Introduction
Diabetes mellitus (DM) is a disorder of glucose
metabolism characterized by hyperglycemia due to insufficient
insulin or insulin resistance, and it is estimated to afflict 439
million individuals worldwide by 2030 due to lifestyle changes,
such as extensive sitting and high fat diet (1). Chronic DM is associated with
dysfunction and failure of most organs in the body such as the
eyes, feet, nervous system, kidneys and liver (2).
Diabetic nephropathy (DN), a complication of
diabetic microangiopathy, is a major cause of end-stage renal
disease (3,4). Patients with DN have sustained
proteinuria and exhibit a glomerular filtration rate in progressive
decline when it is measured twice with an interval of 3-6 months,
which is often associated with increased blood pressure and can
eventually lead to end-stage renal disease (5,6).
Liver fibrosis is another common complication that is associated
with DM (7,8). It has been reported that the rate of
advanced liver fibrosis is higher in individuals with diabetes
compared with in those without diabetes (9). Furthermore, there is an intricate
association between DM and non-alcoholic fatty liver disease
(NAFLD) (10). NAFLD is
characterized by a wide range of liver diseases, from benign
steatosis, to inflammation, fibrosis, cirrhosis, liver failure and
finally, hepatocellular carcinoma. However, this series of
pathological processes is not associated with excessive alcohol,
drugs or viral factors (11-14).
Evidence has suggested that patients diagnosed with NAFLD have a
two-fold increased risk of developing DM, particularly in those
with tumors, cardiovascular disease and renal disease (15,16).
Furthermore, studies have indicated that diabetes is an independent
risk factor for NAFLD (17), and
women with a history of gestational diabetes have an increased risk
of NAFLD (18,19). Conversely, remission of hepatic
steatosis may prevent the development of diabetes (20,21).
From the aforementioned evidence, it was hypothesized that there
may be sex differences in liver and kidney injury induced by
diabetes. Therefore, db/db mice are spontaneous type 2 diabetic
mice caused by Leptin receptor gene deficiency, and its
pathogenesis is very similar to that of human type 2 diabetes so
they were selected as a model of experimental diabetes in the
present study to observe liver and kidney impairment by
pathological sectioning to assess whether there are sex differences
in tissue injury.
Materials and methods
Equipment and reagents
An advantage blood glucose meter and strips from
Roche Diagnostics GmbH, Harris's hematoxylin and eosin (H&E)
stain from Zhuhai Besso Biotechnology Co., Ltd. and a Masson
staining kit (cat. no. G1340) from Beijing Solarbio Science &
Technology Co., Ltd. were used in the present study. Furthermore,
an optical microscope from Olympus Soft Imaging Solutions GmbH
(BX53), the Hitachi H-7650 transmission electron microscope
(Hitachi High-Technologies Corporation), the Leica EG1150H paraffin
embedding machine (Leica Microsystems GmbH) and the Leica Leitz
1512 microtome (Leica Microsystems GmbH) were also used.
Animals
Male and female db/db (37-46 and 36-44 g,
respectively) and db/m C57BLKS/J (BKS) (18-21 and 17-20 g,
respectively) mice (8-9 weeks; total no., 32; n=8 for each group)
were purchased from Changzhou Cavens Experimental Animal Co., Ltd.
[experimental animal production license: : scxk (Su) 2016-0010] and
housed in the Medical Experimental Animal Center of North China
University of Science and Technology (Tangshan, China). db/db
(C57BL/KSJ) mice with Leptin receptor point mutation are homozygous
(-/-), db/m is heterozygous (+/-), WT is m/m mice (+/+). They were
provided with Co60-irradiated chow and water, ad libitum.
The animal experiments were performed in specific-pathogen-free
barrier laboratory at the Experimental Animal Centre of North China
University of Science and Technology (Tangshan, China). The room
temperature was maintained at 23±1˚C (humidity, 55±10%) and there
was a 12-h light/dark cycle. The diabetic model was defined as a
blood glucose level >16.67 mmol/l. The random blood glucose
concentration was periodically measured from the tail vein blood,
using a scalpel to scrape through a small portion of skin at the
end of the tail of each mouse. The volume of blood taken each time
was 0.6 µl, and thus the damage to the experimental animal was
minimal. Blood glucose testing was conducted at 0, 1, 3, 5, 6,7
weeks. The mice were starved for 8 h to carry out the oral glucose
tolerance test (OGTT) at 4 weeks. Blood glucose levels were
measured after glucose administration (i.g, 200 mg/kg) at time
points of 0, 30, 60 and 120 min. Due to the short interval, that
the tail was only scraped once before the blood samples were
collected at the different time points. After feeding for 8 weeks,
the mice were anaesthetized using ether, and then euthanized by
cervical dislocation. Liver and kidney tissue specimens were
dissected and fixed for 24 h at 4˚C in 4% neutral formaldehyde for
paraffin embedding or in 2.5% glutaraldehyde for electron
microscopy. The present study was approved by The Experimental
Animal Ethics Committee of North China University of Science and
Technology (Tangshan, China).
Histological experiments
Tissue sections of 5-µm thickness were prepared,
stained with Harris's hematoxylin for 20 min, washed three times in
distilled water and differentiated with 1% hydrochloric
acid-alcohol for 3 sec. The sections were then washed an additional
three times in distilled water, stained with eosin for 10 min,
dehydrated using an ethanol gradient, clarified using xylene,
sealed with neutral gum and examined using light microscopy (all
performed at room temperature) (22). All steps were performed at room
temperature and Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.) was used for analysis.
Other sections were dewaxed, fixed using Bouin's
solution (Nanjing SenBeiJia Biological Technology Co., Ltd.) for 10
min and then, following the operation of a Masson staining kit, the
sections were stained with Harris's hematoxylin for 5 min, washed
with tap water for 2 min, differentiated with 0.5% hydrochloric
acid-alcohol for 10 sec and washed with distilled water for 5 min.
The sections were then stained with Masson's complex staining
solution for 5 min, differentiated with 0.2% acetic acid solution
for 5 min, differentiated with 5% phosphomolybdic acid solution for
5 min, rinsed 3 times with 0.2% acetic acid solution and rinsed
with 2% aniline blue solution for 15 sec. Subsequently, the
sections were rinsed with anhydrous ethanol for 15 sec, left for 2
h to dry, sealed with neutral gum and examined using light
microscopy. All steps were performed at room temperature.
Ultrastructural observation of mouse
specimens
Portions of liver and kidney tissues (1 mm³) were
fixed in 2.5% glutaraldehyde for 3 h (4˚C), treated with 1% osmic
acid for 1 h (4˚C), dehydrated using a 50-90% acetone gradient
(4˚C) and embedded in EPON 812 (37˚C). Ultrathin sections (80 nm)
were prepared after orientation under a light microscope. The
sections were examined by transmission electron microscopy after
dual staining with uranium acetate and lead citrate (for 15 min,
respectively) at room temperature (23).
Statistical analysis
SPSS 21.0 software (IBM Corp.) was used to analyze
the data. Normally distributed data are expressed as the mean ±
standard deviation (n=8 for body mass, food and water intake, blood
glucose and OGTT experiment, respectively; n=3 for histology of the
liver and kidney tissues) and comparisons between groups were made
using two-way ANOVA and Tukey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Body mass, food and water intake, and
blood glucose
The weight and food intake of the mice over 7 weeks
were recorded and there were no significant differences between
db/m female and male mice (P>0.05), or db/db female and male
mice (P<0.05) (Tables I and
II). The body mass and food
intake of the db/db mice were significantly higher compared with
those of the db/m mice, irrespective of sex (P<0.05; Tables I and II). In addition, there were no
significant differences in water intake or blood glucose
concentrations between db/m female and male mice. However,
differences in water intake and blood glucose were found between
db/db female and male mice malefemale P<0.05;
Tables III and IV).
 | Table IResults of body weight of mice in
each group (n=8). |
Table I
Results of body weight of mice in
each group (n=8).
| | Body weight
(g) |
|---|
| Group | 0 weeks | 1 week | 3 weeks | 5 weeks | 6 weeks | 7 weeks |
|---|
| db/m male | 21.3±1.12 | 22.8±1.04 | 23.9±1.13 | 25.3±1.21 | 25.0±0.85 | 26.3±0.76 |
| db/m female | 18.6±0.84 | 20.9±1.91 | 23.3±0.90 | 24.1±0.76 | 24.6±0.94 | 25.3±1.36 |
| db/db male |
42.0±2.48a,b |
43.0±2.60a,b |
46.9±1.93a,b |
48.8±1.62a,b |
51.4±1.44a,b |
50.3±2.70a,b |
| db/db female |
40.7±2.02a,b |
43.2±2.46a,b |
46.8±2.34a,b |
50.2±4.58a,b |
50.1±3.28a,b |
49.9±3.53a,b |
 | Table IIResults of daily food intake of mice
in each group (n=8). |
Table II
Results of daily food intake of mice
in each group (n=8).
| | Daily food intake
(g) |
|---|
| Group | 0 weeks | 1 week | 3 weeks | 5 weeks | 6 weeks | 7 weeks |
|---|
| db/m male | 3.8±0.28 | 3.2±0.12 | 3.7±0.23 | 4.0±0.21 | 3.8±0.25 | 3.9±0.18 |
| db/m female | 3.5±0.25 | 3.0±0.18 | 3.4±0.18 | 3.4±0.22 | 3.5±0.24 | 3.6±0.18 |
| db/db male |
8.0±0.21a,b |
7.3±0.54a,b |
7.7±0.23a,b |
7.3±0.32a,b |
7.5±0.35a,b |
7.6±0.40a,b |
| db/db female |
7.6±0.43a,b |
6.7±0.07a,b |
6.6±0.34a,b |
6.5±0.48a,b |
6.8±0.20a,b |
6.3±0.50a,b |
 | Table IIIResults of daily water intake of mice
in each group (n=8). |
Table III
Results of daily water intake of mice
in each group (n=8).
| | Daily water intake
(ml) |
|---|
| Group | 0 weeks | 1 week | 3 weeks | 5 weeks | 6 weeks | 7 weeks |
|---|
| db/m male | 4.9±0.29 | 4.6±0.30 | 4.6±0.39 | 4.9±0.30 | 4.7±0.27 | 4.7±0.32 |
| db/m female | 4.0±0.27 | 3.4±0.26 | 3.8±0.32 | 3.7±0.41 | 3.6±0.31 | 3.6±0.30 |
| db/db male |
15.2±0.15a,b |
14.4±1.01a,b |
14.6±0.63a,b |
14.9±0.21a,b |
14.9±0.46a,b |
14.6±0.83a,b |
| db/db female |
10.1±0.16a,b,c |
8.8±0.46a,b,c |
9.3±0.18a,b,c |
8.6±0.56a,b,c |
9.0±0.29a,b,c |
9.0±0.23a,b,c |
 | Table IVResults of random blood glucose of
mice in each group (n=8). |
Table IV
Results of random blood glucose of
mice in each group (n=8).
| | Random blood
glucose (mmol/l) |
|---|
| Group | 0 weeks | 1 week | 3 weeks | 5 weeks | 6 weeks | 7 weeks |
|---|
| db/m male | 6.71±0.41 | 6.43±0.53 | 6.59±0.49 | 6.31±0.41 | 7.49±0.82 | 6.23±1.18 |
| db/m female | 6.49±0.45 | 6.38±0.58 | 6.30±0.68 | 6.41±0.59 | 6.08±0.46 | 5.86±0.77 |
| db/db male |
20.26±1.36a,b |
21.79±1.55a,b |
26.25±0.86a,b |
27.14±0.57a,b |
27.01±1.24a,b |
26.86±2.31a,b |
| db/db female |
18.30±0.79a,b |
17.33±1.26a,b,c |
17.55±1.25a,b,c |
20.04±0.58a,b,c |
20.68±1.30a,b,c |
20.40±1.77a,b,c |
OGTT
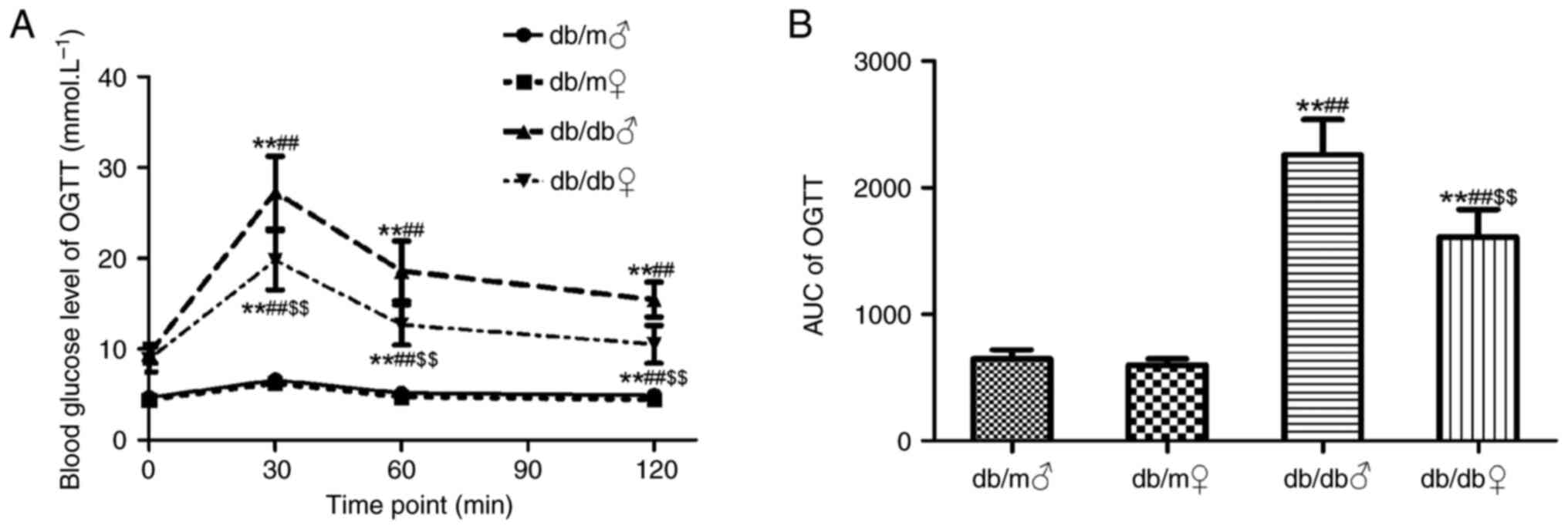
OGTT was performed at the end of the 7th week. Blood
glucose levels in both female and male db/m mice were significantly
lower compared with those in db/db mice at 30 and 60 min (Fig. 1A). Furthermore, the area under the
curve calculated for db/m mice was significantly smaller compared
with that for db/db mice P<0.05; Fig. 1B). From the results of the present
study, abnormal glucose tolerance in db/db mice was demonstrated;
there was a notable sex difference (malefemale;
P<0.05).
Histology of the liver and kidney
tissues
H&E staining demonstrated that both male and
female db/m mice had well-arranged liver lobules and normal
structures of liver lobules, hepatic cords and sinusoids, and the
hepatocytes were regular in shape, uniform in size and distributed
radially around the central vein (Fig.
2). However, both male and female db/db mice had irregular
hepatic lobules, disordered hepatocytes and ballooning of a number
of hepatocytes, and these changes were further marked in male db/db
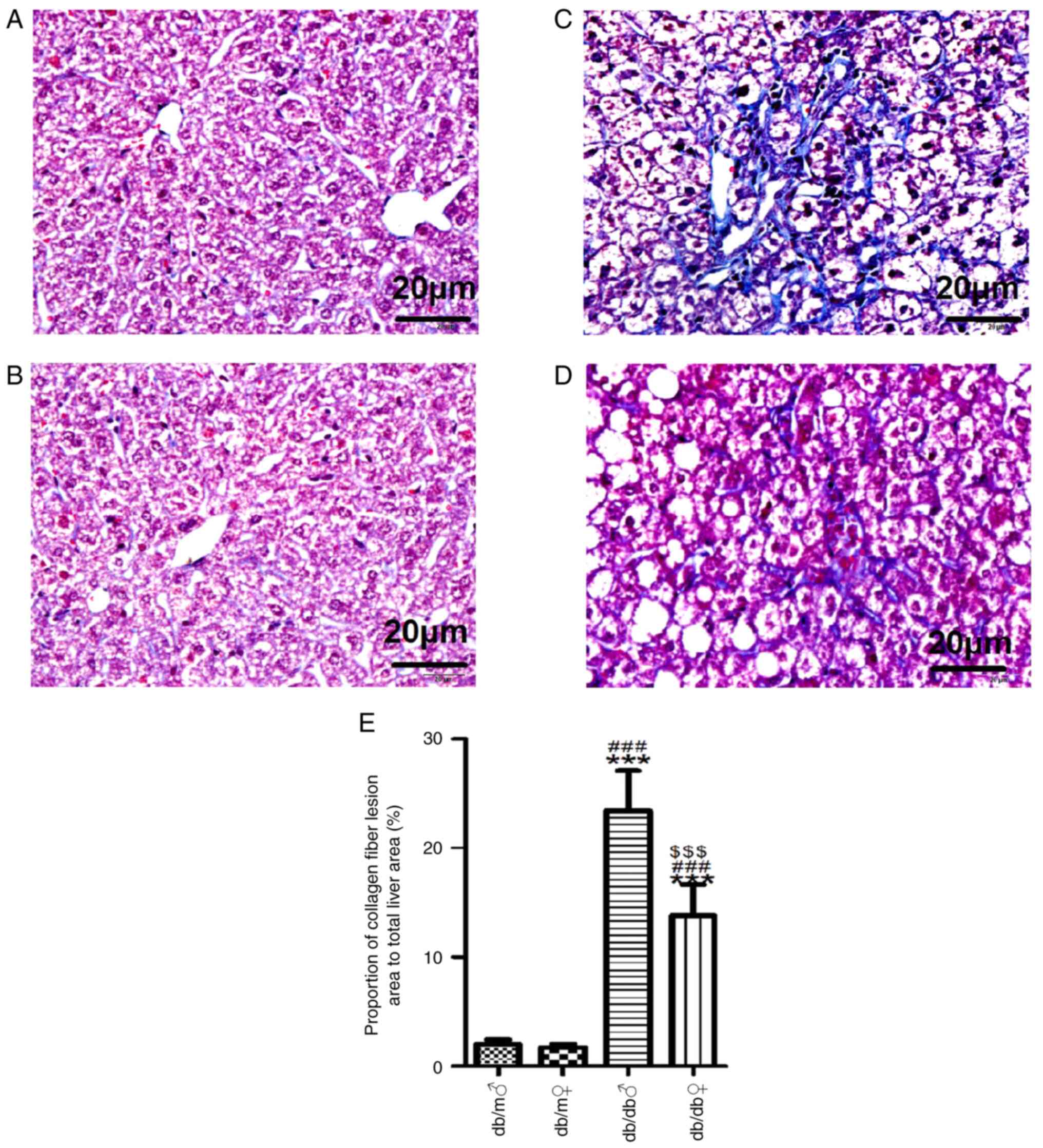
mice (Fig. 2). Masson's trichrome
staining demonstrated that there was a small amount of collagen
deposition in the hepatic interstitium of male and female db/m
mice. Conversely, there were numerous collagen fibers in male db/db
mice but significantly less in female db/db mice (Fig. 3).
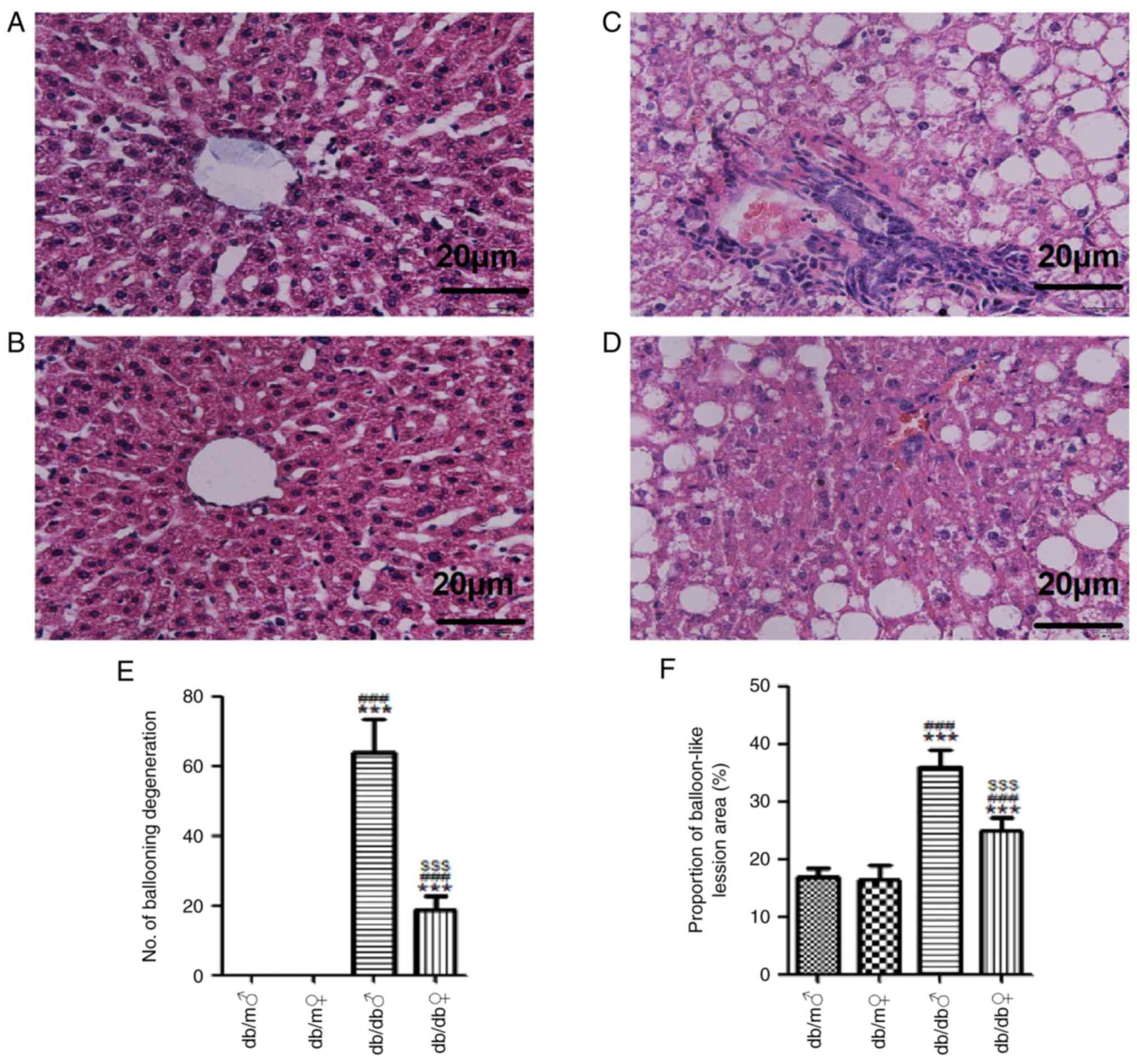 | Figure 2Representative images of liver tissue
stained with H&E. H&E stained liver tissues of (A) db/m
male, (B) db/m female, (C) db/db male and (D) db/db female mice
(magnification, x400). (E) Mean number of ballooning degenerations
and (F) proportion of balloon-like lesion area (%) in each group.
Scale bar, 20 µm. All values are expressed as the mean ± SD (n=3).
t=32.19, ***P<0.001 vs. db/m male; t=29.01,
###P<0.001 vs. db/m female; and t=26.76,
$$$P<0.001 vs. db/db male. H&E, hematoxylin and
eosin. ♂, male; ♀, female. |
H&E staining demonstrated that the renal
structure of db/m mice of both sexes was normal, whereby the
glomerular outlines were clear, the glomerular basement membrane
was not thickened, the mesangial matrix indicated no sign of
proliferation, the tubular epithelial cells were ordered and
regularly shaped, and the renal interstitium had no abnormal
features. Conversely, male db/db mice had larger glomeruli (92.32
µm) and wider renal capsular space (16.11 µm) compared with db/m
mice (36.33 and 5.57 µm, respectively). In female db/db mice, the
glomerular volume (65.36 µm) was larger compared with db/m mice
(38.46 µm) values, but the difference from the db/m mice was less
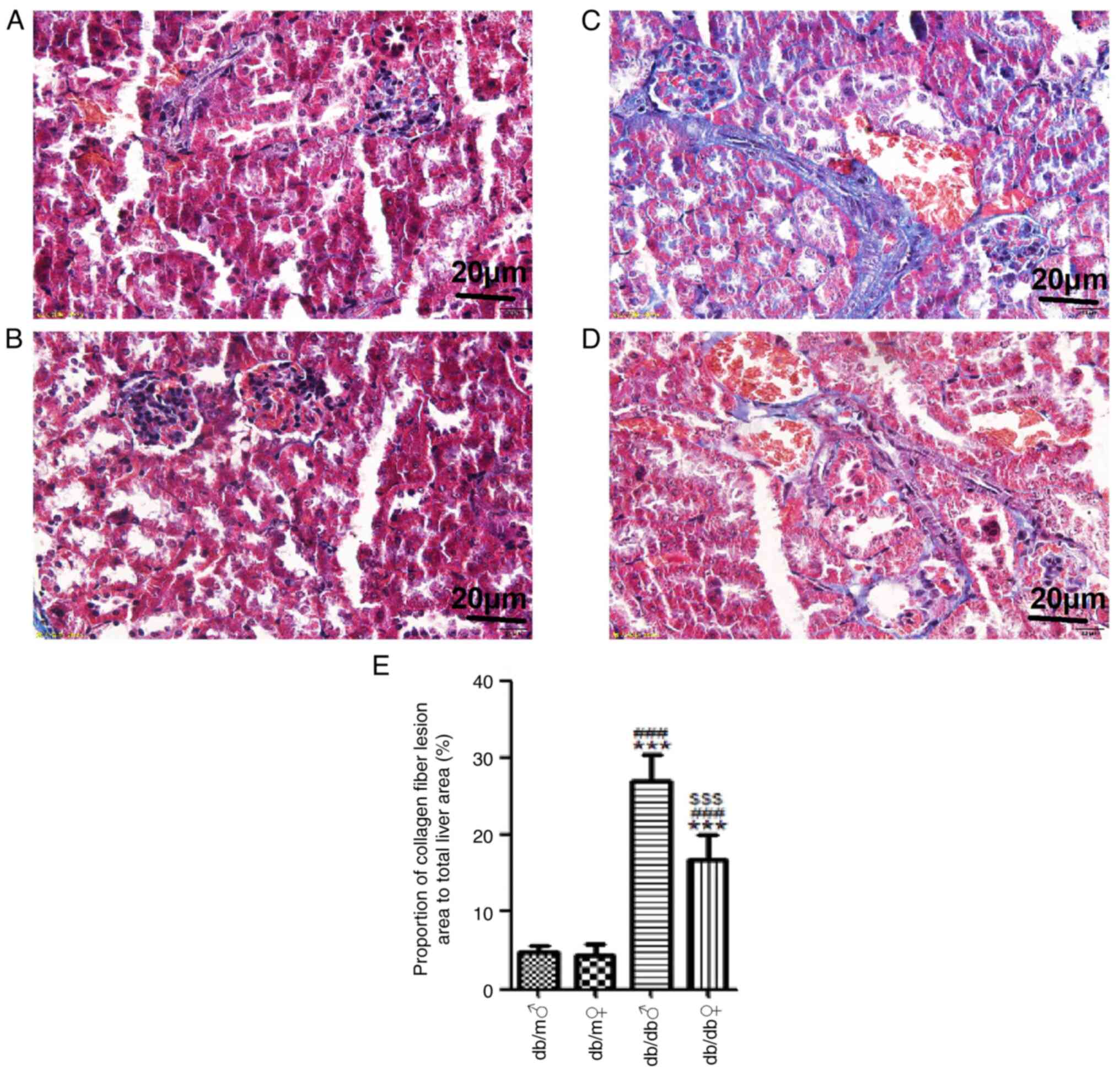
marked compared with that in the male db/db mice (Fig. 4). Masson's trichrome staining
demonstrated that there was a small amount of collagen deposition
in the renal interstitium of db/m mice, whereas there were large
numbers of collagen fibers in the renal interstitium of db/db mice.
However, the renal interstitial fibrosis in the male db/db mice was
significantly increased compared with that in the female db/db mice
(Fig. 5). The results of the
present study showed that male db/db mice had more severe liver and
kidney damage compared with female db/db mice.
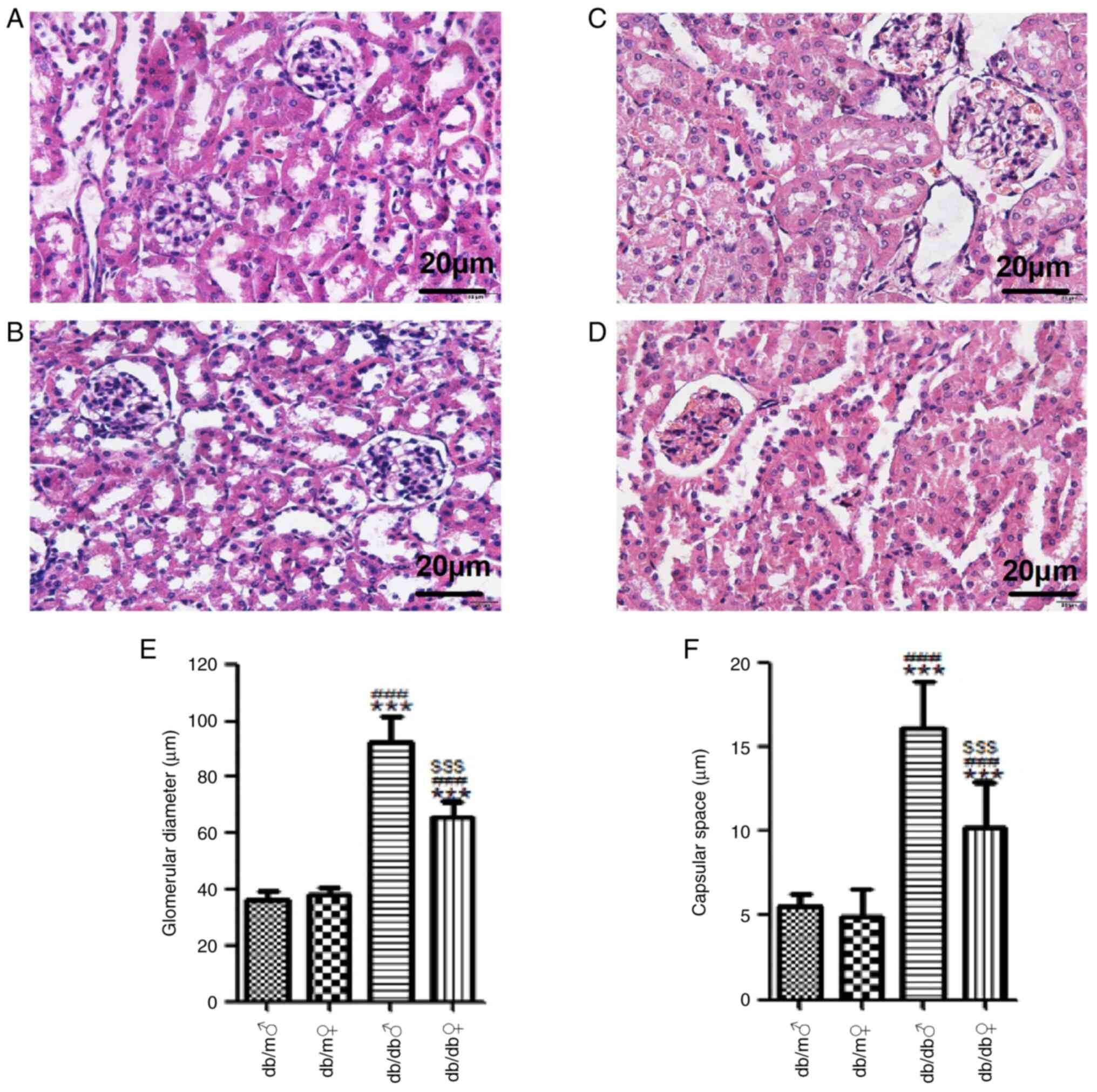 | Figure 4Representative images of renal tissue
stained with H&E. H&E stained renal tissues of (A) db/m
male, (B) db/m female, (C) db/db male and (D) db/db female mice
(magnification, x400). (E) Mean glomerular diameter (µm) and (F)
capsular space (µm) in each group. Scale bar, 20 µm. All values are
expressed as the mean ± SD (n=3). t=18.25, ***P<0.001
vs. db/m male; t=15.29, ###P<0.001 vs. db/m female;
and t=27.84, $$$P<0.001 vs. db/db male. H&E,
hematoxylin and eosin; ♂, male; ♀, female. |
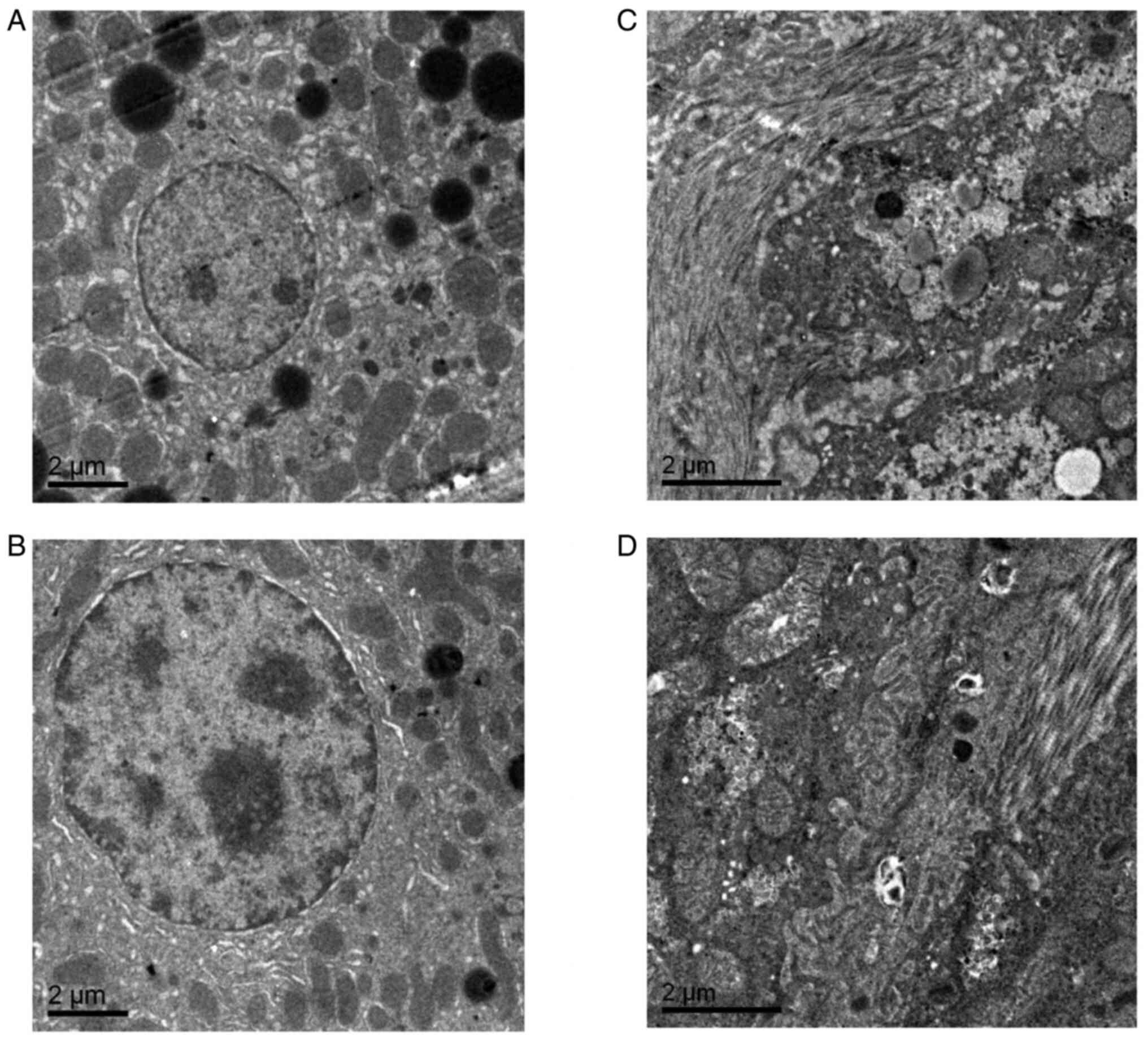
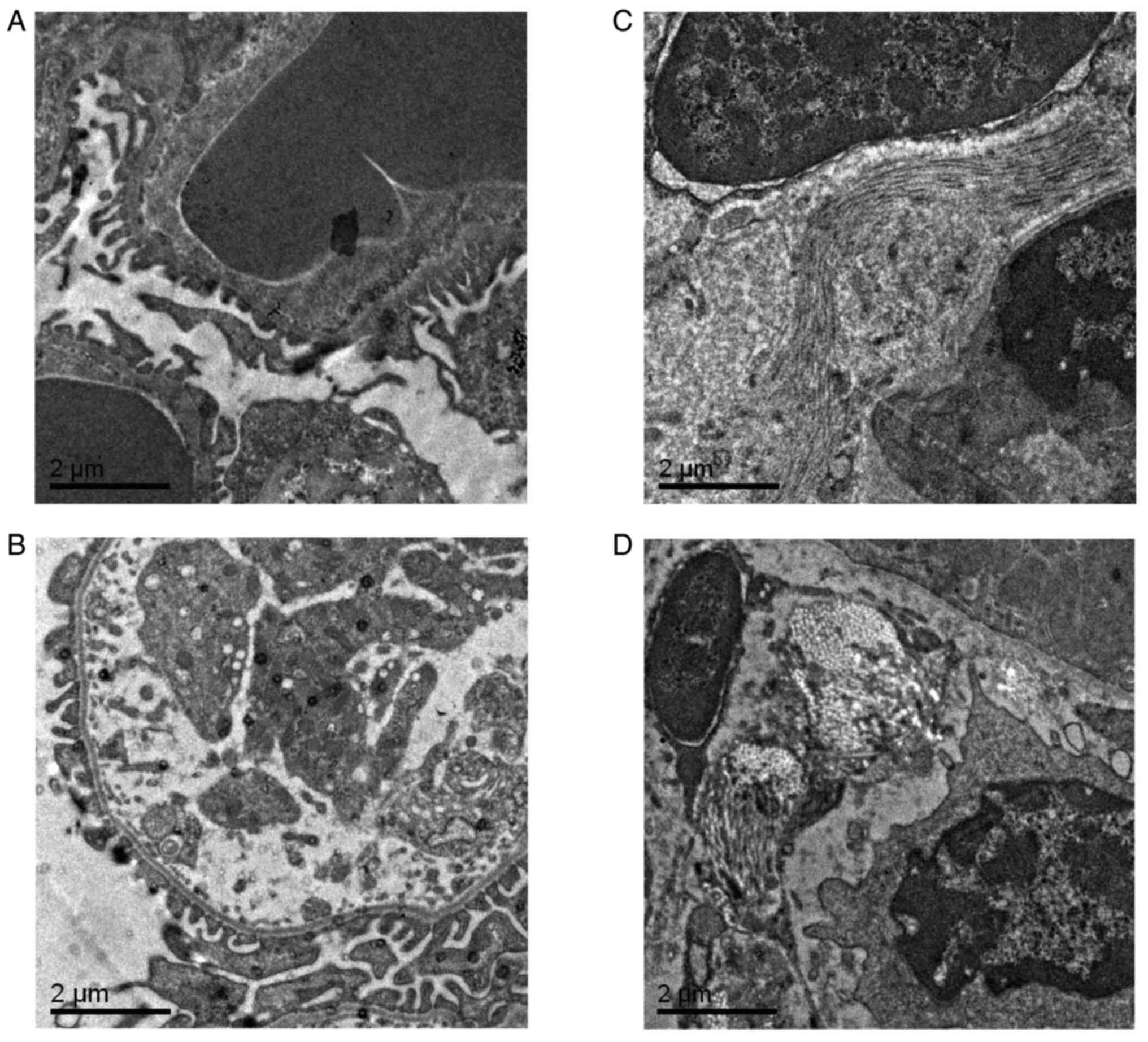
Electron microscopy findings in the
liver and kidney tissues
Electron microscopy demonstrated that the podocyte
nuclei of db/m mice were regular in shape and that the chromatin
was evenly distributed. In addition, normal nucleoli, mitochondria,
endoplasmic reticulum and lysosomes were observed. However, in
female db/db mice, a number of the mitochondria were enlarged and
collagen deposition was observed compared with db/m mice.
Furthermore, in male db/db mice, the mitochondria indicated oedema
and vacuolar degeneration and a large number of collagen fibers
were present (Fig. 6).
Transmission electron microscopy demonstrated that the glomerular
basement membranes of db/m mice of both sexes were intact and
indicated no thickening, and the foot processes were evenly
distributed. Conversely, there was collagen deposition in the
stroma of the kidneys of female db/db mice, with a larger amount of
collagen present in the stroma of male db/db mice (Fig. 7). These results indicated that
there are sex differences in liver and kidney ultrastructure damage
in db/db mice, and male db/db mice have more severe liver and
kidney ultrastructure damage compared with female db/db mice.
Discussion
To investigate whether there are sex differences in
liver and kidney damage in diabetic mice, db/db mice were selected
as diabetic model animals in the present study. The db/db mouse is
an autosomal recessive leptin receptor gene-deficient mouse that
was selected in the C57BL/KSJ strain (24). The homozygous db/db mouse
spontaneously develops diabetes and shows hyperphagia and obesity
from 4 weeks of age (25). Thus,
the pathogenesis of its phenotype resembles that of human type 2 DM
(26,27). Homozygous db/db mice are infertile,
but heterozygous db/m mice can be bred by introducing the ‘misty’
gene (28). The latter has normal
body mass, blood glucose and insulin concentrations, such that it
can be used as a control strain in studies of db/db mice (29).
In the present study, the effects of diabetes on the
liver and kidneys of db/db mice of both sexes were characterized
with reference to db/m controls. As expected, the body masses and
blood glucose concentrations of the db/db mice were significantly
higher compared with those of the db/m mice of both sexes. However,
the results of the present study demonstrated that there were sex
differences in body weight and food intake of mice, but the
differences were not statistically significant. This result may
have been caused by the small sample size of the present study.
Future studies should use an increased sample size to track sex
differences in body weight. Compared with the control group, db/db
mice showed hepatocyte swelling, vacuolization and collagen
deposition in the liver and kidney tissues, but the degree of
pathological changes in the kidney tissue was milder. In previous
pathological studies conducted in humans, patients with diabetic
kidney disease also showed glomerular basement membrane thickening
and interstitial fibrosis (30),
and those with diabetes complicated by NAFLD showed steatosis and
fibrosis (31). Thus, the present
findings are consistent with those in humans, which implies that
the db/db mouse is a suitable model for the study of diabetes and
its complications.
In the present study, there were significant
differences in the body mass or blood glucose concentration of the
male and female db/db mice except for week 0, and the severity of
hepatic and renal fibrosis in male db/db mice was significantly
higher compared with that in female db/db mice. There is
controversy regarding sex differences in diabetic complications
(32). However, the experimental
results of the present study observed a number of sex differences
in diabetic mice. The present study was a preliminary
investigation, and in future studies sex differences and organ
damage will be investigated more thoroughly and over a longer
period of time. In addition, lipids accumulate in the liver, and
the accompanying vacuolation was more pronounced in male compared
with in female db/db mice. Thus, the liver and kidney fibrosis in
male db/db mice also appeared to be more pronounced compared with
that in female db/db mice. Consistent with this, previous studies
have demonstrated that the level of proteinuria in male db/db mice
is usually twice that of female mice (33), female mice demonstrate less
proteinuria and vascular remodeling in their kidneys compared with
male mice (34) and mice lacking
estrogen receptor-α demonstrate obvious proteinuria and
glomerulonephritis (35). This
suggests that estrogen may limit the renal injury that is caused by
diabetes. A previous clinical study has also shown that male
patients with diabetes are at a higher risk of microalbuminuria and
are more likely to have multiple microvascular diseases (36), which suggests that among patients
with diabetes, men are more likely to develop more serious kidney
disease compared with women. Engin (37) found that menopausal hormone therapy
with estrogen can delay the onset of type 2 diabetes in women and
can improve β-cell insulin secretion, blood glucose utilization and
insulin sensitivity. Thus, the protective effect of estrogen may
explain why the complications of female patients with diabetes are
less severe compared with those of male patients.
A small sample size was used in the present study,
and follow-up experiments should aim to determine whether estrogen
is the cause of the difference in the severity of the secondary
pathological changes between male and female db/db mice. However,
the results of the present study suggested that the db/db mouse
represents a useful model of the pathogenesis of human type 2
diabetes and secondary liver and kidney pathology. Furthermore, the
lesions in the male db/db mice were significantly increased
compared with those in the female mice. In future studies, physical
and chemical indicators such as proteinuria should be obtained, to
more specifically analyze liver and kidney injury in mice.
Furthermore, the potential impact of sex should be considered when
animal models are selected for the study of diabetes and other
diseases, so that research can be appropriately targeted.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by grants from The Natural
Science Foundation of Hebei Province (grant no. H2018209260).
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, LGa and SH designed the study. GW made
substantial contributions to acquisition of data. XW and LGu
performed the experiments. WZ conducted the analysis of data. YZ
and SH confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by The Animal
Experimental Ethical Inspection Form of North China University of
Science and Technology (approval no. LX201935; Tangshan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zheng Y, Ley SH and Hu FB: Global
aetiology and epidemiology of type 2 diabetes mellitus and its
complications. Nat Rev Endocrino. 14:88–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hellstrom HR: The altered homeostatic
theory: A hypothesis proposed to be useful in understanding and
preventing ischemic heart disease, hypertension, and
diabetes-including reducing the risk of age and atherosclerosis.
Med Hypotheses. 68:415–433. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Maggiore U, Budde K, Heemann U, Hilbrands
L, Oberbauer R, Oniscu GC, Pascual J, Schwartz Sorensen S, Viklicky
O and Abramowicz D: ERA-EDTA DESCARTES working group. Long-term
risks of kidney living donation: Review and position paper by the
ERA-EDTA DESCARTES working group. Nephrol Dial Transplant.
32:216–223. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sagoo MK and Gnudi L: Diabetic
nephropathy: An overview. Methods Mol Biol. 2067:3–7.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sternlicht H and Bakris GL: Management of
hypertension in diabetic nephropathy: How low should we go? Blood
Purif. 41:139–143. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen G, Yang JC, Zhang GX, Cheng Z and Du
X: Evaluation of six noninvasive methods for the detection of
fibrosis in Chinese patients with obesity and nonalcoholic fatty
liver disease. Obes Surg. 32:3619–3626. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cigrovski Berkovic M, Virovic-Jukic L,
Bilic-Curcic I and Mrzljak A: Post-transplant diabetes mellitus and
preexisting liver disease-a bidirectional relationship affecting
treatment and management. World J Gastroenterol. 26:2740–2757.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Younossi ZM: Non-alcoholic fatty liver
disease-a global public health perspective. J Hepatol. 70:531–544.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Collins FS and Tabak LA: Policy: NIH plans
to enhance reproducibility. Nature. 505:612–613. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Kautzky-Willer A, Abrahamian H, Weitgasser
R, Fasching P, Hoppichler F and Lechleitner M: Sex- and
gender-aspects in regard to clinical practice recommendations for
pre-diabetes and diabetes. Wien Klin Wochenschr. 128 (Suppl
2):S151–S158. 2016.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
12
|
Kautzky-Willer A, Harreiter J and Pacini
G: Sex and gender differences in risk, pathophysiology and
complications of type 2 diabetes mellitus. Endocr Rev. 37:278–316.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tanase DM, Gosav EM, Costea CF, Ciocoiu M,
Lacatusu CM, Maranduca MA, Ouatu A and Floria M: The intricate
relationship between type 2 diabetes mellitus (T2DM), insulin
resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J
Diabetes Res. 2020(3920196)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Machado MV and Diehl AM: Pathogenesis of
nonalcoholic steatohepatitis. Gastroenterology. 150:1769–1777.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Benedict M and Zhang X: Non-alcoholic
fatty liver disease: An expanded review. World J Hepatol.
9:715–732. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ioannou GN, Green P, Kerr KF and Berry K:
Models estimating risk of hepatocellular carcinoma in patients with
alcohol or NAFLD-related cirrhosis for risk stratification. J
Hepatol. 71:523–533. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Younossi ZM, Otgonsuren M, Henry L,
Venkatesan C, Mishra A, Erario M and Hunt S: Association of
nonalcoholic fatty liver disease (NAFLD) with hepatocellular
carcinoma (HCC) in the United States from 2004 to 2009. Hepatology.
62:1723–1730. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hua S, Qi Q, Kizer JR, Williams-Nguyen J,
Strickler HD, Thyagarajan B, Daviglus M, Talavera GA, Schneiderman
N, Cotler SJ, et al: Association of liver enzymes with incident
diabetes in US Hispanic/Latino adults. Diabet Med.
38(e14522)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fujiwara N, Qian T, Koneru B and Hoshida
Y: Omics-derived hepatocellular carcinoma risk biomarkers for
precision care of chronic liver diseases. Hepatol Res. 50:817–830.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes: Standards of medical care
in diabetes-2018. Diabetes Care. 41 (Suppl 1):S13–S27.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sattari M, Bril F, Egerman R,
Kalavalapalli S and Cusi K: Relationship between non-alcoholic
fatty liver disease during pregnancy and abnormal glucose
metabolism during and after pregnancy. J Investig Med. 68:743–747.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hu ZH, Kong YY, Ren JJ, Huang TJ, Wang YQ
and Liu LX: Kidney and lung tissue modifications after BDL-induced
liver injury in mice are associated with increased expression of
IGFBPrP1 and activation of the NF-κB inflammation pathway. Int J
Clin Exp Pathol. 13:192–202. 2020.PubMed/NCBI
|
|
23
|
Liu YK, Xu H, Liu F, Tao R and Yin J:
Effects of serum cobalt ion concentration on the liver, kidney and
heart in mice. Orthop Surg. 2:134–140. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Roesler WJ, Pugazhenthi S and Khandelwal
RL: Hepatic glycogen metabolism in the db/db mouse. Mol Cell
Biochem. 92:99–106. 1990.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Michurina SV, Ishchenko IY, Arkhipov SA,
Cherepanova MA, Vasendin DV and Zavjalov EL: Apoptosis in the liver
of male db/db mice during the development of obesity and type 2
diabetes. Vavilovskii Zhurnal Genet Selektsii. 24:435–440.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao W, Chen L, Zhou H, Deng C, Han Q,
Chen Y, Wu Q and Li S: Protective effect of carvacrol on liver
injury in type 2 diabetic db/db mice. Mol Med Rep.
24(741)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Berger C, Heyne HO, Heiland T, Dommel S,
Höfling C, Guiu-Jurado E, Lorenz J, Roßner S, Dannemann M, Kelso J,
et al: A novel compound heterozygous leptin receptor mutation
causes more severe obesity than in Leprdb/db mice. J
Lipid Res. 62(100105)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Blokh KO, Poltorak VV, Brindak OI and Tur
MI: Importance of db gene for the development of low dose
streptozotocin diabetes in C57BL/KsJ mice. Probl Endokrinol (Mosk).
34:73–75. 1988.PubMed/NCBI(In Russian).
|
|
29
|
Klessens CQF, Woutman TD, Veraar KAM,
Zandbergen M, Valk EJ, Rotmans JI, Wolterbeek R, Bruijn JA and
Bajema IM: An autopsy study suggests that diabetic nephropathy is
underdiagnosed. Kidney Int. 90:149–156. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lal A, Parai JL and Milroy CM: Liver
pathology in first presentation diabetic ketoacidosis at autopsy.
Acad Forensic Pathol. 6:271–280. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ma Y, Li W, Yazdizadeh Shotorbani P,
Dubansky BH, Huang L, Chaudhari S, Wu P, Wang LA, Ryou MG, Zhou Z
and Ma R: Comparison of diabetic nephropathy between male and
female eNOS-/-db/db mice. Am J Physiol Renal Physiol.
316:F889–F897. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lonardo A, Nascimbeni F, Ballestri S,
Fairweather D, Win S, Than TA, Abdelmalek MF and Suzuki A: Sex
differences in nonalcoholic fatty liver disease: State of the art
and identification of research gaps. Hepatology. 70:1457–1469.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kang DH, Yu ES, Yoon KI and Johnson R: The
impact of gender on progression of renal disease: Potential role of
estrogen-mediated vascular endothelial growth factor regulation and
vascular protection. Am J Pathol. 164:679–688. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shim GJ, Kis LL, Warner M and Gustafsson
JA: Autoimmune glomerulonephritis with spontaneous formation of
splenic germinal centers in mice lacking the estrogen receptor
alpha gene. Proc Natl Acad Sci USA. 101:1720–1724. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Singh SS, Roeters-van Lennep JE, Lemmers
RFH, van Herpt TTW, Lieverse AG, Sijbrands EJG and van Hoek M: Sex
difference in the incidence of microvascular complications in
patients with type 2 diabetes mellitus: A prospective cohort study.
Acta Diabetol. 57:725–732. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mauvais-Jarvis F, Manson JE, Stevenson JC
and Fonseca VA: Menopausal hormone therapy and type 2 diabetes
prevention: Evidence, mechanisms, and clinical implications. Endocr
Rev. 38:173–188. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Engin A: Non-alcoholic fatty liver
disease. Adv Exp Med Biol. 960:443–467. 2017.PubMed/NCBI View Article : Google Scholar
|