Introduction
Approximately 10% of patients with human
immunodeficiency virus (HIV) develop intracerebral mass lesions
during the course of the infection. Most of these lesions are found
to be either primary central nervous system lymphoma (PCNSL) or
toxoplasma encephalitis (TE), both of which are considered acquired
immune deficiency syndrome (AIDS)-defining diseases by the Centers
for Disease Control (1). However,
HIV-independent cerebral tumors can also arise, albeit less
commonly. Since 1996, the survival time after HIV infection has
been prolonged as the use of highly active antiretroviral therapy
(HAART) can reduce the occurrence of opportunistic infections
(2). Thus, an increasing
population may live to experience non-AIDS-defining malignancies,
including brain glial tumors. Glioma, particularly glioblastoma
multiforme (GBM), is the most common primary brain tumor in general
adults, which also accounts for a large number of intracerebral
masses in HIV-positive patients (3,4). It
has been reported that the incidence of glioma is higher in
HIV-positive patients than in the general population (5). However, the pathogenesis is unclear
and an insufficient number of patients with such an occurrence has
been previously studied and details published. The immune system in
HIV-positive patients is defective, which may cause the tumor
development processes of elimination, equilibrium and/or escape to
be ineffective. This may result in the more frequent clinical
presentation of tumors in patients at a younger age. HIV itself can
also lead to tumor formation. Specifically, transcriptional
transactivator may upregulate transforming growth factor-β (TGF-β)
and platelet-derived growth factor (PDGF) signaling to induce cell
proliferation. In addition, TGF-β signaling may be increased by the
envelope protein gp120. The downstream effect of PDGF signaling may
be enhanced by matrix protein p17 through activation of C-X-C motif
chemokine receptors 1 and 2. Finally, tumor protein p53 may be
inhibited by accessory protein negative factor, leading to
increased mutagenesis and impaired DNA repair (6).
The present study reports on 10 HIV-positive
patients with intracranial glial tumors, preliminarily explores the
pathogenesis of these tumors, and summarizes previously reported
cases in the literature, to further characterize their clinical and
pathological features and identify the prognostic factors in this
group of patients.
Materials and methods
Patient recruitment and data
collection
Participants were recruited consecutively between
April 2016 and December 2021 at the Department of Neurosurgery of
Beijing Ditan Hospital, an infectious disease hospital affiliated
with Capital Medical University (Beijing, China). A total of 10
HIV-infected patients with concurrent cerebral gliomas were
retrospectively analyzed in the study. Their presentations, scans,
CD4+ T cell counts, HIV loads, pathology, treatments and
prognoses were collected and analyzed. Immunohistochemical
assessment of CD31, CD68 and CD163 was performed in the 10
HIV-positive patients with glioma and 18 HIV-negative patients with
glioma (Table SI). The
CD4+ T cell counts and HIV load data of 33 patients with
AIDS-related PCNSL (AR-PCNSL) and CD4+ T cell count data
of another 17 HIV-negative patients with glioma (Tables SII and SIII) were compared with those of the
HIV-positive patients with glioma. The study was approved by the
Ethics Committee of Beijing Ditan Hospital.
Immunohistochemical staining
The protein expression levels of CD31, CD68, CD163,
isocitrate dehydrogenase 1 (IDH1) and Ki-67 were assessed by
immunohistochemistry. The tumor tissues excised during surgery were
immediately placed in 10% formalin for fixation for 12 h at room
temperature, processed by the automation-tissue-dehydrating machine
according to the operation manual (HistoCore PEGASUS; Leica
Microsystems, Inc.), followed by paraffin embedding and sectioning
(4-µm). The tissues were then immersed in blocking reagent
(H2O2) for 30 min at room temperature
(diluted to 3% with distilled water). The primary antibodies CD31
(cat. no. ZA-0568), CD68 (cat. no. ZM-0060), CD163 (cat. no.
ZM-0428), IDH1 R132H mutation-specific (cat. no. ZM-0447) and Ki-67
(cat. no. ZM-0166) were purchased from ZSGB-BIO (OriGene
Technologies, Inc.) and used at a dilution of 1:100. All tissue
sections were incubated with primary antibody at 4˚C overnight. A
BOND Polymer Refine Detection secondary antibody (cat. no. DS9800;
Leica Microsystems, Inc.) was then incubated with the tissue
sections at room temperature for 30 min. Immunohistochemistry
experiments were performed with a LEICA BOND-MAX automatic
immunohistochemistry staining system. Immunostaining was assessed
based on the number of positive cells in the tissues
(magnification, x200 for CD31; magnification, x400 for IDH1, Ki-67,
CD68 and CD163). A total of three different fields of view were
randomly selected and observed under a Leica SP8 confocal
microscope. The average of the combined counts was then calculated.
The results were interpreted by two neuropathologists
independently.
Data collection from PubMed
To gain an improved understanding of the
relationship between clinical characteristics, including
CD4+T cell count and HAART treatment, and the prognosis
of this disease, the PubMed database was searched for articles
published before February 2022 and the available literature on
gliomas in patients with HIV was reviewed. The search strategy was
[(HIV) OR (AIDS)] AND [(glioma) OR (astrocytoma) OR
(oligodendroglioma) OR (glioblastoma multiforme)]. Cases eligible
for further analysis were required to meet the following criteria:
i) History of HIV before the diagnosis of glioma; and ii) confirmed
histopathological diagnosis of glioma. The selection flow chart of
the literature search is presented in Fig. S1.
The demographics (age and sex), CD4+ T
cell count, the time interval between tumor and HIV diagnosis,
lesion location, treatment [surgical resection (SR), stereotactic
biopsy (SB), radiotherapy (RT) and chemotherapy (CTh)],
pathological diagnosis, World Health Organization (WHO) tumor
grade, critical events (alive or dead and the cause of death), and
overall survival (OS) were extracted from the included articles.
For analysis, the CD4+ T cell count was classified into
two groups with a cut-off value of 200 cells/µl for AIDS diagnosis,
and the WHO glioma grade was classified into two groups,
specifically low-grade for WHO grades 1 and 2, and high-grade for
WHO grades 3 and 4. Five cases with no recorded survival time were
excluded from the survival analysis.
Statistical analysis
SPSS statistical package (version 24.0; IBM Corp.)
was used to perform the statistical analysis. The Shapiro-Wilk
method was used to determine the normality of the continuous data.
Unpaired Student's t-test was used to analyze the normally
distributed continuous CD31+, CD68+ and
CD163+ cell counts. Kruskal-Wallis H test followed by
Dunn-Bonferroni tests was used to analyze the non-normally
distributed continuous CD4+ T cell counts of the
AR-PCNSL, HIV-glioma and non-HIV-glioma cohorts. Mann-Whitney U
two-sample test was used to analyze the non-normally distributed
continuous variable of HIV load between the AR-PCNSL and HIV-glioma
cohorts. Spearman correlation was used to test associations between
CD4+ T cell count, HIV load and the histological WHO
grade of tumors, and between the CD163+ cell count and
the IDH1, Ki-67, WHO grade and the OS. The Kaplan-Meier method was
used to estimate survival curves and the log-rank test was used for
comparison. Univariate and multivariate analyses were performed
using Cox regression models to screen potential predictive factors.
Statistically significant risk factors (P<0.05) in the
univariate analysis and clinically important factors were
considered for further multivariate analysis. Finally, the
significant risk factors based on the multivariate analysis were
included in the nomogram construction. The 0.5 and 1-year OS
probabilities were estimated using the nomogram. Concordance index
(C-index) and area under the receiver operating characteristic
curve (AUC) were used to evaluate discriminative ability.
Calibration plots were used to evaluate calibrating ability. R
software (version 3.6.2; http://www.r-project.org/) was used to establish the
nomogram. P<0.05 was considered to indicate a statistically
significant difference, and all tests were two-sided.
Results
Characteristics of patients
The 10 HIV-positive patients with glioma who were
enrolled in the present study comprised 5 patients with GBM, 2
patients with anaplastic astrocytomas (AAs), 2 patients with
anaplastic oligodendrogliomas and 1 case of astrocytoma. These
patients represented 2.8% (10/3,631) of all patients infected with
HIV, 12.5% (10/80) of all patients with HIV-associated focal mass
lesions and 3.4% (10/294) of all patients with cerebral gliomas at
the Department of Neurosurgery of Beijing Ditan Hospital. At the
time of diagnosis, the patients comprised 9 men and 1 woman with a
mean age of 36.7 years (range, 23-57 years). Seizures were reported
by 4 patients at their initial presentation. The others presented
with intracranial hypertension, limb hemiplegia or aphasia. All the
brain lesions were supratentorial, with the majority located in the
cerebral hemisphere, and only one in the paraventricular region and
two at the basal ganglia. Lymphocyte profiles were available for
all the patients. The median CD4+ T-cell count and HIV
load at glioma presentation were 441 cells/µl (range, 96-925
cells/µl) and 10 copies/ml (range, 0-9,704 copies/ml),
respectively. None of the patients had any pre-existing systemic
tumors or had developed AIDS-defining diseases, such as Kaposi's
sarcoma, PCNSL, TE or PML. In a number of these cases, the
intracranial tumors were detected several years after HIV
diagnosis, with an interval ranging from 50 days to 10 years, with
a median of 4 years. All the patients received regular HAART
treatment after the detection of HIV, with the exception of one who
privately withdrew from the use of drugs after 2 years of regular
medication and had a high HIV load of 9,704 copies/ml at glioma
detection. The radiological and pathological images of the patients
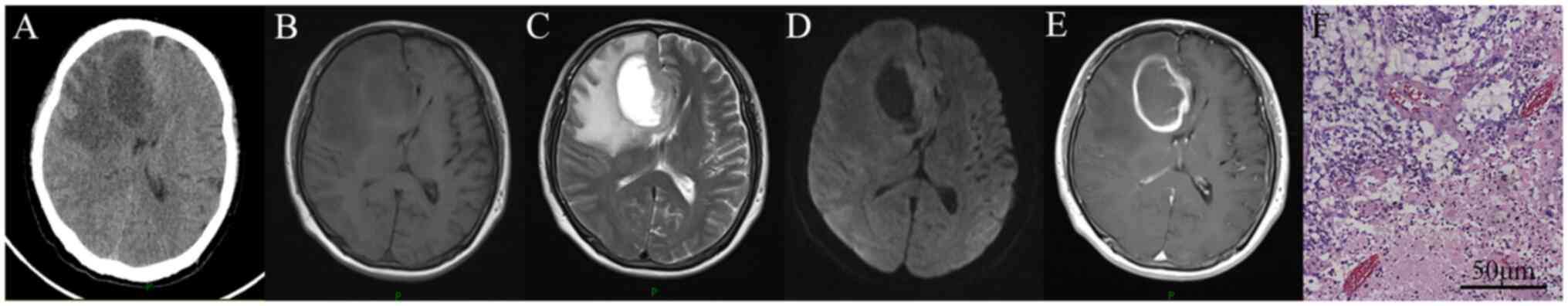
appeared consistent with immunocompetent glioma (Fig. 1). The treatments received included
SR, SB, RT and CTh, which differed from the standard algorithm for
AIDS-defining diseases, such as the treatment of Toxoplasma
gondii for TE and high-dose methotrexate-based chemotherapy for
PCNSL. The therapeutic regimens of RT and CTh were applied
according to the guidelines of the Chinese Glioma Cooperative
Group, Society for Neuro-Oncology of China and Chinese Brain Cancer
Association, and varied by taking into consideration the tumor
grade, patient age, performance status and tumor molecular features
(7). At the time of follow-up,
half of the patients had died due to tumor progression. The median
survival time was 7.5 months after tumor diagnosis. Patient
characteristics, management and outcomes are summarized in Table I.
 | Table ICharacteristics of HIV-positive
patients with brain glial tumors treated at Beijing Ditan
Hospital. |
Table I
Characteristics of HIV-positive
patients with brain glial tumors treated at Beijing Ditan
Hospital.
| No. | Age, years/
sex |
CD4+T-cell count, cells/µl | HIV load,
copies/ml |
Intervala/ HAART, years | Tumor location | Clinical
manifestation | Surgery | Tumor type | WHO grade | IDH1 | Ki-67, % | RT | CTh | Survival, days | Cause of death |
|---|
| 1 | 28/M | 373 | <20 | 5/1 | R. fronto-temporal
lobe | Intracranial
hypertension | SR | AO | 3 | + | 5 | Y | Y | 225 | Tumor |
| 2 | 33/M | 96 | 228 | 3/3 | L. basal
ganglia | Limb hemiplegia and
aphasia | SB | AA | 3 | - | 5 | N | N | 31 | Tumor |
| 3 | 25/M | 925 | 0 | <1/<1 | L. temporo-parietal
lobe | Limb hemiplegia and
aphasia | SR | GBM | 4 | - | 40 | N | N | 30 | Tumor |
| 4 | 43/M | 454 | 80 | 5/5 | L. basal
ganglia | Seizure and
intracranial hypertension | SR | GBM | 4 | - | 20 | N | N | 18 | Tumor |
| 5 | 57/M | 137 | 0 | 6/6 | L. paraventricular
region | Limb hemiplegia and
aphasia | SR | GBM | 4 | - | 30 | Y | Y | 189 + | Alive |
| 6 | 53/M | 195 | 0 | 10/10 | R. frontal
lobe | Limb
hemiplegia | SR | GBM | 4 | - | 80 | Y | Y | 75+ | Alive |
| 7 | 40/M | 602 | <20 | 7/7 | L. frontal
lobe | Seizure | SR | AO | 3 | + | 20 | Y | Y | 106+ | Alive |
| 8 | 23/M | 428 | 9,704 | 3/2 | L. frontal
lobe | Seizure | SB | AA | 4 | + | 25 | Y | N | 90+ | Alive |
| 9 | 36/F | 545 | 0 | 3/2 | R. fronto-temporal
lobe | Intracranial
hypertension | SR | GBM | 4 | + | 10 | N | N | 30 | Tumor |
| 10 | 29/M | 649 | 0 | 1/1 | R. frontal
lobe | Seizure | SR | A | 2 | + | 3 | Y | N | 1,941+ | Alive |
In the pathological analysis, there was a trend of
longer survival in IDH1-positive patients compared with
IDH1-negative patients (log-rank, P=0.219; Fig. S2A and B). In addition, there was no difference
between the Ki-67 high expression group (≥30%) and low expression
group (<30%) (log-rank, P=0.778; Fig. S2C and D). The HIV-positive patients with glioma
had a lower count of CD163+ cells compared with the 18
HIV-negative patients with glioma (P=0.039); however, no
significant difference in CD68+ and CD31+
counts was detected (P=0.162 and P=0.148, respectively; Figs. S3 and S4). In further correlation analysis, no
association between the CD163+ cell count and IDH1,
Ki-67, WHO grade or OS was detected (P=0.496, P=0.853, P=0.186 and
P=0.159, respectively). Compared with the 33 patients with
AR-PCNSL, the HIV-positive patients with glioma had a higher
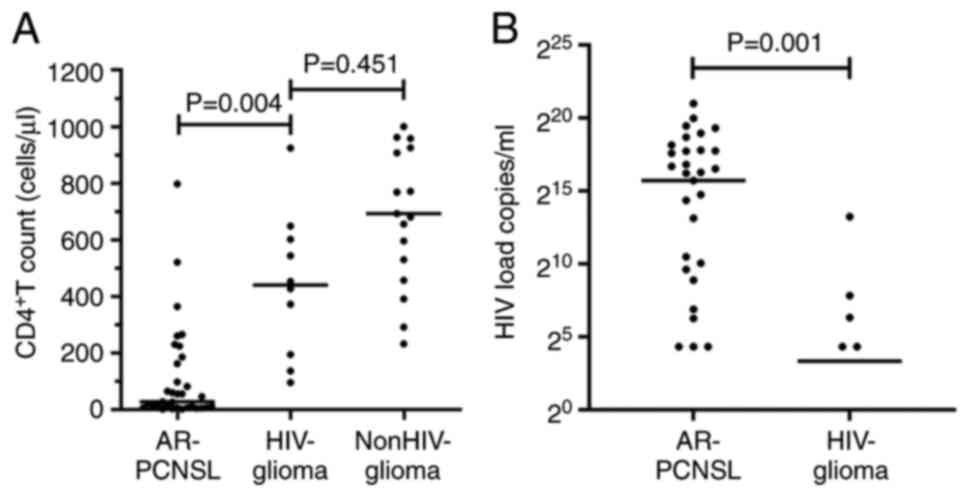
CD4+ T-cell count (adjusted P=0.004; Fig. 2A) and lower HIV load (P=0.001;
Fig. 2B). However, no significant
difference in the CD4+ T-cell count of the HIV-positive
patients with glioma and the 17 HIV-negative patients with glioma
was observed (adjusted P=0.451; Fig.
2A). In addition, no significant association was identified
between the CD4+ T-cell count and either the WHO
histological grade of the tumor (P=0.790) or HIV load (P=0.728).
Moreover, no association between CD4+ T-cell count and
tumor outcome was identified (P=0.815), possibly because of the
limited sample size. To explore the impact of CD4+
T-cell count on the prognosis of such patients and identify the
other prognostic factors, the literature was searched to identify
previous reports of HIV-positive patients with glioma.
Characteristics of previously
published cases
A total of 658 articles were identified by a
literature search for evaluation. Following selection according to
the PRISMA guidelines, 21 publications from 1988 to 2018 were
included (1,3-5,8-24),
including 45 cases of gliomas with HIV infection (Table II). Among these tumors, there were
21 (46.6%) GBMs, 16 (35.6%) astrocytomas, 7 (15.6%) AAs and 1
(2.2%) oligodendroglioma. The mean age of the patients was 38.4
years (range, 16-60 years). Among the 40 patients whose sex was
reported, 33 (82.5%) were male and the other 7 were female (17.5%).
Patients were diagnosed HIV-positive a mean of 3.8 years (range,
0-16 years) prior to tumor presentation. At the time of glioma
diagnosis, the mean CD4+ T-cell count was 316.2±189.9
cells/µl (range, 42-610 cells/µl). Most of the gliomas were located
in the cerebral hemisphere. More than half of these patients
(29/45, 64.4%) died during the follow-up period. Among the patients
for which the cause of death was recorded, all but 5 died from the
progressive effect of the brain tumor (20/25, 80%). In addition, 2
patients died from surgical complications, specifically,
postoperative pulmonary embolism and intracerebral hematoma. The
other 3 patients died due to opportunistic AIDS-associated
infections: Pneumocystis carinii pneumonia in 2 cases and
cytomegalovirus in 1 case. The 45 reviewed cases are summarized in
Table II.
 | Table IIHIV-positive patients with glioma
reported in the literature. |
Table II
HIV-positive patients with glioma
reported in the literature.
| First author/s,
year | Age, years/sex |
Intervala, years | CD4 count,
cells/µl | HAART | Tumor location | Surgery | RT | CTh | Tumor type | WHO grade | Survival, days | Cause of death | (Refs.) |
|---|
| Gasnault et
al, 1988 | 43/M | - | - | N | L. occipital
lobe | SB | N | N | A | 2 | 12 | Intracerebral
hematoma | (8) |
| | 19/M | - | - | N | L. parietal
lobe | SB | Y | N | GBM | 4 | 365 | Tumor | |
| Carrana et
al, 1990 | 34/M | - | - | N | R. parietal
lobe | SB | Y | N | AA | 3 | 1,095 | Tumor | (9) |
| Ho et al,
1991 | 37/M | 2 | - | N | L. temporal
lobe | SB | N | N | AA | 3 | 30 | CMV | (10) |
| Kasantikul et
al, 1992 | 32/M | 0.17 | 257 | N | L.
parieto-occipital lobe | SB | N | N | AA | 3 | 3 | Tumor | (11) |
| Chappell et
al, 1992 | - | - | - | N | - | SB | - | - | A | 2 | 555 | - | (12) |
| Chamberlain,
1994 | 32/M | 2 | - | N | R. temporal
lobe | SR | Y | Y | AA | 3 | 570 | Tumor | (1) |
| | 38/M | 4 | - | N | R. frontal
lobe | SR | Y | Y | GBM | 4 | 300+ | Alive | |
| Moulignier et
al, 1994 | 48/M | 0 | 80 | N | Bilateral frontal
lobe | SB | N | N | GBM | 4 | 180 | Tumor | (3) |
| | 30/M | 1.08 | 605 | N | R. parietal
parasagittal | SB | N | N | A | 2 | -b | - | |
| | 43/M | 0.25 | 200 | N | L.
parieto-occipital lobe | SB | N | N | A | 2 | 11 | Pulmonary
embolism | |
| | 34/M | 0 | 417 | N | Subthalamic | SB | Y | N | A | 2 | 210 | Tumor | |
| Gervasoni et
al, 1995 | - | - | 54 | N | L. parietal
lobe | SB | N | N | AA | 4 | 10 | Tumor | (13) |
| | - | - | 54 | N | L. parietal lobe
and corpus callosum | SB | Y | N | A | 2 | 270 | Tumor | |
| | - | - | 405 | N | L. thalamus | SB | N | N | A | 1 | 365 | PCP | |
| | - | - | 405 | N | L. paraventricular
parietooccipital region | SB | Y | N | A | 2 | 540+ | Alive | |
| Neal et al,
1996 | 35/M | 2 | 280 | N | L. temperal
lobe | SB | N | N | GBM | 4 | 60 | Tumor | (14) |
| Tacconi et
al, 1996 | 22/M | - | - | N | L.temperal
lobe | SB | Y | N | A | 2 | 600 | Tumor | (4) |
| | 32/M | - | - | N | L. frontal
lobe | SB | Y | N | A | 2 | 300+ | Alive | |
| | 44/M | - | - | N | R. basal
ganglia | SB | Y | Y | AA | 3 | 210 | PCP | |
| | 38/M | - | - | N | R. frontal
lobe | SB | Y | N | A | 2 | 180+ | Alive | |
| Monforte et
al, 1997 | 28/M | - | 270 | N | - | SB | Y | N | A | 2 | 270 | Tumor | (15) |
| | 34/M | - | 436 | N | - | SB | Y | N | A | 2 | 450+ | Alive | |
| Blumenthal et
al, 1999 | 36/M | 6 | 112 | N | - | SRx2 | Y | Y | GBM | 4 | 270 | - | (5) |
| | 60/M | 11 | - | N | - | SB | Y | Y | GBM | 4 | 450+ | Alive | |
| | 38/F | 0 | 490 | N | - | Necropsy? | N | N | GBM | 4 | 1 | Tumor | |
| | 44/M | 4 | - | Y | - | SR | Y | Y | GBM | 4 | 270 | - | |
| | 41/M | 11 | 408 | N | - | SR | Y | Y | GBM | 4 | 90 | Tumor | |
| | 38/M | 0 | 225 | N | - | SR | Y | N | A | 2 | 4,320+ | Alive | |
| Vannemreddy et
al, 2000 | 29/M | - | - | N | Corpus
callosum | SB | Y | N | GBM | 4 | 120 | Tumor | (16) |
| Wolff et al,
2002 | 31/M | - | - | N | Brainstem | SB | N | N | GBM | 4 | 60 | Tumor | (17) |
| Corti et al,
2004 | 31/F | - | 42 | Y | R. frontal
lobe | SR | N | N | O | 2 | 840+ | Alive | (18) |
| Hall and Short,
2009 | 33/M | 3 | 610 | Y | L. frontal
lobe | SRx2 | Y | Y | GBM | 4 | 390 | Tumor | (19) |
| | 50/F | 10 | 600 | Y | R. frontal
lobe | SR | N | N | GBM | 4 | 780+ | Alive | |
| | 43/M | - | 400 | Y | R. temporal
lobe | SRx2 | Y | Y | GBM | 4 | 365 | Tumor | |
| | 42/F | - | - | N | L. basal
ganglia | SB | N | N | GBM | 4 | 60 | Tumor | |
| Chaudry et
al, 2013 | 55/M | 3 | 423 | Y | Brainstem | SB | N | N | AA | 3 | 30+ | Alive | (20) |
| Brassesco et
al, 2013 | 16/F | 16 | - | - | Both frontal
lobes | SB | Y | Y | GBM | 4 | 90 | Tumor | (21) |
| de Oliveira et
al, 2014 | 42/F | 0.5 | 66 | Y | L. fronto-temporal
lobe | Necropsy | N | N | GBM | 4 | 30 | Tumor | (22) |
| Wang et al,
2018 | 53/M | - | - | - | L. temperal
lobe | SR | N | Y | GBM | 4 | 240 | - | (23) |
| | 46/M | 4 | - | Y | R. parietal
lobe | SR | Y | Y | GBM | 4 | 450+ | Alive | |
| Jokonya et
al, 2018 | 29/F | - | 81 | Y | - | - | - | - | A | 2 | -b | - | (24) |
| | 47/M | - | 530 | Y | - | - | - | - | A | 2 | -b | - | |
| | 52/M | - | 530 | Y | - | - | - | - | GBM | 4 | -b | - | |
| | 57/M | - | 240 | N | - | - | - | - | GBM | 4 | -b | - | |
The mean OS of these patients was 23.7 months,
whereas the median survival time was 9 months. This was slightly
higher than that of the primary patient cohort, perhaps due to the
lower proportion of high-grade gliomas (27/40, 67.5% vs. 9/10,
90%). A total of 50 cases, including the 10 cases reported in the
present study and 40 cases in the published articles, excluding the
5 cases for which no survival time was reported, were further
subjected to a survival analysis. Seven parameters, namely the age
of onset, CD4+ T-cell count (0-200 and >200
cells/µl), HAART treatment (no/yes), surgery (SB/SR), RT (no/yes),
CTh (no/yes), and WHO grade (low-grade/high-grade) were analyzed
for their association with OS.
CD4+ T-cell count and
HAART
Patients were classified into two cohorts based on
CD4+ T-cell count (0-200 and >200 cells/µl) and HAART
(no/yes). The median survival was 180±89 days for patients with a
low CD4+ T-cell count (n=10) and 270±75 days for
patients with a high CD4+ T-cell count (n=21). The
median survival was 365±109 days for patients treated with HAART
(n=18) and 270±47 days for patients not treated with HAART (n=30).
There was a trend of increased median survival with higher
CD4+ T-cell count and HAART treatment; however, no
statistically significant difference was detected between the
subgroups according to the log-rank analysis of Kaplan-Meier curves
(P=0.366 and P=0.303, respectively; Fig. 3A and B). The additional univariate analysis
also found no statistically significant relationship of
CD4+ T-cell count and HAART with OS (P=0.381 and
P=0.318, respectively; Table
III). However, in clinical practice, HAART is considered an
important therapy for such patients. Therefore, this parameter was
included in further multivariate Cox regression models.
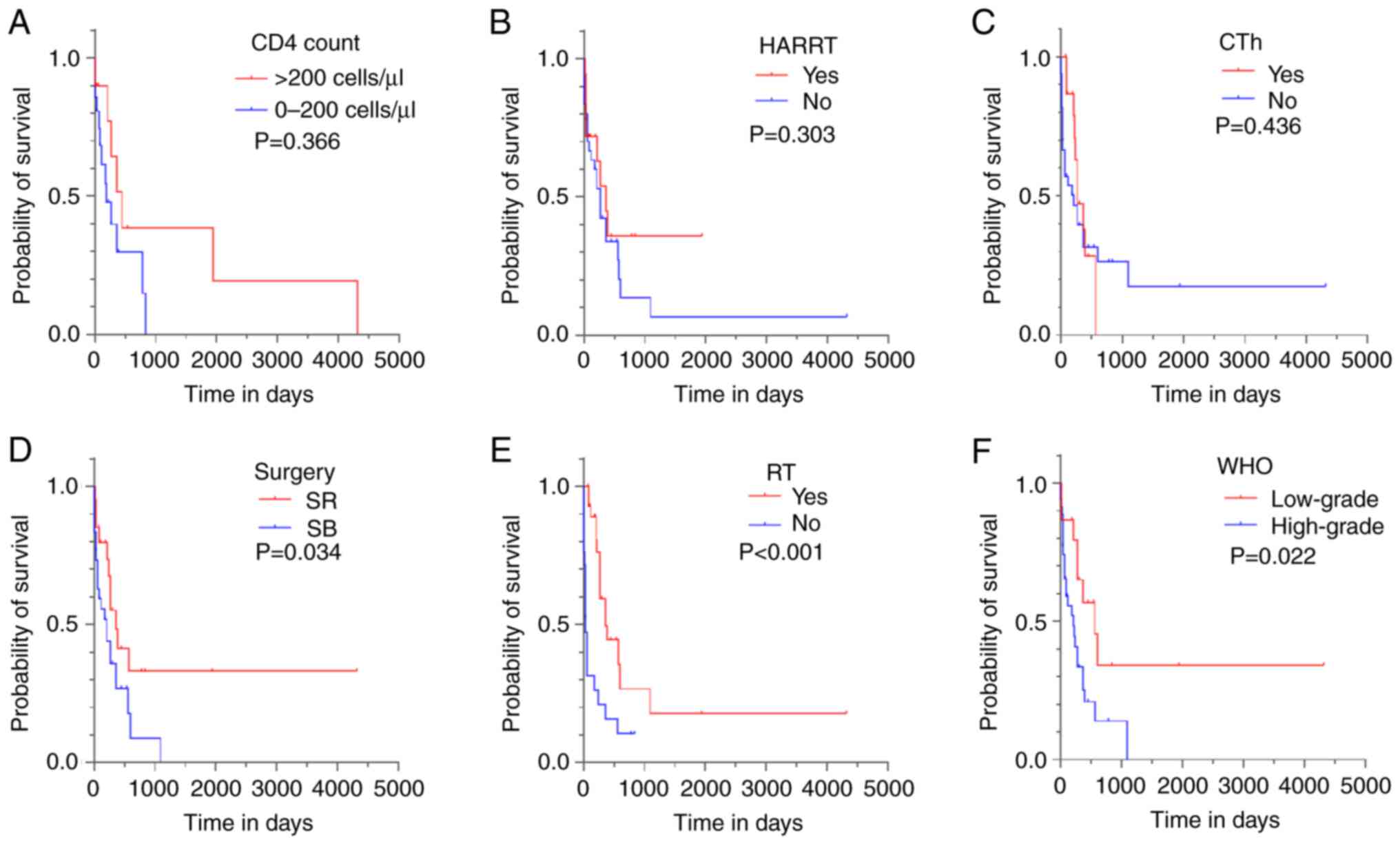 | Figure 3Kaplan-Meier curves for OS based on
clinical parameters. Effects of different clinical parameters on
the OS of human immunodeficiency virus-positive patients with
glioma. Log-rank tests and Kaplan-Meier curves were used to compare
differences between subgroups with regard to (A) CD4+
T-cell count, (B) HAART, (C) CTh, (D) surgery type, (E) RT and (F)
WHO grade. OS, overall survival; HAART, highly active
antiretroviral therapy; CTh, chemotherapy; SR, surgical resection;
SB, stereotactic biopsy; RT, radiotherapy; WHO, World Health
Organization. |
 | Table IIIUnivariate and multivariate analyses
for risk factors of overall survival. |
Table III
Univariate and multivariate analyses
for risk factors of overall survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | Subgroup | Frequency, n
(%) | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Onset age | - | - | 0.988
(0.952-1.026) | 0.534 | - | - |
| CD4 | 0-200 | 10 (32.3) | 0.659
(0.259-1.675) | 0.381 | - | - |
| | >200 | 21 (67.7) | | | - | - |
| HAART | No | 30 (62.5) | 0.674
(0.318-0.674) | 0.318 | 0.582
(0.189-1.795) | 0.346 |
| | Yes | 18 (37.5) | | | | |
| Surgery | SB | 28 (58.3) | 0.460
(0.217-0.976) | 0.043 | 0.548
(0.185-1.623) | 0.278 |
| | SR | 20 (41.7) | | | | |
| RT | No | 28 (57.1) | 0.257
(0.124-0.532) | <0.001 | 0.210
(0.092-0.480) | <0.001 |
| | Yes | 21 (42.9) | | | | |
| CTh | No | 33 (67.3) | 0.739
(0.337-1.622) | 0.451 | - | - |
| | Yes | 16 (32.7) | | | - | - |
| WHO grade | Low | 15 (30.0) | 2.455
(1.089-5.535) | 0.030 | 3.079
(1.270-7.464) | 0.013 |
| | High | 35 (70.0) | | | | |
Onset age, surgery, RT, CTh and WHO
grade
Univariate analysis showed that onset age and CTh
did not influence OS (P=0.534 and P=0.451, respectively; Table III). In addition, no trend of
longer survival was observed in patients who received adjuvant
therapy with CTh (log-rank P=0.436; Fig. 3C). The log-rank analysis of
Kaplan-Meier curves based on surgery, RT and WHO grade showed
significant differences between the subgroups (P=0.034, P<0.001
and P=0.022, respectively; Fig.
3D-F). In the univariate analysis, these three parameters also
exhibited significant differences (P=0.043, P<0.001 and P=0.030,
respectively).
Parameters related to OS
The three significant parameters, along with HAART,
were included in the multivariate Cox regression model. Only RT and
WHO grade were independent prognostic parameters (P<0.001 and
P=0.013, respectively). High WHO tumor grade was identified as a
factor that increased the risk of death by 3.079-fold with a 95% CI
of 1.270-7.464. In addition, RT substantially reduced the risk of
death by 0.210-fold (95% CI, 0.092-0.480).
Nomogram construction
A nomogram (Fig.
4A) for predicting the 0.5- and 1-year survival rates of such
patients was established based on HAART (no/yes), surgery (SB/SR),
RT (no/yes) and WHO grade (low-grade/high-grade). The nomogram had
a high C-index of 0.774 (95% CI, 0.703-0.845) for assessment of the
predictive model. Calibration plots and receiver operating
characteristic curves for the nomogram model were established. The
calibration curves indicated that the predicted 0.5- and 1-year
survival rates were consistent with the actual results in the
cohort (Fig. 4B and C). The AUC value of the nomogram for
0.5-year survival was 0.931 and for 1-year survival was 0.787
(Fig. 4D), suggesting that the
model had good predictive ability.
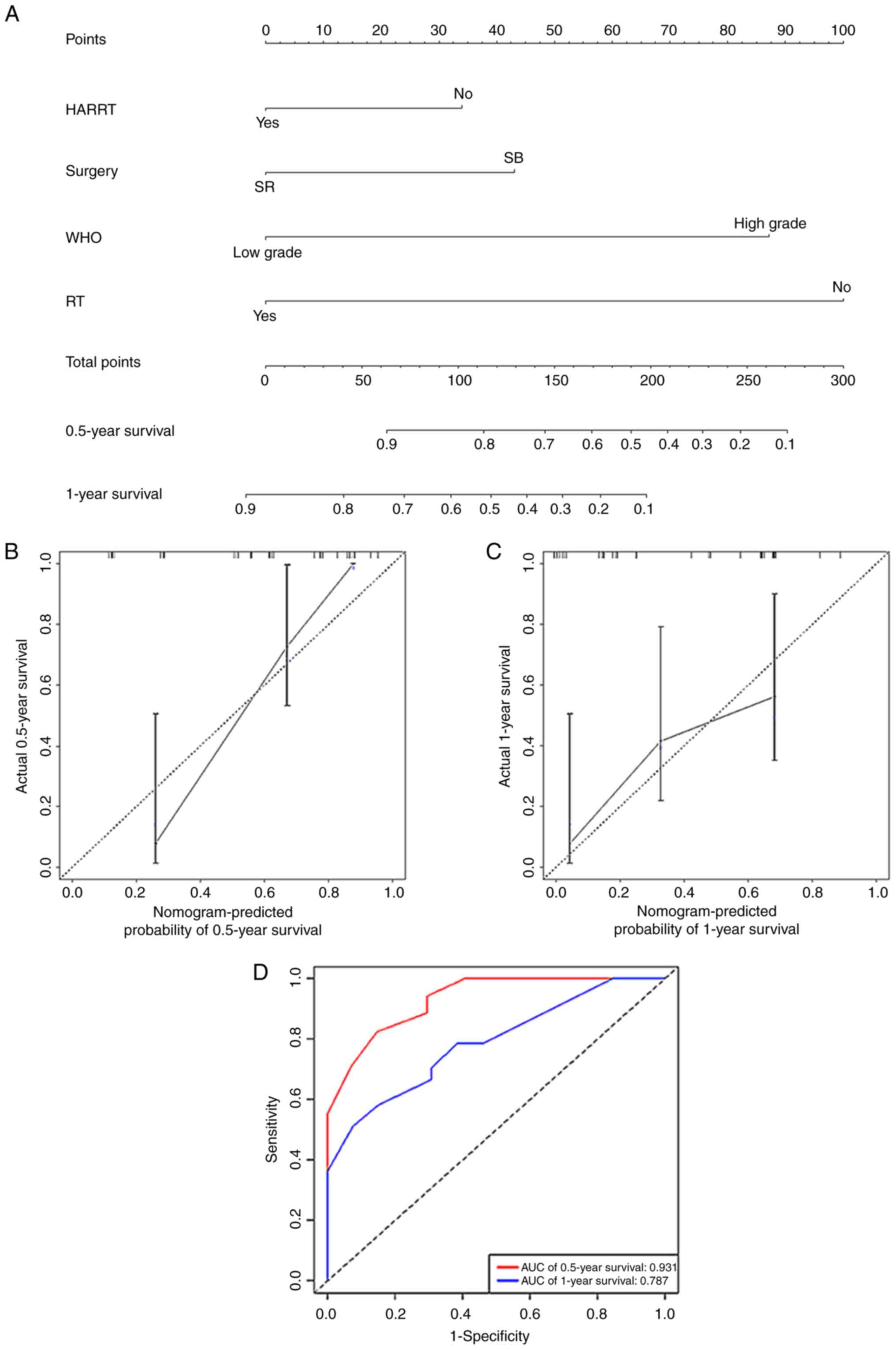 | Figure 4Nomogram construction, calibration
plot and ROC curves of the nomogram model for human
immunodeficiency virus-positive patients with glioma. (A) Nomogram
for the prediction of 0.5- and 1-year survival rates of patients
based on four independent prognostic factors: HAART, surgery type,
WHO grade and RT. Calibration plots of the nomogram model for (B)
0.5 and (C) 1-year survival. (D) ROC curves of the nomogram model.
ROC, receiver operating characteristic; HAART, highly active
antiretroviral therapy; WHO, World Health Organization; SR,
surgical resection; SB, stereotactic biopsy; RT, radiotherapy; AUC,
area under the receiver operating characteristic curve. |
Discussion
Neurological involvement is increasingly common in
HIV-positive patients. It has been reported that 40-60% of patients
with AIDS have concurrent neurological disorders (25). In addition to the most commonly
identified focal intracerebral pathological entities, which include
cryptococcosis, tuberculoma, PML, PCNSL and TE, other entities
should be considered in the differential diagnosis of lesions that
affect the central nervous system (CNS) in HIV-positive patients
due to divergent treatment options and prognosis. The survival
duration of patients with HIV has increased by a large margin due
to the use of HAART which can reduce opportunistic infections. In
2017, the incidence rate of HIV infection in China was estimated to
be 0.025-0.50 per 1,000 population (26). The prevalence of glioma in
individuals who are not infected with HIV was reportedly 0.05-0.06%
worldwide from 2010 to 2014 (7,27);
by comparison, its prevalence in HIV-positive individuals in the
present study was ~2.8%, which is considerably higher than that in
the immunocompetent population. Among patients with HIV-related
focal mass lesions, glioma was detected 6% in two cohorts in the
1990s before the utilization of HAART (3,4), and
in 12.5% of the patients enrolled in the current study. These data
indicate that the presence of glioma in HIV-positive patients with
focal mass lesions may be more common than is currently recognized,
particularly in the era after the introduction of HAART. This may
be due to the survival time after infection with HIV being
prolonged (2).
The pathogenesis of glioma in HIV-positive
individuals was explored in the present study via analysis of the
macrophage markers CD68 and CD163, and the endothelial cell marker
CD31. The gliomas of HIV-positive patients had a lower cell count
of CD163+ cells but no difference in CD68+
and CD31+ cell counts compared with those of
HIV-negative patients. CD163 is a differentiation marker of M2
phenotype tumor-associated macrophages (TAMs). Therefore, the
results suggest that HIV-positive glioma may have a lower
proportion of M2-phenotype TAMs which are considered to create a
supportive stroma for tumor growth (28). In a previous study, CD163
expression was found to have a positive association with the
malignant grade of glioma and IDH wild-type glioma, and higher
CD163 expression indicated a significantly poor survival in
patients with gliomas (28). In
the patients of the present study, however, no association between
CD163+ cell count and IDH1, Ki-67, WHO grade or OS was
detected, possibly because of the limited sample size. Further
analysis with a larger sample size is necessary to further
investigate this.
In the total cohort in the present study, the
majority of deaths were tumor-related (25/30, 83.3%), and the
median survival time was only 9 months, which is much lower than
that of immunocompetent patients with glioma. The poor prognosis of
these patients may be due to the presumptive diagnosis of PCNSL or
TE for HIV-positive patients with focal cerebral mass lesions.
These patients received empiric treatment, instead of undergoing
diagnosis by biopsy or resection surgery, particularly when the
CD4+ T lymphocyte count was <200 cells/µl. The poor
prognosis of these cases may be associated with a delay in the
establishment of the correct diagnosis and ineffective empiric
treatment, which caused the optimal opportunity for the most
appropriate therapy to be missed. The establishment of a correct
diagnosis is crucial before the initiation of further treatment.
Therefore, in atypical cases or cases with no clinical or
neuroradiological remission following empirical treatment for TE or
PCNSL, an immediate aggressive approach is required to confirm the
diagnosis.
Among all the cases in the present study, more
patients who underwent conservative surgery via SB than SR (58.3
vs. 41.7%), According to log-rank analysis, SR resulted in a longer
survival than SB (P=0.034). However, univariate and multivariate
logistic regression analysis showed that surgery type was a
predictive rather than an independent factor (P=0.043 and P=0.278,
respectively). Postoperative RT was found to be an independent
predictor (P<0.001) for OS. However, there was no trend of
longer survival in patients who received CTh, possibly due to the
limited number of samples from patients who received CTh (16
cases). These results indicate that SR followed by RT should be
recommended for HIV-positive patients with glioma, as it is in the
general population, particularly those with high-grade glioma,
since a high tumor grade is also an independent risk factor for a
shorter survival time. It is well known that the WHO grade in
glioma plays an important role in prognosis prediction. However, it
has not been well studied in HIV patients with concurrent glioma.
Furthermore, not enough samples are available for a separate
analysis for the rare concurrence of these two diseases. Also,
previous studies have analyzed different graded of glioma together
when looking for prognostic factors (29,30).
Most of the patients who participated in the
survival analysis were younger than non-HIV infected glioma
patients, with a mean age of ~37 years. At the time of glioma
diagnosis, patients had a low CD4+ T-cell count with a
median interval from HIV diagnosis of 3 years (50 days-11 years).
This suggests that gliomas may occur earlier and more frequently in
this population. Hajjar et al (31) showed that the incidence of
non-AIDS-defining malignancies in patients with AIDS was 5.4-fold
higher than that of the general population. This phenomenon may be
due to the inhibitory effect of HIV infection on immune
surveillance accelerating the development of malignant tumors
(32). However, in the current
univariate logistic regression analysis, CD4+ T-cell
count and HAART status did not affect OS, and only a trend of
increased median OS with high CD4+ T-cell count and
HAART treatment was observed. This may be due to the majority of
the patients succumbing to tumor-related factors (25/30, 83.3%)
before lethal AIDS-related complications developed. However, there
were some patients (3/50, 6%) who died due to AIDS complications.
Therefore, it remains strongly recommended to regularly administer
HAART, particularly protease inhibitors, to such patients to
inhibit HIV replication and restore their immune function.
Moreover, protease inhibitors can promote the inhibition of
vascular endothelial growth factors and reduce angiogenesis, which
is the main mechanism of malignant tumor growth (33).
The present study reports the largest series of
patients with concurrent HIV infection and glioma. The clinical
characteristics, management and prognosis of 10 patients were
evaluated and the relevant literature on gliomas in HIV/AIDS
patients was also reviewed. A total of 45 cases were extracted from
21 different articles and survival analysis with a sample of 50
cases was conducted. The analysis found that early age of onset and
CTh were not associated with OS in this population, although these
factors are commonly recognized as being beneficial in
immunocompetent patients with glioma; this may be due to the
limited sample size. At the initial diagnosis of glioma, the
CD4+ T-cell count and HAART status were not found to
affect OS, but a trend of increased median survival with high
CD4+ T-cell count and HAART treatment was identified.
Adjuvant RT and the WHO grade of the glioma were found to be
independent prognostic factors. The results also indicate that
glioma may occur more commonly than is currently recognized in this
population. Therefore, in addition to the most common AIDS-defining
diseases, glioma should be included in the differential diagnosis
of contrast-enhanced CNS lesions in patients with HIV. When the
diagnosis remains uncertain, SB or direct SR should be performed in
selective cases to avoid inappropriate treatment. When the
diagnosis of glioma is confirmed, the approach to the management of
the tumor should be the same as that in the general population. SR
followed by RT plus regular HAART is recommended for the treatment
of HIV-positive patients with glioma. Tumor progression, as opposed
to AIDS-related complications, determines patient survival and is
the leading cause of mortality. Despite multiple aggressive
therapies, the median survival time after diagnosis is <1 year.
In the present study, a nomogram containing four factors that
performed well in predicting the 0.5- and 1-year survival rates was
developed. As evidenced by the calibration plot and its
discriminative ability, the nomogram provides accurate survival
probability, which can assist clinicians, patients and families in
decision-making and prognosis prediction for cases with concurrent
HIV infection and glioma.
There are several limitations of the present study.
This retrospective study was based on a small sample population due
to the rare incidence of the concurrence of HIV and glioma.
Patients who succumbed to HIV infection and surgical complications
were not excluded, which may have contributed to some statistical
bias. Future studies with larger cohorts and molecular pathological
analyses are required to corroborate the effect of HIV infection on
the development of gliomas. Furthermore, the nomogram model was
based only on treatment strategies and WHO grade risk factors.
Other promising risk factors, such as molecular features observed
in immunocompetent glioma patients, may be included to further
improve the performance of the model.
Supplementary Material
Flow chart representation of the
systematic literature review performed according to PRISMA
guidelines.
Kaplan-Meier curves for OS based on
(A) IDH1 status, alongside representative immunohistochemical
imaging of (B) IDH1+ and (C) IDH1-. Kaplan-Meier curves for OS
based on (D) Ki-67 level, alongside representative
immunohistochemical imaging of (E) Ki-67 20% and (F) Ki-67 80%.
IDH, isocitrate dehydrogenase 1. OS, overall survival.
Comparison of CD31+,
CD68+ and CD163+ cell counts between gliomas
from HIV-positive and -negative patients. Gliomas from HIV-positive
patients had a lower count of CD163+ cells, but no
difference in CD31+ and CD68+ counts.
*P<0.05. HIV, human immunodeficiency virus; HPF,
high-power field at a magnification of x400; ns, not
significant.
Immunohistochemical imaging of CD31,
CD68 and CD163 in gliomas from HIV-positive and -negative patients
(magnification, x400). HIV, human immunodeficiency virus.
Immunohistochemical staining results
for CD31, CD68 and CD163 in 10 HIV-positive patients with glioma
(ID no. 1-10) and 18 HIV-negative patients with glioma (ID no.
11-28).
CD4+T cell count and HIV
load of 33 patients with acquired immunodeficiency syndromerelated
primary central nervous system lymphoma.
CD4+T cell count of 17
HIV-negative patients with glioma.
Acknowledgements
Not applicable.
Funding
Funding: The study was funded by The Seedling Plan of Beijing
Ditan Hospital Research Fund (grant no. DTYM-202106), Key
Laboratory Open Research of Beijing Ditan Hospital (grant no.
DTKF-202203) and Beijing Municipal Administration of Hospitals
Incubating Program (grant no. PX-2024065).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XD, TL and EF were responsible for
conceptualization. XD and BL curated the data. TL and EF acquired
funding. BL, TL and EF performed the investigations. XD and XZho
were responsible for methodology. TL and EF performed project
administration. XZhe and FW contributed to the acquisition of
clinical data. XZho, HG and JC contributed to the acquisition of
experimental data. XD was responsible for software. TL and EF
supervised the study. EF validated and visualized the results. XD
wrote the original draft of the manuscript, and BL and TL reviewed
and edited the manuscript. EF and TL confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
Beijing Ditan Hospital (approval no. KY 2022-029-001). Written
informed consent was obtained from all the patients included in the
study.
Patient consent for publication
The patient or their parent, guardian or next of kin
provided written informed consent for the publication of any
associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chamberlain MC: Gliomas in patients with
acquired immune deficiency syndrome. Cancer. 74:1912–1914.
1994.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Engels EA, Pfeiffer RM, Goedert JJ, Virgo
P, McNeel TS, Scoppa SM and Biggar RJ: HIV/AIDS Cancer Match Study.
Trends in cancer risk among people with AIDS in the United States
1980-2002. AIDS. 20:1645–1654. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Moulignier A, Mikol J, Pialoux G,
Eliaszewicz M, Thurel C and Thiebaut JB: Cerebral glial tumors and
human immunodeficiency virus-1 infection. More than a coincidental
association. Cancer. 74:686–692. 1994.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tacconi L, Stapleton S, Signorelli F and
Thomas DG: Acquired immune deficiency syndrome (AIDS) and cerebral
astrocytoma. Clin Neurol Neurosurg. 98:149–151. 1996.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Blumenthal DT, Raizer JJ, Rosenblum MK,
Bilsky MH, Hariharan S and Abrey LE: Primary intracranial neoplasms
in patients with HIV. Neurology. 52:1648–1651. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mendez Valdez MJ, Lu VM, Kim E, Rivas SR,
Govindarajan V, Ivan M, Komotar R, Nath A, Heiss JD and Shah AH:
Glioblastoma multiforme in patients with human immunodeficiency
virus: An integrated review and analysis. J Neurooncol.
159:571–579. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jiang T, Nam DH, Ram Z, Poon WS, Wang J,
Boldbaatar D, Mao Y, Ma W, Mao Q, You Y, et al: Clinical practice
guidelines for the management of adult diffuse gliomas. Cancer
Lett. 499:60–72. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gasnault J, Roux FX and Vedrenne C:
Cerebral astrocytoma in association with HIV infection. J Neurol
Neurosurg Psychiatry. 51:422–424. 1988.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Carrana EJ, Rossitch E, Moore MR and
Funkenstein HH: Anaplastic astrocytoma in association with human
immunodeficiency virus type 1 infection. Am J Emerg Med. 8:565–567.
1990.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ho KL, Gottlieb C and Zarbo RJ:
Cytomegalovirus infection of cerebral astrocytoma in an AIDS
patient. Clin Neuropathol. 10:127–133. 1991.PubMed/NCBI
|
|
11
|
Kasantikul V, Kaoroptham S and Hanvanich
M: Acquired immunodeficiency syndrome associated with cerebral
astrocytoma. Clin Neuropathol. 11:25–27. 1992.PubMed/NCBI
|
|
12
|
Chappell ET, Guthrie BL and Orenstein J:
The role of stereotactic biopsy in the management of HIV-related
focal brain lesions. Neurosurgery. 30:825–829. 1992.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gervasoni C, Ridolfo AL, Rocca A, Vago L
and d'Arminio Monforte A: Cerebral astrocytoma in HIV-infected
patients. AIDS. 9:403–404. 1995.PubMed/NCBI
|
|
14
|
Neal JW, Llewelyn MB, Morrison HL, Jasani
B and Borysiewicz LK: A malignant astrocytoma in a patient with
AIDS: A possible association between astrocytomas and HIV
infection. J Infect. 33:159–162. 1996.PubMed/NCBI View Article : Google Scholar
|
|
15
|
d'Arminio Monforte A, Cinque P, Vago L,
Rocca A, Castagna A, Gervasoni C, Terreni MR, Novati R, Gori A,
Lazzarin A and Moroni M: A comparison of brain biopsy and CSF-PCR
in the diagnosis of CNS lesions in AIDS patients. J Neurol.
244:35–39. 1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vannemreddy PS, Fowler M, Polin RS, Todd
JR and Nanda A: Glioblastoma multiforme in a case of acquired
immunodeficiency syndrome: Investigation a possible oncogenic
influence of human immunodeficiency virus on glial cells. Case
report and review of the literature. J Neurosurg. 92:161–164.
2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wolff R, Zimmermann M, Marquardt G,
Lanfermann H, Nafe R and Seifert V: Glioblastoma multiforme of the
brain stem in a patient with acquired immunodeficiency syndrome.
Acta Neurochir (Wien). 144:941–944; discussion 944-5.
2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Corti ME, Yampolsky C, Metta H, Valerga M,
Sevlever G and Capizzano A: Oligodendroglioma in a patient with
AIDS: Case report and review of the literature. Rev Inst Med Trop
Sao Paulo. 46:195–197. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hall JR and Short SC: Management of
glioblastoma multiforme in HIV patients: A case series and review
of published studies. Clin Oncol (R Coll Radiol). 21:591–597.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chaudhry NS, Ahmad FU, Blieden C and
Benveniste RJ: Brainstem anaplastic glioma in patients with AIDS: A
case report and review of the literature. BMJ Case Rep.
2013(bcr2012008384)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Brassesco MS, Darrigo LG Jr, Valera ET,
Oliveira RS, Yamamoto YA, de Castro Barros MV and Tone LG:
Giant-cell glioblastoma of childhood associated with HIV-1 and JC
virus coinfection. Childs Nerv Syst. 29:1387–1390. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Oliveira VC, Gomes T, Ferreira LC, Damian
MM, Silva VM, Araújo JR, Safe IP and Ramasawmy R: Glioblastoma
multiforme in an HIV-Infected patient: An unexpected diagnosis. J
Int Assoc Provid AIDS Care. 13:411–413. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang T, Gao T, Niu X, Xing X, Yang Y, Liu
Y and Mao Q: Clinical characteristics and prognostic analysis of
glioma in human immunodeficiency virus-infected patients. World
Neurosurg. 114:e218–e223. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jokonya L, Musara A, Esene IN, Kabulo KDM,
Kabeya CM and Kalangu KKN: Prevalence of human immunodeficiency
virus infection in brain glioma patients: Is the virus protective
from gliomas? Surg Neurol Int. 9(103)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Simpson DM and Tagliati M: Neurologic
manifestations of HIV infection. Ann Intern Med. 121:769–785.
1994.PubMed/NCBI View Article : Google Scholar
|
|
26
|
GBD 2017 HIV collaborators: Global,
regional, and national incidence, prevalence, and mortality of HIV,
1980-2017, and forecasts to. 2030, for 195 countries and
territories: A systematic analysis for the Global Burden of
Diseases, Injuries, and Risk Factors Study 2017. Lancet. HIV 6:
e831-e859, 2019.
|
|
27
|
Ostrom QT, Cote DJ, Ascha M, Kruchko C and
Barnholtz-Sloan JS: Adult glioma incidence and survival by race or
ethnicity in the United States From 2000 to 2014. JAMA Oncol.
4:1254–1262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu S, Zhang C, Maimela NR, Yang L, Zhang
Z, Ping Y, Huang L and Zhang Y: Molecular and clinical
characterization of CD163 expression via large-scale analysis in
glioma. Oncoimmunology. 8(1601478)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu L, Liu Z, Wang H, Lu J, Xu J, Meng Y,
Huang K and Liu B: SESN2 could be a potential marker for diagnosis
and prognosis in glioma. Genes (Basel). 14(701)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang M, Cheng Y, Xue Z, Sun Q and Zhang
J: A novel pyroptosis-related gene signature predicts the prognosis
of glioma through immune infiltration. BMC Cancer.
21(1311)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hajjar M, Lacoste D, Brossard G, Morlat P,
Dupon M, Salmi LR and Dabis F: Non-acquired immune deficiency
syndrome-defining malignancies in a hospital-based cohort of human
immunodeficiency virus-infected patients: Bordeaux, France,
1985-1991. Groupe d'Epidémiologie Clinique du SIDA en Aquitaine. J
Natl Cancer Inst. 84:1593–1595. 1992.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rabkin CS and Blattner WA: HIV infection
and cancers other than non-Hodgkin lymphoma and Kaposi's sarcoma.
Cancer Surv. 10:151–160. 1991.PubMed/NCBI
|
|
33
|
Pore N, Gupta AK, Cerniglia GJ and Maity
A: HIV protease inhibitors decrease VEGF/HIF-1alpha expression and
angiogenesis in glioblastoma cells. Neoplasia. 8:889–895.
2006.PubMed/NCBI View Article : Google Scholar
|