Introduction
Lung cancer refers to a malignant tumor originating
from the bronchial mucosal epithelium (1). Lung cancer is one of the most common
and fastest-growing type of malignant tumor at present (2). Currently, it is the main cause of
human cancer deaths, accounting for ~30% of all cancer deaths
worldwide (3). Radiotherapy and
chemotherapy remain effective treatments for early, middle and
advanced stages of lung cancer (4). The 5-year survival rate of patients
with lung cancer after radiotherapy and chemotherapy is 15%
(5). In the treatment of patients
with lung cancer, standard platinum-based doublet therapy, which is
the traditional chemotherapy regimen, has not made a breakthrough
in the improvement of the survival rate (6). In addition, there can be serious
adverse reactions, including cutaneous adverse reactions and
diabetic ketoacidosis, to certain drugs, including poziotinib and
pembrolizumab (7,8). Therefore, the development of
alternative effective treatments is an important topic in lung
cancer research.
Glycolysis, also known as Warburg effect, refers to
the transformation of glucose into lactate in cancer cells under
the aerobic conditions (9).
Increasing studies have unveiled the significance of glycolysis in
tumor progression of lung cancer (10,11).
Traditional Chinese Medicine (TCM) combined with
chemotherapy and radiotherapy forms an important part of the lung
cancer treatment system in China (12). Increasing numbers of TCM
preparations, including Liqi, Ganoderma lucidum and
Prunella vulgaris have been reported to exert anticancer
effects with few side effects. Nowadays, TCM has also been
extensively applied to the clinical treatment of lung cancer and
TCM has great potential to provide candidate drugs for the disease
(13,14). Britannin compound is a key
bioactive component from the Chinese herb Inula, which has
traditionally been used for the effects of eliminating phlegm and
being an anti-emetic. Britannin has been reported to reduce the
activity of the acute lymphoblastic leukemia cell line MOLT-4 by
decreasing the proliferative capacity of cells whilst promoting
cell cycle arrest (15). In
addition, Britannin can inhibit the invasion and migration of 4T1
breast cancer cells, thereby inhibiting lung metastasis of these
cells (16). However, to the best
of our knowledge, a study on the effects of Britannin in lung
cancer has not yet been reported.
Kruppel-like factor 5 (KLF5) is a transcription
factor that is expressed in various tissues and functions as a
pivotal regulator in cell proliferation, differentiation and
survival (17). Research has
revealed that abnormal expression of KLF5 is observed in a number
of different types of cancer, such as breast, prostate and bladder
cancer (17). Notably, increasing
evidence has shown that KLF5 also exerts tumor-promoting activities
in lung cancer (18-21).
Therefore, in the present study, the influence of
Britannin on the proliferation, migration, and glycolysis of lung
cancer cells were analyzed, before its mechanism of action was
investigated.
Materials and methods
Molecular docking
The three-dimensional (3D) structure of the
Kruppel-like factor 5 (KLF5) protein (PDB ID: 2EBT) was obtained
using the PDB database (https://www.rcsb.org/). Thereafter, the KLF5 protein
structure file was edited using the PyMOL software (version 2.2.0;
Delano Scientific LLC) to remove the excess water molecules and
delete small ligands so that only the protein structure remained.
Since the downloaded protein structure contained ligands, the
original ligands such as water molecules and other bound ligands
were deleted from the structure and the original ligand positions
were set as docking sites. The PubChem database (http://pubchem.ncbi.nlm.nih.gov) was used to
obtain the 3D structure of Britannin, which was then imported into
OpenBabel software (version 2.2.1; Open Babel development team) for
hydrogenation and conversion into the ‘mol2’ file format (6). AutoDock software (version 4.2;
Scripps Research Institute) was utilized to display the specific
docking energy value. The position with the lowest binding energy
(-6.3 kcal/mol) was selected for visualization. Finally, the
results were analyzed using the Protein-Ligand Interaction Profiler
(version 2.3.0; https://plip-tool.biotec.tu-dresden.de/plip-web).
Cell culture
Lung carcinoma cell A549 cells (cat. no. B211337)
and bronchial epithelial 16HBE cells (cat. no. MZ-1420) purchased
from Ningbo Mingzhou Biotechnology Co., Ltd. Cells were cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C with 5%
CO2. Different concentrations of Britannin (5, 10 and 20
µM; purity, 99.90%; cat. no. HY-N3005; MedChemExpress) dissolved in
DMSO were used to treat A549 or 16HBE cells for 24 h at 37˚C and
untreated cells were used as negative controls.
Cell counting kit-8 (CCK-8) assay
A549 cells were seeded into 96-well plates
(8x104 cells/well) for 24 h at 37˚C. Different
concentrations of Britannin (5, 10 and 20 µM) were then added to
the cells and incubated for 24 h at 37˚C. Subsequently, 10 µl CCK-8
reagent (Beyotime Institute of Biotechnology) was added for 3 h of
incubation after the removal of the original culture medium. The
optical density of the samples were measured at 450 nm using a
plate reader (Infinite® 200 PRO; Tecan Group, Ltd.).
EdU staining
Cell proliferation capacity was determined by
5-ethynyl-2'-deoxyuridine (EdU) staining (Beyotime Institute of
Biotechnology). A549 cells were seeded into 12-well chambers,
treated with the aforementioned concentrations of Britannin and
incubated for 24 h at 37˚C. The cells were then incubated with 200
µl EdU (500 µM) at 37˚C for 2 h. Next, cells were fixed with 4%
paraformaldehyde for 15 min at room temperature and permeabilized
with 0.3% Triton X-100 for 10 min. Cells were then labeled with 2
µg/ml Hoechst 33342 (Beyotime Institute of Biotechnology) for 30
min at room temperature. EdU-positive cells were imaged using a
fluorescence microscope (Leica Microsystems GmbH).
Colony formation assay
A549 cells were seeded into 6-well plates
(5x103 cells/well) before the aforementioned
concentrations of Britannin were added to the cells. The culture
medium was replaced every 3 days. After 14 days at 37˚C, the cells
were washed with PBS, fixed in 4% paraformaldehyde at room
temperature for 15 min and then stained with 0.05% crystal violet
at room temperature for 20 min to visualize cell colonies under a
BX53 light microscope (Olympus Corporation). Colonies (≥50 cells)
were counted with Image J software v.1.52 (National Institutes of
Health).
Wound healing assay
A549 cells were seeded into 6-well plates
(5x105 cells/well) before the aforementioned
concentrations of Britannin were added to the cells. Subsequently,
the samples were scratched using a 200-µl pipette tip. Following
removal of the floating cells using PBS, the cells were cultured in
serum-free DMEM containing the aforementioned treatments. Cells
were imaged at 0 and 24 h under a light microscope. ImageJ software
v.1.52 (National Institutes of Health) was used to measure the
scratch areas. The recovery rate of the wound was calculated using
the following equation: [(Width at 0 h-width at 24 h)/width at 0 h]
x100.
Transwell migration assay
A549 cells at a density of 3x104/well in
serum-free medium were added to the upper Transwell chamber (8-µm
pore diameter; Merck KGaA) whilst the bottom chamber was filled
with complete DMEM containing 7% FBS. The cells were the incubated
for 24 h at 37˚C before cells on the upper side of the filter
membrane were removed using a cotton swab. Cells that passed
through the membrane were fixed with 70% methanol for 15 min at
room temperature, stained with 0.1% crystal violet at room
temperature for 30 min and imaged using a light microscope (Olympus
Corporation). Cell migration rates were determined using ImageJ
software (version 1.48; National Institutes of Health).
Western blotting
A549 cells (1x105 cells/well) were seeded
into 6-well plates. After the cells were treated with the
aforementioned concentrations of Britannin, the proteins were
extracted using RIPA buffer (Beyotime Institute of Biotechnology).
The supernatant was used to determine protein concentration by
bicinchoninic acid protein concentration determination kit (cat.
no. RTP7102; Real-Times (Beijing) Biotechnology Co., Ltd.).
Proteins (6 µg/lane) were subjected to SDS-PAGE on 10% gels and
then transferred onto a PVDF membrane (0.45 µm; MilliporeSigma).
Membranes were blocked with 5% BSA (Beyotime Institute of
Biotechnology) for 2 h at room temperature and incubated with
primary antibodies overnight at 4˚C, followed by incubation with
secondary antibodies for 1 h at room temperature, specifically Goat
Anti-Rabbit IgG H&L (HRP; 1:2,000; cat. no. ab6721; Abcam).
Primary antibodies used were as follows: E-cadherin (1:1,000; cat.
no. ab227639; Abcam); N-cadherin (1:1,000; cat. no. ab76011;
Abcam); Snail (1:1,000; cat. no. ab216347; Abcam); KLF5 (1:1,000;
cat. no. ab137676; Abcam); and GAPDH (1:1,000; cat. no. ab181602;
Abcam). Bands were visualized using ECL Western Blotting Detection
Reagent (cat. no. RPN2106; GE Healthcare) and ImageJ software
(version 1.8.0; National Institutes of Health) was used for the
semi-quantification of protein expression levels.
RNA isolation and reverse
transcriptase-quantitative PCR (RT-qPCR)
Total RNA was extracted from Britannin-treated 16HBE
cells or A549 cells subjected to Britannin treatment and
transfection of Oe-KLF5 or Oe-NC using the RNeasy Kit®
and DNase Set (DNAse H; Qiagen China Co., Ltd.) assay kits
according to the manufacturer's protocols. RNA concentration was
measured using a NanoDrop photometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.). A total of 1 µg RNA was
reverse-transcribed to cDNA using the iScript™ RT kit (Bio-Rad
Laboratories, Inc.). The temperature protocol was 15 min at 37˚C, 5
sec at 85˚C and 30 min at 4˚C. Amplification of cDNA was the
performed using a CFX96 Touch™ Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.) with the iQ SYBR® Green
Supermix (Bio-Rad Laboratories, Inc.) according to the
manufacturer's instructions. The thermal cycling conditions used
were as follows: Initial denaturation at 95˚C for 5 min; followed
by 40 cycles of 95˚C for 10 sec, 57-60.5˚C for 10 sec and 72˚C for
30 sec. GAPDH served as the internal control gene for normalization
and the expression levels were quantified using the
2-ΔΔCq method (22).
The PCR primers used were as follows: KLF5 forward (F),
5'-AGCTACAATACGCTTGGCCT-3' and reverse (R).
5'-ATGTGTGTTACGCACGGTCT-3' and GAPDH F, 5'-AATGGGCAGCCGTTAGGAAA-3'
and R, 5'-GCGCCCAATACGACCAAATC-3'.
Detection of oxygen consumption rate
(OCR) and extracellular acidification rate (ECAR)
A Seahorse Bioscience XF96 Extracellular Flux
Analyzer (Agilent Technologies, Inc.) was used to detect the
cellular ECAR and OCR. In brief, A549 cells (2x104
cells/well) subjected to Britannin treatment and transfection of
Oe-KLF5 or Oe-NC were seeded into 96-well cell culture XF
microplates and incubated overnight at 37˚C for further testing
according to the manufacturer's instructions.
Lactate production and glucose uptake
assay
A549 cells subjected to Britannin treatment and
transfection of Oe-KLF5 or Oe-NC were seeded into a 24-well plate
(2x105 cells/ml). Aliquots of media from each well were
assessed for the concentration of lactate present using a lactate
test kit (cat. no. A019-2; Nanjing Jiancheng Bioengineering
Institute) according to the manufacturer's instructions. For
glucose uptake assay, A549 cells were cultured under normoxic
conditions in glucose-free DMEM (Gibco; Thermo Fisher Scientific,
Inc.) for 16 h at 37˚C and were then incubated with 25 mmol/l
high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.) for 24 h
at 37˚C. Then, the intracellular glucose levels were measured using
a fluorescence-based glucose assay kit (cat. no. ab136956;
BioVision, Inc.) according to the manufacturer's instructions.
Values were normalized accordingly to calculate lactic acid
production and glucose consumption.
Cell transfection
Cells were transfected with pcDNA3.1 containing KLF5
(Oe-KLF5; GeneChem, Inc.) and negative control plasmid (Oe-NC;
GeneChem, Inc.) using the Lipofectamine® 2000
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
at final concentration of 50 nM according to the manufacturer's
instructions. After 48 h at 37˚C, RT-qPCR and western blotting were
used to evaluate the transfection efficiency. Further experiments
were performed after 48 h.
Statistical analysis
All data were expressed as mean ± standard
deviation. One-way analysis of variance with Tukey's post hoc test
was performed for multiple comparisons using GraphPad Prism
software (version 5; Dotmatics). A two-tailed unpaired Student's
t-test was applied for comparisons between two groups. P<0.05
was considered to indicate a statistically significant difference.
Each experiment was conducted at least three times.
Results
Britannin inhibits the proliferation
of lung cancer cells
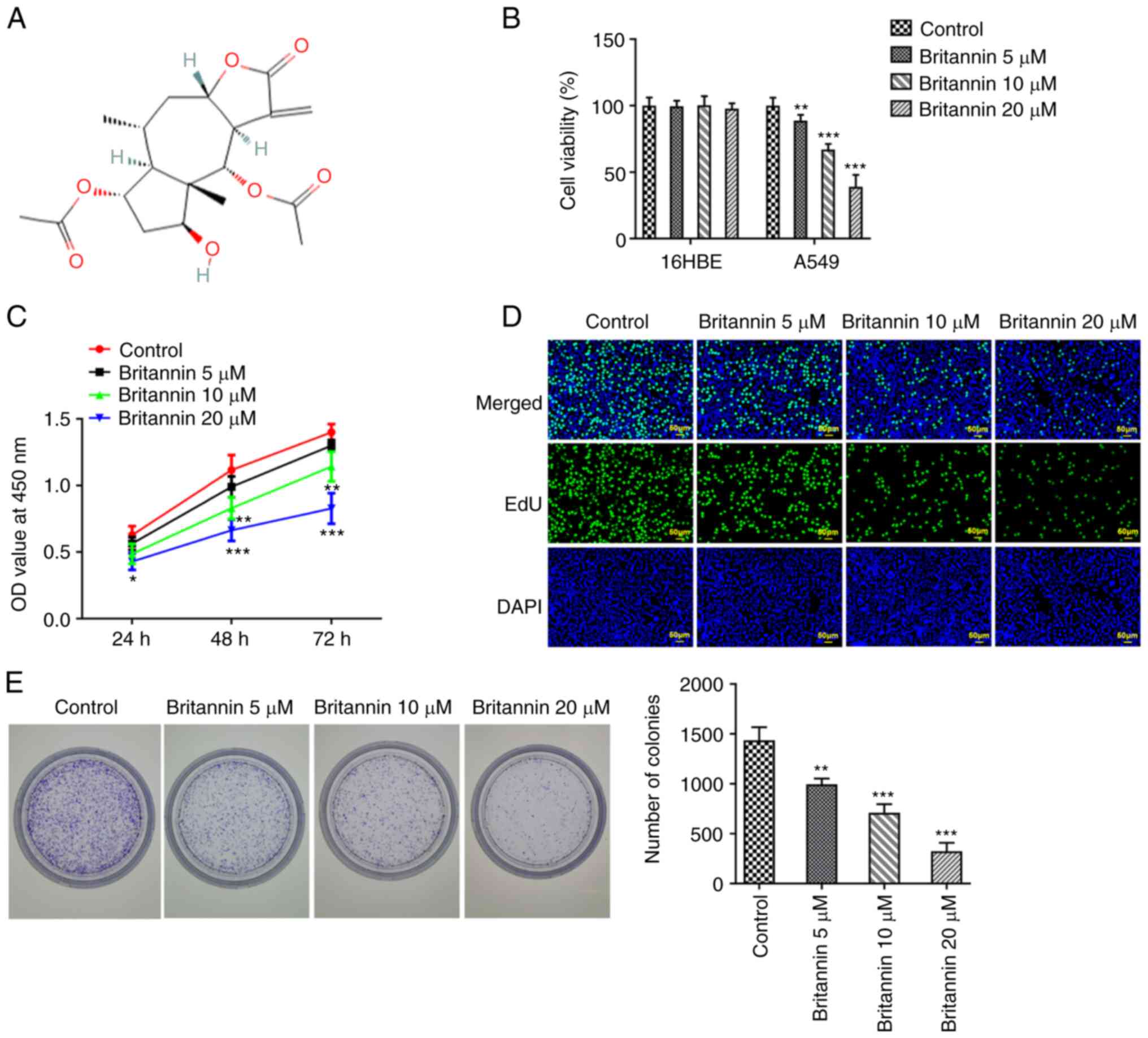
The chemical formula for Britannin was shown in
Fig. 1A. Different concentrations
of Britannin were used to treat 16HBE and A549 cells for 24 h, then
a CCK-8 assay was used to detect whether Britannin had an effect on
cell viability. The results demonstrated that Britannin was not
toxic to 16HBE cells, but the viability of A549 cells was
significantly decreased with the increase of Britannin
concentrations compared with that in the control group (Fig. 1B). Considering that Britannin
exerted no effect on the viability of 16HBE cells, A549 cells whose
viability was greatly diminished by Britannin were selected for the
ensuing experiments to further work out the role of Britannin in
lung cancer. A549 cells were then treated with different
concentrations of Britannin for 24, 48 and 72 h before cell
proliferation was analyzed using CCK-8 assay. The results
demonstrated that with the increase of Britannin treatment time,
the inhibitory ability of Britannin on the viability of A549 cells
was significantly enhanced compared with that in the untreated
cells (Fig. 1C). EdU staining and
colony formation assay were next performed to measure the extent of
cell proliferation, where it was demonstrated that the
proliferation of A549 cells treated with Britannin was markedly
decreased in a dose-dependent manner compared with that in the
control group (Fig. 1D and
E).
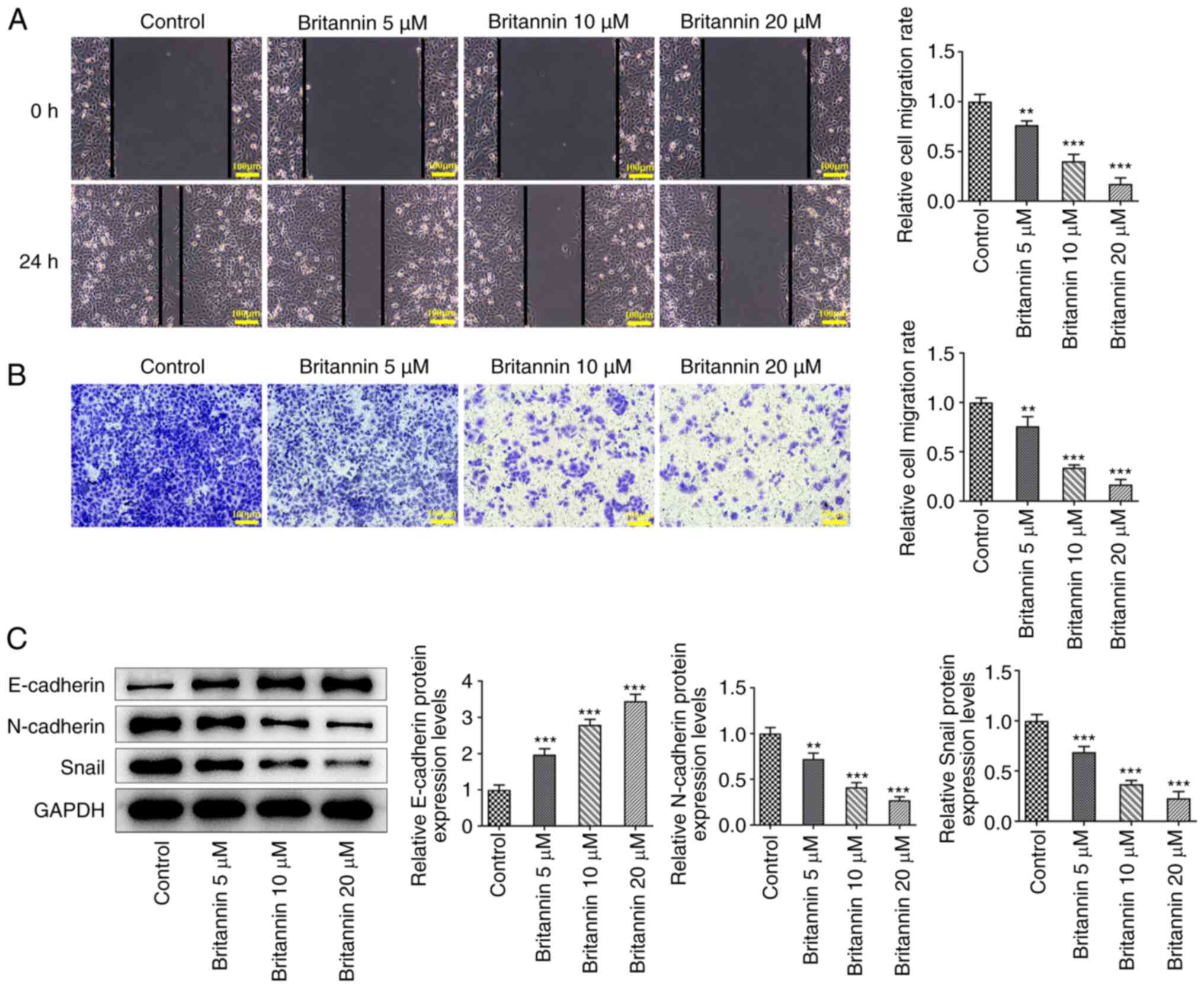
Britannin inhibits the migration of
lung cancer cells
The migratory ability of A549 cells was subsequently
detected by wound healing and Transwell assays. It was demonstrated
that the migratory ability of cells was significantly decreased
with increasing concentrations of Britannin treatment (Fig. 2A and B). The expression of
epithelial-mesenchymal transition (EMT)-related proteins
E-cadherin, N-cadherin and Snail was then detected by western
blotting. Compared with those in the control group, the protein
expression levels of E-cadherin were increased significantly in
Britannin-treated cells, whilst those of N-cadherin and Snail were
decreased significantly (Fig.
2C).
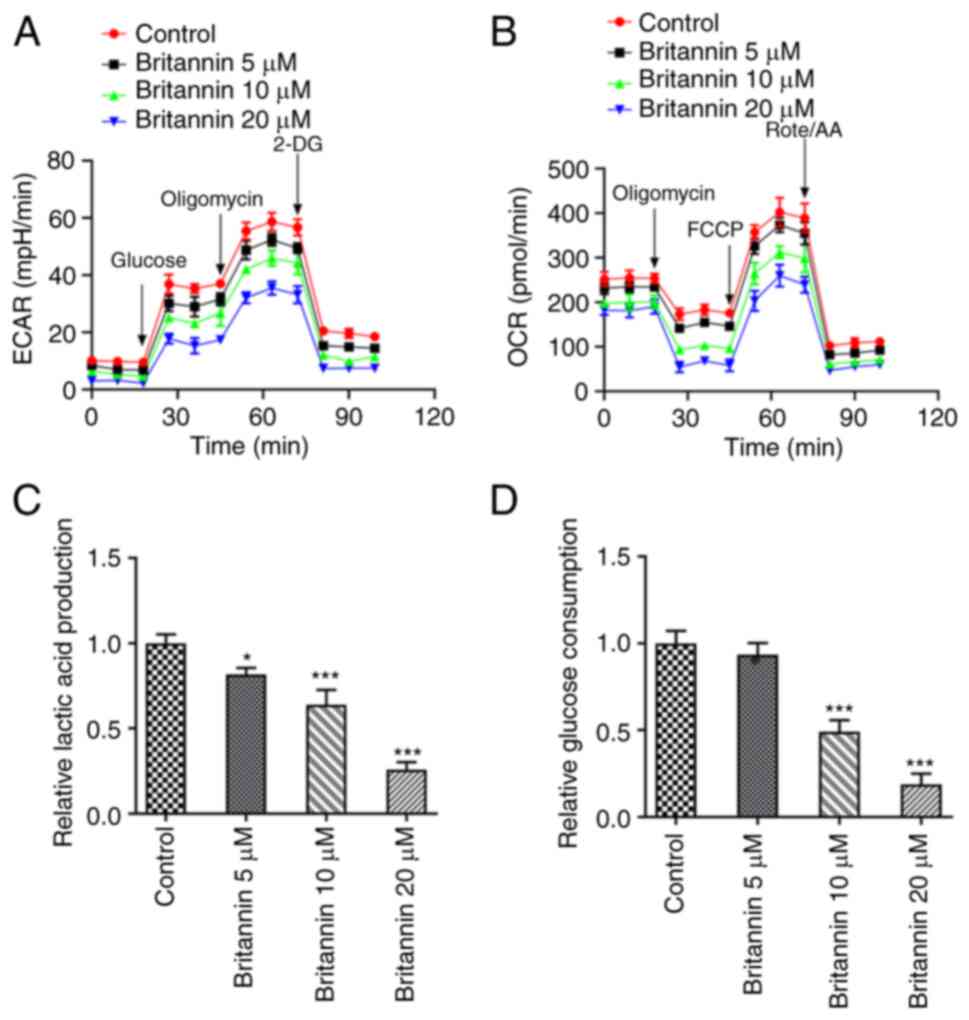
Britannin inhibits glycolysis in lung
cancer cells
The XF96 extracellular flux analyzer was used to
detect ECAR and OCR in Britannin-treated cells. These results
demonstrated that compared with those in the control group, ECAR
and OCR levels in the Britannin-treated groups were decreased in a
dose-dependent manner (Fig. 3A and
B). The lactic acid production and
glucose consumption levels in the cell supernatant were next
measured using assay kits, where the results demonstrated that with
the increase in the treatment dose of Britannin, the lactic acid
and glucose consumption levels were decreased in a dose-dependent
manner (Fig. 3C and D). Compared with those in the untreated
cells, lactic acid production was significantly decreased by all
doses of Britannin treatment whereas the glucose consumption level
was significantly decreased by 10 and 20 µM of Britannin.
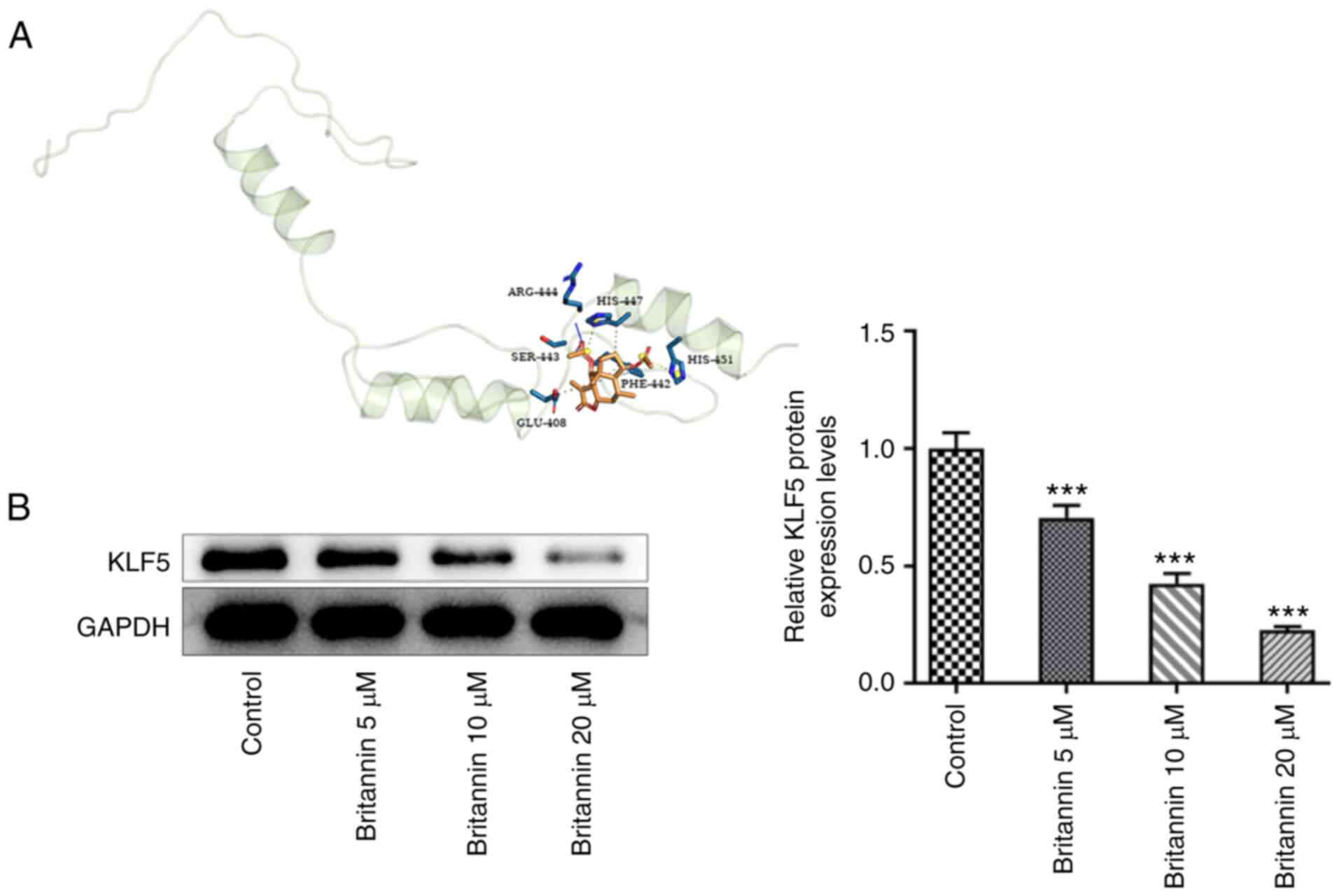
Britannin downregulates the protein
expression level of KLF5
KLF5 has been increasingly identified as an oncogene
in lung cancer. A molecular docking strategy was used to study the
interaction between ligand and receptor and to predict the binding
mode and affinity between Britannin and KLF5. It was demonstrated
that Britannin could target KLF5 (Fig.
4A). In addition, after Britannin treatment, the expression of
the KLF5 protein in A549 cells was decreased significantly in a
dose-dependent manner, compared with that in untreated cells
(Fig. 4B).
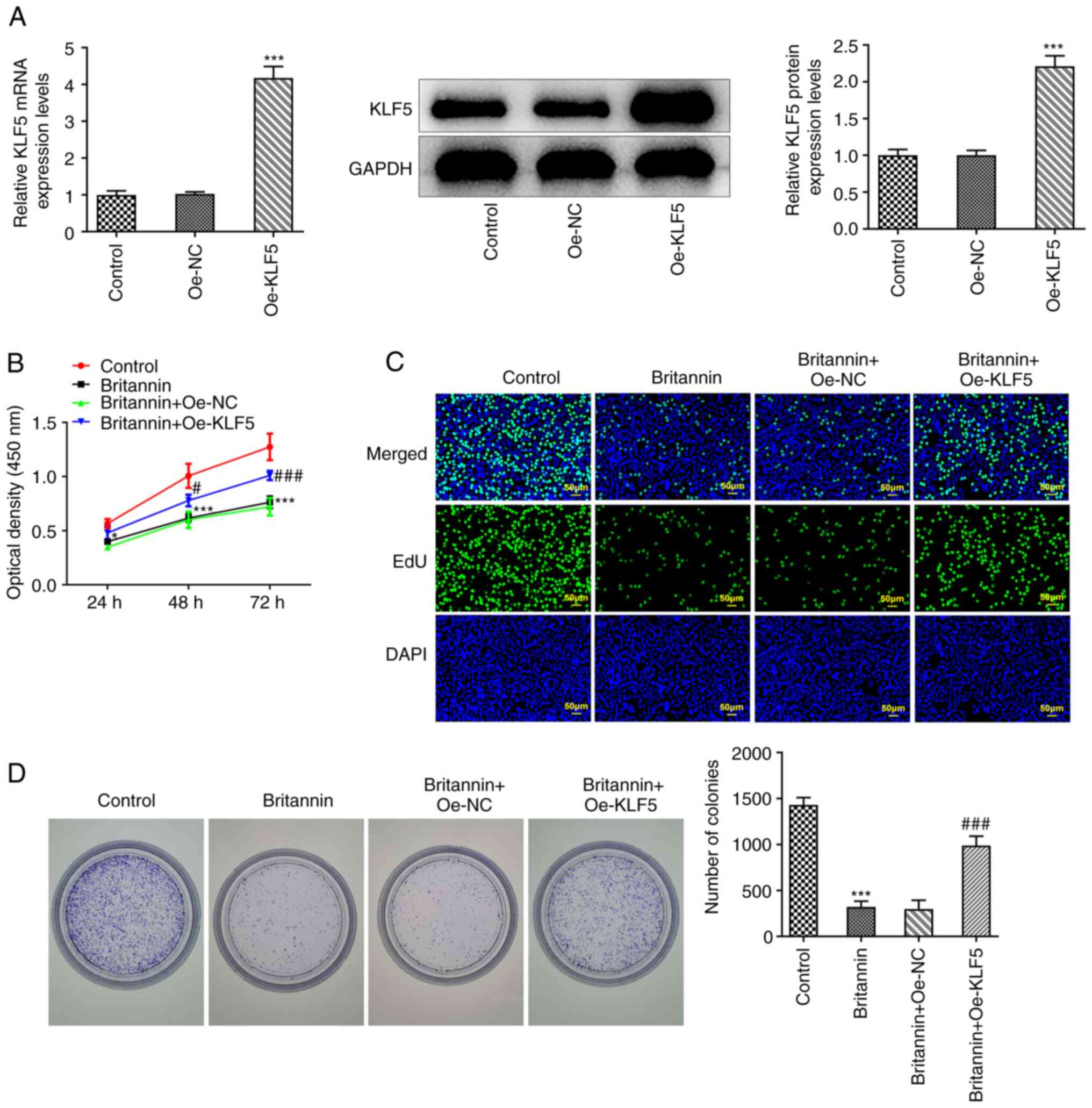
Overexpression of KLF5 reverses the
inhibitory effects of Britannin on the proliferation, migration and
glycolysis of lung cancer cells
The regulatory mechanism of Britannin in A549 cells
was then explored. A KLF5 overexpression plasmid was constructed
and the successful transfection of Oe-KLF5 was verified by RT-qPCR
and western blotting (Fig. 5A).
The cells were then divided into the following treatment groups: i)
Control; ii) Britannin; iii) Britannin + Oe-NC; and iv) Britannin +
Oe-KLF5. CCK-8, EdU staining and colony formation assays were used
to analyze the degree of cell proliferation and the results
demonstrated that compared with that in the Britannin + Oe-NC
group, cell proliferation was found to be markedly increased in the
Britannin + Oe-KLF5 group (Fig.
5B-D).
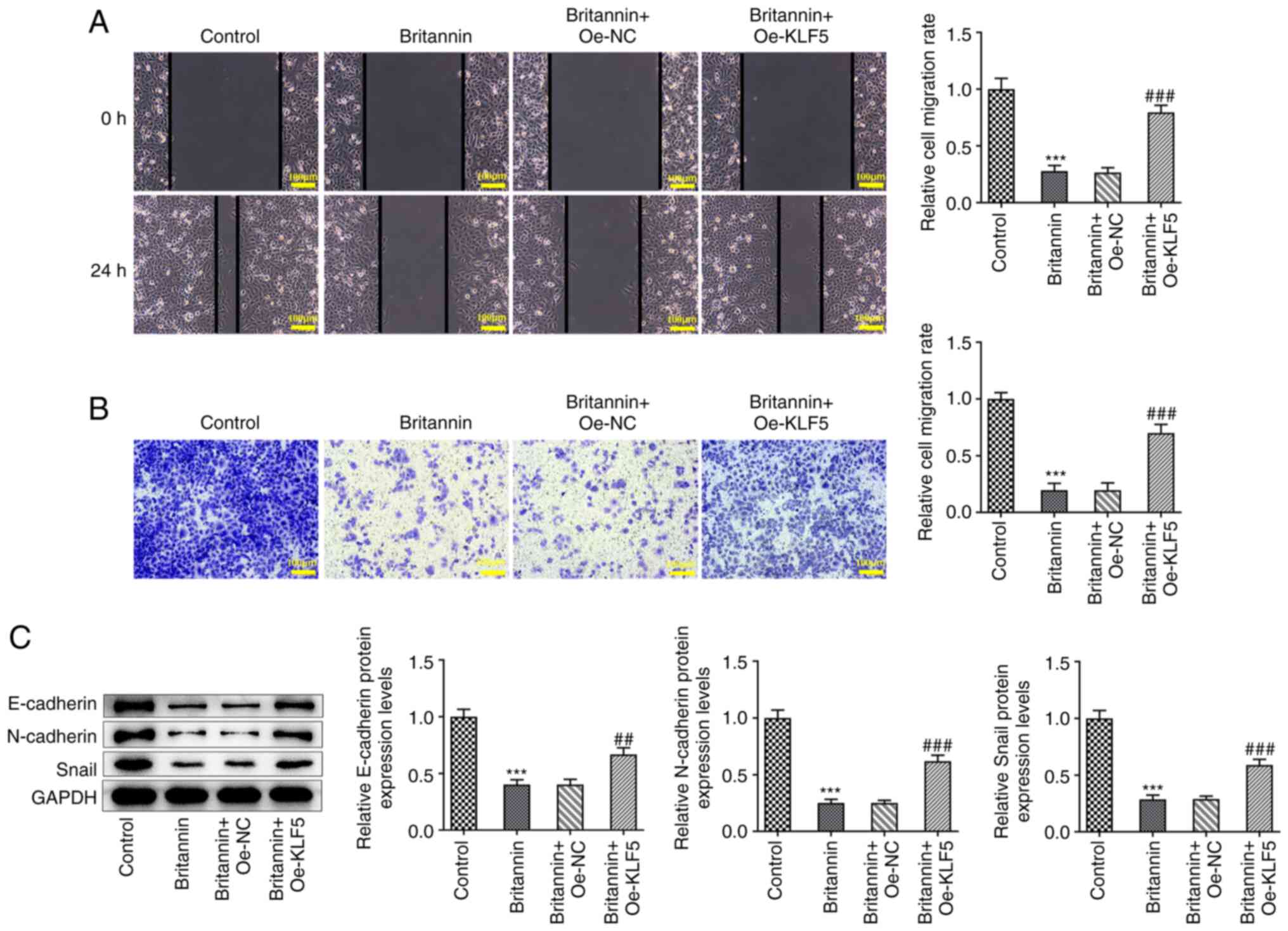
Wound healing and Transwell assay results
demonstrated that the overexpression of KLF5 significantly reversed
the inhibition of A549 cell migration caused by Britannin treatment
(Fig. 6A and B). Western blotting results demonstrated
that compared with those in the Britannin + Oe-NC group, the
protein expression levels of E-cadherin, N-cadherin and Snail were
significantly increased in the Britannin + Oe-KLF5 group (Fig. 6C).
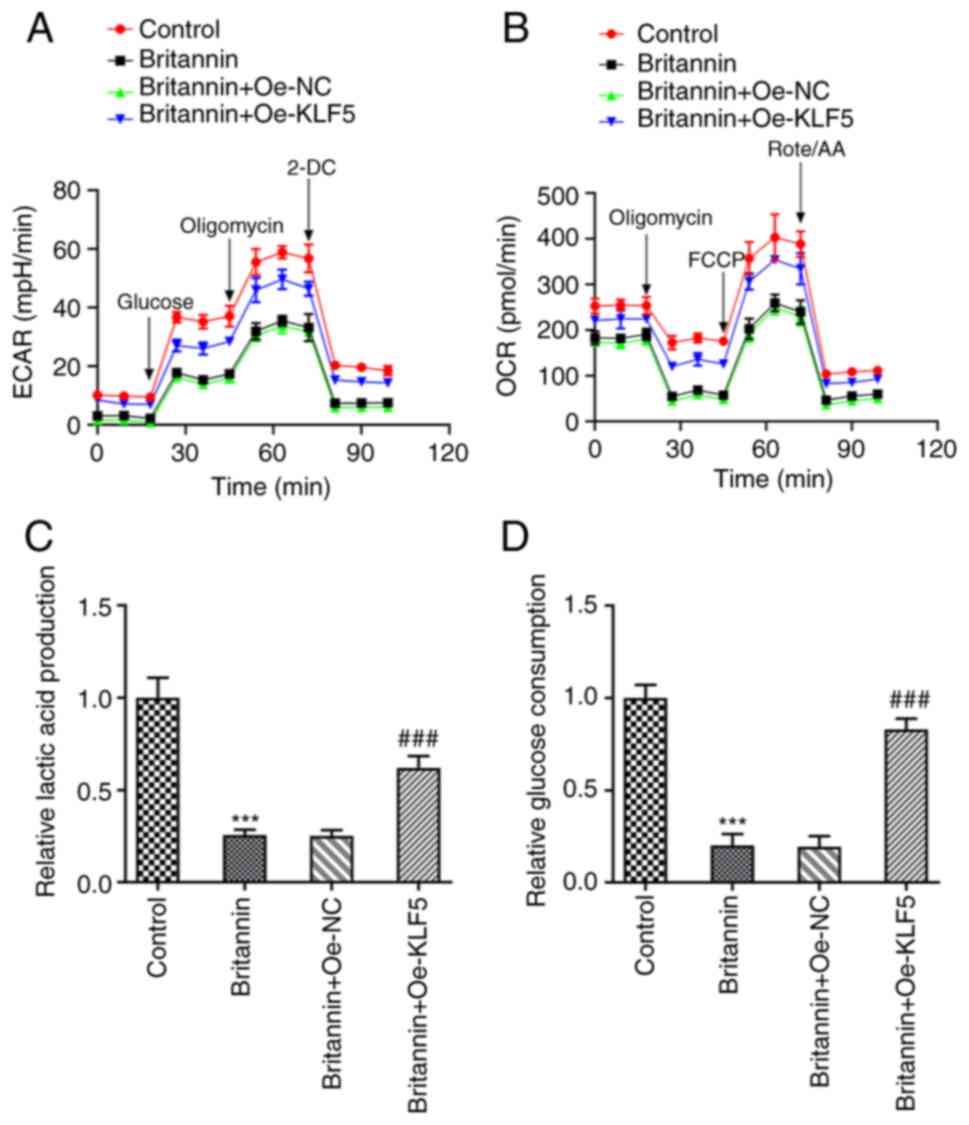
The results of ECAR and OCR indices detected using
the XF96 extracellular flux analyzer demonstrated that
overexpression of KLF5 markedly reversed the inhibitory effect of
Britannin on ECAR and OCR (Fig. 7A
and B). In addition, lactate
production and glucose consumption levels were significantly
increased in the Britannin + Oe-KLF5 group compared with those in
the Britannin + Oe-NC group (Fig.
7C and D). These results
suggest that overexpression of KLF5 reversed the inhibitory effects
of Britannin on the proliferation, migration and glycolysis of lung
cancer cells.
Discussion
Britannin, which can be extracted from the plant
Inula japonica Thunb. and has been used in TCM, has been
previously reported to have anti-inflammatory, anti-oxidative
stress, antitumor and organ protective effects (23). In the present study, Britannin was
demonstrated to inhibit the viability and proliferation of A549
lung cancer cells. It was also demonstrated that Britannin can
significantly inhibit cell migration and the expression levels of
EMT-related proteins. To the best of our knowledge, there have been
no previous studies on the effects of Britannin on lung cancer,
though Britannin reportedly has been demonstrated to exert
therapeutic effects in other types of cancer. In particular, a
previous study reported that Britannin inhibited the proliferation
and migration of pancreatic cancer cells whilst inhibiting the
NF-κB pathway, thereby inhibiting pancreatic cancer tumor growth
(24). In liver cancer cells,
Britannin has been found to induce apoptosis and autophagy through
the activation of AMP-activated protein kinase regulated by
reactive oxygen species, thereby inhibiting the growth and
metastasis of liver cancer cells (25). These previous findings, together
with the results of the present study, suggest that Britannin can
serve an inhibitory role in the growth and migration of a number of
tumor types.
It has previously been reported that KLF5 is
expressed in paracancer and lung cancer tissues in patients with
lung cancer. Specifically, the expression levels of KLF5 are
abnormally elevated in lung cancer tissues, indicating that KLF5 is
an oncogene in lung cancer (18).
Furthermore, α-Catulin has been reported to promote cancer stemness
by antagonizing the WW domain-containing E3 ubiquitin protein
ligase 1-mediated degradation of KLF5 in lung cancer (19). Testis development-related 1 serves
a carcinogenic role in non-small cell lung cancer by targeting the
microRNA (miR)-214-5p/KLF5 axis (20). KLF5-induced γ-butyrobetaine
hydroxylase 1-antisense RNA 1 has been found to upregulate maternal
embryonic leucine zipper kinase expression to activate focal
adhesion kinase (FAK) signaling, by sponging miR-27a-5p,
contributing to the malignant phenotype of non-small cell lung
cancer (21). These results
suggest that KLF5 is an oncogene in lung cancer. The regulatory
mechanism of Britannin was next explored. Through the use of
molecular docking technology, it was demonstrated that Britannin
targeted the KLF5 protein. Therefore, this may explain how
Britannin inhibited the expression of KLF5 and the development of
lung cancer in the present study. Overexpression of KLF5 in A549
cells in the present study significantly reversed the inhibitory
effects of Britannin on the proliferation and migration of lung
cancer cells.
Metabolic reprogramming is an important
characteristic of tumor cells, which is mainly caused by increased
glycolysis and decreased oxidative phosphorylation, in a process
called ‘aerobic glycolysis’ or the ‘Warburg effect’ (9). Such metabolic changes have been
reported in primary and metastatic cancers, and accelerates the
various stages of tumor occurrence and development (26). The Warburg effect is considered a
marker of advanced malignant tumors (27). A previous study reported that
Britannin mediated the apoptosis and glycolysis of T cell
lymphoblastic lymphoma cells through AMPK-dependent autophagy
(28). In addition, KLF5 knockdown
was found to inhibit hypoxia-induced cisplatin resistance by
inhibiting hypoxia inedible factor-1α-dependent glycolysis in lung
cancer cells (29). KLF5 can also
directly bind to the phospholipase A and acyltransferase 3 promoter
to activate its transcription, which promotes glycolysis in
pancreatic cancer (30).
Therefore, this suggests that KLF5 is associated with glycolysis,
such that Britannin may serve a regulatory role in the glycolysis
of lung cancer cells through KLF5. In the present study, Britannin
was demonstrated to inhibit the ECAR and OCR in A549 cells, in
addition to the level of lactic acid production and glucose
consumption in the cell supernatant, suggesting that Britannin can
inhibit the glycolysis of A549 lung cancer cells. Furthermore,
overexpression of KLF5 reversed the inhibitory effect of Britannin
on the glycolysis of lung cancer cells.
The present study has certain limitations. All the
experiments were conducted in only one lung cancer cell line.
Additional lung cancer cell lines will need to be used in future
experiments. In addition, KLF5 expression in normal lung cells was
not analyzed in the present study and the impact of Britannin on
the protein expression levels of KLF5 in normal cells was not
determined. Since this was not the focus of the present study,
whether KLF5 is affected by Britannin in normal lung cells should
be analyzed in future experiments.
To conclude, it was found in the present study that
Britannin inhibited cell proliferation, metastasis and glycolysis
by downregulating KLF5 expression in lung cancer cells. These
findings offer a reference for the further investigation of the
mechanism underlying the therapeutic effect of Britannin in lung
cancer.
Acknowledgements
Not applicable.
Funding
Funding: Funding was obtained from the Medical Health Science
and Technology Development Project of Shandong Province (grant no.
202103020759).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW designed the experiments. YW, BY and MQ performed
the experiments and wrote the article. FL and XW analyzed the
experimental data and confirm the authenticity of all the raw data.
All the authors agreed to the publication of the article. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Greenberg AK, Yee H and Rom WN:
Preneoplastic lesions of the lung. Respir Res. 3(20)2002.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Mao Y, Yang D, He J and Krasna MJ:
Epidemiology of Lung Cancer. Surg Oncol Clin N Am. 25:439–445.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36
Cancers in 185 Countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung cancer: Biology and treatment options. Biochim
Biophys Acta. 1856:189–210. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bilfinger T, Keresztes R, Albano D and
Nemesure B: Five-Year Survival Among Stage IIIA Lung Cancer
Patients Receiving Two Different Treatment Modalities. Med Sci
Monit. 22:2589–2594. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Johnson ML and Patel JD: Chemotherapy and
targeted therapeutics as maintenance of response in advanced
non-small cell lung cancer. Semin Oncol. 41:93–100. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hui H, Wang M, Guo H, Wang Y and Shi B:
Poziotinib-induced cutaneous adverse reactions in the treatment of
non-small cell lung cancer: A case report. Int J Dermatol.
61:769–770. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Leonardi GC, Oxnard GR, Haas A, Lang JP,
Williams JS and Awad MM: Diabetic Ketoacidosis as an Immune-related
Adverse Event from Pembrolizumab in Non-Small Cell Lung Cancer. J
Immunother. 40:249–251. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ganapathy-Kanniappan S and Geschwind JF:
Tumor glycolysis as a target for cancer therapy: progress and
prospects. Mol Cancer. 12(152)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang L, Zhang Z and Yu Z: Identification
of a novel glycolysis-related gene signature for predicting
metastasis and survival in patients with lung adenocarcinoma. J
Transl Med. 17(423)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Smolle E, Leko P, Stacher-Priehse E, Brcic
L, El-Heliebi A, Hofmann L, Quehenberger F, Hrzenjak A, Popper HH,
Olschewski H and Leithner K: Distribution and prognostic
significance of gluconeogenesis and glycolysis in lung cancer. Mol
Oncol. 14:2853–2867. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li X, Yang G, Li X, Zhang Y, Yang J, Chang
J, Sun X, Zhou X, Guo Y, Xu Y, Liu J and Bensoussan A: Traditional
Chinese medicine in cancer care: a review of controlled clinical
studies published in chinese. PLoS One. 8(e60338)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Su XL, Wang JW, Che H, Wang CF, Jiang H,
Lei X, Zhao W, Kuang HX and Wang QH: Clinical application and
mechanism of traditional Chinese medicine in treatment of lung
cancer. Chin Med J (Engl). 133(24):2987–2997. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wei Z, Chen J, Zuo F, Guo J, Sun X, Liu D
and Liu C: Traditional Chinese Medicine has great potential as
candidate drugs for lung cancer: A review. J Ethnopharmacol.
300(115748)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mohammadlou H, Hamzeloo-Moghadam M, Yami
A, Feizi F, Moeinifard M and Gharehbaghian A: Britannin a
Sesquiterpene Lactone from Inula aucheriana Exerted an
Anti-leukemic Effect in Acute Lymphoblastic Leukemia (ALL) Cells
and Enhanced the Sensitivity of the Cells to Vincristine. Nutr
Cancer. 74:965–977. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lu H, Wu Z, Wang Y, Zhao D, Zhang B and
Hong M: Study on inhibition of Britannin on triple-negative breast
carcinoma through degrading ZEB1 proteins. Phytomedicine.
104(154291)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Luo Y and Chen C: The roles and regulation
of the KLF5 transcription factor in cancers. Cancer Sci.
112:2097–2117. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang H, Shao F, Guo W, Gao Y and He J:
Knockdown of KLF5 promotes cisplatin-induced cell apoptosis via
regulating DNA damage checkpoint proteins in non-small cell lung
cancer. Thorac Cancer. 10:1069–1077. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tung CH, Huang MF, Liang CH, Wu YY, Wu JE,
Hsu CL, Chen YL and Hong TM: alpha-Catulin promotes cancer stemness
by antagonizing WWP1-mediated KLF5 degradation in lung cancer.
Theranostics. 12:1173–1186. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lu X, Zhao N, Duan G, Deng Z and Lu Y:
Testis developmental related gene 1 promotes non-small-cell lung
cancer through the microRNA-214-5p/Kruppel-like factor 5 axis.
Bioengineered. 13:603–616. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shi J, Yang C, An J, Hao D, Liu C, Liu J,
Sun J and Jiang J: KLF5-induced BBOX1-AS1 contributes to cell
malignant phenotypes in non-small cell lung cancer via sponging
miR-27a-5p to up-regulate MELK and activate FAK signaling pathway.
J Exp Clin Cancer Res. 40(148)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang YF, Zhang ZH, Li MY, Wang JY, Xing
Y, Ri M, Jin CH, Xu GH, Piao LX, Zuo HX, Jin HL, Ma J and Jin X:
Britannin stabilizes T cell activity and inhibits proliferation and
angiogenesis by targeting PD-L1 via abrogation of the crosstalk
between Myc and HIF-1alpha in cancer. Phytomedicine.
81(153425)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li K, Zhou Y, Chen Y, Zhou L and Liang J:
A novel natural product, britanin, inhibits tumor growth of
pancreatic cancer by suppressing nuclear factor-kappaB activation.
Cancer Chemother Pharmacol. 85:699–709. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cui YQ, Liu YJ and Zhang F: The
suppressive effects of Britannin (Bri) on human liver cancer
through inducing apoptosis and autophagy via AMPK activation
regulated by ROS. Biochem Biophys Res Commun. 497:916–923.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Karlstaedt A, Barrett M, Hu R, Gammons ST
and Ky B: Cardio-Oncology: Understanding the Intersections Between
Cardiac Metabolism and Cancer Biology. JACC Basic Transl Sci.
6:705–718. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Danhier P, Banski P, Payen VL, Grasso D,
Ippolito L, Sonveaux P and Porporato PE: Cancer metabolism in space
and time: Beyond the Warburg effect. Biochim Biophys Acta Bioenerg.
1858:556–572. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hong H, Luo B, Xie Z, Li M, Xu Q, He Z and
Peng Z: Retracted: Britannin mediates apoptosis and glycolysis of
T-cell lymphoblastic lymphoma cells by AMPK-dependent autophagy. J
Biochem Mol Toxicol. e23211:2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gong T, Cui L, Wang H, Wang H and Han N:
Knockdown of KLF5 suppresses hypoxia-induced resistance to
cisplatin in NSCLC cells by regulating HIF-1α-dependent glycolysis
through inactivation of the PI3K/Akt/mTOR pathway. J Transl Med.
16(164)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xia W, Bai H, Deng Y and Yang Y: PLA2G16
is a mutant p53/KLF5 transcriptional target and promotes glycolysis
of pancreatic cancer. J Cell Mol Med. 24:12642–12655.
2020.PubMed/NCBI View Article : Google Scholar
|