Introduction
Diabetic kidney disease (DKD) is a serious
microvascular complication that can progress to severe end-stage
renal failure (1). A previous
epidemiological survey between 2015 and 2017 reported that there
are a large number of individuals with diabetes, with a prevalence
of 11.2% among individuals aged ≥18 years (2). Of the total number of patients with
diabetes, ~33.6% develop DKD, which can seriously affect their
quality of life (3). However, its
pathogenesis is still unclear. Clinically, DKD can be
quantitatively diagnosed based on urinary microalbumin levels.
However, the severity and prognosis of the disease cannot be
accurately assessed based on the degree of proteinuria (4). Therefore, attention should be paid to
the importance of pathological changes observed in patients with
DKD. Renal fibrosis is the main pathological feature closely
associated with DKD progression (5). Therefore, determining the occurrence
and progression of renal fibrosis is important.
Tubular epithelial-mesenchymal transition (EMT) is
widely regarded as the underlying mechanism of renal fibrosis
(6). One of the important
profibrotic cytokines that induces EMT is TGF-β1(7). In children with type 1 diabetes, the
level of serum TGF-β1 was significantly higher compared with that
in healthy children (8). Qiao
et al (9) reported that
serum and urinary TGF-β1 levels were significantly increased in
patients with type 2 diabetes mellitus (T2DM) and DKD. A number of
previous studies reported that TGF-β1 levels increased in
streptozotocin-induced diabetic mouse models and inhibition of
TGF-β1 ameliorated renal fibrosis in DKD (10-12).
The TGF-β1 pathway is also closely related to tubulointerstitial
fibrosis in rats with DKD (13).
The aforementioned reports suggest that TGF-β1 could be a potential
target for the treatment of renal fibrosis in DKD.
Apelin, an adipokine secreted by adipocytes, is an
endogenous peptide that was first isolated from the bovine stomach
in 1998(14). Apelin stimulates
glucose use, enhances the sensitivity of target cells to insulin
and serves a role in the pathogenesis and complications of diabetes
(15). A previous study reported
that circulating apelin levels increased in patients with T2DM
(16), while another study
reported that serum apelin levels decreased in patients with T2DM
(17). The aforementioned study
also reported that apelin might be a biomarker for early detection
of diabetic nephropathy in patients with T2DM. However, serum
apelin levels were not correlated with the urine protein to
creatinine ratio (17). Therefore,
the expression of apelin in DKD is yet to be fully elucidated.
Fibrosis represents the final step of DKD and TGF-β1 has been
established as the main mediator of renal fibrosis (18). However, the relationship between
serum apelin levels and renal fibrosis in patients with DKD is not
currently understood. Additionally, to the best of our knowledge,
the relationship between serum apelin and TGF-β1 expression levels
has not yet been reported.
The apelin gene encodes a 77 amino acid
prepropeptide and can be cleaved into different active forms
including apelin-12, -13, -15, -16, -17, -19, -28, -31 and
-36(19). All isoforms of apelin
can bind to the G protein-coupled receptor, APJ (14). Apelin-13, the shorter isomer of
apelin, exhibits the strongest biological activity of all the
apelin isoforms (14). Apelin-13
is widely expressed in many tissues and organs including the
cardiovascular, respiratory, digestive endocrine and neurological
systems and it is particularly abundant in blood (20). Therefore, in the present study,
serum apelin-13 and TGF-β1 levels were measured and their
relationship in diabetic patients with and without DKD was
analyzed.
Materials and methods
Participants
PASS 2021 software (NCSS LLC) was used to calculate
the number of patients included in the present study. Based on the
pre-experimental data, the sample size required for each group was
≥25 cases by taking 1-β=0.9 and α=0.05. In the present study, 70
patients with T2DM with or without DKD, who were admitted to The
Jinan Fifth People's Hospital (Jinan, China) between February 18,
2021 and April 30, 2022, were enrolled. Additionally, 30 age- and
sex-matched healthy controls from the examination center of The
Jinan Fifth People's Hospital between March 1, 2021 and July 31,
2021 were selected for the study.
Ethics approval and patient
consent
The present study was designed according to The
Strengthening the Reporting of Observational Studies in
Epidemiology guidelines (21). The
present study was registered in the Chinese Clinical Trial Registry
(https://www.chictr.org.cn/bin/userProject; trial
registration no. ChiCTR2200060945) and was approved by the Ethics
Committee of The Fifth People's Hospital of Jinan (Jinan, China;
approval no. 20-ke-01). A total of 100 subjects including patients
in the DKD, non-DKD and healthy control groups were included in the
present study. Written informed consent was obtained from all
participants.
Inclusion and exclusion criteria
The clinical diagnostic criteria for DKD were as
follows: A 24-h urinary total protein level ≥30 mg/24 h and/or a
prolonged decrease in eGFR <60 ml/min (1.73
m2)-1 without other types of kidney disease,
based on The Kidney Disease Improving Global Outcomes 2020 Clinical
Practice Guideline (22). The
inclusion criteria of patients were as follows: i) Female or male
patients who were hyperglycemic and being treated with
glucose-lowering drugs; ii) aged 28-75 years; iii) majority ~55
years old; iv) a history of diabetes for ≥6 months; v) negative
insulin autoantibodies, islet cell antibodies and glutamate
decarboxylase antibodies; vi) fasting blood glucose (FBG) levels
after treatment <7.0 mmol/l; and vii) postprandial 2-hour plasma
glucose levels after treatment <10.0 mmol/l. Patients with type
1 diabetes, gestational diabetes, serious cardiovascular diseases,
cerebrovascular diseases, respiratory disease, digestive disease,
inflammatory diseases, malignant tumors, urinary tract infections,
other kidney diseases, recent nephrotoxic drug exposure and
proteinuria caused by other factors were excluded. The inclusion
criteria of the controls were as follows: i) Healthy males and
females; ii) aged 30-65 years; and iii) no history of diabetes.
Exclusion criteria of the controls were any history of
hypertension, tumors and a variety of serious acute and chronic
diseases including brain, heart, lung, liver and kidney
disease.
Control of bias
The methods to control confounding factors in the
present study were as follows: i) Limiting the inclusion and
exclusion conditions of subjects during the study design; and ii)
taking some characteristics that interfere with the results as
matching factors, which was a limiting method to maintain the same
matching factors between the cases and controls. The confounding
factors, such as BMI, FBG, cholesterol, triglyceride (TG),
low-density lipoprotein cholesterol (LDL-c) and high-density
lipoprotein cholesterol (HDL-c) levels were matched in the DKD
group and the non-DKD group, which may potentially have resulted in
selection bias due to the necessity to discard patients that did
not have matching confounding factors. In order to reduce selection
bias, random sampling was performed. By the strict selection of
representative subjects, the cases and controls were balanced.
Moreover, the criteria for inclusion and exclusion were strictly
limited. When analyzing the final results, the blind method and
objective statistical indicators such as FBG, blood pressure and
serum lipids were applied. Good patient compliance and preventing
loss in follow-up of patients also served a role in bias
reduction.
Measurements of indicators
Sex, age, systolic blood pressure, diastolic blood
pressure, mean disease duration, weight and BMI were recorded at
the time of patient enrollment. The levels of FBG, serum TG, LDL-c,
HDL-c, total cholesterol, blood urea nitrogen (BUN) and creatinine
were detected using the TBA-FX8 automatic biochemical analyzer
(Shenzhen Mindray Bio-Medical Electronics Co., Ltd.). The 24 h
urinary total protein (24-h UTP) was measured using the BS-2000M
automatic biochemical analyzer (Shenzhen Mindray Bio-Medical
Electronics Co., Ltd.). Glycosylated hemoglobin A1c (HbA1c) levels
were analyzed using the Tosoh Automated Glycohemoglobin Analyzer
HLC-723G11 (Tosoh Bioscience Co., Ltd.). Fasting C-peptide (FCP)
levels were measured using a MAGLUMI X8 automatic chemiluminescence
immunoanalyzer (Shenzhen New Industry Biomedical Engineering Co.,
Ltd.).
Serum samples were collected, centrifuged at 1,000 x
g at room temperature for 20 min and stored at -80˚C until
analysis. Serum apelin-13 concentration was measured using an ELISA
kit (cat. no. E-EL-H0458c; Wuhan Elabscience Biotechnology Co.,
Ltd.). Serum TGF-β1 concentration was measured using an ELISA kit
(cat. no. CSB-E04725h; Wuhan Huamei Biological Engineering Co.,
Ltd.). The standards and samples were added to the coated assay
plates. After incubation at 37˚C for 1 h (apelin ELISA kit) or 2 h
(TGF-β1 ELISA kit), horseradish peroxidase-avidin was added to
biotin-labeled antibodies (1:100). Then, the assay plate containing
apelin-13 was incubated at 37˚C for 30 min and the assay plate
containing TGF-β1 was incubated at 37˚C for 1 h . After
5'-tetramethylbenzidine substrate incubation for 15 min at 37˚C,
stop solution was injected into each well and the absorbance of
each sample was measured at 450 nm.
Statistical analysis
Statistical analyses were performed using SPSS
(version 22.0; IBM Corp.). The Shapiro-Wilk test was used to
determine the normal distribution of the quantitative parametric
data. An unpaired t-test was used to analyze the quantitative
parametric data which were presented as the mean ± standard
deviation. Variables with non-normal distributions were evaluated
using the Mann-Whitney U test and were presented as the median with
the 25th and 75th percentiles. The χ2 test was used to
analyze qualitative variables and the data were presented as
percentages of frequency. Pearson's correlation was used to
calculate the correlation between continuous data. Spearman's
correlation analysis was used to evaluate potential correlations
between rank variables. P<0.05 was considered to indicate
a statistically significant difference.
Results
Comparison of indicators between the
diabetic groups and the controls
The groups of patients with diabetes consisted of 40
males and 30 females, with an average age of 57.91±8.42 years. The
control group consisted of 14 males and 16 females, with an average
age of 48.20±4.59 years. There were no significant differences
observed in sex, weight, BMI, LDL-c, HDL-c, TG, blood creatinine
and the estimated glomerular filtration rate (eGFR) of patients
with diabetes and the control group (P>0.05). Serum apelin and
TGF-β1 levels, age, systolic and diastolic pressure, FBG, total
cholesterol and BUN were significantly higher in the diabetic group
compared with the controls (P<0.05; Table I).
 | Table ICharacteristics of patients with
diabetes compared with healthy controls. |
Table I
Characteristics of patients with
diabetes compared with healthy controls.
| Patient
characteristic | Healthy control group
(n=30) | Patients with
diabetes (n=70) | P-value |
|---|
| Sex, n (%) | | | 0.335 |
|
Male | 14 (25.9) | 40 (74.1) | |
|
Female | 16 (34.8) | 30 (65.2) | |
| Proteinuria, n
(%) | | | <0.001 |
|
Yes | 0 (0.0) | 25 (100.0) | |
|
No | 30 (40.0) | 45 (60.0) | |
| Age, years | 48.20±4.59 | 57.91±8.42 | <0.001 |
| Weight, kg | 67.19±11.96 | 68.34±12.26 | 0.666 |
| BMI, kg/m2 | 24.31±3.83 | 24.85±3.84 | 0.517 |
| Systolic blood
pressure, mmHg | 122.60±13.68 | 141.39±21.26 | <0.001 |
| Diastolic blood
pressure, mmHg | 77.00±12.89 | 84.31±10.82 | 0.004 |
| Fasting blood
glucose, mmol/l | 5.06±0.53 | 8.46±2.56 | <0.001 |
| Serum low-density
lipoprotein cholesterol, mmol/l | 2.86±0.67 | 3.01±1.13 | 0.406 |
| Serum high-density
lipoprotein cholesterol, mmol/l | 1.23
(1.06/1.48) | 1.17
(0.99/1.31) | 0.112 |
| Serum triglyceride,
mmol/l | 1.13
(0.79/1.49) | 1.34
(0.93/2.06) | 0.093 |
| Cholesterol,
mmol/l | 4.70±0.74 | 5.14±1.43 | 0.046 |
| Creatinine,
µmol/l | 63.00
(52.75/69.25) | 62.50
(54.00/109.50) | 0.250 |
| Blood urea
nitrogen, mmol/l | 4.55
(3.90/5.40) | 6.05
(5.08/8.75) | <0.001 |
| Estimated
glomerular filtration rate, ml/min (1.73
m2)-1 | 122.02
(104.55/131.94) | 116.31
(58.18/146.43) | 0.339 |
| Apelin-13,
pg/ml | 14,415.16
(10,285.52/18,011.01) | 29,716.03
(17,278.46/49,354.44) | <0.001 |
| TGF-β1, ng/ml | 79.32
(48.20/103.00) | 103.02
(64.45/150.92) | 0.003 |
Comparison of indicators between the
DKD group and the non-DKD groups of patients
Subgroup analysis was performed for diabetic groups
with and without DKD. Age, sex, weight, BMI, diastolic pressure,
FBG, HbA1c, FCP, LDL-c, HDL-c, TG and total cholesterol levels were
evaluated and no significant differences were demonstrated between
the DKD and non-DKD groups of patients. However, the mean disease
duration was significantly longer in the DKD group compared with
the non-DKD group (P<0.05). Additionally, a statistically
significant increase was demonstrated in serum apelin-13 and TGF-β1
levels, systolic pressure, blood creatinine, BUN and 24-h UTP in
the DKD group compared with the non-DKD group (P<0.05). By
contrast, eGFR was significantly decreased in the DKD group
compared with the non-DKD group of patients (P<0.05; Table II).
 | Table IICharacteristics of patients in the
DKD and non-DKD groups. |
Table II
Characteristics of patients in the
DKD and non-DKD groups.
| Patient
characteristic | DKD group
(n=31) | Non-DKD group
(n=39) | P-value |
|---|
| Sex, n (%) | | | 0.728 |
|
Male | 17 (42.5) | 23 (57.5) | |
|
Female | 14 (46.7) | 16 (53.3) | |
| Proteinuria, n
(%) | | | <0.001 |
|
Yes | 25 (100.00) | 0 (0.00) | |
|
No | 6 (13.33) | 39 (86.67) | |
| Age, years | 59.74±10.08 | 56.46±6.60 | 0.106 |
| Duration of
disease, years | 15.00
(10.00/20.00) | 4.00
(1.00/10.00) | <0.001 |
| Weight, kg | 68.27±12.43 | 68.38±12.29 | 0.970 |
| BMI,
kg/m2 | 25.00±4.03 | 24.73±3.73 | 0.769 |
| Systolic blood
pressure, mmHg | 151.52±24.46 | 133.33±14.08 | 0.001 |
| Diastolic blood
pressure, mmHg | 86.74±12.26 | 82.38±9.24 | 0.106 |
| Fasting blood
glucose, mmol/l | 8.32±2.74 | 8.58±2.44 | 0.670 |
| Glycosylated
hemoglobin A1c, % | 9.00±1.88 | 8.87±2.25 | 0.800 |
| Fasting C peptide,
ng/ml | 2.13
(1.31/3.59) | 1.85
(1.40/2.50) | 0.226 |
| Serum low-density
lipoprotein cholesterol, mmol/l | 3.09±1.38 | 2.95±0.90 | 0.650 |
| Serum high-density
lipoprotein cholesterol, mmol/l | 1.15
(0.97/1.28) | 1.18
(0.99/1.37) | 0.603 |
| Serum triglyceride,
mmol/l | 1.62
(1.05/2.39) | 1.27
(0.85/1.84) | 0.170 |
| Cholesterol,
mmol/l | 5.22±1.78 | 5.08±1.09 | 0.695 |
| Creatinine,
µmol/l | 111.00
(67.00/175.00) | 56.00
(48.00/64.00) | <0.001 |
| Blood urea
nitrogen, mmol/l | 8.30
(5.80/15.40) | 5.30
(4.40/6.80) | <0.001 |
| 24 h urinary total
protein, mg/24 h | 681.90
(332.00-3,603.60) | 58.88
(36.45-100.20) | <0.001 |
| Estimated
glomerular filtration rate, ml/min (1.73
m2)-1 | 66.91±42.82 | 138.57±39.62 | <0.001 |
| Apelin-13,
pg/ml | 50,720.36
(26,954.76/78,880.05) | 20,490.58
(16,008.67/33,997.97) | <0.001 |
| TGF-β1, ng/ml | 141.37±71.93 | 96.47±43.81 | 0.004 |
Correlation analysis of apelin-13 and
parameters in the DKD and non-DKD groups
The Pearson and Spearman's correlation analyses
demonstrated a significant negative correlation between serum
apelin-13 and TGF-β1 levels in the DKD and non-DKD groups
(P<0.05); however, no significant correlation was demonstrated
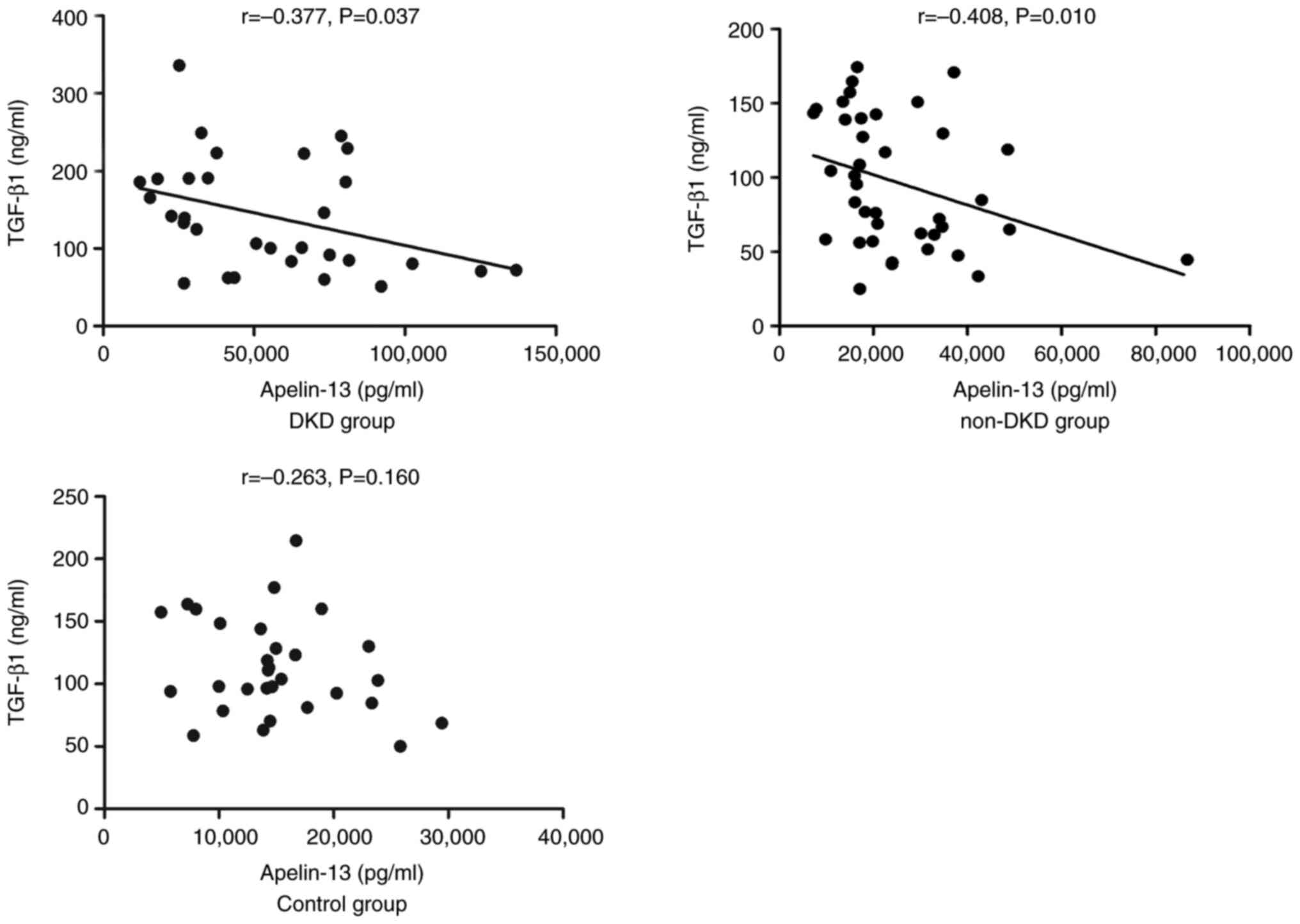
in the control group (Fig. 1).
Serum apelin-13 levels were associated with FCP, blood creatinine,
BUN and 24-h UTP in the DKD group (Table III; P<0.05). A significant
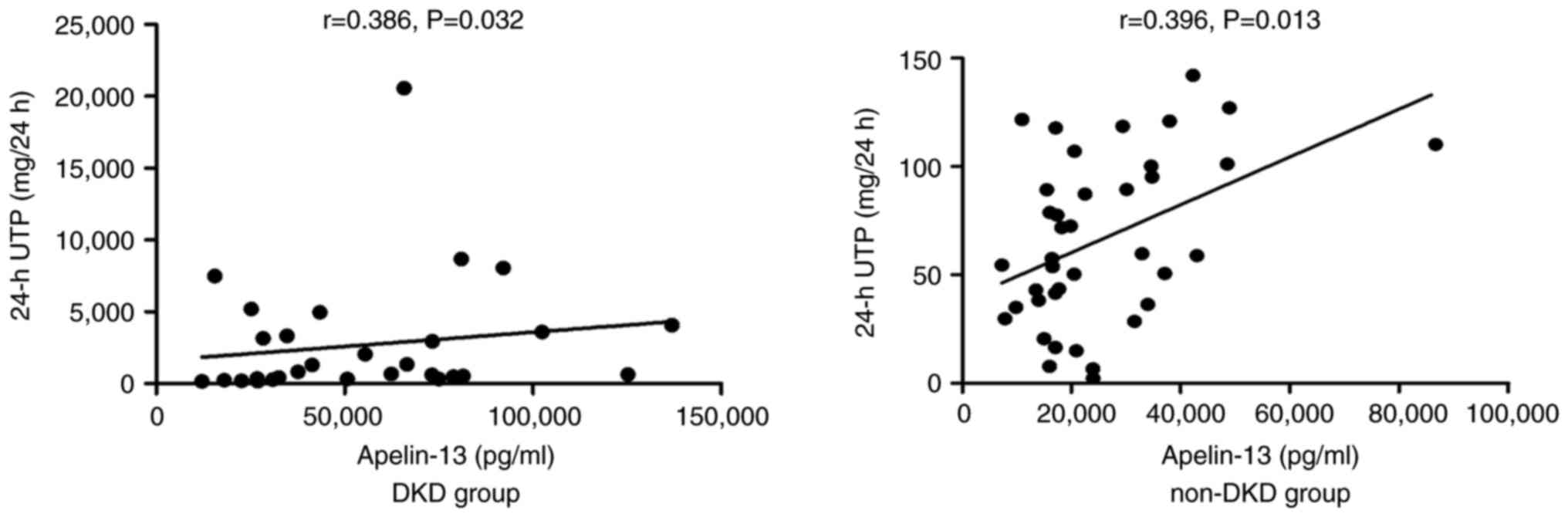
positive correlation was demonstrated between apelkin-13 and 24-h
UTP levels in both the DKD and non-DKD groups (P<0.05; Fig. 2). In addition, apelin-13 levels
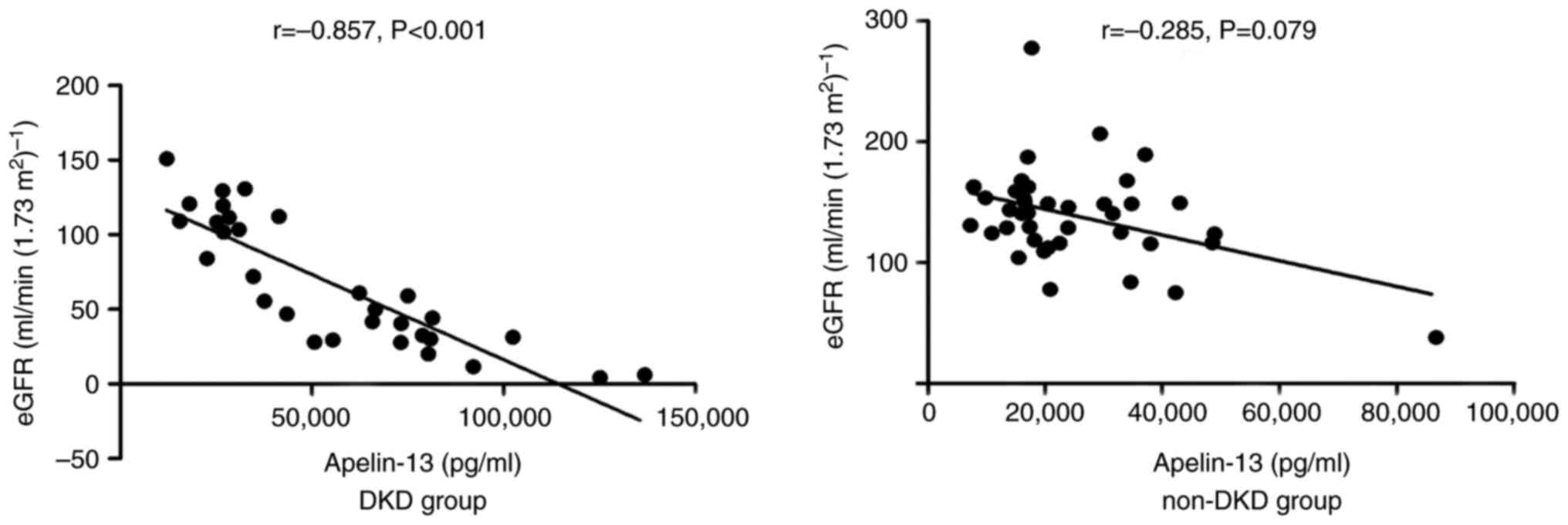
were significantly negatively correlated with eGFR in the DKD group
(P<0.05; Fig. 3) and a marked
negative correlation was demonstrated between eGFR and apelin-13
levels in the non-DKD group (P>0.05; Fig. 3). No significant correlation was
observed between serum apelin-13 levels and age, disease duration,
weight, BMI, blood pressure, FBG, HbA1c, LDL-c, HDL-c, TG or total
cholesterol levels in the DKD and non-DKD groups (P>0.05;
Table III).
 | Table IIICorrelation between apelin-13
expression levels and characteristics of patients in the DKD and
non-DKD groups. |
Table III
Correlation between apelin-13
expression levels and characteristics of patients in the DKD and
non-DKD groups.
| | DKD group | Non-DKD group |
|---|
| Patient
characteristic | r | P-value | r | P-value |
|---|
| Age, years | -0.149 | 0.423 | 0.032 | 0.847 |
| Disease duration,
years | 0.082 | 0.663 | -0.076 | 0.645 |
| Weight, kg | -0.122 | 0.513 | -0.004 | 0.980 |
| BMI,
kg/m2 | -0.108 | 0.565 | 0.029 | 0.862 |
| Systolic blood
pressure, mmHg | 0.088 | 0.636 | 0.176 | 0.283 |
| Diastolic blood
pressure, mmHg | 0.195 | 0.293 | 0.246 | 0.131 |
| Fasting blood
glucose, mmol/l | -0.284 | 0.122 | -0.108 | 0.513 |
| Glycosylated
hemoglobin A1c, % | -0.201 | 0.279 | -0.008 | 0.960 |
| Fasting C peptide,
ng/ml | 0.405 | 0.024 | 0.225 | 0.168 |
| Serum low-density
lipoprotein cholesterol, mmol/l | -0.029 | 0.876 | 0.094 | 0.568 |
| High-density
lipoprotein cholesterol, mmol/l | -0.029 | 0.878 | -0.038 | 0.817 |
| Serum triglyceride,
mmol/l | -0.005 | 0.980 | -0.023 | 0.889 |
| Cholesterol,
mmol/l | -0.017 | 0.927 | 0.081 | 0.622 |
| Creatinine,
µmol/l | 0.848 | <0.001 | 0.152 | 0.354 |
| Blood urea
nitrogen, mmol/l | 0.810 | <0.001 | 0.023 | 0.891 |
Discussion
With a worldwide increase in the incidence of DKD in
recent decades, DKD has become one of the most serious
complications of diabetes. Hyperglycemia is an important factor in
the development of DKD (23). The
potential mechanism by which this occurs may involve an increase in
angiotensin II, growth factors and advanced glycation end products
causing hyperfiltration, which leads to an increase in capillary
pressure. Subsequently, the basement membrane thickens, the
mesangium expands and the extracellular matrix thickens, which
triggers fibrosis (24). In the
present study, FPG was higher in patients with diabetes compared
with the controls, which supported the previously published finding
that hyperglycemia ultimately damages the kidney (24).
Reduced eGFR and increased albuminuria are
indicators of DKD diagnosis and progression. In the present study,
eGFR was lower and 24-h UTP was higher in the DKD group compared
with the non-DKD patients. Moreover, serum eGFR and apelin-13
levels were negatively correlated and 24-h UTP was positively
correlated with apelin-13 levels in the DKD group, which is in
accordance with a previous study that reported that apelin-13
caused a significant increase in the ratio of microalbuminuria in
diabetic mice (25). Another study
reported that pyr-apelin-13 had the opposite effect on albuminuria,
showing a tendency to reduce renal swelling and inflammation
(26). These results indicate that
the effects of apelin on albuminuria in the kidneys may be related
to the morphology of apelin or its differential pathophysiological
states.
Apelin is an endogenous peptide that may serve as an
important biomarker for the detection of DKD (17). The results of the present study
demonstrated that the serum levels of apelin-13 in patients in the
DKD and non-DKD groups were significantly higher compared with
those in the controls. This finding was consistent with a previous
study that reported that circulating apelin concentration was
significantly elevated in patients with diabetes compared to those
in the control group (27).
However, there are conflicting reports on decreased apelin levels
in T2DM (17), possibly because
different stages of DKD development may initiate different
signaling pathways to regulate the expression of apelin, thus
affecting serum apelin levels.
TGF-β1 is a recognized fibrogenic factor and may be
considered a driver of renal fibrosis, which is an important
pathological feature of DKD, as a previous study reported that
TGF-β1 signaling pathways were key regulators of renal fibrosis
(28). In the present study,
TGF-β1 expression levels in the serum of patients with DKD were
increased, which was consistent with the previous study conducted
by Sawires et al (8) that
reported higher serum levels of TGF-β1 in children with DKD. The
grouping was similar in the two studies, but the present study
focused on the relationship between apelin-13 and disease
indicators in T2DM nephropathy, while the previously published
study reported the relationship between TGF-β1 and other related
indicators in type 1 diabetic nephropathy in children.
Apelin has previously been reported to serve a
crucial role in heart, liver and kidney fibrosis (29). Therefore, a relationship may exist
between apelin and TGF-β1 expression levels under specific
physiological and pathological conditions. In a previous study by
Kocer et al (30), apelin
was negatively correlated to TGF-β in patients with polycystic
kidney disease. Animal and cell studies have also reported that
apelin treatment served an inhibitory role on TGF-β1, prevented
acute cell damage and improved the outcome of ischemia/reperfusion
injury to the kidneys (31).
Although it was observed that both apelin-13 and TGF-β1 expression
levels increased in the DKD and non-DKD groups of patients, the
increase was not the same in the present study as the values
previously reported. The present study demonstrated that an
increase in apelin-13 levels inhibited serum TGF-β1 levels. This
phenomenon has also been reported by Lu et al (32), who suggested a negative correlation
of axial length and peak distance in myopia, emmetropia, and
hyperopic groups, although both axial length and peak distance
increased in myopia group than emmetropia and hyperopia groups.
Therefore, the results of the present study demonstrated that serum
apelin-13 level were negatively associated with TGF-β1, which
suggested there may be an inverse association between apelin-13 and
renal fibrosis.
In the present study, apelin-13 was positively
correlated with 24-h UTP and negatively correlated with eGFR, which
served a detrimental role in DKD. However, apelin-13 was also
negatively correlated with the fibrotic factor TGF-β1, which served
a favorable role in DKD. This has been reported in several animal
studies which showed that apelin-13 caused a significant increase
in the ratio of microalbuminuria in diabetic KK-Ay mice (25) and inhibited autophagy in podocytes,
which led to podocyte apoptosis and massive proteinuria in diabetic
KK-Ay mice (33). Apelin-13 may be
positively correlated with urinary protein and promote the progress
of DKD by increasing fat mass, promoting angiogenesis in glomeruli
to form abnormal vessels, increasing permeability or inducing
podocyte apoptosis (18). However,
it has also been reported that apelin inhibited the process of EMT
in podocytes in diabetic KK-Ay mice (34), decreased the levels of TGF-β and
suppressed kidney tissue fibrosis in Sprague Dawley rats with
diabetic nephropathy (35).
Therefore, apelin-13 may be relevant to increased fat mass,
increased glomerular permeability and induced podocyte apoptosis
during the early stages of DKD, while it may inhibit renal fibrosis
in the advanced stage of DKD.
Confounding factors may interfere with the results
in the present study. A number of studies reported that apelin may
be associated with BMI, FBG, cholesterol, TG, LDL and HDL. Zaki M
et al. concluded that serum apelin levels were positively
correlated with BMI (36). FBG,
cholesterol, TG, LDL and HDL are indicators of glycolipid
metabolism and Bertrand et al (37) reported that apelin improved hepatic
lipid metabolism in obese and insulin-resistant mice. This is in
accordance with a previous study that reported that apelin-13
treatment resulted in the significant decreases in the FBG levels
and the serum levels of cholesterol, TG and LDL in mice with
gestational diabetes mellitus (38). Therefore, case-control matching is
helpful for known confounders. BMI, FBG, LDL-c, HDL-c, TG and
cholesterol were not statistically significant between the DKD
group and the non-DKD group in the present study. On this basis,
the expression and correlation of apelin-13 in DKD were
studied.
In conclusion, the present study demonstrated that a
significant negative relationship between apelin-13 and TGF-β1 in
both DKD and non-DKD groups was observed. This phenomenon has laid
a foundation for the future study of the roles of apelin and
fibrosis in DKD. Apelin-13 may potentially be a negative indicator
of renal fibrosis in patients with DKD. More studies are required
to assess the association and the mechanism between apelin-13 and
the fibrosis in renal disease from both cellular and animal
experiments. Future studies will use high-glucose-treated human
renal tubular epithelial cells with different dosages of apelin-13
treatment to observe the morphological changes of the cells and
detect the levels of fibrosis-related markers such as E-cadherin,
vimentin and α-SMA. Furthermore, rat models of DKD may be
constructed by streptozocin injection. Upon injecting apelin-13
into diabetic rats, the weight, blood glucose levels, urinary
protein content, levels of serum apelin-13 and TGF-β1 could be
detected. Staining of renal tissues and analysis of RNA and protein
expression levels of E-cadherin, vimentin and α-SMA should be
performed. Performing these studies would aid in the understanding
of how apelin-13 may potentially serve as a useful marker for renal
fibrosis.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Shandong Provincial
Natural Science Foundation (grant no. ZR2021QH106), Jinan Municipal
Science and Technology Project (grant nos. 202019083 and 202019091)
and The Dean Foundation of the First Affiliated Hospital of
Shandong First Medical University (grant no. QYPY2019NSFC0805).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW contributed to the conception and design of the
study and drafting of the manuscript. XL and SM acquired, analyzed
and interpreted the data. HX was responsible for data measurement
and curation. AZ and YL were responsible for the study concept,
manuscript editing and supervision of the manuscript. QW and YL
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Fifth People's Hospital of Jinan (approval no.
20-ke-01; Jinan, China) and written informed consent was obtained
from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmad J: Management of diabetic
nephropathy: Recent progress and future perspective. Diabetes Metab
Syndr. 9:343–58. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H,
Shi B, Sun H, Ba J, Chen B, et al: Prevalence of diabetes recorded
in mainland China using 2018 diagnostic criteria from the American
diabetes association: National cross sectional study. BMJ.
369(m997)2020.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Weng JP and Bi Y: Epidemiological status
of chronic diabetic complications in China. Chin Med J (Engl).
128:3267–3269. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Radcliffe NJ, Seah JM, Clarke M, MacIsaac
RJ, Jerums G and Ekinci EI: Clinical predictive factors in diabetic
kidney disease progression. J Diabetes Investig. 8:6–18.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang Y, Jin D, Kang X, Zhou R, Sun Y,
Lian F and Tong X: Signaling pathways involved in diabetic renal
fibrosis. Front Cell Dev Biol. 9(696542)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Carew RM, Wang B and Kantharidis P: The
role of EMT in renal fibrosis. Cell Tissue Res. 347:103–116.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sisto M, Lorusso L, Ingravallo G, Tamma R,
Ribatti D and Lisi S: The TGF-β1 signaling pathway as an attractive
target in the fibrosis pathogenesis of Sjögren's syndrome.
Mediators Inflamm. 2018(1965935)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sawires H, Botrous O, Aboulmagd A, Madani
N and Abdelhaleem O: Transforming growth factor-β1 in children with
diabetic nephropathy. Pediatr Nephrol. 34:81–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Qiao YC, Chen YL, Pan YH, Ling W, Tian F,
Zhang XX and Zhao HL: Changes of transforming growth factor beta 1
in patients with type 2 diabetes and diabetic nephropathy. A
PRISMA-compliant systematic review and meta-analysis. Medicine
(Baltimore). 96(e6583)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zheng ZC, Zhu W, Lei L, Liu XQ and Wu YG:
Wogonin ameliorates renal inflammation and fibrosis by inhibiting
NF-κB and TGF-β1/Smad3 signaling pathways in diabetic nephropathy.
Drug Des Devel Ther. 14:4135–4148. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun Z, Ma Y, Chen F, Wang S, Chen B and
Shi J: miR-133b and miR-199b knockdown attenuate TGF-β1-induced
epithelial to mesenchymal transition and renal fibrosis by
targeting SIRT1 in diabetic nephropathy. Eur J Pharmacol.
837:96–104. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Geng XQ, Ma A, He JZ, Wang L, Jia YL, Shao
GY, Li M, Zhou H, Lin SQ and Ran JH: Ganoderic acid hinders renal
fibrosis via suppressing the TGF-β/Smad and MAPK signaling
pathways. Acta Pharmacol Sin. 41:670–677. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Miao XJ, Bi TT, Tang JM, Lv R, Gui DK and
Yang XF: Regulatory mechanism of TGF-β1/SGK1 pathway in
tubulointerstitial fibrosis of diabetic nephropathy. Eur Rev Med
Pharmacol Sci. 23:10482–10488. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tatemoto K, Hosoya M, Habata Y, Fujii R,
Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et
al: Isolation and characterization of a novel endogenous peptide
ligand for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chapman FA, Nyimanu D, Maguire JJ,
Davenport AP, Newby DE and Dhaun N: The therapeutic potential of
apelin in kidney disease. Nat Rev Nephrol. 17:840–853.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Habchi M, Duvillard L, Cottet V, Brindisi
MC, Bouillet B, Beacco M, Crevisy E, Buffier P, Baillot-Rudoni S,
Verges B and Petit JM: Circulating apelin is increased in patients
with type 1 or type 2 diabetes and is associated with better
glycaemic control. Clin Endocrinol (Oxf). 81:696–701.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Demirpence M, Yilmaz H, Colak A, Pamuk BO,
Karakoyun I and Basok B: Apelin: A potential novel serum biomarker
for early detection of diabetic nephropathy in patients with type 2
diabetes. North Clin Istanb. 6:151–155. 2118.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Iwano M: EMT and TGF-beta in renal
fibrosis. Front Biosci (Schol Ed). 2:229–238. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Lv SY, Yang YJ and Chen Q: Regulation of
feeding behavior, gastrointestinal function and fluid homeostasis
by apelin. Peptides. 44:87–92. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Y, Jiang W, Sun W, Guo W, Xia B,
Shen X, et al: Neuroprotective Roles of Apelin-13 in Neurological
Diseases. Neurochem Res. 48:1648–1662. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC, Vandenbroucke JP and STROBE Initiative: The
strengthening the reporting of observational studies in
epidemiology (STROBE) statement: Guidelines for reporting
observational studies. Lancet. 370:1453–1457. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kidney Disease: Improving Global Outcomes
(KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline
for diabetes management in chronic kidney disease. Kidney Int.
98(4S):S1–S115. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625.
2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stevens PE and Levin A: Evaluation and
management of chronic kidney disease: Synopsis of the kidney
disease: Improving global outcomes 2012 clinical practice
guideline. Ann Intern Med. 158:825–830. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang BH, Wang W, Wang H, Yin J and Zeng
XJ: Promoting effects of the adipokine, apelin, on diabetic
nephropathy. PLoS One. 8(e60457)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Day RT, Cavaglieri RC and Feliers D:
Apelin retards the progression of diabetic nephropathy. Am J
Physiol Renal Physiol. 304:F788–F800. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Meral C, Tascilar E, Karademir F, Tanju
IA, Cekmez F, Ipcioglu OM, Ercin CN, Gocmen I and Dogru T: Elevated
plasma levels of apelin in children with type 1 diabetes mellitus.
J Pediatric Endocrinol Metab. 23:497–502. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang LY, Diao ZL, Zhang DL, Zheng JF,
Zhang QD, Ding JX and Liu WH: The regulatory peptide apelin: A
novel inhibitor of renal interstitial fibrosis. Amino Acids.
46:2693–2704. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kocer D, Karakukcu C, Ozturk F, Eroglu E
and Kocyigit I: Evaluation of fibrosis markers: Apelin and
transforming growth factor-β1 in autosomal dominant polycystic
kidney disease patients. Ther Apher Dial. 20:517–522.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen H, Wan D, Wang L, Peng A, Xiao H,
Petersen RB, Liu C, Zheng L and Huang K: Apelin protects against
acute renal injury by inhibiting TGF-β1. Biochim Biophys Acta.
1852:1278–1287. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lu LL, Hu XJ, Yang Y, Xu S, Yang SY, Zhang
CY and Zhao QY: Correlation of myopia onset and progression with
corneal biomechanical parameters in children. World J Clin Cases.
10:1548–1556. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu Y, Zhang J, Wang YJ and Zeng XJ:
Apelin involved in progression of diabetic nephropathy by
inhibiting autophagy in podocytes. Cell Death Dis.
8(e3006)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yin J, Wang Y, Chang J, Li B, Zhang J, Liu
Y, Lai S, Jiang Y, Li H and Zeng X: Apelin inhibited
epithelial-mesenchymal transition of podocytes in diabetic mice
through downregulating immunoproteasome subunits β5i. Cell Death
Dis. 9(1031)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gao Z, Zhong X, Tan YX and Liu D:
Apelin-13 alleviates diabetic nephropathy by enhancing nitric oxide
production and suppressing kidney tissue fibrosis. Int J Mol Med.
48(175)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zaki M, Kamal S, Ezzat W, Hassan N, Yousef
W, Ryad H, Mohamed R, Youness E, Basha W and Elhosary Y: Serum
apelin levels and metabolic risk markers in obese women. J Genet
Eng Biotechnol. 15:423–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bertrand C, Pradère JP, Geoffre N,
Deleruyelle S, Masri B, Personnaz J, Le Gonidec S, Batut A, Louche
K, Moro C, et al: Chronic apelin treatment improves hepatic lipid
metabolism in obese and insulin-resistant mice by an indirect
mechanism. Endocrine. 60:112–121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zheng XD, Huang Y and Li H: Regulatory
role of Apelin-13-mediated PI3K/AKT signaling pathway in the
glucose and lipid metabolism of mouse with gestational diabetes
mellitus. Immunobiology. 226(152135)2021.PubMed/NCBI View Article : Google Scholar
|