediators, initiating inflammatory damage to hepatic cells
(9). Since LPS can induce
inflammation in various animal species and cell lines, and can
mimic the typical pathophysiological process of inflammation, LPS
stimulation has been widely used in a number of experimental models
to investigate the implicated mechanisms and possible treatments of
inflammatory diseases (10).
Toll-like receptors (TLRs) are considered to be
important components of the innate immune response (11). They are expressed by both
hepatocytes and Kupffer cells and serve important roles in hepatic
inflammation (12-14).
LPS, as a TLR ligand, can bind to TLRs and trigger the release of
multiple inflammatory mediators, leading to activation of the
inflammatory cascade and liver injury (15). Among the several TLRs, TLR4 has
been widely investigated in a variety of diseases, such as
pneumonia (16), hepatitis
(17) and diabetes mellitus
(18). Activation of TLR4 by LPS
may activate inflammatory signaling via the NF-κB pathway (19). NF-κB is expressed in nearly all
types of cells (10). Under normal
conditions, NF-κB is inactivated in the cytoplasm by binding to
IκB. The activation of TLR4 by LPS can trigger the degradation of
IκB through phosphorylation, resulting in the translocation of
NF-κB into the nucleus, where it can trigger the expression of
specific inflammatory genes (20).
Therefore, NF-κB activation regulated by TLR4 serves a key role in
regulating inflammation (21).
Several agents, such as aucubin (22) and vildagliptin (23), exhibit hepatoprotective effects by
interfering with TLR4-NF-κB signaling.
Medicinal plants have a history of medical use in
hepatic diseases. A number of natural products, such as sea
buckthorn polysaccharide (24),
artesunate (25) and annona
squamosa seed extract (26), have
been indicated to exhibit hepatoprotective effects in several
hepatic cellular and animal models. Bilobalide (BB) is a
sesquiterpene trilactone compound extracted from the leaves of
Ginkgo biloba that has been reported in the literature to
exhibit anti-inflammatory and cytoprotective properties (27,28).
A previous study has demonstrated that BB abated inflammation in
3T3-L1 adipocytes, partially via inhibiting NF-κB signaling
(29). Another study reported that
BB attenuated oxygen-glucose deprivation/reoxygenation (OGD/R)
injury in BV2 microglia cells via regulating TLR4 signaling
(30). However, the role of BB in
LPS-induced inflammatory response and cell injury in the liver and
the role of TLR4-NF-κB signaling in this process remain
unclear.
Thus, the present study was undertaken to
investigate the cytoprotective effects of BB in HepG2 cells and
elucidate the underlying mechanism.
Materials and methods
Reagents and antibodies
BB (purity≥99%) was purchased from Shanghai Winherb
Medical Technology Co., Ltd. and dissolved in DMSO as a stock
solution. MTT was purchased from Sigma-Aldrich; Merck KGaA. DMEM
was purchased from Cytiva and FBS was from Gibco; Thermo Fisher
Scientific, Inc. BCA assay kit (cat. no. CW0014S) and RIPA buffer
(cat. no. CW2334S) were from CoWin Biosciences. IL-6 (cat. no.
CSB-E04638h), IL-1β (cat. no. CSB-E08053h) and TNF-α (cat. no.
CSB-E04740h) ELISA kits were obtained from CUSABIO TECHNOLOGY LLC.
β-actin (cat. no. 4970S), IκBα (cat. no. 4812S), NF-κB p65 (cat.
no. 8242S), phosphorylated (p)-IκBα (cat. no. 2859S) and p-p65
(cat. no. 3033S) antibodies were purchased from Cell Signaling
Technology, Inc. Horseradish peroxidase-conjugated anti-rabbit IgG
secondary antibody (cat. no. BA1054) was purchased from Wuhan
Boster Biological Technology, Ltd. TransAM NF-κB p65 kit (cat. no.
40096) was obtained from Active Motif, Inc. The chemiluminescent
horseradish peroxidase substrate kit was purchased from
MilliporeSigma. TRIzol® reagent was purchased from
Invitrogen; Thermo Fisher Scientific, Inc. The ReverTra Ace qPCR
Kit (cat. no. FSQ-101) was obtained from Toyobo Life Science.
CLI-095 and LY294002 were obtained from MedChemExpress and
dissolved in DMSO. QuantiNova SYBR Green PCR kit (cat. no. 208054)
was purchased from Qiagen GmbH. Primers were purchased from Sangon
Biotech Co., Ltd. LPS (cat. no. S11060) from Escherichia
coli O55:B5 was purchased from Shanghai Yuanye Bio-Technology
Co., Ltd.
Cell line
Liver cancer cell line HepG2 (cat. no. CBP60199;
CoBioer Biosciences Co., Ltd.) were cultured with DMEM containing
10% FBS and 1% penicillin-streptomycin at 37˚C with 5%
CO2. HepG2 cells were precultured in DMEM complete
medium for 24 h, then subjected to different treatments in the
subsequent experiments.
Experimental design
The study consisted of four experiments as follows:
Experiment 1 aimed to exclude the possible cytotoxicity of BB.
HepG2 cells were seeded in 96-well plates (5x103
cells/well). Cells were grown in DMEM for 24 h, followed by
incubation with various concentrations of BB (0, 5, 10, 20 and 40
µM) for 2, 24, 48 and 72 h. The viability of HepG2 cells was
detected using an MTT assay. Experiment 2 aimed to detect whether
BB could protect HepG2 cells against LPS-induced damage. HepG2
cells were incubated with BB (10 and 20 µM) for 2 h, then
stimulated with LPS at the concentration of 50 µg/ml (31-34)
for 24 h. Cell viability was detected using an MTT assay. The
levels of TLR4 mRNA, p-IκBα, p-p65, p65 DNA-binding activity,
IL-1β, IL-6 and TNF-α were measured. Experiment 3 aimed to explore
whether TLR4 was involved in mediating LPS-induced cell injury and
inflammatory response in HepG2 cells. HepG2 cells were incubated
with LPS and the TLR4 inhibitor CLI-095 (10 µM) for 24 h. Cell
viability and inflammatory response were assessed as
aforementioned. Finally, experiment 4 aimed to confirm the role of
the PI3K/Akt pathway in mediating the effects of BB. Cells were
pretreated with BB (20 µM) with the presence of the PI3K/Akt
inhibitor LY294002 (20 µM) for 2 h, followed by a 24-h LPS
incubation. All incubations were performed at 37˚C.
MTT assay
Following each treatment, cells were incubated with
20 µl MTT solution (5 mg/ml) for 4 h at 37˚C. Subsequently, 100 µl
DMSO were added to dissolve the formazan crystals after removing
the supernatant from the wells. The absorbance of each well was
measured at 570 nm using a microplate reader (Bio-Rad Laboratories,
Inc.). Cell viability was expressed as a percentage of the control
cells (considered to be 100%).
Measurement of inflammatory
cytokines
Following each aforementioned treatment, the culture
medium was collected. The concentrations of IL-1β, IL-6 and TNF-α
were measured using commercially available ELISA kits according to
the manufacturer's instructions.
Western blot assay
HepG2 cells were harvested separately, washed in
cold PBS and lysed using RIPA buffer. The protein concentrations of
the samples were detected via BCA assay. Briefly, 25 µl of each
sample and BSA standard solution (0, 0.125, 0.25, 0.5, 1 and 2
µg/µl) from the BCA assay kit were added to 96-well plates. Then,
25 µl BCA solution was added to each well and the plates were
incubated at 37˚C for 30 min. The absorbance of each well was
measured at 570 nm using a microplate reader. Ultimately, the
protein concentration of the samples was calculated according to
the standard curve. After the measurement of the protein
concentrations, 40 µg protein/sample were loaded on 10% SDS-PAGE
gels, separated and subsequently transferred to PVDF membranes.
Following blocking with 5% skimmed milk buffer for 1 h at 37˚C, the
membranes were incubated overnight at 4˚C with rabbit antibodies
(p65, p-p65, IκBα, p-IκBα and β-actin) diluted 1:1,000.
Subsequently, the membranes were incubated with 1:5,000
HRP-conjugated anti-rabbit IgG secondary antibody for 1 h at room
temperature. Protein expression was visualized with a
chemiluminescent HRP substrate kit. The intensity of the bands was
measured using ImageJ software v1.52a (National Institutes of
Health). The ratio of phosphorylated/total protein was
evaluated.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was obtained from HepG2 cells with TRIzol
reagent and the extracted RNA in each group was reversely
transcribed into cDNA using the ReverTra Ace qPCR kit according to
the manufacturer's instructions. qPCR was then carried out on a
Real Time system machine using the cDNA samples and QuantiNova SYBR
Green PCR kit. The PCR amplification conditions were as follows:
95˚C for 30 sec, followed by 40 cycles at 95˚C for 10 sec and at
60˚C for 60 sec. The primer sequences use for the qPCR were as
listed follows: TLR4 forward, 5'-TGAGCAGTCGTGCTGGTATC-3' and
reverse, 5'-CAGGGCTTTTCTGAGTCGTC-3'; β-actin forward,
5'-CACACTGTGCCCATCTACGA-3' and reverse,
5'-CTCAGTGAGGATCTTCATGAGGTAGT-3'. The expression of the target gene
was determined using the 2-ΔΔCq method (35) and was normalized to the endogenous
control β-actin.
Measurement of p65 DNA-binding
activity
Nuclear extracts were obtained from HepG2 cells and
used to measure p65 DNA-binding activity with TransAM NF-κB p65 kit
strictly according to the manufacturer's protocol. Following
completion of the reactions, the absorbance of each sample was
measured at 450 nm using a plate reader. The DNA-binding activity
was expressed as a percentage of the control cells (considered to
be 100%).
Statistical analysis
Data are presented as the mean ± SEM and were
analyzed with SPSS software version 21.0 (IBM Corp.). Differences
between groups were compared using one-way ANOVA followed by
Tukey's honestly significant difference test. All the experiments
were performed in triplicates and repeated three times.
P<0.05 was considered to indicate a statistically
significant difference.
Results
BB exerts no significant toxic effects
on HepG2 cells
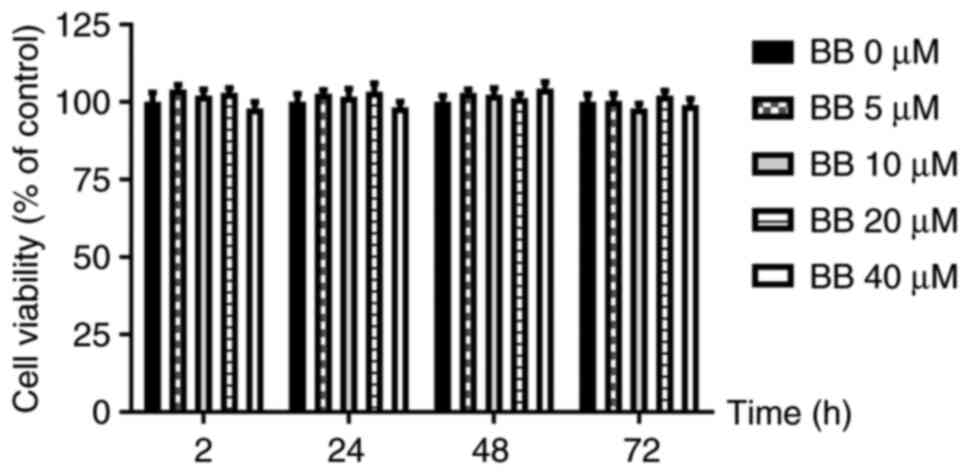
In experiment 1, there were no significant
differences in the viability of HepG2 cells incubated with
different concentrations of BB (0, 5, 10, 20 and 40 µM) for 2, 24,
48 and 72 h. This indicated that BB at concentrations of 0-40 µM
exerted no significant cytotoxic effects (Fig. 1).
BB attenuates LPS-induced HepG2 cell
injury
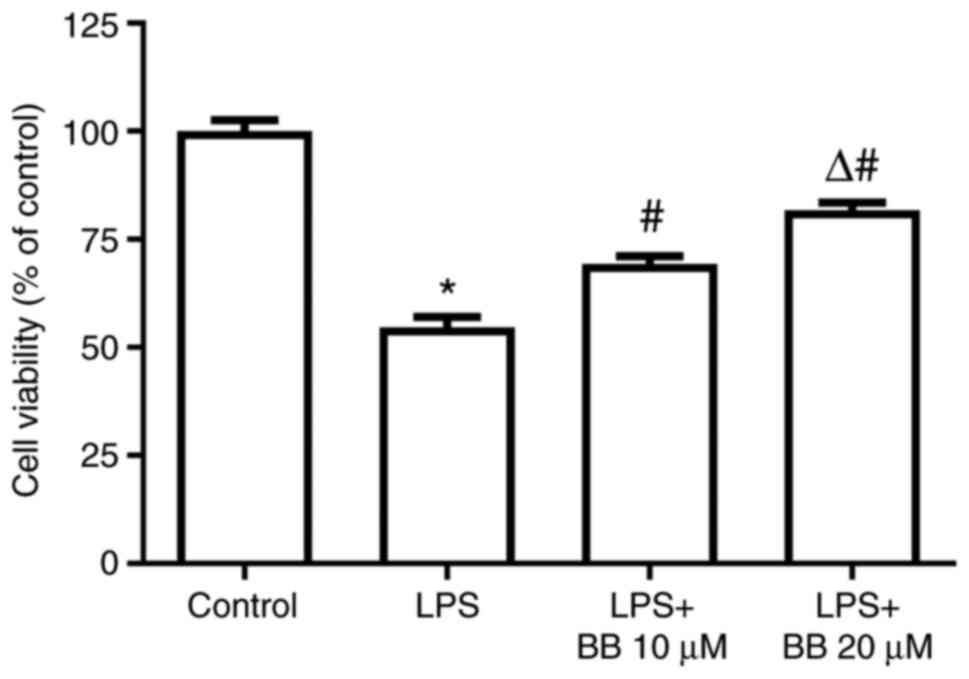
In experiment 2, the MTT assay results revealed that
LPS stimulation significantly decreased the viability of HepG2
cells, but BB (10 and 20 µM) dose-dependently increased the
viability of the LPS-stimulated HepG2 cells, indicating that BB may
protect HepG2 cells from LPS-induced injury (Fig. 2).
BB inhibits LPS-induced activation of
NF-κB signaling
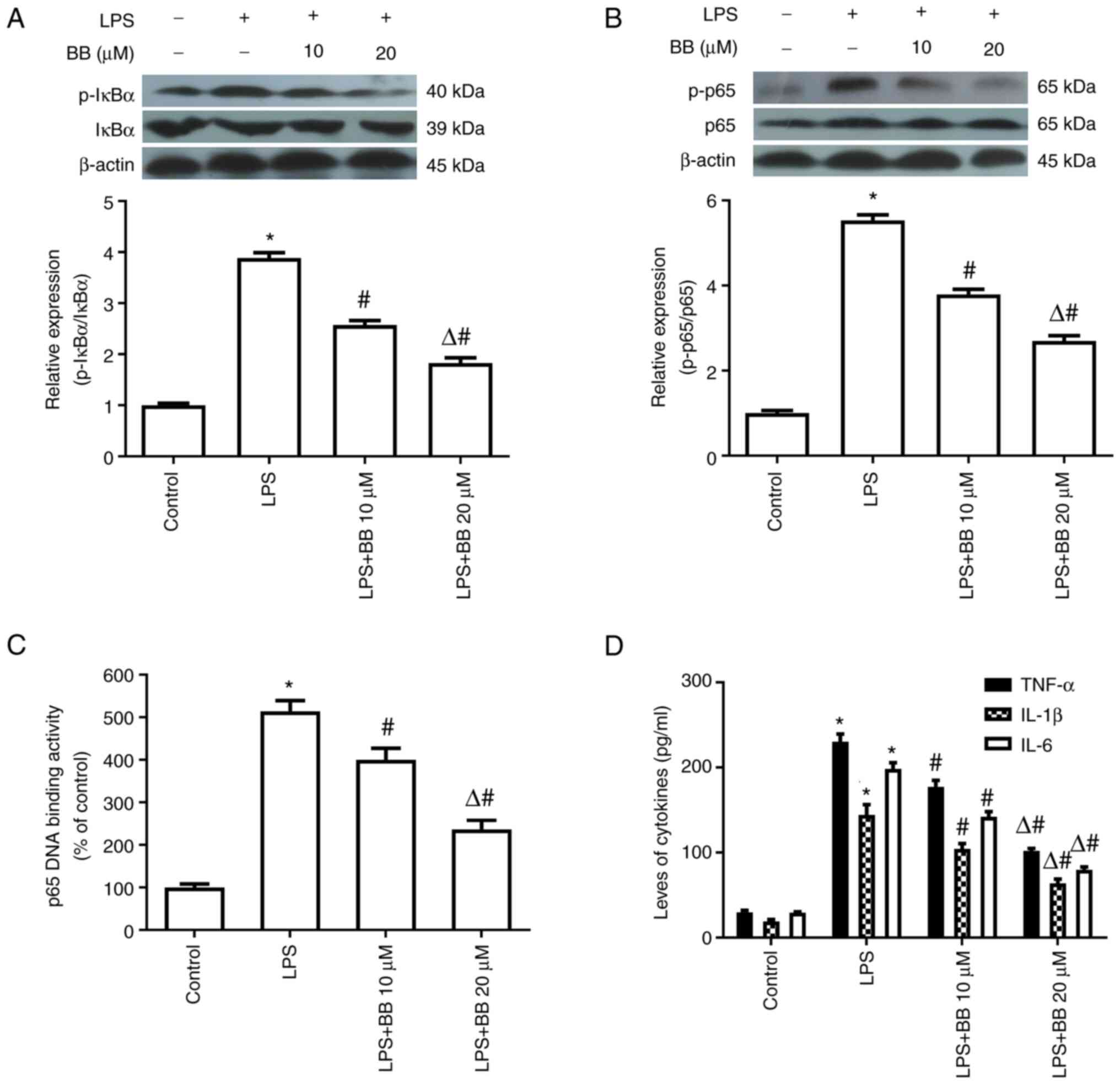
In experiment 2, LPS markedly increased the
phosphorylation of IκBα and p65 and elevated p65 DNA-binding
activity. In addition, LPS promoted the production of TNF-α, IL-1β
and IL-6. However, preincubation with BB diminished the NF-κB
activation and inflammatory cytokine release induced by LPS
(Fig. 3).
BB inhibits LPS-induced TLR4 mRNA
expression
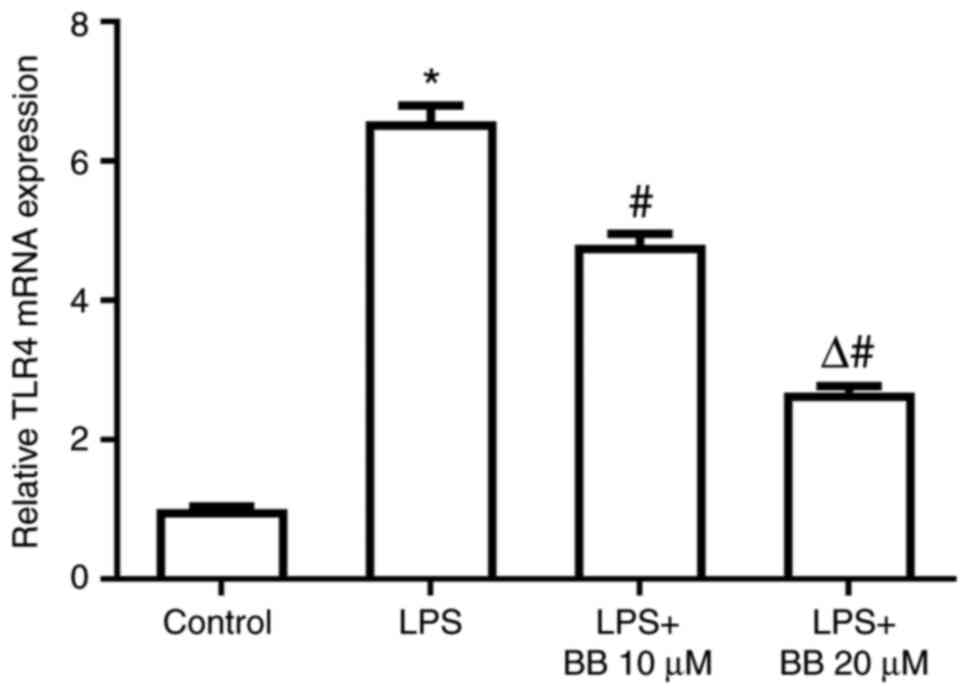
In experiment 2, LPS markedly increased TLR4 mRNA
expression, whereas BB abolished the LPS-induced TLR4 mRNA increase
in a dose-dependent manner (Fig.
4).
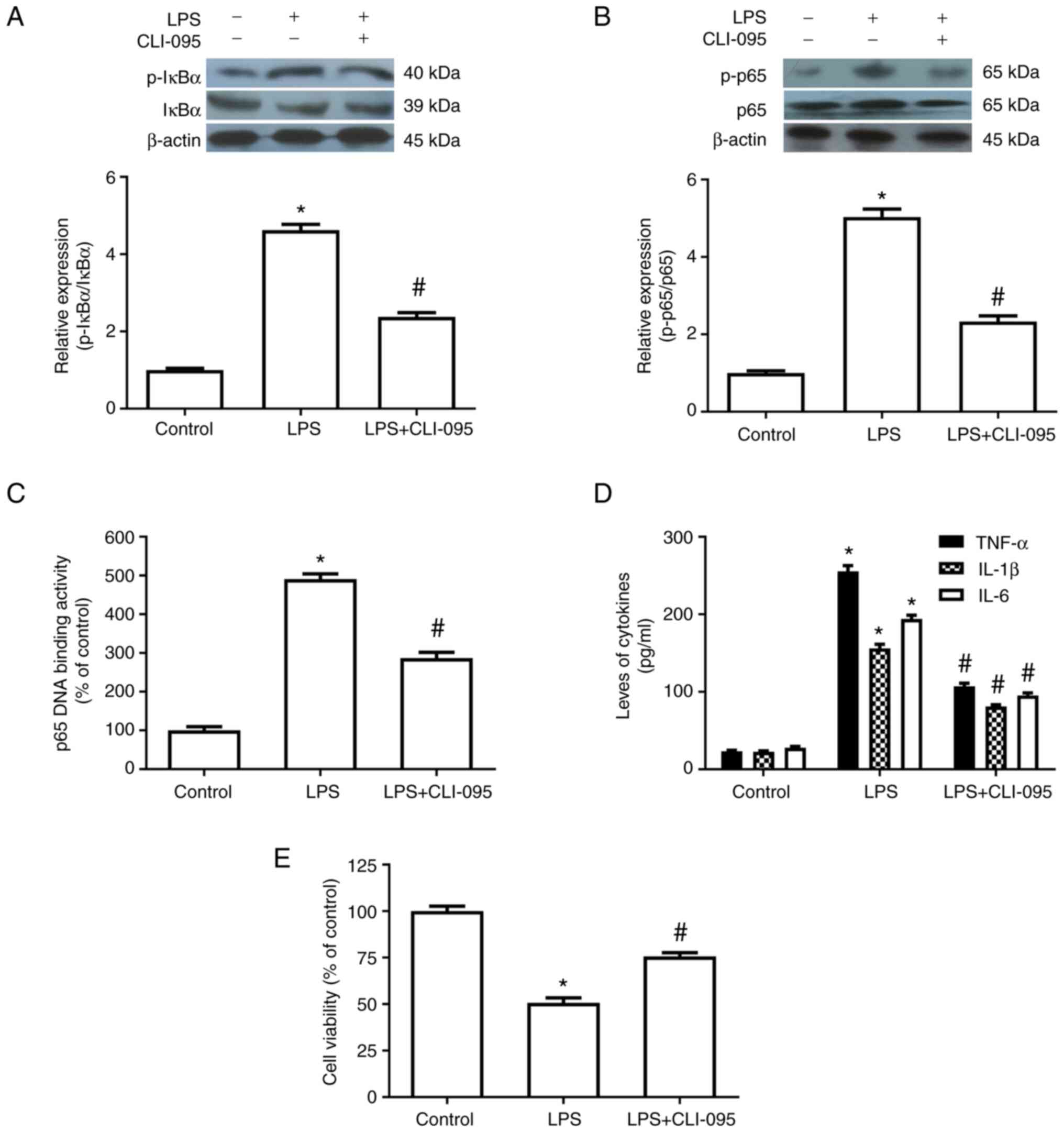
TLR4 serves an important role in
LPS-induced HepG2 cell injury, NF-κB activation and inflammatory
cytokine release
In experiment 3, the TLR4 inhibitor CLI-095 (10 µM)
significantly inhibited the LPS-induced increase in the
phosphorylation of IκBα and p65, p65 DNA-binding activity and the
production of inflammatory cytokines. CLI-095 also attenuated the
LPS-induced decrease in cell viability. These results suggested
that TLR4 may be involved in LPS-induced cell injury and NF-κB
activation in HepG2 cells (Fig.
5).
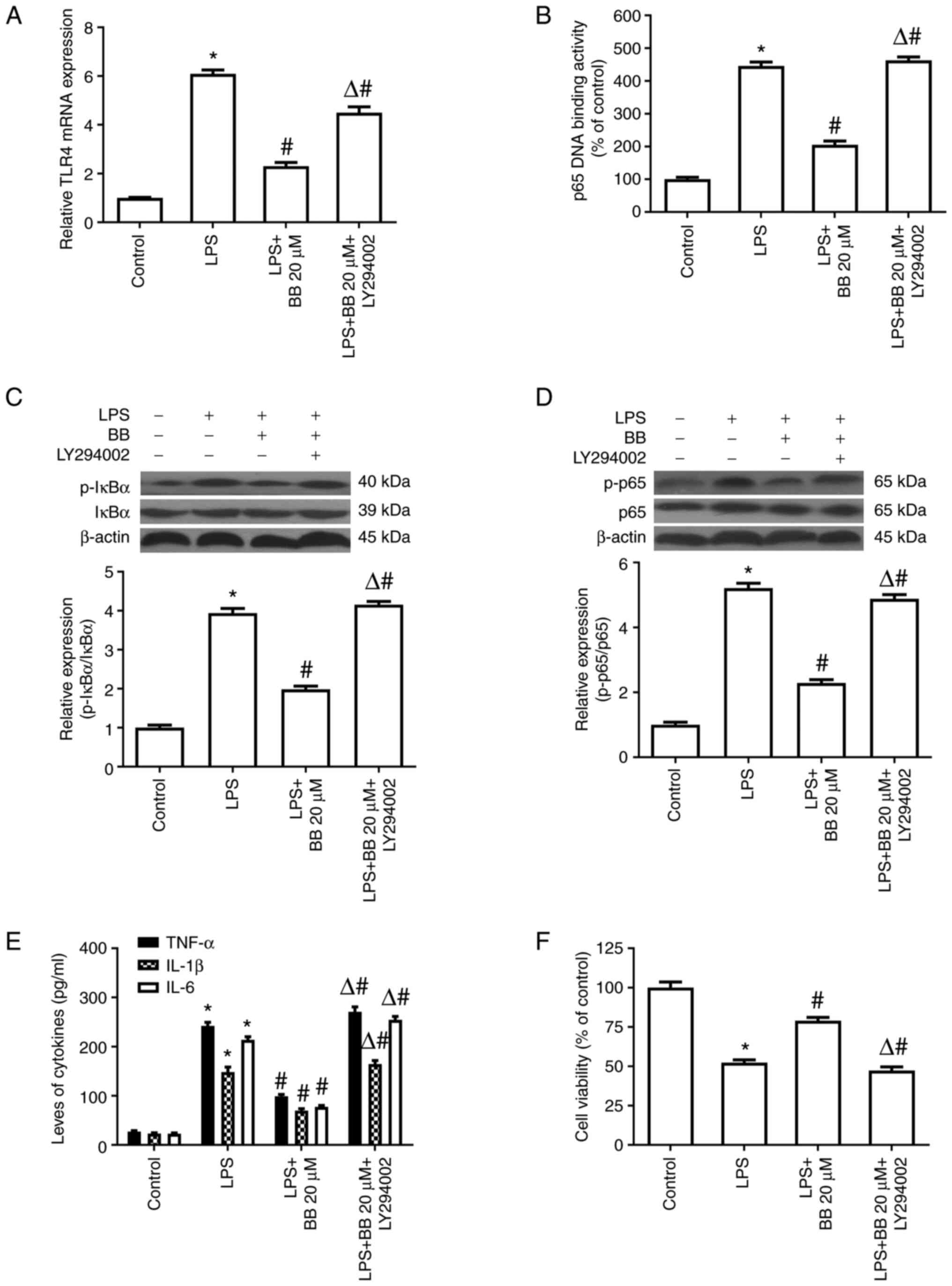
BB inhibits LPS-induced TLR4 mRNA
expression, NF-κB activation and inflammatory cytokine release
through the PI3K/Akt pathway
The results of experiment 4 demonstrated that the
PI3K/Akt inhibitor LY294002 abolished the inhibition of TLR4 mRNA
by BB in the LPS-stimulated HepG2 cells (Fig. 6A). Consistently, LY294002 also
abolished the inhibitory effects of BB on the phosphorylation of
IκBα and p65, p65 DNA-binding activity (Fig. 6B-D) and inflammatory cytokine
release (Fig. 6E) in
LPS-stimulated HepG2 cells. Moreover, LY294002 diminished the
cytoprotective effects of BB after LPS treatment (Fig. 6F). These results strongly suggested
that the effects of BB on LPS-stimulated HepG2 cells may be
mediated via the PI3K/Akt pathway.
Discussion
An increasing amount of experimental and clinical
studies have focused on the regulation of inflammatory response by
natural products (36-39).
A number of natural agents have been indicated to exhibit
hepatoprotective properties (24-26,40).
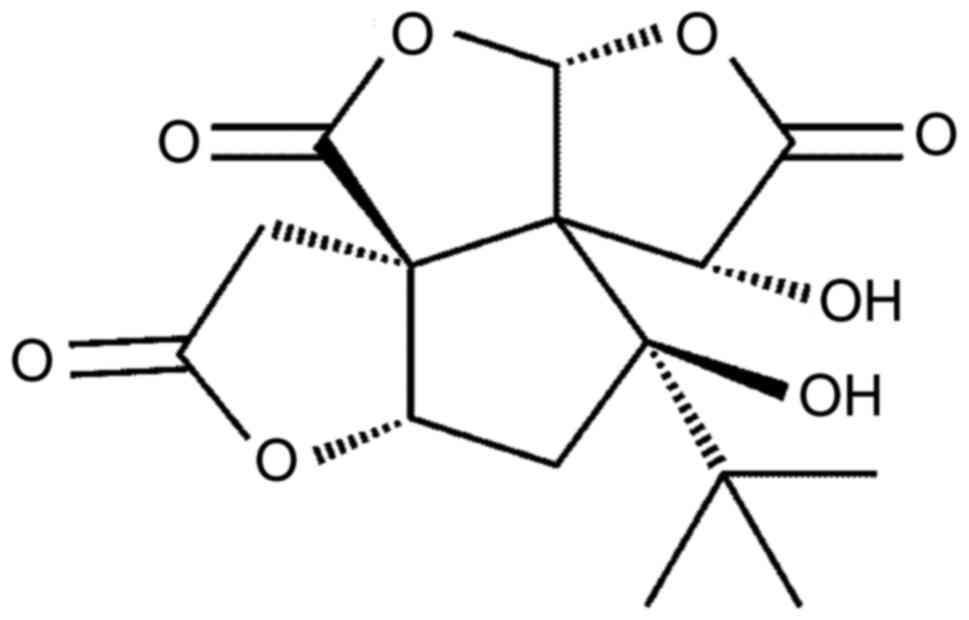
BB (Fig. 7), which is a
sesquiterpene trilactone extracted from the Ginkgo biloba
leaf, has been indicated to exhibit multiple pharmacological
effects (41-43),
and it has been investigated as a therapeutic agent for
cardiovascular diseases (44),
neurological disorders (41,45),
ethanol-induced gastric ulcer (46) and cuprizone-induced demyelination
(47). Previous studies also
demonstrated that BB exerts anti-inflammatory effects (27,28).
However, whether BB exhibits beneficial effects on hepatic injury
following an inflammatory response has yet to be elucidated.
The liver cancer cell line HepG2 has been widely
used in evaluating the hepatoprotective activities of novel agents
(48), as these cells retain
several morphological and biochemical characteristics of normal
hepatocytes (49). In the present
study, the possible cytotoxic effects of BB on HepG2 cells were
firstly excluded. HepG2 cells were incubated with various
concentrations of BB for 2, 24, 48 and 72 h, and the viability of
HepG2 cells was evaluated. The results revealed that incubation
with BB at concentrations of 0-40 µM demonstrated no adverse
effects on cell viability, indicating that BB was not cytotoxic to
HepG2 cells. In order to observe whether BB could protect HepG2
cells from inflammatory damage, HepG2 cells were stimulated with
LPS, which has been widely used to induce inflammation in animal
models and cell lines (50). The
results demonstrated that LPS stimulation resulted in decreased
cell viability, indicating that LPS caused HepG2 cell injury,
whereas 10 and 20 µM BB treatment dose-dependently attenuated the
LPS-induced cell damage. Our previous pilot study demonstrated that
10 and 20 µM BB exhibited a relevant potency on inhibiting
LPS-induced damage; therefore, 10 and 20 µM BB were used in the
current study (data not shown). Similar to the findings of the
present study, the cytoprotective effects of BB have also been
demonstrated in other cell lines. Hua et al (51) revealed that BB could protect neural
cells against aggregated α-synuclein-induced apoptosis. Cao and Li
(52) observed that BB ameliorated
OGD-induced injury in H9c2 cells. A study by Mao et al
(53) demonstrated that BB
alleviated IL-17-induced inflammatory injury in ATDC5 cells. In
addition to the cytoprotective effects of BB, the present study
also demonstrated that BB treatment markedly suppressed the
production of TNF-α, IL-1β and IL-6 induced by LPS in HepG2 cells.
The role of these cytokines in exacerbating hepatocyte apoptosis
(54,55), hepatic fibrosis (56,57),
hepatic stellate cell activation (58,59)
and Kupffer cell activation (60)
has been demonstrated in numerous studies. Considering the
important role of these cytokines in hepatic injury, inhibiting the
overproduction of these cytokines has been regarded as an important
therapeutic strategy in the management of hepatic disorders in both
clinical and experimental studies (61-63).
Therefore, it was hypothesized that the downregulation of
inflammatory cytokines by BB should contribute to the attenuation
of the LPS-induced HepG2 cell injury in the present study.
Consistent with the findings of the current study, BB has been
reported to attenuate inflammatory injury in different disease
models. In a previous study performed by Zhou et al
(30), BV2 cells were subjected to
oxygen-glucose deprivation/reoxygenation (OGD/R) and treated with
BB. The results of the study revealed that OGD/R induced
inflammatory response and cellular damage in BV2 cells in
vitro, but BB treatment suppressed the production of IL-1β,
IL-6, IL-8 and IL-10, and attenuated cell damage. In another study
by Jiang et al (27), BB
treatment alleviated brain damage and inhibited the expression of
the inflammatory cytokines TNF-α and IL-1β in rats with middle
cerebral artery occlusion/reperfusion. Simultaneously, Jiang et
al (27) observed that BB
significantly downregulated TNF-α and IL-1β expression in rat
cortical neurons after OGD/R-induced injury in vitro. Hui
and Fangyu (46) reported that BB
decreased TNF-α, IL-6 and IL-1β levels in mice with gastric ulcers.
Additionally, other studies also demonstrated the protective
effects of BB in several disorders through various mechanisms. For
example, Liu et al (64)
demonstrated that bilobalide acts against cerebral ischemia injury
by activating the Akt/Nrf2 pathway in vitro and in
vivo; Qin et al (65)
reported that bilobalide alleviates neuroinflammation in
Alzheimer's disease by upregulating lincRNA-p21; and Zheng et
al (66) demonstrated that
bilobalide inhibits autophagy and promotes angiogenesis following
focal cerebral ischemia reperfusion by activating Akt/eNOS.
The regulation of inflammatory gene transcription
has been demonstrated to be regulated by specific signaling
pathways and transcriptional regulators (67,68).
NF-κB is one of most important inflammatory regulators, and serves
a key role in determining cytokine expression (69). After being released from its
inhibitor, IκB, the activated p65 subunit translocates to the
nucleus to regulate the expression of inflammatory genes (70). NF-κB has been considered to be an
important therapeutic target in treating inflammatory disorders
(71,72). In the present study, it was
observed that LPS stimulation activated NF-κB signaling in HepG2
cells by enhancing the phosphorylation of IκBα and p65 and
increasing p65 DNA-binding activity. However, BB significantly
abolished NF-κB activation induced by LPS. These inhibitory effects
on NF-κB activation were in accordance with its effects on
inflammatory cytokine release in the present study. In a similar
manner, a number of other natural agents, such as raspberry ketone,
grape-leaf extract and fraxetin, have also been demonstrated to
exert hepatoprotective effects through suppressing NF-κB activation
(73-75).
Although this is, to the best of our knowledge, the first study to
demonstrate the effects of BB on NF-κB activation in HepG2 cells, a
recent study by Priyanka et al (28) demonstrated that BB abated
inflammation and inhibited NF-κB activation in 3T3-L1 adipocytes.
In addition, Zhang et al (76) also reported that BB attenuated
colitis by inhibiting NF-κB signaling in mice.
As one of the receptors of LPS, TLR4 serves a key
function in hepatic diseases (77,78).
Certain agents, such as raspberry ketone (73) and monotropein (79), have been demonstrated to attenuate
liver injury by acting on the TLR4 signaling pathway. In the
present study, BB was indicated to abolish LPS-induced TLR4 mRNA
overexpression. To confirm the involvement of TLR4 in mediating the
LPS-induced alterations in HepG2 cells, the TLR4 inhibitor CLI-095
was used to inhibit TLR4, as described previously (80). The results demonstrated that
CLI-095 reduced LPS-induced cell injury and suppressed the
activation of NF-κB signaling. These results suggested the
involvement of TLR4 in the LPS-induced damage to HepG2 cells. This
is in line with the conclusions of previous studies reporting that
LPS primarily induces inflammatory response through TLR4-NF-κB
signaling (79,81). Therefore, the inhibition of TLR4 by
BB in the present study may contribute to the suppressive effects
of BB on NF-κB activation and cytokine release. In addition to the
inhibitory effects of BB on TLR4 expression observed in the present
study, a previous study also demonstrated that BB inhibited TLR4
expression in BV2 microglia cells (30).
It has been demonstrated that the PI3K/Akt pathway
is involved in regulating TLR4 expression (82,83).
It has been reported that PI3K/Akt activation can inhibit
LPS-induced IL-6 and TNF-α production from microglia and bone
marrow macrophages (84,85). Notably, a previous study
demonstrated that BB could activate PI3K/Akt signaling in SH-SY5Y
cells (86). Therefore, it was
hypothesized that the effects of BB on HepG2 cells may be mediated
via the PI3K/Akt pathway. To confirm this hypothesis, the PI3K/Akt
inhibitor LY294002 was applied to block the PI3K/Akt pathway. The
use of specific inhibitors is considered to be the optimal method
for assessing the pharmacological action of a drug. When assays
with inhibitors are used, three possible results may be observed:
No alteration, total inhibition or partial inhibition. The results
of the present study demonstrated that LY294002 partially abolished
the downregulation of TLR4 mRNA expression by BB. In addition,
LY294002 significantly diminished the effects of BB on NF-κB
activation and cytokine release. Furthermore, the beneficial
effects of BB on cell viability were also abolished by LY294002.
These results suggested that the PI3K/Akt pathway was involved in
mediating the effects of BB on HepG2 cells, as most of the
aforementioned effects were entirely inhibited by LY294002. Similar
to BB, other agents such as lithium, hypaphorine and ginkgolide A
have also been indicated to inhibit TLR4-associated cytokine
release through the PI3K/Akt pathway in microglia and human
vascular endothelial cells (85,87,88).
In conclusion, the results demonstrated that BB may
attenuate LPS-induced HepG2 cell injury by regulating TLR4/NF-κB
signaling through the PI3K/Akt pathway. Therefore, BB may be of
value as a potential agent for the treatment of
inflammation-associated hepatic injury.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Shandong Province (CN) (grant nos. ZR2020MH381 and
ZR2015CL029), the Shandong Provincial Education Department (grant
nos. J15LL54 and J16LM11) and Weifang Science and Technology Bureau
(grant no. 2021GX062).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WT and CL designed the study and confirm the
authenticity of all the raw data. SM, JY, TZ and XZ performed the
experiments. SM and CL were major contributors to the writing of
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weifang Medical University (approval no. 2019SDL029;
Weifang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Szabo G and Csak T: Inflammasomes in liver
diseases. J Hepatol. 57:642–654. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yu S, Wang Y, Jing L, Claret FX, Li Q,
Tian T, Liang X, Ruan Z, Jiang L, Yao Y, et al: Autophagy in the
‘inflammation-carcinogenesis' pathway of liver and HCC
immunotherapy. Cancer Lett. 411:82–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lam P, Cheung F, Tan HY, Wang N, Yuen MF
and Feng Y: Hepatoprotective effects of Chinese medicinal herbs: A
focus on anti-inflammatory and anti-oxidative activities. Int J Mol
Sci. 17(465)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li H, Huang MH, Jiang JD and Peng ZG:
Hepatitis C: From inflammatory pathogenesis to
anti-inflammatory/hepatoprotective therapy. World J Gastroenterol.
24:5297–5311. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Seki E and Schwabe RF: Hepatic
inflammation and fibrosis: Functional links and key pathways.
Hepatology. 61:1066–1079. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu J, Abate W, Xu J, Corry D, Kaul B and
Jackson SK: Three-dimensional spheroid cultures of A549 and HepG2
cells exhibit different lipopolysaccharide (LPS) receptor
expression and LPS-induced cytokine response compared with
monolayer cultures. Innate Immun. 17:245–255. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ju C and Mandrekar P: Macrophages and
alcohol-related liver inflammation. Alcohol Res. 37:251–262.
2015.PubMed/NCBI
|
|
8
|
Park BJ, Lee YJ and Lee HR: Chronic liver
inflammation: Clinical implications beyond alcoholic liver disease.
World J Gastroentero. 20:2168–2175. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Y, Zhou X, Zhao D, Wang X, Gurley EC,
Liu R, Li X, Hylemon PB, Chen W and Zhou H: Berberine inhibits free
fatty acid and LPS-induced inflammation via modulating ER stress
response in macrophages and hepatocytes. PLoS One.
15(e0232630)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lu S, Duan M, Guo Z, Zhou Y, Wu D, Zhang
X, Wang Y, Ye C, Ju R, Li J, et al: Carboxyamidotriazole exerts
anti-inflammatory activity in lipopolysaccharide-induced RAW264.7
macrophages by inhibiting NF-κB and MAPKs pathways. Exp Ther Med.
20:1455–1466. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang XY, Ansari AR, Huang HB, Zhao X, Li
NY, Sun ZJ, Peng KM, Zhong J and Liu HZ: Lipopolysaccharide
mediates immuno-pathological alterations in young chicken liver
through TLR4 signaling. BMC Immunol. 18(12)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang J, Wieser A, Lin H, Li H, Hu M,
Behrens IK, Schiergens TS, Gerbes AL and Steib CJ: Kupffer cell
activation by different microbial lysates: Toll-like receptor-2
plays pivotal role on thromboxane A2 production in mice and humans.
Eur J Immunol. 50:1988–1997. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang X, Jiang W, Zhou AL, Zhao M and
Jiang DR: Inhibitory effect of oxymatrine on hepatocyte apoptosis
via TLR4/PI3K/Akt/GSK-3β signaling pathway. World J Gastroenterol.
23:3839–3849. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim MS, Lee S, Jung N, Lee K, Choi J, Kim
SH, Jun J, Lee WM, Chang Y and Kim D: The vitamin D analogue
paricalcitol attenuates hepaticischemia/reperfusion injury through
down-regulation of Toll-like receptor 4 signaling in rats. Arch Med
Sci. 13:459–469. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cho HI, Hong JM, Choi JW, Choi HS, Kwak
JH, Lee DU, Kook Lee S and Lee SM: β-Caryophyllene alleviates
D-galactosamine and lipopolysaccharide-induced hepatic injury
through suppression of the TLR4 and RAGE signaling pathways. Eur J
Pharmacol. 764:613–621. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Standiford LR, Standiford TJ, Newstead MJ,
Zeng X, Ballinger MN, Kovach MA, Reka AK and Bhan U: TLR4-dependent
GM-CSF protects against lung injury in Gram-negative bacterial
pneumonia. Am J Physiol Lung Cell Mol Physiol. 302:L447–L454.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li Y, Yin S, Chen Y, Zhang Q, Huang R, Jia
B, Jie W, Yao K, Wang J, Tong X, et al: Hepatitis B virus-induced
hyperactivation of B cells in chronic hepatitis B patients via
TLR4. J Cell Mol Med. 24:6096–6106. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tian J, Zhao Y, Wang L and Li L: Role of
TLR4/MyD88/NF-κB signaling in heart and liver-related complications
in a rat model of type 2 diabetes mellitus. J Int Med Res.
49(300060521997590)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Qu Y, Li X, Xu F, Zhao S, Wu X, Wang Y and
Xie J: Kaempferol alleviates murine experimental colitis by
restoring gut microbiota and inhibiting the LPS-TLR4-NF-κB axis.
Front Immunol. 12(679897)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mitchell S, Vargas J and Hoffmann A:
Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med.
8:227–241. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun X, Li P, Qu X and Liu W: Isovitexin
alleviates acute gouty arthritis in rats by inhibiting inflammation
via the TLR4/MyD88/NF-κB pathway. Pharm Biol. 59:1326–1333.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang S, Feng Z, Gao W, Duan Y, Fan G,
Geng X, Wu B, Li K, Liu K and Peng C: Aucubin attenuates liver
ischemia-reperfusion injury by inhibiting the HMGB1/TLR-4/NF-κB
signaling pathway, oxidative stress, and apoptosis. Front
Pharmacol. 11(544124)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sherif IO and Al-Shaalan NH: Vildagliptin
attenuates hepatic ischemia/reperfusion injury via the TLR4/NF-κB
signaling pathway. Oxid Med Cell Longev.
2018(3509091)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu H, Zhang W, Dong S, Song L, Zhao S, Wu
C, Wang X, Liu F, Xie J, Wang J and Wang Y: Protective effects of
sea buckthorn polysaccharide extracts against LPS/d-GalN-induced
acute liver failure in mice via suppressing TLR4-NF-κB signaling. J
Ethnopharmacol. 176:69–78. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lai L, Chen Y, Tian X, Li X, Zhang X, Lei
J, Bi Y, Fang B and Song X: Artesunate alleviates hepatic fibrosis
induced by multiple pathogenic factors and inflammation through the
inhibition of LPS/TLR4/NF-κB signaling pathway in rats. Eur J
Pharmacol. 765:234–241. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zahid M, Arif M, Rahman MA and Mujahid M:
Hepatoprotective and antioxidant activities of Annona squamosa seed
extract against alcohol-induced liver injury in Sprague Dawley
rats. Drug Chem Toxicol. 43:588–594. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jiang M, Li J, Peng Q, Liu Y, Liu W, Luo
C, Peng J, Li J, Yung KK and Mo Z: Neuroprotective effects of
bilobalide on cerebral ischemia and reperfusion injury are
associated with inhibition of pro-inflammatory mediator production
and down-regulation of JNK1/2 and p38 MAPK activation. J
Neuroinflammation. 11(167)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Priyanka A, Nisha VM, Anusree SS and Raghu
KG: Bilobalide attenuates hypoxia induced oxidative stress,
inflammation, and mitochondrial dysfunctions in 3T3-L1 adipocytes
via its antioxidant potential. Free Radic Res. 48:1206–1217.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Priyanka A, Sindhu G, Shyni GL, Preetha
Rani MR, Nisha VM and Raghu KG: Bilobalide abates inflammation,
insulin resistance and secretion of angiogenic factors induced by
hypoxia in 3T3-L1 adipocytes by controlling NF-κB and JNK
activation. Int Immunopharmacol. 42:209–217. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou JM, Gu SS, Mei WH, Zhou J, Wang ZZ
and Xiao W: Ginkgolides and bilobalide protect BV2 microglia cells
against OGD/reoxygenation injury by inhibiting TLR2/4 signaling
pathways. Cell Stress Chaperones. 21:1037–1053. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Norikura T, Mukai Y, Fujita S, Mikame K,
Funaoka M and Sato S: Lignophenols decrease oleate-induced
apolipoprotein-B secretion in HepG2 cells. Basic Clin Pharmacol
Toxicol. 107:813–817. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wollborn J, Wunder C, Stix J, Neuhaus W,
Bruno RR, Baar W, Flemming S, Roewer N, Schlegel N and Schick MA:
Phosphodiesterase-4 inhibition with rolipram attenuates
hepatocellular injury in hyperinflammation in vivo and in vitro
without influencing inflammation and HO-1 expression. J Pharmacol
Pharmacother. 6:13–23. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu M, Fang G, Yin S, Zhao X, Zhang C, Li
J and Liu Z: Caffeic acid prevented LPS-induced injury of primary
bovine mammary epithelial cells through inhibiting NF-κB and MAPK
activation. Mediators Inflamm. 2019(1897820)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xiong W, Ma H, Zhang Z, Jin M, Wang J, Xu
Y and Wang Z: The protective effect of icariin and phosphorylated
icariin against LPS-induced intestinal epithelial cells injury.
Biomed Pharmacother. 118(109246)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Latruffe N: Natural products and
inflammation. Molecules. 22(120)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Amaral-Machado L, Oliveira WN, Rodrigues
VM, Albuquerque NA, Alencar ÉN and Egito EST: Could natural
products modulate early inflammatory responses, preventing acute
respiratory distress syndrome in COVID-19-confirmed patients?
Biomed Pharmacother. 134(111143)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Beutler JA: Natural products as a
foundation for drug discovery. Curr Protoc Pharmacol.
86(e67)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
LeBlanc JF, Wiseman D, Lakatos PL and
Bessissow T: Elderly patients with inflammatory bowel disease:
Updated review of the therapeutic landscape. World J Gastroenterol.
25:4158–4171. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chale-Dzul J, Pérez-Cabeza de Vaca R,
Quintal-Novelo C, Olivera-Castillo L and Moo-Puc R:
Hepatoprotective effect of a fucoidan extract from Sargassum
fluitans Borgesen against CCl4-induced toxicity in rats.
Int J Biol Macromol. 145:500–509. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Feng Z, Sun Q, Chen W, Bai Y, Hu D and Xie
X: The neuroprotective mechanisms of ginkgolides and bilobalide in
cerebral ischemic injury: A literature review. Mol Med.
25(57)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bu S, Yuan CY, Xue Q, Chen Y and Cao F:
Bilobalide suppresses adipogenesis in 3T3-L1 adipocytes via the
AMPK signaling pathway. Molecules. 24(3503)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li Y, Jiang J, Tong L, Gao T, Bai L, Xue
Q, Xing J, Wang Q, Lyu H, Cai M and Sun Z: Bilobalide protects
against ischemia/reperfusion-induced oxidative stress and
inflammatory responses via the MAPK/NF-κB pathways in rats. BMC
Musculoskelet Disord. 21(449)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Maerz S, Liu CH, Guo W and Zhu YZ:
Anti-ischaemic effects of bilobalide on neonatal rat cardiomyocytes
and the involvement of the platelet-activating factor receptor.
Biosci Rep. 31:439–447. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang Y and Zhai H: Bilobalide assuages
morphine-induced addiction in hippocampal neuron cells through
upregulation of microRNA-101. J Biochem Mol Toxicol.
34(e22493)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hui S and Fangyu W: Protective effects of
bilobalide against ethanol-induced gastric ulcer in vivo/vitro.
Biomed Pharmacother. 85:592–600. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sui RX, Miao Q, Wang J, Wang Q, Song LJ,
Yu JW, Cao L, Xiao W, Xiao BG and Ma CG: Protective and therapeutic
role of Bilobalide in cuprizone-induced demyelination. Int
Immunopharmacol. 66:69–81. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Al-Oqail MM, Farshori NN, Al-Sheddi ES,
Al-Massarani SM, Siddiqui MA and Al-Khedhairy AA: Petroselinum
sativum protects HepG2 cells from cytotoxicity and oxidative stress
induced by hydrogen peroxide. Mol Biol Rep. 47:2771–2780.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Dey D, Chaskar S, Bhatt N and Chitre D:
Hepatoprotective activity of BV-7310, a proprietary herbal
formulation of phyllanthus niruri, tephrosia purpurea, boerhavia
diffusa, and andrographis paniculata, in alcohol-induced HepG2
cells and alcohol plus a haloalkane, CCl4, induced liver
damage in rats. Evid Based Complement Alternat Med.
2020(6428906)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ou TT, Kuo CY, Chyau CC, Lee HJ, Peng JS
and Wang CJ: Improvement of lipopolysaccharide-induced hepatic
injuries and inflammation with mulberry extracts. J Sci Food Agric.
93:1880–1886. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hua J, Yin N, Yang B, Zhang J, Ding J, Fan
Y and Hu G: Ginkgolide B and bilobalide ameliorate neural cell
apoptosis in α-synuclein aggregates. Biomed Pharmacother.
96:792–797. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Cao A and Li X: Bilobalide protects H9c2
cell from oxygen-glucose-deprivation-caused damage through
upregulation of miR-27a. Artif Cells Nanomed Biotechnol.
47:2980–2988. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Mao D, Li H, Zhang L, Xu J, Yu C and Zhang
Q: Bilobalide alleviates IL-17-induced inflammatory injury in ATDC5
cells by downregulation of microRNA-125a. J Biochem Mol Toxicol.
33(e22405)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhan X, Zhang J, Chen H, Liu L, Zhou Y,
Zheng T, Li S, Zhang Y, Zheng B and Gong Q: . Capsaicin alleviates
acetaminophen-induced acute liver injury in mice. Clin Immunol.
220(108578)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Du YC, Lai L, Zhang H, Zhong FR, Cheng HL,
Qian BL, Tan P, Xia XM and Fu WG: Kaempferol from Penthorum
chinense Pursh suppresses HMGB1/TLR4/NF-κB signaling and NLRP3
inflammasome activation in acetaminophen-induced hepatotoxicity.
Food Funct. 11:7925–7934. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Del Campo JA, Gallego P and Grande L: Role
of inflammatory response in liver diseases: Therapeutic strategies.
World J Hepatol. 10:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Xu R, Zhang Z and Wang FS: Liver fibrosis:
Mechanisms of immune-mediated liver injury. Cell Mol Immunol.
9:296–301. 2012.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Liu M, Xu Y, Han X, Yin L, Xu L, Qi Y,
Zhao Y, Liu K and Peng J: Dioscin alleviates alcoholic liver
fibrosis by attenuating hepatic stellate cell activation via the
TLR4/MyD88/NF-κB signaling pathway. Sci Rep.
5(18038)2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Chen Y, Zeng Z, Shen X, Wu Z, Dong Y and
Cheng JC: MicroRNA-146a-5p negatively regulates pro-inflammatory
cytokine secretion and cell activation in lipopolysaccharide
stimulated human hepatic stellate cells through inhibition of
toll-Like receptor 4 signaling pathways. Int J Mol Sci.
17(1076)2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wu G, Yang Q, Yu Y, Lin S, Feng Y, Lv Q,
Yang J and Hu J: Taurine inhibits Kupffer cells activation induced
by lipopolysaccharide in alcoholic liver damaged rats. Adv Exp Med
Biol 975 Pt. 2:789–800. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Song S, Chu L, Liang H, Chen J, Liang J,
Huang Z, Zhang B and Chen X: Protective effects of dioscin against
doxorubicin-induced hepatotoxicity via regulation of
Sirt1/FOXO1/NF-κb signal. Front Pharmacol. 10(1030)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Fu T, Wang S, Liu J, Cai E, Li H, Li P and
Zhao Y: Protective effects of alpha-mangostin against
acetaminophen-induced acute liver injury in mice. Eur J Pharmacol.
827:173–180. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lu L, Wu C, Lu BJ, Xie D, Wang Z, Bahaji
Azami NL, An YT, Wang HJ, Ye G and Sun MY: BabaoDan cures hepatic
encephalopathy by decreasing ammonia levels and alleviating
inflammation in rats. J Ethnopharmacol. 249(112301)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Liu Q, Jin Z, Xu Z, Yang H, Li L, Li G, Li
F, Gu S, Zong S, Zhou J, et al: Antioxidant effects of ginkgolides
and bilobalide against cerebral ischemia injury by activating the
Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones.
24:441–452. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Qin YR, Ma CQ, Wang DP, Zhang QQ, Liu MR,
Zhao HR, Jiang JH and Fang Q: Bilobalide alleviates
neuroinflammation and promotes autophagy in Alzheimer's disease by
upregulating lincRNA-p21. Am J Transl Res. 13:2021–2040.
2021.PubMed/NCBI
|
|
66
|
Zheng Y, Wu Z, Yi F, Orange M, Yao M, Yang
B, Liu J and Zhu H: By Activating Akt/eNOS Bilobalide B inhibits
autophagy and promotes angiogenesis following focal cerebral
ischemia reperfusion. Cell Physiol Biochem. 47:604–616.
2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Adcock IM: Transcription factors as
activators of gene transcription: AP-1 and NF-kappa B. Monaldi Arch
Chest Dis. 52:178–186. 1997.PubMed/NCBI
|
|
68
|
Mitchell JP and Carmody RJ: NF-κB and the
transcriptional control of inflammation. Int Rev Cell Mol Biol.
335:41–84. 2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866.
1999.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Freitas RHCN and Fraga CAM: NF-κB-IKKβ
pathway as a target for drug development: Realities, challenges and
perspectives. Curr Drug Targets. 19:1933–1942. 2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Lin TH, Pajarinen J, Lu L, Nabeshima A,
Cordova LA, Yao Z and Goodman SB: NF-κB as a therapeutic target in
inflammatory-associated bone diseases. Adv Protein Chem Struct
Biol. 107:117–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Fouad D, Badr A and Attia HA:
Hepatoprotective activity of raspberry ketone is mediated via
inhibition of the NF-κB/TNF-α/caspase axis and mitochondrial
apoptosis in chemically induced acute liver injury. Toxicol Res
(Camb). 8:663–676. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Amen Y, Sherif AE, Shawky NM, Abdelrahman
RS, Wink M and Sobeh M: Grape-leaf extract attenuates
alcohol-induced liver injury via interference with NF-κB signaling
pathway. Biomolecules. 10(558)2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Wu B, Wang R, Li S, Wang Y, Song F, Gu Y
and Yuan Y: Antifibrotic effects of Fraxetin on carbon
tetrachloride-induced liver fibrosis by targeting NF-κB/IκBα, MAPKs
and Bcl-2/Bax pathways. Pharmacol Rep. 71:409–416. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhang H, Cao N, Yang Z, Fang X, Yang X, Li
H, Hong Z and Ji Z: Bilobalide alleviated dextran sulfate
sodium-induced experimental colitis by inhibiting M1 macrophage
polarization through the NF-κB signaling pathway. Front Pharmacol.
11(718)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820.
2010.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Guven-Maiorov E, Keskin O, Gursoy A and
Nussinov R: A structural view of negative regulation of the
toll-like receptor-mediated inflammatory pathway: Biophys. J.
109:1214–1226. 2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Chen Y, Lu Y, Pei C, Liang J, Ding P, Chen
S and Hou SZ: Monotropein alleviates secondary liver injury in
chronic colitis by regulating TLR4/NF-κB signaling and NLRP3
inflammasome. Eur J Pharmacol. 883(173358)2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zhang M, Xue Y, Chen H, Meng L, Chen B,
Gong H, Zhao Y and Qi R: Resveratrol inhibits MMP3 and MMP9
expression and secretion by suppressing TLR4/NF-κB/STAT3 activation
in Ox-LDL-greated HUVECs. Oxid Med Cell Longev.
2019(9013169)2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Tao Y, Wang Y, Wang X, Wang C, Bao K, Ji
L, Jiang G and Hong M: Calycosin suppresses epithelial derived
initiative key factors and maintains epithelial barrier in allergic
inflammation via TLR4 mediated NF-κB pathway. Cell Physiol Biochem.
44:1106–1119. 2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ke B, Shen XD, Ji H, Kamo N, Gao F,
Freitas MC, Busuttil RW and Kupiec-Weglinski JW: HO-1-STAT3 axis in
mouse liver ischemia/reperfusion injury: Regulation of TLR4 innate
responses through PI3K/PTEN signaling. J Hepatol. 56:359–366.
2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Kim SY, Jeong E, Joung SM and Lee JY:
PI3K/Akt contributes to increased expression of Toll-like receptor
4 in macrophages exposed to hypoxic stress. Biochem Biophys Res
Commun. 419:466–471. 2012.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Tsukamoto K, Hazeki K, Hoshi M, Nigorikawa
K, Inoue N, Sasaki T and Hazeki O: Critical roles of the p110 beta
subtype of phosphoinositide 3-kinasein lipopolysaccharide-induced
Akt activation and negative regulation of nitrite production in RAW
264.7 cells. J Immunol. 180:2054–2061. 2008.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Dong H, Zhang X, Dai X, Lu S, Gui B, Jin
W, Zhang S, Zhang S and Qian Y: Lithium ameliorates
lipopolysaccharide-induced microglial activation via inhibition of
toll-like receptor 4 expression by activating the PI3K/Akt/FoxO1
pathway. J Neuroinflammation. 11(140)2014.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Shi C, Wu F, Yew DT, Xu J and Zhu Y:
Bilobalide prevents apoptosis through activation of the PI3K/Akt
pathway in SH-SY5Y cells. Apoptosis. 15:715–727. 2010.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Sun H, Zhu X, Cai W and Qiu L: Hypaphorine
attenuates lipopolysaccharide-induced endothelial inflammation via
regulation of TLR4 and PPAR-γ dependent on PI3K/Akt/mTOR signal
pathway. Int J Mol Sci. 18(844)2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Zhaocheng J, Jinfeng L, Luchang Y, Yequan
S, Feng L and Kai W: Ginkgolide A inhibits
lipopolysaccharide-induced inflammatory response in human coronary
artery endothelial cells via downregulation of TLR4-NF-κB signaling
through PI3K/Akt pathway. Pharmazie. 71:588–591. 2016.PubMed/NCBI View Article : Google Scholar
|