Introduction
Mesenchymal stem cells (MSCs) are immunologically
tolerable cells that exhibit low expression levels of major
histocompatibility complex (MHC) I and negligible expression levels
of MHC II and co-stimulatory molecules B7-1, B7-2, CD40 or CD40L
(1). Through direct interactions
with target cells or paracrine signalling molecules, such as
indoleamine 2, 3 dioxygenase, prostaglandin E2 (PGE2) and IL-10,
MSCs exert regulatory effects on immune cell activation (1). Previous studies have reported that
the therapeutic functions of transplanted MSCs is based on the
secretion of angiogenesis stimulating and inflammatory alleviating
factors (2-6),
therefore, MSC injection has been widely used in the treatment of
ischemic heart disease (7-9).
MSCs reside in various adult tissues (10); however, MSC populations isolated
from these tissues are heterogeneous and MSC preparations are also
affected by the type of tissue that these cells are isolated from
and the age of donor (11,12). MSC proliferation capacity decreases
with donor age and multiple rounds of in vitro culture
expansion can cause replicative senescence and a change in cell
function (12). Furthermore, the
potency of human MSCs is limited compared with that of embryonic
stem cells (ESCs) or induced pluripotent stem cells (iPSCs).
Therefore, obtaining MSCs with enhanced proliferation and
differentiation potentials to improve their clinical utility is of
primary importance for their use to treat certain diseases.
Currently, stem cell derived MSCs are regarded as an
important cell source to replace tissue derived MSCs. Given that
human ESCs are derived from human embryos, there are ethical issues
associated with using human ESCs as therapeutic cell source.
However, the application of human somatic cell derived iPSCs
(hiPSCs) for patient-specific autologous regenerative treatment is
promising with fewer ethical concerns, and differentiating hiPSCs
into multipotent progenitors prior to transplantation is one of the
most suitable, safe and effective approaches for the use of iPSCs
(13). This approach could
eliminate the issue of immune incompatibility and provide a unified
starting point for generating high quality iPSC derived MSCs at a
large scale (13).
Epithelial-mesenchymal transition (EMT) is a
biological process in embryonic development that serves an
important role in human ESC mesoderm commitment (13). hiPSC spontaneous mesoderm
differentiation can be initiated by subsequent passaging of cells
at a low cell density or during cell embryoid body formation. A
time period of ≥30 days is required to produce cells with increased
expression levels of MSC markers. Hence, there is an urgent need to
develop a more rapid method of obtaining MSCs from hiPSCs, such as
prompting EMT though the addition of soluble active agents.
TGF-β is a secreted growth and differentiation
factor that exerts a verity of biological activities depending on
the type of cellular target. During the process of embryonic
development, TGF-β expression is often detected at the site of
epithelial and mesenchymal interaction (14). TGF-β, through binding with its
receptor, activates a series of intracellular signal transduction
factors, including sentinel node invasion level 1/2 and zinc finger
E-box binding homeobox 1/2, which promote epithelial cell EMT by
upregulating the mesenchymal specification related marker
N-cadherin (15,16).
GSK-3 was initially reported to be a glycogen
synthase inhibitor. However, additional studies have since reported
that GSK-3 was involved with the regulation of >100 substrates
(17,18). Previously, it was reported that
SNAIL repressed the expression of E-cadherin and induced EMT as
constitutive activation of GSK-3 induced SNAIL degradation and
stabilization of SNAIL through inhibition of GSK-3β resulted in
suppression of E-cadherin expression during TGF-β induced EMT
(19,20). After co-culturing with Xenopus
tropicalis immature Sertoli cells for 3 days, the GSK-3
inhibitor CHIR99021 increased the expression of EMT transcription
factors and the number of cells with an MSC-like morphology
(21).
To date, a number of reports indicate that the
experimental and clinical studies using MSCs to treat myocardium
infarction have used an intramyocardial or intracoronary cell
delivery system (7-9).
However, the operation of intramyocardial or intracoronary cell
delivery is complicated and invasive, as a result, repeated
intromyocardial or introcoronary cell delivery is not acceptable
for most of these patients (22).
In comparison with intramyocardial or intracoronary cell delivery,
it is more convenient for patients to be administered with multiple
intravenous (IV) MSC injections. Nevertheless, it has been reported
that only a small portion of the injected cells integrate into the
infarcted area, as most of the injected cells were found in lung,
kidney, liver or spleen tissues and have the potential to form
lethal thromboemboli in these organs (22,23).
The peritoneal cavity is also an appropriate place
for cell injection because there are active fluid and cellular
exchanges occurring between the cavity and the general circulatory
system (23,24). The exchange takes place through
three possible channels: i) ‘Milky spots’, which are located on the
surface of the colicomentum and the fatty tissues; ii) the draining
lymphatic system; and iii) punctate regions (24). Intraperitoneal (IP) injection
permits the administration of higher cell doses without causing
embolization in any organs (24).
In addition, IP injections of MSCs were reported to have more
beneficial effects compared with IV injections in the treatment of
experimental autoimmune encephalomyelitis, cisplatin-induced renal
injury, cornea sterile inflammation and zymosan-induced peritonitis
(25-30).
EMT can be initiated by activation of multiple
extracellular signals, including the TGF-β superfamily and the Wnt
signalling pathway (14,15,18,19).
In the present study, the combined treatment of GSK-3 inhibitor
CHIR99021 and TGF-β was tested to determine if this treatment would
promote hiPSC EMT and facilitate the derivation of MSCs. Secondly,
the hiPSC derived MSCs (hiPSC-MSCs) were intraperitoneally
administrated into a rat model of acute myocardial infarction (AMI)
at a high dosage to evaluate the safety and efficacy of hiPSC-MSCs
for the treatment of a rat model of AMI.
Materials and methods
hiPSC cell line and culture
The hiPSC cell line PBMC-5 (at passage 10) was
donated by Dr Tiancheng Zhou. hiPSCs were prepared from peripheral
blood mononuclear cells of a healthy Han Chinese female donor (age,
31 years) in CAS Key Laboratory of Regenerative Biology, Guangzhou
Institutes of Biomedicine and Health (Guangzhou, China). Cells were
separated using a Ficoll-Paque gradient (Shenzhen DAKEWE
Bio-Engineering Co., Ltd.) and reprogramed using the CTS™
CytoTune™-iPS Sendai virus reprograming kit (Thermo Fisher
Scientific, Inc.). The hiPSCs were maintained as large cell
colonies on Matrigel-coated plates (BD Biosciences) in serum free
mTeSR1 medium (STEMCELL Technologies) at 37˚C, in 5%
CO2.
MSC differentiation of hiPSCs
Dispersed single hiPSC cells at passage 15 were
seeded onto a Matrigel-coated 12-well plate in mTeSR1 medium at a
density of 2x104 cells/cm2 2 days before
induction. Cells were treated with the following different MSC
differentiation mediums for 6 days: i) DMEM basal medium
supplemented with 10% knockout serum replacement (Invitrogen;
Thermo Fisher Scientific, Inc.), 1 mM L-glutamine, 10 mM
non-essential amino acids, 50 U/ml penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) and 0.5 µl/ml DMSO
(MilliporeSigma; Merck KGaA); ii) DMEM containing 5 or 10 µM
CHIR99021 (Stemgent, Inc.; REPROCELL), iii) DMEM containing 10 or
20 µg/ml TGF-β (Creative BioMart); or iv) DMEM medium containing 5
µM CHIR99021 and 10 µg/ml TGF-β. The selected concentrations of
CHIR99021 and TGF-β used in the present study were selected on the
basis of previous reports (20,30-33)
and the induction medium was changed every other day.
At 6 days after cell induction, the differentiated
cells were dispersed into single cells through TrypleSelect
(Invitrogen; Thermo Fisher Scientific, Inc.) digestion at 37˚C for
5 min and collected by centrifugation at 500 x g for 5 min. The
collected cells were suspended in MSC medium [DMEM basal medium
supplemented with 10% FBS (HyClone; Cytiva), 1 mM L-glutamine, 50
U/ml penicillin/streptomycin and 10 mM non-essential amino acids
(Invitrogen; Themo Fisher Scientific, Inc.)] and seeded at a
density of 4x104 cells/cm2. In subsequent
passages, the cell concentration was reduced to 2x104
cells/cm2. The cell induction experiment was repeated
thrice.
Reverse transcriptase-quantitative PCR
(RT-qPCR)
Total RNA isolation from the induced cells was
conducted using the RNase mini kit (Qiagen, Inc.) and a
high-capacity RNA-to-cDNA kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to reverse transcribe RNA into cDNA
according to the manufacturer's instructions (incubation at 85˚C
for 5 sec and 37˚C for 1 h). For quantification of gene expression,
RT-qPCR was performed using specific primers (Table I) and SYBR-Green RT-PCR reagents
(Applied Biosystems; Thermo Fisher Scientific, Inc.) under the
following thermocycling conditions: denaturation at 94˚C for 30
sec; annealing at 60˚C for 35 sec and extension at 72˚C for 40 sec,
30 cycles. The 2-ΔΔCq method was used to analyse
relative gene expression levels and GAPDH was used as the
normalisation control (34). The
quantification of gene transcription was repeated three times.
 | Table IPrimers used for reverse
transcriptase-quantitative PCR. |
Table I
Primers used for reverse
transcriptase-quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| E-cadherin | F:
TTCTGCTGCTCTTGCTGTTT |
| | R:
TGGCTCAAGTCAAAGTCCTG |
| N-cadherin | F:
CCTGCGCGTGAAGGTTTGCC |
| | R:
CCAAGCCCCGCACCCACAA |
| Vimentin | F:
GCCCTTAAAGGAACCAATGA |
| | R:
AGCTTCAACGGCAAAGTTCT |
| Oct3/4 | F:
GACAGGGGGAGGGGAGGAGCTAGG |
| | R:
CTTCCCTCCAACCAGTTGCCCCAAAC |
| Sox2 | F:
GGGAAATGGGAGGGGTGCAAAAGAGG |
| | R:
TTGCGTGAGTGTGGATGGGATTGGTG |
| Nanog | F:
CAGCCCCGATTCTTCCACCAGTCCC |
| | R:
CGGAAGATTCCCAGTCGGGTTCACC |
| GAPDH | F:
CATCAATGGAAATCCCATCA |
| | R:
TTCTCCATGGTGGTGAAGAC |
Western blotting
The EpiQuik whole cell extraction kit (AmyJet
Scientific, Inc.) was used to extract total protein from the
induced cells and protein concentration was measured using the
Bradford DC protein assay (Bio-Rad Laboratories). Protein samples
(30 µg) were separated by electrophoresis on 12% Bis-Tris
polyacrylamide gel and then transferred to nitrocellulose membranes
(MilliporeSigma; Merck KGaA). The membranes were blocked with 5%
BSA (Wuhan Servicebio Technology Co., Ltd.) at room temperature for
1.5 h and incubated overnight at 4˚C with the following primary
antibodies: SMAD 2/3 (cat. no. #3102S), ERK1/2 (cat. no. #4695T)
(Cell Signalling Technology, Inc.), GSK-3 (cat. no. #PA532440;
Thermo Fisher Scientific, Inc.) and SNAIL (cat. no. #ABD38;
MilliporeSigma) primary antibodies at 1:1,000 dilution at 4˚C for
12 h, followed by incubation with goat anti-rabbit polyclonal HRP
conjugated secondary antibodies (1:10,000 dilution in 1% milk/TBS;
cat. no. #A16110; Thermo Fisher Scientific, Inc.) for 2 h at room
temperature. Protein bands were visualized using a
chemiluminescence detection kit (Amersham; Cytiva) and analyzed
using Tanon 5220S image analysis system (Tanon Image software
version 1.0; Tanon Science and Technology Co., Ltd.). Analysis of
protein expression levels was repeated thrice.
MSC specific marker detection and cell
differentiation assay
At six passages after the initial MSC induction from
hiPSCs, the adherent cells with a spindle-like morphology were
collected by 0.25% trypsin treatment at 37˚C for 5 min and
centrifugation at 500 x g for 5 min. Then the cells were
resuspended in PBS at 1x106 cells/ml for determining the
expression levels of typical MSC surface markers (CD29, CD44, CD73,
CD90 and CD105) and hematopoietic cell lineages markers [CD34, CD45
and human leukocyte antigen-DR isotype (HLA-DR)] by
fluorescence-activated cell sorting. The antibodies used were as
follows: fluorescent conjugated anti-CD29, -CD44, -CD73, -CD90 and
-CD105 antibodies (cat. nos. #354603, #338807, #344003, #328109 and
#323205, respectively; BioLegend, Inc.); primary anti-CD45, -CD34,
-HLA-DR antibody and CY3 conjugated goat anti-rabbit IgG secondary
antibody (cat. nos. #A0055-3, #BM4082, #BM4546 and #BA1032,
respectively; Wuhan Boster Biological Technology, Ltd.). The
primary antibodies (1:200 dilution) and secondary antibody (1:5,000
dilution) were incubated respectively with the cells for 30 min at
room temperature, and then the cell surface marker expression was
measured by BD-FACScan (Becton, Dickinson and Company), and the
data were analysed using FlowJo (V10; FlowJo LLC). Measurements
were repeated three times.
The induction of osteogenic, chondrogenic and
adipocytic differentiation and subsequent evaluation of the induced
cells was conducted as previously described (33). Osteogenic differentiation was
initiated by culturing cells with osteo-inductive medium [DMEM
basal medium supplemented with 10% FBS (HyClone; Cytiva), 10 mM
glycerol phosphate, 50 µM ascorbate phosphate, 10-7 M
dexamethasone (MilliporeSigma; Merck KGaA) and 100 ng/ml
recombinant human bone morphogenic protein-2 (Invitrogen; Thermo
Fisher Scientific, Inc.)] for 2 weeks. For chondrogenic
differentiation, cells were cultured in chondrogenic induction
medium [DMEM basal medium supplemented with 10% FBS, 6.25 µg/ml of
insulin (Fisher Scientific, Thermo Fisher Scientific, Inc.), 50 nM
of ascorbate phosphate and 10 ng/ml of TGF-β] for 2 weeks. The
adipocytic differentiation potential was analysed using adipogenic
induction medium (DMEM supplemented with 10% FBS, 10-7 M
of dexamethasone and 10 µg/ml of insulin) to culture the cells for
≥2 weeks.
The cells growing on 60 mm culture dish (Corning)
were fixed by 4% formaldehyde for 10 min at room temperature, and
then stained respectively by 0.1% Alizarin red, 1% Alcian blue and
0.5% Oil-Red-O at room temperature for 30 min to visualize calcium
nodule formation, sulphated proteoglycan formation and
intracellular lipid accumulation in the cells under light
microscope at magnification x200 (Olympus).
Animal acute myocardium infarction
(AMI) model
A total of 30 male Wister rats (age, 4 weeks;
weight, 230-270 g) were purchased from The Medical Experimental
Animal Centre of Guangdong Province. The rats were housed under
constant 20˚C temperature, 45% humidity and a 12 h light-dark cycle
and had free access to standard laboratory chow and tap water.
Approval for the animal experimentation was granted
by The Animal Ethics Committee of The Peking University & Hong
Kong Science and Technology University Medical Centre (approval no.
2020-027). Chinese laws relating to the care and treatment of
animals and the Animal Research: Reporting of In Vivo Experiments
guidelines were followed throughout the study (35).
The rats were randomly separated into MSC injection
group, PBS injection group and sham group, with 10 rats in each
group. In order to construct the AMI model, rats were first
anaesthetised by pentobarbital sodium (40 mg/kg) intraperitoneal
injection and then the heart was exposed via a left thoracotomy
between the 4th and 5th ribs of the animal. A tight suture was made
around the left anterior descending coronary artery and the
resulted ischemia was maintained for 40 min and then the suture was
removed to restore blood flow. In the sham control group, the
suture was immediately removed without any further maintaining.
Before sacrifice, the rats were administered an IP
injection of pentobarbital sodium (40 mg/kg). When the rats were
anaesthetised and unresponsive to stimuli, cervical dislocation was
used to sacrifice each animal. In order to confirm death, the
absence of breath sounds and heart beat was monitored by
observation and echocardiography.
IP administration and distribution of
hiPSC-MSCs in AMI model rats
Immediately after AMI, model rats were divided
randomly into hiPSC-MSC injection group (n=10) and PBS injection
control groups (n=10). An IP injection of 1x107
hiPSC-MSCs (passage 6 after the initial MSC induction from hiPSC)
was administered to rats in the hiPSC-MSC treated group each week
for 4 weeks, with PBS injections used as a control.
To determine the distribution of the IP injected
hiPSC-MSCs, 1x107 hiPSC-MSCs were labelled with Xeno
Light Dir (PerkinElmer, Inc.) and injected into the peritoneal
cavity of rats (n=3). The signals of the injected cells were
detected using the Lumina IVIS II System (PerkinElmer, Inc.) and
the fluorescence images of the peritoneal cavity and individual
colicomenta were obtained using the Living Image Software (version
4.1; PerkinElmer, Inc.) immediately after the injection, 3 days
after the injection, 1 week after the injection and 3 weeks after
the injection.
Peritoneal lavage fluid cytokine
analysis
For in vivo cytokine assay analysis, 1 week
after the IP cell injection, 10 ml of PBS was injected into the
peritoneal cavity of the anesthetised (40 mg/kg pentobarbital
sodium) hiPSC-MSC injected (n=3) and control rats (n=3) with a
10-gauge syringe and the peritoneal lavage was collected. The
concentrations of insulin-like growth factor (IGF), VEGF,
hepatocyte growth factor (HGF), prostaglandin E2 (PGE2), IL-4 and
IL-10 in the lavage were measured using ELISA kits following the
manufacturer's instructions. The kits used were as follows: Human
IGF-1 ELISA kit (cat. no. #EK1131-48), PGE2 Competitive ELISA kit
(cat. no. #EK8103/2-48) (Hangzhou Lianke Biotechnology Co., Ltd.),
human VEGF ELISA Kit (cat. no. #ab100663), hepatocyte growth factor
ELISA kit (cat. no. #ab275901) (Abcam), human IL-10 ELISA kit (cat.
no. KIT10947A), human IL-4 ELISA kit (cat. no. KIT11846) (Sinopharm
Chemical Reagent Co., Ltd.). A 1510 spectrophotometer (Thermo
Fisher Scientific, Inc.) was used to analyse the samples and the
measurements were repeated three times.
Echocardiography evaluation of cardiac
function
A 2D echocardiography system (Vevo 2100; Visual
Sonics) with a MS250 transducer (13-24 MHz) was used to evaluate
the cardiac function of the AMI model rats. The parameter changes
pertaining to left ventricular ejection fraction (LVEF) and left
ventricular fractional shortening (LVFS) were measured and
analysed.
Scar size quantification
The area of myocardial scars of the AMI model rats
was assessed using Masson's trichrome staining 11 weeks after AMI
induction. Briefly, tissue sections of the infarcted area were
fixed in 4% formaldehyde, embedded in paraffin and cut into 5 µm
slices. The slices were stained with Masson's trichrome to detect
fibrosis and three tissue slices taken from each rat were assessed
by a pathologist who was blinded to the samples. The scar area
percentage of total tissue area was quantified using a computerised
morphometry system with a light microscope (Cell Sens; Ver.1.16;
Olympus Corporation).
Infarcted area vascularisation
evaluation
Heart tissue was fixed with 4% paraformaldehyde
(Beyotime Institute of Biotechnology) for 72 h at room temperature,
embedded in paraffin and cut into sections (4 µm). Tissue slices
(n=3) from each rat were deparaffinized, rehydrated and
pressure-cooked at 121˚C for 4 min in citrate buffer (10 mM, pH
6.0) for antigen retrieval. The sections were incubated in 1%
H2O2/methanol at room temperature for 10 min
and then blocked in 4% bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.) for 30 min at room temperature.
Immunohistochemistry detection of α-SMA expressing cells was
conducted with primary mouse anti-rat α-SMA antibodies (1:50
dilution; incubation 2 h at 4˚C; cat. no. #614852; BioLegend, Inc.)
and secondary HRP conjugated anti-mouse IgG antibodies (1:100
dilution, incubation 30 min at room temperature, Wuhan Boster
Biological Technology, Ltd. Cat.# BA1051). α-SMA positive
capillaries in the infarcted regions were manually counted under
light microscope using high-power fields (hpf) of magnification
(x200) in each tissue section.
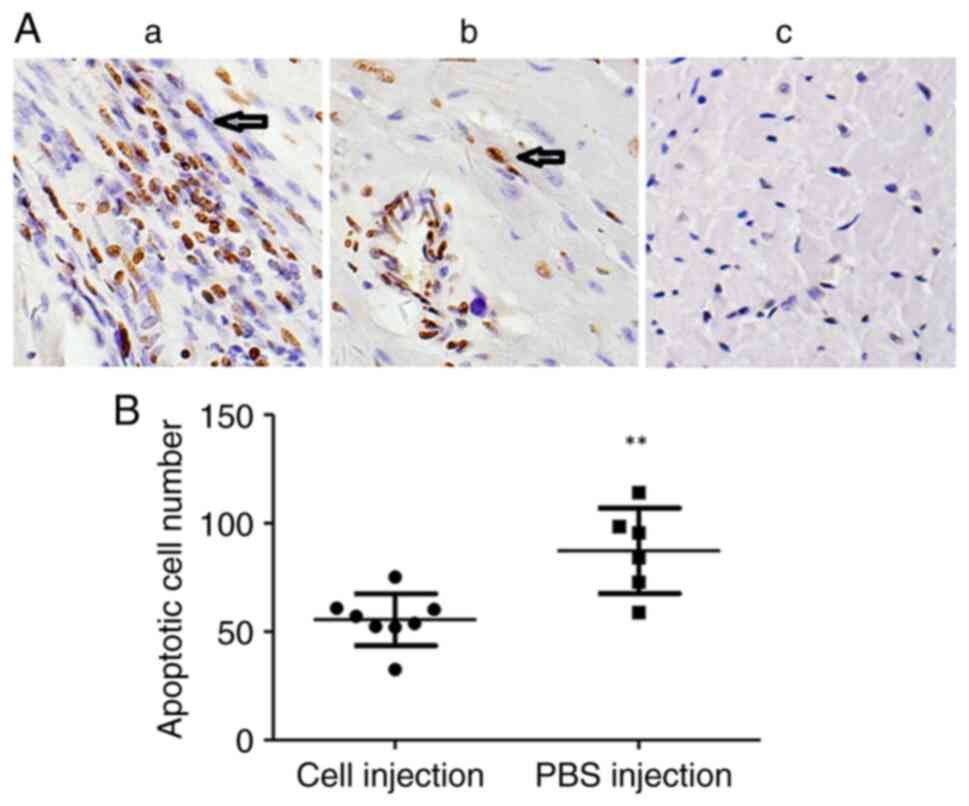
Apoptosis assay
To quantify the extent of cardiomyocyte apoptosis, a
TUNEL kit (Nanjing KeyGen BioTech Co., Ltd.) was used to stain
apoptotic cells present in heart tissue sections (prepared the same
way as in the scar size quantification) according to the
manufacturer's recommendations. 0.5% Hematoxylin counterstaining
was conducted to better visualize normal nuclei. Apoptotic cells
were counted in 5 randomly selected hpf of magnification (x400) in
the infarcted area of three tissue sections from each rat and the
mean count calculated.
Statistical analysis
Data were presented as mean ± standard deviation and
analysed using GraphPad Prism (version 5; GraphPad; Dotmatics). A
one-way ANOVA with Dunnett's test was used for multiple comparisons
and an unpaired t-test was used for comparisons between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CHIR99021 and TGF-β treatment promoted
EMT of human iPSCs
To examine whether CHIR99021 or TGF-β treatment
could initiate mesoderm commitment, hiPSCs were treated with
CHIR99021, TGF-β or a CHIR99021 + TGF-β combination for 6 days. The
mRNA expression levels of EMT-related genes N-cadherin and
Vimentin, mesenchymal to epithelial transition (MET)-related gene
E-cadherin and pluripotency-related transcription factors OCT4,
SOX2 and NANOG were evaluated using RT-qPCR (Fig. 1A).
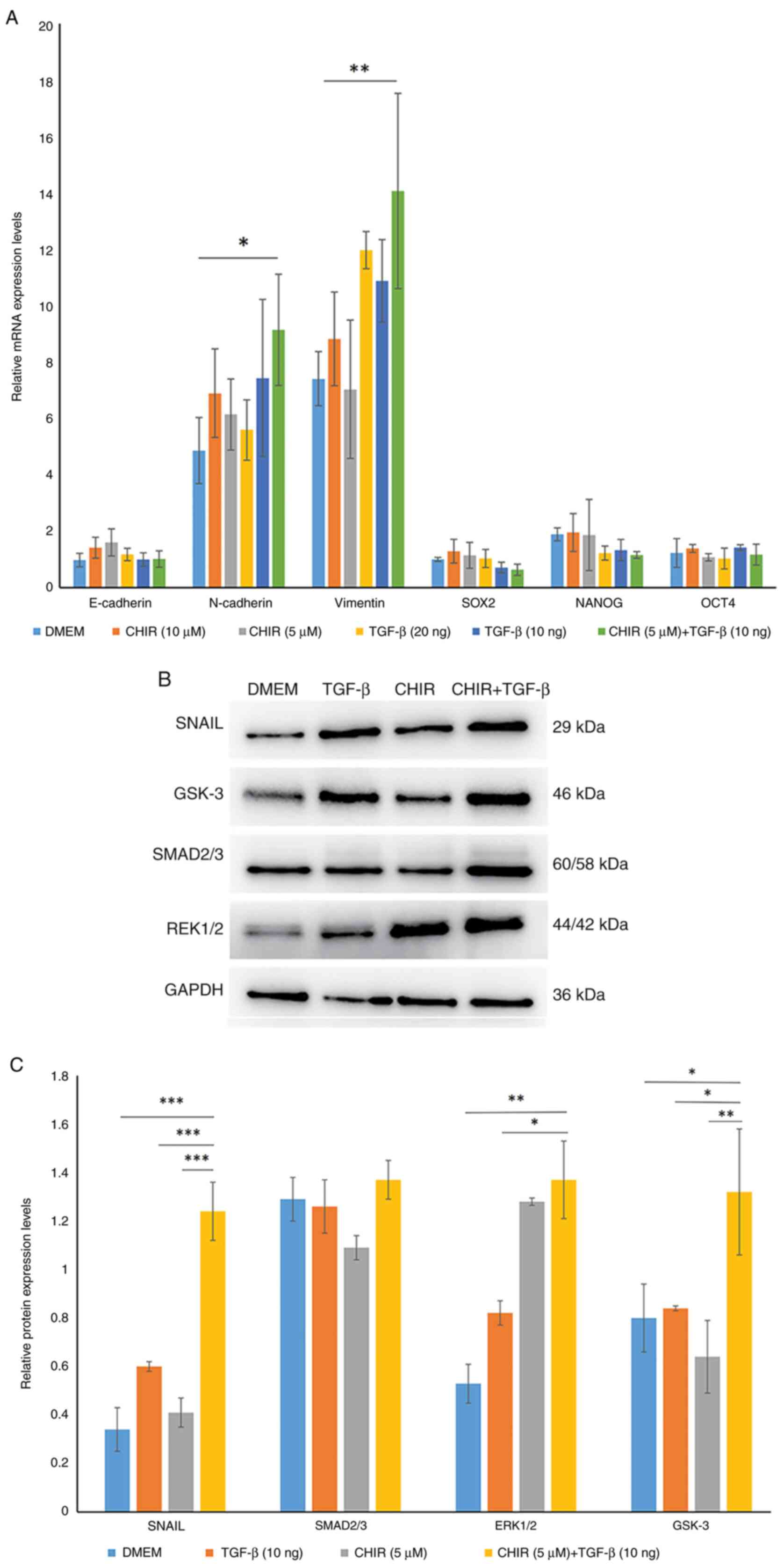 | Figure 1CHIR99021 and TGF-β treatment
promoted EMT of human iPSCs. (A) Treatment of iPSCs with CHIR99021,
TGF-β or CHIR99021 + TGF-β markedly upregulated the expression of
EMT-related genes N-cadherin and Vimentin compared with untreated
controls. Significant upregulation of N-cadherin was demonstrated
in the CHIR99021 + TGF-β treated cells compared with untreated
controls. The expression levels of E-cadherin, Oct4, Sox2 and Nanog
were not significantly altered. (B) Treatment of cells with
CHIR99021, TGF-β or CHIR99021 + TGF-β increased the protein
expression levels of ERK1/2, SNAIL and GSK-3 in the transformed
cells compared with control cells. (C) In comparison with the
untreated and TGF-β treated cells, the protein expression levels of
ERK1/2, SNAIL and GSK-3 in CHIR99021 + TGF-β treated cells was
significantly increased. *P<0.05,
**P<0.01, ***P<0.001. CHIR, CHIR99021;
iPSC, induced pluripotent stem cells; EMT, epithelial-mesenchymal
transition. |
After treatment with CHIR99021, TGF-β or the
CHIR99021 + TGF-β combination for 6 days, compared with the
untreated control cells, the mRNA expression levels of EMT related
genes N-cadherin and Vimentin in the CHIR99021 or TGF-β treated
cells was markedly upregulated and significant upregulation was
demonstrated in the 5 µM CHIR99021 +10 µg/ml TGF-β treated cells
(N-cadherin, P<0.05; Vimentin, P<0.01). A decrease in the
cell growth rate and apoptosis were observed when the concentration
of CHIR99021 and TGF-β was increased to 10 µM and 20 µg/ml,
respectively (data not shown). As a result, the combination of 5 µM
CHIR99021 +10 µg/ml TGF-β was used in the subsequent experiments.
The mRNA expression levels of OCT4, SOX2, NANOG and
E-cadherin in the CHIR99021 or TGF-β treated cells were not
significantly altered compared with that of the untreated control
cells.
To further investigate the potential mechanism
involved in the CHIR99021 and TGF-β induced EMT of hiPSCs, analysis
of SNAIL, SMAD2/3, ERK1/2 and GSK-3 protein expression levels was
conducted by western blotting in the control, CHIR99021, TGF-β and
CHIR99021 + TGF-β treated hiPSCs (Fig.
1B and C). These results
demonstrated that after 6 days of induction, the protein expression
levels of SNAIL and ERK1/2 in the 5 µM CHIR99021 +10 µg/ml TGF-β
treated cells were significantly increased compared with that of
the DMEM or single reagent treated cells. There was no significant
difference in the protein expression levels of SMAD2/3 in control
and treated cells. Furthermore, CHIR99021 + TGF-β treatment
significantly increased the GSK-3 protein expression levels
compared with the control and single reagent treatments.
Morphology, surface-marker expression
and differentiation of hiPSC-MSCs
The morphology of hiPSC-MSCs was analysed (Fig. 2A). At passage 3, the spontaneously
differentiating cells mainly had a pleomorphic morphology. In the
cells treated with 10 or 20 µg/ml TGF-β, <20% of cells exhibited
a short spindle and olive-shaped morphology. In the cells treated
with 5 µM CHIR99021, >30% of the cells exhibited a short spindle
shape, whereas in the 10 µM CHIR99021 treated cells, the percentage
of spindle shaped cells was close to 50%. In cells treated with 5
µM CHIR99021 +10 µg/ml TGF-β, ≥70% of the adherent cells exhibited
a spindle-shaped morphology.
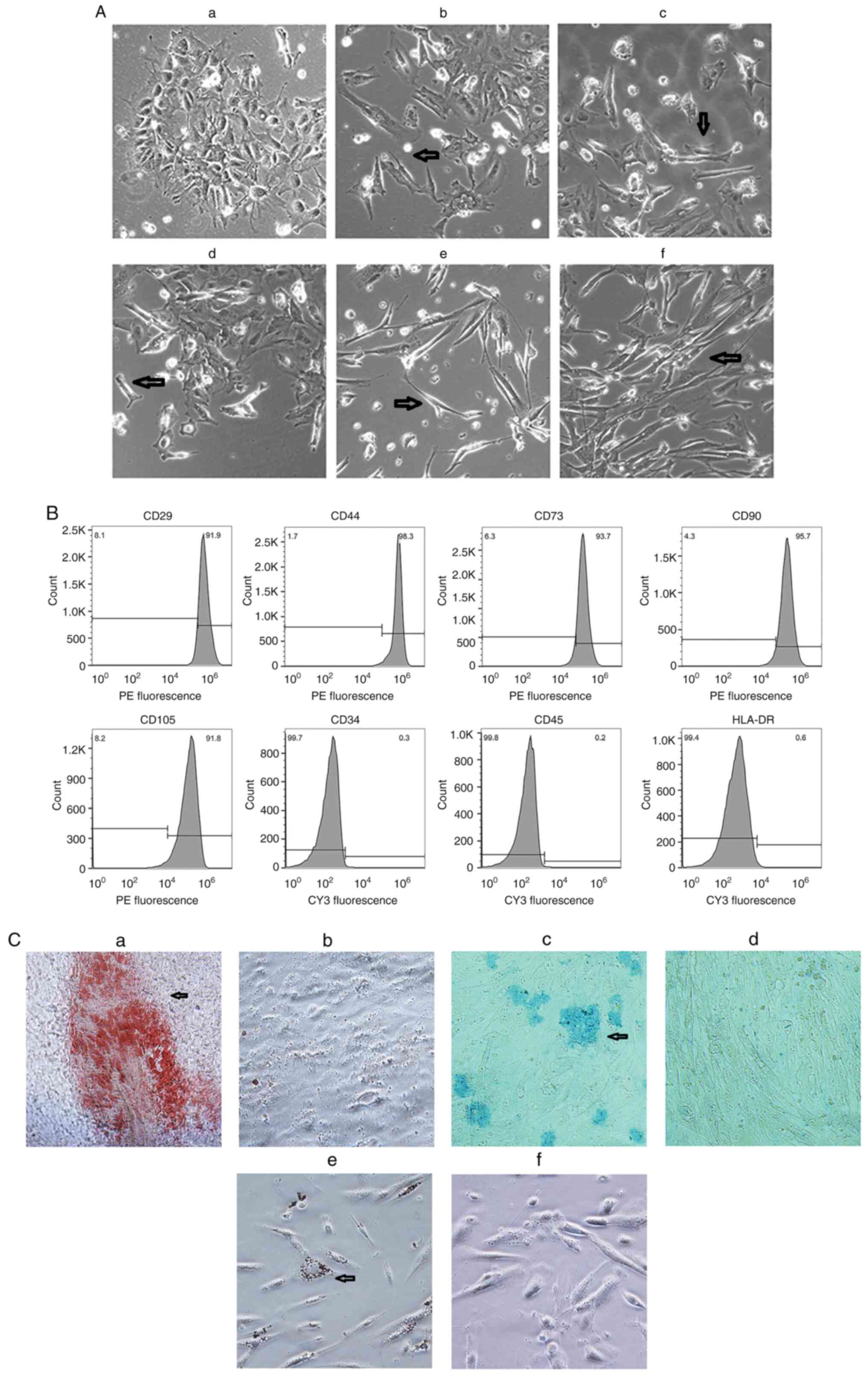 | Figure 2Cell morphology, surface-marker
expression and differentiation of hiPSC-MSCs. (A) Cells with
spindle morphology appeared (arrow) at passage 3 in cells treated
with (A-a) DMEM, (A-b) TGF-β (20 µg/ml), (A-c) TGF-β (10 µg/ml),
(A-d) CHIR99021 (10 µM), (A-e) CHIR99021 (5 µM) and (A-f) CHIR99021
(5 µM)+TGF-β (10 µg/ml). Magnification, x100. (B) Surface-marker
expression analysis demonstrated >90% of cells expressed typical
MSC surface markers (CD29, CD44, CD73, CD90 and CD105), whilst
≤0.6% of cells expressed hematopoietic cell lineage markers (CD34,
CD45 and HLA-DR). (C) hiPSC-MSCs demonstrated (C-a) osteogenic,
(C-c) chondrogenic and (C-e) adipogenic differentiation, whereas
uninduced cells (C-b, C-d and C-f) did not. Magnification, x400.
hiPSC-MSCs, human induced pluripotent stem cells-mesenchymal stem
cells; HLA-DR, human leukocyte antigen-DR isotype. |
MSC surface marker expression and osteogenic,
chondrogenic and adipogenic differentiation analysis was conducted
on the cells at passage 6. MSC surface marker expression analysis
demonstrated that >90% of cells expressed typical MSC surface
markers (CD29, CD44, CD73, CD90 and CD105), while the percentage of
cells expressing hematopoietic cell lineage markers (CD34, CD45 and
HLA-DR) was ≤0.6% (Fig. 2B).
After 2 weeks osteogenic, chondrogenic and
adipogenic differentiation, alizarin red staining positive calcium
nodules and alcian blue staining positive sulphated proteoglycans
were detected in osteogenic and chondrogenic differentiating cells
respectively. In adipogenic differentiated cells, intercellular
lipid vacuoles were identified by Oil-Red-O staining. No typical
positive stained cell was found among the three corresponding
un-induced control cells (Fig.
2C).
IP injected hiPSC-MSCs remained on the
colicomentum and produced angiogenic and immune regulatory
factors
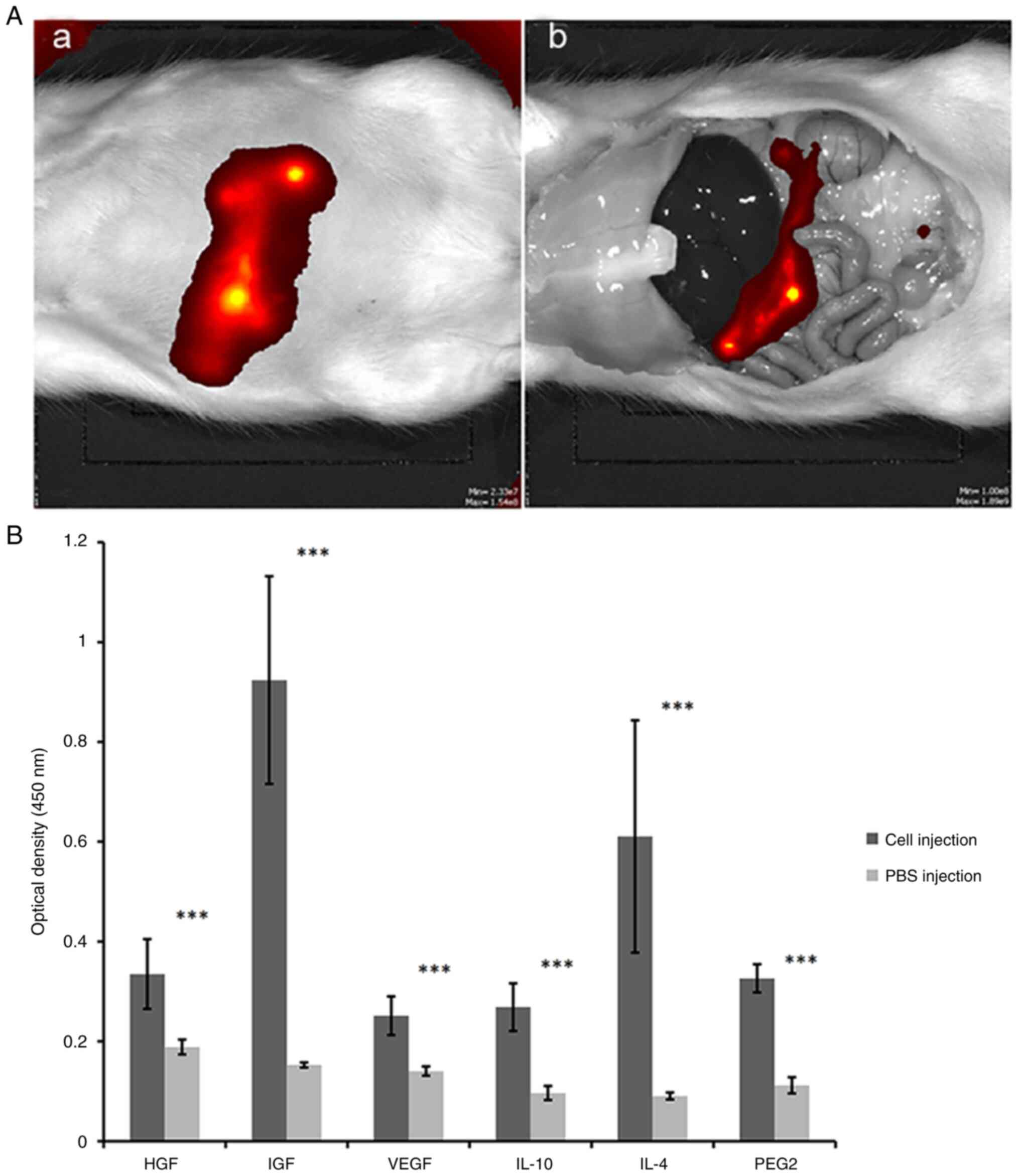
The distribution of hiPSC-MSCs was monitored after
IP injection (Fig. 3A). At 1 h
after the labelled hiPSC-MSCs injection, the fluorescent signal was
detected around the injection site and 3 days after cell injection,
the fluorescent signal moved from the injection site to
colicomentum. Fluorescent signals could be detected for up to 3
weeks on the colicomentum and no fluorescent signals were detected
in the liver, spleen or kidneys. There was no obvious evidence of
an associated inflammatory response or tumour formation in the
peritoneal cavity.
Angiogenic and immune regulatory factors were
measured 1 week after hiPSC-MSC injections using the peritoneal
lavage of hiPSC-MSC injected rats and control rats (Fig. 3B). The levels of IGF, VEGF, HGF,
PGE2, IL-4 and IL-10 in the lavage of hiPSC-MSC injected rats were
significantly increased compared with that in the control rats.
hiPSC-MSC injections improved cardiac
function and survival of AMI model rats
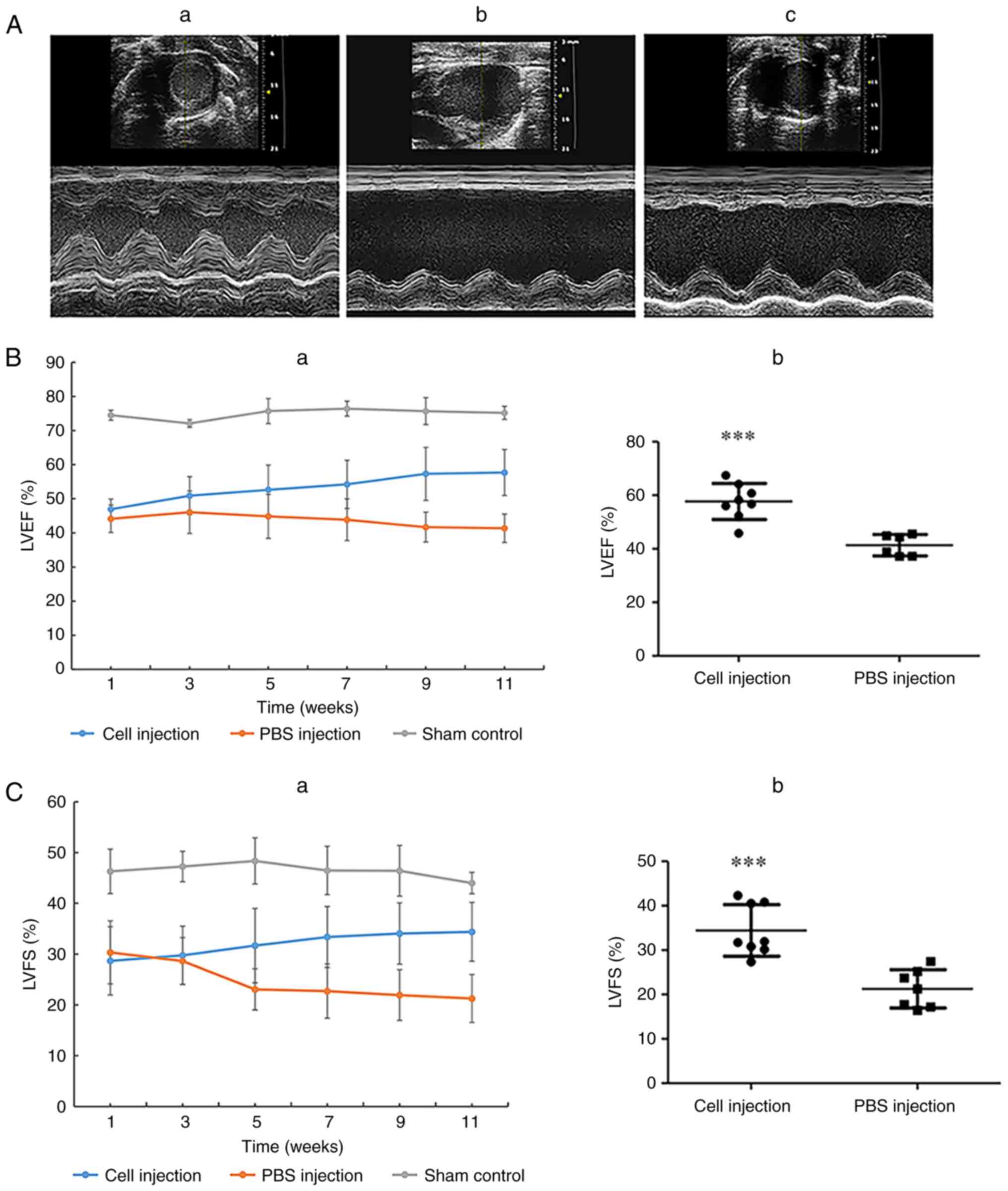
Cardiac function was monitored by measuring LVEF and
LVFS at 2-week intervals from the initial hiPSC-MSC injection to
the end of the experiment. Representative echocardiogram images
taken 11 weeks after AMI was induced demonstrated that the
parameters reflecting the structure and function of heart such as
anterior and posterior wall thickness, ventricular dimension
enlargement and ventricular contraction of the sham group remained
normal (Fig. 4A-a). In the PBS
injection group, anterior and posterior wall thickness, ventricular
dimension enlargement and ventricular contraction decrease were
demonstrated (Fig. 4A-b), whereas
in the hiPSC-MSC injection group, anterior and posterior wall
thickness, ventricular dimension enlargement and ventricular
contraction decrease were improved compared with that of the PBS
injection group (Fig. 4A-c).
Following AMI induction, the LVEF of the sham group
was stable from 74.50±1.46% at week 1 to 75.19±1.93% at week 11
(Fig. 4B-a). In the PBS injection
control group, LVEF declined from 44.14±4.02% at week 1 to
41.35±4.16% at week 11. In the cell injection group, LVEF increased
from 46.94±2.89% at week 1 to 57.70±6.76% at week 11. A significant
increase in LVEF was demonstrated when comparing the cell injection
group with the PBS control group at the 11-week time point
(Fig. 4B-b).
The LVFS of the sham group was stable from
46.30±4.38% at week 1 to 44±2.10% at week 11 (Fig. 4C-a). In the PBS injection control
group, LVFS declined from 30.35±6.23% in week 1 to 21.27±4.73% in
week 11. In the cell injection group, LVFS increased from
28.68±6.73% in week 1 to 34.45±5.79% in week 11. A significant
increase was demonstrated in the LVFS of the cell injection group
compared with the PBS control group at week 11 (Fig. 4C-b).
Over the 11-week experiment, four rats died in the
PBS injection control group (death rate, 40%) and two rats died in
the cell injection group (death rate, 20%).
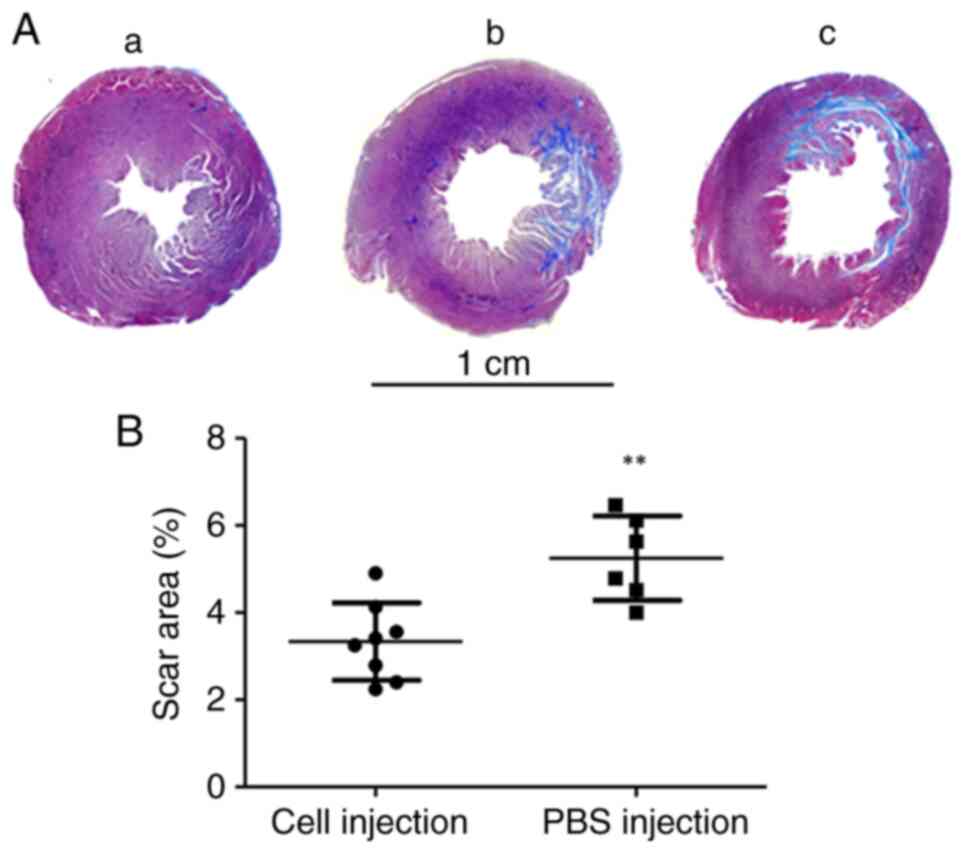
hiPSC-MSC injection reduced scar
size
Histological analysis was conducted 11 weeks after
AMI to evaluate the percentage of scar tissue formation in the
infarct area (Fig. 5A). The scar
area in the PBS control group was 5.25±0.96%, which was
significantly higher compared with the scar area in the cell
injection group (3.33±0.88%) (Fig.
5B).
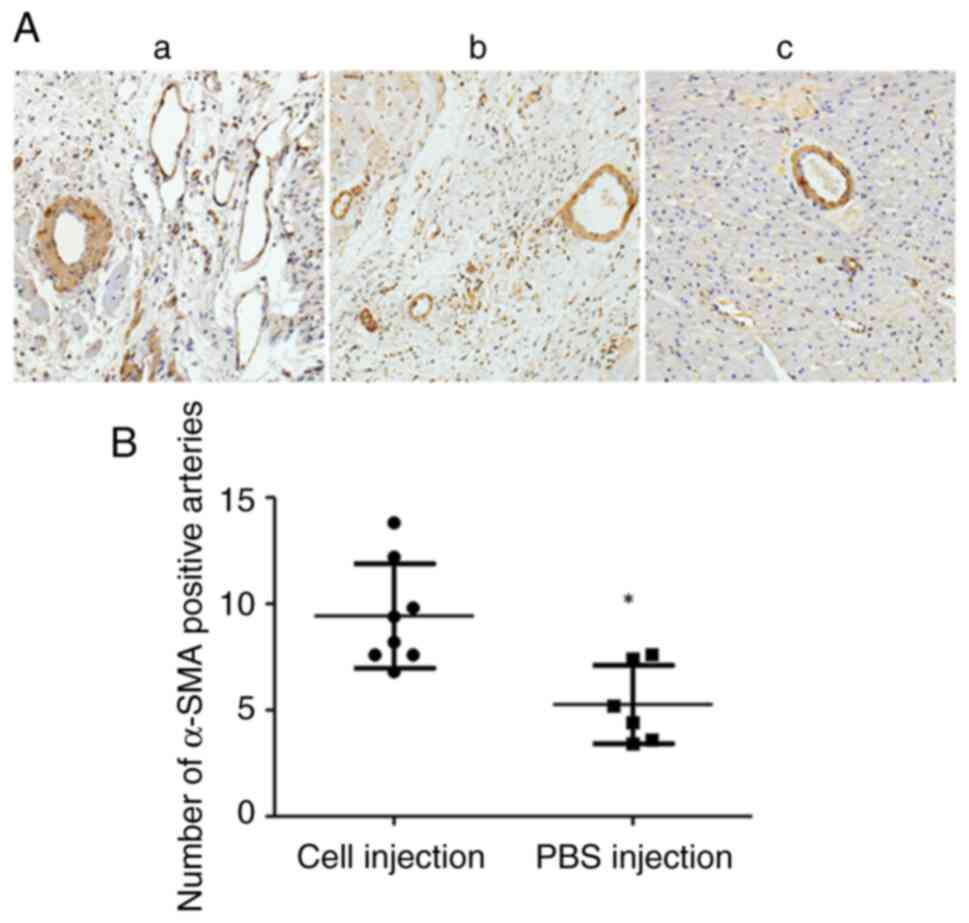
hiPSC-MSC injection enhanced
vascularisation in the infarcted area
Immunohistology staining demonstrated that hiPSC-MSC
injections significantly increased the number of α-SMA positive
arteries in the infarcted area of the hiPSC-MSC injection group
(9.42±2.45) compared with that of the PBS injection group
(5.26±1.84) (Fig. 6A and B).
hiPSC-MSC injection inhibited cell
apoptosis in the infarcted area
The results of the TUNEL assay demonstrated that the
apoptotic cell number in the infarcted area was significantly
reduced in the cell injection group (55.5±11.98 cells/hpf) compared
with the PBS injection control group (87.26±19.68 cells/hpf)
(Fig. 7A and B).
Discussion
Due to their ability to release soluble immune
modulators and angiogenic factors, MSCs have been investigated as a
candidate for the treatment of ischemic myocardial disease for
>10 years. However, there are still a number of obstacles
hampering the use of MSCs in clinic treatment, such as the lack of
methods for the rapid, high quality production of MSCs and issues
concerning the safe and effective administration of MSCs to
patients without the therapeutic efficacy being affected. hiPSCs
have been regarded as a potential stem cell source for cell
therapies. hiPSCs can be obtained without the consideration of
ethical constraints as they are not primary cells isolated from
human tissue and can be rapidly propagated in vitro, thus
providing a unified starting point for downstream cell induction at
a large scale (32). In the
present study, MSC-like cells were rapidly obtained from hiPSCs
using CHIR99021 and TGF-β combined induction and IP injections of
these cells improved the cardiac function of AMI model rats.
EMT is a three state transition process in which
there is an intermediate partial EMT state where cells retain the
characteristics of both epithelial and mesenchymal cells. Cells in
this partial EMT state are more pluripotent than those which have
progressed through the whole EMT process (14). A similar phenomenon has previously
been reported during ETM induction of breast cancer cells (36). Consistent with the aforementioned
studies, the present study demonstrated that during CHIR99021,
TGF-β or CHIR99021 + TGF-β induction, the expression levels of
EMT-related genes N-cadherin and Vimentin were significantly
elevated, but the expression levels of the MET-related gene
E-cadherin and pluripotency-related transcription factors OCT4,
SOX2 and NANOG did not decrease significantly compared
with the spontaneously differentiating hiPSCs. These results
suggested that CHIR99021 or TGF-β treatment initiated the process
of EMT through upregulating the expression of EMT-promoting genes
and these cells may be in the state of partial EMT.
TGF-β initiates EMT through SMAD-dependent or
-independent signalling pathways. In the SMAD-dependent pathway,
the binding of TGF-β to its receptor induces phosphorylation of
SMAD-2 and SMAD-3, then the phosphorylated SMADs form complexes
with SMAD-4 to transactivate the expression of SNAIL and zinc
finger protein SNAI2, which represses transcription of the MET
related gene E-cadherin (37). In
addition, SMAD-4 can specifically bind to the promoter region of
N-cadherin and Vimentin and this binding is required for the
reduction of gene expression (37,38).
In the SMAD-independent pathways, the TGF-β type I receptor can
activate ERKs, Akt, p38 and small G proteins (39).
GSK-3 serves a key role in the regulation of EMT and
inhibition of GSK-3 by CHIR99021 activation of Wnt signalling
initiated human ESC differentiation (40). GSK-3 has been previously reported
to participate in SMAD-3 and SMAD-4 phosphorylation and degradation
(41). STAT3 promotes SNAIL
degradation through activation of GSK-3 phosphorylation, which
suppresses EMT. GSK-3 inhibitors block STAT3 DNA binding activity
and upregulate SNAIL expression (20).
To further clarify the mechanism involved in the EMT
induction process, western blotting was performed to analyse the
protein expression levels of SNAIL, SMAD-2/3, ERK-1/2 and GSK-3 in
the differentiating cells. These results demonstrated that compared
with the untreated control cells, the protein expression levels of
SNAIL and ERK-1/2 were elevated in CHIR99021 or TGF-β treated
cells. The protein expression levels of SNAIL and ERK-1/2 were
significantly increased by the CHIR99021 + TGF-β combination
treatment when compared with control and single reagent treated
cells. These results could indicate that both SMAD-dependent and
-independent singling pathways were activated in the EMT induction
and the combination treatment was more effective at EMT induction
compared with the single reagents tested.
Although increased protein expression levels of
GSK-3 were detected in the combination treated cells, perhaps due
to the significant upregulation of SNAIL and ERK-1/2, EMT promotion
in the differentiating cells was not affected. Similar results have
previously been reported Li et al (41) who showed that a GSK-3β inhibitor
preserved the activity of SNAIL and prevented Vimentin degradation.
Vincent et al (20) previously reported that a GSK-3β
inhibitor alone did not affect the expression levels of E-cadherin
but increased the protein expression levels of SNAIL and
SMAD3/4.
During the process of TGF-β induced EMT, TGF-β
causes cell cycle arrest that leads to the inhibition of cell
proliferation (43). In the
present study, when compared with the single reagent treatments,
the CHIR99021 + TGF-β combined treatment was more efficient in
driving hiPSC cells to transform from compact large cell colonies
to single migrating spindle shaped cells. However, when the
concentration of CHIR99021 and TGF-β was increased to 10 µM and 20
µg/ml respectively, a decrease in the rate of cell growth and
apoptosis was demonstrated in the induced cells (data not shown).
As a result, a lower dosage of CHIR99021 and TGF-β was used for
cell induction and the duration of induction was limited to 6
days.
In our previous study, we reported that four rounds
of IV infusions of 1x106 MSCs to AMI rats could improve
cardiac function. The higher dosage of 2-3x106 cells
caused severe dyspnoea in a number of the model rats and resulted
in the death of 20-50% of the animals within a short time frame,
which was potentially related to pulmonary embolism (44). In the present study, in order to
avoid the potential risk of small blood vessel cell embolism, IP
injections were used and cell dosage was increased to
1x107 cells, 10x as high as the previous IV injections
in order to test the safety of high dosage IP injections. Based on
our previous study, four injections were adopted in the present
study and during the period from the first cell injection to the
end of the experiment, no evidence of severe inflammation or tumour
formation in the peritoneal cavity was observed.
Fluid exchange between the peritoneal cavity and the
circulatory system is dynamic and cytokines which have been
secreted into the peritoneal fluid by the intraperitoneal injected
MSCs can theoretically reach every organ of the body after entering
the blood circulation, including the heart. Yousefi et al
(26) compared the therapeutic
effects of IV and IP injected MSCs in treating experimental
autoimmune encephalitis and reported that IP injected MSCs were
more effective than IV injected MSCs. Roddy et al (29) reported that human MSCs were
effective in reducing corneal opacity and inflammation without
engraftment after either IP or IV administration following chemical
injury to the rat cornea. These aforementioned studies, taken
together with the present study, may indicate that IP injected MSCs
exert their effects from a distance.
In the present study, the level of cytokines in the
blood of rats after IP MSC injection was performed using ELISA
kits, but the level of cytokines in the blood was too low to obtain
reliable data (data not shown). The cytokines produced by the MSCs
residing in the peritoneum were released into peritoneal fluid,
exchanged into the blood circulation and were largely diluted by
the blood, thus, detection of these cytokines was difficult. As a
result, peritoneal fluid was collected to measure the level of the
cytokines. However, the present results showed that although the
serum cytokine level was low in the MSC treated rats, increased
artery number and reduced apoptosis extent of myocardium cells in
the infarcted area were significant comparing with the PBC
injection group. Bazhanov et al (24) previously reported that after MSC IP
injection, the level of cytokines in mouse peritoneal cavity lavage
was higher compared with than that in serum.
A previous study conducted by van Dijk A et
al (6) reported that during
the first week after AMI, the resulting large scale myocardial
necrosis and inflammatory cell infiltration could greatly reduce
the survival rate of the injected cells, therefore, the suggested
optimal time point for the administration of stem cell therapy was
1 week after the acute inflammation period. As the peritoneal
cavity is separated from the infarcted heart, it is unlikely that
the harsh inflammatory environment created by AMI would affect the
survival of the cells injected in the peritoneal cavity to a
serious extent. A previous study by Bazhanov et al (24) showed that the injected cells are
mainly retained on the colicomentum, a result also demonstrated in
the present study. Therefore, IP cell injection could be
administered immediately after the AMI to cope with the onset of
the tissue inflammation and cell apoptosis without inducing a
pulmonary embolism.
In conclusion, the present study demonstrated that
CHIR99021 and TGF-β combination treatment was a rapid and effective
method to obtain MSC-like cells from hiPSCs. Additionally, multiple
high dose IP injections of hiPSC-derived MSCs were a safe and
effective treatment to restore the reduced cardiac function of an
AMI rat model.
Acknowledgements
The authors wish to thank Dr Tiancheng Zhou at The
CAS Key Laboratory of Regenerative Biology, South China Institute
for Stem Cell Biology and Regenerative Medicine, Guangzhou
Institutes of Biomedicine and Health, Chinese Academy of Sciences,
Guangzhou 510530, P.R. China for kindly providing the PBMC-5 human
iPSC cell line.
Funding
Funding: This work was supported by The National Natural Science
Foundation of China (grant no. 82001654), The Shenzhen Science and
Technology Innovation Committee Basic Science Research Grant (grant
no. JCYJ20190809094819102), The Guangzhou Sijiahui Tumour
Prevention Foundation (grant no. GASTO-22-01-002), The Shenzhen
Biomedical Industry Major Public Service Platform and Core
Technology Research Special Support Plan (grant no.
XMHT20220104048) and The Shenzhen Key Medical Discipline
Construction Fund (grant no. SZXK051).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YuZ and YaZ confirm the authenticity of all the raw
data. YuZ prepared the acute myocardium infarction model and tissue
specimens and conducted cardiac function evaluation. YaZ performed
EMT-induction, flow cytometry analysis, western blotting, RT-qPCR
and immunohistochemistry. AH monitored cell distribution and
cytokine secretion. FM aided with acute myocardium infarction model
preparation and cell injections. PC performed hiPSC culture. TL and
GC designed the project, performed statistical analysis and drafted
and revised the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Approval for the animal experiments was granted by
the Animal Ethics Committee of the Peking University & Hong
Kong Science and Technology University Medical Centre (approval no.
2020-027).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gebler A, Zabel O and Seliger B: The
immunomodulatory capacity of mesenchymal stem cells. Trends Mol
Med. 18:128–134. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kinnaird T, Stabile E, Burnett MS and
Epstein SE: Bone-marrow-derived cells for enhancing collateral
development: Mechanisms, animal data, and initial clinical
experiences. Circ Res. 95:354–363. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kinnaird T, Stabile E, Burnett MS, Lee CW,
Barr S, Fuchs S and Epstein SE: Marrow-derived stromal cells
express genes encoding a broad spectrum of arteriogenic cytokines
and promote in vitro and in vivo arteriogenesis through paracrine
mechanisms. Circ Res. 94:678–685. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
van den Akker F, de Jager SC and Sluijter
JP: Mesenchymal stem cell therapy for cardiac inflammation:
Immunomodulatory properties and the influence of toll-like
receptors. Mediators Inflamm. 2013(181020)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kyurkchiev D, Bochev I, Ivanova-Todorova
E, Mourdjeva M, Oreshkova T, Belemezova K and Kyurkchve S:
Secretion of immunoregulatory cytokines by mesenchymal stem cells.
World J Stem Cells. 6:552–570. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
van Dijk A, Naaijkens BA, Jurgens WJ,
Nalliah K, Sairras S, van der Pijl RJ, Vo K, Vonk AB, van Rossum
AC, Paulus WJ, et al: Reduction of infarct size by intravenous
injection of uncultured adipose derived stromal cells in a rat
model is dependent on the time point of application. Stem Cell Res.
7:219–229. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vela DC, Silva GV, Assad JA, Sousa AL,
Coulter S, Fernandes MR, Perin EC, Willerson JT and Buja LM:
Histopathological study of healing after allogenic mesenchymal stem
cell delivery in myocardial infarction in dogs. J Histochem
Cytochem. 57:167–176. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Perin EC, Tian M, Marini FC III, Silva GV,
Zhang Y, Baimbridge F, Quan X, Fernandes MR, Gahremanpour A, Young
D, et al: Imaging long-term fate of intramyocardially implanted
mesenchymal stem cells in a porcine myocardial infarction model.
PLoS One. 6(e22949)2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Williams AR and Hare JM: Mesenchymal stem
cells: Biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kern S, Eichler H, Stoeve J, Klüter H and
Bieback K: Comparative analysis of mesenchymal stem cells from bone
marrow, umbilical cord blood, or adipose tissue. Stem Cells.
24:1294–1301. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou S, Greenberger JS, Epperly MW, Goff
JP, Adler C, Leboff MS and Glowacki J: Age-related intrinsic
changes in human bonemarrow-derived mesenchymal stem cells and
their differentiation to osteoblasts. Aging Cell. 7:335–343.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang L, Wei Y, Chi Y, Liu D, Yang S, Han
Z and Li Z: Two-step generation of mesenchymal stem/stromal cells
from human pluripotent stem cells with reinforced efficacy upon
osteoarthritis rabbits by HA hydrogel. Cell Biosci.
11(6)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Katsuno Y and Derynck R: Epithelial
plasticity, epithelial-mesenchymal transition, and the TGF-β
family. Dev Cell. 56:726–746. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tian XJ, Zhang H and Xing J: Coupled
reversible and irreversible bistable switches underlying
TGFβ-induced epithelial to mesenchymal transition. Biophys J.
105:1079–1089. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bhatia S, Monkman J, Blick T, Pinto C,
Waltham M, Nagaraj SH and Thompson EW: Interrogation of phenotypic
plasticity between epithelial and mesenchymal states in breast
cancer. J Clin Med. 8(893)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tripathi S, Chakraborty P, Herbert L and
Jolly MK: A mechanism for epithelial-mesenchymal heterogeneity in a
population of cancer cells. PLoS Comput Biol.
16(e1007619)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fan X, Zhao Z, Wang D and Xiao J: Glycogen
synthase kinase-3 as a key regulator of cognitive function. Acta
Biochim Biophys Sin (Shanghai). 52:219–230. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Vincent T, Neve EP, Johnson JR, Kukalev A,
Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL,
et al: A SNAIL1-SMAD3/4 transcriptional repressor complex promotes
TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol.
11:943–950. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nguyen TMX, Vegrichtova M, Tlapakova T,
Krulova M and Krylov V: Epithelial-mesenchymal transition promotes
the differentiation potential of xenopus tropicalis immature
sertoli cells. Stem Cells Int. 2019(8387478)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zanetti A, Grata M, Etling EB, Panday R,
Villanueva FS and Toma C: Suspension-expansion of bone marrow
results in small mesenchymal stem cells exhibiting increased
transpulmonary passage following intravenous administration. Tissue
Eng Part C Methods. 21:683–692. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Galipeau J and Sensébé L: Mesenchymal
stromal cells: Clinical challenges and therapeutic opportunities.
Cell Stem Cell. 22:824–833. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bazhanov N, Ylostalo JH, Bartosh TJ,
Tiblow A, Mohammadipoor A, Foskett A and Prockop DJ:
Intraperitoneally infused human mesenchymal stem cells form
aggregates with mouse immune cells and attach to peritoneal organs.
Stem Cell Res Ther. 7(27)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lee RH, Pulin AA, Seo MJ, Kota DJ,
Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P and Prockop
DJ: Intravenous hMSCs improve myocardial infarction in mice because
cells embolized in lung are activated to secrete the
anti-inflammatory protein TSG-6. Cell Stem Cell. 5:54–63.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yousefi F, Ebtekar M, Soleimani M, Soudi S
and Hashemi SM: Comparison of in vivo immunomodulatory effects of
intravenous and intraperitoneal administration of adipose-tissue
mesenchymal stem cells in experimental autoimmune encephalomyelitis
(EAE). Int Immunopharmacol. 17:608–616. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cheng K, Rai P, Plagov A, Lan X, Kumar D,
Salhan D, Rehman S, Malhotra A, Bhargava K, Palestro CJ, et al:
Transplantation of bone marrow-derived MSCs improves
cisplatinum-induced renal injury through paracrine mechanisms. Exp
Mol Pathol. 94:466–473. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Castelo-Branco MT, Soares ID, Lopes DV,
Buongusto F, Martinusso CA, do Rosario A Jr, Souza SA, Gutfilen B,
Fonseca LM, Elia C, et al: Intraperitoneal but not intravenous
cryopreserved mesenchymal stromal cells home to the inflamed colon
and ameliorate experimental colitis. PLoS One.
7(e33360)2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Roddy GW, Oh JY, Lee RH, Bartosh TJ,
Ylostalo J, Coble K, Rosa RH Jr and Prockop DJ: Action at a
distance: Systemically administered adult stem/progenitor cells
(MSCs) reduce inflammatory damage to the cornea without engraftment
and primarily by secretion of TNF-α stimulated gene/protein 6. Stem
Cells. 29:1572–1579. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Choi H, Lee RH, Bazhanov N, Oh JY and
Prockop DJ: Anti-inflammatory protein TSG-6 secreted by activated
MSCs attenuates zymosan-induced mouse peritonitis by decreasing
TLR2/NF-κB signaling in resident macrophages. Blood. 118:330–338.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang J, Tian XJ, Zhang H, Teng Y, Li R,
Bai F, Elankumaran S and Xing J: TGF-β-induced
epithelial-to-mesenchymal transition proceeds through stepwise
activation of multiple feedback loops. Sci Signal.
7(ra91)2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen Q, Yang W, Wang X, Li X, Qi S, Zhang
Y and Gao MQ: TGF-β1 induces EMT in bovine mammary epithelial cells
through the TGFβ1/smad signaling pathway. Cell Physiol Biochem.
43:82–93. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jin W, He Y, Li T, Long F, Qin X, Yuan Y,
Gao G, Shakhawat HM, Liu X, Jin G and Zhou Z: Zhou. Rapid and
robust derivation of mesenchymal stem cells from human pluripotent
stem cells via temporal induction of neuralized ectoderm. Cell
Biosci. 12(31)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG: NC3Rs Reporting Guidelines Working Group. Animal
research: Reporting in vivo experiments: The ARRIVE guidelines. Br
J Pharmacol. 160:1577–1579. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang S, Tang Z, Qing B, Tang R, Duan Q,
Ding S and Deng D: Valproic acid promotes the
epithelial-to-mesenchymal transition of breast cancer cells through
stabilization of Snail and transcriptional upregulation of Zeb1.
Eur J Pharmacol. 865(172745)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang H, Wang L, Zhao J, Chen Y, Lei Z, Liu
X, Xia W, Guo L and Zhang HT: TGF-β-activated SMAD3/4 complex
transcriptionally upregulates N-cadherin expression in non-small
cell lung cancer. Lung Cancer. 87:249–257. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rogel MR, Soni PN, Troken JR, Sitikov A,
Trejo HE and Ridge KM: Vimentin is sufficient and required for
wound repair and remodeling in alveolar epithelial cells. FASEB J.
25:3873–3883. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dubon MJ, Yu JY, Choi S and Park KS:
Transforming growth factor β induces bone marrow mesenchymal stem
cell migration via noncanonical signals and N-cadherin. J Cell
Physiol. 233:201–213. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Setiawan M, Tan XW, Goh TW, Hin-Fai Yam G
and Mehta JS: Inhibiting glycogen synthase kinase-3 and
transforming growth factor-β signaling to promote epithelial
transition of human adipose mesenchymal stem cells. Biochem Biophys
Res Commun. 490:1381–1388. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Patel P and Woodgett JR: Glycogen synthase
kinase 3: A kinase for all pathways? Curr Top Dev Biol.
123:277–302. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li CH, Liu CW, Tsai CH, Peng YJ, Yang YH,
Liao PL, Lee CC, Cheng YW and Kang JJ: Cytoplasmic aryl hydrocarbon
receptor regulates glycogen synthase kinase 3 beta, accelerates
vimentin degradation, and suppresses epithelial–mesenchymal
transition in non-small cell lung cancer cells. Arch Toxicol.
91:2165–2178. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Song J and Shi W: The concomitant
apoptosis and EMT underlie the fundamental functions of TGF-β. Acta
Biochim Biophys Sin (Shanghai). 50:91–97. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Guo S, Zhang Y, Zhang Y, Meng F, Li M, Yu
Z, Chen Y and Cui G: Multiple intravenous injections of valproic
acid-induced mesenchymal stem cell from human-induced pluripotent
stem cells improved cardiac function in an acute myocardial
infarction rat model. Biomed Res Int. 2020(2863501)2020.PubMed/NCBI View Article : Google Scholar
|