1. Introduction
Coronavirus disease 2019 (COVID-19), as the cause of
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a
multi-organ disease with subacute and long-term effects and a wide
range of clinical manifestations, including in the central nervous
system (CNS) (1,2). Following a 4-month follow-up of
patients hospitalized due to COVID-19, 30-40% of patients were
found to have memory dysfunction, attention, or dysexecutive
syndrome (3). Currently, ‘COVID-19
brain fog’ is defined as a non-specific mental syndrome following
infection with COVID-19, that consists of fatigue, low attention
span, memory disability, a loss of motivation and difficulty with
long working hours. A young age has been found to increase the
likelihood of COVID-19-related cognitive decline, regardless of
severe disease (4). Thus far, the
progression of cognitive symptoms is associated with
COVID-19(5). However, it remains
obscure whether the neurological deficits of patients with COVID-19
can be long-lasting (>6 months) or even gradual, conceivably
expanding the likelihood of cognitive damage. Another unanswered
issue is whether COVID-19 infection accentuates the nature of
dementia in individuals with pre-existing conditions. Direct brain
infection may be a potential pathway through which SARS-CoV-2
affects cognitive ability (6).
A main unresolved issue that persists is whether the
cognitive symptoms reported in patients with COVID-19 are explained
by (7) i) the exacerbation of a
systemic inflammatory response/cytokine storm; ii) encephalitis
after the SARS-CoV-2 attack in the brain, or both; iii) COVID-19
may provoke tissue hypoxia and microvascular lesions, destroying
the cerebral perfusion and the integrity of the blood-brain
barrier, which may impair the function and cognition of brain areas
such as the hippocampus; iv) the onset of the autoimmune cascade;
or v) peripheral organ deterioration. Defective cognition may
affect lifetime occupation and physical function following recovery
from COVID-19, initiating a vicious cycle of adverse mental health
and cognitive decline. The investigation of cognitive decline
related to COVID-19 mechanisms is crucial to adopt the proper
strategies with which to prevent subsequent cognitive decline. This
evidence underlines the necessity for the evolution of biological
indicators to evaluate therapeutic interventions in the disease
course.
A biomarker is defined as ‘a characteristic that can
be objectively measured and evaluated as an indicator of normal
biological processes, pathogenic processes, or pharmacological
responses to a therapeutic intervention’ (8). Taking account of i) the invasive
procedures for in vivo brain specimens; and ii) the
reflection of brain-related events in the cerebrospinal fluid
(CSF), the latter may be the perfect origin for biomarkers for
uncovering and surveilling diverse pathophysiological procedures.
Consequently, it is imperative to discover slightly invasive and
disease-specific diagnostic biomarkers that will permit the prompt
recognition of pathologic protein aggregation (9). Thus far, previous meta-analyses with
protein biomarkers (α-synuclein, Aβ42, Tau and pTau 181) in the
CSF, blood and saliva of patients with Parkinson's disease (PD)
have yielded inconsistent conclusions (10-14).
MicroRNAs (miRNAs/miRs) are small, phylogenetically
conserved non-coding RNAs (19-23 nucleotides in length) that
regulate protein expression by interacting with complementary
sequences in the 3-untranslated region (3'-UTR) of their target
mRNAs and then exerting their functions by degrading mRNAs or
inhibiting protein translation (9,15).
miRNAs are involved in RNA silencing and the post-transcriptional
regulation of gene expression. In cells from humans and other
animals, miRNAs have been shown to primarily function by
destabilizing mRNAs. This RNA silencing consists of the following
steps: i) The cleavage of the mRNA strand into two fragments; ii)
the destabilization of the mRNA by shortening its poly(A) tail; or
iii) reducing the translation of the mRNA into proteins (16). To date, >2,500 miRNAs have been
found in humans (miRbase), controlling vital functions, such as
lipid metabolism, apoptosis, differentiation, organ development and
cell death (9,17). The dysregulated expression of
miRNAs has been found to be associated with inflammatory,
degenerative and autoimmune disorders, cancer, cardiovascular
diseases, diabetes mellitus, and rheumatic and neurodegenerative
diseases such as PD (18-20).
Essentially, miRNAs have recently been designated as novel
mediators of cell-cell communications, being cell-secreted in
different biological fluids (blood, CSF, saliva and urine)
(21-23).
These features also characterize miRNAs as plausible biomarkers for
PD (13,23,24).
Several miRNAs have been associated with PD, as they modulate the
expression of critical proteins implicated in pathophysiology, such
as synuclein alpha, leucine-rich repeat kinase 2,
glucosylceramidase beta and nuclear receptor-related-17 (24,25).
A recent meta-analysis identified several miRNAs with a highly
significant differential expression in the brain and blood of
patients with PD (26).
The present review discusses the underlying
mechanisms that link COVID-19 to cognitive decline. miRNAs in
serum, plasma, CSF, extracellular vesicles and exosomes may have an
impact on disease pathogenesis and may be useful as biomarkers or
therapeutic targets. There is an unmet need to unravel
post-COVID-19-associated factors in the early stages in order to
substantially improve the quality of care and therapy. It is
crucial to detect the changes in plasma biomarkers in diverse
cognitive types, since cognitive decline is one of the most
disabling post-COVID effects. Hence, the present review provides an
update the role of circulating miRNAs as diagnostic biomarkers for
COVID-19-related cognitive damage as future therapeutic tools and
prognostic predictors in clinical practice for neurologists.
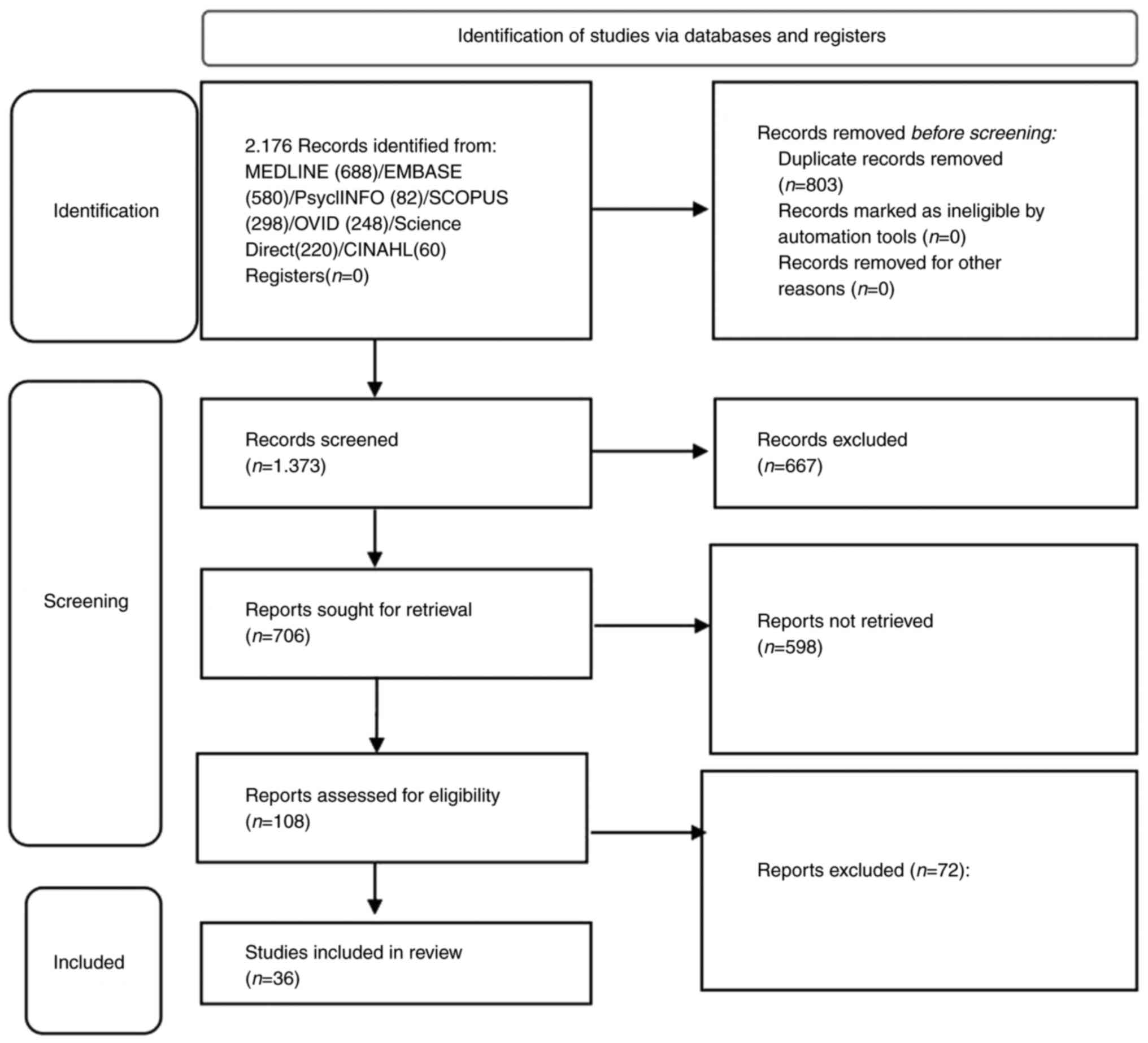
2. Data collection methods
The present study was conducted following the
Patient, Intervention, Comparison, and Outcome (PICO) and Preferred
Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)
guidelines (27). The PRISMA
flowchart used herein (Fig.
1).
Search strategy
A search was performed for related studies using the
Scopus, MEDLINE/PubMed, Embase, OVID, Science Direct, LILACS and
EBSCO databases, using these medical subject headings (MeSH Terms):
‘COVID-19’ OR ‘SARS-CoV-2’ OR ‘post-COVID-19 effects’ OR ‘cognitive
decline’ OR ‘neurodegeneration’ OR ‘miRNAs’ OR ‘Lt-7b’, OR
‘miR-31’, OR ‘miR-155’, OR ‘miR-21’. The articles derived from this
search were subjected to a further selection process for
relevancy.
Inclusion and exclusion criteria,
study quality and risk of bias
The quality of scientific evidence was classified as
high, moderate, low, or very low, according to the GRADE scale
(28). The inclusion criteria were
as follows: 1) Human biological samples; ii) a sample size >15
biological samples; iii) studies with an accuracy (%) of
quantitative polymerase chain reaction (qPCR) measurements >50%;
iv) studies controlled by biological samples from patients with
Alzheimer's disease (AD), mild cognitive impairment (MCI),
frontotemporal lobar degeneration and dementia with Lewy bodies.
The exclusion criteria included reviews, meta-analyses, theses and
case reports. Of note, only qualitative syntheses was performed,
due to the high heterogeneity and small sample size of the studies,
exposure assessment methods and covariate adjustments. The main
miRNAs in cognitive decline related to COVID-19 and the relevant
pathophysiological mechanisms are summarized in Fig. 2.
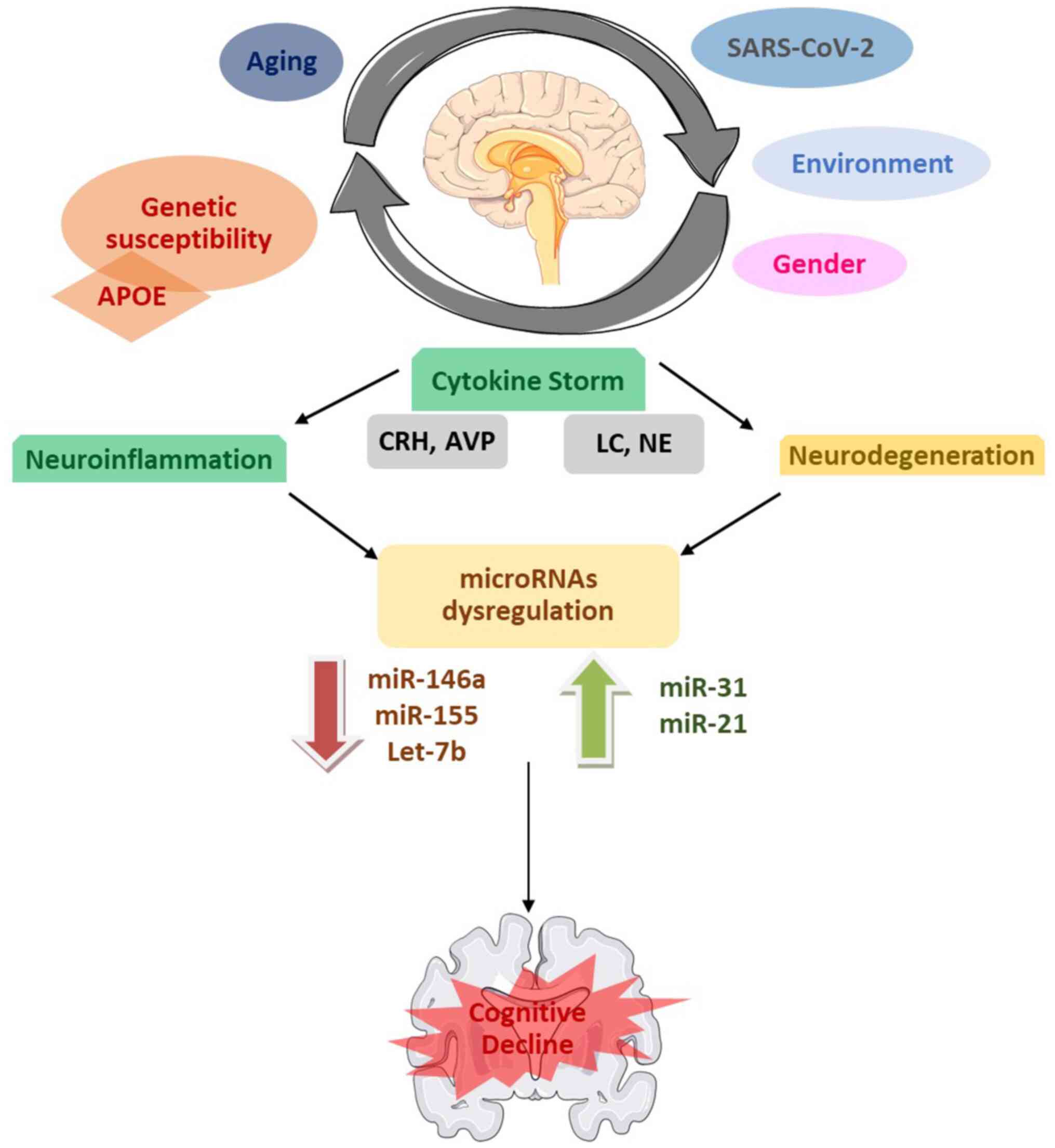 | Figure 2Schematic diagram providing a visual
summary of the key findings, including the main miRNAs studied, and
their associations with cognitive dysfunction and other diseases
and co-existing factors. Parts of this image were derived from the
free medical site http://smart.servier.com/ (accessed on November 15,
2023) by Servier, licensed under a Creative Commons Attribution 3.0
Unported Licence. miRNA/miR, microRNA; APOE, apolipoprotein E; CRH,
corticotrophin-releasing hormone; AVP, arginine-vasopressin; LC,
locus coeruleus; NE, norepinephrine; SARS-CoV-2, severe acute
respiratory syndrome coronavirus 2. |
3. miR-146a
Exosomal miR-146a (Fig.
3) originates from bone marrow-derived mesenchymal stem cells
and is then taken up by activated astrocytes of the hippocampal
region, indicating a neuroprotective role in seizure-associated
cognitive damage (29). Lower
blood levels of circulating miR-146a in AD cause neuroinflammation
(30). The overexpression of
miR146a in microglia has been found to play a neuroprotective role
in learning/memory issues, diminished neuroinflammation and amyloid
plaque by lessening NF-κB nuclear translocation and therefore,
T-cell adhesion molecules. miR-146a also affects microglial
phenotype switching, decreasing the levels of pro-inflammatory
cytokines, and enhancing the clearance of β-amyloid and tau
(31). Thus, serum miR-146a may be
considered a biomarker for the progression of AD (32,33).
The downregulation of microRNA-146a in chronic diseases, such as
diabetes, obesity and hypertension may be associated with severe
COVID-19(34). The overproduction
of IL-6 inactivates anti-COVID drugs, such as tocilizumab (35). However, larger longitudinal studies
are required to elucidate the pathogenetic paths of the function of
miR-146a in the COVID-19-mediated cognitive decline as a
circulating prognostic biomarker, given its overexpression in
dementia and SARS-CoV-2 infection, as well as its role as an
anti-neuroinflammatory, therefore, therapeutic tool.
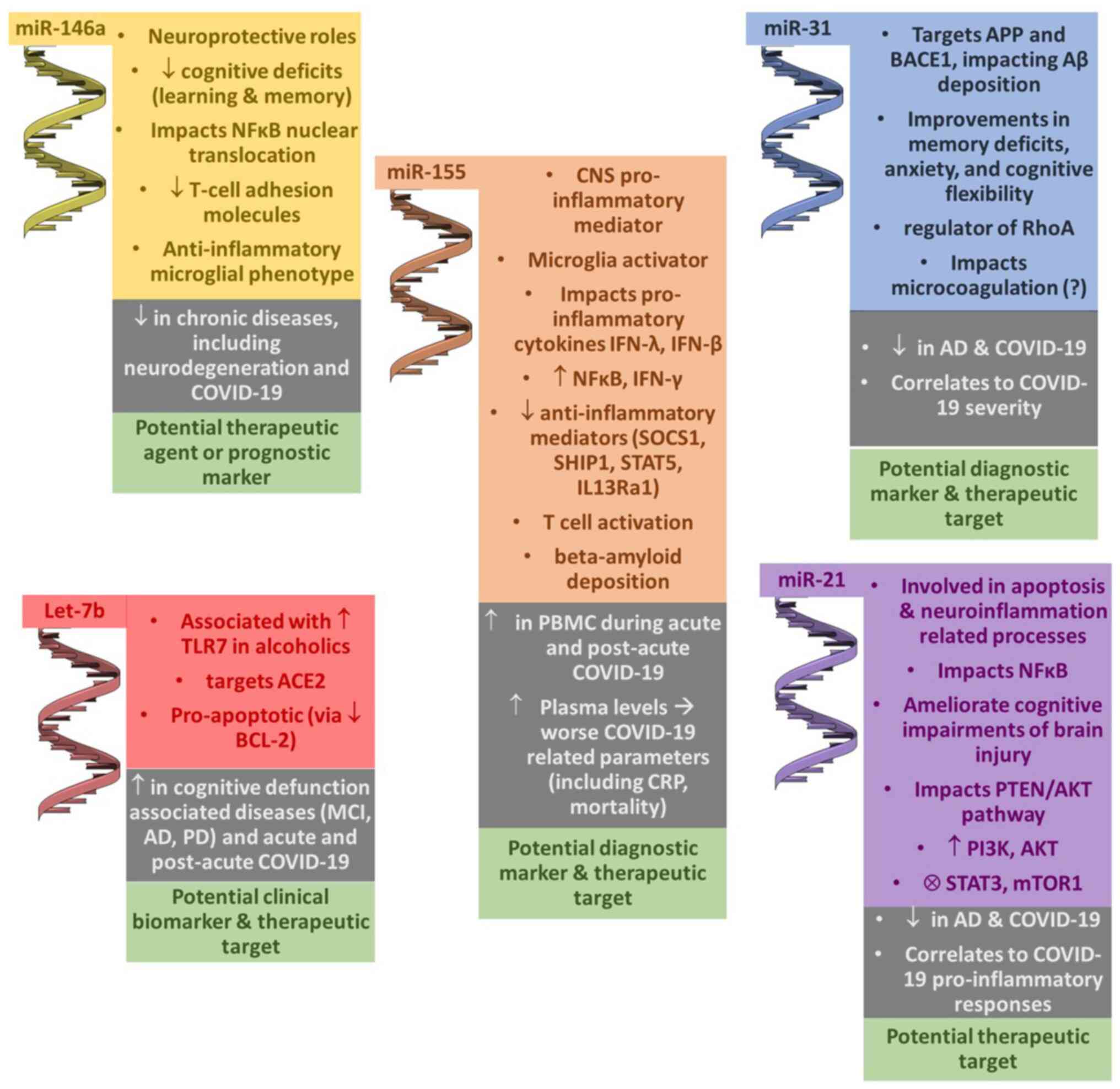 | Figure 3Schematic diagram of the main miRNAs
studied and their associations with cognitive dysfunction and
physiopathological and molecular processes, and other diseases and
observations in patients with COVID-19; potential uses as of miRNAs
as markers or therapeutic targets. Please refer to the text for
further details. Parts of this image derived from the free medical
site http://smart.servier.com/ (accessed on
November 15, 2023) by Servier, licensed under a Creative Commons
Attribution 3.0 Unported Licence. ↓, decrease; ↑, increase; Ⓧ,
inhibition; ACE2, angiotensin-converting enzyme 2; AD, Alzheimer's
disease; AKT, protein kinase B; BACE1, beta-site amyloid precursor
protein cleaving enzyme 1; BCL2, B-cell lymphoma 2; CNS, central
nervous system; IFN, interferon; IL13Ra1, IL-13 receptor alpha 1;
MCI, mild cognitive impairment; mTOR1, mammalian target of
rapamycin 1; NF-κB, nuclear factor κ-light-chain-enhancer of
activated B cells; PD, Parkinson's disease; PI3K, phosphoinositide
3-kinase; PTEN, phosphatase and tensin homolog; RHoA, Ras homolog
family member A; SHIP1, SH2 domain-containing inositol
5'-phosphatase 1; SOCS1, suppressor of cytokine signaling 1; STAT5,
signal transducer and activator of transcription 5; TLR7, Toll-like
receptor 7. |
4. miR-155
In addition to hyperphosphorylated tau protein, the
primary expressed chromosome 21 miRNA in Down's syndrome-related
dementia is miR-155 overexpression in the CNS (36). By regulating inflammatory
cytokines, such as interferon (IFN)-λ and IFN-β, miR-155 functions
as a crucial modulator of pro-inflammatory responses in the CNS and
activates microglia (Fig. 3). The
inflammatory effect of miR-155 in the CNS is mediated in
macrophages and microglia by NF-κB following the releasee of IFN-γ.
Targeting anti-inflammatory regulators, such as SH2
domain-containing inositol-5'-phosphatase 1, activator protein 1,
signal transducer and activator of transcription 5, suppressor of
cytokine signaling 1 and IL-13 receptor alpha 1 may exacerbate
inflammation and compromise the anti-inflammatory response
(37). Additionally linked to the
advancement of cognitive impairment, is the stimulation of CNS
T-cell responses by miR-155. β-amyloid accumulation and consequent
cognitive impairment are caused by T-cell activation, IFN-γ
production and CNS infiltration (38). In mouse models of traumatic brain
injury, knockout experiments have demonstrated that miR-155 exerts
a pro-neuroinflammatory effect by reducing neuroinflammation,
improving cognition and promoting rapid recovery (39).
Moreover, increased concentrations of miR-155 have
been found in blood-derived monocytes and monocyte-derived
macrophages, demonstrating an inadequate cell migration and the
clearance of β-amyloid in patients with AD (40). Promoting neuroinflammation and
blood-brain barrier membrane permeability to neuroinflammatory
cells, miR-155 likely leads to brain atrophy and progressive
cognitive impairment (41).
Elevated levels of serum miR-155 in peripheral blood mononuclear
cells (PBMCs) from individuals experiencing acute COVID-19
infection suggest its potential as a COVID-19 diagnostic biomarker
(42). Similarly, during the
post-acute COVID-19 phase, miR-155 expression has been found to be
elevated in PBMCs from patients with COVID-19(43). Furthermore, there appears to be a
substantial association between plasma miR-155 levels and clinical
markers of COVID, including biochemical results and chest computed
tomography scans. miR-155 has been shown to have a high sensitivity
(90%) and specificity (100%) as a biomarker for the identification
of COVID-19, and a 76% sensitivity and specificity for the severity
of COVID-19(44).
On the whole, the overexpression of miR-155 in
SARS-CoV-2 infection may partially define the abnormal immune
response provoking CNS dysfunction in the frame of COVID-related
cognitive decline.
5. Let-7b
Increased Let-7b is a multipurpose miRNA that is
differentially expressed in different types of cognitive decline,
such as MCI (45), AD (46) and PD (47) (Fig.
3). Increased Toll-like receptor 7 and Let-7b expression have
been linked to the postmortem hippocampal atrophy of alcoholics
(48). As a potential clinical
biomarker for COVID-19 infection, let-7b levels have been found to
be higher in peripheral blood samples during both the acute and
post-acute phases of SARS-CoV-2 infection when compared to healthy
controls (43). Let-7b is a
possible therapeutic weapon against SARS-CoV-2 infection, since it
dysregulates angiotensin-converting enzyme 2 and does not increase
susceptibility to the virus (49).
The overexpression of the Let-7b may result in the downregulation
of BCL-2 duringCOVID-9 by adjusting the immune responses between
chronic inflammatory disease, type 2 diabetes, COVID-19 and
cognitive impairment (50), which
may explain the cognitive dysfunction in these patients. These
upregulations of Let-7b in SARS-CoV-2 infection are signs of the
possible association with cognitive decline; however, small samples
and a lack of other respiratory virus-infected groups are major
limitations.
6. miR-31
Lower serum levels of miR-31 may be useful as a
novel non-invasive biomarker with miR-93 and miR-146a to
distinguish AD from vascular dementia (51) (Fig.
3). The lentiviral delivery of miR-31 has been shown to
attenuate AD neuropathology by decreasing β-amyloid deposition in
both the hippocampus and subiculum of transgenic mouse models
(52). miR-31 targets amyloid
precursor protein (APP) and β-secretase beta-site amyloid precursor
protein cleaving enzyme 1, which further abolishes the pathological
alterations in AD. These findings are indicative of memory
amelioration, reduced anxiety and improved cognitive flexibility,
proposing miR-31 as a therapeutic option of AD. RhoA has been
demonstrated to adjust synaptic plasticity, and the inhibition of
the RhoA pathway prevent damage in synapses and dendritic spines
(53,54). Low serum miR-31 levels have been
noted in patients with COVID-19(55). Low miR31 levels are also indices of
severe COVID-19, increasing microcoagulation, thus functioning as
an essential prognostic biomarker of COVID-19 and severity
(56). Consequently, low levels
ofmiR-31 appear to exert a complex effect on cognitive dysfunction
and SARS-CoV-2 infection, indicating a possible association between
the two disease processes. Conversely, machine learning analysis
has revealed that a three-miRNA signature (miR-423-5p, miR-23a-3p
and miR-195-5p) can independently classify COVID-19 cases with an
accuracy of 99.9% (57). Larger
well-designated studies with a more sophisticated mechanistic
approach are required however, to elucidate the precise role of
miR-31 in the COVID-19-associated cognitive decline.
7. miR-21
miR-21 has been shown to significantly modulate
apoptosis and neuroinflammation in cognitive decline (59) (Fig.
3). The level of miR-21 in plasma-derived extracellular
vesicles is decreased in patients with AD in comparison to those
with Lewy body dementia and healthy individuals (60). By functioning as a negative
feedback modulator of NF-κB in the anticipation of pro-inflammatory
signaling, miR-21 exhibits anti-inflammatory miRNA behavior
(61). miR-21 improves dementia
associated with brain injury from subarachnoid hemorrhage by
modulating the PTEN/AKT pathway and apoptosis in the hippocampus
and prefrontal cortex (62). Cell
culture AD studies of miR-21 mimics found that miR-21 reduced
β-amyloid-induced apoptosis by augmenting the expression of PI3K,
AKT and glycogen synthase kinase 3beta (63,64).
Through the inhibition of the mTOR1 pathway, miR-21
restores neurogenesis and reverses cellular senescence in models of
vascular dementia, rendering it a promising therapeutic agent for
vascular dementia and related cognitive impairment (65). In patients with COVID-19, the
relative expression of miR-21 has been found to be downregulated,
while that of target pro-inflammatory genes is upregulated
(66). The downregulation of
miR-21 in patients with COVID-19 aggravates systemic inflammation
due to the hyperactive immune response and a lack of T-cell
function (67). Increased systemic
inflammation may destroy the blood-brain barrier, generating
increased neuroinflammation and leading to neurodegeneration.
Hence, low miR-21levels may affect the onset and advancement of
COVID-associated cognitive decline or may aggravate pre-existing
dementia following infection with SARS-CoV-2. The main aspects of
the roles of miRNAs included in the present review are summarized
in Table I.
 | Table IMain aspects of the role of miRNAs
included in the present review. |
Table I
Main aspects of the role of miRNAs
included in the present review.
| miRNA | Origin | Role | (Refs.) |
|---|
| miR-146a | Βone marrow
mesenchymal stem cells activated astrocytes | Neuroprotection
anti-neuroinflammatory clearance of β-amyloid and tau | (28-34) |
| miR-155 | Αs a gene that was
transcriptionally activated by promoter insertion in B-cell
lymphomas | Modulator of
inflammatory cytokines (IFN-λ and IFN-β) clearance of
β-amyloid | (35-43) |
| Let-7b | Caenorhabditis
elegans | Postmortem
hippocampal atrophy of alcoholics; ACE2 dysregulation of BCL-2
downregulation | (44-49) |
| miR-31 | As gene is located
on chromosome band 9p21.3 | Abolition of
β-amyloid pathology by targeting APP and BACE1 | (50-56) |
| miR-21 | Human glioblastoma
cells | Inhibitor mTOR1
pathway and β-amyloid-induced apoptosis; neurogenesis | (59-64,67) |
8. Limitations to current research
Lack of comprehensive miRNA profiling
studies
One of the limitations of current research on the
role of miRNAs in cognitive decline related to COVID-19 is the lack
of comprehensive miRNA profiling studies. Rigorously designated
clinical cohorts with convenient matched controls provide
measurements of the whole repertoire, such as serum, plasma, CSF,
urine, saliva and exosomes, if feasible. The majority of the
existing studies investigating the dysregulation of miRNAs in
COVID-19-related cognitive impairment have focused on a limited
number of miRNAs. It is crucial to conduct comprehensive profiling
studies to identify specific miRNAs that are dysregulated in the
context of cognitive decline related to COVID-19. Such studies
should encompass a wide range of miRNAs, considering their
potential role in neuronal function and neuroinflammation. There is
a paucity of comprehensive biofluids analyses assessing CSF, blood
levels of multiple inflammatory markers along with CSF levels of
other neurodegenerative RNA proteins (apart from miRNAs) as
potential biomarkers, such as circular RNAs for comparison.
Inadequate animal models
Another limitation is the inadequacy of relevant
animal models for studying the neurological consequences of
COVID-19. Current animal models may not fully replicate the complex
neurological symptoms and cognitive decline experienced by patients
with COVID-19. Therefore, it is essential to develop more accurate
animal models that can more closely mimic the long-term
neurological complications observed in patients with COVID-19,
facilitating a better understanding of the molecular mechanisms
underlying cognitive impairment.
Limited longitudinal studies
The limited number of longitudinal studies focusing
on the long-term consequences of COVID-19 is also a significant
barrier. Rigorously designated clinical cohorts with convenient
matched controls provide measurements of the whole repertoire, such
as serum, plasma, CSF, urine, saliva and exosomes, if feasible.
Longitudinal studies are vital for tracing the natural history of
COVID-19-related cognitive decline, monitoring changes in cognitive
function over time, and identifying potential biomarkers for
cognitive impairment. Such studies would provide valuable insight
into the progression and persistence of cognitive decline in
COVID-19 survivors.
Challenges in miRNA detection and
quantification
The methodologies used for miRNA detection and
quantification present challenges in terms of standardization and
accuracy. Current techniques used, such as RT-qPCR and small RNA
sequencing have limitations in terms of sensitivity and
specificity. The methods of RNA and exosome isolation, and
downstream miRNA detection, quantification and normalization
methods vary between studies, such as enzyme-linked immunosorbent
assay, western blotting and mass spectrometry, leading to
conflicting results. Standardizing methods is essential for
ensuring reliable and reproducible results across different
studies. Robust and validated miRNA detection and quantification
methods are imperative for identifying reliable biomarkers of
cognitive decline related to COVID-19.
9. Recommendations for future research
Comprehensive miRNA profiling
studies
Future research should prioritize conducting
comprehensive miRNA profiling studies in patients with COVID-19
with cognitive impairment. These studies can be performed on
biological samples, such as plasma, CSF, or brain tissue and should
encompass a wide array of miRNAs. Utilizing advanced
high-throughput sequencing techniques, such as small RNA
sequencing, will enable the identification of specific miRNAs that
are dysregulated in COVID-19-related cognitive decline, allowing
for a more detailed characterization of the molecular pathways
involved.
Functional role studies
To enhance the understanding of the molecular
mechanisms underlying cognitive decline related to COVID-19, future
research should focus on elucidating the functional roles of
dysregulated miRNAs in the pathogenesis of cognitive impairment.
Utilizing in vitro and in vivo models, researchers
can assess the impact of miRNA dysregulation on neuroinflammation,
neurodegeneration and cognitive function. Understanding the
functional roles of miRNAs may lead to the identification of
potential therapeutic targets and the development of miRNA-based
interventions to mitigate cognitive decline.
Development of accurate animal
models
Efforts should be directed towards establishing
accurate animal models that accurately mimic the neurological
consequences of COVID-19. Such models should replicate the
neuroinflammatory and neurodegenerative processes observed in
patients with COVID-19. The utilization of genetically modified
animal models or non-human primates infected with SARS-CoV-2 may
provide a more thorough understanding of the pathophysiology
underlying COVID-19-related cognitive decline, facilitating the
evaluation of potential therapeutic strategies.
Longitudinal studies on COVID-19
survivors
Conducting well-designed longitudinal studies on
COVID-19 survivors to investigate the long-term neurological
sequelae is imperative. These studies should involve comprehensive
neurological assessments, including cognitive function tests, brain
imaging and biomarker analysis. Human studies in cohorts with
cognitive decline and genetic predisposition (e.g., APOE) or the
prodromal type of dementia are in their infancy, without any
longitudinal studies reported to date, at least to the best of our
knowledge. The data collected from longitudinal studies may provide
valuable insight into the persistence of cognitive decline, the
nature of cognitive deterioration and the identification of
potential prognostic factors or biomarkers.
Standardization of miRNAs
Researchers should work towards standardizing and
validating miRNA detection and quantification methods. To detect
miRNAs, sensitive assays need to be standardized, validated and
developed quantitatively, so that they can possibly be used for the
assessment of disease progression and the response to
disease-modifying therapies. The development and adoption of
standardized protocols for miRNA analysis, as well as the
implementation of quality control measures, will enhance the
accuracy and reproducibility of miRNA-based studies. Robustly
validated protocols are required to measure miRNAs in PD samples,
taking into account iteration and intra-variability. This
standardization is critical for generating robust and reliable
miRNA biomarkers for cognitive decline related to COVID-19.
10. Conclusions
The notion related to cognitive damage and COVID-19
is multifactorial; however, large studies to explore this notion
are lacking. The discovery of miRNAs as promising biomarkers could
accumulate with crucial data for classifying these patients,
ameliorating primary care, and providing state-of-the-art
personalized therapy in relation to the cognitive decline of the
patient. The present review provides an update of the
neuropathological COVID-19 related mechanisms and highlights the
importance of using circulating miRNAs biomarkers to elucidate
these mechanisms. It should be emphasized that thus far, there are
no miRNAs that are specific for the different types of
dementia.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AB and VEG conceptualized the study. IGL, VEG, PP,
EA, NT, PS, GF, AB and DAS made a substantial contribution to the
interpretation and analysis of data from studies to be included in
the present review and wrote and prepared the draft of the
manuscript. DAS and AB analyzed the data from the studies to be
included in the present review, and provided critical revisions.
All authors contributed to manuscript revision and have read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Georgakopoulou VE, Gkoufa A, Damaskos C,
Papalexis P, Pierrakou A, Makrodimitri S, Sypsa G, Apostolou A,
Asimakopoulou S, Chlapoutakis S, et al: COVID-19-associated acute
appendicitis in adults. A report of five cases and a review of the
literature. Exp Ther Med. 24(482)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khatoon F, Prasad K and Kumar V: COVID-19
associated nervous system manifestations. Sleep Med. 91:231–236.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gordon MN, Heneka MT, Le Page LM,
Limberger C, Morgan D, Tenner AJ, Terrando N, Willette AA and
Willette SA: Impact of COVID-19 on the onset and progression of
Alzheimer's disease and related dementias: A roadmap for future
research. Alzheimers Dement. 18:1038–1046. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Graham EL, Clark JR, Orban ZS, Lim PH,
Szymanski AL, Taylor C, DiBiase RM, Jia DT, Balabanov R, Ho SU, et
al: Persistent neurologic symptoms and cognitive dysfunction in
non-hospitalized Covid-19 ‘long haulers’. Ann Clin Transl Neurol.
8:1073–1085. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
de Erausquin GA, Snyder H, Carrillo M,
Hosseini AA, Brugha TS and Seshadri S: CNS SARS-CoV-2 Consortium.
The chronic neuropsychiatric sequelae of COVID-19: The need for a
prospective study of viral impact on brain functioning. Alzheimers
Dement. 17:1056–1065. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Manzo C, Serra-Mestres J, Isetta M and
Castagna A: Could COVID-19 anosmia and olfactory dysfunction
trigger an increased risk of future dementia in patients with
ApoE4? Med Hypotheses. 147(110479)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li Z, Zhang Z, Zhang Z, Wang Z and Li H:
Cognitive impairment after long COVID-19: Current evidence and
perspectives. Front Neurol. 14(1239182)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Biomarkers Definitions Working Group.
Biomarkers and surrogate endpoints: Preferred definitions and
conceptual framework. Clin Pharmacol Ther. 69:89–95.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bougea A: MicroRNA as candidate biomarkers
in atypical parkinsonian syndromes: Systematic literature review.
Medicina (Kaunas). 58(483)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Parnetti L, Farotti L, Eusebi P,
Chiasserini D, De Carlo C, Giannandrea D, Salvadori N, Lisetti V,
Tambasco N, Rossi A, et al: Differential role of CSF
alpha-synuclein species, tau, and Aβ42 in Parkinson's disease.
Front Aging Neurosci. 6(53)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vivacqua G, Latorre A, Suppa A, Nardi M,
Pietracupa S, Mancinelli R, Fabbrini G, Colosimo C, Gaudio E and
Berardelli A: Abnormal Salivary Total and Oligomeric
Alpha-Synuclein in Parkinson's Disease. PLoS One.
11(e0151156)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Eusebi P, Giannandrea D, Biscetti L,
Abraha I, Chiasserini D, Orso M, Calabresi P and Parnetti L:
Diagnostic utility of cerebrospinal fluid α-synuclein in
Parkinson's disease: A systematic review and meta-analysis. Mov
Disord. 32:1389–1400. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bougea A: New markers in Parkinson's
disease. Adv Clin Chem. 96:137–178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bougea A, Koros C and Stefanis L: Salivary
alpha-synuclein as a biomarker for Parkinson's disease: A
systematic review. J Neural Transm (Vienna). 126:1373–1382.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kuo MC, Liu SC, Hsu YF and Wu RM: The role
of noncoding RNAs in Parkinson's disease: Biomarkers and
associations with pathogenic pathways. J Biomed Sci.
28(78)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gebert LFR and MacRae IJ: Regulation of
microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kozomara A, Birgaoanu M and
Griffiths-Jones S: miRBase: From microRNA sequences to function.
Nucleic Acids Res. 47(D1):D155–D162. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Barbagallo C, Mostile G, Baglieri G,
Giunta F, Luca A, Raciti L, Zappia M, Purrello M, Ragusa M and
Nicoletti A: Specific signatures of serum miRNAs as potential
biomarkers to discriminate clinically similar neurodegenerative and
vascular-related diseases. Cell Mol Neurobiol. 40:531–546.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Candido S, Lupo G, Pennisi M, Basile MS,
Anfuso CD, Petralia MC, Gattuso G, Vivarelli S, Spandidos DA, Libra
M and Falzone L: The analysis of miRNA expression profiling
datasets reveals inverse microRNA patterns in glioblastoma and
Alzheimer's disease. Oncol Rep. 42:911–922. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pierouli K, Papakonstantinou E,
Papageorgiou L, Diakou I, Mitsis T, Dragoumani K, Spandidos DA,
Bacopoulou F, Chrousos GP, Goulielmos GΝ, et al: Role of non-coding
RNAs as biomarkers and the application of omics technologies in
Alzheimer's disease (Review). Int J Mol Med. 51(5)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Conti I, Varano G, Simioni C, Laface I,
Milani D, Rimondi E and Neri LM: miRNAs as influencers of cell-cell
communication in tumor microenvironment. Cells.
9(220)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ramaswamy P, Yadav R, Pal PK and
Christopher R: Clinical application of circulating MicroRNAs in
Parkinson's Disease: The challenges and opportunities as diagnostic
biomarker. Ann Indian Acad Neurol. 23:84–97. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ravanidis S, Bougea A, Papagiannakis N,
Koros C, Simitsi AM, Pachi I, Breza M, Stefanis L and Doxakis E:
Validation of differentially expressed brain-enriched microRNAs in
the plasma of PD patients. Ann Clin Transl Neurol. 7:1594–1607.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Roser AE, Caldi Gomes L, Schünemann J,
Maass F and Lingor P: Circulating miRNAs as diagnostic biomarkers
for Parkinson's disease. Front Neurosci. 12(625)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ravanidis S, Bougea A, Papagiannakis N,
Maniati M, Koros C, Simitsi AM, Bozi M, Pachi I, Stamelou M,
Paraskevas GP, et al: Circulating Brain-enriched MicroRNAs for
detection and discrimination of idiopathic and genetic Parkinson's
disease. Mov Disord. 35:457–467. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schulz J, Takousis P, Wohlers I, Itua IOG,
Dobricic V, Rücker G, Binder H, Middleton L, Ioannidis JPA,
Perneczky R, et al: Meta-analyses identify differentially expressed
micrornas in Parkinson's disease. Ann Neurol. 85:835–851.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Atkins D, Best D, Briss PA, Eccles M,
Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry
D, et al: Grading quality of evidence and strength of
recommendations. BMJ. 328(1490)2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kong H, Yin F, He F, Omran A, Li L, Wu T,
Wang Y and Peng J: The Effect of miR-132, miR-146a, and miR-155 on
MRP8/TLR4-Induced astrocyte-related inflammation. J Mol Neurosci.
57:28–37. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lukiw WJ: microRNA-146a Signaling in
Alzheimer's Disease (AD) and Prion Disease (PrD). Front Neurol.
11(462)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liang C, Zou T, Zhang M, Fan W, Zhang T,
Jiang Y, Cai Y, Chen F, Chen X, Sun Y, et al: MicroRNA-146a
switches microglial phenotypes to resist the pathological processes
and cognitive degradation of Alzheimer's disease. Theranostics.
11:4103–4121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kumar S and Reddy PH: Are circulating
microRNAs peripheral biomarkers for Alzheimer's disease? Biochim
Biophys Acta. 1862:1617–1627. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Maffioletti E, Milanesi E, Ansari A,
Zanetti O, Galluzzi S, Geroldi C, Gennarelli M and
Bocchio-Chiavetto L: miR-146a Plasma Levels Are Not Altered in
Alzheimer's Disease but Correlate With Age and Illness Severity.
Front Aging Neurosci. 11(366)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Roganović J: Downregulation of
microRNA-146a in diabetes, obesity and hypertension may contribute
to severe COVID-19. Med Hypotheses. 146(110448)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sabbatinelli J, Giuliani A, Matacchione G,
Latini S, Laprovitera N, Pomponio G, Ferrarini A, Svegliati Baroni
S, Pavani M, Moretti M, et al: Decreased serum levels of the
inflammaging marker miR-146a are associated with clinical
non-response to tocilizumab in COVID-19 patients. Mech Ageing Dev.
193(111413)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tili E, Mezache L, Michaille JJ, Amann V,
Williams J, Vandiver P, Quinonez M, Fadda P, Mikhail A and Nuovo G:
microRNA 155 up regulation in the CNS is strongly correlated to
Down's syndrome dementia. Ann Diagn Pathol. 34:103–109.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zingale VD, Gugliandolo A and Mazzon E:
MiR-155: An important regulator of neuroinflammation. Int J Mol
Sci. 23(90)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Song J and Lee JE: miR-155 is involved in
Alzheimer's disease by regulating T lymphocyte function. Front
Aging Neurosci. 7(61)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Henry RJ, Doran SJ, Barrett JP, Meadows
VE, Sabirzhanov B, Stoica BA, Loane DJ and Faden AI: Inhibition of
miR-155 limits neuroinflammation and improves functional recovery
after experimental traumatic brain injury in mice.
Neurotherapeutics. 16:216–230. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Guedes JR, Santana I, Cunha C, Duro D,
Almeida MR, Cardoso AM, de Lima MC and Cardoso AL: MicroRNA
deregulation and chemotaxis and phagocytosis impairment in
Alzheimer's disease. Alzheimers Dement (Amst). 3:7–17.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Varma-Doyle AV, Lukiw WJ, Zhao Y, Lovera J
and Devier D: A hypothesis-generating scoping review of miRs
identified in both multiple sclerosis and dementia, their protein
targets, and miR signaling pathways. J Neurol Sci.
420(117202)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Abbasi-Kolli M, Sadri Nahand J, Kiani SJ,
Khanaliha K, Khatami A, Taghizadieh M, Torkamani AR, Babakhaniyan K
and Bokharaei-Salim F: The expression patterns of MALAT-1, NEAT-1,
THRIL, and miR-155-5p in the acute to the post-acute phase of
COVID-19 disease. Braz J Infect Dis. 26(102354)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Donyavi T, Bokharaei-Salim F, Baghi HB,
Khanaliha K, Alaei Janat-Makan M, Karimi B, Sadri Nahand J, Mirzaei
H, Khatami A, Garshasbi S, et al: Acute and post-acute phase of
COVID-19: Analyzing expression patterns of miRNA-29a-3p, 146a-3p,
155-5p, and let-7b-3p in PBMC. Int Immunopharmacol.
97(107641)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Haroun RA, Osman WH, Amin RE, Hassan AK,
Abo-Shanab WS and Eessa AM: Circulating plasma miR-155 is a
potential biomarker for the detection of SARS-CoV-2 infection.
Pathology. 54:104–110. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kenny A, McArdle H, Calero M, Rabano A,
Madden SF, Adamson K, Forster R, Spain E, Prehn JHM, Henshall DC,
et al: Elevated Plasma microRNA-206 levels predict cognitive
decline and progression to dementia from mild cognitive impairment.
Biomolecules. 9(734)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Leidinger P, Backes C, Deutscher S,
Schmitt K, Mueller SC, Frese K, Haas J, Ruprecht K, Paul F, Stähler
C, et al: A blood based 12-miRNA signature of Alzheimer disease
patients. Genome Biol. 14(R78)2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Huang Y, Liu Y, Huang J, Gao L, Wu Z, Wang
L and Fan L: Let-7b-5p promotes cell apoptosis in Parkinson's
disease by targeting HMGA2. Mol Med Rep. 24(820)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Coleman LG Jr, Zou J and Crews FT:
Microglial-derived miRNA let-7 and HMGB1 contribute to
ethanol-induced neurotoxicity via TLR7. J Neuroinflammation.
14(22)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bellae Papannarao J, Schwenke DO, Manning
P and Katare R: Upregulated miR-200c is associated with
downregulation of the functional receptor for severe acute
respiratory syndrome coronavirus 2 ACE2 in individuals with
obesity. Int J Obes (Lond). 46:238–241. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Islam MB, Chowdhury UN, Nain Z, Uddin S,
Ahmed MB and Moni MA: Identifying molecular insight of synergistic
complexities for SARS-CoV-2 infection with pre-existing type 2
diabetes. Comput Biol Med. 136(104668)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dong H, Li J, Huang L, Chen X, Li D, Wang
T, Hu C, Xu J, Zhang C, Zen K, et al: Serum MicroRNA profiles serve
as novel biomarkers for the diagnosis of Alzheimer's disease. Dis
Markers. 2015(625659)2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Barros-Viegas AT, Carmona V, Ferreiro E,
Guedes J, Cardoso AM, Cunha P, Pereira de Almeida L, Resende de
Oliveira C, Pedro de Magalhães J, Peça J and Cardoso AL: miRNA-31
improves cognition and abolishes amyloid-β pathology by targeting
APP and BACE1 in an animal model of Alzheimer's disease. Mol Ther
Nucleic Acids. 19:1219–1236. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Pearn ML, Schilling JM, Jian M, Egawa J,
Wu C, Mandyam CD, Fannon-Pavlich MJ, Nguyen U, Bertoglio J, Kodama
M, et al: Inhibition of RhoA reduces propofol-mediated growth cone
collapse, axonal transport impairment, loss of synaptic
connectivity, and behavioural deficits. Br J Anaesth. 120:745–760.
2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Qian H, Shang Q, Liang M, Gao B, Xiao J,
Wang J, Li A, Yang C, Yin J, Chen G, et al: MicroRNA-31-3p/RhoA
signaling in the dorsal hippocampus modulates
methamphetamine-induced conditioned place preference in mice.
Psychopharmacology (Berl). 238:3207–3219. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bautista-Becerril B, Pérez-Dimas G,
Sommerhalder-Nava PC, Hanono A, Martínez-Cisneros JA,
Zarate-Maldonado B, Muñoz-Soria E, Aquino-Gálvez A,
Castillejos-López M, Juárez-Cisneros A, et al: miRNAs, from
evolutionary junk to possible prognostic markers and therapeutic
targets in COVID-19. Viruses. 14(41)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Keikha R, Hashemi-Shahri SM and Jebali A:
The relative expression of miR-31, miR-29, miR-126, and miR-17 and
their mRNA targets in the serum of COVID-19 patients with different
grades during hospitalization. Eur J Med Res. 26(75)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Farr RJ, Rootes CL, Rowntree LC, Nguyen
THO, Hensen L, Kedzierski L, Cheng AC, Kedzierska K, Au GG, Marsh
GA, et al: Altered microRNA expression in COVID-19 patients enables
identification of SARS-CoV-2 infection. PLoS Pathog.
17(e1009759)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bai X and Bian Z: MicroRNA-21 Is a
versatile regulator and potential treatment target in central
nervous system disorders. Front Mol Neurosci.
15(842288)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Gámez-Valero A, Campdelacreu J, Vilas D,
Ispierto L, Reñé R, Álvarez R, Armengol MP, Borràs FE and Beyer K:
Exploratory study on microRNA profiles from plasma-derived
extracellular vesicles in Alzheimer's disease and dementia with
Lewy bodies. Transl Neurodegener. 8(31)2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ma X, Becker Buscaglia LE, Barker JR and
Li Y: MicroRNAs in NF-kappaB signaling. J Mol Cell Biol. 3:159–166.
2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Gao X, Xiong Y, Li Q, Han M, Shan D, Yang
G, Zhang S, Xin D, Zhao R, Wang Z, et al: Extracellular
vesicle-mediated transfer of miR-21-5p from mesenchymal stromal
cells to neurons alleviates early brain injury to improve cognitive
function via the PTEN/Akt pathway after subarachnoid hemorrhage.
Cell Death Dis. 11(363)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Feng MG, Liu CF, Chen L, Feng WB, Liu M,
Hai H and Lu JM: MiR-21 attenuates apoptosis-triggered by amyloid-β
via modulating PDCD4/PI3K/AKT/GSK-3β pathway in SH-SY5Y cells.
Biomed Pharmacother. 101:1003–1007. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wang Y and Chang Q: MicroRNA miR-212
regulates PDCD4 to attenuate Aβ25-35-induced
neurotoxicity via PI3K/AKT signaling pathway in Alzheimer's
disease. Biotechnol Lett. 42:1789–1797. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Blount GS, Coursey L and Kocerha J:
MicroRNA networks in cognition and dementia. Cells.
11(1882)2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Keikha R, Hashemi-Shahri SM and Jebali A:
The miRNA neuroinflammatory biomarkers in COVID-19 patients with
different severity of illness. Neurologia (Engl Ed). 38:e41–e51.
2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Tang H, Gao Y, Li Z, Miao Y, Huang Z, Liu
X, Xie L, Li H, Wen W, Zheng Y and Su W: The noncoding and coding
transcriptional landscape of the peripheral immune response in
patients with COVID-19. Clin Transl Med. 10(e200)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Nersisyan S, Shkurnikov M, Turchinovich A,
Knyazev E and Tonevitsky A: Integrative analysis of miRNA and mRNA
sequencing data reveals potential regulatory mechanisms of ACE2 and
TMPRSS2. PLoS One. 15(e0235987)2020.PubMed/NCBI View Article : Google Scholar
|