Introduction
Mesenchymal stem cells (MSCs) represent a promising
source for cell-based clinical applications (1,2).
MSCs are adult pluripotent stem cells present in almost every
tissue and organ and the bone marrow is an important site for MSC
isolation (1,2). Adipose tissue is a promising source
of MSCs with a comprehensive impact on various clinical
applications such as the treatment of osteoarthritis (2,3).
Adipose tissue is relatively abundant in the body and can be
collected in large amounts with minimally invasive procedures,
resulting in low rates of morbidity (3). Human adipose-derived stem cells
(hASCs) can differentiate into multiple lineages, including
osteogenic (4), chondrogenic
(5), adipogenic (6) and neurogenic (7) lineages. Based on their
differentiation abilities, hASCs have been used in regenerative
medicine to promote bone regeneration (8), wound healing and age suppression
(9), and to facilitate nerve
regeneration (8). Additionally,
their high proliferation rate as well as their immune-privileged
and -tolerant properties make hASCs effective and a potential novel
therapy in the field of regenerative medicine (8,9).
Human platelet lysates (PLTs) contain several
mitogenic growth factors, including platelet-derived growth factor
(PDGF), fibroblast growth factor (FGF), epidermal growth factor
(EGF) and transforming growth factor (TGF)-β (10,11).
PLT can be used in MSC cultures without adversely affecting the
immunophenotype or metabolism of MSCs, and can replace fetal bovine
serum (FBS) for cell proliferation (12,13).
The superiority of PLT over human AB serum (14), human plasma (15) and human autologous serum (15) for ex vivo expansion of MSCs
is due to its high growth factor content, low cost and ease of
large-scale production (12-15).
Therefore, this method can be applied in clinical studies
concerning hASCs using PLTs. PLTMax, a derivative of platelet-rich
plasma (PRP), is a commercialized PLT product created from the
whole blood of American donors, which has been tested for
infections. Alonso-Camino and Mirsch (16) demonstrated that PLTMax exhibited
proliferative effects similar to those of PRP. Furthermore, our
group previously reported that culturing hASCs with PLTMax resulted
in a superior effect on cell proliferation compared to culturing
with FBS (17).
PDGF is a major growth factor in PLT that stimulates
the proliferation, survival and motility of MSCs (18). PDGF belongs to the family of
cationic homo- and disulfide-linked dimers of A- and B-polypeptide
chains (19). PDGF isoforms bind
to α- and β-tyrosine kinase receptors, and promote
autophosphorylation and kinase activity (19). Previous studies on the PDGF
signaling pathway and target genes have demonstrated that the
activation of PDGF receptor (PDGFR) downstream molecules
contributes to PDGF-promoted cell proliferation and migration
(19,20). However, PDGF does not account for
the mitogenic ability of PLT (20). In addition, PLTMax (or PLT)
contains proliferation and differentiation factors released by
platelets, such as vascular endothelial growth factor (VEGF),
hepatocyte growth factor (HGF) and EGF (16). These factors act as mitogens and
motogens for diverse cell types and angiogenesis promoters
(18). Previous in vitro
studies have demonstrated that hASCs cultured with individual or
combinations of growth factors differentiate into endothelial,
chondrogenic, myogenic, osteogenic and neural cells (21,22).
However, the cooperative role of the individual factors present in
PLTMax in the proliferation and differentiation of hASCs remains
unknown, and the precise mechanisms involved in the recruitment of
PDGF and its receptor to other signaling pathways involved in the
proliferation and migration of hASCs are unclear.
In the present study, the contribution of
proliferative factors to hASC potency was systematically
investigated. Our group previously reported the positive effects of
PLT (or PRP), PDGF-BB and FGF-2 on the proliferation and migration
of hASCs (20,23,24).
The present study aimed to optimize hASC proliferation in
2-dimensional (D) and 3-D cultures by supplementing hASCs with
growth factors. PDGF-BB combined with VEGF, HGF or EGF had a
synergistic effect on the PDGF-BB-dependent migration and
proliferation of hASCs. PDGF-BB/PDGFR signaling predominantly
controlled different signaling pathways to activate the ERK1/2 and
p38 MAPK mitogenic enzymes, followed by cell proliferation and
migration.
Materials and methods
Ethics approval
The present study was approved by The Ethics Review
Board of Kansai Medical University (Hirakata, Japan; approval no.
2017094) in accordance with the ethical guidelines of the
Declaration of Helsinki of 1975.
Reagents
Human platelet lysate (PLTMax) was purchased from
MilliporeSigma (cat. no. SCM141). PDGF-BB was purchased from
PeproTech EC Ltd. VEGF-A (VEGF) and HGF were obtained from Takara
Biotechnology Co., Ltd. FBS was purchased from HyClone (Cytiva).
Trypsin, trypsin inhibitor and imatinib (a PDGFR inhibitor) were
purchased from FUJIFILM Wako Pure Chemical Corporation. VEGFR
tyrosine kinase inhibitor II (a VEGFR inhibitor) and tivantinib [a
MET proto-oncogene, receptor tyrosine kinase (c-Met) inhibitor]
were purchased from Cayman Chemical Company. All chemicals used in
the present study were of analytical grade.
Isolation of hASCs
The present study complies with the International
Society for Stem Cell Research guidelines (https://www.isscr.org/guidelines). Adipose tissue was
obtained from a 42-year-old male patient, who had provided informed
oral and written consent, whilst undergoing plastic surgery at
Kansai Medical University in 2017. hASCs were isolated as
previously described (20,24). Briefly, adipose tissue was cut into
small pieces and digested with collagenase (MilliporeSigma). After
the addition of Dulbecco's modified Eagle's medium (DMEM; Nissui
Pharmaceutical Co., Ltd.; cat. no. 05915) containing 10% FBS
(Hyclone; Cytiva) and 2% penicillin/streptomycin (Gibco; Thermo
Fisher Scientific, Inc.), the tissue was centrifuged at 400 x g for
3 min at room temperature. The obtained supernatant was passed
through a 100-µm nylon mesh (Falcon; Corning Life Sciences) to
remove cellular debris. The resulting primary hASCs were cultured
for 4-5 days until the cells reached 90% confluency. These cells
were defined as passage ‘0’. Cells from passages 7-9 were used for
all the experiments. hASCs were identified through the presence
(CD73, CD90 and CD105) or absence (CD14 and CD31) of cell surface
markers by flow cytometric analysis (6,8,20).
Cell proliferation assay
hASCs were seeded into 96-well cell culture plates
at a density of 3.0x103 cells/well and incubated in
complete medium overnight at 37˚C. The cell culture medium was then
replaced with serum-free DMEM (control medium). After incubation
for 18 h, hASCs were cultured in control medium supplemented with
PLTMax (1-5%), PDGF-BB (20 ng/ml), PDGF-BB (20 ng/ml)/VEGF (1
ng/ml), VEGF (1 ng/ml) and PDGF-BB (20 ng/ml)/HGF (1 ng/ml) for 48
h at 37˚C. For pharmacological inhibition assays, imatinib (Cayman
Chemical Company; cat. no. 13139), VEGFR inhibitor II (Cayman
Chemical Company; cat no. 17654) and tivantinib (Cayman Chemical
Company; cat. no. 17135) were added to the control medium 1 h
before incubation at 37˚C with medium containing growth factors.
Cell proliferation was examined using a Cell Counting Kit-8
(Dojindo Molecular Technologies, Inc.) according to the
manufacturer's instructions as described previously (17,20,24).
The optical absorbance was measured at 450 nm using a multi-well
plate reader (EnSpire 2300 Multilabel Reader; PerkinElmer,
Inc.).
Immunofluorescent analysis
hASCs (1x104) were treated with PDGF-BB
(20 ng/ml), VEGF (1 ng/ml), HGF (1 ng/ml), PDGF-BB (20 ng/ml)/VEGF
(1 ng/ml) and PDGF-BB (20 ng/ml)/HGF (1 ng/ml) for 24 h. The cells
were fixed in 4% formaldehyde solution for 20 min at room
temperature and permeabilized with 0.3% Triton X-100 for 15 min
(17). After blocking with
phosphate-buffered saline (PBS) containing 3% FBS (Hyclone; Cytiva)
for 2 h at room temperature, cells were incubated with a monoclonal
antibody against Ki-67 (1:800; cat. no. #9449; Cell Signaling
Technology, Inc.) for 2 h at room temperature, followed by
incubation with FITC-conjugated anti-rabbit immunoglobulin (IgG;
1:100; cat. no. SA00003-1; Proteintech Group, Inc.) and DAPI
(1:1,000; cat. no. D523; Dojindo Molecular Technologies, Inc.) for
1 h at room temperature. Images of the antigens were captured using
a fluorescence microscope (BZ9000; Keyence Corporation).
Western blot analysis
hASCs (5x105) were treated without or
with PDGF-BB (20 ng/ml), VEGF (1 ng/ml), HGF (1 ng/ml), PDGF-BB (20
ng/ml)/VEGF (1 ng/ml) and PDGF-BB (20 ng/ml)/HGF (1 ng/ml) for 20
min. Cells were lysed in M-PER solution (cat. no. 78501; Thermo
Fisher Scientific, Inc.) supplemented with a protease and
phosphatase inhibitor cocktail (cat. no. 78440; Thermo Fisher
Scientific, Inc.) (20). Protein
concentration was estimated using a BCA assay kit (cat. no. A53225;
Thermo Fisher Scientific, Inc.). Cellular proteins (20 µg/lane)
were separated by SDS-PAGE on 4-15% gradient gels (cat. no.
NP0321BOX; Thermo Fisher Scientific, Inc.) and electroblotted onto
polyvinylidene difluoride membranes (cat. no. IPVH00010; Thermo
Fisher Scientific, Inc.). After blocking with Blocking One-P
reagent (cat. no. 05999-84; Nacalai Tesque, Inc.) for 60 min at
room temperature, the membranes were incubated overnight at 4˚C
with the following primary antibodies: Anti-phospho-Erk1/2
(1:1,000; cat. no. #4370; Cell Signaling Technology, Inc.),
anti-Erk1/2 (1:1,000; cat. no. #4695; Cell Signaling Technology,
Inc.), anti-phospho-PDGFRb (1:1,000; cat. no. GTX133525; GeneTex,
Inc.), anti-PDGFRb (1:1,000; cat. no. 134491AP; Proteintech Group,
Inc.), anti-phospho-c-Met (1:1,000; cat. no. 600401989S; Rockland
Immunochemicals Inc.), anti-c-Met (1:1,000; cat. no. GTX631992;
GeneTex, Inc.), anti-phospho-VEGFR2 (1:1,000; cat. no. CSBPA000703;
Cusabio Technology, LLC), anti-VEGFR2 (1:1,000; cat. no.
CSBPA008334; Cusabio Technology, LLC), anti-phospho-p38 MAPK
(1:1,000; cat. no. #4511; Cell Signaling Technology, Inc.),
anti-p38 MAPK (1:1,000; cat. no. #8690; Cell Signaling Technology,
Inc.) and anti-β-actin (1:1,000; cat. no. #4970; Cell Signaling
Technology, Inc.). Next, the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10,000;
cat. no. SC2357; Santa Cruz Biotechnology, Inc.) or rabbit
anti-mouse IgG (1:10,000; cat. no. SC2031; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Immunoreactive
bands were visualized using an enhanced chemiluminescence reagent
(cat. no. 296-69901; FUJIFILM Wako Pure Chemical Corporation), and
signals were quantified using ImageJ software (version 1.53t;
National Institutes of Health) with β-actin as the loading control
for normalization.
Wound healing assay
hASCs were seeded into 24-well cell culture plates
at 6.0x104 cells/well and incubated in complete medium
at 37˚C overnight, followed by incubation with serum-free medium
for 4 h at 37˚C. The cell monolayer was then scratched using a
sterilized 200-µl disposable pipette tip. Subsequently, the cells
were washed twice with PBS and cultured in DMEM containing PDGF-BB
(20 ng/ml), VEGF (1 ng/ml), HGF (1 ng/ml), PDGF-BB (20 ng/ml)/VEGF
(1 ng/ml), and PDGF-BB (20 ng/ml)/HGF (1 ng/ml). Images of the
scratched areas from three independent experiments were compared at
0, 12 and 24 h using an optical microscope (Primovert; Zeiss, AG),
and the area between the two edges of the wound was analyzed using
ImageJ software (National Institutes of Health; version 1.53t).
Gene expression
Total RNA was isolated from the hASCs using a
Maxwell RSC kit (cat. no. AS1340; Promega Corporation) (25). Reverse transcription-quantitative
PCR (RT-qPCR) was performed using SYBR Green RT-qPCR Master Mix
(cat. no. 204243; Qiagen GmbH), according to the manufacturer's
protocol, on a Rotor-Gene Q HRM Real-Time PCR System (Qiagen GmbH).
The PCR thermocycling conditions were 40 cycles of 10 sec at 95˚C
and 20 sec at 60˚C. Relative gene expression changes were
calculated using the 2-IICq method (26) with GAPDH as the internal reference
gene. The PCR primers used in the present study are listed in
Table I.
 | Table IPrimer sequences used for
quantitative PCR analysis. |
Table I
Primer sequences used for
quantitative PCR analysis.
| Gene | Forward primer,
5'-3' | Reverse primer,
5'-3' |
|---|
| GAPDH |
GTCTCCTCTGACTTCAACAGCG |
ACCACCCTGTTGCTGTAGCCAA |
| COL10A1 |
CGCTGAACGATACCAAATGCCC |
TGGACCAGGAGTACCTTGCTCT |
| RUNX2 |
CCCAGTATGAGAGTAGGTGTCC |
GGGTAAGACTGGTCATAGGACC |
| PPARG |
AGCCTGCGAAAGCCTTTTGGTG |
GGCTTCACATTCAGCAAACCTGG |
| ACTA1 |
AGGTCATCACCATCGGCAACGA |
GCTGTTGTAGGTGGTCTCGTGA |
| SOX2 |
GCTACAGCATGATGCAGGACCA |
TCTGCGAGCTGGTCATGGAGTT |
| CD73 |
AGTCCACTGGAGAGTTCCTGCA |
TGAGAGGGTCATAACTGGGCAC |
Spheroid formation assays
hASCs were suspended in DMEM containing PDGF-BB (20
ng/ml), VEGF (1 ng/ml), HGF (1 ng/ml), PDGF-BB (20 ng/ml)/VEGF (1
ng/ml) and PDGF-BB (20 ng/ml)/HGF (1 ng/ml) at a density of
1x104 cells/100 µl and seeded in a Corning
ultra-low-attachment 96-well plate (Corning, Inc.). The cells were
cultured in the indicated medium at 37˚C for 6 days, and 50 µl DMEM
containing PDGF-BB (20 ng/ml), VEGF (1 ng/ml), HGF (1 ng/ml),
PDGF-BB (20 ng/ml)/VEGF (1 ng/ml) and PDGF-BB (20 ng/ml)/HGF (1
ng/ml) was added every 2 days. Phase-contrast images were acquired
using an optical microscope (Primovert; Zeiss GmbH). Quantification
of spheroid diameters was performed using ImageJ software (version
1.53t; National Institutes of Health).
Statistical analysis
Data are expressed as the mean ± standard deviation
(n=3/4). All statistical analyses were performed using
JMP® Pro v.16.2 (JMP Statistical Discovery LLC). Data
homogeneity was examined using the Shapiro-Wilk test. Significant
differences were evaluated using one-way ANOVA followed by Tukey's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Proliferative effects of PLTMax,
PDGF-BB, VEGF, HGF and EGF on hASCs
When cells were cultured with PLTMax, hASC viability
increased gradually with 1-3% PLTMax in a dose-dependent manner,
and the greatest stimulation was observed at 3% PLTMax with 3%
PLTMax showing higher viability of the cells than 10% FBS (Fig. S1A). Trypsin treatment, but not
trypsin inhibitor (TI) treatment, significantly reduced hASC
proliferation, indicating that growth factors and other proteins in
PLTMax played essential roles in cell proliferation (Fig. S1B).
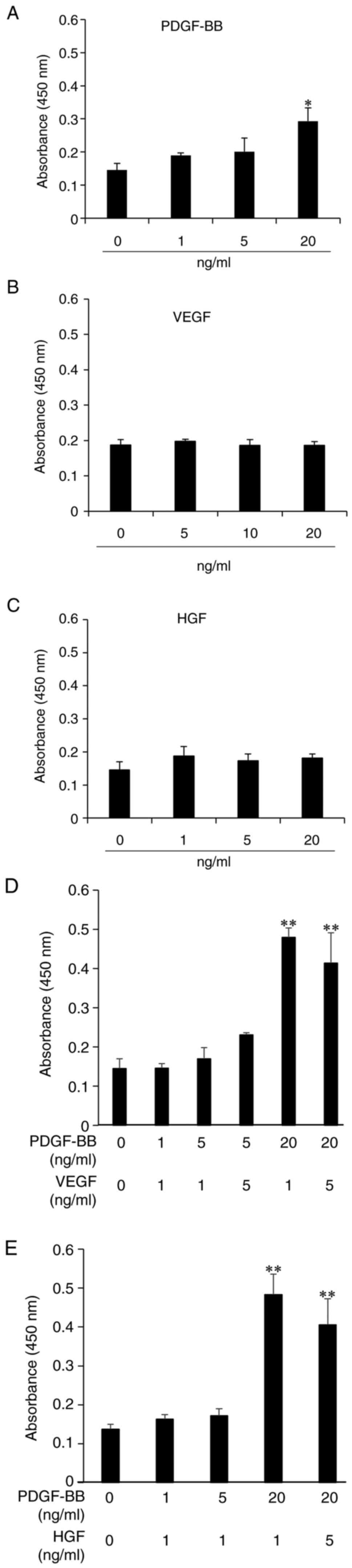
Next, the proliferative effects of PDGF-BB, a major
growth factor in PLTMax, were examined. PDGF-BB caused a
dose-dependent stimulation of hASC proliferation at 0-20 ng/ml
(Fig. 1A). At >20 ng/ml PDGF-BB
concentrations, the extent of the proliferation curve decreased
(Fig. S2). Conversely, other
growth factors such as VEGF and HGF did not exhibit any significant
proliferative effects on hASCs (Fig.
1B and C). When cells were
treated with VEGF and PDGF-BB, the addition of 1 ng/ml VEGF to 20
ng/ml PDGF-BB-containing medium further enhanced PDGF-dependent
cell proliferation. Cells treated with PDGF-BB and higher
concentrations of VEGF showed a decrease in cell viability,
compared with the lower concentration. The potency of stimulation
with PDGF-BB and VEGF appeared to be stronger than stimulation with
PDGF-BB alone (Fig. 1D). Treatment
with 20 ng/ml PDGF-BB and 1 ng/ml HGF resulted in the greatest cell
proliferation, similar to that observed with the combination of
PDGF and VEGF (Fig. 1E).
Furthermore, EGF alone did not enhance cell
viability but the enhancement of the proliferation with PDGF-BB (20
ng/ml) and EGF (1 ng/ml) in combination was ~2.1-fold (Fig. S3A). The extent of enhancement by
EGF combined with PDGF-BB was lower than that induced by PDGF-BB
combined with either VEGF or HGF. Thus, in the presence of PDGF-BB,
growth factors such as VEGF, HGF and EGF showed a synergistic
effect on hASC proliferation. The combined use of growth factors
resulted in maximal hASC proliferation, similar to that achieved by
PLTMax.
Effects of PDGF-BB, VEGF and HGF on
the number of Ki-67+ cells
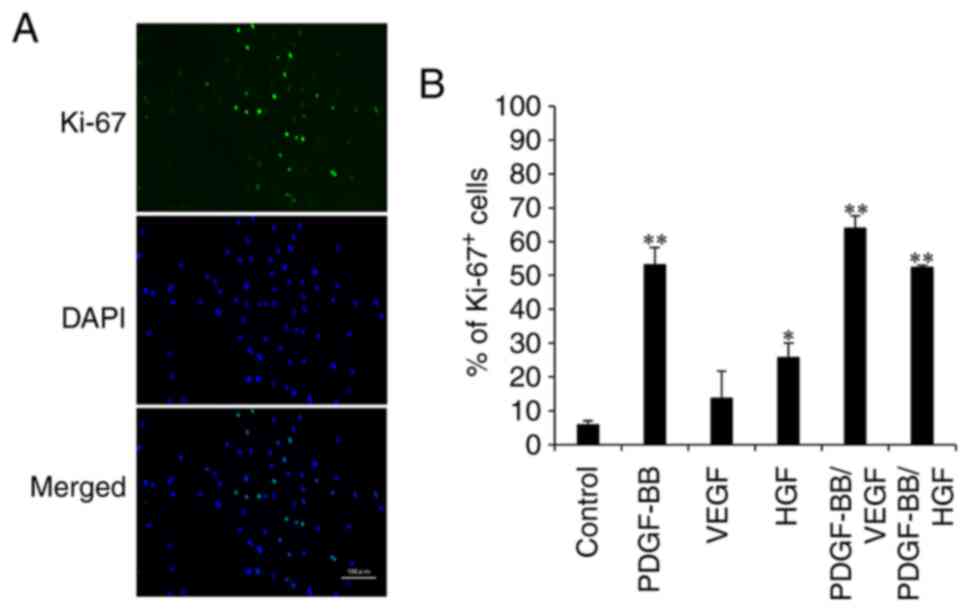
To examine proliferative markers of cell
proliferation, fluorescent immunostaining of hASCs with anti-Ki-67
antibody was performed (Fig. 2A).
The percentage of Ki-67+ cells in the control was low
(8%). However, the percentage of Ki-67+ cells in the
VEGF and HGF groups were 12 and 26%, respectively. The addition of
PDGF-BB increased the number of Ki-67-positive cells by 52%, which
was similar to that observed after PDGF-BB/HGF treatment. The
highest expression level was observed in the PDGF-BB/VEGF group
(Fig. 2B). These results confirmed
that PDGF-BB combined with VEGF resulted in an improved
proliferative ability of hASCs.
Pharmacological effects on hASC
viability
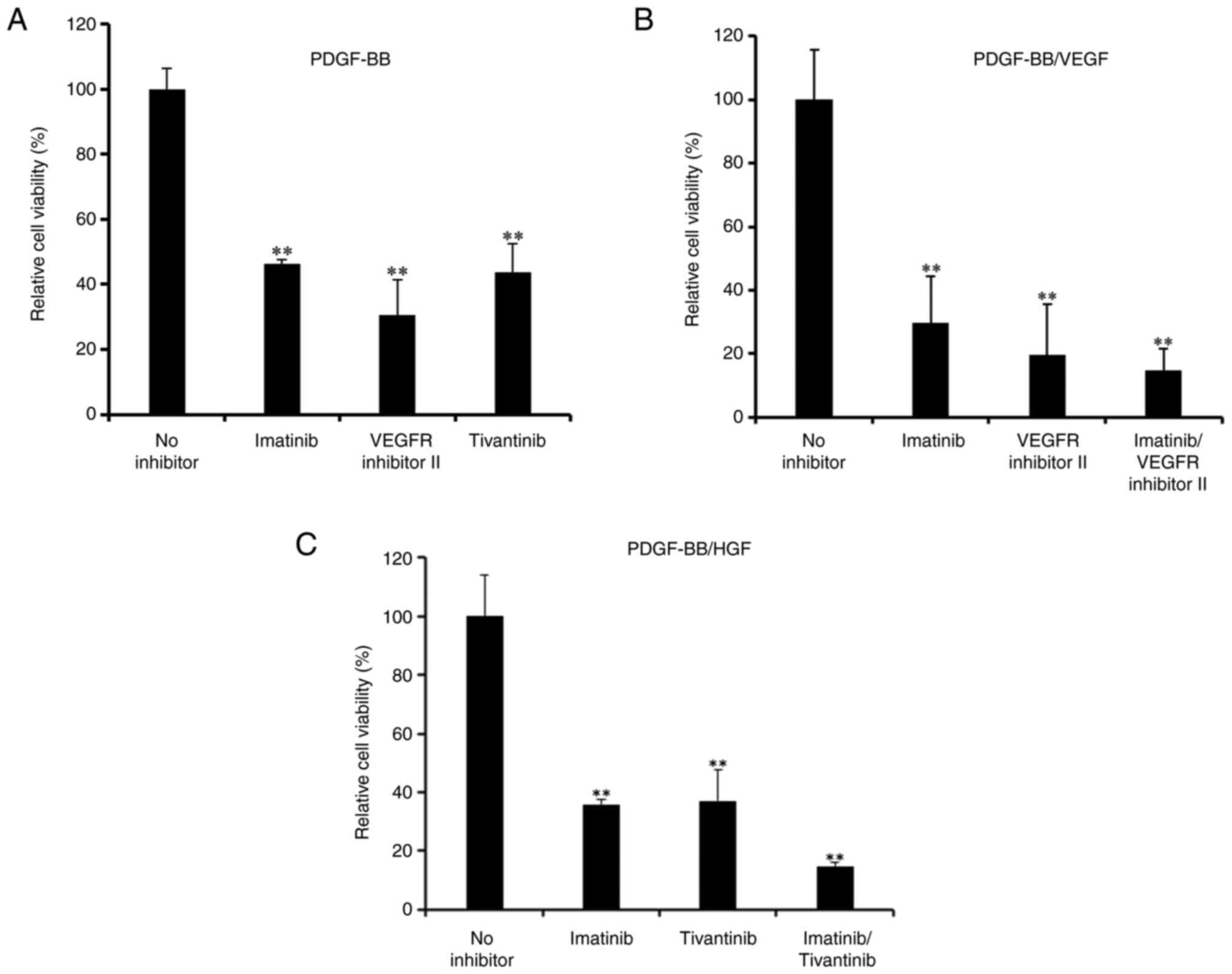
The effects of pharmacological inhibitors of PDGF,
VEGF and HGF receptors on cell viability were investigated.
Proliferation of hASCs induced by PDGF-BB was significantly
decreased when cells were treated with imatinib (a PDGFR
inhibitor), tivantinib (a c-Met inhibitor) and VEGFR inhibitor II
(Fig. 3A).
Imatinib significantly inhibited the proliferation
of PDGF-BB/VEGF-treated hASCs (Fig.
3B), and further inhibition was observed with a combination of
imatinib and the aforementioned VEGFR inhibitor. Furthermore,
inhibition by imatinib and/or tivantinib was observed after
treatment with PDGF-BB/HGF (Fig.
3C).
PD153035, an EGFR inhibitor, inhibited the
proliferation induced by PDGF-BB and EGF (Fig. S3B). These results indicated that
all growth factor receptor inhibitors investigated in the present
study exhibited a similar potent effect on the inhibition of hASC
proliferation.
Activation of receptors for growth
factors and signaling enzymes by treatment with PDGF-BB,
PDGF-BB/VEGF and PDGF-BB/HGF
To clarify the involvement of receptors in PDGF-,
VEGF- and HGF-mediated signaling pathways, immunoblotting was
performed with hASCs. Phospho-PDGFR levels increased after
treatment with PDGF-BB (Fig. 4).
Treatment with a combination of PDGF-BB and VEGF resulted in
significantly higher phosphorylation level of PDGFRβ compared with
other treatment groups. Furthermore, PDGF-BB stimulated the
phosphorylation of VEGFR2 but VEGF did not significantly affect
phosphorylated protein levels. A marked increase in phospho-VEGFR2
levels was observed in PDGF-BB/VEGF-treated cells. The increased
phosphorylation of c-Met was not observed in HGF-treated cells, but
it was increased by PDGF-BB/HGF, compared with HGF-only treatment.
In response to the increase in phospho-PDGFRβ, phospho-VEGFR2 and
phospho-c-Met, the levels of phospho-ERK1/2 and phospho-p38 MAPK
increased in PDGF-BB, PDGF-BB/VEGF and PDGF-BB/HGF groups. These
results indicated that the activation of PDGF/PDGFR signaling
played a predominant role in the stimulation of VEGFR and c-Met,
subsequently triggering the phosphorylation of ERK and p38,
followed by the enhancement of hASC proliferation.
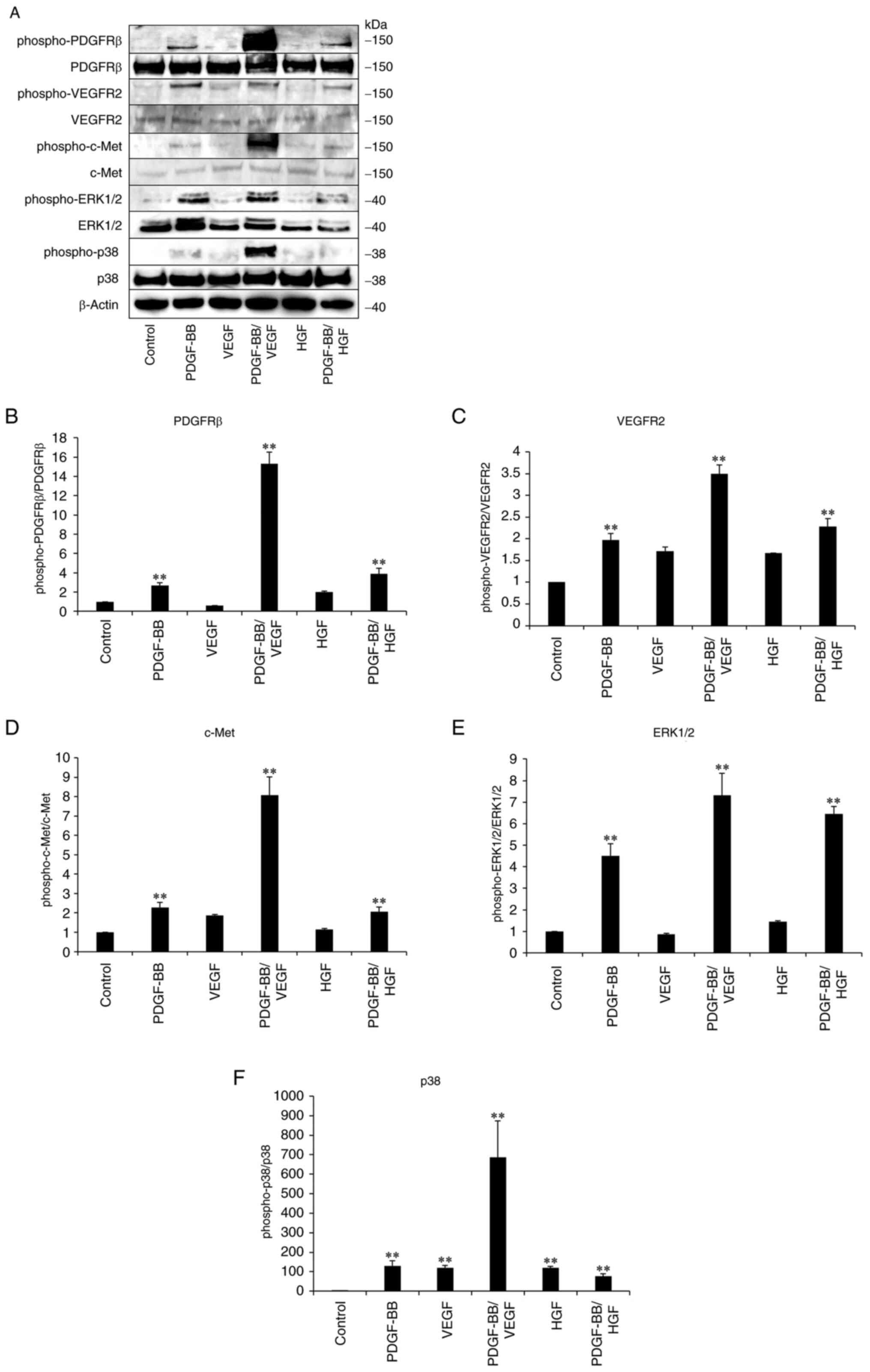 | Figure 4Activation of growth factor receptors
and signal enzymes in growth factor-treated hASCs. hASCs were
cultured in DMEM containing 10% FBS, followed by starvation for 16
h. The cells were then incubated in DMEM containing PDGF-BB (20
ng/ml), VEGF (1 ng/ml), HGF (1 ng/ml), PDGF-BB (20 ng/ml)/VEGF (1
ng/ml) or PDGF-BB (20 ng/ml)/HGF (1 ng/ml) for 20 min. The cells
then were washed, collected and lysed. Next, cellular proteins were
analyzed by SDS-PAGE using 4-15% gels, followed by (A)
immunoblotting with the indicated primary antibodies. (B) Ratio of
phospho-PDGFRb versus total PDGFRb, (C) ratio of phospho-VEGFR2
versus total VEGFR2, (D) ratio of phospho-c-Met versus total c-Met,
(E) ratio of phospho-ERK1/2 versus total ERK1/2 and (F) ratio of
phospho-p38 versus total p38 were calculated. Data are presented as
the mean ± SD (n=3). **P<0.01 vs. control. hASCs,
human adipose-derived stem cells; PDGF, platelet-derived growth
factor; VEGF, vascular endothelial growth factor; HGF, hepatocyte
growth factor. |
Effects of PDGF-BB, VEGF and HGF on
hASC migration
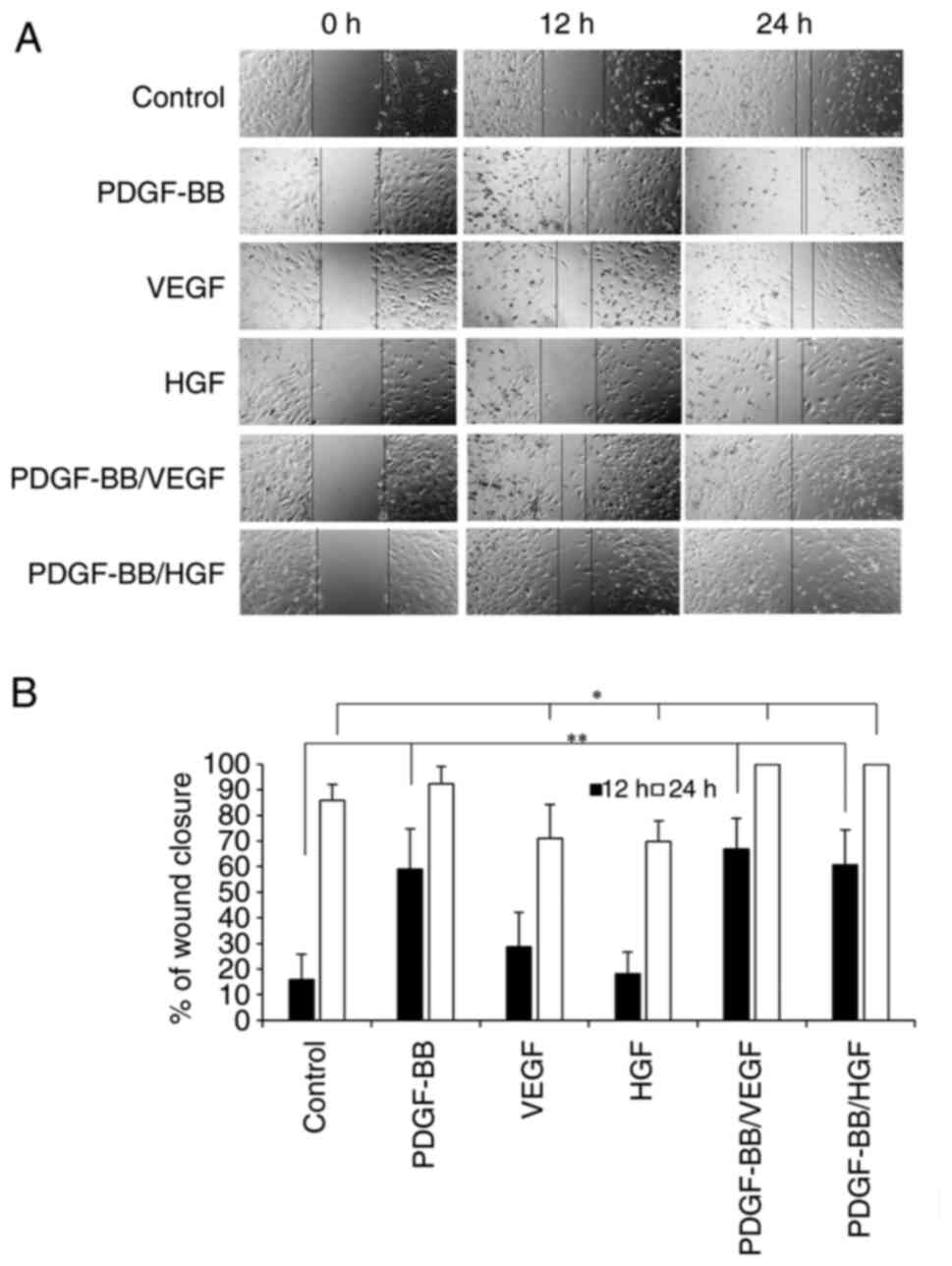
To investigate the effects of growth factors on hASC
migration, wound closure was measured after 12 h of incubation.
PDGF-BB, PDGF-BB/VEGF and PDGF-BB/HGF markedly increased cell
migration compared with the control (Fig. 5A and B). PDGF-BB/VEGF and PDGF-BB/HGF
treatments showed 100% wound closure by 24 h of incubation.
PDGF-BB/VEGF and PDGF-BB/HGF appeared to be the most effective
combination to induce cell migration. Treatment with VEGF or HGF
alone had no effects on cell migration.
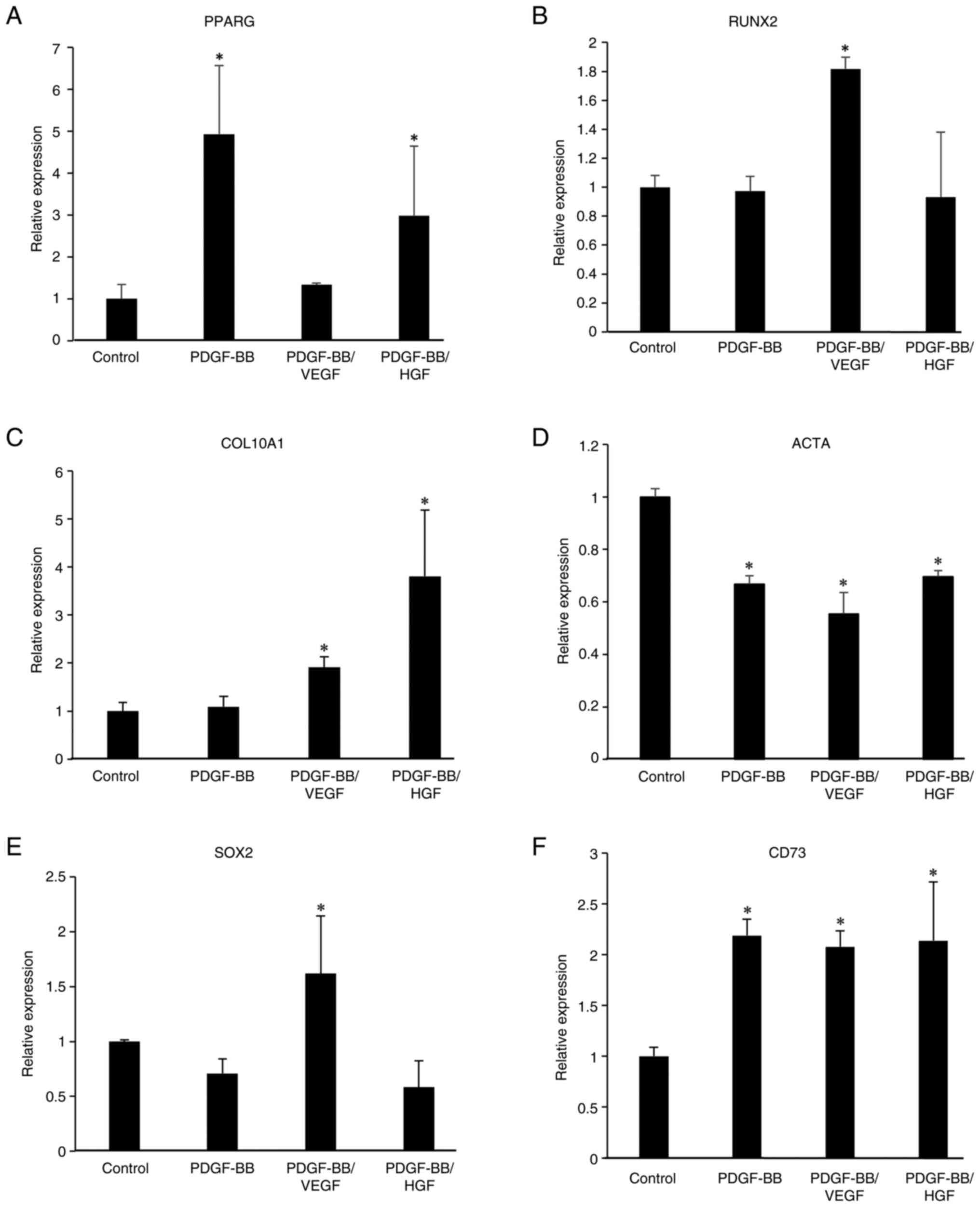
Gene expression in PDGF-BB-, VEGF- and
HGF-treated cells
To examine the effects of PDGF-BB, VEGF and HGF on
the gene expression of stem cell and differentiation markers,
RT-qPCR was performed on growth factor-treated cells for 6 days. As
shown in Fig. 6, PDGF-BB,
PDGF-BB/VEGF and PDGF-BB/HGF increased the mRNA levels of
peroxisome proliferator-activated receptor γ (PPARG), an adipogenic
marker, indicating that these growth factors could be involved in
adipocyte differentiation. Conversely, the levels of RUNX family
transcription factor 2 (RUNX2), an osteogenic marker, and actin α1
(ACTA), a myogenic marker, were decreased in PDGF-BB-treated cells.
Additionally, the expression level of collagen type X α1 chain
(COL10A1), a chondrogenesis-related gene, was decreased by
treatment with PDGF-BB, but was restored in PDGF-BB/VEGF- and
PDGF-BB/HGF-treated cells. An increase in the levels of SRY-box
transcription factor 2 (SOX2), a stem cell marker, was observed in
the PDGF-BB/VEGF and PDGF-BB/HGF groups, but not in the PDGF-BB
group. The levels of CD73, a mesenchymal stem cell marker, were
decreased by treatment with PDGF-BB and PDGF-BB/VEGF.
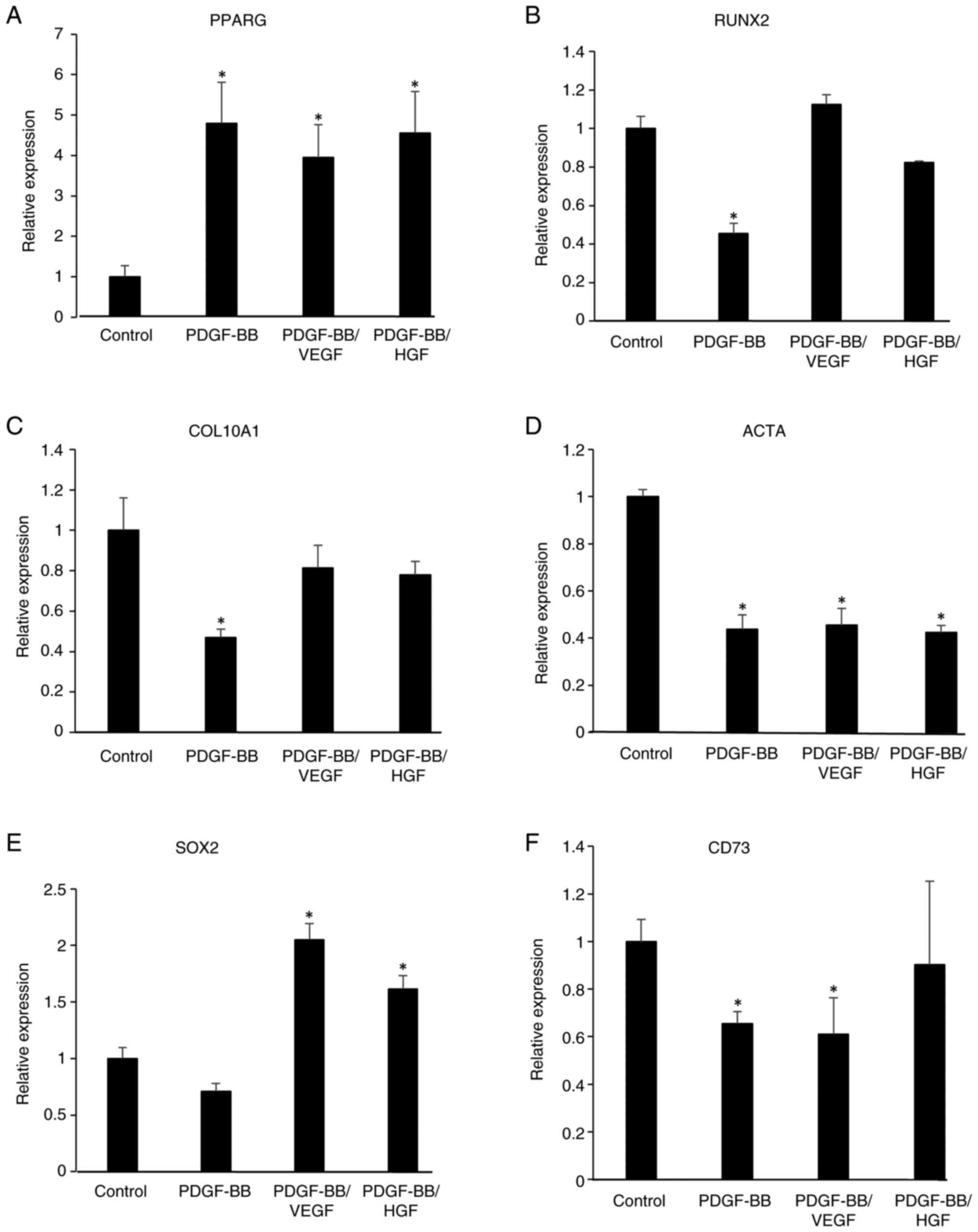 | Figure 6Gene expression of
differentiation-related genes, including (A) PPARG, (B) RUNX2, (C)
COL10A1 and (D) ACTA, and stem cell-related genes, including (E)
SOX2 and (F) CD73 in growth factor-treated hASCs. hASCs were
incubated in DMEM without or with PDGF-BB (20 ng/ml), VEGF (1
ng/ml), HGF (1 ng/ml), PDGF-BB (20 ng/ml)/VEGF (1 ng/ml) and
PDGF-BB (20 ng/ml)/HGF (1 ng/ml), and the cells were cultured for 6
days. The individual medium was changed every 2 days. Total RNA in
the cells was isolated and reverse transcription-quantitative PCR
was performed. mRNA levels were normalized to GAPDH mRNA
expression. Data are presented as the mean ± SD (n=3).
*P<0.05 vs. control. hASCs, human adipose-derived
stem cells; PPARG, peroxisome proliferator-activated receptor γ;
RUNX2, RUNX family transcription factor 2; COL10A1, collagen type X
α1 chain; ACTA, actin α1; SOX2, SRY-box transcription factor 2. |
Effects of PDGF-BB, VEGF and HGF on
hASC spheroid formation
Cell aggregates were observed when suspended cells
were cultured in a low-attachment culture plate with notably few
adherent cells on day 2 (Fig. 7A).
The cells formed spheroids of various sizes and the number of
suspended cells decreased after further incubation. Treatment of
cells with PDGF-BB, PDGF-BB/VEGF and PDGF-BB/HGF increased the
number of spheroids (diameter >100 µm) with incubation time.
Since the spheroid number in all groups was zero at the start of
the experiment, following incubation there was a significant
formation of spheroids in all groups. At day 6, the number of
spheroids 100-200 µm was high after treatment with PDGF-BB or
PDGF-BB/VEGF, and spheroids >200 µm were observed in PDGF-BB-,
PDGF-BB/VEGF- and PDGF-BB/HGF-treated cells (Fig. 7B). Thus, PDGF plays a dominant role
in spheroid formation.
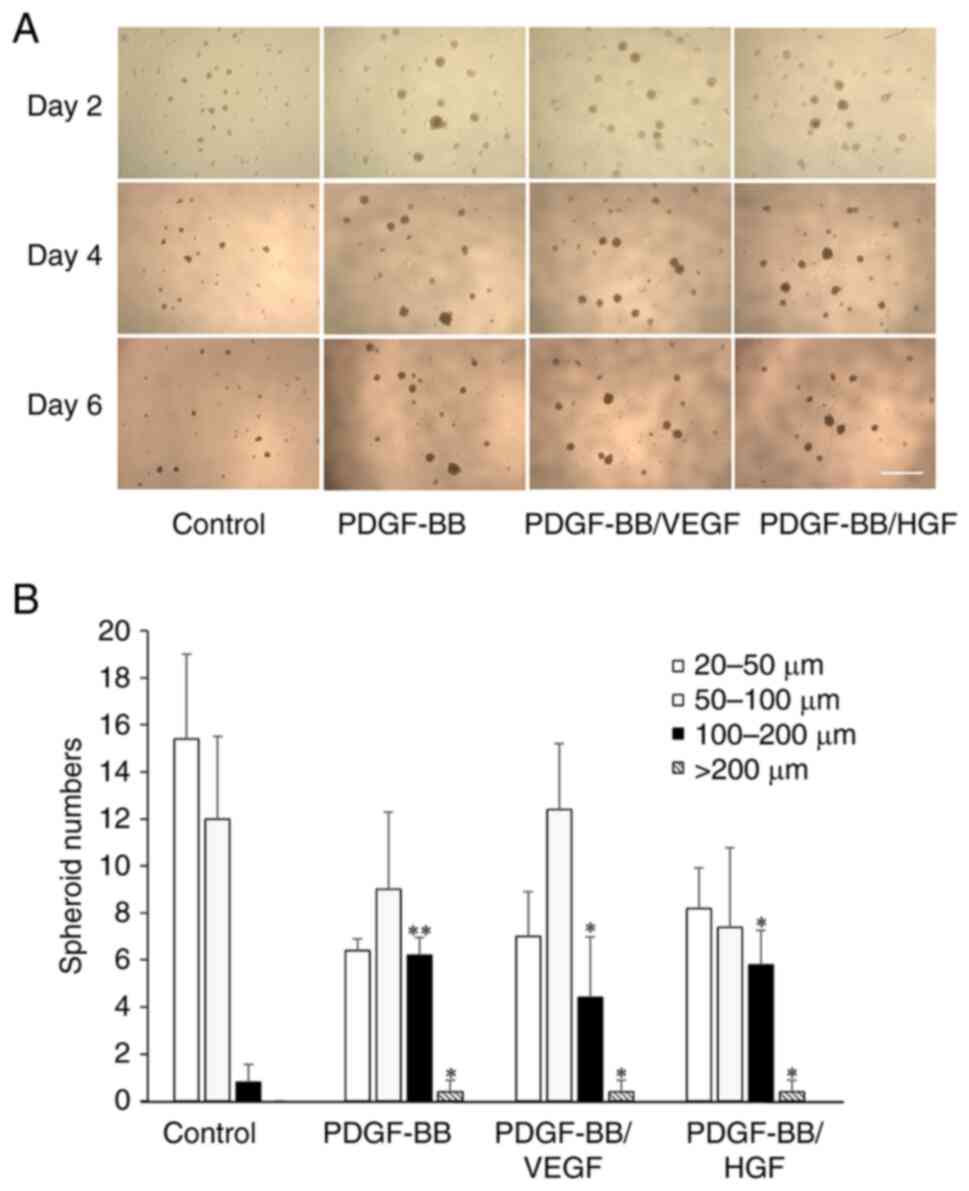 | Figure 7Spheroid formation of hASCs with
PDGF-BB, PDGF-BB/VEGF and PDGF-BB/HGF. (A) Representative
microscopy images of spheroids under the indicated conditions.
Cells were seeded at 1,000 cells/well in low-attachment plates
(96-wells), and cultured with DMEM containing PDGF-BB (20 ng/ml),
VEGF (1 ng/ml), HGF (1 ng/ml), PDGF-BB (20 ng/ml)/VEGF (1 ng/ml)
and PDGF-BB (20 ng/ml)/HGF (1 ng/ml) for 6 days. The cells were
also cultured with serum-free DMEM as a control (scale bar, 1 mm).
(B) Quantification of total spheroids distributed by size. The
shapes of the spheroids were analyzed by ImageJ software, and
spheres <20 µm were excluded. The diameter of spheroids was
divided into 20-50, 50-100, 100-200 and >200 µm, and the number
of spheroid in each group was shown. Data are presented as the mean
± SD (n=3). *P<0.05; **P<0.01 vs.
control. hASCs, human adipose-derived stem cells; PDGF,
platelet-derived growth factor; VEGF, vascular endothelial growth
factor; HGF, hepatocyte growth factor. |
Gene expression in hASCs from day 6 spheroids was
examined. RT-qPCR data showed that COL10A1 and RUNX2 mRNA levels
were increased by treatment with PDGF-BB/VEGF compared with the
control group, and PDGF-BB/HGF treatment also increased COL10A1
mRNA levels. Furthermore, treatment with PDGF-BB md PDGF-BB/HGF led
to an increase in PPARG mRNA, but ACTA mRNA levels were decreased
in PDGF-BB, PDGF-BB/VEGF, and PDGF-BB/HGF groups (Fig. 8). SOX2 mRNA levels were increased
in PDGF-BB/VEGF-treated cells, and CD73 mRNA levels increased with
all treatments. These results indicated that the expression of stem
cell markers in the spheroids was high.
 | Figure 8Gene expression of
differentiation-related genes, including (A) PPARG, (B) RUNX2, (C)
COL10A1 and (D) ACTA, and stem cell-related genes, including (E)
SOX2 and (F) CD73 in growth factor-treated hASC spheroids.
Spheroids treated with the indicated growth factors for 6 days were
collected by centrifugation at 1,000 x g for 5 min at room
temperature. Total RNA in the cells was isolated and reverse
transcription-quantitative PCR was performed. Data are presented as
the mean ± SD (n=3). *P<0.05 vs. control. hASCs,
human adipose-derived stem cells; PPARG, peroxisome
proliferator-activated receptor γ; RUNX2, RUNX family transcription
factor 2; COL10A1, collagen type X α1 chain; ACTA, actin α1; SOX2,
SRY-box transcription factor 2. |
Discussion
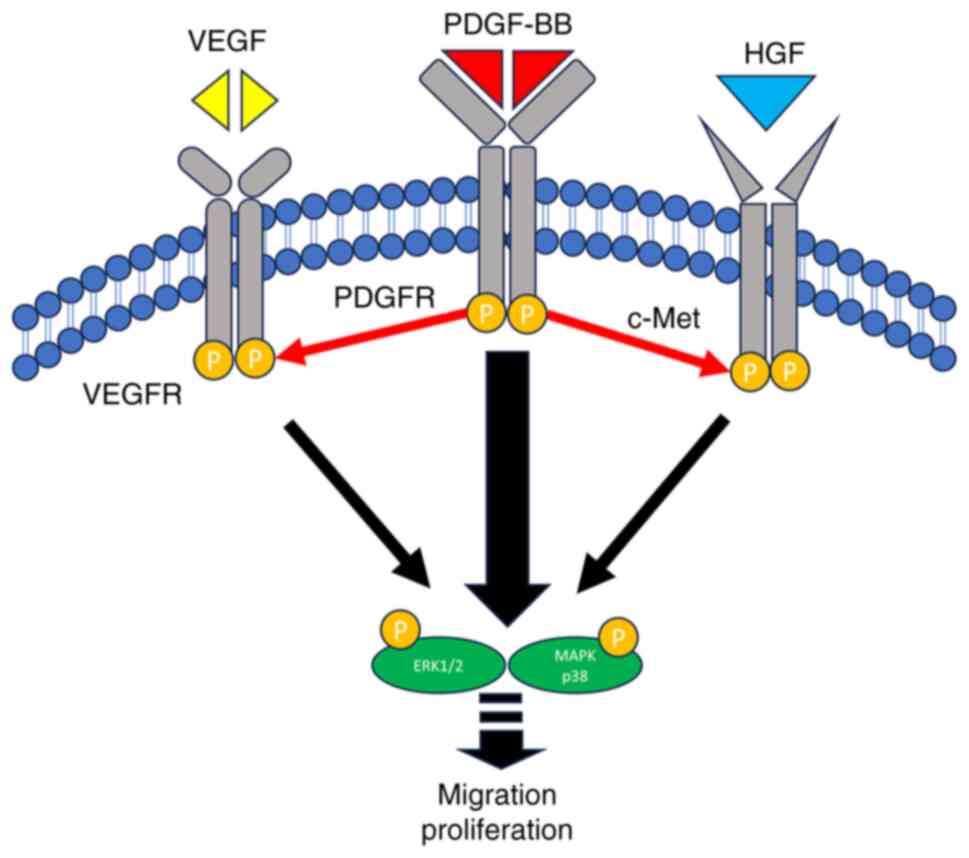
To the best of our knowledge, the present study is
the first to demonstrate that PDGF/PDGFR signaling predominantly
stimulates hASC migration and proliferation (Fig. 9). PDGF-BB is a major growth factor
present in both PLTMax and PRP (17,20).
Proteolytic treatment with PLTMax reduced the enhancement of hASCs
proliferation, suggesting that proteins, including growth factors
and adhesion molecules, were involved in such stimulation. Our
group previously reported that PDGF treatment induced the
proliferation of hASCs (20).
However, the effect of PDGF on cell proliferation was lower than
that of PRP (or PLTMax) (20). In
the present study, although it was examined whether growth factors
such as VEGF, HGF and EGF stimulated hASC proliferation, treatment
with VEGF, HGF or EGF alone did not exert any proliferative effect.
When hASCs were incubated with PLTMax for 48 h, the highest
proliferative effect was observed with 3-5% PLTMax. A stimulatory
effect similar to that of PLTMax was observed with the combined use
of PDGF-BB, VEGF and HGF. Furthermore, the number of
Ki-67+ cells increased significantly after treatment
with PDGF-BB, PDGF-BB/VEGF and PDGF-BB/HGF, but not with VEGF or
HGF alone. EGF or insulin-like growth factor (IGF) combined with
PDGF was less effective for hASC proliferation than PRP alone
(20). Thus, VEGF and HGF
synergistically enhanced the PDGF-dependent proliferation of hASCs.
In addition, as shown in the present study PDGF is a primary factor
responsible for cell proliferation and can draw on the potential of
other growth factors including VEGF, HGF and EGF in PLTMax when
hASCs are cultured with PLTMax (or PRP).
The addition of 20 ng/ml PDGF-BB resulted in
significantly higher stimulation of hASC proliferation (Fig. 1). Kang et al (27) also found that PDGF induced the
chemotactic migration of human adipose tissue-derived MSCs in a
dose-dependent manner (5-25 ng/ml) and increased the number of
cells after incubation for 48 h. Additionally, PDGF isoforms
stimulate the migration and proliferation of equine epithelial
cells, keratinocytes (28), and
mesoderm-derived epithelial and glial cells (29) to different extents, indicating that
the extent of the PDGF proliferative effect is dependent on
different cell types owing to different quantities of receptors.
Thus, the distribution of PDGFR and the concentration of PDGF
isoforms are important determinants of the fate of different cell
types.
Accompanied by the stimulation of cell
proliferation, the wound-healing assays revealed that the migration
of hASCs was enhanced by treatment with PDGF and VEGF. Growth
factors released after activation could stimulate the chemotaxis of
fibroblasts into wound tissues (30). Among these growth factors, PDGF is
a potent chemotactic factor that activates cell adhesion molecules,
including integrins (31). HGF,
EGF and TGF-β positively regulate focal adhesion molecules and
integrins (32-34).
During inflammation, PDGF induces the migration and proliferation
of monocytes, fibroblasts and vascular smooth muscle cells;
attracts monocytes to sites of vascular injury; and limits
proinflammatory events through autocrine feedback inhibition of
platelet aggregation (35).
Furthermore, PDGF is a strong pro-angiogenic factor that stimulates
the migration and proliferation of endothelial cells (36).
PDGFR consists of α and β subunits (37). PDGFR-α can bind to either PDGF-A or
PDGF-B, while PDGFR-β can bind only to PDGF-B. PDGF-BB binds to the
two subunits of PDGFR and is ~10-fold more mitogenic than PDGF-AA
(37). Since hASCs express high
levels of PDGFR-β, which is fundamental in cell proliferation,
adhesion and migration at the commencement of angiogenesis
(38,39), it is possible that the
PDGF-B/PDGFR-β signaling may play an important role in cell
stimulation. On the other hand, PDGF-B is not expressed by hASCs,
whereas PDGF-D and PDGFR-β are, and PDGF-D also upregulates growth
factor expression and exerts a strong mitogenic effect on hASCs
(40). PDGF-D is a minor component
of platelets (41). Therefore, it
mainly acts as a mitogenic factor in response to inflammation and
tissue injury regeneration.
The present study demonstrated that rapid
phosphorylation of VEGFR2 and c-Met occurred upon treatment of
hASCs with PDGF-BB. This indicated that transactivation of these
receptors by PDGF-BB induced the phosphorylation of the mitogenic
enzymes ERK1/2 and p38MAPK, leading to cell migration and
proliferation. Conversely, neither VEGF nor HGF activated any
receptor for PDGF, HGF or VEGF in hASCs, which was consistent with
the lack of stimulation on cell proliferation. VEGFR and c-Met may
be located intracellularly in hASCs under resting conditions. By
contrast, phosphorylation of VEGFR2 and c-Met occurs without
binding of the corresponding ligands once PDGFR-β activation
occurs. Interactions between PDGFR and EGFR have also been reported
(42). Increased PDGFR-β kinase
activity leads to upregulation of VEGF and VEGFR2 mRNAs in bone
marrow MSCs, acting directly on endothelial cells and leading to
increased vessel formation (43).
To the best of our knowledge, no previous studies have examined the
transactivation of PDGFR to VEGFR and c-Met in any cell type.
Although the mechanisms underlying VEGFR2 and c-Met activation by
PDGF are unclear, it is possible that the activation signal of
PDGFR-β transactivates other receptors in a manner mediated by
intracellular molecules. G protein-coupled receptors and
sphingosine 1-phosphate receptor can transfer activating signals to
cell surface receptors (44).
Thus, the predominant control of PDGF/PDGFR signaling to activate
other growth factor receptors is essential for promoting hASC
migration and proliferation.
In the presence of PDGF-BB, VEGF further increased
the levels of phospho-VEGFR and phospho-c-Met, and subsequently
markedly activated ERK1/2 and p38 MAPK. These events were ascribed
to the synergistic effect of VEGF and c-Met on PDGF-dependent
enhancement of hASC migration and proliferation. Considering that
all inhibitors of PDGFR, VEGFR and c-Met reduced the proliferation
of hASCs in the presence of PDGF-BB, the activation of these
receptors by PDGF appears to be required for cellular activation.
Among the growth factors present in PLTs, PDGF, IGF and FGF promote
proliferation and cell cycle transition in human MSCs (27,41).
However, the effects of TGF-β and EGF on MSCs remain unknown.
Treatment of hASCs with EGF and basic FGF promotes the cell
proliferation and differentiation of neural lineage (45). When hASCs were treated with a
combination of FGF2 and VEGF, the promotion of cell proliferation
and endothelial differentiation was accompanied by an increase in
the expression of the endothelial markers CD31, von Willebrand
factor and CD144(46). The present
data demonstrated that VEGF and HGF promoted the PDGF-dependent
proliferation of hASCs, with high mRNA expression of the stem cell
markers SOX2 and CD73. Similarly, PDGF-BB combined with VEGF or HGF
enhanced the formation of spheroids in which hASCs abundantly
expressed SOX2 and CD73 mRNAs. These results indicated that PDGF
may be an essential growth factor that promoted maintenance of hASC
stemness.
Several studies have shown that MSCs do not express
VEGFR (37) and that VEGF can bind
to PDGFR (47), suggesting that
VEGF induces MSC proliferation by activating the PDGF/PDGFR axis.
By contrast, the present data clearly demonstrated that no PDGFR-β
activation occurred by treating hASCs with VEGF alone. The reason
for this discrepancy is not clear, but it is possible that PDGFR is
localized on the cell surface of MSCs. However, growth hormone
receptors are generally translocated to the plasma membrane upon
phosphorylation (48). As shown in
the present study, once PDGFR-β and PDGF-BB phosphorylate VEGFR2
and c-Met in hASCs, the activated receptors are translocated to the
cell surface and bind to their cognate ligands. Thus, the
activation of PDGF/PDGFR signaling is indispensable for the
stimulation of other signaling pathways in hASCs, improving the
wound healing properties of hASCs.
PDGF has multiple effects on the differentiation of
hASCs. PDGF-B enhances vascular network stability and osteogenic
differentiation, resulting in the development of vascularized bone
tissues by hASCs (49). PDGF
promotes the tenogenic differentiation of hASCs (49,50).
RT-qPCR analysis showed that mRNA expression of the osteogenic
marker RUNX2 was slightly increased upon treatment with PDGF-BB and
VEGF, and this effect was potent in spheroids. The mRNA levels of
the chondrogenic marker COL10A1 increased in spheroids treated with
all the evaluated growth factors. Although PDGF treatment of hASCs
reduced the expression of adipogenesis-related genes in a previous
study (51), the present data
showed an increase in the mRNA expression of the adipogenic marker
PPARG by PDGF-BB, PDGF-BB/VEGF and PDGF-BB/HGF treatment compared
to the control. The reasons for such differences in gene expression
between previous and present data are unclear. hASCs produce
various paracrine factors that are dependent on different culture
conditions (52,53) and therefore the cells may develop
along different lineages. In the present study, SOX2 and CD73 were
selected as stem cell markers to examine the maintenance of stem
cell self-renewal. SOX2 mRNA was increased by treatment with
PDGF-BB/VEGF under monolayer and spheroid culture conditions, and
was virtually unchanged with PDGF-BB exposure. CD73 mRNA levels
increased in all spheroid treatment groups, but remained unchanged
in monolayer cells. Thus, the supplementation of hASC spheroids
with PDGF-BB, VEGF and HGF positively affected stem cell
pluripotency. Furthermore, VEGF and HGF synergistically enhanced
the PDGF-dependent migration and proliferation of hASCs. However,
other additional effects of VEGF and HGF on the fate of hASCs were
not observed. The proliferation and differentiation potential of
hASC spheroids differs depending on the culture conditions,
including supplementation with growth factors, scaffold environment
and cell density, and therefore further systematic gene expression
studies are required to elucidate the roles of VEGF, HGF and PDGF
in the differentiation potential of hASCs.
Several studies have reported the
proliferation-promoting effects of PLTs on bone marrow-derived MSCs
(12,54). Additionally, Huang et al
(55) reported that PLTs enhanced
neuronal proliferation and differentiation to a greater extent than
FBS. Additionally, PLTs contain higher concentrations of various
growth factors other than FBS (17). Collectively, PLTs are a promising
xenogeneic-free substitute for FBS in hASC cultures, with an
underlying mechanism of growth factor-induced proliferation. The
present study demonstrated that the combination of the growth
factors PDGF-BB, VEGF and HGF promoted the migration and
proliferation of hASCs. It is unclear whether the cells could
acquire multiple functions for tissue regeneration or senescence,
although this may be induced upon long-term culture (53). This may be further explained by the
fact that PLTs (or PRP), which also contain adhesion molecules,
chemokines and various plasma proteins, share similar importance
with platelets and leukocytes during wound healing (56). The combination of other factors,
including chemokines and adhesion molecules, with VEGF and HGF
could further enhance the proliferative effect on hASCs through
PDGF/PDGFR signaling. Improving the viability and stability of
hASCs by preserving their homogeneity may contribute to the
development of stem cell therapeutics using biofunctional
materials.
In conclusion, the present study demonstrated that
VEGF and HGF treatment synergistically enhanced the
PDGF-BB-dependent proliferation of hASCs. hASC migration after
PDGF-BB/VEGF and PDGF-BB/HGF treatment was greater than that after
PDGF-BB treatment alone. These enhancements were accompanied by the
phosphorylation of PDGFR, VEGFR2 and c-Met. RT-qPCR analysis
revealed high expression of stem cell markers in growth
factor-treated cells. During hASC spheroid formation, PDGF-BB
played a predominant role in the synergistic effects of VEGF and
HGF. These observations provide new insights for future
investigations surrounding the beneficial effects of supplementing
cultured hASCs with PDGF-BB and VEGF to repair injured tissues.
Supplementary Material
Proliferation of hASCs with PLTMax.
(A) Dosedependent viability of hASCs with PLTMax. Cells were
incubated with the indicated concentrations of PLTMax for 48 h.
Cell viability was examined using CCK-8 assays and measuring the
optical density at 450 nm. Data are expressed as the mean ± SD of
three experiments. *P<0.05; **P<0.01
vs. no addition. (B) Effect of trypsin treatment of PLTMax on hASC
proliferation. PLTMax was incubated with trypsin (1 mg/ml) at 37˚C
for 30 min, and the reaction was stopped by the addition of TI (at
a final concentration of 2 mg/ml). The cells were cultured with or
without 3% PLTMax, 3% trypsintreated PLTMax and 3% PLTMax plus TI
(60 μg/ml), for 48 h, and CCK-8 assays were performed. Data
are expressed as the mean ± SD (n=4). *P<0.05 vs.
PLTMax treatment. hASCs, human adipose-derived stem cells; CCK-8,
Cell Counting Kit-8; PLT, platelet lysate; TI, trypsin
inhibitor.
Effect of PDGF-BB on the proliferation
of hASCs. Cells were cultured in DMEM with or without the indicated
concentrations of PDGF-BB for 48 h. Cell viability was measured
using Cell Counting Kit-8 assays. Data are shown as the mean ± SD
(n=3). **P<0.01 vs. no addition. hASCs, human
adipose-derived stem cells; PDGF, platelet-derived growth
factor.
Synergistic effect of EGF on the
PDGF-dependent viability of hASCs. (A) hASCs were incubated with
PDGF (1, 5 and 20 ng/ml), EGF (1 and 5 ng/ml) and PDGF (20
ng/ml)/EGF (1 ng/ml) for 48 h. Cell viability was then assayed
using Cell Counting Kit-8 assays. The cell viability was examined
by optical absorbance at 450 nm using a multi-well plate reader
(EnSpire 2300 Multilabel Reader; PerkinElmer, Inc.). Data are
presented as the mean ± SD (n=3). *P<0.05 vs. no
addition. (B) Pharmacological inhibition of hASC proliferation when
cultured with PDGF (20 ng/ml)/EGF (1 ng/ml). The cells were
cultured with or without PD153035 (10 μM; Cayman Chemical
Company; cat. no. 18080), imatinib (5 μM) and PD153035 plus
imatinib. Data are expressed as the mean ± SD (n=4).
**P<0.01 vs. no inhibitor. hASCs, human
adipose-derived stem cells; PDGF, platelet-derived growth factor;
EGF, epidermal growth factor.
Acknowledgements
The authors would like to thank the members of The
Life Science Research Laboratory at Kansai Medical University
(Hirakata, Japan) for their technical assistance.
Funding
Funding: The present study was supported by a Grant-in-Aid for
Scientific Research (C) from the Ministry of Education, Science,
Sports, Japan (grant no. 22K0989).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS conducted all the laboratory work, acquired data
and drafted the manuscript. NK and ST designed this study and
revised the manuscript. MF, AK and SK acquired the RT-qPCR data.
The first draft of the manuscript was written by ZS, MF, ST and NK.
ZS, MF, ST and NK confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Board of Kansai Medical University in accordance with the ethical
guidelines of the Helsinki Declaration of 1975 (approval no.
2017094; Hirakata, Japan). All specimens were collected from one
donor and informed oral and written consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pittenger MF, Discher DE, Péault BM,
Phinney DG, Hare JM and Caplan AI: Mesenchymal stem cell
perspective: Cell biology to clinical progress. NPJ Regen Med.
4(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Koźlik M and Wójcicki P: The use of stem
cells in plastic and reconstructive surgery. Adv Clin Exp Med.
23:1011–1017. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang M, Zhang P, Liu Y and Zhou Y: GSK3
inhibitor AR-A014418 promotes osteogenic differentiation of human
adipose-derived stem cells via ERK and mTORC2/Akt signaling
pathway. Biochem Biophys Res Commun. 490:182–188. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Galeano-Garces C, Camilleri ET, Riester
SM, Dudakovic A, Larson DR, Qu W, Smith J, Dietz AB, Im HJ, Krych
AJ, et al: Molecular validation of chondrogenic differentiation and
hypoxia responsiveness of platelet-lysate expanded adipose
tissue-derived human mesenchymal stromal cells. Cartilage.
8:283–299. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Paul NE, Denecke B, Kim BS, Dreser A,
Bernhagen J and Pallua N: The effect of mechanical stress on the
proliferation, adipogenic differentiation and gene expression of
human adipose-derived stem cells. J Tissue Eng Regen Med.
12:276–284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jahromi M, Razavi S, Amirpour N and
Khosravizadeh Z: Paroxetine can enhance neurogenesis during
neurogenic differentiation of human adipose-derived stem cells.
Avicenna J Med Biotechnol. 8:152–158. 2016.PubMed/NCBI
|
|
8
|
Gaur M, Dobke M and Lunyak VV: Mesenchymal
stem cells from adipose tissue in clinical applications for
dermatological indications and skin aging. Int J Mol Sci.
18(208)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takahashi H, Ishikawa H and Tanaka A:
Regenerative medicine for Parkinson's disease using differentiated
nerve cells derived from human buccal fat pad stem cells. Hum Cell.
30:60–71. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Iudicone P, Fioravanti D, Bonanno G,
Miceli M, Lavorino C, Totta P, Frati L, Nuti M and Pierelli L:
Pathogen-free, plasma-poor platelet lysate and expansion of human
mesenchymal stem cells. J Transl Med. 12(28)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang CJ, Sun YC, Christopher K, Pai AS,
Lu CJ, Hu FR, Lin SY and Chen WL: Comparison of corneal
epitheliotrophic capacities among human platelet lysates and other
blood derivatives. PLoS One. 12(e0171008)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Doucet C, Ernou I, Zhang Y, Llense JR,
Begot L, Holy X and Lataillade JJ: Platelet lysates promote
mesenchymal stem cell expansion: A safety substitute for animal
serum in cell-based therapy applications. J Cell Physiol.
205:228–236. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Crespo-Diaz R, Behfar A, Butler GW, Padley
DJ, Sarr MG, Bartunek J, Dietz AB and Terzic A: Platelet lysate
consisting of a natural repair proteome supports human mesenchymal
stem cell proliferation and chromosomal stability. Cell Transplant.
20:797–811. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Díez JM, Bauman E, Gajardo R and Jorquera
JI: Culture of human mesenchymal stem cells using a candidate
pharmaceutical grade xeno-free cell culture supplement derived from
industrial human plasma pools. Stem Cell Res Ther.
6(28)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stute N, Holtz K, Bubenheim M, Lange C,
Blake F and Zander AR: Autologous serum for isolation and expansion
of human mesenchymal stem cells for clinical use. Exp Hematol.
32:1212–1225. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alonso-Camino V and Mirsch B: Rapid
expansion of mesenchymal stem/stromal cells using optimized media
supplemented with human platelet lysate PLTMax® or
PLTGold®, suitable for cGMP expansion at large scale.
Cytotherapy. 21 (Suppl)(S85)2019.
|
|
17
|
Kakudo N, Morimoto N, Ma Y and Kusumoto K:
Differences between the proliferative effects of human platelet
lysate and fetal bovine serum on human adipose-derived stem cells.
Cells. 8(1218)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang JM, Feng FE, Wang QM, Zhu XL, Fu HX,
Xu LP, Liu KY, Huang XJ and Zhang XH: Platelet-derived growth
factor-bb protects mesenchymal stem cells (MSCs) derived from
immune thrombocytopenia patients against apoptosis and senescence
and maintains MSC-mediated immunosuppression. Stem Cells Transl
Med. 5:1631–1643. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Papadopoulos N and Lennartsson J: The
PDGF/PDGFR pathway as a drug target. Mol Aspects Med. 62:75–88.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lai F, Kakudo N, Morimoto N, Taketani S,
Hara T, Ogawa T and Kusumoto K: Platelet-rich plasma enhances the
proliferation of human adipose stem cells through multiple
signaling pathways. Stem Cell Res Ther. 9(107)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Imam SS, Al-Abbasi FA, Hosawi S, Afzal M,
Nadeem MS, Ghoneim MM, Alshehri S, Alzarea SI, Alquraini A, Gupta G
and Kazmi I: Role of platelet rich plasma mediated repair and
regeneration of cell in early stage of cardiac injury. Regen Ther.
19:144–153. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chun SY, Lim JO, Lee EH, Han MH, Ha YS,
Lee JN, Kim BS, Park MJ, Yeo M, Jung B and Kwon TG: Preparation and
characterization of human adipose tissue-derived extracellular
matrix, growth factors, and stem cells: A concise review. Tissue
Eng Regen Med. 16:385–393. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ma Y, Kakudo N, Morimoto N, Lai F,
Taketani S and Kusumoto K: Fibroblast growth factor-2 stimulates
proliferation of human adipose-derived stem cells via Src
activation. Stem Cell Res Ther. 10(350)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kakudo N, Minakata T, Mitsui T, Kushida S,
Notodihardjo FZ and Kusumoto K: Proliferation-promoting effect of
platelet-rich plasma on human adipose-derived stem cells and human
dermal fibroblasts. Plast Reconstr Surg. 122:1352–1360.
2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fukui M, Matsuoka Y, Taketani S, Higasa K,
Hihara M, Kuro A and Kakudo N: Accelerated angiogenesis of human
umbilical vein endothelial cells under negative pressure was
associated with the regulation of gene expression involved in the
proliferation and migration. Ann Plast Surg. 89:e51–e59.
2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kang YJ, Jeon ES, Song HY, Woo JS, Jung
JS, Kim YK and Kim JH: Role of c-Jun N-terminal kinase in the
PDGF-induced proliferation and migration of human adipose
tissue-derived mesenchymal stem cells. J Cell Biochem.
95:1135–1145. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Haber M, Cao Z, Panjwani N, Bedenice D, Li
WW and Provost PJ: Effects of growth factors (EGF, PDGF-BB and
TGF-beta 1) on cultured equine epithelial cells and keratocytes:
Implications for wound healing. Vet Ophthalmol. 6:211–217.
2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Knorr M, Völker M, Denk PO, Wunderlich K
and Thiel HJ: Proliferative response of cultured human tenon's
capsule fibroblasts to platelet-derived growth factor isoforms.
Graefes Arch Clin Exp Ophthalmol. 235:667–671. 1997.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Seppä H, Grotendorst G, Seppä S,
Schiffmann E and Martin GR: Platelet-derived growth factor in
chemotactic for fibroblasts. J Cell Biol. 92:584–588.
1982.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Donovan J, Abraham D and Norman J:
Platelet-derived growth factor signaling in mesenchymal cells.
Front Biosci (Landmark Ed). 18:106–119. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Bellas RE, Bendori R and Farmer SR:
Epidermal growth factor activation of vinculin and beta 1-integrin
gene transcription in quiescent Swiss 3T3 cells. Regulation through
a protein kinase C-independent pathway. J Biol Chem.
266:12008–12014. 1991.PubMed/NCBI
|
|
33
|
Celotti F, Colciago A, Negri-Cesi P,
Pravettoni A, Zaninetti R and Sacchi MC: Effect of platelet-rich
plasma on migration and proliferation of SaOS-2 osteoblasts: Role
of platelet-derived growth factor and transforming growth
factor-beta. Wound Repair Regen. 14:195–202. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li JF, Yin HL, Shuboy A, Duan HF, Lou JY,
Li J, Wang HW and Wang YL: Differentiation of hUC-MSC into
dopaminergic-like cells after transduction with hepatocyte growth
factor. Mol Cell Biochem. 381:183–190. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lopatina T, Favaro E, Grange C, Cedrino M,
Ranghino A, Occhipinti S, Fallo S, Buffolo F, Gaykalova DA, Zanone
MM, et al: PDGF enhances the protective effect of adipose stem
cell-derived extracellular vesicles in a model of acute hindlimb
ischemia. Sci Rep. 8(17458)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lopatina T, Bruno S, Tetta C, Kalinina N,
Porta M and Camussi G: Platelet-derived growth factor regulates the
secretion of extracellular vesicles by adipose mesenchymal stem
cells and enhances their angiogenic potential. Cell Commun Signal.
12(26)2014.PubMed/NCBI View Article : Google Scholar : Veevers-Lowe J,
Ball SG, Shuttleworth A and Kielty CM: Mesenchymal stem cell
migration is regulated by fibronectin through
α5β1-integrin-mediated activation of PDGFR-β and potentiation of
growth factor signals. J Cell Sci 124: 1288-1300, 2011.
|
|
37
|
Ball SG, Shuttleworth CA and Kielty CM:
Mesenchymal stem cells and neovascularization: Role of
platelet-derived growth factor receptors. J Cell Mol Med.
11:1012–1030. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Veevers-Lowe J, Ball SG, Shuttleworth A
and Kielty CM: Mesenchymal stem cell migration is regulated by
fibronectin through α5β1-integrin-mediated activation of PDGFR-β
and potentiation of growth factor signals. J Cell Sci.
124:1288–1300. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jung KH, Chu K, Lee ST, Bahn JJ, Jeon D,
Kim JH, Kim S, Won CH, Kim M, Lee SK and Roh JK: Multipotent
PDGFRβ-expressing cells in the circulation of stroke patients.
Neurobiol Dis. 41:489–497. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gao Z, Daquinag AC, Su F, Snyder B and
Kolonin MG: PDGFRα/PDGFRβ signaling balance modulates progenitor
cell differentiation into white and beige adipocytes. Development.
145(dev155861)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yan L, Zhou L, Yan B, Zhang L, Du W, Liu
F, Yuan Q, Tong P, Shan L and Efferth T: Growth factors-based
beneficial effects of platelet lysate on umbilical cord-derived
stem cells and their synergistic use in osteoarthritis treatment.
Cell Death Dis. 11(857)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Brizzi MF, Tarone G and Defilippi P:
Extracellular matrix, integrins, and growth factors as tailors of
the stem cell niche. Curr Opin Cell Biol. 24:645–651.
2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Magnusson PU, Looman C, Ahgren A, Wu Y,
Claesson-Welsh L and Heuchel RL: Platelet-derived growth factor
receptor-beta constitutive activity promotes angiogenesis in vivo
and in vitro. Arterioscler Thromb Vasc Biol. 27:2142–2149.
2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kilpatrick LE and Hill SJ: Transactivation
of G protein-coupled receptors (GPCRs) and receptor tyrosine
kinases (RTKs): Recent insights using luminescence and fluorescence
technologies. Curr Opin Endocr Metab Res. 16:102–112.
2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hu F, Wang X, Liang G, Lv L, Zhu Y, Sun B
and Xiao Z: Effects of epidermal growth factor and basic fibroblast
growth factor on the proliferation and osteogenic and neural
differentiation of adipose-derived stem cells. Cell Reprogram.
15:224–232. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Khan S, Villalobos MA, Choron RL, Chang S,
Brown SA, Carpenter JP, Tulenko TN and Zhang P: Fibroblast growth
factor and vascular endothelial growth factor play a critical role
in endotheliogenesis from human adipose-derived stem cells. J Vasc
Surg. 65:1483–1492. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mamer SB, Chen S, Weddell JC, Palasz A,
Wittenkeller A, Kumar M and Imoukhuede PI: Discovery of
high-affinity pdgf-vegfr interactions: Redefining RTK dynamics. Sci
Rep. 7(16439)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bergeron JJ, Di Guglielmo GM, Dahan S,
Dominguez M and Posner BI: Spatial and temporal regulation of
receptor tyrosine kinase activation and intracellular signal
transduction. Annu Rev Biochem. 85:573–597. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kim WS, Park HS and Sung JH: The pivotal
role of PDGF and its receptor isoforms in adipose-derived stem
cells. Histol Histopathol. 30:793–799. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Younesi Soltani F, Javanshir S, Dowlati G,
Parham A and Naderi-Meshkin H: Differentiation of human
adipose-derived mesenchymal stem cells toward tenocyte by
platelet-derived growth factor-BB and growth differentiation
factor-6. Cell Tissue Bank. 23:237–246. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Artemenko Y, Gagnon A, Aubin D and Sorisky
A: Anti-adipogenic effect of PDGF is reversed by PKC inhibition. J
Cell Physiol. 204:646–653. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hye Kim J, Gyu Park S, Kim WK, Song SU and
Sung JH: Functional regulation of adipose-derived stem cells by
PDGF-D. Stem Cells. 33:542–556. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lee CS, Nicolini AM, Watkins EA, Burnsed
OA, Boyan BD and Schwartz Z: Adipose stem cell microbeads as
production sources for chondrogenic growth factors. J Stem Cells
Regen Med. 10:38–48. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Carrancio S, López-Holgado N,
Sánchez-Guijo FM, Villarón E, Barbado V, Tabera S, Díez-Campelo M,
Blanco J, San Miguel JF and Del Cañizo MC: Optimization of
mesenchymal stem cell expansion procedures by cell separation and
culture conditions modification. Exp Hematol. 36:1014–1021.
2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Huang CT, Chu HS, Hung KC, Chen LW, Chen
MY, Hu FR and Chen WL: The effect of human platelet lysate on
corneal nerve regeneration. Br J Ophthalmol. 105:884–890.
2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Verma R, Kumar S, Garg P and Verma YK:
Platelet-rich plasma: A comparative and economical therapy for
wound healing and tissue regeneration. Cell Tissue Bank.
24:285–306. 2023.PubMed/NCBI View Article : Google Scholar
|