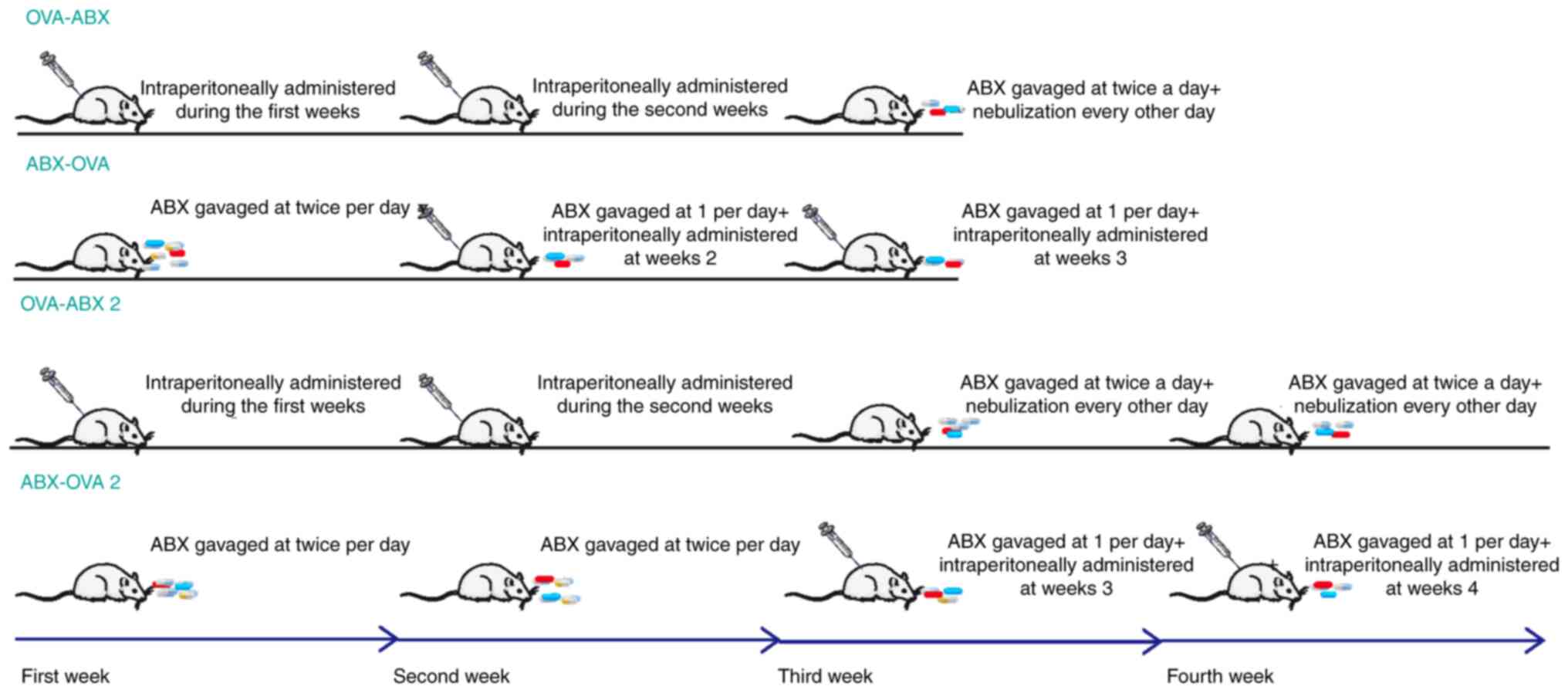
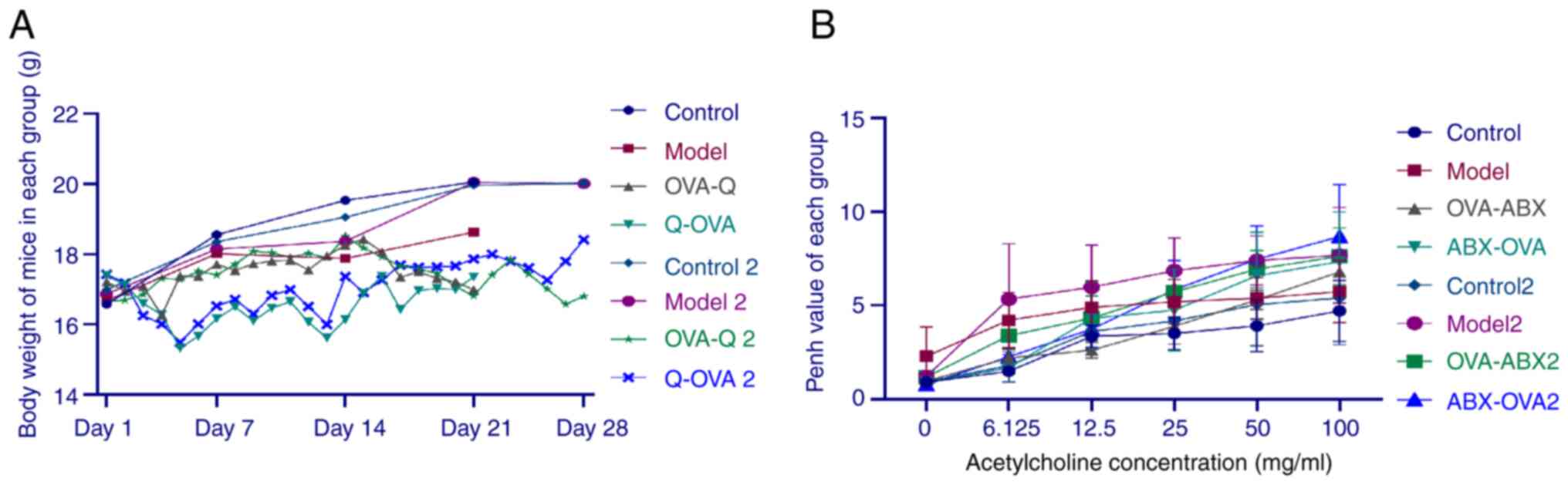
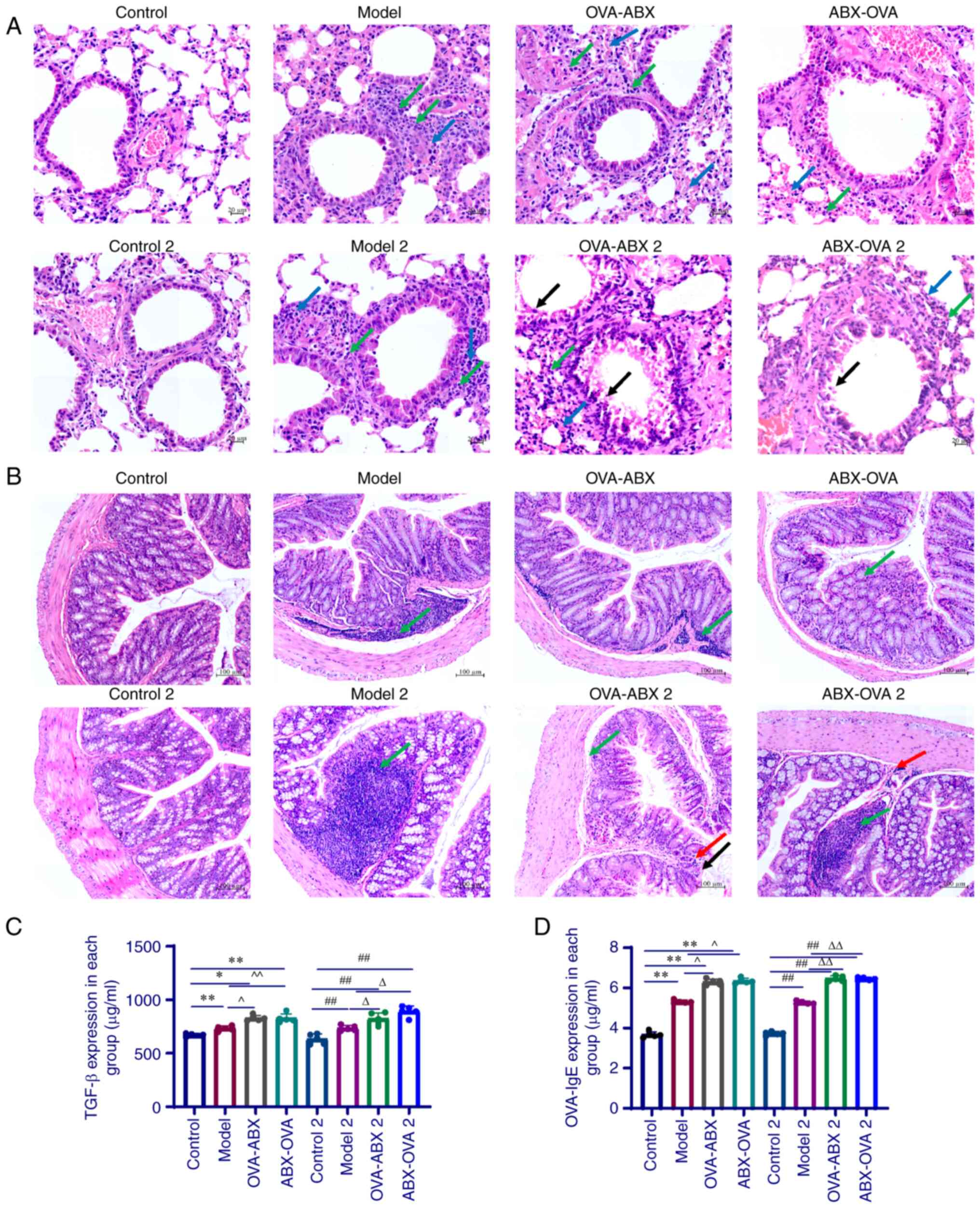
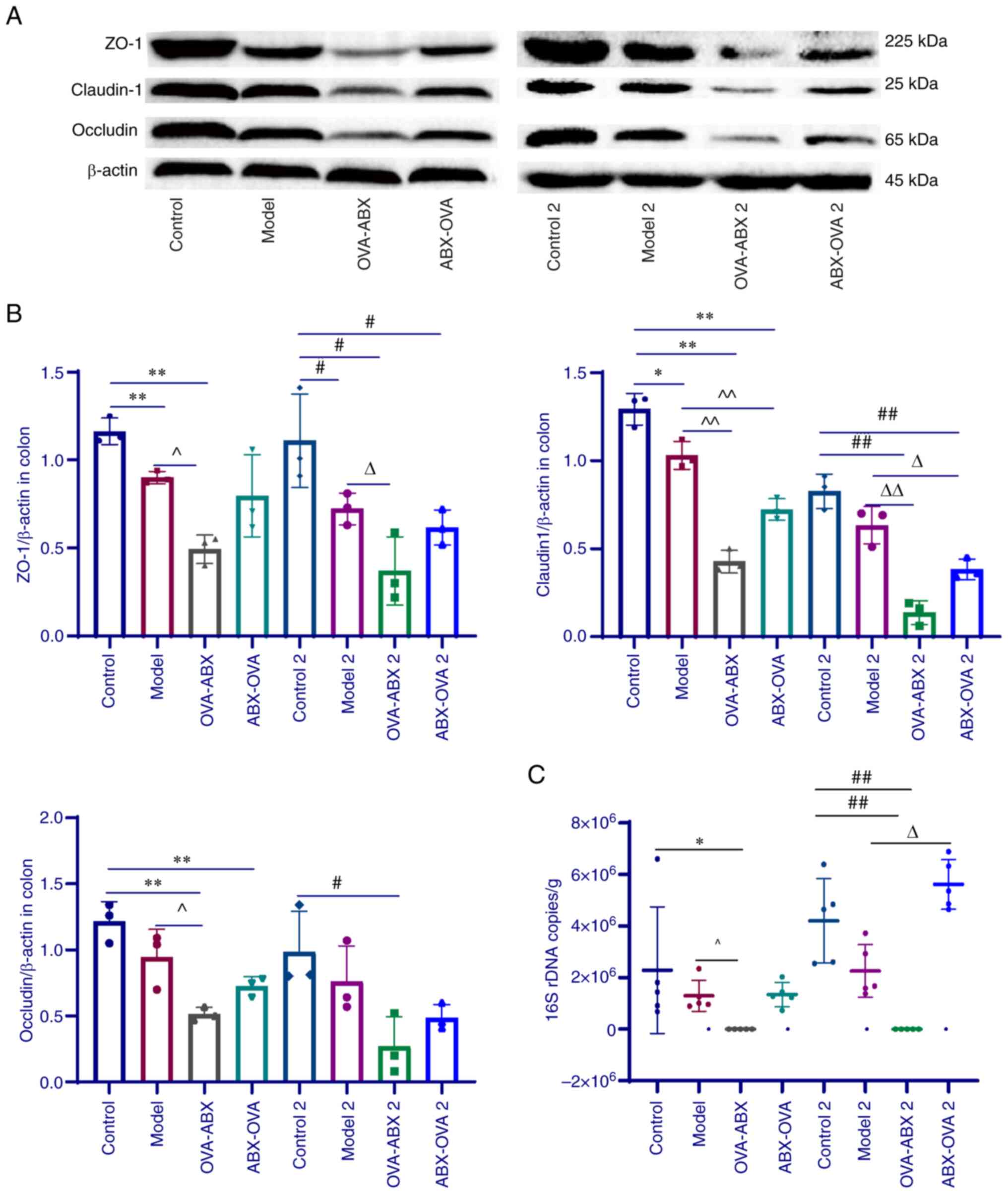
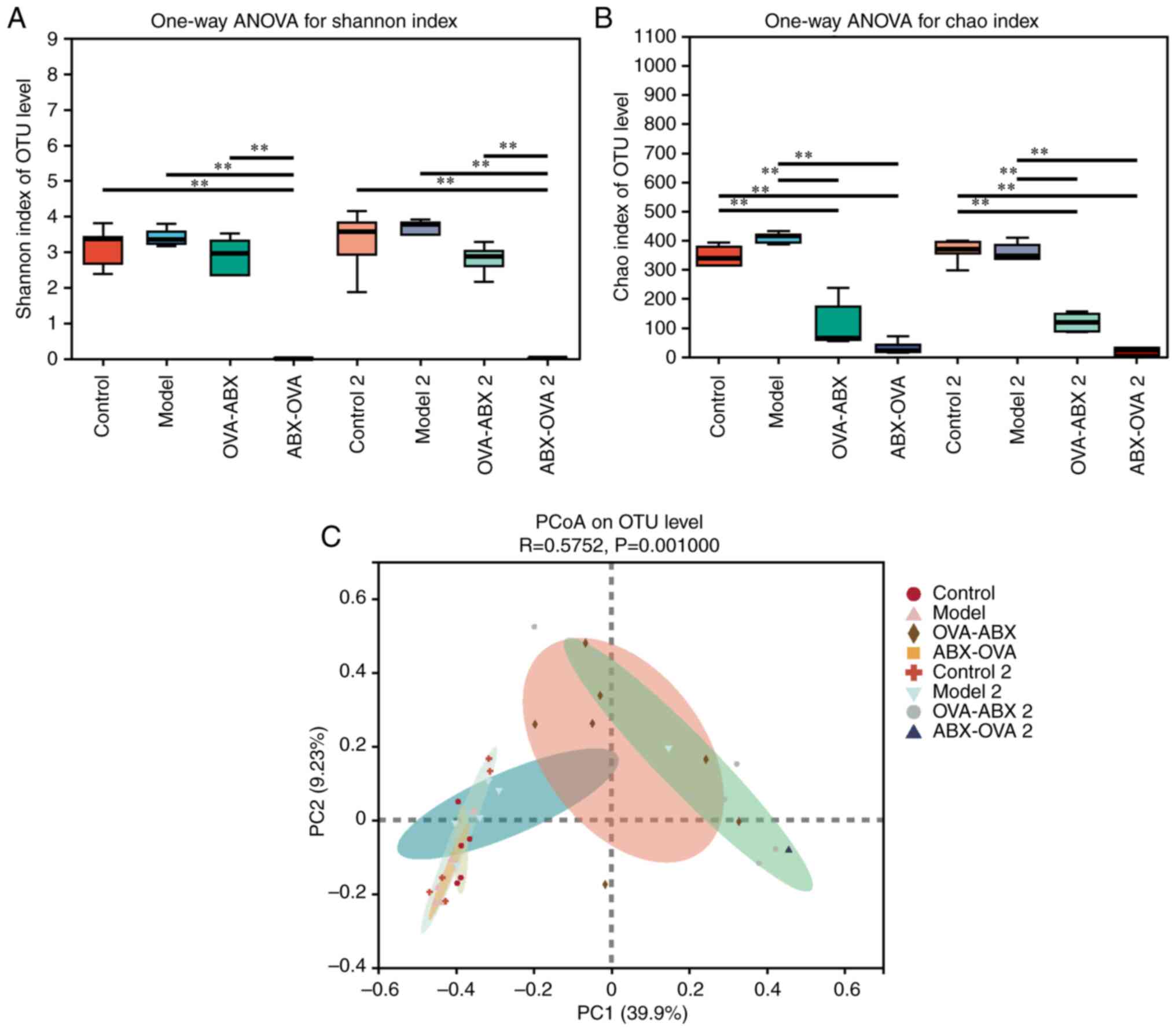
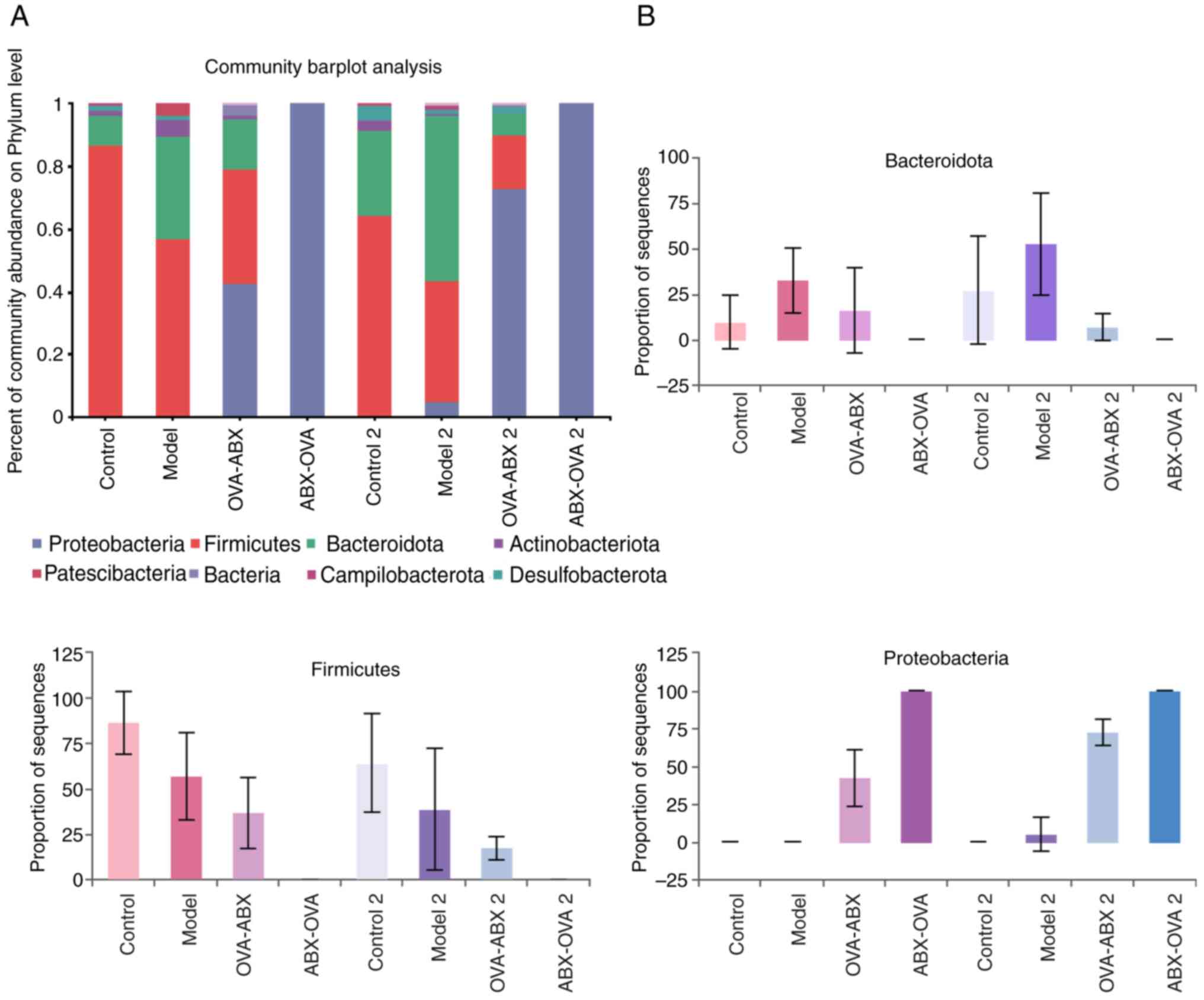
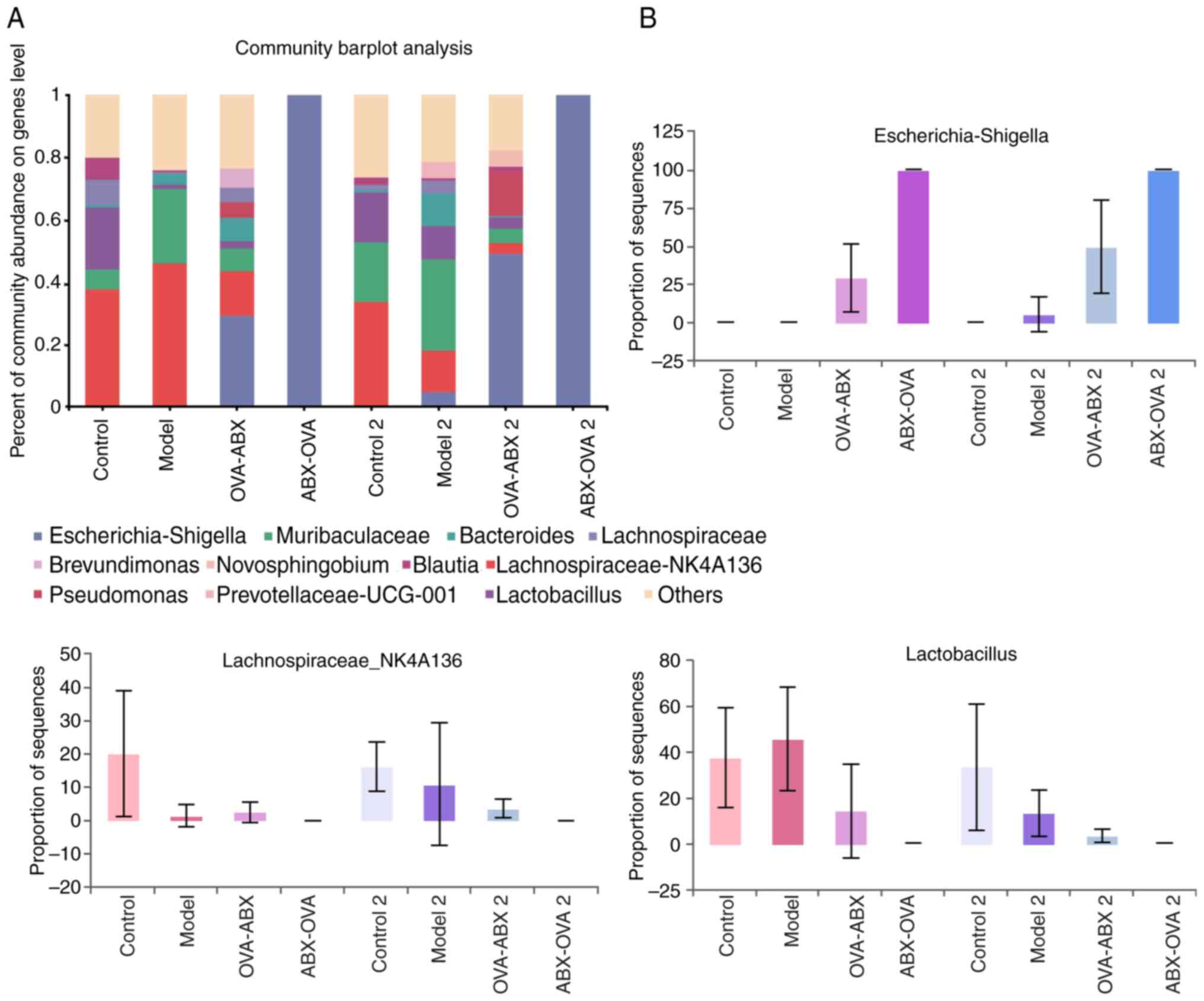
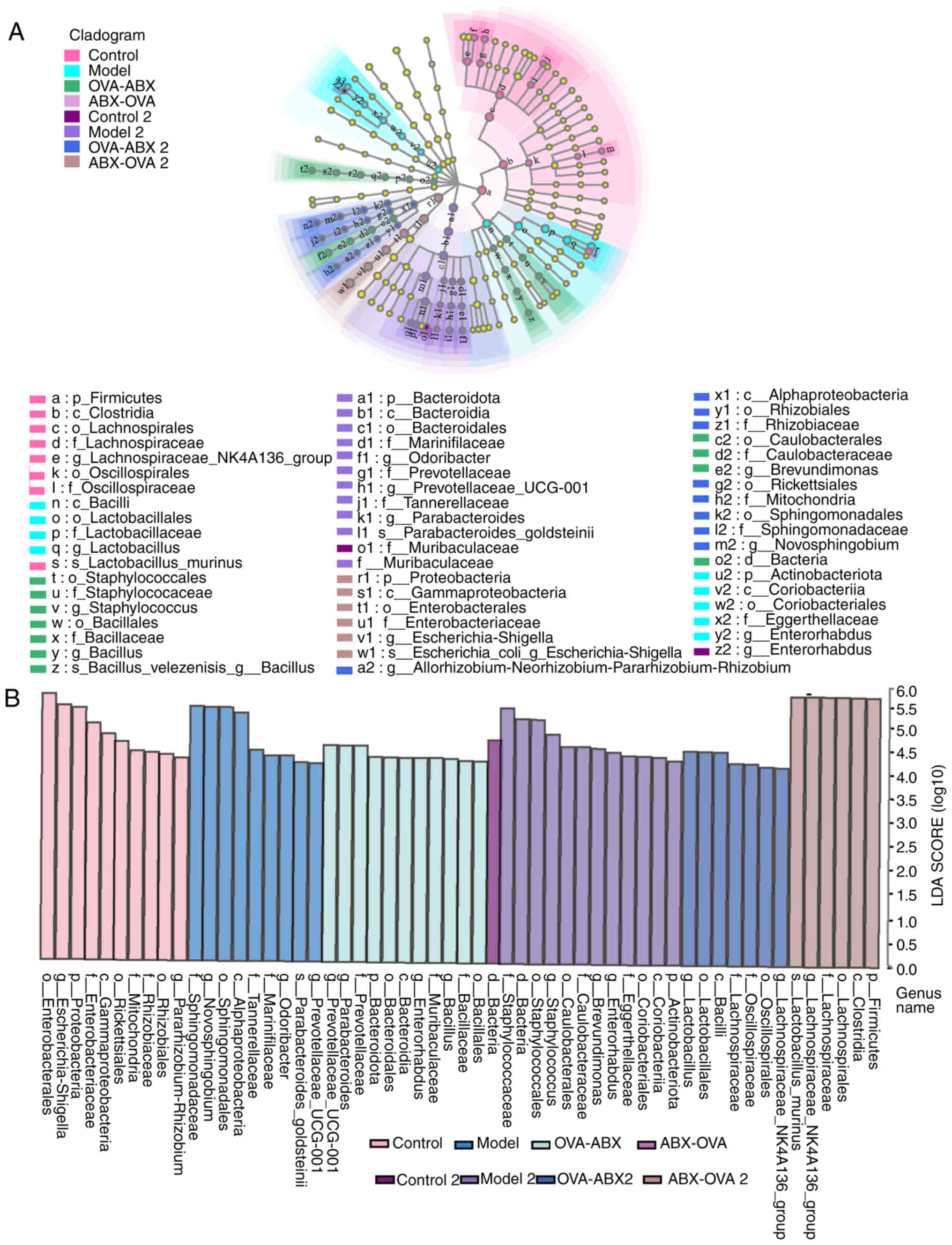
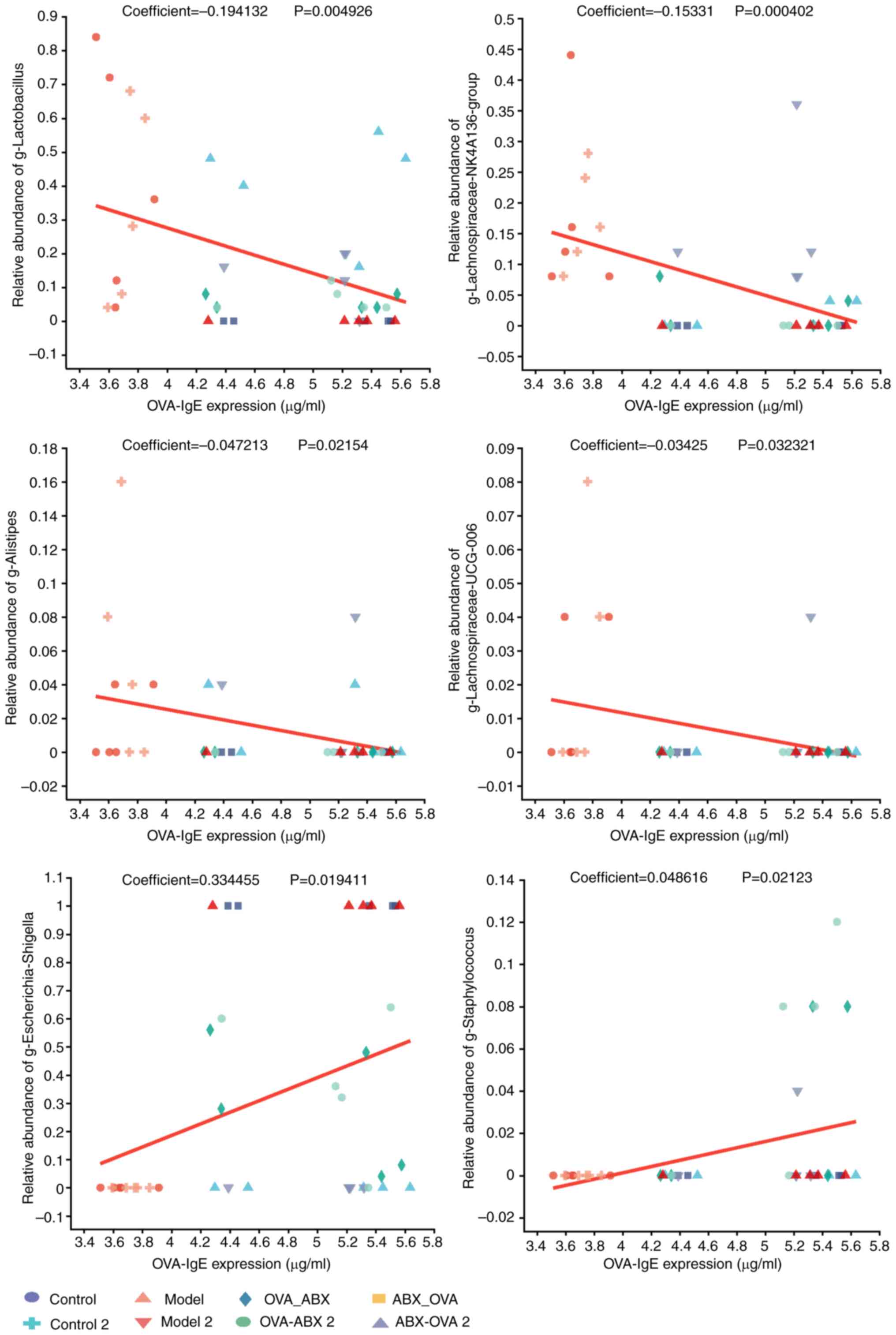
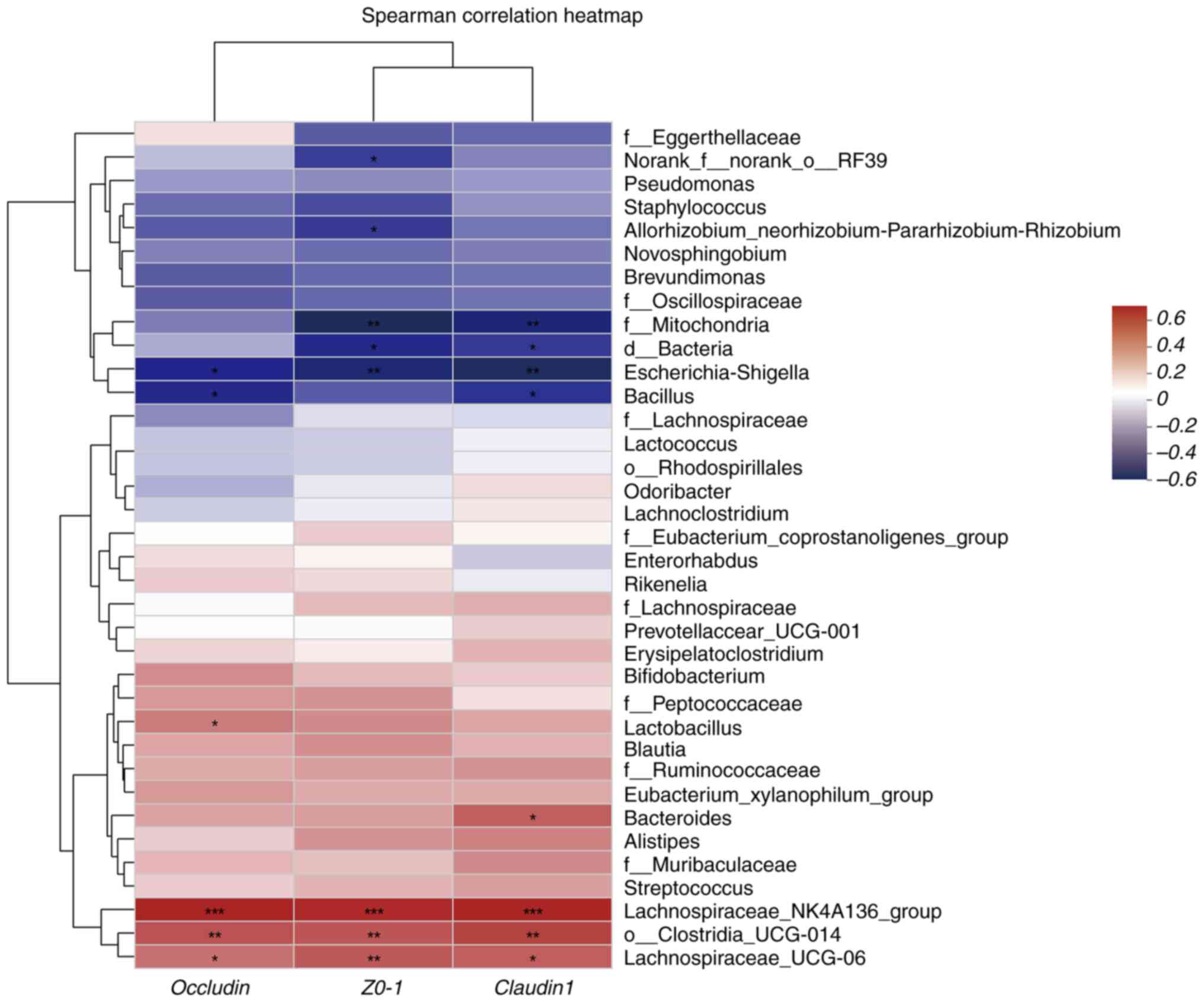
|
1
|
Shipp CL, Gergen PJ, Gern JE, Matsui EC
and Guilbert TW: Asthma management in children. J Allergy Clin
Immunol Prac. 11:9–18. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Voskamp AL, Kormelink TG, van Wijk RG,
Hiemstra PS, Taube C, de Jong EC and Smits HH: Modulating local
airway immune responses to treat allergic asthma: Lessons from
experimental models and human studies. Semin Immunopathol.
42:95–110. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Trivedi R and Barve K: Gut microbiome a
promising target for management of respiratory diseases. Biochem J.
477:2679–2696. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ye X, Wang A, Lin W, Xu Y, Dong X, Zhou Y,
Tian K and Xu X: The role of intestinal flora in anti-tumor
antibiotic therapy. Front Biosci (Landmark Ed).
27(281)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Francino MP: Antibiotics and the human gut
microbiome: Dysbioses and accumulation of resistances. Front
Microbiol. 6(1543)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu
KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling
C, Golubeva AV, et al: The microbiota-gut-brain axis. Physiol Rev.
99:1877–2013. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
McAleer JP and Kolls JK: Contributions of
the intestinal microbiome in lung immunity. Eur J Immunol.
48:39–49. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ottman N, Reunanen J, Meijerink M, Pietilä
TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skurnik M,
Boeren S, et al: Pili-like proteins of Akkermansia muciniphila
modulate host immune responses and gut barrier function. PLoS One.
12(e0173004)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fan H, Wang A, Wang Y, Sun Y, Han J, Chen
W, Wang S, Wu Y and Lu Y: Innate lymphoid cells: Regulators of gut
barrier function and immune homeostasis. J Immunol Res.
2019(2525984)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Turner JR: Molecular basis of epithelial
barrier regulation: From basic mechanisms to clinical application.
Am J Pathol. 169:1901–1909. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kuo WT, Odenwald MA, Turner JR and Zuo L:
Tight junction proteins occludin and ZO-1 as regulators of
epithelial proliferation and survival. Ann N Y Acad Sci.
1514:21–33. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hufnagl K, Pali-Schöll I, Roth-Walter F
and Jensen-Jarolim E: Dysbiosis of the gut and lung microbiome has
a role in asthma. Semin Immunopathol. 42:75–93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jia W, Xu C, Zhao T, Fan Q, Qiao B, Wu Y,
Yuan J and Chen J: Integrated network pharmacology and gut
microbiota analysis to explore the mechanism of sijunzi decoction
involved in alleviating airway inflammation in a mouse model of
Asthma. Evid Based Complement Alternat Med.
2023(1130893)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo W, Zhou X, Li X, Zhu Q, Peng J, Zhu B,
Zheng X, Lu Y, Yang D, Wang B and Wang J: Depletion of gut
microbiota impairs gut barrier function and antiviral immune
defense in the liver. Front Immunol. 12(636803)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Reikvam DH, Erofeev A, Sandvik A, Grcic V,
Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA and
Johansen FE: Depletion of murine intestinal microbiota: Effects on
gut mucosa and epithelial gene expression. PLoS One.
6(e17996)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Edgar RC: UPARSE: Highly accurate OTU
sequences from microbial amplicon reads. Nat Methods. 10:996–998.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Brodin P: Immune-microbe interactions
early in life: A determinant of health and disease long term.
Science. 376:945–950. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Leonardi I, Gao IH, Lin WY, Allen M, Li
XV, Fiers WD, De Celie MB, Putzel GG, Yantiss RK, Johncilla M, et
al: Mucosal fungi promote gut barrier function and social behavior
via Type 17 immunity. Cell. 185:831–846.e814. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Han P, Gu JQ, Li LS, Wang XY, Wang HT,
Wang Y, Chang C and Sun JL: The association between intestinal
bacteria and allergic diseases-cause or consequence? Front Cell
Infect Microbiol. 11(650893)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Brodin P: Immune-microbe interactions
early in life: A determinant of health and disease long term.
Science. 376:945–950. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Barcik W, Boutin RCT, Sokolowska M and
Finlay BB: The role of lung and gut microbiota in the pathology of
asthma. Immunity. 52:241–255. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lambrecht BN, Hammad H and Fahy JV: The
cytokines of asthma. Immunity. 50:975–991. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zou XL, Wu JJ, Ye HX, Feng DY, Meng P,
Yang HL, Wu WB, Li HT, He Z and Zhang TT: Associations between gut
microbiota and asthma endotypes: A cross-sectional study in south
china based on patients with newly diagnosed asthma. J Asthma
Allergy. 14:981–992. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Depner M, Taft DH, Kirjavainen PV,
Kalanetra KM, Karvonen AM, Peschel S, Schmausser-Hechfellner E,
Roduit C, Frei R, Lauener R, et al: Maturation of the gut
microbiome during the first year of life contributes to the
protective farm effect on childhood asthma. Nat Med. 26:1766–1775.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Raftery AL, Tsantikos E, Harris NL and
Hibbs ML: Links between inflammatory bowel disease and chronic
obstructive pulmonary disease. Front Immunol.
11(2144)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nieuwdorp M, Gilijamse PW, Pai N and
Kaplan LM: Role of the microbiome in energy regulation and
metabolism. Gastroenterology. 146:1525–1533. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Satokari R: High intake of sugar and the
balance between pro- and anti-inflammatory gut bacteria. Nutrients.
12(1348)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dicker AJ, Huang JTJ, Lonergan M, Keir HR,
Fong CJ, Tan B, Cassidy AJ, Finch S, Mullerova H, Miller BE, et al:
The sputum microbiome, airway inflammation, and mortality in
chronic obstructive pulmonary disease. J Allergy Clin Immunol.
147:158–167. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Guo MY, Chen HK, Ying HZ, Qiu FS and Wu
JQ: The role of respiratory flora in the pathogenesis of chronic
respiratory diseases. Biomed Res Int. 2021(6431862)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wan J, Song J, Lv Q, Zhang H, Xiang Q, Dai
H, Zheng H, Lin X and Zhang W: Alterations in the gut microbiome of
young children with airway allergic disease revealed by
next-generation sequencing. J Asthma Allergy. 16:961–972.
2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu Y, Chen Y, Li Q, Ye X, Guo X, Sun L,
Zou J, Shen Y, Mao Y, Li C and Yang Y: Tetrahydrocurcumin
alleviates allergic airway inflammation in asthmatic mice by
modulating the gut microbiota. Food Funct. 12:6830–6840.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Arrieta MC, Stiemsma LT, Dimitriu PA,
Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ,
Britton HM, Lefebvre DL, et al: Early infancy microbial and
metabolic alterations affect risk of childhood asthma. Sci Transl
Med. 7(307ra152)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Arrieta MC, Sadarangani M, Brown EM,
Russell SL, Nimmo M, Dean J, Turvey SE, Chan ES and Finlay BB: A
humanized microbiota mouse model of ovalbumin-induced lung
inflammation. Gut Microbes. 7:342–352. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang J, McDowell A, Seo H, Kim S, Min TK,
Jee YK, Choi Y, Park HS, Pyun BY and Kim YK: Diagnostic models for
atopic dermatitis based on serum microbial extracellular vesicle
metagenomic analysis: A pilot study. Allergy Asthma Immunol Res.
12:792–805. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tulstrup MV, Christensen EG, Carvalho V,
Linninge C, Ahrné S, Højberg O, Licht TR and Bahl MI: Antibiotic
treatment affects intestinal permeability and gut microbial
composition in wistar rats dependent on antibiotic class. PLoS One.
10(e0144854)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hoedt EC, Hueston CM, Cash N, Bongers RS,
Keane JM, van Limpt K, Amor KB, Knol J, MacSharry J and van
Sinderen D: A synbiotic mixture of selected oligosaccharides and
bifidobacteria assists murine gut microbiota restoration following
antibiotic challenge. Microbiome. 11(168)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rouaud F, Vasileva E, Spadaro D, Tsukita S
and Citi S: R40.76 binds to the α domain of ZO-1: Role of ZO-1 (α+)
in epithelial differentiation and mechano-sensing. Tissue Barriers.
7(e1653748)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fan L, Qi Y, Qu S, Chen X, Li A, Hendi M,
Xu C, Wang L, Hou T, Si J and Chen S: B. adolescentis ameliorates
chronic colitis by regulating Treg/Th2 response and gut microbiota
remodeling. Gut Microbes. 13:1–17. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wu X, Wei S, Chen M, Li J, Wei Y, Zhang J
and Dong W: P2RY13 Exacerbates intestinal inflammation by damaging
the intestinal mucosal barrier via activating IL-6/STAT3 pathway.
Int J Biol Sci. 18:5056–5069. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ahmad R, Sorrell MF, Batra SK, Dhawan P
and Singh AB: Gut permeability and mucosal inflammation: Bad, good
or context dependent. Mucosal Immunol. 10:307–317. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Baltazar-Díaz TA, González-Hernández LA,
Aldana-Ledesma JM, Peña-Rodríguez M, Vega-Magaña AN, Zepeda-Morale
ASM, López-Ro RI, Toro-Arreola SD, Martínez-López E, Salazar-Montes
AM and Bueno-Topete MR: Escherichia/Shigella, SCFAs, and metabolic
pathways-the triad that orchestrates intestinal dysbiosis in
patients with decompensated alcoholic cirrhosis from Western
Mexico. Microorganisms. 10(1231)2022.PubMed/NCBI View Article : Google Scholar
|