Introduction
As the proportion of the population aged ≥65 years
is globally rapidly increasing, the number of patients with
dementia is expected to double within the next 20 years (1-4).
With this aging global population, a number of studies have
predicted an upcoming dementia epidemic, based on the frequently
observed notion that cognitive decline is associated with advancing
age (3-5).
Dementia is one of the most incapacitating diseases in elderly
patients, representing 1/6 of all disability adjusted life years in
older individuals (4,5). Alzheimer's disease (AD) is the most
prevalent type of dementia, accounting for 60-70% of the ~55
million individuals with dementia worldwide (1-4).
Therefore, early detection of dementia would allow the prompt
implementation of measures that can potentially delay the cognitive
impairment or even prevent the associated neurodegeneration.
Cognitive impairment and dementia are widely
considered to be the biggest contributors to the drain of resources
associated with patient care compared with that incurred by other
chronic diseases (such as cardiovascular) and impairments (6). The associated burden of care has been
previously found to be related to the severity of dementia. The
cognitive and functional decline this disease causes, negatively
affects the patient's ability to be independent and to engage in
daily activities. The rising prevalence rates of dementia due to
population aging and growth, in addition to the absence of curative
therapies, cast a somber picture for the future of patients with
dementia (3).
Depression has been increasingly reported as being
one of the leading causes of psychiatric and medical morbidity and
mortality amongst the elderly population. It is estimated that
10-15% of the AD cases may be attributed to depression, whereas 25%
reduction in the prevalence rate of depression may result in the
decrease in global cases of AD by 827,000(7). In addition, available data are
suggesting that depression occurs in 20-30% patients with AD,
though this proportion is likely to be even higher in patients
diagnosed with vascular dementia or Lewy body dementia (8). In particular, late-life depression
has been associated with a 2X increase in the risk of developing
different types of dementia (9).
The majority of previous studies that focused on the relationship
between depression and dementia, especially that caused by AD,
reported that there is a positive association between the two
entities. There is accumulating evidence supporting both hypotheses
that early life depression is a risk factor for dementia in later
life and later-life depression is a prodrome to dementia (1).
Age of onset of the first depressive episode has
been reported to be associated highly with depression length and
burden, meaning that it may be applied as a useful marker for
phenotypic distinction. The etiopathogenicity of depression is
multifactorial, with various factors observed to influence both the
severity and age of debut. Early-onset depression is considered to
be associated with family history and genetic predisposition,
whilst late-life depression appears to be more associated with the
vascular burden and other pathological degenerative processes.
Patients suffering from depression early in life may experience
cognitive impairment due to lengthier depressive episodes, leading
to hippocampal atrophy, increased allostatic load and reduced brain
reserve (10,11). In addition, several studies have
revealed that patients with late-life depression exhibited more
significant cognitive impairment at baseline followed by
significant subsequent decline, whilst patients with early-onset
depression tended to suffer from substantial impairment later,
followed by an important consecutive decline (11-13).
There is also substantiation from previous clinical
studies demonstrating that both conditions exhibit similar
neurobiological changes, such as white matter disease, suggesting
either shared risk factors or shared pattern of neuronal damage.
Various biomarkers that can be assessed by neuroimaging or
laboratory testing of biological samples, such as functional
impairment, neuronal loss and protein deposition, were previously
compared. Several neuropathological abnormalities were found to be
commonly associated with depression, including amyloid depositions,
cerebral or hippocampal atrophy, reduced volume in the basal
ganglia and prefrontal regions and high levels cerebrovascular
injury-inducing inflammatory plasma markers and glucocorticoids
(13,14). These findings suggest similarities
between the two clinical entities, although different cognitive
stages or manifestations may be present among patients with
depression with similar biomarker profiles (2,15).
However, the current clinical assessment methods lack measurements
for daily clinical practice that can be used to identify the early
risk of dementia in patients manifesting unspecific symptoms. The
present study aimed at validating the hypothesis of the link
between early depression and consecutive dementia, providing an
in-depth analisys of the correlations between relevant clinical
parameters measured comparatively at baseline when depression was
diagnosed and later in life when dementia was diagnosed.
Materials and methods
The present study is a retrospective study designed
to analyze the relevant demographic and clinical parameters of
depression in a study group of 103 patients aged >60 years (35
men and 78 women, mean age -74.77 years with standard deviation
7.305) who were hospitalized within a period of 9 years (January
2013-December 2021) in two centers, namely ‘Elisabeta Doamna’
Psychiatric Hospital of Galati (Galati, Romania) and in the
Geriatric Clinic ‘St. Apostle Andrei’ Clinical Emergency County
Hospital in Galati (Galati, Romania). These patients were diagnosed
with AD during the follow-up period, using the definitions of
Diagnostic and Statistical Manual of Mental Disorders, Fifth
Edition (1,12). Inclusion criteria referred to: age
>60 years old, patients previously diagnosed with depression,
and valid cognitive assessment performed both at depression onset
and at the moment AD was diagnosed. Exclusion criteria contained
age <60 years old, lack of complete cognitive assessment or lack
of patient consent for research use of data.
In total, the following two main milestones were
defined: The baseline was set as the onset of the depressive
disorder, whereas the comparison threshold was the moment of AD
diagnosis. The assessment was performed by the hospital
geriatrician, psychiatrist and psychologist based on the standard
clinical protocols (like the Geriatric Comprehensive
Assessment).
In the study group, cognitive function was assessed
using the following basic standard clinical tools: Mini Mental
State Evaluation (MMSE); Clock Drawing Test and Montreal Cognitive
Assessment (16-18).
The MMSE score values were used as a basis for the statistical
analysis.
The majority of patients (54.2%) included in the
present cohort study were evaluated using widely available cerebral
imaging techniques, namely native computed tomography (CT)-scanning
and/or magnetic resonance imaging (MRI), with all available imaging
data retrospectively analyzed.
Software used for statistical analysis was SPSS
Statistics 23 (IBM Corp.). Statistical analyses, such as
independent samples test-t-test for Equality of Means/Levene's test
for equality of variances, descriptive statistics for group
evaluation and the ANOVA method.
For ordinal values the Spearman correlation
coefficient (ρ) was calculated, in addition to the associated
probability, using the significance threshold α=0.05. A correlation
between the variables would be considered if P<0.05 was found
between the variables, whereas the P-value provides the correlation
degree, which range between +1 and -1. The correlation level closer
to +1 or -1 reflects a higher degree of correlation in direct
proportion for positive values (+) or inversed for negative values
(-), respectively. To check if the values are normally distributed,
the Kolmogorov Smirnov test was utilized. The values of φ, C, V
correlation coefficients were calculated for the nominal data
analysis in addition to their associated probabilities. A
χ2 test on these data was applied to see if there is an
association between variables.
Paired T-test for two independent samples was used
to test for statistically significant differences between mean
values of the same variable (cognitive function defined by the MMSE
score at the two set thresholds, namely the baseline of the
depression onset and the moment of AD diagnosis) in 2 subgroups
defined by sex (male and female), living background of patients
(rural/urban) or the manifestation of a certain symptom (defining
the subgroups of patients showing that particular symptom or not).
The equality of the variances was tested using the Levene's test,
with a statistical significance threshold at P<0.05.
The present retrospective analysis and subsequent
study publication was approved by the Hospital Ethics Committee
‘Elisabeta Doamna’ Psychiatric Hospital of Galati, (approval no.
4/11.03.2019); Hospital Ethics Committee of the ‘St. Apostle
Andrei’ Clinical Emergency County Hospital in Galati, (approval no.
29955/25.11.2022) and was conducted according to the Declaration of
Helsinki (19). No Artificial
Intelligence (AI) software was used for data collection,
statistical analysis or the preparation of the manuscript.
Results
Baseline characteristics
The study group included 103 patients diagnosed with
AD, with 35 (34%) men and 68 (66%) women and 59 (57.3%) of which
living in urban areas. The median age of the entire study group was
74.7 years old (60-89 years old range). From the perspective of
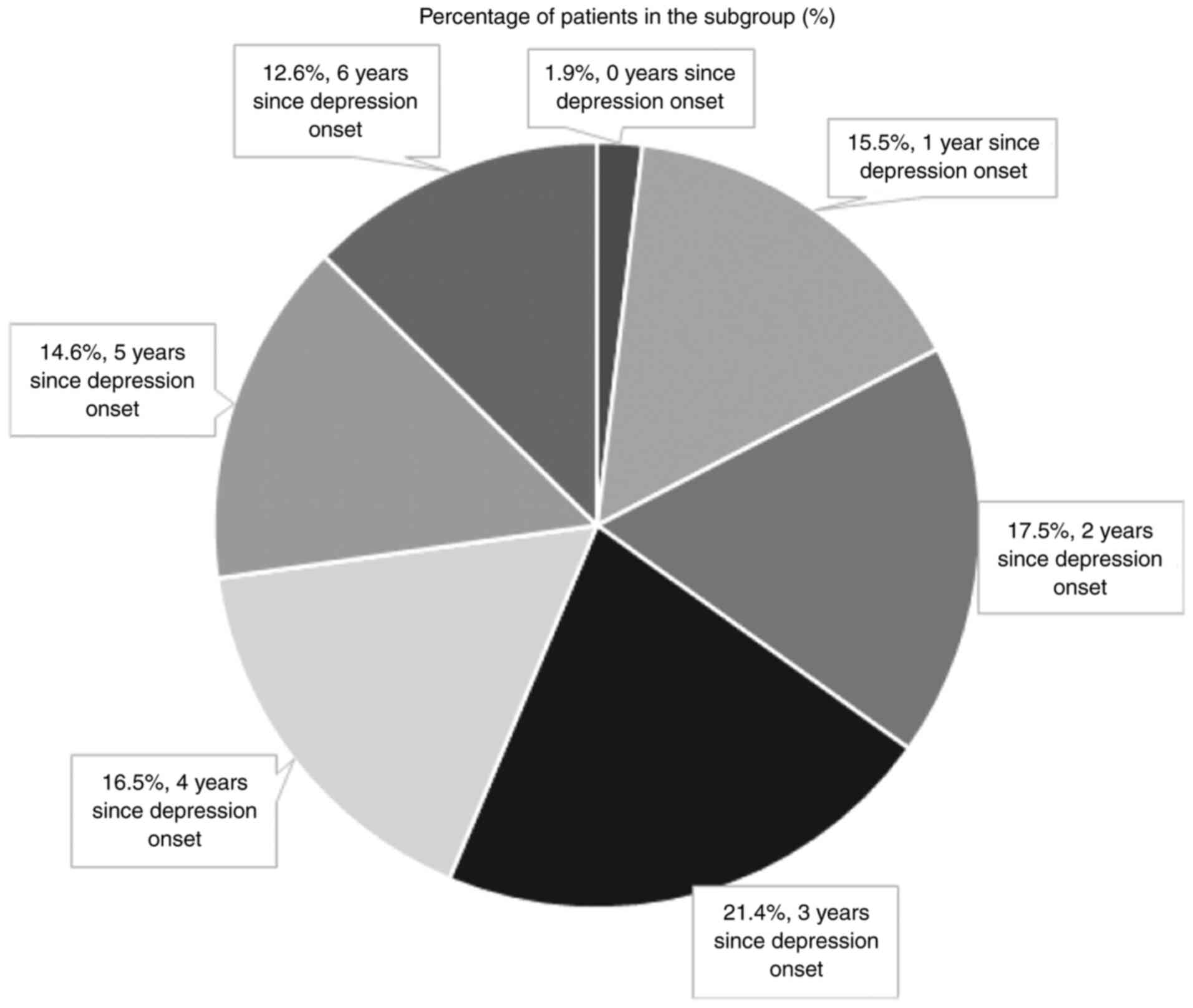
depressive symptoms onset, for the majority of patients this took
place <5 years before their AD diagnosis (75 cases, 72.8% of the
study sample; Fig. 1). These
patients were frequently admitted to hospital multiple times in
between the date of first depression onset and AD diagnosis, 50.5%
of whom being hospitalized ≥5 times for various
psycho-neurocognitive complaints. Specifically, the average number
of hospitalizations in the trial cohort was 6.5 (standard deviation
-4.8), with a total of 1,168 days of hospital stay (average, 11.3
days; range, 1-44 days, standard deviation -59.854). Regarding the
deterioration of cognitive deficiency in the present cohort study,
the mean MMSE scores varied from 25.39±1.981 at the moment of
depressive disorder diagnosis (range, 21-30) to 16.76±3.142 when
dementia was diagnosed (range, 8-22). The Kolmogorov Smirnov
confirmed that the MMSE scores follow a normal distribution.
Correlations identified between set
parameters
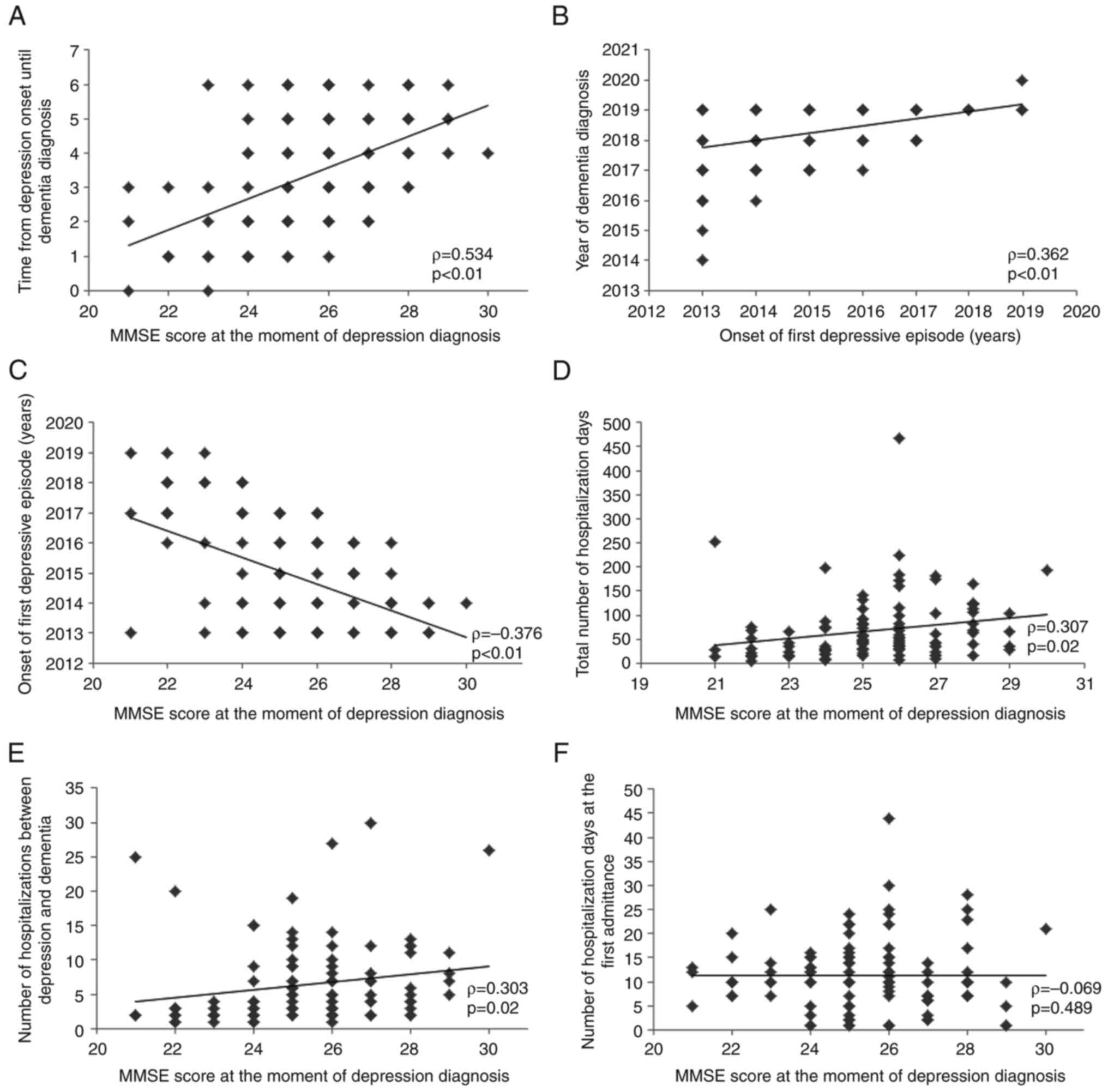
A strong correlation was identified between the
duration (years) from the onset of depression to dementia diagnosis
and the value of the MMSE score at the moment of depression
diagnosis (ρ=0.534; P<0.01; Fig.
2A). The onset of the first depressive episode was found to
correlate with the year of dementia diagnosis (ρ=0.362; P<0.01;
Fig. 2B), suggesting an earlier
onset of dementia if the depression onset was also earlier.
The onset of the first depressive episode was
correlated with the MMSE score at the moment of depressive
diagnosis (ρ=-0.376; P<0.01; Fig.
2C). Because this correlation index has a negative value, this
indicates an inversed proportionality between the two variables.
This suggests that a lower MMSE score is correlated with an earlier
onset of depression, therefore a more important cognitive
deficit.
The MMSE score at the moment of depression diagnosis
was found to be directly correlated with the total number of days
of hospitalization (ρ=0.307; P=0.02; Fig. 2D) and the number of
hospitalizations between depression onset and dementia diagnosis
(ρ=0.303; P=0.02; Fig. 2E), but
not with the duration of hospitalization at the first admittance
into a healthcare service (ρ=-0.069; P=0.489; Fig. 2F).
Analyzing the relationship between age and cognitive
function, no correlation could be found between these two
parameters. Age is not correlated with either the MMSE score at the
moment of depression diagnosis (ρ=0.066; P=0.507; data not shown)
or dementia (ρ=-0.159; P=0.109; data not shown). This suggests that
although cognitive decline is possibly associated with age, the
impact of age is limited in determining the evolution of cognition
between first depressive symptoms and the actual diagnosis of
dementia.
Symptoms associated to the onset of
the depression, analyzed by sex
Anhedonia and depressive mood were presented by all
study subjects, irrespective of sex. A difference was found with
regards to the tendency to frequent crying spells and tearfulness,
manifesting in ~50% of the study cohort but with different
incidences between men and women (20% of men and 66% of women).
With P<0.001, an association was found between crying and sex
(χ2=19.710; φ=0.437; Cramer's V=0.431; Contingency
coefficient=0.401; Table I).
 | Table ICorrelation analysis of easy crying
spells by sex. |
Table I
Correlation analysis of easy crying
spells by sex.
| A, Crosstabulation
for sex and easy crying spells |
|---|
| | Easy crying spells
and tearfulness | |
|---|
| Parameters | No | Yes | Total |
|---|
| Sex | | | |
|
Male | 28 | 7 | 35 |
|
Female | 23 | 45 | 68 |
| Total | 51 | 52 | 103 |
| B, Correlation
parameters for nominal variables |
| Parameters | | Value | P-value |
| Pearson chi-square
(χ2) | | 19.710 | <0.001 |
| Phi | | 0.437 | <0.001 |
| Cramer's V | | 0.431 | <0.001 |
| Contingency
coefficient | | 0.401 | <0.001 |
In addition, a direct but weak association was found
between sex and the loss of appetite as a depressive symptom
(χ2=13.352; φ=0.360; V=0.360; C=0.339; P-value <0.001
< α=0.05; data not shown). Specifically, 76.47% women manifested
a loss of their appetite, whilst only 40.0% men presented with this
symptom.
No association between sex and insomnia as a common
symptom for depression (χ2=2.046; P=0.145 and φ=0.141;
V=0.141; C=0.140; P=0.15305; data not shown) or psychomotor unrest
(χ2=0.281; P=0.596 and φ=V=C=0.052; P=0.59605; data not
shown) could be found.
Association analysis of cerebral
imaging
A significant association of sex with cerebral
abnormalities identified by native CT-scan/MRI at the moment of
dementia diagnosis was found (χ2=7.094; φ=0.262;
V=0.262; C=0.254; P=0.029). In the subgroup of women with modified
cerebral imaging, 25% presented cortical atrophy whereas 69.1% had
both cortical and subcortical atrophy. There is a different split
in the subgroup of men with cerebral imaging disturbances.
Specifically, 48.5% presented with cortical atrophy, none showing
only white matter abnormalities, whilst 51.4% presented both
generalized cortical and subcortical atrophy (Table II).
 | Table IICrosstabulation for sex and cerebral
abnormalities identified by native CT-scan/MRI imaging at the
moment of dementia diagnosis. |
Table II
Crosstabulation for sex and cerebral
abnormalities identified by native CT-scan/MRI imaging at the
moment of dementia diagnosis.
| A, Cerebral
abnormalities (native CT-scan/MRI imaging) at the moment of
dementia diagnosis |
|---|
| Sex | Cortical
atrophy | Subcortical
atrophy | Both cortical and
subcortical atrophy | Total |
|---|
|
Male | 17 | 0 | 18 | 35 |
|
Female | 17 | 4 | 47 | 68 |
| Total | 34 | 4 | 65 | 103 |
| B, Correlation
parameters for nominal variables |
| Parameters | | | Value | P-value |
| Pearson chi-square
(χ2) | | | 7.094 | 0.029 |
| Phi | | | 0.262 | 0.029 |
| Cramer's V | | | 0.262 | 0.029 |
| Contingency
coefficient | | | 0.254 | 0.029 |
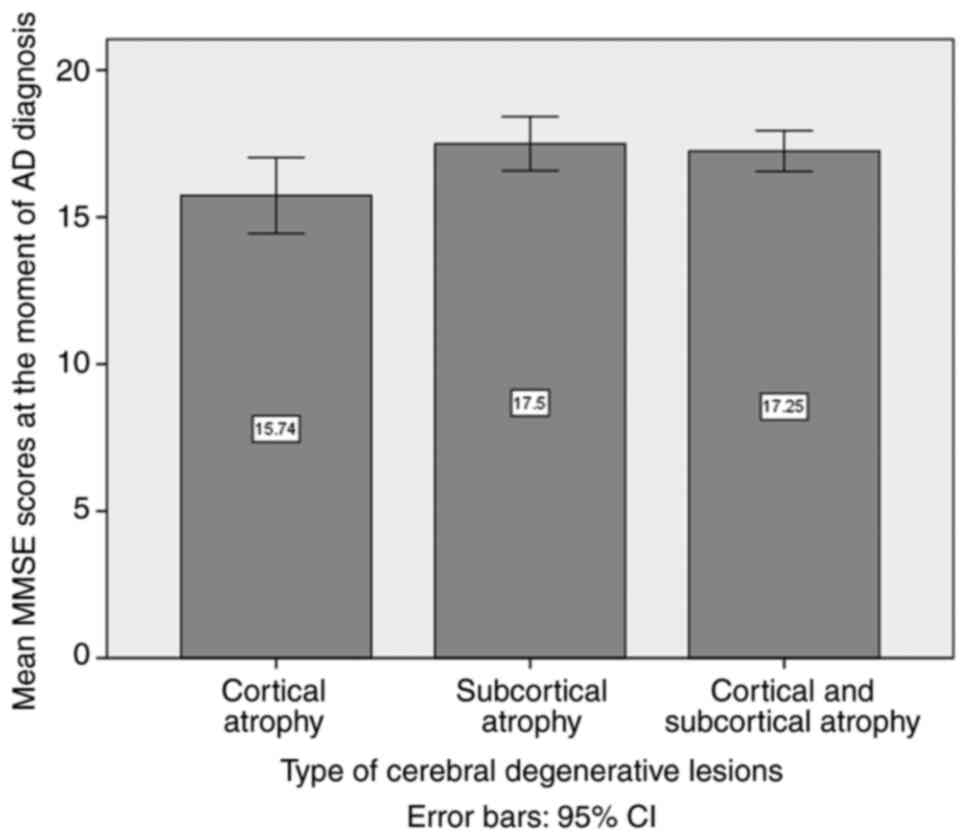
ANOVA of the mean MMSE scores at the moment of AD
diagnosis, comparing the three defined subgroups based on the type
of cerebral degenerative lesions, namely clustered as cortical,
subcortical and both cortical and subcortical atrophy, revealed no
statistically significant difference among the three patient
subgroups (P=0.066; Fig. 3).
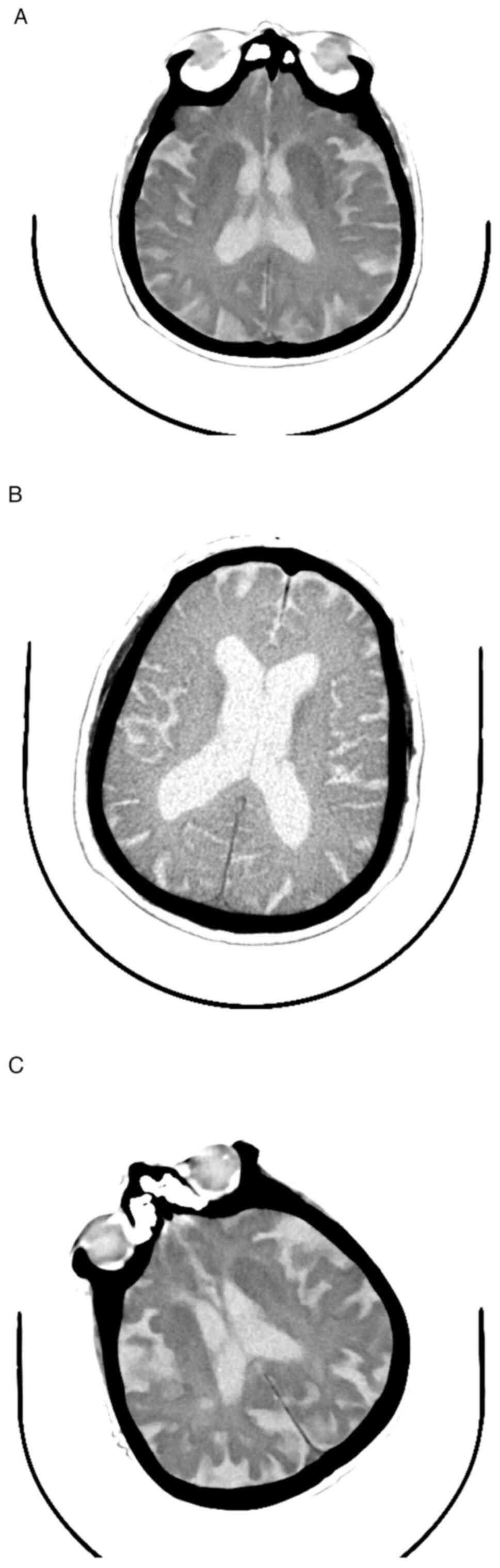
In the present patient sample lot, analysis of
cerebral atrophy was used as the main structural abnormality
detected by imaging (native CT-scanning or MRI), which revealed
that the majority of patients examined at the moment of AD
diagnosis were already presenting both cortical and white matter
atrophy (63.1% of the patients; Fig.
4A-C).
Cognitive function analysis using
t-test for two independent samples
Levene's test confirmed the equality of variances
for the MMSE score values at the moment of depression onset for the
two groups determined by sex. Variances were not equal in the case
of the MMSE scores the moment of AD diagnosis. The t-test for the
MMSE score at the moment of depression onset, considering the two
independent subgroups defined by sex, indicated that there was no
statistically significant difference between men and women (P=0.316
> α=0.05). However, at the moment of AD diagnosis, the situation
changed, with significantly higher MMSE values in the women
subgroup compared with those in the men subgroup (P=0.022; Table III).
 | Table IIIGroup statistics of cognitive status
by sex (unpaired t-test for equal variance; Welch's t-test for
unequal variance). |
Table III
Group statistics of cognitive status
by sex (unpaired t-test for equal variance; Welch's t-test for
unequal variance).
| A, Descriptive
statistics |
|---|
| Parameters | | Sex | N | Mean | Std. deviation | Std. error
mean | | | |
|---|
| MMSE score values
at the moment of depression onset | | Male | 35 | 25.11 | 2.069 | 0.350 | | | |
| | | Female | 68 | 25.53 | 1.935 | 0.235 | | | |
| MMSE score values
at the moment of dementia diagnosis | | Male | 35 | 15.66 | 3.694 | 0.624 | | | |
| | | Female | 68 | 17.32 | 2.673 | 0.324 | | | |
| B, Independent
samples t-test data analysis |
| | T-test for equality
of means |
| | Levene's test for
equality of variances | | 95% confidence
interval of the difference |
| Parameters | | F | P-value | t | P-value | Mean
difference | Std. error
difference | Upper | Lower |
| MMSE score values
at the moment of depression onset | Equal variances
assumed | 0.072 | 0.789 | -1.007 | 0.316 | -0.415 | 0.412 | -1.233 | 0.403 |
| MMSE score values
at the moment of dementia diagnosis | Equal variances not
assumed | 7.773 | 0.006 | -2.369 | 0.022 | -1.666 | 0.704 | -3.078 | -0.255 |
Clinical symptoms analysis using
t-test for two independent samples
Regarding clinical symptoms, a comparative t-test
could not be applied for anhedonia and depressive mood, as all
patients presented them when the depressive disorder occurred. For
crying and tearfulness, there was no statistically significant
difference between patients presenting these and those who did not
at the moment of depressive symptom onset regarding the MMSE
scores. Incidence of insomnia at the moment of the occurrence of
depression had no influence on the mean MMSE score value (P=0.254
> α=0.05; Table IV), stating
the same for psychomotor unrest (P=0.459 > α=0.05; Table V).
 | Table IVGroup statistics of cognitive status
by symptoms at the moment of depression onset; insomnia (unpaired
t-test for equal variance; Welch's t-test for unequal
variance). |
Table IV
Group statistics of cognitive status
by symptoms at the moment of depression onset; insomnia (unpaired
t-test for equal variance; Welch's t-test for unequal
variance).
| A, Descriptive
statistics |
|---|
| Parameters | | Manifesting
insomnia | N | Mean | Std. deviation | Std. error
mean | | | |
|---|
| MMSE score values
at the moment of depression onset | | No | 9 | 26.11 | 1.269 | 0.423 | | | |
| | | Yes | 94 | 25.32 | 2.028 | 0.209 | | | |
| B, Independent
samples t-test data analysis |
| | T-test for equality
of means |
| | Levene's test for
equality of variances | | 95% confidence
interval of the difference |
| Parameters | | F | P-value | t | P-value | Mean
difference | Std. error
difference | Upper | Lower |
| MMSE score values
at the moment of depression onset | Equal variances
assumed | 2.073 | 0.153 | 1.147 | 0.254 | 0.792 | 0.690 | 2.161 | -0.577 |
| | Equal variances not
assumed | 7.773 | 0.006 | 1.678 | 0.119 | 0.792 | 0.472 | 1.817 | -0.233 |
 | Table VGroup statistics of cognitive status
by symptoms at the moment of depression onset; psychomotor unrest
(unpaired t-test for equal variance; Welch's t-test for unequal
variance). |
Table V
Group statistics of cognitive status
by symptoms at the moment of depression onset; psychomotor unrest
(unpaired t-test for equal variance; Welch's t-test for unequal
variance).
| A, Descriptive
statistics |
|---|
| Parameters | | Manifesting
psychomotor unrest | N | Mean | Std. deviation | Std. error
mean | | | |
|---|
| MMSE score values
at the moment of depression onset | | No | 29 | 25.62 | 1.522 | 0.283 | | | |
| | | Yes | 74 | 25.30 | 2.137 | 0.248 | | | |
| B, Independent
samples t-test data analysis |
| | T-test for equality
of means |
| | Levene's test for
equality of variances | | 95% confidence
interval of the difference |
| Parameters | | F | P-value | t | P-value | Mean
difference | Std. error
difference | Upper | Lower |
| MMSE score values
at the moment of depression onset | Equal variances
assumed | 3.658 | 0.059 | 0.743 | 0.459 | 0.323 | 0.435 | 1.186 | -0.540 |
| | Equal variances not
assumed | | | 0.860 | 0.393 | 0.323 | 0.376 | 1.074 | -0.427 |
Regarding the MMSE scores at the moment of
depression occurrence were found to be higher for patients
reporting maintained appetite compared with those in patients who
lost appetite (P=0.032; Table
VI).
 | Table VIGroup statistics of cognitive status
by symptoms at the moment of depression onset; loss of appetite
(unpaired t-test for equal variance; Welch's t-test for unequal
variance). |
Table VI
Group statistics of cognitive status
by symptoms at the moment of depression onset; loss of appetite
(unpaired t-test for equal variance; Welch's t-test for unequal
variance).
| A, Descriptive
statistics |
|---|
| Parameters | | Manifesting loss of
appetite | N | Mean | Std. deviation | Std. error
mean | | | |
|---|
| MMSE score values
at the moment of depression onset | | No | 37 | 25.95 | 1.632 | 0.268 | | | |
| | | Yes | 66 | 25.08 | 2.100 | 0.258 | | | |
| B, Independent
samples t-test data analysis |
| | T-test for equality
of means |
| | Levene's test for
equality of variances | | 95% confidence
interval of the difference |
| Parameters | | F | P-value | t | P-value | Mean
difference | Std. error
difference | Upper | Lower |
| MMSE score values
at the moment of depression onset | Equal variances
assumed | 2.409 | 0.124 | 2.177 | 0.032 | 0.870 | 0.400 | 1.663 | 0.077 |
| | Equal variances not
assumed | | | 2.336 | 0.022 | 0.870 | 0.373 | 1.610 | 0.130 |
Independent paired t-test between the patient
subgroups with or without a certain clinical feature at the moment
of AD diagnosis revealed significantly lower MMSE scores in
patients presenting with aphasia (P<0.001; Table VII), agnosia (P<0.001;
Table VIII), temporal-spatial
disorientation (P=0.008; Table
IX), apraxia (P<0.001; Table
X), psychomotor agitation (P<0.001; Table XI) and hallucinations (P<0.001;
Table XII).
 | Table VIIGroup statistics of cognitive status
by symptoms at the moment of dementia diagnosis; aphasia (unpaired
t-test for equal variance; Welch's t-test for unequal
variance). |
Table VII
Group statistics of cognitive status
by symptoms at the moment of dementia diagnosis; aphasia (unpaired
t-test for equal variance; Welch's t-test for unequal
variance).
| A, Descriptive
statistics |
|---|
| Parameters | | Manifesting
aphasia | N | Mean | Std. deviation | Std. error
mean | | | |
|---|
| MMSE score values
at the moment of dementia diagnosis | | No | 9 | 19.44 | 1.424 | 0.475 | | | |
| | | Yes | 94 | 16.50 | 3.144 | 0.324 | | | |
| B, Independent
samples t-test data analysis |
| | T-test for equality
of means |
| | Levene's test for
equality of variances | | 95% confidence
interval of the difference |
| Parameters | | F | P-value | t | P-value | Mean
difference | Std. error
difference | Upper | Lower |
| MMSE score values
at the moment of dementia diagnosis | Equal variances
assumed | 5.697 | 0.019 | 2.772 | 0.007 | 2.944 | 1.062 | 5.051 | 0.838 |
| | Equal variances not
assumed | | | 5.122 | <0.001 | 2.944 | 0.575 | 4.158 | 1.731 |
 | Table VIIIGroup statistics of cognitive status
by symptoms at the moment of dementia diagnosis; agnosia (unpaired
t-test for equal variance; Welch's t-test for unequal
variance). |
Table VIII
Group statistics of cognitive status
by symptoms at the moment of dementia diagnosis; agnosia (unpaired
t-test for equal variance; Welch's t-test for unequal
variance).
| A, Descriptive
statistics |
|---|
| Parameters | | Manifesting
agnosia | N | Mean | Std. deviation | Std. error
mean | | | |
|---|
| MMSE score values
at the moment of dementia diagnosis | | No | 40 | 18.60 | 2.098 | 0.332 | | | |
| | | Yes | 63 | 15.59 | 3.145 | 0.396 | | | |
| B, Independent
samples t-test data analysis |
| | T-test for equality
of means |
| | Levene's test for
equality of variances | | 95% confidence
interval of the difference |
| Parameters | | F | P-value | t | P-value | Mean
difference | Std. error
difference | Upper | Lower |
| MMSE score values
at the moment of depression onset | Equal variances
assumed | 10.394 | 0.002 | 5.346 | <0.001 | 3.013 | 0.564 | 4.131 | 1.895 |
| | Equal variances not
assumed | | | 5.830 | <0.001 | 3.013 | 0.517 | 4.038 | 1.988 |
 | Table IXGroup statistics of cognitive status
by symptoms at the moment of dementia diagnosis; temporal-spatial
disorientation (unpaired t-test for equal variance; Welch's t-test
for unequal variance). |
Table IX
Group statistics of cognitive status
by symptoms at the moment of dementia diagnosis; temporal-spatial
disorientation (unpaired t-test for equal variance; Welch's t-test
for unequal variance).
| A, Descriptive
statistics |
|---|
| Parameters | | Manifesting
temporalspatial disorientation | N | Mean | Std. deviation | Std. error
mean | | | |
|---|
| MMSE score values
at the moment of dementia diagnosis | | No | 20 | 18.15 | 2.277 | 0.509 | | | |
| | | Yes | 83 | 16.42 | 3.239 | 0.356 | | | |
| B, Independent
samples t-test data analysis |
| | T-test for equality
of means |
| | Levene's test for
equality of variances | | 95% confidence
interval of the difference |
| Parameters | | F | P-value | t | P-value | Mean
difference | Std. error
difference | Upper | Lower |
| MMSE score values
at the moment of dementia diagnosis | Equal variances
assumed | 6.432 | 0.013 | 2.252 | 0.026 | 1.728 | 0.767 | 3.251 | 0.206 |
| | Equal variances not
assumed | | | 2.783 | 0.008 | 1.728 | 0.621 | 2.984 | 0.473 |
 | Table XGroup statistics of cognitive status
by symptoms at the moment of dementia diagnosis; apraxia (unpaired
t-test for equal variance; Welch's t-test for unequal
variance). |
Table X
Group statistics of cognitive status
by symptoms at the moment of dementia diagnosis; apraxia (unpaired
t-test for equal variance; Welch's t-test for unequal
variance).
| A, Descriptive
statistics |
|---|
| Parameters | | Manifesting
apraxia | N | Mean | Std. deviation | Std. error
mean | | | |
|---|
| MMSE score values
at the moment of dementia diagnosis | | No | 53 | 17.94 | 2.735 | 0.376 | | | |
| | | Yes | 50 | 15.50 | 3.079 | 0.435 | | | |
| B, Independent
samples t-test data analysis |
| | T-test for equality
of means |
| | Levene's test for
equality of variances | | 95% confidence
interval of the difference |
| Parameters | | F | P-value | t | P-value | Mean
difference | Std. error
difference | Upper | Lower |
| MMSE score values
at the moment of dementia diagnosis | Equal variances
assumed | 2.539 | 0.114 | 4.264 | <0.001 | 2.443 | 0.573 | 3.580 | 1.307 |
| | Equal variances not
assumed | | | 4.294 | <0.001 | 2.443 | 0.575 | 3.585 | 1.302 |
 | Table XIGroup statistics of cognitive status
by symptoms at the moment of dementia diagnosis-psychomotor
agitation (unpaired t-test for equal variance; Welch's t-test for
unequal variance). |
Table XI
Group statistics of cognitive status
by symptoms at the moment of dementia diagnosis-psychomotor
agitation (unpaired t-test for equal variance; Welch's t-test for
unequal variance).
| A, Descriptive
statistics |
|---|
| Parameters | | Manifesting
psychomotor agitation | N | Mean | Std. deviation | Std. error
mean | | | |
|---|
| MMSE score values
at the moment of dementia diagnosis | | No | 47 | 17.89 | 2.513 | 0.367 | | | |
| | | Yes | 56 | 15.80 | 3.316 | 0.443 | | | |
| B, Independent
samples t-test data analysis |
| | T-test for equality
of means |
| | Levene's test for
equality of variances | | 95% confidence
interval of the difference |
| Parameters | | F | P-value | t | P-value | Mean
difference | Std. error
difference | Upper | Lower |
| MMSE score values
at the moment of dementia diagnosis | Equal variances
assumed | 7.365 | 0.008 | 3.549 | 0.001 | 2.090 | 0.589 | 3.258 | 0.922 |
| | Equal variances not
assumed | | | 3.634 | <0.001 | 2.090 | 0.575 | 3.231 | 0.949 |
 | Table XIIGroup statistics of cognitive status
by symptoms at the moment of dementia diagnosis; hallucinations
(unpaired t-test for equal variance; Welch's t-test for unequal
variance). |
Table XII
Group statistics of cognitive status
by symptoms at the moment of dementia diagnosis; hallucinations
(unpaired t-test for equal variance; Welch's t-test for unequal
variance).
| A, Descriptive
statistics |
|---|
| Parameters | | Manifesting
hallucinations | N | Mean | Std. deviation | Std. error
mean | | | |
|---|
| MMSE score values
at the moment of dementia diagnosis | | No | 62 | 17.63 | 2.638 | 0.335 | | | |
| | | Yes | 40 | 15.30 | 3.330 | 0.526 | | | |
| B, Independent
samples t-test data analysis |
| | T-test for equality
of means |
| | Levene's test for
equality of variances | | 95% confidence
interval of the difference |
| Parameters | | F | P-value | t | P-value | Mean
difference | Std. error
difference | Upper | Lower |
| MMSE score values
at the moment of dementia diagnosis | Equal variances
assumed | 4.254 | 0.042 | 3.923 | <0.001 | 2.329 | 0.594 | 3.507 | 1.151 |
| | Equal variances not
assumed | | | 3.732 | <0.001 | 2.329 | 0.624 | 3.574 | 1.084 |
Comorbidities analysis using t-test
for two independent samples
Another characteristic assessed was comorbidities
associated at the moment of AD diagnosis, yielding data consistent
with the previous hypothesis of etiopathological mechanisms of AD.
Whilst associations with high blood pressure were found (P<0.028
< α=0.05; data not shown), no such associations could be found
with dyslipidemia (P=0.591 > α=0.05; data not shown), cardiac
rhythm disorders (such as atrial fibrillation; P=0.545 > α=0.05;
data not shown) or diabetes (P=0.059 > α=0.05; data not shown).
From the analysis, diabetes mellitus, hypertension, cardiac rhythm
disorders, anemia, dyslipidemia, anxiety were found to be the most
frequently associated comorbidities with the cognitive
dysfunction.
In the present study, most likely due to small
sample size, there was no statistically significant differences
found following group division by the presence of diabetes
(P=0.059), hypercholesterolemia (P=0.591), anemia (P=0.894),
appetite dysregulation (P=0.246), cardiac rhythm disorders
(P=0.545), anxiety (P=0.370) and chronic alcohol abuse (P=0.384);
data not shown.
Discussion
There is accumulating evidence on the association
between depression and subsequent dementia diagnosis, suggesting an
increased risk of dementia following depression. A 2020 study of
the Lancet Commission on dementia (20) previously listed depression as one
of the 12 modifiable risk factors that can be addressed, resulting
in the prevention or delay of ≤40% of dementias.
In AD, amyloid-β plaques and neurofibrillary tangles
are extensively reported substrates that can induce disruption of
neural function, culminating in neuronal cell death. A relative
increase in non-neuronal cell numbers, both in the cerebral cortex
and subcortical white matter, can contribute to the reactive glial
cell response to neuronal death (21,22).
A number of previous studies have suggested that neurodegenerative
diseases are caused by cortical network dysfunction instead of
dysregulation in any isolated brain region. However, the isolated
brain regions that are selectively damaged are likely to act as
‘nodes’ in the pathological brain network (21-24).
This was proposed as the ‘network degradation hypotheses. According
to this hypothesis, the functional circuits may disconnect or
weaken due to misfolded protein aggregates, resulting in small and
selectively vulnerable neuron populations. Consequently, their
aberrant excitability disrupts neuronal homeostasis and function,
triggering a progressive degeneration of the entire functional
network (21-24).
In older adults, a deficiency in energy metabolism
may translate into chronic stress and depression. Stress-related
decreases in brain-derived neurotrophic factor levels, in addition
to other neurotrophic factors (for example, neurotrophins), can
aggravate the atrophy of main limbic structures, including the
hippocampus and prefrontal cortex, further impairing cognition and
awareness (23,24). Several brain proinflammatory
cytokines such as interleukin 1β (IL-1β) have been reported to
contribute to the pathogenesis of both major depressive disorder
(MDD) and AD. Specifically, these cytokines can activate the same
signaling pathways, leading to neuronal damage by triggering a
cascade of cellular and tissue alterations leading to various
conditions, such as chronic depression, neurodegenerative diseases
and other chronic immune diseases-for instance, rheumatoid
arthritis (23-27).
Although there are broad genetic data available for
AD, for genes such as Apolipoprotein E, MPKA and amyloid precursor
protein/presenilin-I, the majority of patients typically undergo
genetic testing already at the late stages of the disease, where no
intervention can prevent the occurrence of the disease and the
cognitive dysfunction has already become irreversible. Studies have
identified co-expressed genes for AD, type 2 diabetes mellitus
(T2DM) and MDD using bioinformatics analysis (27-29).
A total of seven co-deregulated genes, namely structural
maintenance of chromosomes 4, cell division cycle 27, hepatocyte
nuclear factor 1 homeobox A, RhoD, Cut-like homeobox 1, PDZ and LIM
Domain 5 and transthyretin, were considered to have a diagnostic
value for AD, T2DM and MDD (27).
Another genomic study previously provided evidence for a
significant causal genetic effect of depression on AD using the
analysis of single nucleotide polymorphism profiles (30,31).
AD is an insidious, age-related neurodegenerative
disorder that is clinically characterized by a variety of
neuropsychological alterations, such as impaired thinking ability,
cognitive decline, memory loss and behavioral impairment. By
contrast, depressive disorder can manifest as sadness that is
severe enough or persistent enough to interfere with a person's
function by reducing the affected individual's interest or pleasure
in activities (also known as anhedonia). While its diagnosis is
mainly clinical, depression may be triggered by hereditary,
neurotransmitter profile or neuroendocrine changes or different
psychosocial factors. In particular, changes in a number of
neurotransmitter levels can include the abnormal regulation of
cholinergic, catecholaminergic (noradrenergic or dopaminergic),
glutamatergic and serotonergic (5-hydroxytryptamine)
neurotransmission. Neuroendocrine dysregulation may also be a
pathological factor, with particular emphasis on the following
three axes: Hypothalamic-pituitary-adrenal,
hypothalamic-pituitary-thyroid and hypothalamic-pituitary-growth
hormone (32,33). A number of these pathways were
added to the therapeutic arsenal that clinicians use to manage
depression at the earliest possible opportunity, where proof of
changing the course towards cognitive impairment exists.
The main objective of the present study was to
assess if there were any correlations between different demographic
or clinical parameters to characterize the associations between the
time of dementia onset and depressive symptom onset. Results from
the present study partially support those previous reported
studies, such as the direct correlation of the level of cognitive
alteration with an earlier onset of depression and the direct
association between the moment of depression onset and the one of
first AD manifestations (34-44).
By contrast, some conclusions from the present statistical analysis
were found to be contradictory to those reported by previous
studies, such as those regarding association with certain
comorbidities (cardiovascular or metabolic). This is likely to be
due to the small sample size of the study group and limited
information on the earlier medical history of the patients.
AD is associated with multiple comorbidities and
bio-medical conditions. A number of which are associated with
advanced age, whilst others may be triggered by various
pathological condition, such as hypercholesterolemia, hypertension,
T2DM, atherosclerosis, psychosis, depression, epilepsy and sleep
disorders (45,46). The comorbidities affecting patients
with AD can be regarded as either risk factors for AD, or
conditions arising as a consequence of the pathological processes
that occur during AD. Therefore, understanding the mechanism
underlying AD development and the influence of comorbidities on its
pathogenesis may be crucial for creating an individualized,
effective and comprehensive set of interventions for patients with
AD.
Drastic alterations in structural and functional
neuroimaging features are one of the main biomarkers in AD. AD
neuroimaging initiative (ADNI) is a currently ongoing, naturalistic
study designed to understand the structural, biochemical and
cognitive changes that occur during AD progression (47). ADNI provides evidence of increased
brain atrophy in several frontal brain areas. In addition, there is
available data suggesting a direct association between the
neuropsychiatric symptoms manifested by patients with AD and brain
morphology changes, mainly atrophy of the frontotemporal brain
structures (48-53).
The complex relationship linking dementia and
depression has several underlying neuropathological mechanisms,
such as increased hippocampal alterations and tangle formation in
patients with AD and a history of depression (49-53).
In addition, decreased cortical thickness, white matter loss and
alterations in the hippocampal region were associated with both
depressive symptoms and impairment of cognitive functions (49-53).
Regardless of the age at onset, patients with AD
will typically require lifelong treatment. Therefore, early
diagnosis and treatment will likely improve their long-term
prognosis and quality of life. Exploring how interventions can
effect the modifiable risk factors and how efficiently novel
biomarkers can be exploited to provide objective evidence for early
diagnosis, are in urgent demand. Furthermore, identification of
sensitive and specific novel biomarkers, in addition to actionable
and modifiable risk factors is currently a topic of intense
research. Effective dementia biomarkers aid in the early
identification and differential diagnosis, whilst also contribute
an essential step towards developing personalized approaches and
new drug therapies.
The present study has certain limitations that
should be considered when interpreting the results, especially when
validating previously accepted hypotheses regarding comorbidities.
The relatively small sample size, the type of the present
study-namely retrospective- and the lack of longitudinality, should
be considered. In addition, all patient cases were analyzed only at
the level of one academic center although the cases were gathered
from two hospitals. Nevertheless, a comprehensive set of clinical
characteristics was used to compare the two relevant thresholds in
the pathogenic progress of depression towards dementia to
thoroughly characterize the suggestive clinical features. The
analysis was performed on widely available, brief standard
examinations, where measures were taken to ensure the
generalizability of results from the perspective of patient lot
heterogenity. Therefore, the findings of the present study should
be suitable for clinicians in any type of clinic and not only
research centers.
Despite the wealth of research evidence over the
past two decades, the relationship between AD and depression
remains completely understood, where a highly complex interplay
involving multifactorial and diverse influences likely exists. If
aging, AD and depressive disorders share a common biological basis
in pathophysiology, any common therapeutic tools could- and
should-be investigated for their effects on prevention and
treatment.
To concude, the results of the present comprehensive
analysis on the association between AD dementia and
early-manifesting depression in geriatrically hospitalized patients
support the idea of standardizing a clinical assessment protocol
for evaluating the cognitive dysfunction in all patients with
depression and monitoring their progress as they age. Since
effective treatment methods for AD dementia remain scarce, patients
would benefit from the clinicians' preventive approach using the
identification of high-risk individuals and potentially modifiable
risk factors, namely depression.
Acknowledgements
Not applicable.
Funding
Funding: The article processing charges were supported by
‘Dunarea de Jos’ University of Galati (Romania) as academic support
with no influence on the research.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FS and VDO conceived the study. ML and AR developed
methodology and validated the data. VDO conducted formal analysis
and project administration, performed data visualization and wrote
the original draft of the manuscript. FS performed data curation
and conducted investigation. AR and ML wrote, reviewed and edited
the manuscript. ML supervised the study. AR acquired funding. All
authors have discussed the results and read and approved the final
version of the manuscript. FS, VDO and AR confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and was approved (approval no.
4/11.03.2019) as doctoral research by ‘Elisabeta Doamna’
Psychiatric Hospital Ethics Committee (Galati, Romania) and
additionally also approved by (approval no. 29955/25.11.2022)
Hospital Ethics Committee of the ‘St. Apostle Andrei’ Clinical
Emergency County Hospital (Galati, Romania). Due to the
retrospective nature of this study, individual patient consent for
use of their data was not necessary and was waived by the ethics
committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brzezińska A, Bourke J, Rivera-Hernández
R, Tsolaki M, Woźniak J and Kaźmierski J: Depression in dementia or
dementia in depression? Systematic review of studies and
hypotheses. Curr Alzheimer Res. 17:16–28. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Byers A and Yaffe K: Depression and risk
of developing dementia. Nat Rev Neurol. 7:323–331. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
WHO data-updated March 2023. Available
from: https://www.who.int/health-topics/dementia#tab=tab_2.
Accessed on 28 April 2023.
|
|
4
|
GBD 2016 Dementia Collaborators. Global,
regional, and national burden of Alzheimer's disease and other
dementias, 1990-2016: A systematic analysis for the global burden
of disease study 2016. Lancet Neurol. 18:88–106. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Javaid SF, Giebel C, Khan MA and Hashim
MJ: Epidemiology of Alzheimer's disease and other dementias: Rising
global burden and forecasted trends [version 1; peer review: 1
Approved with reservations]. F1000Res. 10(425)2021.
|
|
6
|
Sousa RM, Ferri CP, Acosta D, Albanese E,
Guerra M, Huang Y, Jacob KS, Jotheeswaran AT, Rodriguez JJ,
Pichardo GR, et al: Contribution of chronic diseases to disability
in elderly people in countries with low and middle incomes: A 10/66
dementia research group population-based survey. Lancet.
374:1821–1830. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Barnes DE and Yaffe K: The projected
effect of risk factor reduction on Alzheimer's disease prevalence.
Lancet Neurol. 10:819–828. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Enache D, Winblad B and Aarsland D:
Depression in dementia: Epidemiology, mechanisms, and treatment.
Curr Opin Psychiatry. 24:461–472. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Diniz BS, Butters MA, Albert SM, Dew MA
and Reynolds CF III: Late-life depression and risk of vascular
dementia and Alzheimer's disease: Systematic review and
meta-analysis of community-based cohort studies. Br J Psychiatry.
202:329–335. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Aizenstein HJ, Baskys A, Boldrini M,
Butters MA, Diniz BS, Jaiswal MK, Jellinger KA, Kruglov LS,
Meshandin IA, Mijajlovic MD, et al: Vascular depression consensus
report-a critical update. BMC Med. 14(161)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Taylor WD, Aizenstein HJ and Alexopoulos
GS: The vascular depression hypothesis: Mechanisms linking vascular
disease with depression. Mol Psychiatry. 18:963–974.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Riddle M, Potter GG, McQuoid DR, Steffens
DC, Beyer JL and Taylor WD: Longitudinal cognitive outcomes of
clinical phenotypes of late-life depression. Am J Geriatr
Psychiatry. 25:1123–1134. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Muliyala KP and Varghese M: The complex
relationship between depression and dementia. Ann Indian Acad
Neurol. 13 (Suppl 2):S69–S73. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Leonard BE: Inflammation, depression and
dementia: Are they connected? Neurochem Res. 32:1749–1756.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ly M and Andreescu C: Advances and
barriers for clinical neuroimaging in late-life mood and anxiety
disorders. Curr Psychiatry Rep. 20(7)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Palsetia D, Rao GP, Tiwari SC, Lodha P and
De Sousa A: The clock drawing test versus mini-mental status
examination as a screening tool for dementia: A clinical
comparison. Indian J Psychol Med. 40:1–10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kørner A, Lauritzen L, Abelskov K, Gulmann
N, Marie Brodersen A, Wedervang-Jensen T and Marie Kjeldgaard K:
The geriatric depression scale and the cornell scale for depression
in dementia. A validity study. Nord J Psychiatry. 60:360–364.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ismail Z, Rajji TK and Shulman KI: Brief
cognitive screening instruments: An update. Int J Geriatr
Psychiatry. 25:111–120. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
WMA Declaration of Helsinki-Ethical
Principles for Medical Research Involving Human Subjects-7th
revision, 2013. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
Accessed on 28.04.2023.
|
|
20
|
Livingston G, Huntley J, Sommerlad A, Ames
D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J,
Cooper C, et al: Dementia prevention, intervention, and care: 2020
Report of the lancet commission. Lancet. 396:413–446.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rastad S, Barjaste N, Lanjanian H, Moeini
A, Kiani F and Masoudi-Nejad A: Parallel molecular alteration
between Alzheimer's disease and major depressive disorder in the
human brain dorsolateral prefrontal cortex: An insight from gene
expression and methylation profile analyses. Genes Genet Syst.
97:311–324. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Błaszczyk JW: Pathogenesis of dementia.
Int J Mol Sci. 24(543)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Du F, Yu Q, Swerdlow RH and Waites CL:
Glucocorticoid-driven mitochondrial damage stimulates Tau
pathology. Brain. 146:4378–4394. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Phillips C: Brain-derived neurotrophic
factor, depression, and physical activity: Making the neuroplastic
connection. Neural Plast. 2017(7260130)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rapp MA, Schnaider-Beeri M, Wysocki M,
Guerrero-Berroa E, Grossman HT, Heinz A and Haroutunian V:
Cognitive decline in patients with dementia as a function of
depression. Am J Geriatr Psychiatry. 19:357–363. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Almeida OP, Hankey GJ, Yeap BB, Golledge J
and Flicker L: Depression as a modifiable factor to decrease the
risk of dementia. Transl Psychiatry. 7(e1117)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang Y, Geng R, Liu M, Deng S, Ding J,
Zhong H and Tu Q: Shared peripheral blood biomarkers for
Alzheimer's disease, major depressive disorder, and type 2 diabetes
and cognitive risk factor analysis. Heliyon.
9(e14653)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Réus GZ, Titus SE, Abelaira HM, Freitas
SM, Tuon T, Quevedo J and Budni J: Neurochemical correlation
between major depressive disorder and neurodegenerative diseases.
Life Sci. 158:121–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fanelli G, Franke B, De Witte W, Ruisch
IH, Haavik J, van Gils V, Jansen WJ, Vos SJB, Lind L, Buitelaar JK,
et al: Insulinopathies of the brain? Genetic overlap between
somatic insulin-related and neuropsychiatric disorders. Transl
Psychiatry. 12(59)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Harerimana NV, Liu Y, Gerasimov ES, Duong
D, Beach TG, Reiman EM, Schneider JA, Boyle P, Lori A, Bennett DA,
et al: Genetic evidence supporting a causal role of depression in
Alzheimer's disease. Biol Psychiatry. 92:25–33. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ni H, Xu M, Zhan GL, Fan Y, Zhou H, Jiang
HY, Lu WH, Tan L, Zhang DF, Yao YG and Zhang C: The GWAS risk genes
for depression may be actively involved in Alzheimer's disease. J
Alzheimers Dis. 64:1149–1161. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ghasemi M, Phillips C, Fahimi A, McNerney
MW and Salehi A: Mechanisms of action and clinical efficacy of NMDA
receptor modulators in mood disorders. Neurosci Biobehav Rev.
80:555–572. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yatawara C, Lim L, Chander R, Zhou J and
Kandiah N: Depressive symptoms influence global cognitive
impairment indirectly by reducing memory and executive function in
patients with mild cognitive impairment. J Neurol Neurosurg
Psychiatry. 87:1375–1383. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Holmquist S, Nordström A and Nordström P:
The association of depression with subsequent dementia diagnosis: A
Swedish nationwide cohort study from 1964 to 2016. PLoS Med.
17(e1003016)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gurgu M, Zamfirescu A, Stroie AM and Aurel
R: Cognitive impairment prevalence and correlations with subjective
memory impairment: Findings from Brasov, Romania. J Nutr Health
Aging. 16(862)2012.
|
|
36
|
Kane RL, Butler M, Fink HA, Brasure M,
Davila H, Desai P, Jutkowitz E, McCreedy E, Nelson VA, McCarten JR,
et al: Interventions to prevent age-related cognitive decline, mild
cognitive impairment, and clinical Alzheimer's-type dementia.
Comparative Effectiveness Review No. 188. (Prepared by the
Minnesota Evidence-based Practice Center under Contract No.
290-2015-00008-I.) AHRQ Publication No. 17-EHC008-EF. Rockville,
MD: Agency for Healthcare Research and Quality; March 2017.
www.effectivehealthcare.ahrq.gov/reports/final.cfm.
|
|
37
|
Amidfar M, Garcez ML and Kim YK: The
shared molecular mechanisms underlying aging of the brain, major
depressive disorder, and Alzheimer's disease: The role of circadian
rhythm disturbances. Prog Neuropsychopharmacol Biol Psychiatry.
123(110721)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Morgan SL, Naderi P, Koler K, Pita-Juarez
Y, Prokopenko D, Vlachos IS, Tanzi RE, Bertram L and Hide WA: Most
pathways can be related to the pathogenesis of Alzheimer's disease.
Front Aging Neurosci. 14(846902)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bou Khalil R, Khoury E and Koussa S:
Linking multiple pathogenic pathways in Alzheimer's disease. World
J Psychiatr. 6:208–214. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Du Y, Yan F, Zhao L, Fang Y, Qiu Q, Wei W,
Wang J, Tang Y, Lin X and Li X: Depression symptoms moderate the
relationship between gray matter volumes and cognitive function in
patients with mild cognitive impairment. J Psychiatr Res.
151:516–522. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Botto R, Callai N, Cermelli A, Causarano L
and Rainero I: Anxiety and depression in Alzheimer's disease: A
systematic review of pathogenetic mechanisms and relation to
cognitive decline. Neurol Sci. 43:4107–4124. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bature F, Guinn BA, Pang D and Pappas Y:
Signs and symptoms preceding the diagnosis of Alzheimer's disease:
A systematic scoping review of literature from 1937 to 2016. BMJ
Open. 7(e015746)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ismail Z, Gatchel J, Bateman DR,
Barcelos-Ferreira R, Cantillon M, Jaeger J, Donovan NJ and Mortby
ME: Affective and emotional dysregulation as pre-dementia risk
markers: Exploring the mild behavioral impairment symptoms of
depression, anxiety, irritability, and euphoria. Int Psychogeriatr.
30:185–196. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Morozova A, Zorkina Y, Abramova O, Pavlova
O, Pavlov K, Soloveva K, Volkova M, Alekseeva P, Andryshchenko A,
Kostyuk G, et al: Neurobiological highlights of cognitive
impairment in psychiatric disorders. Int J Mol Sci.
23(1217)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Oprea VD, Marinescu M, Rișcă Popazu C,
Sârbu F, Onose G and Romila A: Cardiovascular comorbidities in
relation to the functional status and vitamin D levels in elderly
patients with dementia. Diagnostics (Basel).
12(2994)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang JH, Wu YJ, Tee BL and Lo RY: Medical
comorbidity in Alzheimer's disease: A nested case-control study. J
Alzheimers Dis. 63:773–781. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Weber CJ, Carrillo MC, Jagust W, Jack CR
Jr, Shaw LM, Trojanowski JQ, Saykin AJ, Beckett LA, Sur C, Rao NP,
et al: The worldwide Alzheimer's disease neuroimaging initiative:
ADNI-3 updates and global perspectives. Alzheimer's Dement.
7(e12226)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li H, Tan CC, Tan L and Xu W: Predictors
of cognitive deterioration in subjective cognitive decline:
Evidence from longitudinal studies and implications for SCD-plus
criteria. J Neurol Neurosurg Psychiatry. 94:844–854.
2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sinclair LI and Ballard CG: Alzheimer's
Disease Neuroimaging Initiative. Persistent depressive symptoms are
associated with frontal regional atrophy in patients with
Alzheimer's disease. Int J Geriatr Psychiatry.
38(e5858)2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Walia N, Eratne D, Loi SM, Farrand S, Li
QX, Malpas CB, Varghese S, Walterfang M, Evans AH, Parker S, et al:
Cerebrospinal fluid neurofilament light and cerebral atrophy in
younger-onset dementia and primary psychiatric disorders. Intern
Med J. 53:1564–1569. 2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dintica CS, Habes M, Erus G, Simone T,
Schreiner P and Yaffe K: Long-term depressive symptoms and midlife
brain age. J Affect Disord. 320:436–441. 2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gerritsen L, Sigurdsson S, Jonsson PV,
Gudnason V, Launer LJ and Geerlings MI: Depressive symptom profiles
predict dementia onset and brain pathology in older persons. The
AGES-Reykjavik study. Neurobiol Aging. 111:14–23. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Siafarikas N, Alnæs D, Monereo-Sanchez J,
Lund MJ, Selbaek G, Stylianou-Korsnes M, Persson K, Barca ML,
Almdahl IS, Fladby T, et al: Neuropsychiatric symptoms and brain
morphology in patients with mild cognitive impairment and
Alzheimer's disease with dementia. Int Psychogeriatr. 33:1217–1228.
2021.PubMed/NCBI View Article : Google Scholar
|