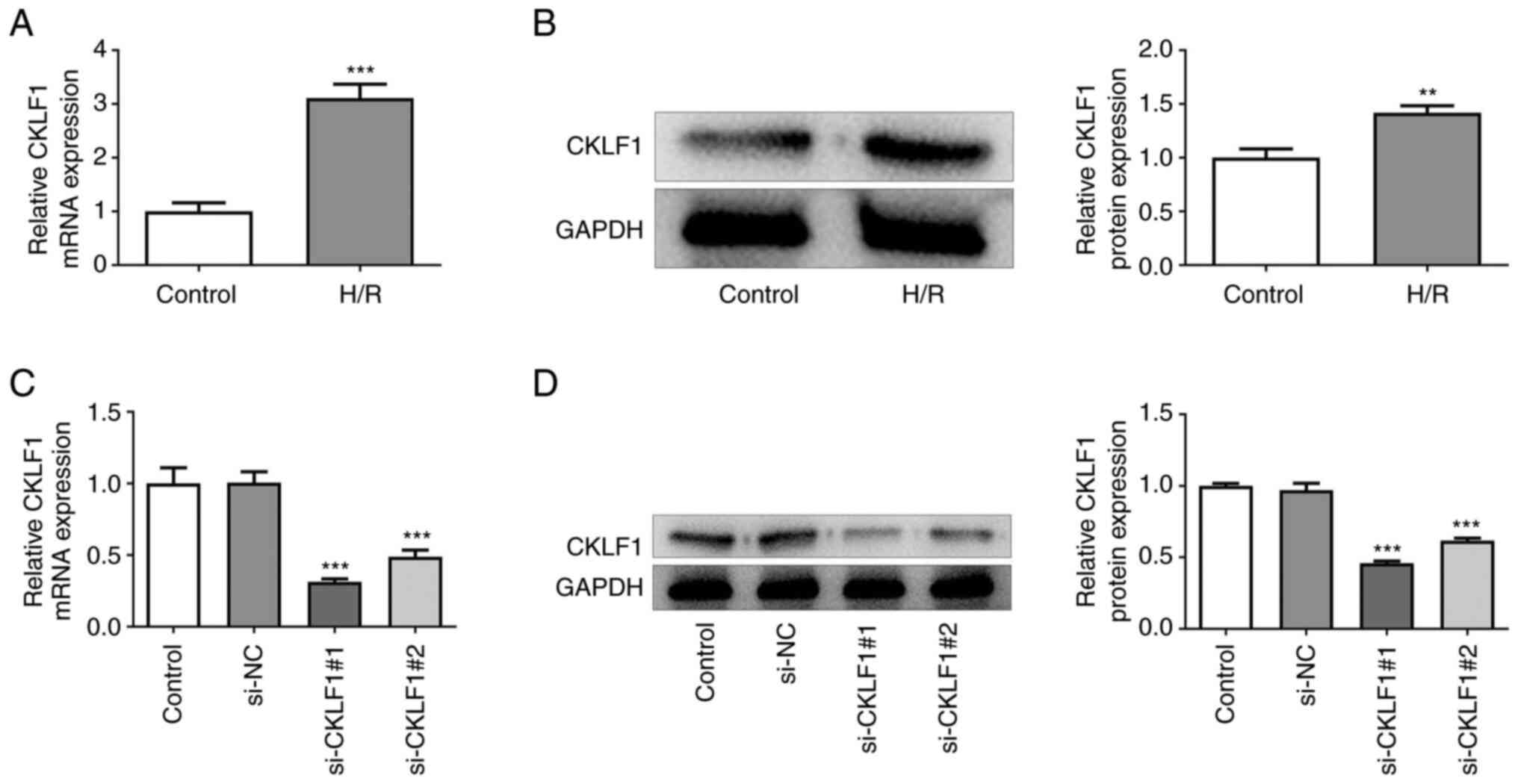
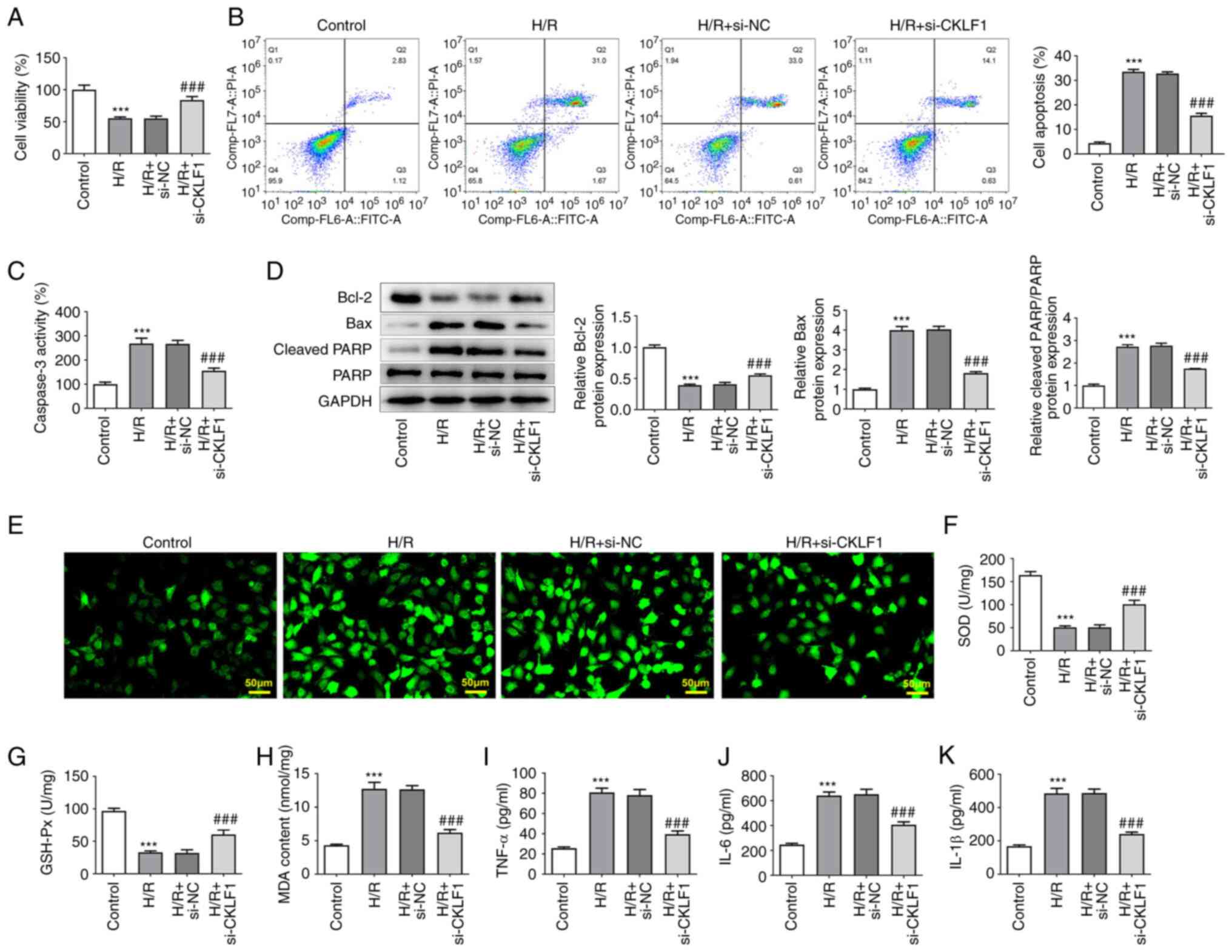
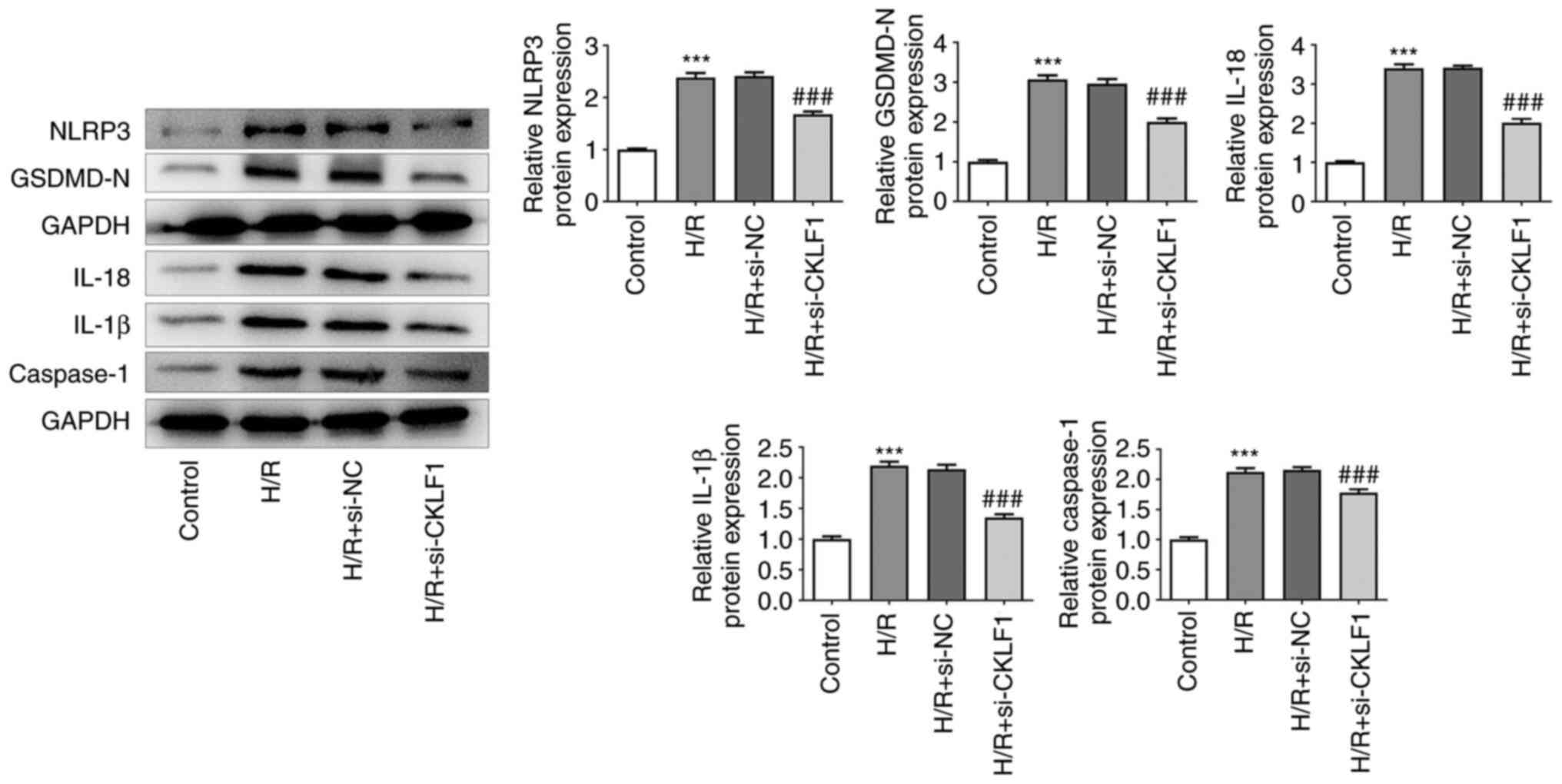
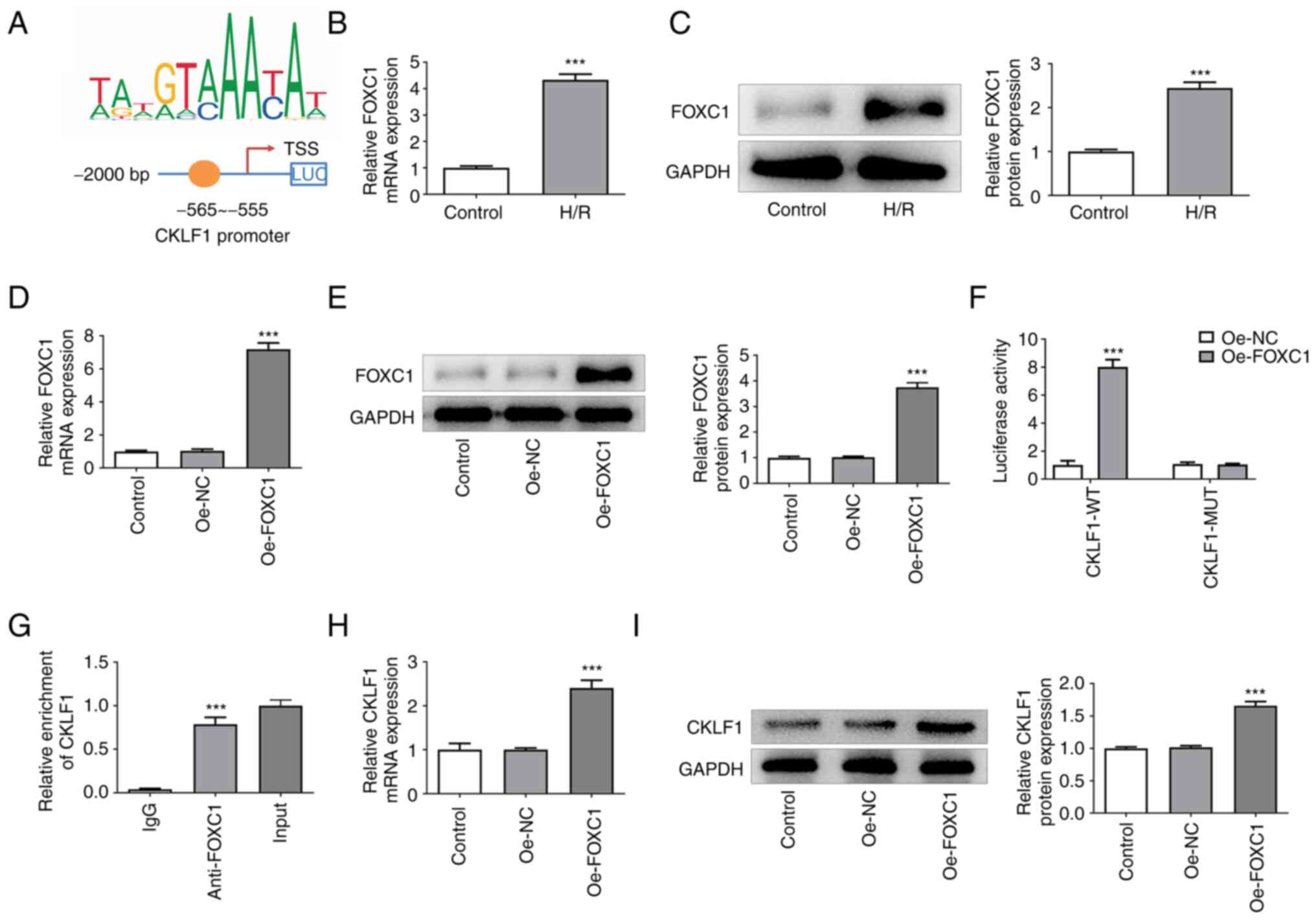
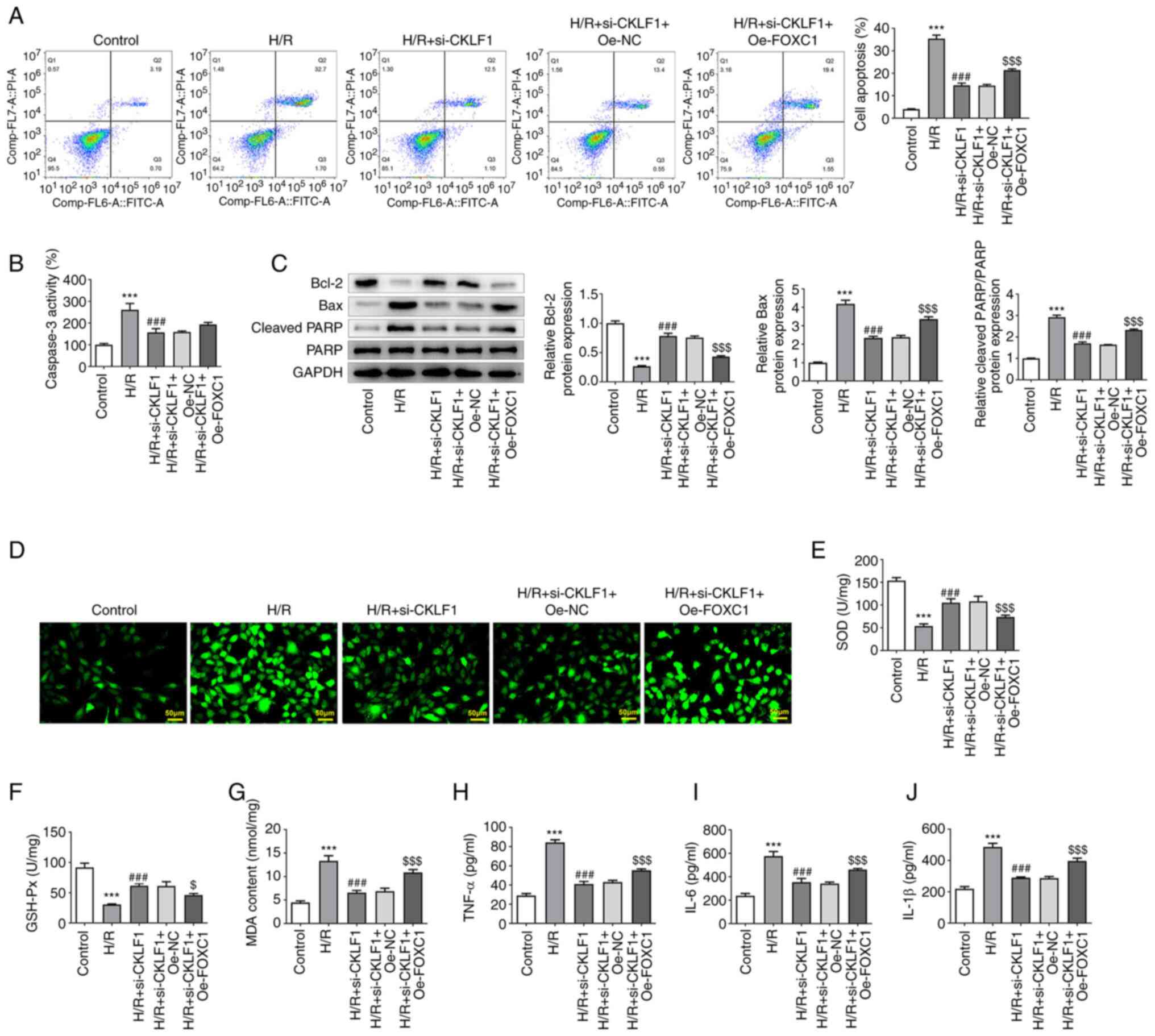
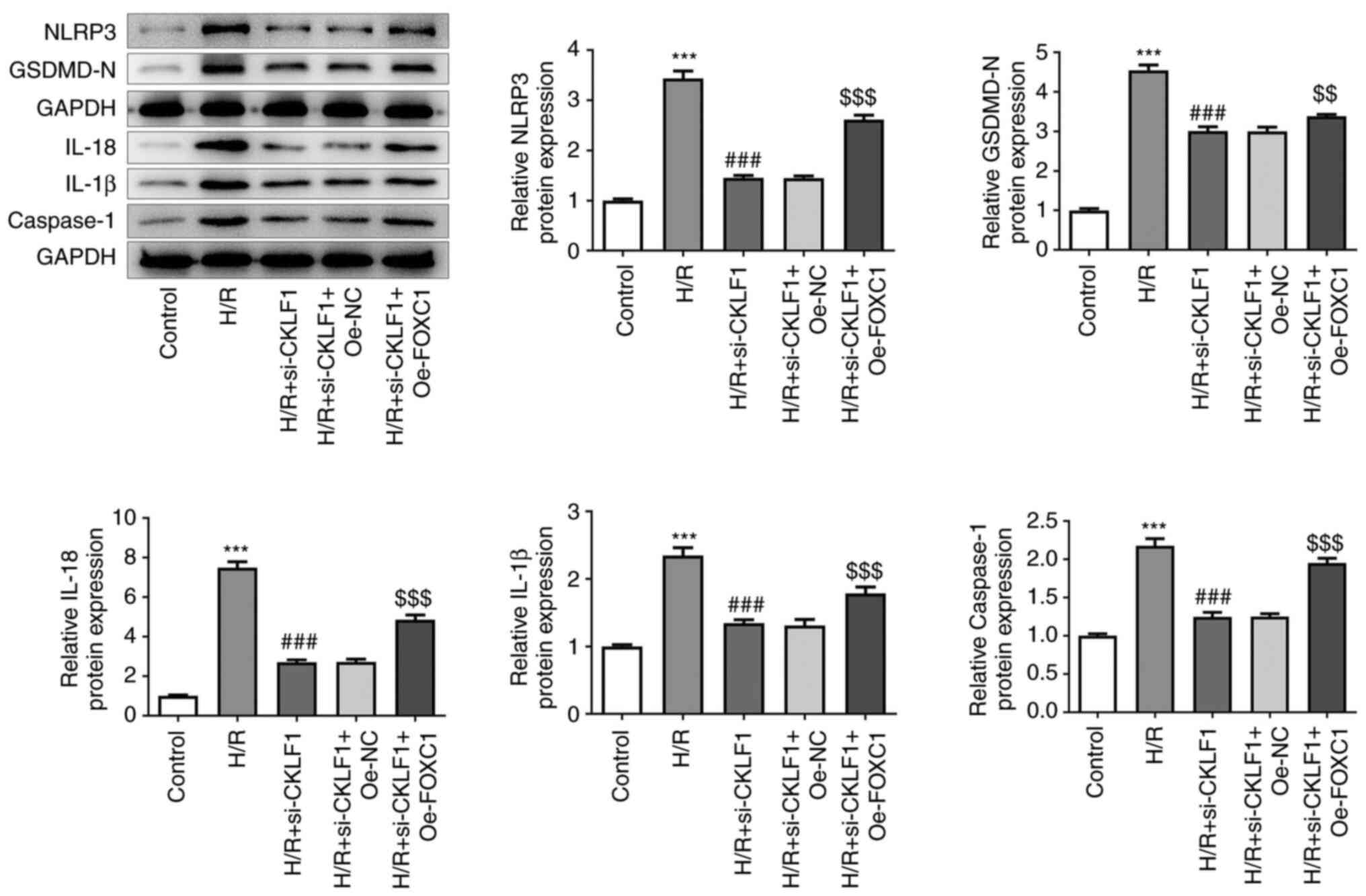
|
1
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart disease and stroke statistics-2018 Update:
A report from the american heart association. Circulation.
137:e67–e492. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jennings RB: Historical perspective on the
pathology of myocardial ischemia/reperfusion injury. Circ Res.
113:428–438. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gonzalez-Montero J, Brito R, Gajardo AI
and Rodrigo R: Myocardial reperfusion injury and oxidative stress:
Therapeutic opportunities. World J Cardiol. 10:74–86.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Ischemia/Reperfusion. Compr Physiol. 7:113–170.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mokhtari-Zaer A, Marefati N, Atkin SL,
Butler AE and Sahebkar A: The protective role of curcumin in
myocardial ischemia-reperfusion injury. J Cell Physiol.
234:214–222. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Han W, Lou Y, Tang J, Zhang Y, Chen Y, Li
Y, Gu W, Huang J, Gui L, Tang Y, et al: Molecular cloning and
characterization of chemokine-like factor 1 (CKLF1), a novel human
cytokine with unique structure and potential chemotactic activity.
Biochem J. 357:127–135. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tan Y, Wang Y, Li L, Xia J, Peng S and He
Y: Chemokine-like factor 1-derived C-terminal peptides induce the
proliferation of dermal microvascular endothelial cells in
psoriasis. PLoS One. 10(e0125073)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang T, Zhang X, Yu W, Chen J, Li Q, Jiao
Y, He P and Shen C: Effects of chemokine-like factor 1 on vascular
smooth muscle cell migration and proliferation in vascular
inflammation. Atherosclerosis. 226:49–57. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen C, Chu SF, Ai QD, Zhang Z, Guan FF,
Wang SS, Dong YX, Zhu J, Jian WX and Chen NH: CKLF1 aggravates
focal cerebral ischemia injury at early stage partly by modulating
Microglia/Macrophage toward M1 polarization through CCR4. Cell Mol
Neurobiol. 39:651–669. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kong LL, Wang ZY, Hu JF, Yuan YH, Han N,
Li H and Chen NH: Inhibition of chemokine-like factor 1 protects
against focal cerebral ischemia through the promotion of energy
metabolism and anti-apoptotic effect. Neurochem Int. 76:91–98.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ai QD, Chen C, Chu S, Zhang Z, Luo Y, Guan
F, Lin M, Liu D, Wang S and Chen N: IMM-H004 therapy for permanent
focal ischemic cerebral injury via CKLF1/CCR4-mediated NLRP3
inflammasome activation. Transl Res. 212:36–53. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li FF, Zhou X, Chu SF and Chen NH:
Inhibition of CKLF1 ameliorates hepatic ischemia-reperfusion injury
via MAPK pathway. Cytokine. 141(155429)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang B, Hong T, Liu QZ, Feng XR, Gong YJ,
Bu DF, Li XM, Xue L, Zhao CY and Huo Y: Effects of in vivo transfer
of human chemokine-like factor 1 gene on cardiac function after
acute myocardial infarction in rats. Beijing Da Xue Xue Bao Yi Xue
Ban. 41:144–147. 2009.PubMed/NCBI(In Chinese).
|
|
14
|
Feng XR, Hong T, Gong YJ, Bu DF, Yuan JY,
Xue L, Zhao CY and Huo Y: In vivo transfer of human chemokine-like
factor 1 gene increases peripheral blood CD34+ stem cells after
myocardial infarction in rats. Beijing Da Xue Xue Bao Yi Xue Ban.
38:592–596. 2006.PubMed/NCBI(In Chinese).
|
|
15
|
Lin YJ, Shyu WC, Chang CW, Wang CC, Wu CP,
Lee HT, Chen LJ and Hsieh CH: Tumor hypoxia regulates forkhead box
C1 to promote lung cancer progression. Theranostics. 7:1177–1191.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang SP, Yang RH, Shang J, Gao T, Wang R,
Peng XD, Miao X, Pan L, Yuan WJ, Lin L and Hu QK: FOXC1
up-regulates the expression of toll-like receptors in myocardial
ischaemia. J Cell Mol Med. 23:7566–7580. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao B, Li GP, Peng JJ, Ren LH, Lei LC, Ye
HM, Wang ZY and Zhao S: Schizandrin B attenuates
hypoxia/reoxygenation injury in H9c2 cells by activating the
AMPK/Nrf2 signaling pathway. Exp Ther Med. 21(220)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu J, Huang J, He X, Hu M, Su S and Liu P:
Myosin 1b Participated in the Modulation of
Hypoxia/Reoxygenation-Caused H9c2 Cell Apoptosis and Autophagy.
Anal Cell Pathol (Amst). 2022(5187304)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Del Re DP, Amgalan D, Linkermann A, Liu Q
and Kitsis RN: Fundamental mechanisms of regulated cell death and
implications for heart disease. Physiol Rev. 99:1765–1817.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Badalzadeh R, Mokhtari B and Yavari R:
Contribution of apoptosis in myocardial reperfusion injury and loss
of cardioprotection in diabetes mellitus. J Physiol Sci.
65:201–215. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Q, Dang YY, Luo X, Fu JJ, Zou ZC,
Jia XJ, Zheng GD and Li CW: Kazinol B protects H9c2 cardiomyocytes
from hypoxia/reoxygenation-induced cardiac injury by modulating the
AKT/AMPK/Nrf2 signalling pathway. Pharm Biol. 61:362–371.
2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang Y, Wang Y, Ma Z, Liang Q, Tang X,
Tan H, Xiao C and Gao Y: Ginsenoside Rb1 inhibits
Doxorubicin-Triggered H9C2 cell apoptosis via aryl hydrocarbon
receptor. Biomol Ther (Seoul). 25:202–212. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen C, Ai Q and Wei Y: Hydroxytyrosol
protects against cisplatin-induced nephrotoxicity via attenuating
CKLF1 mediated inflammation, and inhibiting oxidative stress and
apoptosis. Int Immunopharmacol. 96(107805)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen YE, Yang H, Pang HB and Shang FQ:
Circ-CBFB exacerbates hypoxia/reoxygenation-triggered cardiomyocyte
injury via regulating miR-495-3p in a VDAC1-dependent manner. J
Biochem Mol Toxicol. 36(e23189)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang H, Wang C, Zhang L, Lv J and Ni H:
Rutin alleviates hypoxia/reoxygenation-induced injury in myocardial
cells by up-regulating SIRT1 expression. Chem Biol Interact.
297:44–49. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Radak Z, Zhao Z, Goto S and Koltai E:
Age-associated neurodegeneration and oxidative damage to lipids,
proteins and DNA. Mol Aspects Med. 32:305–315. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cadenas S: ROS and redox signaling in
myocardial ischemia-reperfusion injury and cardioprotection. Free
Radic Biol Med. 117:76–89. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao Q, Liu Z, Huang B, Yuan Y, Liu X,
Zhang H, Qiu F, Zhang Y, Li Y, Miao H, et al: PEDF improves cardiac
function in rats subjected to myocardial ischemia/reperfusion
injury by inhibiting ROS generation via PEDF-R. Int J Mol Med.
41:3243–3252. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou T, Chuang CC and Zuo L: Molecular
characterization of reactive oxygen species in myocardial
Ischemia-Reperfusion Injury. Biomed Res Int.
2015(864946)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Algoet M, Janssens S, Himmelreich U, Gsell
W, Pusovnik M, Van den Eynde J and Oosterlinck W: Myocardial
ischemia-reperfusion injury and the influence of inflammation.
Trends Cardiovasc Med. 33:357–366. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ai Q, Chen C, Chu S, Luo Y, Zhang Z, Zhang
S, Yang P, Gao Y, Zhang X and Chen N: IMM-H004 protects against
cerebral ischemia injury and cardiopulmonary complications via
CKLF1 mediated inflammation pathway in adult and aged rats. Int J
Mol Sci. 20(1661)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lambers E, Arnone B, Fatima A, Qin G,
Wasserstrom JA and Kume T: Foxc1 regulates early cardiomyogenesis
and functional properties of embryonic stem cell derived
cardiomyocytes. Stem Cells. 34:1487–1500. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen HY, Xiao ZZ, Ling X, Xu RN, Zhu P and
Zheng SY: ELAVL1 is transcriptionally activated by FOXC1 and
promotes ferroptosis in myocardial ischemia/reperfusion injury by
regulating autophagy. Mol Med. 27(14)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
He X and Deng L: miR-204-5p inhibits
inflammation of synovial fibroblasts in osteoarthritis by
suppressing FOXC1. J Orthop Sci. 27:921–928. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Oka S, Tsuzuki T, Hidaka M, Ohno M,
Nakatsu Y and Sekiguchi M: Endogenous ROS production in early
differentiation state suppresses endoderm differentiation via
transient FOXC1 expression. Cell Death Discov.
8(150)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fouad AA, Abdel-Aziz AM and Hamouda AAH:
Diacerein downregulates NLRP3/Caspase-1/IL-1beta and IL-6/STAT3
pathways of inflammation and apoptosis in a rat model of cadmium
testicular toxicity. Biol Trace Elem Res. 195:499–505.
2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bian Y, Li X, Pang P, Hu XL, Yu ST, Liu
YN, Li X, Wang N, Wang JH, Xiao W, et al: Kanglexin, a novel
anthraquinone compound, protects against myocardial ischemic injury
in mice by suppressing NLRP3 and pyroptosis. Acta Pharmacol Sin.
41:319–326. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shi H, Gao Y, Dong Z, Yang J, Gao R, Li X,
Zhang S, Ma L, Sun X, Wang Z, et al: GSDMD-mediated cardiomyocyte
pyroptosis promotes Myocardial I/R injury. Circ Res. 129:383–396.
2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Qiu Z, He Y, Ming H, Lei S, Leng Y and Xia
ZY: Lipopolysaccharide (LPS) Aggravates High Glucose- and
Hypoxia/Reoxygenation-Induced Injury through Activating
ROS-Dependent NLRP3 Inflammasome-Mediated pyroptosis in H9C2
cardiomyocytes. J Diabetes Res. 2019(8151836)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Toldo S and Abbate A: The NLRP3
inflammasome in acute myocardial infarction. Nat Rev Cardiol.
15:203–214. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang Y, Yan X, Mi S, Li Z, Wang Y, Zhu H,
Sun X, Zhao B, Zhao C, Zou Y, et al: Naoxintong attenuates
Ischaemia/reperfusion Injury through inhibiting NLRP3 inflammasome
activation. J Cell Mol Med. 21:4–12. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Toldo S, Marchetti C, Mauro AG, Chojnacki
J, Mezzaroma E, Carbone S, Zhang S, Van Tassell B, Salloum FN and
Abbate A: Inhibition of the NLRP3 inflammasome limits the
inflammatory injury following myocardial ischemia-reperfusion in
the mouse. Int J Cardiol. 209:215–220. 2016.PubMed/NCBI View Article : Google Scholar
|