Introduction
As the proportion of the population who are elderly
grows rapidly, the process of aging is of increasing concern to
society, particularly as it is the primary risk factor for numerous
chronic diseases and the primary driver of neurodegeneration
(1). As such, it is essential to
explore the mechanisms of aging and to discover novel anti-aging
drugs. In recent years, a number of natural medicines have been
found to delay senescence, including astragalus (2) and ginseng (3).
Saffron is the dry stigmas of the plant Crocus
sativus L. of the family Iridaceae, which is widespread in
several Asian countries. The active ingredients of saffron have
been identified to include crocin, picrocrocin and safranal
(4,5). Previous studies have reported that
saffron or its active ingredients exert protective effects against
Parkinson's disease (6), cerebral
ischemia (7), tumors (8), and depression (9). In addition, saffron has also been
shown to exert anti-aging effects, which manifest by inhibition of
the loss of skin moisture and the exertion of anti-aging effects on
the skin (10), by the reduction
of β-secretase expression and inhibition GSK3β and ERK1/2 activity
(11), and the alleviation of
D-galactose-induced senescence in rats (12).
Nicotinamide phosphoribosyltransferase (NAMPT), also
known as pre-B-cell colony enhancing factor or visfatin, is a key
enzyme in the synthesis of nicotinamide adenine dinucleotide
(NAD+) (13), which
exists both intracellularly and extracellularly (14). NAMPT is mainly expressed
intracellularly in young mice and extracellularly in old mice.
Regarding the cellular distribution in the brain, NAMPT is
predominantly expressed in neurons and is absent from the
microglia/astrocytes in young and healthy brains. However, it is
mainly expressed in the microglia/astrocytes upon aging (15). The increase in extracellular NAMPT
levels and the change of cellular distribution can induce the
secretion of inflammatory cytokines, including tumor necrosis
factor (TNF)-α, interleukin (IL)-1β, IL-4 and oxidative stress
factors from the microglia (14),
which further promotes NAMPT secretion (16). NAD+ is also an important
substrate for redox processes and is closely associated with
cellular metabolism, energy production and DNA repair (17). As such, it can be concluded that
NAMPT-NAD+ is involved in the senescence process
(18), and may be a novel factor
in the regulation of the aging process (19).
Our previous study showed that crocin ameliorated
depressive-like behaviors induced by chronic restraint stress in
mice by increasing the expression of NAMPT-NAD+
(9). Saffron has also been shown
to exert anti-inflammatory and anti-oxidative effects (20). However, it is unclear whether
saffron exerts senescence-delaying effects by affecting the
NAMPT-NAD+ pathway and the subsequent inflammatory
response. Therefore, in the present study, the senescence-delaying
effects of saffron were explored to elucidate the possible
mechanisms involving the NAMPT-NAD pathway.
Materials and methods
Preparation and detection of saffron
aqueous extract
Saffron was purchased from Saffron Planting Base in
Jiande, China. The saffron (6 g) was subjected to aqueous
extraction five times under ultrasonication. The aqueous extract
was then mixed and concentrated in a rotary evaporator under
reduced pressure. Finally, it was lyophilized, yielding 3.7 g of
aqueous extract (yield 61.6% w/w).
High-performance liquid chromatography (HPLC) was
then carried out on a Waters 600E instrument, equipped with a
Waters 2996 PDA detector (Waters GmbH). An Agilent C18 column
(250x4.6 mm internal diameter; particle size, 5 µm; Agilent
Technologies, Inc.) was used for separation. The HPLC conditions
were as follows: Eluent A, double-distilled H2O; eluent
B, 95% acetonitrile; temperature at 25˚C; quantity of aqueous
extract was 10 µl each time; gradient of A:B, from 87:13 at 0 min
to 77:23 at 20 min; to 75:25 at 23 min; and to 50:50 at 45 min. The
column was then equilibrated with an A:B ratio of 87:13 for 20 min
at a flow rate of 1 ml/min. Peaks were assigned by comparison of
their retention times with that of three reference compounds eluted
in parallel with the same mobile phase, and by spiking the sample
with reference compounds. The calibration curves of picrocrocin,
crocin Ⅰ and crocin Ⅱ were Y=15.932X + 0.00114
(R2=0.9996), Y=39.867X - 54.766 (R2=0.9993)
and Y=49.66X - 50.675 (R2=0.999), respectively. The
contents of the active ingredients were determined according to the
corresponding calibration curves.
Animals and groups
A total of 40 female C57BL/6J mice (3 and 8 months
old, 20-25 g; 16 months old, 25-30 g) were obtained from Shanghai
SLAC Laboratory Animal Co., Ltd. (certificate no. SCXK 2022-0004).
All mice had food and water provided ad libitum, and were
maintained at room temperature controlled at 22-24˚C and humidity
at 50-60%, under a 12/12-h light/dark cycle. The C57BL/6J mice were
randomly divided into the control groups (3-, 8- and 16-month-old),
and the saffron groups (8- and 16-months-old), with 8 mice in each
group. No mice died during this experiment. The Animal Health,
Ethics and Research Committee of Hangzhou Medical College approved
the study procedures.
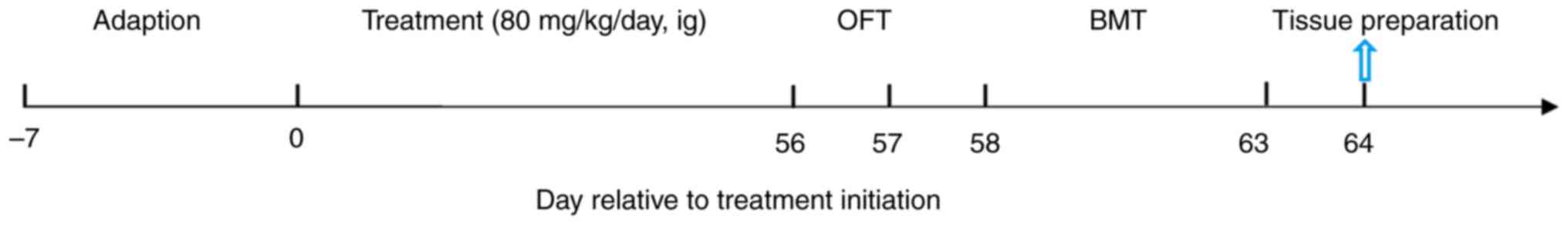
Experimental protocol
The duration of the experiment was 63 days. Aqueous
saffron extract was administered to the mice by oral gavage at a
dose of 80 mg/kg once daily for 8 weeks. The mice in the control
groups were given the same dose of double-distilled H2O
by oral gavage for 8 weeks. The open field test (OFT) and Barnes
maze test (BMT) were performed on days 57, and 58-63, respectively,
after the drug treatments (Fig.
1). Following the behavioral tests, on days 64, 3 mice in each
group were anesthetized by the inhalation of isoflurane (4.5% for
induction and 2% for maintenance). The mice were considered to be
anesthetized when they breathed evenly, did not respond to their
whiskers being touched and exhibited no toe-pinch pain reflex. They
were then perfused with saline and 4% polyformaldehyde followed by
rapid cervical dislocation. Tissue sections including brain, liver,
kidneys and skin were then prepared to evaluate the cellular
distribution of NAMPT and synaptic density by immunofluorescence,
and organ-aging by hematoxylin and eosin (H&E) staining. The
other 5 mice from each group were killed quickly by cervical
dislocation to minimize suffering. No fluctuation in the chest, no
visual response, and the eyelids becoming white confirmed the death
of the mice. The brain tissues were quickly collected directly on
ice. The brain was analyzed to detect the contents of NAMPT,
NAD+, malondialdehyde (MDA) and inflammatory factors,
and the activity of superoxide dismutase (SOD) and
acetylcholinesterase (AChE).
OFT
Briefly, the mice were habituated to the laboratory
environment one day before the test. The next day, the mice were
gently placed in the center of a chamber (40x40x40 cm; Shanghai
Xinruan Information Technology Co., Ltd.). Each mouse was allowed
to habituate to the chamber for 5 min, and then allowed to explore
freely for 5 min while a video recording was made. The total
distance traveled and the mean velocity of movement were
measured.
BMT
Mice were placed in the center of a circular
platform with 20 equally spaced holes, one of which was connected
to a safe chamber (Shanghai Xinruan Information Technology Co.,
Ltd.). Aversive noise (65 dB) was used to encourage the mouse to
locate the safe target. All mice were trained for 5 consecutive
days with 4 min per trial, once daily. Tests were conducted on day
6, with only one chance to reach the target given. The number of
times the mice explored each hole, and the latency to find the
target hole were recorded.
Enzyme-linked immunosorbent assays
(ELISAs)
The brains collected on ice were rapidly dissected,
and a homogenate was obtained by sonication. After the removal of
particulates by centrifugation (1,450 x g, 4˚C, 20 min), the levels
of TNF-α, IL-1β, IL-4 and IL-10 in the brain, and NAMPT in the
brain and serum were measured using the corresponding ELISA kits
(cat. nos. ml002095, ml301814, ml002149 and ml002285, respectively;
Shanghai Enzyme-linked Biotechnology Co., Ltd.), in accordance with
the manufacturer's instructions. The contents of TNF-α, IL-1β,
IL-4, IL-10 and NAMPT were calculated by reference to the
corresponding standard curve.
Western blotting
Brains were dissected and homogenized in ice-cold
RIPA lysis buffer (Beyotime Institute of Biotechnology). Then they
were centrifuged with 8,700 x g at 4˚C for 10 min to obtain total
proteins. After that each sample was quantitatively tested by Nano
Drop, and loaded with the same total protein amount (50 mg). The
proteins were separated by 10% SDS-PAGE, and transferred to PVDF
membranes using an electrophoretic transfer system (Bio-Rad
Laboratories, Inc.). Membranes were blocked against non-specific
binding in 5% skimmed milk for 1 h at room temperature. The
membranes were then incubated with anti-NAMPT (1:5,000; cat. no.
A300-372A; Bethyl Laboratories, Inc.) or anti-GAPDH (1:1,000; cat.
no. AF1186; Beyotime Institute of Biotechnology) primary antibody
overnight at 4˚C. The membranes were washed by TBST (0.1% tween-20;
Beyotime Institute of Biotechnology) and incubated with HRP-labeled
goat anti-rabbit IgG (H+L) (1:200; cat. no. A0208; Beyotime
Institute of Biotechnology for 1 h at room temperature. Then
BeyoECL Moon (cat. no. P0018FS; Beyotime Institute of
Biotechnology) was used for visualization. Finally, the results
were analyzed using the Tanon 5200 Imaging System (Tanon Science
and Technology Co., Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the brain using an RNA
extraction kit (cat. no. R6934-01; Omega Bio-Tek, Inc.). After
measuring the purity and concentration of the RNA, reverse
transcription to cDNA was performed using a Hifair® II 1st Strand
cDNA Synthesis SuperMix for qPCR (gDNA digester plus) (cat. no.
11123ES60; Shanghai Yeasen Biotechnology Co., Ltd.) at 25˚C for 5
min; and 42˚C for 30 min; then at 85˚C for 5 min. qPCR
amplification was then performed using the cDNA as the template by
YeaRed Nucleic Acid Gel Stain (cat. no. 10202ES76; Shanghai Yeasen
Biotechnology Co., Ltd.). The thermocycling conditions were as
follows: Initial denaturation at 95˚C for 2 min; followed by 42
cycles of 95˚C for 10 sec and 55˚C for 30 sec. The relative
expression of Nampt mRNA was calculated using the
2-ΔΔCq method (21) with the following primers: NAMPT
forward: 5'-GCAGAAGCCGAGTTCAACATC-3' and reverse:
5'-TTTTCACGGCATTCAAAGTAGGA-3'; GAPDH forward:
5'-AAGAAGGTGGTGAAGCAGG-3' and reverse:
5'-GAAGGTGGAAGAGTGGGAGT-3'.
Enzyme immunoassays
The enzymatic activity of SOD (cat. no. BC010-2) and
AChE (cat. no. BC2025), and the contents of NAD+ (cat.
no. SH985W) and MDA (cat. no. BC003-2) in the brain were quantified
using corresponding kits (Beijing Solarbio Science & Technology
Co., Ltd.), according to the manufacturer's instructions.
Absorbance values were subsequently determined at the appropriate
wavelength.
Immunofluorescence and H&E
staining
After the last behavioral test, mice were sacrificed
and the brain, liver, kidneys and skin were removed following
perfusion through the left ventricle with saline solution and 4%
paraformaldehyde. The tissues were submerged in 4% paraformaldehyde
for 24 h at 4˚C, and dehydrated with 30% sucrose solution for 2-3
days. Sections were then prepared with a frozen microtome. The 5%
goat serum (cat. no. C0265; Beyotime Institute of Biotechnology)
was used to block the tissue at 37˚C for 2 h. The thickness of
liver and kidneys were 8 µm, skin sections were 6 µm and brain
sections were 25 µm.
For immunofluorescence, the sections were incubated
in anti-NAMPT (1:500; cat. no. A300-372A), anti-neuronal nuclei
(NeuN; 1:100; cat. no. MAB377; Sigma-Aldrich; Merck KGaA),
anti-glial fibrillary acidic protein (GFAP; 1:10; cat. no. BM2287;
OriGene Technologies, Inc.), anti-ionized calcium-binding adapter
molecule 1 (Iba-1; 1:100, cat. no. 019-19741; Wako Pure Chemical
Industries, Ltd.), anti-postsynaptic density protein 95 (PSD95;
1:200; cat. no. 3450S; Cell Signaling Technology, Inc.) and
anti-synaptophysin (1:200; cat. no. 9020S; Cell Signaling
Technology, Inc.). After incubation with Cy3-labeled goat
anti-rabbit IgG (H+L) (1:200, cat. no. A0516; Beyotime Institute of
Biotechnology) and FITC-labeled goat anti-mouse IgG (H+L) (1:200,
cat. no. A0568; Beyotime Institute of Biotechnology) for 2 h at
room temperature, the sections were mounted with DAPI. Images of
the sections were captured using confocal microscope, and the
quantitative assessment was subsequently performed by Image J 1.0
software (National Institutes of Health).
All samples of the liver, kidneys and skin were
stained with H&E at room temperature for 4 min, and to analyze
the pathological changes under a light microscope. The thickness of
the skin was measured at 6 randomly selected regions using Image J
software (National Institutes of Health).
Statistical analysis
All data were analyzed using SPSS 25.0 software (IBM
Corp.), and are presented as the mean ± SEM. One-way analysis of
variance followed by LSD (homogeneity of variance) and Tamhane's
T2 (heterogeneity of variance) test were performed to
analyze differences among groups. P<0.05 was considered to
indicate a statistically significant result.
Results
Contents of active components in
saffron
The HPLC results showed that the average content of
the saffron active components picrocrocin, crocin I and crocin II
was 12.00±0.13, 10.80±0.27 and 8.77±0.19%, respectively (Table I and Fig. S1).
 | Table IContents of the main components of
saffron aqueous extract. |
Table I
Contents of the main components of
saffron aqueous extract.
| Variable | Picrocrocin | Crocin I | Crocin II |
|---|
| Average peak
area | 639.46 | 1,245.36 | 645.06 |
| Average content,
% | 12.00±0.13 | 10.80±0.27 | 8.77±0.19 |
Effects of saffron on the pathological
morphology of the liver, kidneys and skin in mice
Analysis of pathological morphology in the mice
revealed fewer hepatocytes and more pyknosis in the liver tissue in
the 8- and 16-month-old control mice than the 3-month-old control
mice. Additionally, both 8- and 16-month-old groups were
characterized by the irregular arrangement of hepatocytes with
vacuolated cytoplasm. Shrunken central veins with blood congestion
and irregular blood sinusoids were also detected. However, sections
from saffron-treated mice exhibited almost normal hepatocyte
nuclei, and an intact central vein (Fig. 2A). Further, the number of
hepatocytes was 57.03% higher in the 8-month-old mice with saffron
treatment compared with the 8-month-old control mice (P<0.01;
Fig. 2D).
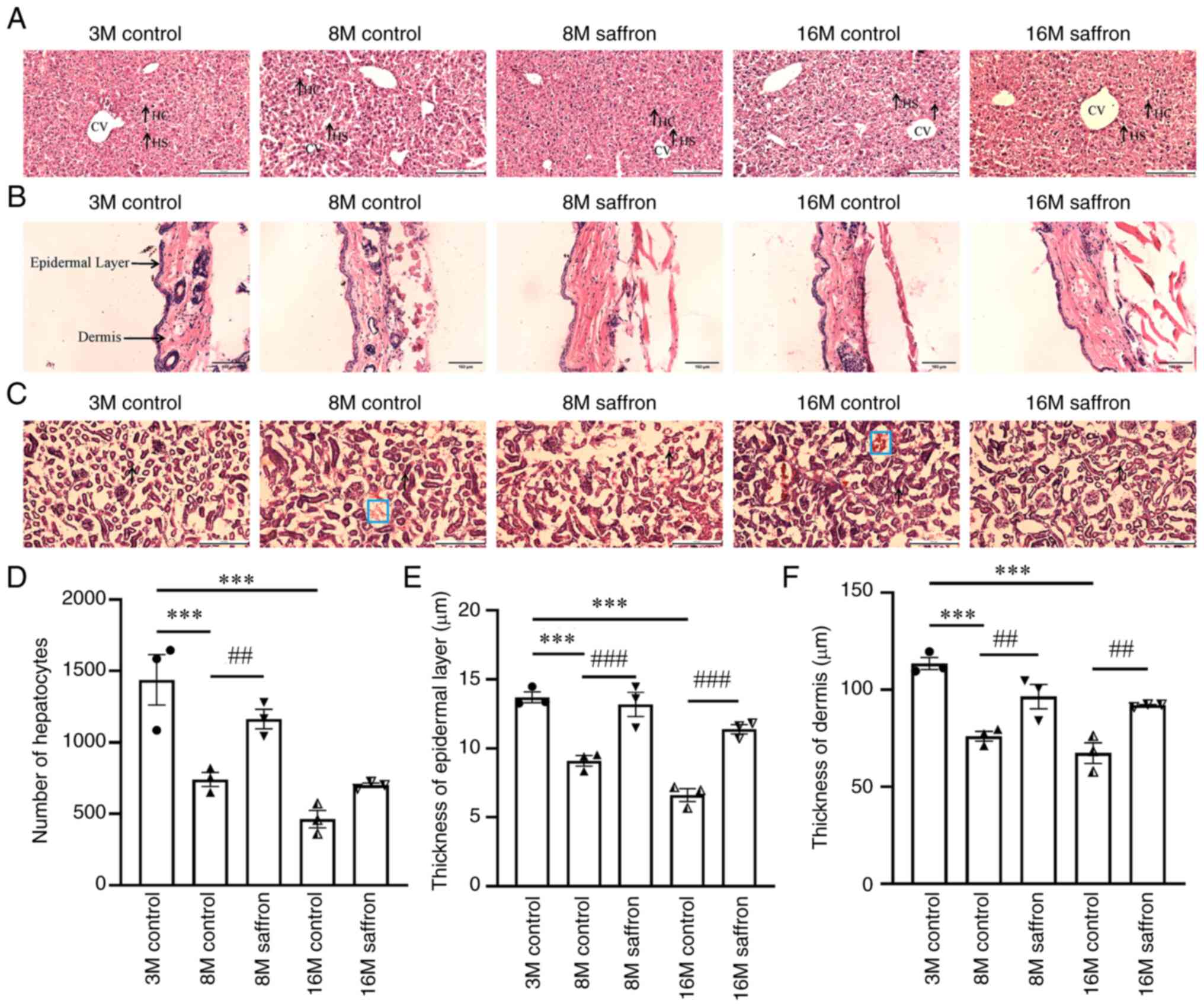 | Figure 2Effects of saffron on the
pathological morphology of the liver, kidneys and skin in mice.
Pathological morphology of the (A) liver (scale bar, 200 µm), (B)
skin (scale bar, 100 µm) and (C) kidneys (scale bar, 200 µm) as
revealed by hematoxylin and eosin staining (blue boxes indicated
bleeding in the area). (D) Hepatocyte number, (E) thickness of the
epidermal layer and (F) thickness of the dermis. Values are
presented as the mean ± SEM. ***P<0.001 vs. the
3-month-old control group; ##P<0.01,
###P<0.001 vs. the age-matched control group. HC,
hepatocyte; HS, hepatic sinusoid; CV, central vein; M, month. |
Fewer and thinner dermal collagen fibers, and the
infiltration of dermal inflammatory cells were observed in the 8-
and 16-month-old control groups (Fig.
2B), while the thickness of the epidermis and dermis were
decreased compared with those in the 3-month-old control group (all
P<0.001; Fig. 2E and F). In the 8- and 16-month-old mice,
saffron treatment increased the thickness of the epidermis (both
P<0.001; Fig. 2E) and dermis
(both P<0.01; Fig. 2F) compared
with those in the age-matched controls.
Increased hemorrhage and ballooning were observed in
the kidneys of the 8- and 16-month-old control groups when compared
with the 3-month-old control group, and saffron treatment
ameliorated these changes (Fig.
2C).
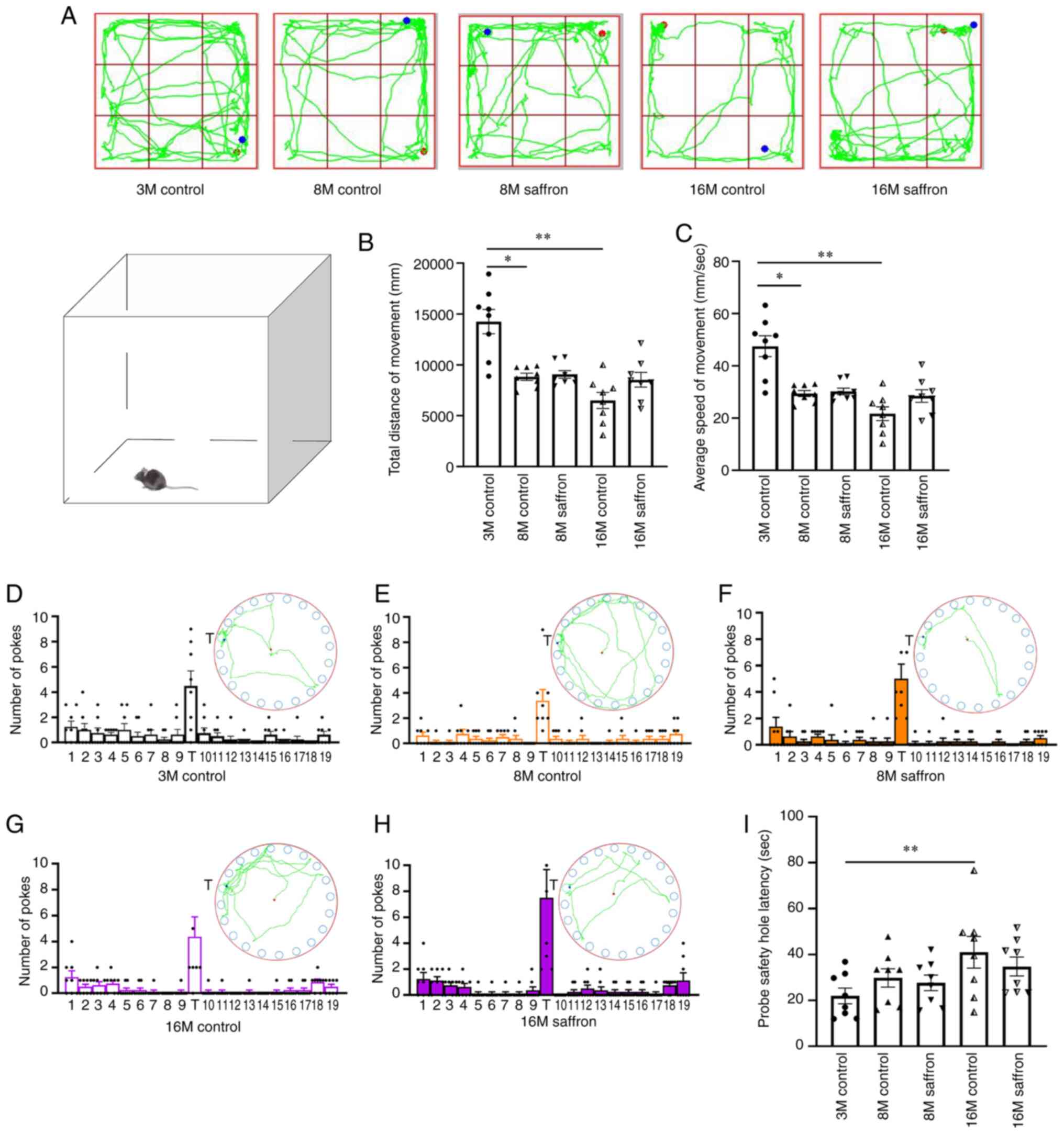
Effects of saffron on the behavior of
the mice
In the OFT, the 8- and 16-month-old control mice had
a shorter total traveled distance (P<0.05 and P<0.01,
respectively) and slower mean velocity (P<0.05 and P<0.01,
respectively) compared with the 3-month-old mice. However, no
difference was detected between the same-aged saffron and control
groups (Fig. 3A-C). Furthermore,
no significant difference in the number of explorations of the
target hole among groups was observed in the BMT (Fig. 3D-H). However, the probe safety hole
latency was significantly increased in the 16-month-old control
mice compared with 3-month-old mice in the BMT (P<0.01; Fig. 3I).
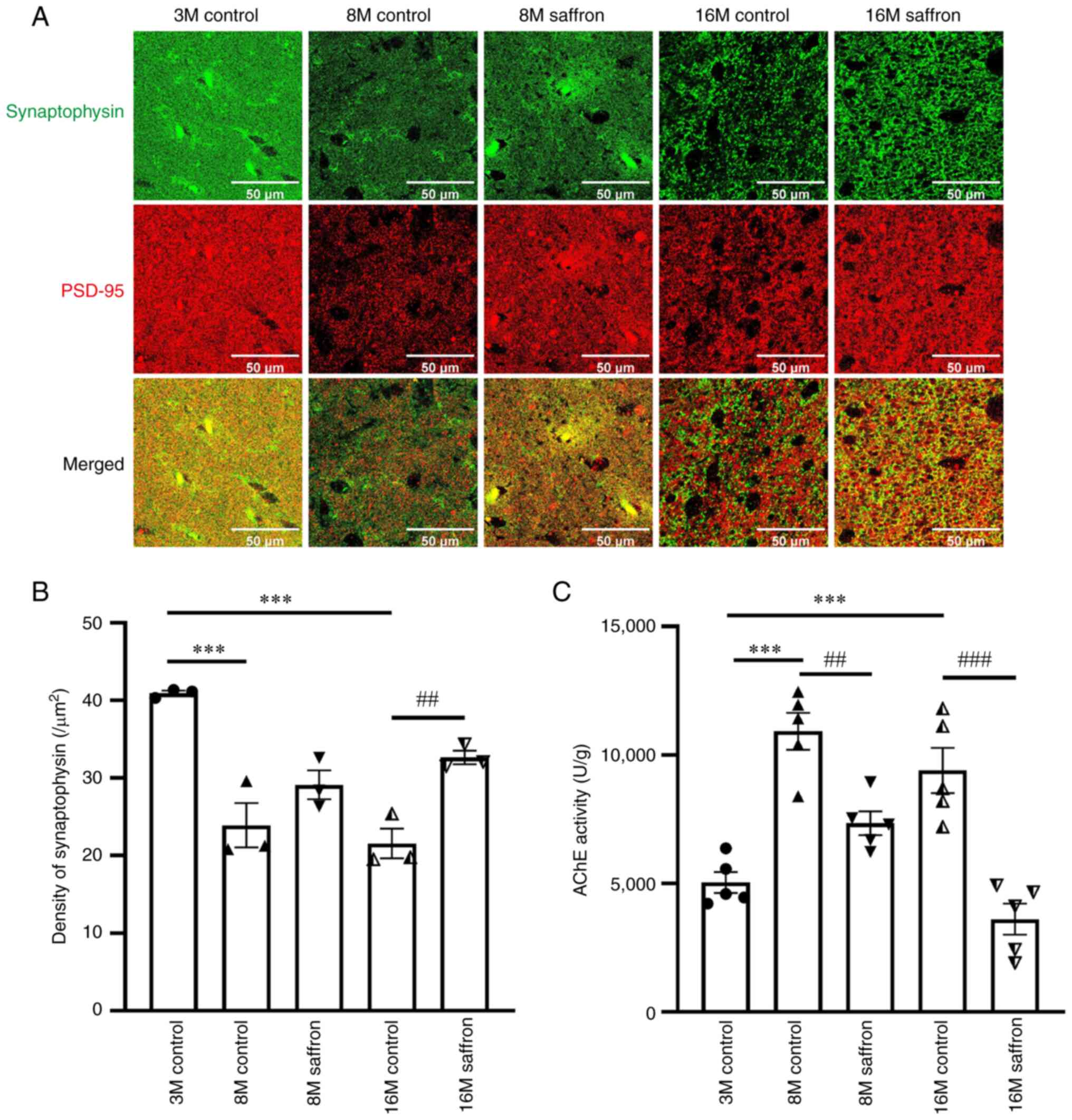
Effects of saffron on synaptic density
and AChE activity in mice
PSD95 and synaptophysin were co-expressed to
determine synaptic density (22).
Compared with the synaptophysin density in the brains of the
3-month-old control group, that in the 8- and 16-month-old control
groups decreased by 41.60 and 47.37%, respectively (both
P<0.001). Following saffron treatment, synaptophysin density
increased by 51.52% in the 16-month-old group when compared with
the same-aged control mice (P<0.01; Fig. 4A and B). In addition, the AChE activity in the
8- and 16-month-old control groups increased by 116.76 and 86.60%
compared with that in the 3-month-old control group (both
P<0.001), and decreased with saffron treatment in the 8- and
16-month-old mice (P<0.01 and P<0.001, respectively; Fig. 4C).
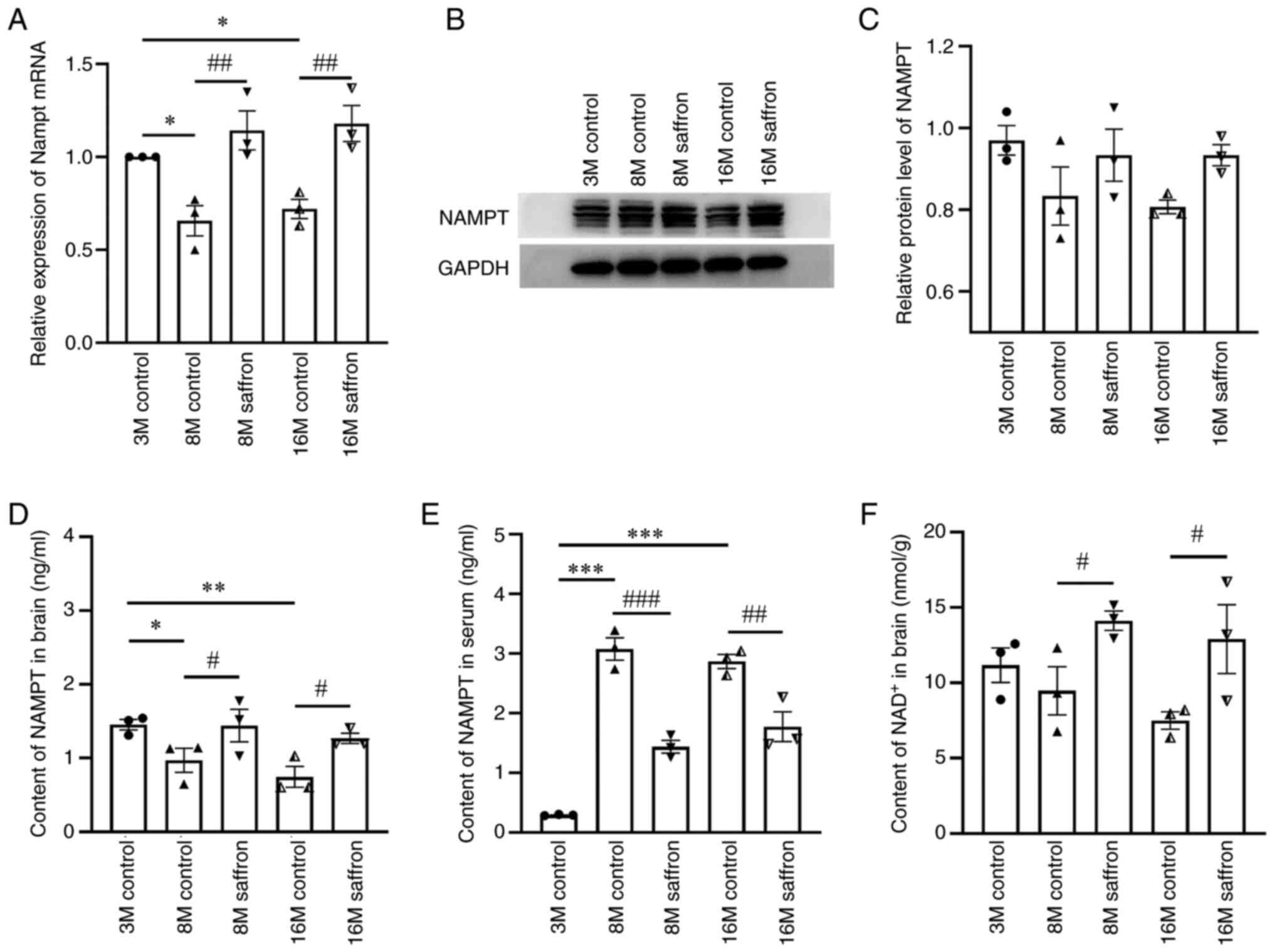
Effects of saffron on NAMPT and
NAD+ content in mice
The relative expression of Nampt mRNA in the
brain was decreased by 34.16 and 28.02% in the 8- and 16-month-old
control groups compared with the 3-month-old group (both P<0.05;
Fig. 5A). Following saffron
treatment, Nampt mRNA expression significantly increased in
the 8- and 16-month-old mice compared with the same-aged control
mice (both P<0.01; Fig. 5A).
Similar results were found regarding NAMPT content in the brain by
ELISA (Fig. 5D). No significant
difference in NAMPT protein levels in the brain was detected among
all groups by western blotting (Fig.
5B and C). However, in the
serum, NAMPT protein increased by 948.15 and 877.78% in the 8- and
16-month-old control groups compared with the 3-month-old group
(both P<0.001), and these increases were attenuated by saffron
treatment in the 8- and 16-month-old mice (P<0.001 and
P<0.01, respectively; Fig.
5E).
The contents of NAD+ in the brain
decreased with aging, albeit not significantly, and were
significantly increased by saffron treatment in the 8- and
16-month-old mice (both P<0.05; Fig. 5F).
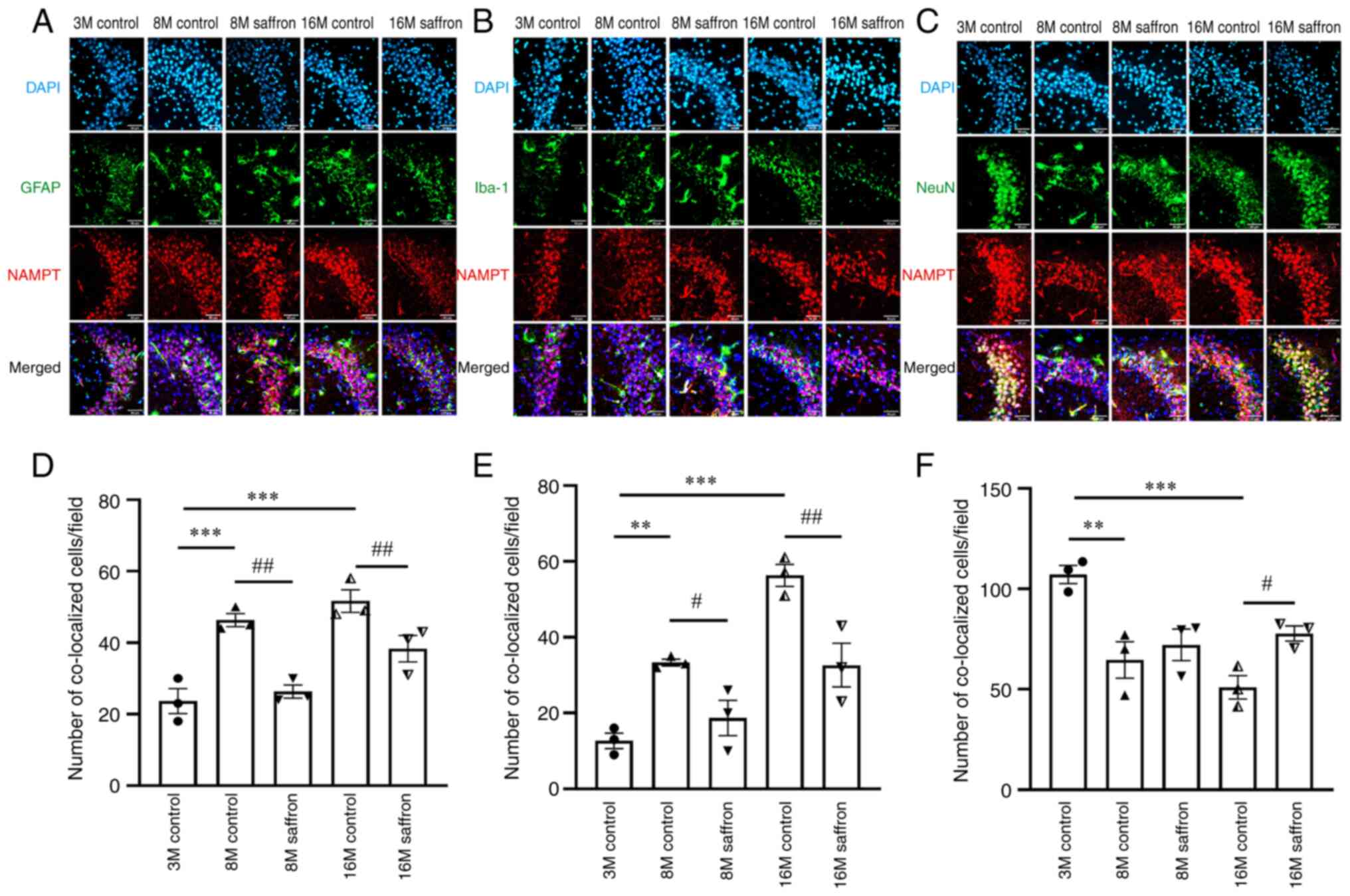
Effects of saffron on the cellular
distribution of NAMPT expression
With aging, the astrocyte cell bodies were observed
to increase in size and become rounder, and the protrusions from
the cell body shrank. In addition, the microglia increased in size
and branching. Regarding the co-distribution of with NAMPT with
other proteins, the number of cells in which NAMPT was co-expressed
with GFAP (both P<0.001) was significantly increased in the 8-
and 16-month-old control groups compared with the 3-month-old
group, as was co-expression with Iba-1 (P<0.01 and P<0.001,
respectively). Further, the co-distribution of GFAP or Iba-1 with
NAMPT decreased in the 8- and 16-month-old saffron groups compared
with the age-matched control groups (GFAP, both P<0.01; Iba-1,
P<0.05 and P<0.01, respectively; Fig. 6A, B, D and
E).
With aging, neural stem cells gradually become
senescent. The number of mature nerve cells decreases, activity
decreases, and the renewal and replenishment ability of neurons
decreases markedly (23). The
co-distribution of NeuN with NAMPT in the 8- and the 16-month-old
control groups decreased by 39.66 and 52.41% compared with that in
the 3-month-old control group (P<0.01 and P<0.001,
respectively). After saffron treatment, a significant increase in
NeuN and NAMPT co-distribution was observed in the 16 month-old
mice compared with the age-matched control (P<0.05); however,
the increase in the 8 month-old mice was not statistically
significant (Fig. 6C and F).
Effects of saffron on inflammatory
reaction and oxidative stress factors in mice
The contents of TNF-α in the brains of the 8- and
16-month old control mice increased by 17.70 and 28.64% compared
with those in the 3-month-old control group (both P<0.001), and
the IL-1β contents were also significantly increased in the 8- and
16-month-old control groups (both P<0.01). After saffron
treatment, significant reductions in TNF-α and IL-1β were observed
in the 8- and 16-month-old groups compared with the corresponding
age-matched controls (TNF-α, both P<0.001; IL-1β, P<0.05 and
P<0.01, respectively; Fig. 7A
and B). Regarding
anti-inflammatory factors, significant reductions with aging in the
8- and 16-month-old control groups compared with the 3-month-old
group were observed in the contents of IL-4 (both P<0.05) and
IL-10 (both P<0.01) (Fig. 7C
and D). The reductions in IL-4
content were reversed by saffron treatment in the 8- and
16-month-old saffron groups (P<0.05 and P<0.01, respectively;
Fig. 7C). However, the content of
IL-10 increased significantly only in the 8-month-old saffron group
(P<0.01), and not in the 16-month-old saffron group (Fig. 7D).
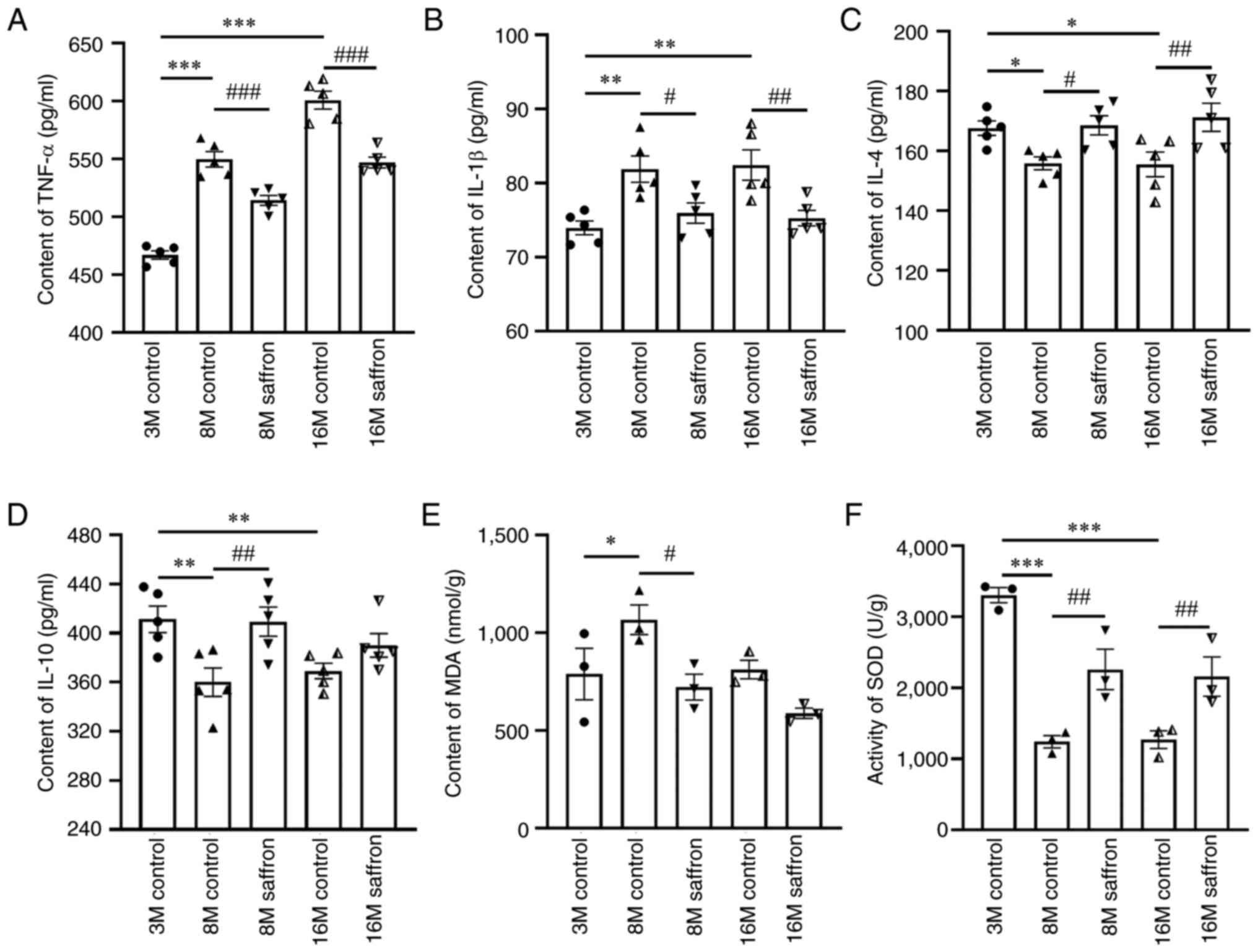 | Figure 7Effects of saffron on inflammatory
and oxidative stress factors in mice. Contents of (A) TNF-α, (B)
IL-1β, (C) IL-4, (D) IL-10 and (E) MDA, and (F) SOD activity in the
brain. Values are presented as the mean ± SEM.
*P<0.05, **P<0.01 and
***P<0.001 vs. the 3-month-old control group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. the age-matched control group. TNF,
tumor necrosis factor; IL, interleukin; MDA, malondialdehyde; SOD,
superoxide dismutase; M, month. |
The content of MDA increased significantly only in
the 8-month-old control group compared with the 3-month-old group
(P<0.05), and was decreased significantly only in the
8-month-old saffron group compared with the age-matched control
(P<0.05; Fig. 7E). However, the
activity of SOD in the 8- and 16-month-old control groups was
significantly decreased compared with the 3-month control (both
P<0.001; Fig. 7F), and the
reduction was attenuated significantly with saffron treatment in
the 8- and 16-month-old mice (both P<0.01; Fig. 7F).
Discussion
In the present study, the senescence-delaying
effects of saffron in mice were investigated. These manifested as
improvements in cognition dysfunction, the pathological morphology
of the tissues, and increased synaptic density and decreased AChE
activity in the brain. We hypothesize that these changes may be
associated with changes in the content and cellular distribution of
NAMPT, followed by an increase in NAD+ production.
In order to evaluate whether saffron met the
requirements of the Chinese Pharmacopoeia, which comprise a total
crocin content of >10%, the contents of picrocrocin and crocin
were detected by HPLC prior to use of the saffron extract. The
results indicated that the quality of saffron used in the present
study was suitable for investigating the senescence-delaying
effects of saffron in aging mice. The quality of the saffron was
similar to that in our previous study and another phytochemical
study (7,24). With consideration of the
pharmacokinetic properties of saffron (25), it is proposed that it may be more
valuable to study the effects of saffron extract than those of its
active components for use in healthcare. However, in order to
clarify the mechanism of the senescence-delaying effects of saffron
extract, it will be essential to use molecular docking techniques
to screen its active components and conduct further experiments to
verify their effects.
Several prior studies have reported that the
morphology of the liver (26),
kidneys (27) and skin (28) all change with aging, including the
development of pycnotic nuclei in the liver, thinner dermal
collagen fibers, lower infiltration of inflammatory cytokines in
the skin, and less hemorrhaging and ballooning in the kidneys. In
the present study, the senescence-delaying effects of saffron were
also indicated by amelioration of the pathophysiological changes in
the liver, skin and kidneys.
Behavioral tests, including the OFT and BMT, are
often applied to measure the spontaneous activity and
learning-memory function of mice, which decrease in an
age-dependent manner (29). In the
present study, the results of these tests showed decreased
locomotor activity in the 8- and 16-month-old control mice and
increased probe safety hole latency in 16-month-old mice, which are
key signs of aging (30). An
increasing tendency in the number of explorations of the target
hole was observed after saffron treatment. We hypothesize that the
insignificance of the behavioral improvements achieved by saffron
could be associated with the ages of the animals. Shoji and
Miyakawa (31) previously reported
that 46- to 72-week-old mice were middle-aged and 98-week-old mice
were aged. However, in the present study 8- and 16-month-old mice
were considered as middle-aged and aged, respectively. The
senescence-delaying effects of saffron may be more pronounced in
older mice, and BMT or other methods should be used in further
studies to investigate this.
Certain indicators associated with learning-memory
function change with aging, such as synaptic density, AChE
activity, and performance in Morris water maze and novel object
recognition tests (32). Several
reports have suggested that the immunoreactivity of synaptophysin
reduces with aging, which would cause learning and memory
dysfunction (33,34). Consistent with these previous
findings, the present study found that synaptophysin density
decreased with aging, while saffron increased synaptophysin density
in 16-month-old mice. AChE decomposes acetylcholine (ACh) and
inhibits ACh-induced signal transmission; ACh is an important
neurotransmitter that is released by cholinergic neuron terminals,
participates in the transmission of neural signals and is closely
associated with learning and memory function (35). The present study found that AChE
activity increased in the 8- and 16-month-old mice, and saffron
reduced its activity in mice of both ages. Furthermore, the
reduction was greater in the 16-month-old mice. These results
suggest that saffron can improve indicators of learning and memory
impairment associated with aging, but this it has not been
significantly reflected in the BMT results, and requires further
verification using other tests.
NAMPT is a rate-limiting enzyme in mammalian
NAD+ biosynthesis and the controller of intracellular
NAD+ levels (13).
NAMPT-mediated NAD+ biosynthesis plays an important role
in energy metabolism, DNA repair, chromatin remodeling, cell aging
and immune cell function regulation (36). The level of NAMPT decreases
significantly with aging, which leads to the development of
age-related diseases including metabolic diseases,
neurodegenerative diseases and cancer (37). NAMPT exists in two forms in
mammals, namely intracellular NAMPT (iNAMPT) and extracellular
NAMPT (eNAMPT). iNAMPT is predominantly found in the brain, while
eNAMPT mainly exists in the serum. iNAMPT protects cells
predominantly by promoting the synthesis of nicotinamide
mononucleotide, a precursor of NAD (17,38,39).
Conversely, eNAMPT can function either as an enzyme for NAD
biosynthesis (40), or a
proinflammatory cytokine (17,41).
It has previously been shown that level of eNAMPT in
the serum increases and that of iNAMPT in the brain decreases upon
aging (15); the latter is
consistent with the ELISA findings for NAMPT in the present study.
The reduction of eNAMPT achieved following saffron treatment
indicates alleviation of the inflammation that occurs upon aging.
Although that mRNA level of nampt was shown to decrease in
the brain with aging, no difference in the protein expression of
NAMPT was observed among the groups. We hypothesize it could be
associated with a change in the cellular distribution of iNAMPT,
resulting in its secretion out of the brain. Ma et al
(42) previously reported similar
findings regarding Nampt mRNA and NAMPT protein
expression.
The cellular distribution of iNAMPT changes with
aging, which manifests as an increase in NAMPT expression in the
microglia and astrocytes, and a reduction in NAMPT expression in
neurons (15,43). Microglia and astrocytes are
inflammatory cells of the central nervous system that possesses a
heightened reactivity in the aged brain (44), and iNAMPT has been shown to play a
role in regulating their inflammatory reaction (45). Furthermore, it has been reported
that oxidative stress (46) and
inflammatory reactions (47,48)
are both associated with aging. As such, the increase in NAMPT in
neurons and reduction in the microglia and astrocytes may be a
mechanism underlying the alleviation of inflammatory and oxidative
stress reactions after saffron treatment in middle-aged and aged
mice (49,50).
Although senescence-delaying effects and changes of
NAMPT-NAD+ contents and cellular distribution induced by
saffron were found in the present study, the association between
these effects is unclear. However, according to the literature
(15,43) and findings on aging from the
present study, it is speculated that NAMPT-NAD+ may have
a certain relationship with the senescence-delaying effects of
saffron. However, the exact association between them requires
further study. In the future,
Namptflox/flox mice
(51) will be used to explore and
clarify the mechanism by which saffron extract and its active
components affect the NAMPT-NAD+ system and induce
senescence-delaying effects.
In summary, the present study showed that saffron
treatment changed the content and cellular distribution of NAMPT in
the brain and serum induced by aging in mice. These changes could
be partly associated with improvements in physiological state of
aging, learning-memory dysfunction, and microglia- and
astrocyte-mediated inflammatory reaction. Therefore, the findings
of the study suggest that saffron has potential as a treatment to
delay senescence.
Supplementary Material
High-performance liquid
chromatography-derived results of the main components of saffron
aqueous extract by comparison with reference standards.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Project of National
Innovation and Entrepreneurship Training Program for College
Students (grant no. 202213023012) and the Natural Science
Foundation of Zhejiang Province (grant no. LY20H310002).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LXiao contributed to the animal model, behavioral
tests, data collection and analysis, as well as writing the
original draft of the manuscript, writing-review and editing. RS
contributed to the animal model, data collection and analysis. YH
and LXia contributed to the animal model and behavioral tests. KL
helped with behavioral testing and funding acquisition. WF also
helped with behavioral testing while KZ contributed to the animal
model and data collection. YY was responsible for project
administration and design, data collection and analysis,
writing-review and editing and funding acquisition. LXiao and YY
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal experiment was approved (approval no.
2021-040) by the Animal Health, Ethics and Research Committee of
Hangzhou Medical College (Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tchkonia T and Kirkland JL: Aging, cell
senescence, and chronic disease: Emerging therapeutic strategies.
JAMA. 320:1319–1320. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li X, Yang S, Wang S, Shi Y, Dai Y, Zhang
X, Liu Y, Guo Y, He J and Xiu M: Regulation and mechanism of
Astragalus polysaccharide on ameliorating aging in Drosophila
melanogaster. Int J Biol Macromol. 234(123632)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin K, Sze SC, Liu B, Zhang Z, Zhang Z,
Zhu P, Wang Y, Deng Q, Yung KK and Zhang S: 20(S)-protopanaxadiol
and oleanolic acid ameliorate cognitive deficits in APP/PS1
transgenic mice by enhancing hippocampal neurogenesis. J Ginseng
Res. 45:325–333. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bostan HB, Mehri S and Hosseinzadeh H:
Toxicology effects of saffron and its constituents: A review. Iran
J Basic Med Sci. 20:110–121. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jose Bagur M, Alonso Salinas GL,
Jimenez-Monreal AM, Chaouqi S, Llorens S, Martínez-Tomé M and
Alonso GL: Saffron: An old medicinal plant and a potential novel
functional food. Molecules. 23(30)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tamegart L, Abbaoui A, Makbal R, Zroudi M,
Bouizgarne B, Bouyatas MM and Gamrani H: Crocus sativus restores
dopaminergic and noradrenergic damages induced by lead in Meriones
shawi: A possible link with Parkinson's disease. Acta Histochem.
121:171–181. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhong K, Wang RX, Qian XD, Yu P, Zhu XY,
Zhang Q and Ye YL: Neuroprotective effects of saffron on the late
cerebral ischemia injury through inhibiting astrogliosis and glial
scar formation in rats. Biomed Pharmacother.
126(110041)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Khorasanchi Z, Shafiee M, Kermanshahi F,
Khazaei M, Ryzhikov M, Parizadeh MR, Kermanshahi B, Ferns GA, Avan
A and Hassanian SM: Crocus sativus a natural food coloring and
flavoring has potent anti-tumor properties. Phytomedicine.
43:21–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang F, Zhu X, Yu P, Sheng T, Wang Y and
Ye Y: Crocin ameliorates depressive-like behaviors induced by
chronic restraint stress via the NAMPT-NAD(+)-SIRT1 pathway in
mice. Neurochem Int. 157(105343)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Madan K and Nanda S: In-vitro evaluation
of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase
activities of safranal and determination of its sun protection
factor in skin photoaging. Bioorg Chem. 77:159–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chalatsa I, Arvanitis DA, Koulakiotis NS,
Giagini A, Skaltsounis AL, Papadopoulou-Daifoti Z, Tsarbopoulos A
and Sanoudou D: The crocus sativus compounds trans-crocin 4 and
trans-crocetin modulate the amyloidogenic pathway and tau
misprocessing in alzheimer disease neuronal cell culture models.
Front Neurosci. 13(249)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Heidari S, Mehri S and Hosseinzadeh H:
Memory enhancement and protective effects of crocin against
D-galactose aging model in the hippocampus of Wistar rats. Iran J
Basic Med Sci. 20:1250–1259. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Koltai E, Szabo Z, Atalay M, Boldogh I,
Naito H, Goto S, Nyakas C and Radak Z: Exercise alters SIRT1,
SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech
Ageing Dev. 131:21–28. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sun Z, Lei H and Zhang Z: Pre-B cell
colony enhancing factor (PBEF), a cytokine with multiple
physiological functions. Cytokine Growth Factor Rev. 24:433–442.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu LY, Wang F, Zhang XY, Huang P, Lu YB,
Wei EQ and Zhang WP: Nicotinamide phosphoribosyltransferase may be
involved in age-related brain diseases. PLoS One.
7(e44933)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q,
Jin Q, Cao F, Tian F and Chen Y: Melatonin protects cardiac
microvasculature against ischemia/reperfusion injury via
suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis.
J Pineal Res. 63(e12413)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Garten A, Petzold S, Korner A, Imai S and
Kiess W: Nampt: Linking NAD biology, metabolism and cancer. Trends
Endocrinol Metab. 20:130–138. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Imai S: The NAD World: A new systemic
regulatory network for metabolism and aging-Sirt1, systemic NAD
biosynthesis, and their importance. Cell Biochem Biophys. 53:65–74.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang F and Zhang WP: Research progress on
nicotinamide phosphoribosyl transferase involved in aging and
age-related diseases. Zhejiang Da Xue Xue Bao Yi Xue Ban.
40:680–684. 2011.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
20
|
Moshfegh F, Balanejad SZ, Shahrokhabady K
and Attaranzadeh A: Crocus sativus (saffron) petals extract and its
active ingredient, anthocyanin improves ovarian dysfunction,
regulation of inflammatory genes and antioxidant factors in
testosterone-induced PCOS mice. J Ethnopharmacol.
282(114594)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Okabe S, Miwa A and Okado H: Spine
formation and correlated assembly of presynaptic and postsynaptic
molecules. J Neurosci. 21:6105–6114. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ibrayeva A, Bay M, Pu E, Jörg DJ, Peng L,
Jun H, Zhang N, Aaron D, Lin C, Resler G, et al: Early stem cell
aging in the mature brain. Cell Stem Cell. 28:955–966 e7.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alavizadeh SH and Hosseinzadeh H:
Bioactivity assessment and toxicity of crocin: A comprehensive
review. Food Chem Toxicol. 64:65–80. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hosseini A, Razavi BM and Hosseinzadeh H:
Pharmacokinetic properties of saffron and its active components.
Eur J Drug Metab Pharmacokinet. 43:383–390. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
El-Sherbiny M, Atef H, Helal GM, Al-Serwi
RH, Elkattawy HA, Shaker GA, Said E, Abulfaraj M, Albalawi MA and
Elsherbiny NM: Vitamin K2 (MK-7) Intercepts Keap-1/Nrf-2/HO-1
pathway and hinders inflammatory/apoptotic signaling and liver
aging in naturally aging rat. Antioxidants (Basel).
11(2150)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dybiec J, Szlagor M, Mlynarska E, Rysz J
and Franczyk B: Structural and functional changes in aging kidneys.
Int J Mol Sci. 23(15435)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee H, Hong Y and Kim M: Structural and
functional changes and possible molecular mechanisms in aged skin.
Int J Mol Sci. 22(12489)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Logan S, Owen D, Chen S, Chen WJ, Ungvari
Z, Farley J, Csiszar A, Sharpe A, Loos M, Koopmans B, et al:
Simultaneous assessment of cognitive function, circadian rhythm,
and spontaneous activity in aging mice. Geroscience. 40:123–137.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Creighton SD, Stefanelli G, Reda A and
Zovkic IB: Epigenetic mechanisms of learning and memory:
Implications for aging. Int J Mol Sci. 21(6918)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shoji H and Miyakawa T: Age-related
behavioral changes from young to old age in male mice of a C57BL/6J
strain maintained under a genetic stability program.
Neuropsychopharmacol Rep. 39:100–118. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu B, Kou J, Li F, Huo D, Xu J, Zhou X,
Meng D, Ghulam M, Artyom B, Gao X, et al: Lemon essential oil
ameliorates age-associated cognitive dysfunction via modulating
hippocampal synaptic density and inhibiting acetylcholinesterase.
Aging (Albany NY). 12:8622–8639. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Alonso-Nanclares L, Merino-Serrais P,
Gonzalez S and DeFelipe J: Synaptic changes in the dentate gyrus of
APP/PS1 transgenic mice revealed by electron microscopy. J
Neuropathol Exp Neurol. 72:386–395. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wan L, Ai JQ, Yang C, Jiang J, Zhang QL,
Luo ZH, Huang RJ, Tu T, Pan A, Tu E, et al: Expression of the
excitatory postsynaptic scaffolding protein, shank3, in human
brain: effect of age and alzheimer's disease. Front Aging Neurosci.
13(717263)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chandar NB and Ganguly B: A first
principles investigation of aging processes in soman conjugated
AChE. Chem Biol Interact. 204:185–190. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Stromland O, Diab J, Ferrario E, Sverkeli
LJ and Ziegler M: The balance between NAD(+) biosynthesis and
consumption in ageing. Mech Ageing Dev. 199(111569)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Garten A, Schuster S, Penke M, Gorski T,
de Giorgis T and Kiess W: Physiological and pathophysiological
roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 11:535–546.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
van der Veer E, Ho C, O'Neil C, Barbosa N,
Scott R, Cregan SP and Pickering JG: Extension of human cell
lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem.
282:10841–10845. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang H, Yang T, Baur JA, Perez E, Matsui
T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A,
et al: Nutrient-sensitive mitochondrial NAD+ levels dictate cell
survival. Cell. 130:1095–1107. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Revollo JR, Korner A, Mills KF, Satoh A,
Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR,
et al: Nampt/PBEF/Visfatin regulates insulin secretion in beta
cells as a systemic NAD biosynthetic enzyme. Cell Metab. 6:363–375.
2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang T, Zhang X, Bheda P, Revollo JR, Imai
S and Wolberger C: Structure of Nampt/PBEF/visfatin, a mammalian
NAD+ biosynthetic enzyme. Nat Struct Mol Biol. 13:661–662.
2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ma C, Pi C, Yang Y, Lin L, Shi Y, Li Y, Li
Y and He X: Nampt expression decreases age-related senescence in
rat bone marrow mesenchymal stem cells by targeting Sirt1. PLoS
One. 12(e0170930)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xie X, Gao Y, Zeng M, Wang Y, Wei TF, Lu
YB and Zhang WP: Nicotinamide ribose ameliorates cognitive
impairment of aged and Alzheimer's disease model mice. Metab Brain
Dis. 34:353–366. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jurgens HA and Johnson RW: Dysregulated
neuronal-microglial cross-talk during aging, stress and
inflammation. Exp Neurol. 233:40–48. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bermudez B, Dahl TB, Medina I, Groeneweg
M, Holm S, Montserrat-de la Paz S, Rousch M, Otten J, Herias V,
Varela LM, et al: Leukocyte overexpression of intracellular NAMPT
attenuates atherosclerosis by regulating PPARү-Dependent monocyte
differentiation and function. Arterioscler Thromb Vasc Biol.
37:1157–1167. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kudryavtseva AV, Krasnov GS, Dmitriev AA,
Alekseev BY, Kardymon OL, Sadritdinova AF, Fedorova MS, Pokrovsky
AV, Melnikova NV, Kaprin AD, et al: Mitochondrial dysfunction and
oxidative stress in aging and cancer. Oncotarget. 7:44879–44905.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Pawelec G: Aging as an inflammatory
disease and possible reversal strategies. J Allergy Clin Immunol.
145:1355–1356. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Uyar B, Palmer D, Kowald A, Murua Escobar
H, Barrantes I, Möller S, Akalin A and Fuellen G: Single-cell
analyses of aging, inflammation and senescence. Ageing Res Rev.
64(101156)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cerda-Bernad D, Valero-Cases E, Pastor JJ
and Frutos MJ: Saffron bioactives crocin, crocetin and safranal:
Effect on oxidative stress and mechanisms of action. Crit Rev Food
Sci Nutr. 62:3232–3249. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Salem M, Shaheen M, Tabbara A and Borjac
J: Saffron extract and crocin exert anti-inflammatory and
anti-oxidative effects in a repetitive mild traumatic brain injury
mouse model. Sci Rep. 12(5004)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang J, Sun R, Xia L, Zhu X, Zhang Q and
Ye Y: Potential therapeutic effects of NAMPT-Mediated NAD
biosynthesis in depression in vivo. Brain Sci.
12(1699)2022.PubMed/NCBI View Article : Google Scholar
|