Introduction
The stress response to the surgery is characterized
by various inflammatory, hormonal and immune changes in the body.
Primarily, the release of cortisol, catecholamines, glucagon, IL-1
and IL-6 leads to the insulin resistance and impaired immune
functions (1). Prolonged
preoperative fasting deteriorates these responses by causing
detrimental catabolic effects including increased glycogenolysis,
proteolysis and lipolysis and decreased insulin sensitivity with
normal insulin levels (2). Several
studies have reported that actual fasting time for patients
undergoing elective surgery is usually longer than 12 h due to the
delays in the operating theatre schedule; meaning that most
patients are exposed to prolonged fasting and dehydration (3,4).
Given the concerns of regurgitation and pulmonary
aspiration, fasting after midnight has traditionally been offered
to all surgical patients. However, it has been shown that there
were no differences in terms of gastric volume and acidity and
pulmonary complications between the patients who were fasted
overnight and those allowed to drink clear liquids up to 2 h before
surgery (5). Following these
results, a paradigm shift has occurred in the timing and
administration of preoperative solids and liquids over the last
decade. The major anesthesiology societies have started to
recommend clear liquids up to 2 h and solids up to 6 h before
anesthesia (6,7). Although these recommendations would
prevent dehydration, they are still insufficient in minimizing
perioperative catabolic stress response.
In recent years, there has been growing interest in
preoperative use of carbohydrate-enriched drinks in order to ensure
a metabolically fed-state and to overcome catabolic stress
response. It has been shown that avoiding prolonged fasting and
giving clear liquids containing sufficient carbohydrates before
surgery induces an anabolic state, promoting tissue healing by
decreasing insulin resistance and inflammatory mediators such as
IL-6(8). Currently, the
consumption of at least 45 g of carbohydrate as a clear liquid 2-3
h preoperatively is considered as one of the key elements of
enhanced recovery after surgery (ERAS) protocols (9).
Despite the theoretical benefits, however, the
favorable effect of carbohydrate loading on postoperative morbidity
has not been consistently demonstrated and remains controversial
(10). Most of the outcomes
reported in the literature were derived from non-gynecologic
surgery data, with only one study involving a limited number of
patients, specifically in gynecological oncology (11). The current study investigated the
effect of preoperative carbohydrate loading as a single element of
ERAS on postoperative course and morbidity in patients undergoing
debulking surgery for epithelial ovarian cancer (EOC).
Materials and methods
Study design and endpoints
The present study was a non-randomized, prospective
cohort trial enrolling consecutive patients with EOC who underwent
debulking surgery, either primary or interval, at Antalya Training
and Research Hospital between June 2018 and December 2021.
Exclusion criteria were: Minimally invasive surgery, surgery for
recurrent disease, hyperthermic intraperitoneal chemotherapy
(HIPEC) and type 1 diabetes mellitus. The present study was
approved by the Ethics Committee of the Antalya Training and
Research Hospital (7 May, 2018; approval no. 021). Written informed
consent was obtained from all participants.
Patients were fasted for solids from 02:00 a.m. the
night before surgery, but were allowed to drink clear liquids until
06:00 a.m. on the day of surgery. An oral carbohydrate supplement
(Nutricia Fantomalt®, Nutricia Turkiye), at a dose of 50
g diluted in 250 ml water, which contained 48 g carbohydrate and
192 kcal of energy was given to patients at 06:00 a.m. on the
morning of surgery. The consumption time for the drink was 10 min.
All patients underwent surgery as the first case of the day and
operations were scheduled to start at 08:30 a.m.
Data regarding age, performance status,
comorbidities, neoadjuvant chemotherapy, stage of the disease,
tumor histotype, surgical resections, blood transfusion, operative
time, need for intensive care unit (ICU), length of ICU stay,
length of hospital stay, postoperative day 1 serum albumin level,
postoperative day 1 to -7 serum c-reactive protein (CRP) levels,
30-day hospital readmission, 30-day relaparotomy, 30-day
postoperative morbidities including deaths and the time interval
between surgery and adjuvant therapy were collected following
ethics committee approval.
Prospectively collected data of patients were
compared with a historical cohort of consecutive patients who
underwent debulking surgery for EOC at the same institution without
a preoperative carbohydrate loading between January 2015 and June
2018.
Based on the results of previous studies (12,13)
indicating a 50% reduction in postoperative morbidity with
carbohydrate loading, it was calculated that a sample size of at
least 68 patients per group was required to detect a 50% reduction
in morbidity, with a two-sided 5% significance level and 80% power.
It was anticipated that 90 patients with carbohydrate treatment
would be recruited in this study based on a 1:1 ratio to the number
of historical control group. However, 18 patients were excluded
from the carbohydrate loading group: 7 had HIPEC, 8 had different
primary tumor origin and 3 withdrew consent before surgery.
The primary endpoint of the present study was the
effect of carbohydrate loading on postoperative course and
morbidity and the secondary endpoint was determination of factors
associated with postoperative morbidities. The postoperative
morbidities were graded according to the Clavien-Dindo
classification (14).
Perioperative management
Perioperative management strategies of patients,
with the exception of fasting time and carbohydrate loading, were
identical between the two cohorts. Patients in the historical
cohort were subjected to conventional overnight fasting for solids
and liquids. Although routinely implementing most of the ERAS
elements in had been in the Antalya Training and Research Hospital
since January 2015, there was not a strict policy in this
regard.
A routine mechanical bowel preparation was not used
in any patient. All patients received thromboembolism prophylaxis
with a low-molecular weight heparin and wore anti-embolism
stockings. An antimicrobial prophylaxis was initiated with 2 g of
intravenous (IV) cefazolin within 60 min before skin incision and
the dose was repeated every 3 h during the surgery. A vertical
midline laparotomy was used in all patients. Perioperative fluid
therapy was individualized according to invasive hemodynamic
monitoring and urine output. The decision for ICU admission was at
the discretion of the anesthesiologists. On postoperative day 1 the
urinary catheter was removed and oral intake of clear liquids was
allowed. The diet was advanced gradually as bowel functions
returned. Drains were removed when the output decreased to <100
ml/24 h. Antibiotics were continued until drains were removed.
Patients were discharged home if they were able to walk without
assistance and tolerated regular diet.
Statistical analysis
Analyses were performed using SPSS version 20.0 (IBM
Corp.). The differences between two cohorts were tested by
independent samples T-test for parametric data and Mann-Whitney
U-test for nonparametric data. Pearson Chi-Square test was used for
comparison of categorical variables. Factors associated with
postoperative morbidities were evaluated by both univariate and
multivariate logistic regression analyses. Variables with a
P<0.05 in univariate analyses were included into multivariate
analyses. The effects of variables on morbidity were reported as
adjusted odds ratios (OR) and 95% confidential intervals (CI). The
predictive mean matching method was used in order to deal with
missing values. Cut-off values of independent scale variables were
calculated using receiver operating characteristic (ROC) analysis.
An area under the curve value of >0.70 was considered
satisfactory. For the ROC curve, the point with the largest sum of
sensitivity and specificity was chosen as a threshold. P<0.05
was considered to indicate a statistically significant
difference.
Results
The final analyses were performed on a total of 162
patients, including 72 patients in the carbohydrate loading group
and 90 patients in the control group. Study groups were comparable
for preoperative characteristics and histopathological findings
(Table I) In both groups, the
majority of patients had International Federation of Gynecology and
Obstetrics stage (15) III-IV
disease (72.3% vs. 67.7%) and received primary debulking surgery
(68.1% vs. 65.6%).
 | Table IPreoperative characteristics and
histopathological findings. |
Table I
Preoperative characteristics and
histopathological findings.
| | Preoperative
carbohydrate loading | |
|---|
| Variable | Yes (n=72) | No (n=90) | P-value |
|---|
| Age, years, median
(range) | 58 (22-80) | 58 (36-82) | 0.426c |
| ECOG performance
status, n (%) | | | 0.388d |
|
0-1 | 57 (79.2) | 66 (73.3) | |
|
≥2 | 15 (20.8) | 24 (26.7) | |
| Comorbidities, n
(%) | 24 (33.3) | 40 (44.4) | 0.151d |
| Cardiac
comorbiditya | 14 (19.4) | 28 (31.1) | 0.092d |
| Pulmonary
comorbidityb | 5 (6.9) | 9 (10.0) | 0.492d |
| Diabetes
mellitus | 9 (12.5) | 15 (16.7) | 0.458d |
| Neoadjuvant
chemotherapy, n (%) | 23 (31.9) | 31 (34.4) | 0.945d |
|
3
cycles | 9 (12.5) | 12 (13.3) | |
|
≥4
cycles | 14 (19.4) | 19 (21.1) | |
| FIGO stage, n
(%) | | | 0.803d |
|
I | 13 (18.1) | 20 (22.2) | |
|
II | 7 (9.7) | 9 (10.0) | |
|
III | 30 (41.7) | 31 (34.4) | |
|
IV | 22 (30.6) | 30 (33.3) | |
| Tumor histotype, n
(%) | | | 0.830d |
|
High-grade
serous | 49 (68.1) | 56 (62.2) | 0.440d |
|
Others | | | |
| Grade 2-3
endometrioid | 2 (2.8) | 2 (2.2) | |
| Carcinosarcoma | 2 (2.8) | 1 (1.1) | |
|
Clear
cell | 6 (8.3) | 5 (5.6) | |
|
Low-grade
serous | 5 (6.9) | 13 (14.4) | |
|
Grade 1
endometrioid | 4 (5.6) | 6 (6.7) | |
|
Mucinous | 3 (4.2) | 4 (4.4) | |
|
Seromucinous | - | 1 (1.1) | |
|
Squamous
cell carcinoma | 1 (1.4) | 1 (1.1) | |
|
Wolffian
adnexal tumor | - | 1 (1.1) | |
Intraoperative findings and surgical characteristics
of study groups are summarized in Table II. There were no statistically
significant differences between the groups in terms of ascites
(36.1% vs. 38.9%), peritoneal carcinomatosis (61.1% vs. 57.8%),
bowel resection (25.0% vs. 20.0%), peritonectomy (55.6% vs. 48.9%),
splenectomy (12.5% vs. 12.2%), lymph node dissection (62.5% vs.
65.6%), rate of maximal cytoreduction (70.8% vs. 70.0%), median
operative time (330 min vs. 330 min), blood transfusion (56.9% vs.
53.3%) and need for ICU (63.9% vs. 55.6%).
 | Table IIIntraoperative findings and surgical
characteristics. |
Table II
Intraoperative findings and surgical
characteristics.
| | Preoperative
carbohydrate loading | |
|---|
| Variable | Yes (n=72) | No (n=90) | P-value |
|---|
| Ascites, n (%) | 26 (36.1) | 35 (38.9) | 0.717a |
| Large volume (seen
on all quadrants) | 13 (18.1) | 21 (23.3) | 0.412a |
| Omental cake, n
(%) | 26 (36.1) | 34 (37.8) | 0.827a |
| Peritoneal
carcinomatosis, n (%) | 44 (61.1) | 52 (57.8) | 0.668a |
| Diffuse,
miliary | 32 (44.4) | 32 (35.6) | 0.250a |
| Diaphragmatic
disease, n (%) | 22 (30.6) | 22 (24.4) | 0.385a |
| Small bowel serosal
and/or mesentery involvement, (diffuse, miliary), n (%) | 18 (25.0) | 22 (24.4) | 0.935a |
| Large bowel serosal
and/or mesentery involvement, (diffuse, miliary), n (%) | 19 (26.3) | 21 (23.3) | 0.654a |
| Spleen metastasis
(any surface/hilar lesion), n (%) | 7 (9.7) | 12 (13.3) | 0.478a |
| Liver metastasis
(parenchymal lesion), n (%) | 5 (6.9) | 6 (6.7) | 0.923a |
| Cytoreduction, n
(%) | | | |
| Maximal (no visible
residual disease) | 51 (70.8) | 63 (70.0) | 0.908a |
| Optimal (residual
tumor nodules <1 cm) | 18 (25.0) | 17 (18.9) | 0.348a |
| Suboptimal
(residual tumor nodules ≥1 cm) | 3 (4.2) | 10 (11.1) | 0.106a |
| Bowel resection
(large and/or small bowel), n (%) | 18 (25.0) | 18 (20.0) | 0.447a |
|
Large
bowel | 15 (20.8) | 15 (16.7) | 0.498a |
|
Colorectal
resection | 12 (16.7) | 13 (14.4) | |
| Right
hemicolectomy | 3 (4.2) | 1 (1.1) | |
| Transverse colon
resection | - | 1 (1.1) | |
|
Small
bowel | 4 (5.6) | 4 (4.4) | 0.746a |
|
Peritonectomy
(partial and/or total), n (%) | 40 (55.6) | 44 (48.9) | 0.399a |
|
Pelvic | 39 (54.2) | 42 (46.7) | 0.343a |
|
Paracolic | 22 (30.6) | 22 (24.4) | 0.385a |
|
Diaphragm | 14 (19.4) | 10 (11.1) | 0.138a |
| Appendectomy, n
(%) | 27 (37.5) | 33 (36.7) | 0.913a |
| Splenectomy ±
distal pancreatectomy, n (%) | 9 (12.5) | 11(12.2) | 0.957a |
| Systematic
pelvic-paraaortic LN dissection, n (%) | 45 (62.5) | 59 (65.6) | 0.687a |
| Number of LNs
removed, median (range) | 55 (29-129) | 60 (27-100) | 0.757b |
| Operative time,
min, median (range) | 330 (240-610) | 330 (195-530) | 0.144c |
| Intraoperative
blood transfusion, n (%) | 41 (56.9) | 48 (53.3) | 0.646a |
| Need for intensive
care unit, n (%) | 46 (63.9) | 50 (55.6) | 0.283a |
The comparison of postoperative course and
morbidities between the study groups are presented in Table III. The median length of ICU stay
(1 day vs. 1 day), length of hospital stay (11 days vs. 11 days),
day 1 serum albumin levels (2.7 g/dl vs. 2.5 g/dl), day 1 to -7
serum CRP levels, 30-day readmission (11.6% vs. 11.5%), 30-day
relaparotomy (2.8% vs. 3.4%) and the time interval between surgery
and adjuvant therapy (35 days vs. 39 days) were comparable between
the groups. At least one postoperative morbidity occurred in 48.6
and 46.7% of patients with and without carbohydrate loading,
respectively (P=0.805). Wound infection was the most common
morbidity in both groups (22.2% vs. 16.7%; P=0.372), followed by
ileus (13.9% vs. 13.3%, P=0.918). No significant differences in
grades of morbidities were identified between the groups
(P=0.511).
 | Table IIIPostoperative course and
morbidities. |
Table III
Postoperative course and
morbidities.
| | Preoperative
carbohydrate loading | |
|---|
| Variable | Yes (n=72) | No (n=90) | P-value |
|---|
| Length of ICU stay,
days, median (range) | 1 (1-69) | 1 (1-14) | 0.091a |
| Length of hospital
stay, days, median (range) | 11 (5-77) | 11 (1-37) | 0.555a |
| Day 1, serum
albumin level, g/dl, median (range) | 2.7 (1.0-3.4) | 2.5 (0.8-3.9) | 0.138b |
| Serum CRP level,
mg/l, median (range) | | | |
|
Day 1 | 63.0
(17.0-306) | 106.5
(18.9-317) | 0.213a |
|
Day 2 | 214.0
(75.0-395) | 224.0
(75.9-435) | 0.675b |
|
Day 3 | 235.5
(32.0-364) | 210.0
(30.3-499) | 0.980b |
|
Day 4 | 154.5
(21.0-443) | 145.5
(33.0-442) | 0.709b |
|
Day 5 | 95.0
(16.0-412) | 98.0
(18.9-417) | 0.608a |
|
Day 6 | 53.0
(10.0-260) | 57.3 (8.2-455) | 0.989a |
|
Day 7 | 57.0 (5.0-319) | 82.3
(17.9-439) | 0.529a |
| 30-day hospital
readmission, n (%) | 8 (11.6) | 10 (11.5) | 0.985c |
| 30-day
relaparotomy, n (%) | 2 (2.8) | 3 (3.4) | 0.809c |
| 30-day
postoperative morbidity, n (%) | 35 (48.6) | 42 (46.7) | 0.805c |
|
Eventration/evisceration | 1 (1.4) | 1 (1.1) | 0.874c |
| Any infectious
morbidity | 21 (29.2) | 21 (23.3) | 0.400c |
| Wound
infection | 16 (22.2) | 15 (16.7) | 0.372c |
| Intra-abdominal
infection/abscess | 10 (13.9) | 6 (6.7) | 0.126c |
| Urinary
infection | 4 (5.6) | 7 (7.8) | 0.576c |
| Sepsis | 3 (4.2) | 1 (1.1) | 0.213c |
| Gastrointestinal
morbidity | 11 (15.3) | 14 (15.6) | 0.961c |
| Ileus | 10 (13.9) | 12 (13.3) | 0.918c |
| Anastomotic
leakage | 1 (1.4) | 1 (1.1) | 0.874c |
| Intestinal
perforation | 1 (1.4) | - | 0.262c |
| Biliary
leakage | - | 1 (1.1) | 0.370c |
| Pulmonary
morbidity | 11 (15.3) | 8 (8.9) | 0.209c |
| Pleural
effusion | 8 (11.1) | 6 (6.7) | 0.317c |
|
Pneumo-mediastinum | 1 (1.4) | - | 0.262c |
| Pulmonary
thromboembolism | 3 (4.2) | 1 (1.1) | 0.213c |
| Pulmonary
edema | 1 (1.4) | 5 (5.6) | 0.163c |
| Transfusion-related
acute lung injury | - | 1 (1.1) | 0.370c |
| Cardiac
morbidity | 2 (2.8) | 5 (5.6) | 0.388c |
| Unexplained sudden
cardiac arrest | 1 (1.4) | 2 (2.2) | 0.696c |
| Atrial
fibrillation | 1 (1.4) | 3 (3.3) | 0.428c |
| Others | | | |
|
Chylous
ascites | 6 (8.3) | 5 (5.6) | 0.485c |
|
Renal artery
thrombosis | 1 (1.4) | 2 (2.2) | 0.696c |
|
Vesico-vaginal
fistula | 1 (1.4) | - | 0.262c |
|
Acute
basilar artery occlusion | 1 (1.4) | - | 0.262c |
| Clavien-Dindo
classification of morbidities, n (%) | | | 0.511c |
|
Grade 1 | 6 (8.3) | 11 (12.2) | 0.422c |
|
Grade 2 | 10 (13.9) | 17 (18.9) | 0.396c |
|
Grade 3 | 10 (13.9) | 5 (5.6) | 0.069c |
|
Grade 4 | 6 (8.3) | 6 (6.7) | 0.687c |
|
Grade 5
(mortality) | 3 (4.2) | 3 (3.3) | 0.780c |
| Time interval
between debulking surgery and adjuvant therapy, days, median
(range) | 35 (14-73) | 39 (19-99) | 0.451b |
A total of six deaths occurred postoperatively, with
three (4.2%) in the carbohydrate loading group and three (3.3%) in
the control group (P=0.780). One patient in the carbohydrate
loading group and two patients in the control group succumbed due
to unexplained sudden cardiac arrest occurring within the first 24
h after surgery. The other two mortalities in the carbohydrate
loading group were due to a neglected small bowel perforation
diagnosed 3 weeks after surgery and due to pulmonary
thromboembolism on postoperative day 5, respectively. The third
mortality in the control group was from a large bowel anastomotic
leak on postoperative day 14.
In univariate analysis, four variables were
significantly associated with any postoperative morbidity:
Peritoneal carcinomatosis (P=0.035), operative time (P=0.001), ICU
admission (P=0.042) and serum albumin level on postoperative day 1
(P=0.030). In multivariate analysis, however, only the ‘operative
time’ remained as an independent factor associated with any
postoperative morbidity after adjustment for other confounders
(Table IV). Optimal cut-off value
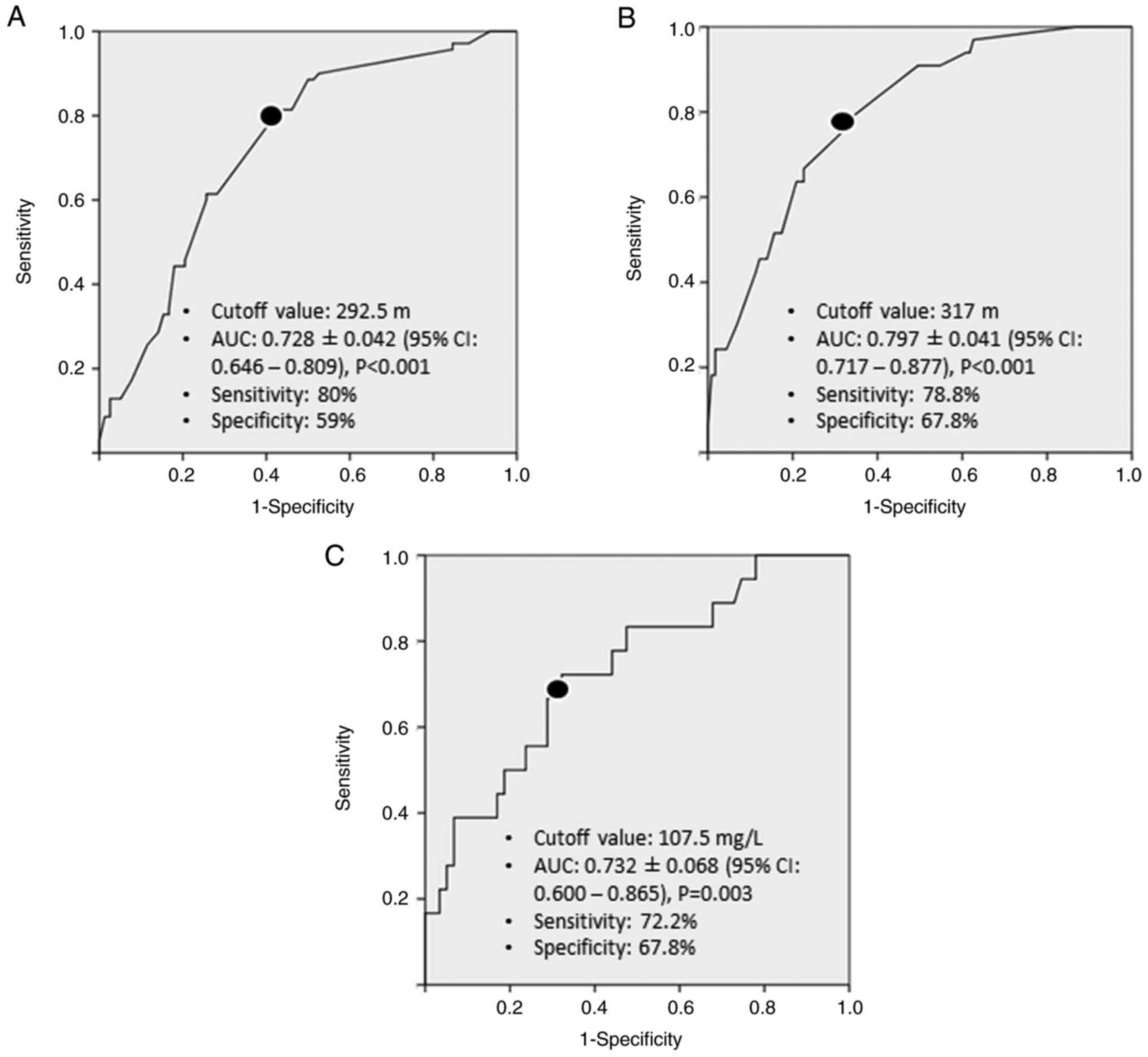
of operative time for predicting any morbidity was found as 292.5
min, with a sensitivity of 80% and specificity of 59% (Fig. 1A). Patients who had an operative
time ≥292.5 min were 3.5 times more likely to experience any
postoperative morbidity (OR: 3.531; 95% CI: 1.326-9402;
P=0.012).
 | Table IVFactors associated with any
postoperative morbidity. |
Table IV
Factors associated with any
postoperative morbidity.
| | Any morbidity |
|---|
| | Unadjusted | Adjusted |
|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Preoperative
carbohydrate loading (no vs. yes) | 1.081 | 0.581-2.011 | 0.805 | - | - | - |
| Age, years | 0.996 | 0.969-1.023 | 0.761 | - | - | - |
| ECOG performance
status (0-1 vs. ≥2) | 1.600 | 0.774-3.307 | 0.204 | - | - | - |
| Diabetes mellitus
(no vs. yes) | 1.123 | 0.472-2.673 | 0.793 | - | - | - |
| Neoadjuvant
chemotherapy (no vs. yes) | 0.928 | 0.482-1.787 | 0.824 | - | - | - |
| Ascites, large
volume (no vs. yes) | 1.779 | 0.827-3.831 | 0.141 | - | - | - |
| Peritoneal
carcinomatosis, diffuse (no vs. yes) | 1.987 | 1.049-3.765 | 0.035 | - | - | 0.826 |
| Maximal
cytoreduction (no vs. yes) | 1.296 | 0.659-2.547 | 0.452 | - | - | - |
| Large bowel
resection (no vs. yes) | 2.204 | 0.972-4.995 | 0.058 | - | - | - |
| Total
peritonectomya
(no vs. yes) | 2.194 | 0.770-6.252 | 0.141 | - | - | - |
| Diaphragm stripping
(no vs. yes) | 2.043 | 0.837-4.984 | 0.116 | - | - | - |
| Splenectomy (no vs.
yes) | 1.119 | 0.439-2.855 | 0.813 | - | - | - |
| Systematic
pelvic-paraaortic LND (no vs. yes) | 1.063 | 0.559-2.023 | 0.852 | - | - | - |
| Operative time (≥
292.5 min)b | 5.750 | 2.745-12.043 | 0.001 | 3.531 | 1.326-9402 | 0.012 |
| Intraoperative
blood transfusion (no vs. yes) | 1.776 | 0.949-3.326 | 0.073 | - | - | - |
| Need for intensive
care unit (no vs. yes) | 1.938 | 1.023-3.673 | 0.042 | - | - | 0.676 |
| Day 1, serum
albumin level, g/dl | 0.487 | 0.254-0.933 | 0.030 | - | - | 0.159 |
| Day 1, serum CRP
level, mg/l | 1.005 | 0.999-1.011 | 0.076 | - | - | - |
When the factors specifically associated with grade
III-V morbidities were analyzed, ascites (P=0.005), peritoneal
carcinomatosis (P=0.001), maximal cytoreduction (P=0.009), colon
resection (P=0.001), total peritonectomy (P=0.031), diaphragmatic
stripping (P=0.007), splenectomy (P=0.025), operative time
(P=0.001), intraoperative blood transfusion (P=0.001), ICU
admission (P=0.001), serum albumin level on postoperative day 1
(P=0.026) and serum CRP level on postoperative day 1 (P=0.005) were
significant factors in univariate analysis; whereas splenectomy
(P=0.049), operative time (P=0.021) and serum CRP level on
postoperative day 1 (P=0.024) were found to be independent factors
in multivariate analysis after adjustment for other confounders
(Table V). Optimal cut-off values
of operative time and day 1 serum CRP level for predicting grade
III-V morbidities were 317 min (sensitivity: 78.8%, specificity:
67.8%) and 107.5 mg/l (sensitivity: 72.2%, specificity: 67.8%),
respectively (Fig. 1B and C).
 | Table VFactors associated with grade III-V
postoperative morbidities. |
Table V
Factors associated with grade III-V
postoperative morbidities.
| | Grade III-V
morbidity |
|---|
| | Unadjusted | Adjusted |
|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Preoperative
carbohydrate loading (no vs. yes) | 1.946 | 0.897-4.221 | 0.092 | - | - | - |
| Age, years | 1.018 | 0.984-1.053 | 0.310 | - | - | - |
| ECOG performance
status (0-1 vs. ≥2) | 2.159 | 0.945-4.933 | 0.068 | - | - | - |
| Diabetes mellitus
(no vs. yes) | 1.034 | 0.355-3.011 | 0.951 | - | - | - |
| Neoadjuvant
chemotherapy (no vs. yes) | 1.184 | 0.532-2.632 | 0.679 | - | - | - |
| Ascites, large
volume (no vs. yes) | 3.343 | 1.443-7.745 | 0.005 | - | - | 0.336 |
| Peritoneal
carcinomatosis, diffuse (no vs. yes) | 7.212 | 2.990-17.392 | 0.001 | - | - | 0.945 |
| Maximal
cytoreduction (no vs. yes) | 2.853 | 1.293-6.293 | 0.009 | - | - | 0.810 |
| Large bowel
resection (no vs. yes) | 6.333 | 2.650-15.138 | 0.001 | - | - | 0.782 |
| Total peritonectomy
(no vs. yes) | 3.204 | 1.116-9.201 | 0.031 | - | - | 0.830 |
| Diaphragm stripping
(no vs. yes) | 3.571 | 1.414-9.023 | 0.007 | - | - | 0.913 |
| Splenectomy (no vs.
yes) | 3.120 | 1.155-8.425 | 0.025 | 14.724 | 1.008-215.008 | 0.049 |
| Systematic
pelvic-paraaortic LND (no vs. yes) | 0.513 | 0.236-1.114 | 0.092 | - | - | - |
| Operative time (≥
317 min)a | 7.830 | 3.115-19.682 | 0.001 | 15.368 | 1.498-157.710 | 0.021 |
| Intraoperative
blood transfusion (no vs. yes) | 4.863 | 1.881-12.570 | 0.001 | - | - | 0.690 |
| Need for intensive
care unit (no vs. yes) | 9.545 | 2.773-32.854 | 0.001 | - | - | 0.304 |
| Day 1, serum
albumin level, g/dl | 0.443 | 0.217-0.907 | 0.026 | - | - | 0.548 |
| Day 1, serum CRP
level (≥ 107.50 mg/l)a | 5.337 | 1.660-17.163 | 0.005 | 7.795 | 1.311-46.340 | 0.024 |
Discussion
The present study compared two cohorts of patients
who underwent debulking surgery for EOC with or without
preoperative carbohydrate loading. It revealed no significant
differences between the cohorts in terms of postoperative course
and morbidities. The length of ICU stay, length of hospital stay,
day 1 serum albumin level, day 1-7 serum CRP levels, the rates of
30-day hospital readmission and 30-day relaparotomy, the rate and
the severity of 30-day postoperative morbidities and the time
interval between surgery and adjuvant therapy were comparable
between the cohorts. The only independent risk factor for any
morbidity after debulking surgery was operative time, while the
independent risk factors specifically for grade III-V morbidities
were operative time, splenectomy and day 1 serum CRP level.
The available literature regarding the effect of
preoperative carbohydrate loading on postoperative morbidity is
confusing and still a matter of debate. Earlier studies reported
that postoperative symptoms, length of hospital stay and
morbidities were significantly reduced with the use of oral
carbohydrates as part of an ERAS protocol in patients undergoing
colorectal resection (12,13). However, a large ERAS registry data
of colorectal resections has shown that shorter hospital stay was
related to carbohydrate loading, whereas reduced morbidities were
only associated with the restrictive perioperative IV fluids
(16). Similarly, a Cochrane
review including all randomized controlled trials (RCT) of
carbohydrate treatment in patients undergoing any elective surgery
has demonstrated that carbohydrate loading was associated with
earlier return of bowel functions and shorter length of hospital
stay, but had no effect on morbidity (10). On the other hand, a more recent
metaanalysis evaluating only abdominal surgeries has revealed that
carbohydrate loading was associated with lower morbidity when
compared with overnight fasting; but morbidity rates were similar
between carbohydrate loading and clean water administration
(17). The main reason of the
discrepancies between the results of the previous studies is the
significant heterogeneity within the patient populations and
surgical procedures.
Although there are various studies reporting ERAS
outcomes in gynecological oncology (18), only one study has specifically
investigated the impact of carbohydrate loading as a single element
of ERAS on clinical outcomes. In a single-center RCT, Al-Hirmizy
et al (11) randomized 75
patients with EOC to receive a carbohydrate-enriched drink (n=37)
or placebo (n=38). The authors initially found that carbohydrate
loading increased the length of hospital stay by one day compared
with the placebo. Although not significant, morbidities were also
found to be higher in the carbohydrate loading group compared with
placebo. However, study groups were unbalanced in terms of type of
surgery, with more patients in the placebo group undergoing
interval debulking surgery. After adjusting factors that may affect
length of hospital stay, the authors found no difference between
the groups in regard to morbidities. The results of the present
study are comparable to those of Al-Hirmizy et al (11). However, despite the lack of a
randomized control group, the present study included a larger
number of patients and more homogenous and consistent data with
respect to baseline patient characteristics compared with
Al-Hirmizy et al (11).
Debulking surgery for EOC is a highly complicated
surgical proceedure involving multiple pelvic and upper abdominal
resections performed concurrently. Postoperative morbidity rates
have been reported to be as high as 67% (19). Similarly, in the current study, 77
of 162 patients (47.5%) developed at least one morbidity. It was
also found that operative time was the only independent risk factor
for any postoperative morbidity. The literature on morbidity after
debulking surgery shows that these morbidities often have a
multifactorial etiology that is not easy to prevent. Some of these
factors include patient-related characteristics such as age,
comorbidities, poor performance status, ascites and high tumor
burden, while others are surgery- and center-related
characteristics such as extensive resections, prolonged operative
time, poor surgical care and low surgical volume (19-21).
This multifactorial etiology may underlie the inability to
demonstrate the theoretical benefit of carbohydrate loading on
postoperative course and morbidity. In addition, the fact that the
present study did not have data on the genetic profiles of its
patients may have led to misinterpretation of the impact of
carbohydrate loading on postoperative course and morbidity.
Although the literature data on this subject are conflicting, some
studies have shown that tumor burden, invasion patterns,
resectability rates and postoperative complications may differ
between EOC patients with and without germline breast cancer gene
(BRCA) mutation. Petrillo et al (22) investigated the association between
BRCA mutation status and disease presentation in a large series of
patients with advanced high-grade serous EOC, including 107
patients with BRCA1/2 mutation and 166 patients without BRCA
mutation. The authors reported that EOC patients with a BRCA
mutation had a significantly higher incidence of peritoneal spread
without an ovarian mass (25.2% vs. 13.9%), bulky lymph nodes (30.8%
vs. 17.5%) and increased tumor burden (42.1% vs. 27.1%) than those
without a BRCA mutation. They concluded that more complex surgical
procedures may be required to achieve complete resection in
BRCA-mutant patients compared with non-mutant patients, which may
lead to longer operation times and more severe postoperative
complications. Kotsopoulos et al (23) examined the clinicopathological
characteristics of 1,421 patients with EOC, of whom 177 had BRCA1/2
mutation. The authors reported a significantly lower complete
resection rate (19% vs. 39%) in patients with a BRCA mutation
compared with non-mutant patients. By contrast, in a more recent
study involving a total of 612 patients with EOC, of whom 134 had a
BRCA1/2 mutation, Ataseven et al (24) found no effect of BRCA status on
disease burden, surgical complexity, complete resection rates (BRCA
mutant: 74.4%; BRCA wild-type: 69.0%; P=0.274) and postoperative
grade III-V complication rates (BRCA mutant: 12.0%; BRCA wild-type:
19.1%; P=0.082).
In the current study, operative time, splenectomy
and postoperative day 1 serum CRP level were independent risk
factors for grade III-V morbidities specifically. The optimal
cut-off value for day 1 serum CRP was found to be 107.5 mg/l, with
a sensitivity of 72.2% and specificity of 67.8%. CRP is an
acute-phase protein that is elevated in the presence of an
inflammatory process. Postoperatively, serum CRP levels increase in
response to surgical stress, peaking within 48-72 h and then
decrease (25). A number of
studies have shown that CRP levels remain elevated in complicated
postoperative conditions (25-28).
Schutz et al (26) studied
the CRP kinetics between postoperative day 1 and day-30 after
orthopedic surgery. The authors reported that serum CRP levels were
significantly higher in patients with a postoperative complication
than in patients without a complication, with a cut-off value of
105 mg/l on the first postoperative day. However, the authors noted
that the sensitivity and specificity of just one CRP value above
the threshold for predicting postoperative complications was only
48%. Nam et al (27)
estimated the first five postoperative day serum CRP cut-off values
for predicting early postoperative complications including
pneumonia, wound infection, intra-abdominal infection and
anastomotic leakage after surgery for colorectal cancer to be 65,
108, 114, 66 and 57 mg/l, respectively. In a meta-analysis of 23
studies with more than 6,600 patients, Yeung et al (28) investigated the association between
anastomotic leakage and serum CRP levels following colorectal
surgery. The authors reported that anastomotic leakage was
associated with higher CRP levels on each postoperative day
compared with no leakage after colorectal surgery. A cut-off value
of 110 mg/l on postoperative day 1 was found to have a sensitivity
of 60% and specificity of 73% in predicting anastomotic leakage.
Our cut-off value for day 1 CRP level is almost similar to the
findings of Yeung et al (28). However, it should be noted that due
to the different definitions of complications used in the
literature and differences in surgical procedures, it may not
always be appropriate to determine and recommend a cut-off value
for CRP as a predictor of postoperative complications.
The main limitation of the current study was its
retrospective design of control group, which may lead to a possible
selection bias. Another limitation is the difficulty of analyzing
postoperative course and morbidities in the historical control
group in a detailed and reliable manner. Lastly, the single-center
nature of the study is a barrier against generalizability of its
findings. However, despite the limitations, the present study is
the second (11) to examine the
exclusive role of carbohydrate loading as a single element of ERAS
in a controversial issue, which makes it valuable.
In conclusion, preoperative carbohydrate loading may
have some benefits, especially in gastrointestinal surgeries where
carbohydrate homeostasis is essential. However, based on the
results of the current study, postoperative course and morbidity
seems to be unaffected by carbohydrate loading in patients
undergoing debulking surgery for EOC. More high-quality evidence is
needed to recommend the routine use of carbohydrate loading in
patients with EOC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TT, IU and SD designed the whole study and agreed to
be accountable for all aspects of it. AK, MuG, AA, NY, MCK, NA, SK
and MeG contributed to the acquisition and curation of data. TT, SK
and MeG analyzed and interpreted the data. TT and AA drafted the
manuscript. IU and SD critically revised the manuscript. TT and IU
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Antalya Training and Research Hospital (7 May,
2018; approval no. 021). Written informed consent was obtained from
all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID IDs, TAYFUN TOPTAS 0000-0002-6706-6915, ISIN
UREYEN 0000-0002-3491-4682, ALPER KAHRAMAN 0000-0002-1689-2782,
MUSTAFA GOKKAYA 0000-0002-0477-157X, NECIM YALCIN
0000-0001-5980-3244, AYSUN ALCI 0000-0002-7912-7375, MERVE CAKIR
KOLE 0000-0002-9330-3363, SELIM KANDEMİR 0000-0002-3951-3503,
MEHMET GOKSU 0000-0001-9330-6241, NEDIM AKGUL 0000-0002-7003-7883,
SELEN DOGAN 0000-0002-4019-5581.
References
|
1
|
Finnerty CC, Mabvuure NT, Ali A, Kozar RA
and Herndon DN: The surgically induced stress response. JPEN J
Parenter Enteral Nutr. 37 (Suppl 5):S21–S9. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pogatschnik C and Steiger E: Review of
preoperative carbohydrate loading. Nutr Clin Pract. 30:660–664.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Abebe WA, Rukewe A, Bekele NA, Stoffel M,
Dichabeng MN and Shifa JZ: Preoperative fasting times in elective
surgical patients at a referral hospital in Botswana. Pan Afr Med
J. 23(102)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Witt L, Lehmann B, Sümpelmann R, Dennhardt
N and Beck CE: Quality-improvement project to reduce actual fasting
times for fluids and solids before induction of anaesthesia. BMC
Anesthesiol. 21(254)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fawcett WJ and Thomas M: Pre-operative
fasting in adults and children: Clinical practice and guidelines.
Anaesthesia. 74:83–88. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Smith I, Kranke P, Murat I, Smith A,
O'Sullivan G, Søreide E, Spies C and in't Veld B: European Society
of Anaesthesiology. Perioperative fasting in adults and children:
Guidelines from the european society of anaesthesiology. Eur J
Anaesthesiol. 28:556–569. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dobson G, Chow L, Flexman A, Hurdle H,
Kurrek M, Laflamme C, Perrault MA, Sparrow K, Stacey S, Swart P and
Wong M: Guidelines to the practice of anesthesia-revised edition
2019. Can J Anaesth. 66:75–108. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Viganò J, Cereda E, Caccialanza R, Carini
R, Cameletti B, Spampinato M and Dionigi P: Effects of preoperative
oral carbohydrate supplementation on postoperative metabolic stress
response of patients undergoing elective abdominal surgery. World J
Surg. 36:1738–1743. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wischmeyer PE, Carli F, Evans DC, Guilbert
S, Kozar R, Pryor A, Thiele RH, Everett S, Grocott M, Gan TJ, et
al: American society for enhanced recovery and perioperative
quality initiative joint consensus statement on nutrition screening
and therapy within a surgical enhanced recovery pathway. Anesth
Analg. 126:1883–1895. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Smith MD, McCall J, Plank L, Herbison GP,
Soop M and Nygren J: Preoperative carbohydrate treatment for
enhancing recovery after elective surgery. Cochrane Database Syst
Rev. 8(CD009161)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Al-Hirmizy D, Wood NJ, Ko S, Henry A,
Nugent D, West R and Duffy S: A single centre randomised control
study to assess the impact of pre-operative carbohydrate loading on
women undergoing major surgery for epithelial ovarian cancer.
Cureus. 12(e10169)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nygren J, Soop M, Thorell A, Hausel J,
Ljungqvist O and ERAS Group: An enhanced-recovery protocol improves
outcome after colorectal resection already during the first year: A
single-center experience in 168 consecutive patients. Dis Colon
Rectum. 52:978–985. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gustafsson UO, Hausel J, Thorell A,
Ljungqvist O, Soop M and Nygren J: Enhanced Recovery After Surgery
Study Group. Adherence to the enhanced recovery after surgery
protocol and outcomes after colorectal cancer surgery. Arch Surg.
146:571–577. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Clavien PA, Barkun J, de Oliveira ML,
Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J,
Slankamenac K, Bassi C, et al: The Clavien-Dindo classification of
surgical complications: Five-year experience. Ann Surg.
250:187–196. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Prat J: FIGO Committee on Gynecologic
Oncology. Staging classification for cancer of the ovary, fallopian
tube, and peritoneum. Int J Gynaecol Obstet. 124:1–5.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
ERAS Compliance Group. The impact of
enhanced recovery protocol compliance on elective colorectal cancer
resection: Results from an international registry. Ann Surg.
261:1153–1159. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ricci C, Ingaldi C, Alberici L, Serbassi
F, Pagano N, De Raffele E, Minni F, Pironi L, Sasdelli AS and
Casadei R: Preoperative carbohydrate loading before elective
abdominal surgery: A systematic review and network meta-analysis of
phase II/III randomized controlled trials. Clin Nutr. 41:313–320.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bisch SP and Nelson G: Outcomes of
enhanced recovery after surgery (ERAS) in gynecologic oncology: A
review. Curr Oncol. 29:631–640. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Castro BGR, Dos Reis R, Cintra GF, Sousa
MMA, Vieira MA and Andrade CEMDC: Predictive factors for surgical
morbidities and adjuvant chemotherapy delay for advanced ovarian
cancer patients treated by primary debulking surgery or interval
debulking surgery. Int J Gynecol Cancer. 28:1520–1528.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu Z, Becerra AZ, Justiniano CF, Aquina
CT, Fleming FJ, Boscoe FP, Schymura MJ, Sinno AK, Chaoul J, Morrow
GR, et al: Complications and survivorship trends after primary
debulking surgery for ovarian cancer. J Surg Res. 246:34–41.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kengsakul M, Nieuwenhuyzen-de Boer GM,
Udomkarnjananun S, Kerr SJ, Niehot CD and van Beekhuizen HJ:
Factors predicting postoperative morbidity after cytoreductive
surgery for ovarian cancer: A systematic review and meta-analysis.
J Gynecol Oncol. 33(e53)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Petrillo M, Marchetti C, De Leo R, Musella
A, Capoluongo E, Paris I, Benedetti Panici P, Scambia G and Fagotti
A: BRCA mutational status, initial disease presentation, and
clinical outcome in high-grade serous advanced ovarian cancer: A
multicenter study. Am J Obstet Gynecol. 217:334.e1–334.e9.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kotsopoulos J, Rosen B, Fan I, Moody J,
McLaughlin JR, Risch H, May T, Sun P and Narod SA: Ten-year
survival after epithelial ovarian cancer is not associated with
BRCA mutation status. Gynecol Oncol. 140:42–47. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ataseven B, Tripon D, Schwameis R, Harter
P, Rhiem K, Schneider S, Heikaus S, Baert T, Francesco AP, Heitz F,
et al: Clinical outcome in patients with primary epithelial ovarian
cancer and germline BRCA1/2-mutation-real life data. Gynecol Oncol.
163:569–577. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Harada K, Matsumoto C, Toihata T, Kosumi
K, Iwatsuki M, Baba Y, Ohuchi M, Eto K, Ogawa K, Sawayama H, et al:
C-Reactive protein levels after esophagectomy are associated with
increased surgical complications and poor prognosis in esophageal
squamous cell carcinoma patients. Ann Surg Oncol. 30:1554–1563.
2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schutz R, Boukebous B, Boutroux P and
Guillon P: C-reactive protein levels for early detection of early
postoperative complications after proximal femoral fracture
surgery. Eur J Orthop Surg Traumatol. 28:907–913. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nam JH, Noh GT, Chung SS, Kim KH and Lee
RA: Validity of C-reactive protein as a surrogate marker for
infectious complications after surgery for colorectal cancer. Surg
Infect (Larchmt). 24:488–494. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yeung DE, Peterknecht E, Hajibandeh S,
Hajibandeh S and Torrance AW: C-reactive protein can predict
anastomotic leak in colorectal surgery: A systematic review and
meta-analysis. Int J Colorectal Dis. 36:1147–1162. 2021.PubMed/NCBI View Article : Google Scholar
|