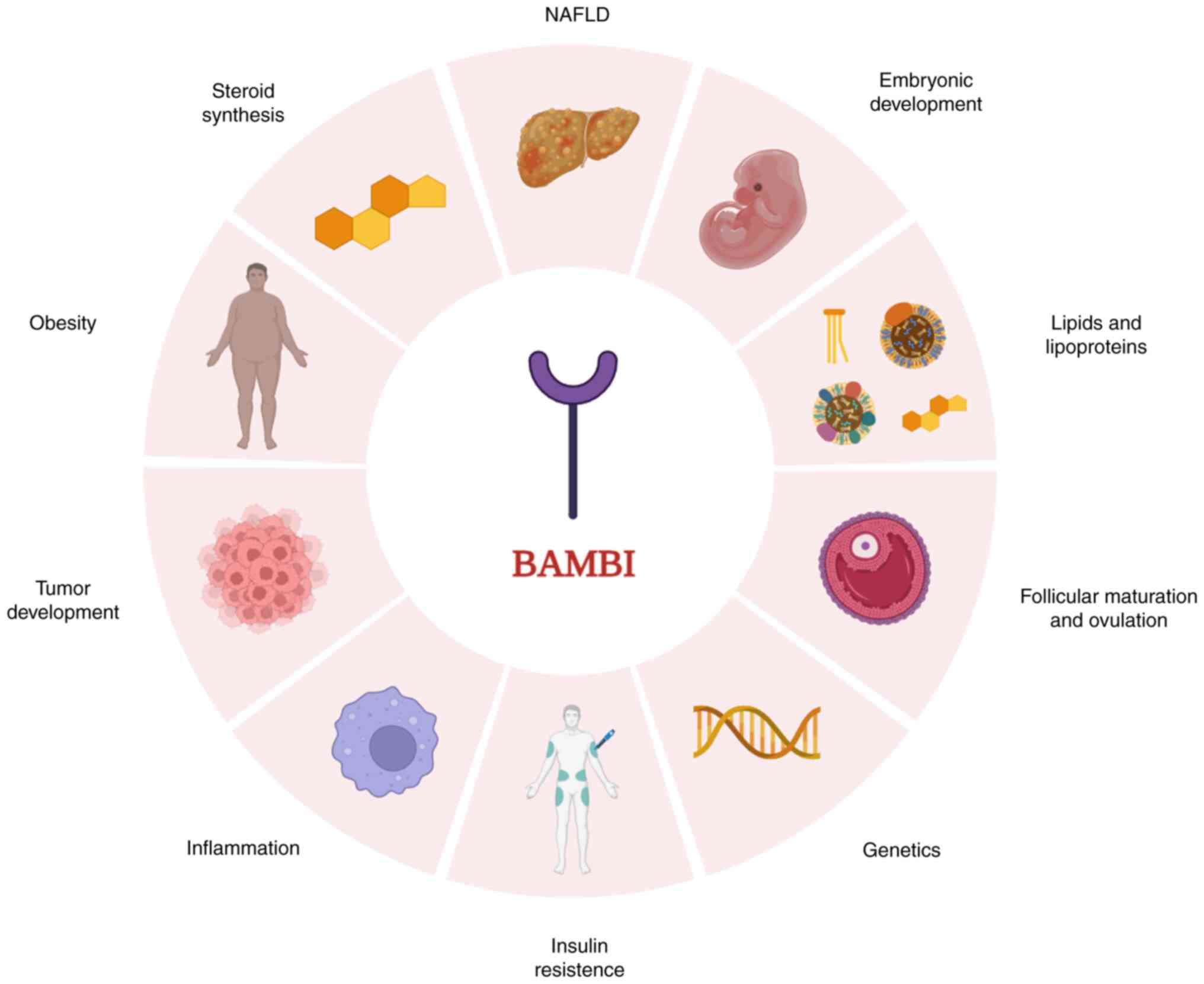
1. Introduction
BMP and activin receptor membrane-bound inhibitor
(BAMBI) was discovered by Onichtchouk et al (1) in 1999, and was considered a homologue
of human non-metastatic gene A due to the strong structural
similarity between the two molecules (2). BAMBI expression is highly
conserved in chordates, from fish to humans; however, its
expression patterns vary significantly among different animals. For
example, the BAMBI gene is highly expressed in human kidney
medulla, placenta and spleen tissues, but not in lung or muscle
tissues (2), whereas in mice,
Bambi is primarily expressed in heart, lung and testis
tissues (3). BAMBI has a broad
spectrum of effects, including effects on lipid metabolism through
inhibition of adipocyte lipid deposition (4), on myogenesis through promotion of
muscle stem cell proliferation and differentiation (5), on ovarian function through regulation
of steroidogenesis and follicle-stimulating hormone (FSH)
expression levels in porcine granulocytes (6), on inflammation through inhibition or
modulation of inflammatory processes (7) and on tumor development through
inhibition of tumor cell motility, invasion and survival (8). Given its important roles in
physiological and pathological conditions, BAMBI has increasingly
become the focus of research over the past two decades (Fig. 1).
2. Structural characteristics of BAMBI
BAMBI is a transmembrane glycoprotein
comprising 260 amino acids with an N-terminal extracellular domain
and a short C-terminal intracellular domain (9,10).
Furthermore, BAMBI contains numerous important
post-translational modification sites, including two protein kinase
C phosphorylation sites, six casein kinase phosphorylation sites,
three cAMP protein kinase sites and three N-acylation sites
(11,12). The structure of the extracellular
ligand-binding domain of BAMBI is similar to that of
transforming growth factor β receptor 1 (TGFβRI)/bone morphogenetic
protein receptor type 1 (BMPRI), while BAMBI lacks an
equivalent intracellular serine/threonine kinase structural domain
(9). Therefore, BAMBI
readily forms heterodimers with TGFβRI/BMPRI, which can
interfere with TGFβ or BMP pathways (10). Thus, BAMBI is considered a
pseudo-receptor in the TGFβ and BMP signaling pathways (1,3).
During binding of TGFβ family members to their receptors, BAMBI can
compete with type I TGFβ receptors for binding to type II TGFβ
receptors. Since the serine/threonine kinase structural domain is
not present in BAMBI, amino acid phosphorylation does not
occur, thus blocking TGFβ signaling pathway transduction (Fig. 2).
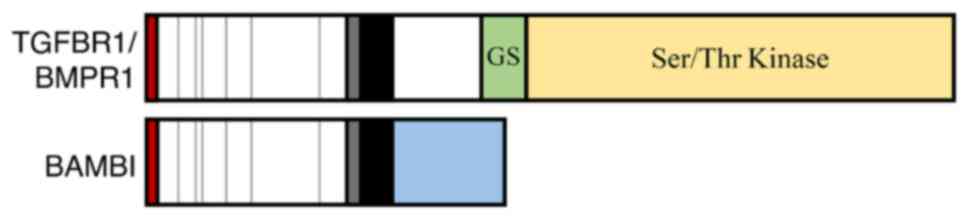 | Figure 2Schematic of BAMBI structure. BAMBI
is a 261-amino acid transmembrane protein involved in the
regulation of the TGFβ and Wnt signaling pathways. The structure of
the extracellular ligand-binding domain of BAMBI is similar to that
of TGFβRI/BMPRI, whereas BAMBI lacks an equivalent intracellular
Ser/Thr kinase domain. From left to right: red box, signal
sequence; white box, seven conserved cysteines (vertical lines) and
the putative extracellular domain of the cysteine box (gray);
black, transmembrane domain; green and yellow, GS and Ser/Thr
kinase domains, respectively, of TGFBRI/BMPRI; blue, intracellular
domain of BAMBI. TGFβ, transforming growth factor β; BAMBI, BMP and
activin receptor membrane-bound inhibitor; BMPR1, bone
morphogenetic protein receptor type 1; GS, glycine- and serine-rich
sequence; Ser/Thr, serine/threonine. |
3. Role of BAMBI in signal transduction
TGFβ signaling
BAMBI, a pseudo-receptor for TGFβ, inhibits
TGFβ signaling pathway transduction, and this inhibition is mainly
associated with the SMAD family molecules (13). Guillot et al (14) demonstrated that deletion of
BAMBI enhances phosphorylation of the TGFβ downstream
proteins, SMAD1/5 and ERK1/2, thereby delineating a
physiological role for BAMBI in endothelial environmental
homeostasis and angiogenesis regulation. In addition, BAMBI
can form a ternary complex with SMAD7 and the TGFβ
type I receptor, ALK5/TGFBRI, thus inhibiting the
interaction between ALK5/TGFBRI and SMAD3, and
ultimately affecting SMAD3 activation (15). Meanwhile, BAMBI and
SMAD7 can inhibit SMAD2 phosphorylation, and
decreased levels of Smad2 phosphorylation are associated
with gastric cancer invasion (15-17).
In addition, natural upregulation of BAMBI and SMAD7
expression affects the prognosis of patients with acute myeloid
leukemia (AML) (18). Hence, data
published to date demonstrate that BAMBI can affect gastric
cancer invasion, as well as serving as a novel biomarker for
predicting prognosis in patients with AML. Notably, although
BAMBI can inhibit TGFβ signaling, TGFβ can directly bind to
the BAMBI transcriptional promoter through SMAD3 and
SMAD4 to regulate BAMBI transcription, and SMAD3 and
SAMD4 can synergistically enhance its transcription (19) (Fig.
3, center panel).
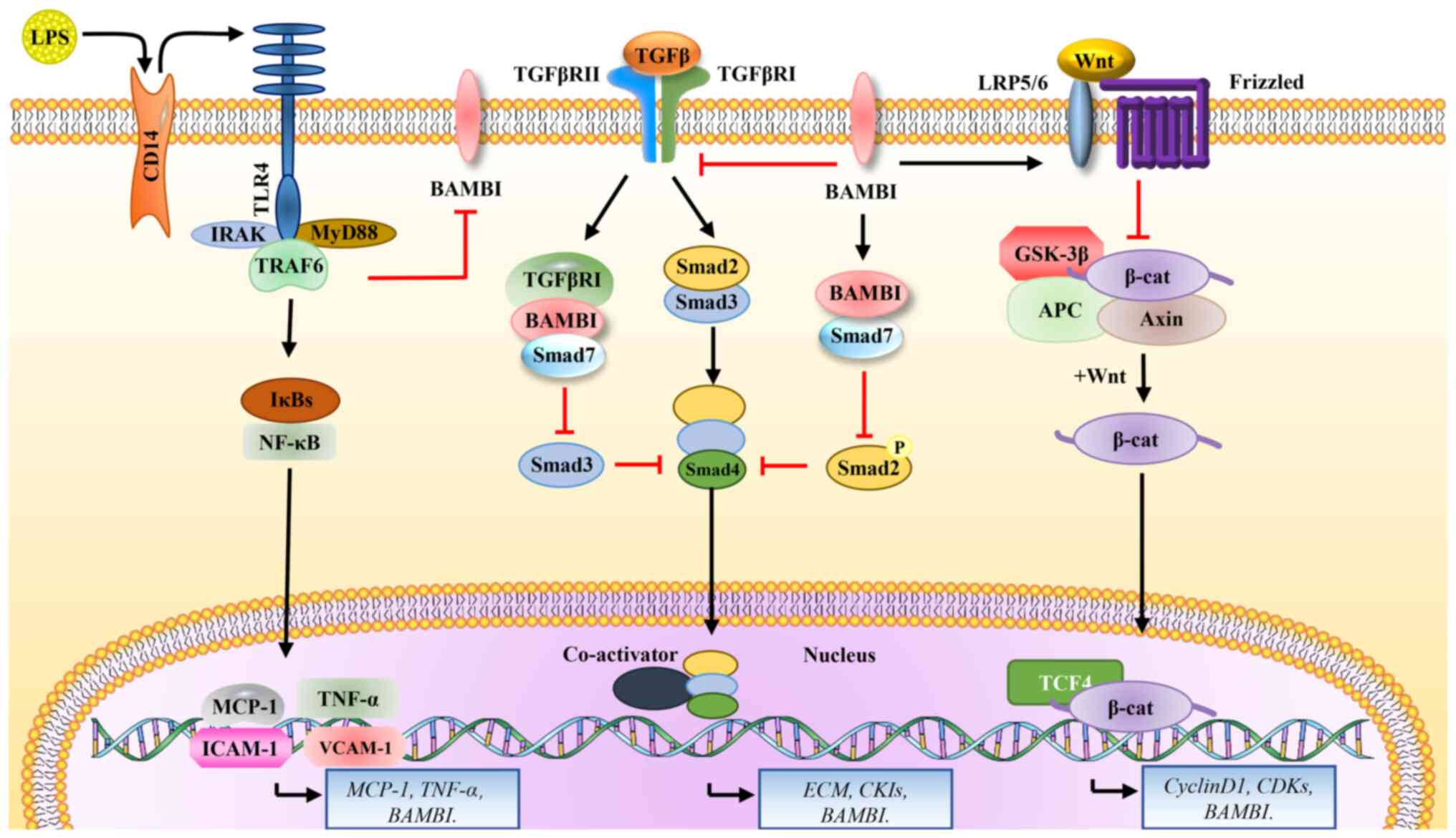 | Figure 3Signaling pathways regulated by
BAMBI. BAMBI binds and affects three receptors on the cell membrane
and activates downstream signaling pathways: i) LPS binds to the
membrane receptor, CD14, to activate TLR4 signaling, and also
downregulates BAMBI through MyD88-NF-κB-induced signaling, which in
turn enhances TGFβ signaling; ii) BAMBI inhibits TGFβ signaling
transduction, and this inhibition is mainly associated with the
SMAD family; iii) BAMBI activates the Wnt pathway by increasing
GSK3β phosphorylation and upregulating levels of unphosphorylated
β-catenin and downstream cyclin D1. LPS, lipopolysaccharide; CD14,
cluster of differentiation 14; TLR4, toll-like receptor 4; IRAK,
interleukin 1 receptor associated kinase 1; MyD88, myeloid
differentiation primary response 88; IκB, inhibitor of nuclear
factor κ B kinase subunit β; NFκB, nuclear factor-kB; MCP1, C-C
motif chemokine ligand 2; TNFα, tumor necrosis factor α; ICAM-1,
intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion
molecule 1; BAMBI, BMP and activin membrane-bound inhibitor; TGFβ,
transforming growth factor β1; TGFβRI, TGFβ receptor 1; Smad2, SMAD
family member 2; CKI, choline kinase α; LRP5, LDL receptor-related
protein 5; GSK-3β, glycogen synthase kinase 3 β; APC, APC regulator
of WNT signaling pathway; TCF4, transcription factor 4; CDKs,
cyclin-dependent kinases; ECM, extracellular matrix. |
Toll-like receptor 4 (TLR4)
signaling
TLRs are a class of pattern recognition receptors
that are mainly expressed on the surface of innate immune cells
(20). In 1997, the first
mammalian TLR, TLR4, was discovered in human monocytes
(21). When TLR4 is
activated, two signaling pathways are induced (22): The myeloid differentiation factor
88 (MyD88)-dependent pathway and the TIR domain bridging
protein-dependent pathway. The two pathways require the NF-κB
signaling pathway, which is a physiological regulator of the
transcription and secretion of pro-inflammatory factors (23) (Fig.
3, left panel).
Lipopolysaccharide (LPS) downregulates BAMBI
through MyD88/NF-κB-induced signaling, which in turn enhances TGFβ
signaling, thereby reducing liver fibrosis in MyD88-deficient mice
(24). It has been shown that the
expression level of BAMBI in the livers of patients with hepatitis
is significantly lower than in the livers of healthy individuals.
Liu et al (25) found that
LPS and tumor necrosis factor-α could further induce NF-κB
p50-histone deacetylase 1 interaction in hepatic stellate cells
(HSCs) to inhibit BAMBI transcription and ultimately enhance the
TGFβ signaling pathway. In addition, LPS promotes miR-942
expression via NF-κB p50, thereby inhibiting BAMBI expression at
the post-transcriptional level (26). He et al (27) found that the TLR4 inhibitor,
clio-095, could eliminate LPS-induced BAMBI downregulation,
suggesting that activation of the LPS/TLR4 axis may
downregulate BAMBI expression at the mRNA and protein levels.
Wanninger et al (28) found
that inhibitors of NF-κB activation partially inhibited metformin-
and lipocalin-mediated upregulation of BAMBI in human hepatocytes.
In another study, it was confirmed, by meta-analysis in publicly
available hepatocellular carcinoma data cohorts, that natural BAMBI
overexpression was present in 78% of patients with HCC (n=803), and
that it was also present and upregulated in cirrhotic samples and
the tumor stroma. Furthermore, upregulated BAMBI expression was
also confirmed in MDR2-KO mice (29). All these results suggest that the
rise and fall of BAMBI expression levels are inextricably linked to
the development of liver diseases.
LPS is also able to downregulate the expression
level of BAMBI (30,31), and at the same time, the activation
of bacterial autophagy is correlated with the LPS-mediated decrease
in BAMBI expression, revealing a correlation between autophagy
induced by bacterial infection and the expression level of BAMBI.
This effect needs to be realized by the activation of the LPS/TLR4
axis (32). BAMBI may therefore be
able to act as a biomarker of bacterial infection.
Wnt/β-catenin signaling
The Wnt/β-catenin pathway is a focus of intense
research in the field of Wnt signaling. In humans, Wnt ligands
comprise a large family of 19 glycoproteins (33). When Wnt signaling is activated, Wnt
ligands first bind to the frizzled class receptor (FZD) structural
domain, including the extracellular N-terminal cysteine-rich
structural region of the Wnt binding domain and a single
transmembrane co-receptor [low-density lipoprotein receptor-related
protein 5/6 (LRP5/LRP6)], to form the FZD-LRP receptor
complex (34,35). Subsequently, LRP6 is phosphorylated
and recruits axin to the cytoplasmic tail of LRP6 (36,37).
Next, LRP6 interacts with axin in the presence of scattered
proteins [dishevelled protein (DVL)] (38,39),
thus preventing β-catenin phosphorylation and proteasome
degradation. Finally, β-catenin accumulates in the cytoplasm and
translocates to the nucleus, thereby activating transcription of a
series of Wnt signaling target genes (40) (Fig.
3, right panel).
BAMBI is highly expressed in melanoma tissues and
may activate the Wnt signaling pathway by negatively regulating
miR-708, thus accelerating melanoma development (41). In gastric cancer cells,
BAMBI downregulation blocks translocation of β-catenin from
the cytoplasm to the nucleus, thus interfering with Wnt signaling
(42). Proper cellular trophoblast
invasion is a prerequisite for normal pregnancy. Inadequate human
trophoblast invasion leads to abnormal placental development,
resulting in a variety of pregnancy-related complications such as
preeclampsia and intrauterine growth restriction, all of which are
detrimental to the health of both the mother and the fetus
(43). Zhao et al (44) found that BMP2 treatment increased
BAMBI mRNA levels and activated Wnt signaling in human trophoblast
cells, including increasing levels of phosphorylated GSK3β
and upregulating unphosphorylated β-catenin and downstream
cytosolic cyclin D1 levels, suggesting that the upregulation of
BAMBI expression levels promotes human trophoblast cell invasion
and thus embryo development.
Numerous studies have linked altered Wnt signaling
to tumorigenesis (e.g., rectal cancer and osteosarcoma), and
BAMBI also plays an important role in tumorigenesis caused
by aberrant Wnt signaling activation. Fritzmann et al
(45) found that BAMBI
regulated metastasis in rectal cancer by linking the canonical Wnt
pathway, and that Wnt/β-catenin was further activated by
coactivators in the nucleus to regulate BAMBI expression.
Sekiya et al (46)
demonstrated that BAMBI expression levels in colorectal
tumor cell lines were inhibited by dominant-negative mutants of
T-cell factor 4 or inhibitors of β-catenin-TCF interactions.
Subramaniam et al (47)
demonstrated that BAMBI promotes cell proliferation and
survival through the Wnt/β-catenin pathway in HSCs, as well as
other cell lines. Zhou et al (48) found that BAMBI
overexpression in human osteosarcoma cells strongly induced the
transcription of catenin and Wnt-induced target genes, including
cyclin D1 and cell cycle protein-dependent kinases, and that
endogenous BAMBI knockdown by siRNA blocked the Wnt pathway.
Therefore, it is evident from the aforementioned results that BAMBI
is involved in the development of the aforementioned diseases
through the Wnt signaling pathway, and targeted intervention of
BAMBI and related factors of the Wnt signaling pathway may be a
novel approach for intervention or treatment of these diseases;
however, further basic and clinical experiments are needed for
specific application.
Overall, BAMBI is upregulated by Wnt signaling and
enhances the activity of this pathway; BAMBI overexpression
upregulates levels of Wnt target genes, while silencing or deletion
of BAMBI has the opposite effect.
4. Role of BAMBI in biological processes and
diseases
Adipogenesis
Obesity occurs due to an increase in the ratio of
caloric intake to energy expenditure, which leads to adipocyte
hypertrophy (49). The role of
BAMBI in lipid metabolism is relevant to research into obesity and
metabolic syndrome, as there is evidence that BAMBI may be involved
in regulating adipocyte differentiation and lipid metabolism
pathways, affecting energy homeostasis and lipid storage (4,50).
One study showed that BAMBI expression was significantly lower in
adipose tissue from patients with obesity than in individuals of
healthy weight, and 12 specific variant sites in BAMBI,
including R151W and H201R, were associated with obesity, indicating
a strong relationship between BAMBI and obesity (50). In this study, involving 677
children and adolescents with obesity and 529 lean control
individuals, researchers conducted mutation analysis of the BAMBI
coding region and intron-exon boundaries, and identified coding
region variants in 21 individuals with obesity compared with 5 lean
controls; the difference in variant frequency (3.1% in subjects
with obesity and 0.9% in lean controls) was significant (P=0.004).
However, there have been few studies on the role of BAMBI in
adiposity to date, and the role of BAMBI in adipogenesis has been
primarily explored at the cellular level. In 2012, Luo et al
(51) found that BAMBI could act
as a proximal effector of fibroblast growth factor 1 (FGF1)
in human adipocytes, and was also regulated by FGF1, with
BAMBI levels decreasing in cells that were treated with
FGF1. In addition, PI3K and ERK signaling pathways can also
regulate BAMBI expression, and TGFβ and Wnt/β-catenin signaling
pathways are affected by BAMBI, thus inhibiting
adipogenesis. In addition, Mai et al (52) found that BAMBI was
downregulated during porcine preadipocyte differentiation.
BAMBI inhibition increased adipogenesis, primarily through
the Wnt/β-catenin signaling pathway. The opposite phenomenon was
observed in BAMBI overexpressing cells, where a significant
increase in nuclear translocation of β-catenin was observed, which
inhibited adipogenic differentiation of adipocytes. By contrast,
Huang et al (53) conducted
dual luciferase reporter assays, which demonstrated that BAMBI was
a target gene of miR-106a during porcine preadipocytes
differentiation, and that miR-106a promotes lipogenic
differentiation of porcine preadipocytes by targeting BAMBI.
Yang et al (54)
demonstrated that BAMBI gene downregulation promoted bovine
preadipocyte differentiation and inhibited the myogenesis of
myoblasts. In our latest study (4), adipose-specific BAMBI knockout
mice (BAMBI AKO mice) were constructed. Phenotypically, the BAMBI
AKO mice exhibited an obese phenotype after high-fat feeding,
accompanied by insulin resistance and a fatty liver phenotype. In
addition, BAMBI knockdown promotes the lipogenic
differentiation of white and brown precursor adipocytes.
Mechanistically, BAMBI deletion caused an increased in
reactive oxygen species (ROS) levels in mitochondria and promoted
the mitotic clonal expansion stage of preadipocyte differentiation,
which in turn increased binding of CCAAT/Enhancer-Binding Protein β
(C/EBPβ) to downstream target genes and ultimately promoted
lipogenesis, representing a possible mechanism of adipogenesis
regulation through modulation of ROS levels in mice (4).
There is compelling evidence that non-alcoholic
fatty liver disease (NAFLD) is more common in individuals who are
overweight/obese, and the condition is closely associated with
lipid metabolism dysfunction in the liver (55,56).
Hepatic steatosis is a relatively benign stage of NAFLD, but can
leave the liver vulnerable to further damage (57). Non-alcoholic steatohepatitis (NASH)
is characterized by liver inflammation and can progress to liver
fibrosis, cirrhosis and hepatocellular carcinoma (58). One study reported that BAMBI
protein levels were low in human hepatic steatosis and that BAMBI
levels in the liver negatively correlated with body mass index
(BMI) (28). By contrast, in a
high-fat diet NASH model, immunohistochemical analysis revealed a
significant decrease in BAMBI protein levels in the liver (59). In addition, researchers reported a
significant increase in BAMBI protein levels in experimental NAFLD,
and improved hepatic oxidative stress and immune cell function
after inhibition of the TLR4 signaling pathway with sparstolonin
(59). Furthermore, Chen et
al (4) showed that BAMBI gene
deficiency may indirectly cause hepatic steatosis by promoting the
release of fatty acids from hypertrophic adipose tissue, rather
than by directly regulating adipogenesis in the liver. In
conclusion, there is a large body of evidence suggesting that
adipose tissue dysfunction is a key factor in NAFLD pathogenesis,
and that NAFLD leads to downregulation of BAMBI, while BAMBI
deficiency also causes NAFLD in high-fat diet models.
Myogenesis
BAMBI is also implicated in myogenesis, muscle
tissue maintenance and repair, possibly through effects on muscle
stem cell proliferation and differentiation (5,60).
Numerous studies have reported that both the TGFβ and the
Wnt/β-catenin pathways have regulatory roles during myogenesis,
while BAMBI can inhibit signal transduction via these
pathways in various cell types. Therefore, investigation of the
contribution of BAMBI to skeletal muscle myogenesis is warranted.
Zhang et al (60) showed
that BAMBI expression levels peaked during the early
differentiation stage of C2C12 myoblasts, while interfering with
BAMBI expression using siRNA inhibited C2C12 myoblast
differentiation and Wnt/β-catenin pathway activity. It was
concluded that BAMBI is required for C2C12 myoblast differentiation
and that its role in myogenesis is mediated by the Wnt/β-catenin
pathway. In general, in vivo studies provide better evidence
for the effects of genes on biological processes. Yao et al
(5) found that BAMBI
expression levels decreased gradually during skeletal muscle
development. Moreover, BAMBI was generally expressed at
higher levels in glycolytic muscle fibers.
Ovarian function
BAMBI may also be associated with germ cell
development and normal function of ovarian tissue, and its aberrant
expression can affect ovary-related reproductive physiological
processes (6,61). In the context of reproduction, the
results of the study by Bai et al (62) elegantly illustrated the role played
by BAMBI in pig and human granulosa cells. First, the
results revealed that BAMBI overexpression promoted the
expression of aromatase and steroidogenic acute regulatory protein
(StAR) in porcine primary granulosa cells, while mRNA and
protein levels of P450scc and 3b-HSD were not significantly
increased. In addition, levels of estradiol and progesterone in the
culture medium were significantly increased. Meanwhile, knockdown
of endogenous BAMBI reduced the mRNA expression levels of
cytochrome P450 family 19 subfamily A member 1 (Cyp19a1) and
StAR, as well as the estradiol and progesterone accumulation
levels. In human granulosa cells, Bai et al (13) reported that BMP2 activated
SMAD1/5/8 and upregulated BAMBI expression,
suggesting that BAMBI is a BMP target gene in human ovarian
granulosa cells that mediates negative feedback regulation of TGFβ
signaling in human ovaries. In another study, Bai et al
(63) found that stimulation of
porcine granulosa cells with FSH reduced BAMBI
expression levels, and concluded that FSH can inhibit
BAMBI expression in porcine luteinized granulosa cells.
Studies in porcine granulosa cells suggested that
BAMBI is involved in steroid synthesis, which is an
important physiological process affecting follicular maturation and
ovulation, and granulosa cells are the main cell type in follicles
that produce steroid hormones in response to FSH and
luteinizing hormone stimulation (64,65),
while TGFβ1 induces SMAD3 phosphorylation in porcine
granulosa cells (62).
Pre-transfection with BAMBI-overexpression adenovirus inhibited
TGFβ1-induced downregulation of estradiol and progesterone
production, and TGFβ1-induced SMAD3 phosphorylation
in porcine granulosa cells (62).
In cattle, TGFβ1 concentration was negatively correlated
with estradiol in follicular fluid, and TGFβ1 decreased
FSH-stimulated estradiol production in cultured granulosa cells
(66). These findings revealed a
potential mechanism by which BAMBI can regulate steroidogenesis in
porcine granulocytes.
Pigs possess genetic and protein variants similar to
those of humans, including genes associated with a number of human
diseases, such as Alzheimer's disease, Parkinson's disease and
obesity (67,68). In addition, pig internal organs are
arranged very similarly to those of humans, and pig hearts are
comparable in size and shape to those of humans. Although there are
similarities between humans and pigs at the genetic level, there
are also some differences (69).
Overall, however, the similarities are helpful for the study of
human disease models, as well as for other aspects of biomedical
research, and the pig as a model animal helps to further
characterize human disease and physiology (70). To conclude, the role of BAMBI in
enhancing porcine reproductive performance was reviewed and, given
the homology between human and porcine genes and proteins, it is
hypothesized that BAMBI is a potential physiological target for
enhancing female fertility and the treatment of infertility.
Inflammation
BAMBI has been found to be involved in regulating
inflammatory responses. It may play a role in inhibiting or
modulating inflammatory processes with potential anti-inflammatory
effects. Yang et al (7)
showed that BAMBI expression was significantly decreased in
a rat model of spinal cord injury. Overexpression of BAMBI
effectively reduced the expression of the mammalian target of
rapamycin gene, interleukin 1β (IL1β), IL6 and
IL10. These results suggested that BAMBI has
neuroprotective effects in rats with spinal cord injury and can
reduce the inflammatory response in rats. If these results are
validated in humans, it will be a new therapeutic option to
alleviate the neurological damage and inflammatory response caused
by spinal cord injury. MCP1 has been recognized as a key mediator
of renal fibrosis in chronic kidney diseases, including diabetic
nephropathy (71). The reduction
of MCP1 expression level implies that the level of inflammation as
well as the level of fibrosis in the kidneys is also further
reduced (72). In rat renal
tubular epithelial cells, interference with BAMBI promoted
ERK1/2 phosphorylation and TGFβ1-induced monocyte
chemotactic protein 1 (MCP1) expression. Conversely,
BAMBI overexpression inhibited ERK1/2 phosphorylation
and TGFβ1-induced MCP1 upregulation. Therefore, Liang et al
(73) suggested that in rat renal
tubular epithelial cells, metformin can inhibit TGFβ1-induced MCP1
expression through a BAMBI-mediated MEK/ERK1/2 signaling
pathway. Studies have also reported on the questionable efficacy of
metformin against cisplatin-induced renal cytotoxicity. The results
of the study by Li et al (74) suggested that metformin may prevent
cisplatin-induced tubular cell apoptosis and acute kidney injury by
stimulating AMPKα activation and inducing tubular cell autophagy.
Given this effect of metformin, the upregulation of BAMBI by
metformin could also alleviate cisplatin-induced renal cytotoxicity
by inhibiting the TGFβ signaling pathway. In addition, BAMBI can
regulate the inflammatory responses in different tissues (e.g.,
glioma and lung) by directly influencing macrophage proliferation
and differentiation (75,76). Furthermore, BAMBI
overexpression reduced the expression of TGFβ, IL1β,
IL6 and IL10 levels, suggesting that BAMBI can
play a neuroprotective role by reducing inflammatory responses in
rats with spinal cord injury (7).
Tumor development
Numerous studies have reported that BAMBI has
an important regulatory role in pathological processes such as
tumorigenesis and fibrogenesis, and is highly expressed in various
tumor cells and tissues, including ovarian cancer cells and
metastatic tumors (45,77). Human BAMBI gene expression
is downregulated in metastatic melanoma cell lines and high-grade
bladder cancer cells (2,78). Furthermore, the inhibitory effect
of BAMBI promoter hypermethylation on BAMBI
expression is an important epigenetic event affecting bladder
cancer cell invasion (78). In
this study, hypermethylation of the BAMBI promoter suppressed the
expression level of BAMBI, whereas a decrease in the expression
level of BAMBI activated the TGFβ pathway, which enabled the
promotion of tumor cell motility, invasion and survival. In an
in vitro study of BAMBI, injection of colon cancer
cells transfected with BAMBI overexpression plasmid into the
spleen tissue of nude mice resulted in rapid tumor formation and
metastasis of colon cancer cells to mouse liver and lymph nodes.
Further investigation of the regulatory mechanisms involved
revealed that high BAMBI expression in colon and liver
cancer cells impaired TGFβ-mediated inhibition of cancer
cell growth (46). BAMBI has also
been reported to promote the growth and invasion of human gastric
cancer cells (42,79,80).
Yuan et al (80) showed
that BAMBI overexpression inhibited the TGFβ/epithelial mesenchymal
transition signaling pathway and suppressed the invasiveness of
gastric tumors, whereas Zhang et al (6) demonstrated that BAMBI and
SMAD7 expression levels were both significantly elevated in
human gastric cancer tissues. Moreover, BAMBI and
SMAD7 inhibited the phosphorylation of SMAD2, and
decreased phosphorylated SMAD2 levels were associated with
tumor invasion and poor prognosis in patients with gastric cancer
(7,11,12).
These findings suggested that BAMBI and SMAD7 may
synergistically inhibit TGFβ signaling, thereby promoting gastric
cancer progression. In addition, Liu et al (42) found that BAMBI could also improve
the prognosis of gastric cancer by regulating the classical
Wnt/β-catenin pathway. To summarize, BAMBI can regulate the growth
and invasion of gastric cancer cells by modulating TGFβ and
Wnt/β-catenin signaling. By contrast, expression levels of the long
non-coding RNA PVT1 and BAMBI were significantly
increased during non-small cell lung cancer development, and
PVT1 could promote cell viability, migration and invasion
through miR-17-5p targeting of BAMBI, thus promoting
non-small cell lung cancer development (81).
Besides being involved in cancer development,
BAMBI is also associated with diseases such as liver
fibrosis. A previous study reported that BAMBI expression
was negatively correlated with donor BMI and was expressed at low
levels in human fibrosis-prone fatty liver lesions (28). Furthermore, BAMBI was
downregulated in rodent models of liver inflammation and fibrosis,
and that BAMBI and TLR4 were downregulated in
LPS-regulated liver fibrosis; however, hepatoprotective adiponectin
induced high BAMBI expression in human primary hepatocytes
(28). BAMBI mRNA and
protein levels were significantly reduced in patients with advanced
liver fibrosis, while BAMBI overexpression decreased the
mRNA levels of the fibrosis markers α-SMA, COL1 and
matrix metalloprotein in human HSCs (26). The aforementioned summarized
studies suggest that BAMBI may contribute to inflammatory
and fibrotic responses in various diseases.
Embryogenesis
Several studies have reported important regulatory
roles for BAMBI in embryonic development and organogenesis
(82-84),
although a previous BAMBI knockout mouse study reported that
BAMBI is not essential for embryonic development and
postnatal survival (84).
Onichtchouk et al (1)
showed that BAMBI was closely co-expressed with BMP4
in the early development of the African toad embryo, while another
group found that BAMBI expression is regulated by
BMP4 in mouse embryonic fibroblasts, and that BAMBI
spatiotemporal expression patterns are consistent with those of
BMP4 during mouse embryonic development (84).
In addition to regulating embryonic development,
BAMBI also regulates tooth formation. Gonzales et al
(85) reported that BAMBI
expression is elevated during tooth formation and is involved in
regulating the expression of the dental matrix proteins in the
MD10-A2 cell line, which normally regulate the control of dentin
mineralization (3). Furthermore,
Xavier et al (86) found
that BAMBI was expressed in kidney endothelial cells and human
umbilical vein endothelial cells, and inhibited endothelial cell
function. Furthermore, BAMBI knockdown increased capillary
generation and migration, while BAMBI overexpression
inhibited capillary generation and migration, and an in vivo
experiment showed that angiogenesis was increased in BAMBI
knockout mice (14).
In conclusion, dysregulation of BAMBI affects the
TGFβ pathway, which may lead to developmental disorders or
diseases; however, to date, no specific syndromes or disorders have
been associated with BAMBI dysregulation during embryonic
development. Therefore, further basic and clinical studies are
needed to determine whether any syndromes or diseases are
associated with BAMBI dysregulation.
5. Conclusions and perspectives
Recent trends in molecular medicine have centered
on the elaboration of the signaling pathways that control the
functions of different tissues. Understanding these pathways can
facilitate treatment, as well as prevention, of a wide range of
pathologies arising from abnormal molecular mechanisms. Since its
discovery in 1998, BAMBI has attracted considerable attention, due
to its involvement in various pathophysiological processes
associated with a number of diseases. In the present review, the
major physiological signaling pathways involving the function of
BAMBI and its associations with a range of diseases were
evaluated. As the role of BAMBI in regulating lipid
metabolism remains controversial, due to its inhibitory effect on
TGFβ, more research is needed to determine whether BAMBI influences
the development of obesity and diabetes through TGFβ signaling.
Although previous studies have reported a key role for BAMBI
in lipid metabolism, some conflicting results require further
discussion and clarification. Firstly, little is known about the
mechanisms that regulate BAMBI in various diseases.
Identification of key regulators may provide new insights into the
physiological functions of BAMBI and has potential to
facilitate development of BAMBI-based therapeutic
approaches. Secondly, the maximum dose of exogenous BAMBI
recombinant protein tolerated by patients has not been studied in
depth, nor has it been determined whether there are uncontrollable
side effects associated with BAMBI administration.
In brief, although BAMBI can inhibit the occurrence
of some tumors and provide protection for human health, at present,
BAMBI is not in use as part of a specific drug treatment in
clinical medicine, and more research is needed to explore this
possibility. Although large, complex and accurate gene regulatory
networks involving BAMBI have been discovered, further
investigation is required to provide an evidence base for the
application of BAMBI as a drug to treat various diseases.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the Key
Scientific Research Project of Education Department of Shaanxi
(grant no. 22JS033), the Natural Science Basic Research Program of
Shaanxi (grant no. 2023-JC-QN-0964), the Youth Innovation Team of
Shaanxi Universities (grant no. 202056) and the Xi'an Medical
University Scientific Research Fund (grant nos. 2021DOC13 and
2020DOC29).
Availability of data and materials
Not applicable.
Authors' contributions
XCC, DZ and QY conceived the manuscript and
summarized the contents of the manuscript. XCC, HG and LSZ were
responsible for the literature search and discussion. XCC and AQX
drafted the manuscript. XCC, JL and PHS wrote the revised
manuscript and prepared the figures. All authors read and approved
the final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Onichtchouk D, Chen YG, Dosch R, Gawantka
V, Delius H, Massagué J and Niehrs C: Silencing of TGF-beta
signalling by the pseudoreceptor BAMBI. Nature. 401:480–485.
1999.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Degen WG, Weterman MA, van Groningen JJ,
Cornelissen IM, Lemmers JP, Agterbos MA, Geurts van Kessel A, Swart
GW and Bloemers HP: Expression of nma, a novel gene, inversely
correlates with the metastatic potential of human melanoma cell
lines and xenografts. Int J Cancer. 65:460–465. 1996.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Knight C, Simmons D, Gu TT,
Gluhak-Heinrich J, Pavlin D, Zeichner-David M and MacDougall M:
Cloning, characterization, and tissue expression pattern of mouse
Nma/BAMBI during odontogenesis. J Dent Res. 80:1895–1902.
2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen X, Zhao C, Xu Y, Huang K, Wang Y,
Wang X, Zhou X, Pang W, Yang G and Yu T: Adipose-specific BMP and
activin membrane-bound inhibitor (BAMBI) deletion promotes
adipogenesis by accelerating ROS production. J Biol Chem.
296(100037)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yao X, Yu T, Xi F, Xu Y, Ma L, Pan X, Chen
S, Han M, Yin Y, Dai X, et al: BAMBI shuttling between cytosol and
membrane is required for skeletal muscle development and
regeneration. Biochem Biophys Res Commun. 509:125–132.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang Y, Guo Z, Du Z, Yao Z, Guo T, Cheng
Y, Wang K, Ma X, Chen C, Kebreab E, et al: Effects of BAMBI on
luteinized follicular granulosa cell proliferation and steroid
hormone production in sheep. Mol Reprod Dev. 90:153–165.
2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang Y, Guo C, Liao B, Cao J, Liang C and
He X: BAMBI inhibits inflammation through the activation of
autophagy in experimental spinal cord injury. Int J Mol Med.
39:423–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Weber F, Treeck O, Mester P and Buechler
C: Expression and function of BMP and activin membrane-bound
inhibitor (BAMBI) in chronic liver diseases and hepatocellular
carcinoma. Int J Mol Sci. 24(3473)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pawlak JB and Blobe GC: TGF-β superfamily
co-receptors in cancer. Dev Dyn. 251:137–163. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nickel J, Ten Dijke P and Mueller TD:
TGF-β family co-receptor function and signaling. Acta Bioch Bioph
Sin (Shanghai). 50:12–36. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kirsch T, Sebald W and Dreyer MK: Crystal
structure of the BMP-2-BRIA ectodomain complex. Nat Struct Biol.
7:492–496. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Luo W and Lin SC: Axin: A master scaffold
for multiple signaling pathways. Neurosignals. 13:99–113.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bai L, Chang HM, Cheng JC, Klausen C, Chu
G, Leung PCK and Yang G: SMAD1/5 mediates bone morphogenetic
protein 2-induced up-regulation of BAMBI expression in human
granulosa-lutein cells. Cell Signal. 37:52–61. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guillot N, Kollins D, Gilbert V, Xavier S,
Chen J, Gentle M, Reddy A, Bottinger E, Jiang R, Rastaldi MP, et
al: BAMBI regulates angiogenesis and endothelial homeostasis
through modulation of alternative TGFβ signaling. PLoS One.
7(e39406)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yan X, Lin Z, Chen F, Zhao X, Chen H, Ning
Y and Chen YG: Human BAMBI cooperates with Smad7 to inhibit
transforming growth factor-beta signaling. J Biol Chem.
284:30097–30104. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Paulsen M, Legewie S, Eils R, Karaulanov E
and Niehrs C: Negative feedback in the bone morphogenetic protein 4
(BMP4) synexpression group governs its dynamic signaling range and
canalizes development. Proc Natl Acad Sci USA. 108:10202–10207.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu Y, Li Q, Zhou X, Yu J, Mu Y, Munker S,
Xu C, Shen Z, Müllenbach R, Liu Y, et al: Decreased levels of
active SMAD2 correlate with poor prognosis in gastric cancer. PLoS
One. 7(e35684)2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shehata MM, Sallam AM, Naguib MG and
El-Mesallamy HO: Overexpression of BAMBI and SMAD7 impacts
prognosis of acute myeloid leukemia patients: A potential TERT
non-canonical role. Cancer Biomark. 31:47–58. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sekiya T, Oda T, Matsuura K and Akiyama T:
Transcriptional regulation of the TGF-beta pseudoreceptor BAMBI by
TGF-beta signaling. Biochem Biophys Res Commun. 320:680–684.
2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kashani B, Zandi Z, Pourbagheri-Sigaroodi
A, Bashash D and Ghaffari SH: The role of toll-like receptor 4
(TLR4) in cancer progression: A possible therapeutic target? J Cell
Physiol. 236:4121–4137. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the Drosophila Toll protein
signals activation of adaptive immunity. Nature. 388:394–397.
1997.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Federico S, Pozzetti L, Papa A, Carullo G,
Gemma S, Butini S, Campiani G and Relitti N: Modulation of the
innate immune response by targeting toll-like receptors: A
perspective on their agonists and antagonists. J Med Chem.
63:13466–13513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Koushki K, Shahbaz SK, Mashayekhi K,
Sadeghi M, Zayeri ZD, Taba MY, Banach M, Al-Rasadi K, Johnston TP
and Sahebkar A: Anti-inflammatory action of statins in
cardiovascular disease: The role of inflammasome and toll-like
receptor pathways. Clin Rev Allergy Immunol. 60:175–199.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Seki E, De Minicis S, Osterreicher CH,
Kluwe J, Osawa Y, Brenner DA and Schwabe RF: TLR4 enhances TGF-beta
signaling and hepatic fibrosis. Nat Med. 13:1324–1332.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Liu C, Chen X, Yang L, Kisseleva T,
Brenner DA and Seki E: Transcriptional repression of the
transforming growth factor β (TGF-β) Pseudoreceptor BMP and activin
membrane-bound inhibitor (BAMBI) by Nuclear Factor κB (NF-κB) p50
enhances TGF-β signaling in hepatic stellate cells. J Biol Chem.
289:7082–7091. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tao L, Xue D, Shen D, Ma W, Zhang J, Wang
X, Zhang W, Wu L, Pan K, Yang Y, et al: MicroRNA-942 mediates
hepatic stellate cell activation by regulating BAMBI expression in
human liver fibrosis. Arch Toxicol. 92:2935–2946. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
He Y, Ou Z, Chen X, Zu X, Liu L, Li Y, Cao
Z, Chen M, Chen Z, Chen H, et al: LPS/TLR4 signaling enhances TGF-β
response through downregulating BAMBI during prostatic hyperplasia.
Sci Rep. 6(27051)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wanninger J, Neumeier M, Bauer S, Weiss
TS, Eisinger K, Walter R, Dorn C, Hellerbrand C, Schäffler A and
Buechler C: Adiponectin induces the transforming growth factor
decoy receptor BAMBI in human hepatocytes. FEBS Lett.
585:1338–1344. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dediulia T: Expression analysis and
functional studies of Bone Morphogenetic Protein and Activin
Membrane-Bound Inhibitor (BAMBI) in hepatocellular carcinoma
(unpublished PhD thesis). Ruperto Carola University Heidelberg,
Heidelberg, 2019.
|
|
30
|
Tomita K, Teratani T, Suzuki T, Shimizu M,
Sato H, Narimatsu K, Okada Y, Kurihara C, Irie R, Yokoyama H, et
al: Free cholesterol accumulation in hepatic stellate cells:
Mechanism of liver fibrosis aggravation in nonalcoholic
steatohepatitis in mice. Hepatology. 59:154–169. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tomita K, Teratani T, Suzuki T, Shimizu M,
Sato H, Narimatsu K, Usui S, Furuhashi H, Kimura A, Nishiyama K, et
al: Acyl-CoA: Cholesterol acyltransferase 1 mediates liver fibrosis
by regulating free cholesterol accumulation in hepatic stellate
cells. J Hepatol. 61:98–106. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen M, Liu J, Yang W and Ling W:
Lipopolysaccharide mediates hepatic stellate cell activation by
regulating autophagy and retinoic acid signaling. Autophagy.
13:1813–1827. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Perugorria MJ, Olaizola P, Labiano I,
Esparza-Baquer A, Marzioni M, Marin JJG, Bujanda L and Banales JM:
Wnt-beta-catenin signalling in liver development, health and
disease. Nat Rev Gastroenterol Hepatol. 16:121–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cong F, Schweizer L and Varmus H: Wnt
signals across the plasma membrane to activate the beta-catenin
pathway by forming oligomers containing its receptors, Frizzled and
LRP. Development. 131:5103–5115. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zeng X, Tamai K, Doble B, Li S, Huang H,
Habas R, Okamura H, Woodgett J and He X: A dual-kinase mechanism
for Wnt co-receptor phosphorylation and activation. Nature.
438:873–877. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Davidson G, Wu W, Shen J, Bilic J, Fenger
U, Stannek P, Glinka A and Niehrs C: Casein kinase 1 gamma couples
Wnt receptor activation to cytoplasmic signal transduction. Nature.
438:867–872. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schwarz-Romond T, Fiedler M, Shibata N,
Butler PJ, Kikuchi A, Higuchi Y and Bienz M: The DIX domain of
Dishevelled confers Wnt signaling by dynamic polymerization. Nat
Struct Mol Biol. 14:484–492. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Vlad A, Rohrs S, Klein-Hitpass L and
Muller O: The first five years of the Wnt targetome. Cell Signal.
20:795–802. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lu HJ, Yan J, Jin PY, Zheng GH, Zhang HL,
Bai M, Wu DM, Lu J and Zheng YL: Mechanism of MicroRNA-708
Targeting BAMBI in cell proliferation, migration, and apoptosis in
mice with melanoma via the Wnt and TGF-β signaling pathways.
Technol Cancer Res Treat. 17(1533034618756784)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu K, Song X, Ma H, Liu L, Wen X, Yu J,
Wang L and Hu S: Knockdown of BAMBI inhibits β-catenin and
transforming growth factor β to suppress metastasis of gastric
cancer cells. Mol Med Rep. 10:874–880. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yuan H, Liu H, Liu Z, Zhu D, Amos CI, Fang
S, Lee JE and Wei Q: Genetic variants in Hippo pathway genes YAP 1,
TEAD 1 and TEAD 4 are associated with melanoma-specific survival.
Int J Cancer. 137:638–645. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhao HJ, Chang HM, Klausen C, Zhu H, Li Y
and Leung PCK: Bone morphogenetic protein 2 induces the activation
of WNT/β-catenin signaling and human trophoblast invasion through
up-regulating BAMBI. Cell Signal. 67(109489)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Fritzmann J, Morkel M, Besser D, Budczies
J, Kosel F, Brembeck FH, Stein U, Fichtner I, Schlag PM and
Birchmeier W: A colorectal cancer expression profile that includes
transforming growth factor beta inhibitor BAMBI predicts metastatic
potential. Gastroenterology. 137:165–175. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sekiya T, Adachi S, Kohu K, Yamada T,
Higuchi O, Furukawa Y, Nakamura Y, Nakamura T, Tashiro K, Kuhara S,
et al: Identification of BMP and activin membrane-bound inhibitor
(BAMBI), an inhibitor of transforming growth factor-beta signaling,
as a target of the beta-catenin pathway in colorectal tumor cells.
J Biol Chem. 279:6840–6846. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Subramaniam N, Sherman MH, Rao R, Wilson
C, Coulter S, Atkins AR, Evans RM, Liddle C and Downes M:
Metformin-mediated Bambi expression in hepatic stellate cells
induces prosurvival Wnt/beta-catenin signaling. Cancer Prev Res
(Phila). 5:553–561. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhou L, Park J, Jang KY, Park HS, Wagle S,
Yang KH, Lee KB, Park BH and Kim JR: The overexpression of BAMBI
and its involvement in the growth and invasion of human
osteosarcoma cells. Oncol Rep. 30:1315–1322. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Piché ME, Tchernof A and Després JP:
Obesity phenotypes, diabetes, and cardiovascular diseases. Circ
Res. 126:1477–1500. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Van Camp JK, De Freitas F, Zegers D,
Beckers S, Verhulst SL, Van Hoorenbeeck K, Massa G, Verrijken A,
Desager KN, Van Gaal LF and Van Hul W: Investigation of common and
rare genetic variation in the BAMBI genomic region in light of
human obesity. Endocrine. 52:277–286. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Luo X, Hutley LJ, Webster JA, Kim YH, Liu
DF, Newell FS, Widberg CH, Bachmann A, Turner N, Schmitz-Peiffer C,
et al: Identification of BMP and activin membrane-bound inhibitor
(BAMBI) as a potent negative regulator of adipogenesis and
modulator of autocrine/paracrine adipogenic factors. Diabetes.
61:124–136. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mai Y, Zhang Z, Yang H, Dong P, Chu G,
Yang G and Sun S: BMP and activin membrane-bound inhibitor (BAMBI)
inhibits the adipogenesis of porcine preadipocytes through
Wnt/β-catenin signaling pathway. Biochem Cell Biol. 92:172–182.
2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Huang K, Shi X, Wang J, Yao Y, Peng Y,
Chen X, Li X and Yang G: Upregulated microRNA-106a promotes porcine
preadipocyte proliferation and differentiation by targeting
different genes. Genes (Basel). 10(805)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yang X, Ning Y, Mei C, Zhang W, Sun J,
Wang S and Zan L: The role of BAMBI in regulating adipogenesis and
myogenesis and the association between its polymorphisms and growth
traits in cattle. Mol Biol Rep. 47:5963–5974. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Polyzos SA, Kountouras J and Mantzoros CS:
Obesity and nonalcoholic fatty liver disease: From pathophysiology
to therapeutics. Metabolism. 92:82–97. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ferguson D and Finck BN: Emerging
therapeutic approaches for the treatment of NAFLD and type 2
diabetes mellitus. Nat Rev Endocrinol. 17:484–495. 2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Pouwels S, Sakran N, Graham Y, Leal A,
Pintar T, Yang W, Kassir R, Singhal R, Mahawar K and Ramnarain D:
Non-alcoholic fatty liver disease (NAFLD): A review of
pathophysiology, clinical management and effects of weight loss.
BMC Endocr Disord. 22(63)2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Younossi Z, Anstee QM, Marietti M, Hardy
T, Henry L, Eslam M, George J and Bugianesi E: Global burden of
NAFLD and NASH: Trends, predictions, risk factors and prevention.
Nat Rev Gastroenterol Hepatol. 15:11–20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Dattaroy D, Seth RK, Sarkar S, Kimono D,
Albadrani M, Chandrashekaran V, Hasson FA, Singh UP, Fan D,
Nagarkatti M, et al: Sparstolonin B (SsnB) attenuates liver
fibrosis via a parallel conjugate pathway involving P53-P21 axis,
TGF-beta signaling and focal adhesion that is TLR4 dependent. Eur J
Pharmacol. 841:33–48. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhang Q, Shi XE, Song C, Sun S, Yang G and
Li X: BAMBI Promotes C2C12 myogenic differentiation by enhancing
Wnt/β-Catenin Signaling. Int J Mol Sci. 16:17734–17745.
2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Loveland KL, Bakker M, Meehan T, Christy
E, von Schönfeldt V, Drummond A and de Kretser D: Expression of
Bambi is widespread in juvenile and adult rat tissues and is
regulated in male germ cells. Endocrinology. 144:4180–4186.
2003.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Bai L, Chu G, Wang W, Xiang A and Yang G:
BAMBI promotes porcine granulosa cell steroidogenesis involving
TGF-β signaling. Theriogenology. 100:24–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Bai L, Chu G, Mai Y, Zheng J, Wang W,
Zhang Q and Yang G: Identification and expression analyses of BAMBI
mediated by FSH in swine luteinizing granulosa cells.
Theriogenology. 82:1094–1101. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Baufeld A and Vanselow J: Increasing cell
plating density mimics an early post-LH stage in cultured bovine
granulosa cells. Cell Tissue Res. 354:869–880. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Matsuda F, Inoue N, Manabe N and Ohkura S:
Follicular growth and atresia in mammalian ovaries: Regulation by
survival and death of granulosa cells. J Reprod Dev. 58:44–50.
2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ouellette Y, Price CA and Carriere PD:
Follicular fluid concentration of transforming growth factor-beta1
is negatively correlated with estradiol and follicle size at the
early stage of development of the first-wave cohort of bovine
ovarian follicles. Domest Anim Endocrinol. 29:623–633.
2005.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Prather RS, Lorson M, Ross JW, Whyte JJ
and Walters E: Genetically engineered pig models for human
diseases. Annu Rev Anim Biosci. 1:203–219. 2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Perleberg C, Kind A and Schnieke A:
Genetically engineered pigs as models for human disease. Dis Model
Mech. 11(dmm030783)2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lunney JK, Van Goor A, Walker KE,
Hailstock T, Franklin J and Dai C: Importance of the pig as a human
biomedical model. Sci Transl Med. 13(eabd5758)2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Hou N, Du X and Wu S: Advances in pig
models of human diseases. Animal Model Exp Med. 5:141–152.
2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Du Q, Fu YX, Shu AM, Lv X, Chen YP, Gao
YY, Chen J, Wang W, Lv GH, Lu JF and Xu HQ: Loganin alleviates
macrophage infiltration and activation by inhibiting the MCP-1/CCR2
axis in diabetic nephropathy. Life Sci. 272(118808)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Tesch GH: MCP-1/CCL2: A new diagnostic
marker and therapeutic target for progressive renal injury in
diabetic nephropathy. Am J Physiol Renal Physiol. 294:F697–F701.
2008.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Liang D, Song Z, Liang W, Li Y and Liu S:
Metformin inhibits TGF-beta 1-induced MCP-1 expression through
BAMBI-mediated suppression of MEK/ERK1/2 signalling. Nephrology
(Carlton). 24:481–488. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Li J, Gui Y, Ren J, Liu X, Feng Y, Zeng Z,
He W, Yang J and Dai C: Metformin protects against
cisplatin-induced tubular cell apoptosis and acute kidney injury
via AMPKα-regulated autophagy induction. Sci Rep.
6(23975)2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Wang D, Chen X and Zhang R: BAMBI promotes
macrophage proliferation and differentiation in gliomas. Mol Med
Rep. 17:3960–3966. 2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Sun SW, Chen L, Zhou M, Wu JH, Meng ZJ,
Han HL, Miao SY, Zhu CC and Xiong XZ: BAMBI regulates macrophages
inducing the differentiation of Treg through the TGF-β pathway in
chronic obstructive pulmonary disease. Respir Res.
20(26)2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Pils D, Wittinger M, Petz M, Gugerell A,
Gregor W, Alfanz A, Horvat R, Braicu EI, Sehouli J, Zeillinger R,
et al: BAMBI is overexpressed in ovarian cancer and co-translocates
with Smads into the nucleus upon TGF-beta treatment. Gynecol Oncol.
117:189–197. 2010.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Khin SS, Kitazawa R, Win N, Aye TT, Mori
K, Kondo T and Kitazawa S: BAMBI gene is epigenetically silenced in
subset of high-grade bladder cancer. Int J Cancer. 125:328–338.
2009.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Sasaki T, Sasahira T, Shimura H, Ikeda S
and Kuniyasu H: Effect of Nma on growth inhibition by TGF-betaa in
human gastric carcinoma cell lines. Oncol Rep. 11:1219–1223.
2004.PubMed/NCBI
|
|
80
|
Yuan CL, Liang R, Liu ZH, Li YQ, Luo XL,
Ye JZ and Lin Y: Bone morphogenetic protein and activin
membrane-bound inhibitor overexpression inhibits gastric tumor cell
invasion via the transforming growth
factor-β/epithelial-mesenchymal transition signaling pathway. Exp
Ther Med. 15:5422–5430. 2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Wang Z, Zhang Q, Sun Y and Shao F: Long
Non-Coding RNA PVT1 Regulates BAMBI to promote tumor progression in
non-small cell lung cancer by sponging miR-17-5p. Onco Targets
Ther. 13:131–142. 2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Grotewold L, Plum M, Dildrop R, Peters T
and Rüther U: Bambi is coexpressed with Bmp-4 during mouse
embryogenesis. Mech Dev. 100:327–330. 2001.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Chen J, Bush JO, Ovitt CE, Lan Y and Jiang
R: The TGF-beta pseudoreceptor gene Bambi is dispensable for mouse
embryonic development and postnatal survival. Genesis. 45:482–486.
2007.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Higashihori N, Song Y and Richman JM:
Expression and regulation of the decoy bone morphogenetic protein
receptor BAMBI in the developing avian face. Dev Dyn.
237:1500–1508. 2008.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Gonzales CB, Simmons D and MacDougall M:
Competing roles of TGFbeta and Nma/BAMBI in odontoblasts. J Dent
Res. 89:597–602. 2010.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Xavier S, Gilbert V, Rastaldi MP, Krick S,
Kollins D, Reddy A, Bottinger E, Cohen CD and Schlondorff D: BAMBI
is expressed in endothelial cells and is regulated by
lysosomal/autolysosomal degradation. PLoS One.
5(e12995)2010.PubMed/NCBI View Article : Google Scholar
|