Introduction
Various forms of ectopic splenic tissue exist,
encompassing splenosis, accessory spleen, wandering spleen and
polysplenism (1-3).
Splenosis is the primary focus of this paper. Splenosis denotes the
detachment and embedding of a segment of the spleen in various
anatomical sites subsequent to traumatic rupture or therapeutic
resection (2). The disseminated
spleen tissue can be propagated through various means, such as
direct dissemination or via splenic venous blood (3-5).
This process leads to the gradual establishment of blood
circulation and subsequent regeneration of splenic tissue.
Statistically, the likelihood of splenic implantation resulting
from splenic trauma or rupture post-splenectomy is ~67%, with
16-17% of patients undergoing elective splenectomy for
hematological disease (6). This
implantation, when observed elsewhere months or years later, is
often misinterpreted as a tumor (7). The present study reported a rare case
of splenosis located on the gastric wall in a female patient.
Initially, gastrointestinal stromal tumor (GIST) was considered
based on imaging findings. The patient exhibited clinical symptoms
and presented with a sizable tumor, prompting the decision to
perform a comprehensive endoscopic resection for tumor removal.
Unexpectedly, the histopathological diagnosis post-resection
revealed splenosis. In terms of splenosis treatment, surgical
resection remains the primary therapeutic option for symptomatic or
large lesions. However, an asymptomatic splenosis should not
receive any treatment, including surgical intervention (8-10).
Asymptomatic splenosis misdiagnosed as tumors can lead to
unnecessary surgery, and real diseases may be overlooked due to
misdiagnosis (11). Recent studies
propose that spleen imaging using Tc-99m sulphur colloid scanning
and Tc-99m tagged heat-damaged autologous red blood cells
(99mTc-DRBC) serves as the ‘gold standard’ for
diagnosing ectopic splenic tissue (12,13).
The objective of the present study was to augment the diagnostic
accuracy of splenosis within the context of the novel diagnostic
modality. Clinicians should aim to individualize treatment
approaches for patients afflicted with splenosis, thereby averting
unnecessary clinical interventions.
Case report
A 16-year-old female patient presented at the Zunyi
Medical University Hospital (Zunyi, China) with persistent
abdominal pain spanning six months in November 2019. The patient's
medical history included thalassemia, and the patient had undergone
laparoscopic splenectomy two years prior at Kunming Children's
Hospital (Kunming, China) due to hypersplenism. The postoperative
recovery was uneventful, evidenced by multiple scattered 1 cm-sized
old surgical scars on the abdominal wall during physical
examination. Upon admission, biochemical analysis revealed abnormal
values in the following parameters: Decreased hemoglobin (74 g/dl;
normal: 114-154 g/l), elevated white blood cells
(12.51x109/l; normal: 3.5-9.5x109/l),
increased total bilirubin (34.2 µmol/l; normal: 5.1-19.8 µmol/l),
slightly elevated direct bilirubin (8.3 µmol/l; normal: 0-6.84
µmol/l) and slightly increased indirect bilirubin (25.9 µmol/l;
normal: 3.0-17.0 µmol/l). Other laboratory indicators, including
renal routine and serum tumor markers, fell within the normal
reference range. Endoscopic ultrasound (EUS) examination for upper
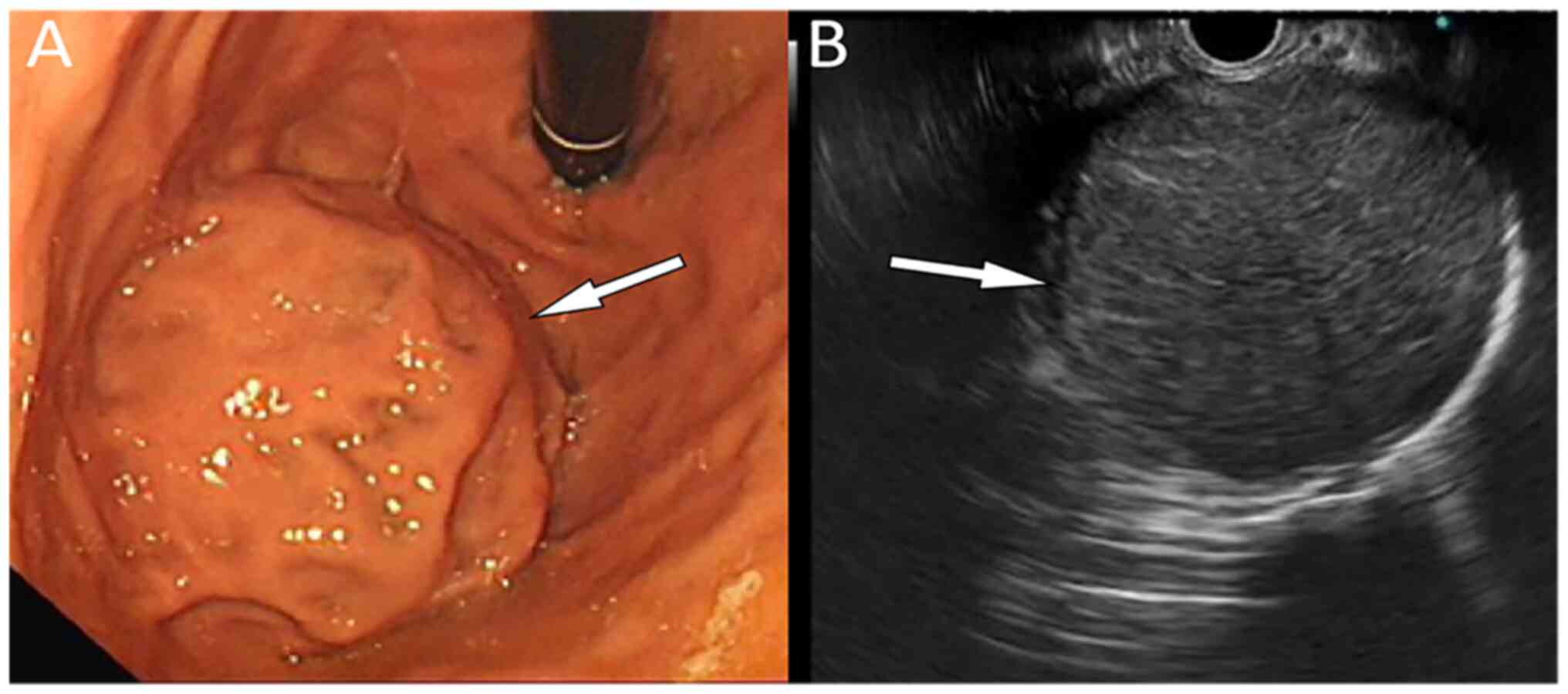
abdominal discomfort revealed a well-defined microhypoechoic mass
at the bulge of the gastric fundus, measuring ~5.9x5.1 cm and
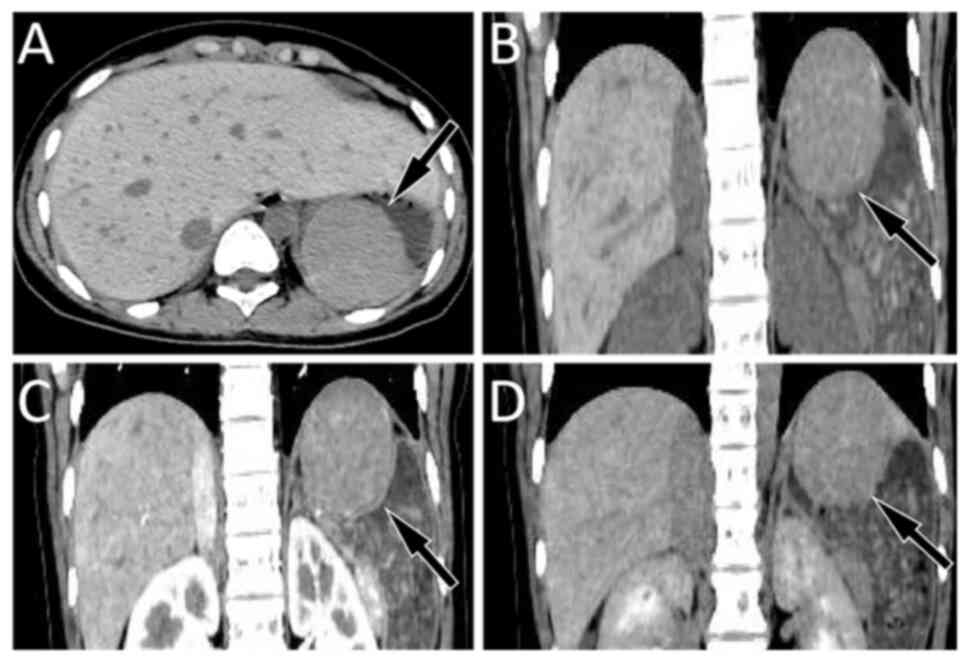
originating from the fourth layer with uniform echo (Fig. 1). As indicated in Fig. 2, abdominal computed tomography (CT)
confirmed a round-like mass shadow with a smooth edge in the
stomach cavity, measuring ~5.7x5.1 cm, and located close to the
back wall of the fundus and stomach, displaying an unclear boundary
with the gastric wall. The contrast-enhanced scan depicted slightly
uniform enhancement in the arterial phase, further enhancement in
the venous phase and attenuation in the delayed phase. Initial
considerations based on these imaging findings leaned towards a
GIST. Preoperative magnetic resonance imaging (MRI) and positron
emission tomography-CT scans were recommended to the patient and
the patient's family in order to further determine the best
treatment plan; however, they refused the examination for financial
reasons. Given the evident clinical symptoms and the substantial
tumor volume, a multidisciplinary oncology consultation, involving
gastroenterology, radiology, pediatrics and anesthesiology, led to
the decision for laparoscopic resection of the gastric fundus
tumor. During surgery, the tumor, observed under gastroscopy and
stratified under EUS, was successfully removed. Titanium clips were
employed to close the incision in the gastric wall, resulting in
minimal blood oozing and a favorable postoperative condition. For
hematoxylin-eosin staining, the specimen was fixed with 10% neutral
formalin, dehydrated at room temperature for ~24 h and paraffin
embedded. Next, 3- to 4-µm thick sections were stained with
hematoxylin-eosin (Fuzhou Maixin Biotech. Co., Ltd.), and viewed at
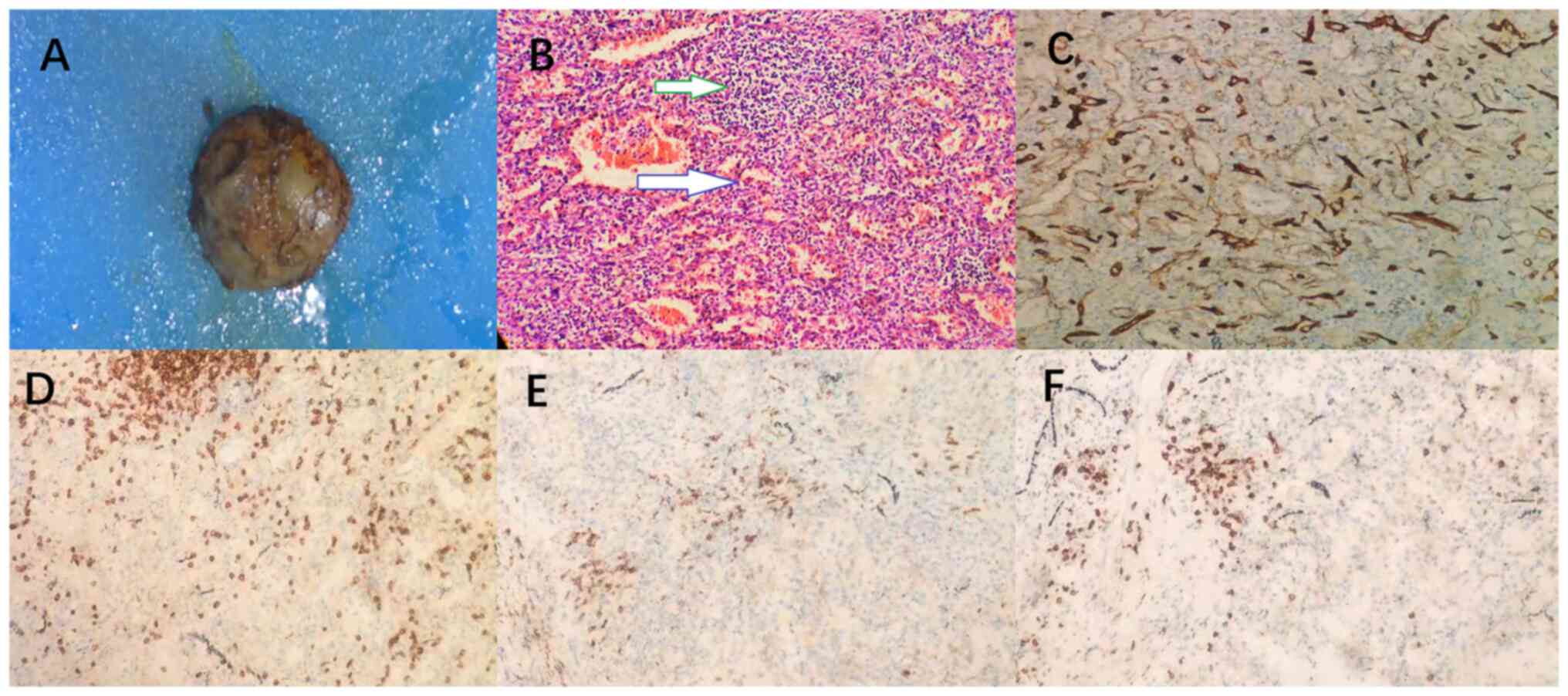
x100 magnification under an optical microscope. The staining
revealed splenic structures, including red and white pulp, focal
fibrous hyperplasia with hyaluronic hyperplasia, abundant blood
vessels and a multinuclear giant cell reaction. Immunohistochemical
tests showed that the white pulp lymphocytes were mostly CD20 and
CD38 positive B cells with fewer numbers of CD3 and CD8 positive T
cells. CD34 positive was closely related to small blood vessels.
Cytokine receptor CD117 was negative for specific markers of GIST
by immunohistochemical examination (Fig. 3). The pathological and
immunohistochemical findings sustained the diagnosis of gastric
splenosis. The patient was discharged on the sixth day
post-operation and underwent an uneventful recovery with a
two-month telephone follow-up, reporting no discomfort during this
period.
Literature review
Case reports and case series on splenosis retrieved
from the PubMed (http://pubmed.ncbi.nlm.nih.zd.hggfdd.top/), Embase
(http://www.embase.zd.hggfdd.top/) and
Web of Science (http://webofscience.zd.hggfdd.top/WOS) databases as of
December 1, 2022, adhered to language restrictions with a
limitation to English. The key words encompassed ‘splenosis’,
‘heterotopic spleen’, ‘stomach’, ‘abdomen’, ‘epigastrium’,
‘gastrectomy’, ‘gastroscopy’, ‘cardia’, ‘gastric’, ‘esophagogastric
junction’, ‘pyloric antrum’ and ‘pylorus’. These terms were
employed individually with the Boolean operator ‘AND’ or ‘OR’. A
systematic search yielded an initial 251 articles. Following the
exclusion of duplicates and irrelevant articles, 21 articles were
ultimately considered for inclusion in this study. A detailed flow
chart of the literature screening process is presented in Fig. S1. The first author, publication
year and country of each case, along with the patient's age,
gender, main clinical symptoms, medical history, lesion size and
findings from gastroscopic, ultrasonic and CT imaging, were
meticulously recorded, as depicted in Table I (4,6,14-31).
Through a systematic search, a total of 22 cases of gastric
splenosis and intragastric splenosis were definitively confirmed
from the published literature. Including the patient of the present
study, the literature review comprised 23 patients for statistical
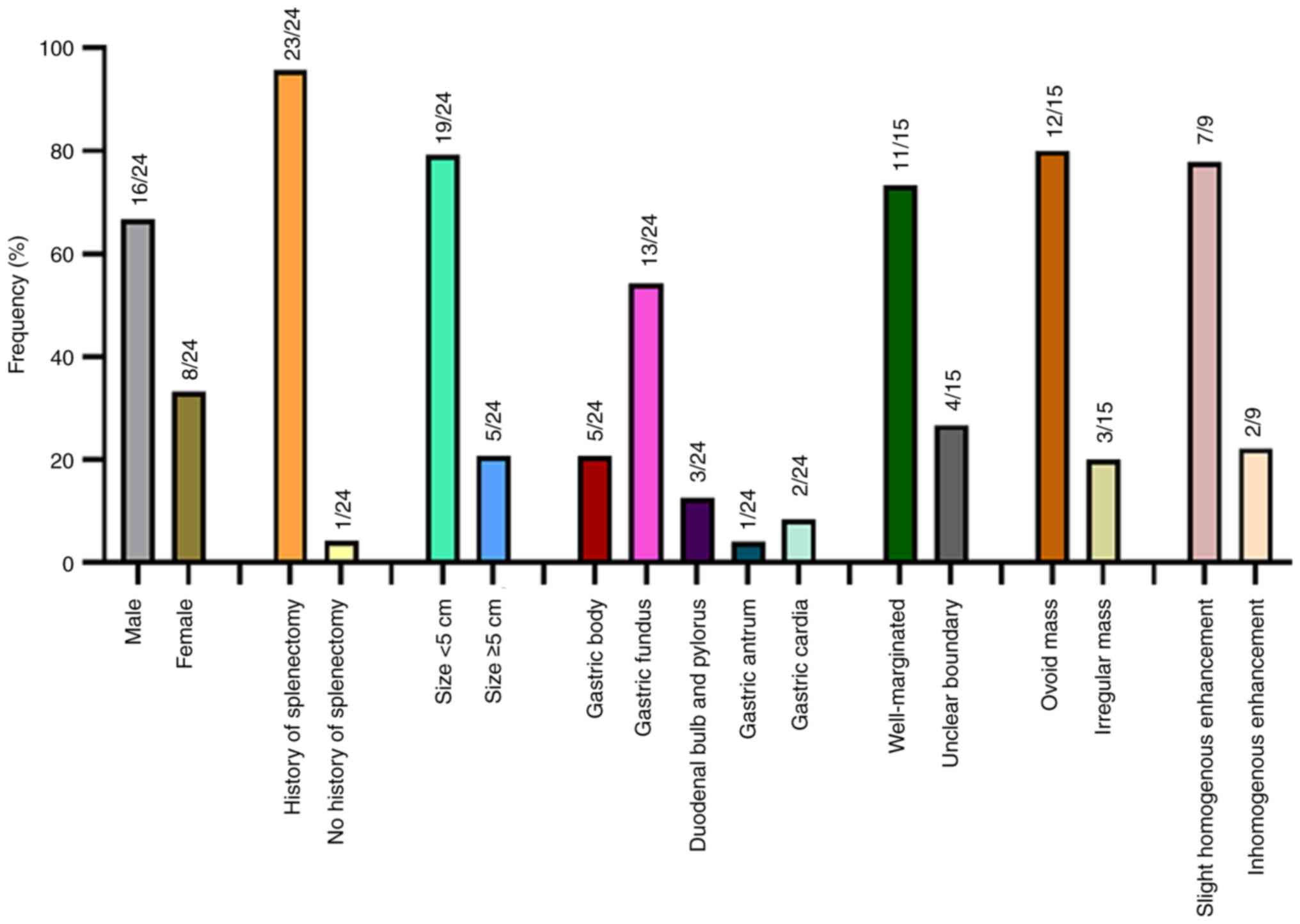
analysis, including 16 males and 8 females, with the median age of
46.8 years. The majority of cases were reported in China (50%),
followed by the US (12.5%) and Italy (12.5%). The detailed
characteristics of gender, medical history, lesion size, location
and CT findings of the 23 patients are illustrated in Fig. 4.
 | Table IClinical and imaging features of the
cases of splenosis in the stomach and duodenum based on the
literature review. |
Table I
Clinical and imaging features of the
cases of splenosis in the stomach and duodenum based on the
literature review.
| | Imaging findings | |
|---|
| Author/s, year | Country | Sex | Age, years | Splenectomy | Presentation | Location | Size (cm) | Endoscopy | Ultrasound | CT | Follow-up | (Refs.) |
|---|
| Fujita et
al, 2020 | Japan | M | 47 | Y (trauma) | Asymptomatic | Gastric body | 2.5 | - | Hypoechoic | Well-marginate
homogeneously enhanced | - | (16) |
| Okano et al,
2021 | Japan | M | 68 | N | Asymptomatic | Gastric fundus | 1.4 | Not apparent | Hypoechoic | Well-marginated
ovoid mass | 3 years | (4) |
| Jing et al,
2022 | China | M | 26 | Y (trauma) | Fatigue | Gastric fundus | 2.6x4.7 | Giant protuberant
lesion | - | Well-marginated
ovoid mass | - | (17) |
| Kanagalingam et
al, 2021 | USA | M | 68 | Y (trauma) | Asymptomatic | Gastric fundus | 0.4 | Smooth submucosal
mass | Hypoechoic | - | 2 years | (18) |
| Li et al,
2015 | China | F | 40 | Y (trauma) | Abdominal pain and
pharyngalgia | Gastric fundus | 2.0x1.5 | Smooth mass with
varicose veins around it | Hypoechoic | Well-marginated
ovoid mass | - | (15) |
| Li et al,
2015 | China | M | 32 | Y (trauma) | Epigastric
discomfort | Gastric fundus | 2.0x2.0 | Smooth and round
mass | Hypoechoi | - | - | (15) |
| Rompou et
al, 2021 | Greece | M | 29 | Y (trauma) | Multiple vomiting
episodes | Pylorus | 3.3x3.2 | - | Hypoechoic | - | - | (19) |
| Guan et al,
2018 | China | F | 74 | Y (cirrhosis) | Asymptomatic | Gastric body | 3.95x2.8 | Smooth and rounded
mass | Hypoechoic | Well-marginated and
low-density ovoid mass | - | (20) |
| Yang et al,
2022 | China | M | 53 | Y (trauma) | Fatigue and
dizziness | Gastric fundus | 4x 5 | Multi-strip
protuberant lesion appearing as varices | Hypoechoic | Thickened gastric
wall of the fundus with unclear boundary | - | (14) |
| Yang et al,
2013 | China | M | 42 | Y (trauma) | Epigastric pain and
melena | Gastric fundus | 5x5 | Smooth submucosal
mass | Hypoechoic | Well-marginated
ovoid mass | - | (6) |
| Isopi et al,
2020 | Italy | F | 47 | Y (trauma) | Epigastric pain and
dyspepsia | Gastric fundus | 3 in diameter | Smooth submucosal
mass | Hypoechoic | Well-marginated and
enhanced rounded mass | - | (21) |
| Zheng et al,
2021 | China | M | 44 | Y (trauma) | Epigastric
pain | Gastric body | 5.6x4.0 | Smooth submucosal
mass | - | Irregular soft
tissue density and slight inhomogeneous enhancement | - | (22) |
| Wang et al,
2016 | China | M | 40 | Y (trauma) | Epigastric
pain | Gastric fundus | 1.8x1.5 | Solid mass | Hypoechoic | Well-marginated and
obviously enhanced ovoid mass | - | (23) |
| Deng et al,
2014 | China | F | 55 | Y (trauma) | Asymptomatic | Duodenal bulb | 6.1x3.6 | - | - | Irregular soft
tissue density and slight inhomogeneous enhancement | 6 months | (24) |
| Fujita | Japan | M | 47 | Y (trauma) | Asymptomatic | Gastric body | 2.5 | - | Hypoechoic | Well-marginate | - | (16) |
| Barbuscio et
al, 2019 | Italy | F | 49 | Y (trauma) | Epigastric
discomfort | Gastric fundus | 2 in diameter | Submucosal
mass | Hypoechoic | - | - | (25) |
| Yang et al,
2015 | China | M | 53 | Y (trauma) | Epigastric
pain | Gastric body | 2.2x1.5 | Submucosal
mass | Hypoechoic | - | - | (26) |
| Falk et al,
2009 | Ireland | F | 64 | Y (pancreatic
cystadenoma) | Asymptomatic | Gastric cardia | 4.3 in
diameter | Smooth submucosal
mass | Hypoechoic | Sub-serosal ovoid
mass | - | (27) |
| Yang et al,
2013 | China | M | 42 | Y (trauma) | Epigastric
pain | Gastric fundus | - | Submucosal
mass | Hypoechoic | Well-marginated and
enhanced ovoid mass | - | (6) |
| Agha, 1984 | USA | M | 52 | Y (trauma) | Asymptomatic | Gastric cardia | 2x3 | Extramucosal
mass | - | No other
abnormalities | - | (28) |
| Carrara et
al, 2016 | Italy | F | 65 | Y (pancreatic
mucinous cystic neoplasm) | Asymptomatic | Gastric fundus | 1.8 in
diameter | Smooth submucosal
mass | Hypoechoic | - | - | (29) |
| Deutsch et
al, 1999 | USA | M | 67 | Y (trauma) | Asymptomatic | Gastric antral | 1 in diameter | Smooth submucosal
mass | Hypoechoic | - | 6 months | (30) |
| Arroja et
al, 2011 | Portugal | M | 68 | Y (trauma) | Asymptomatic | Gastric body | - | Smooth bulge | - | Well-marginated and
enhanced ovoid mass | - | (31) |
Most patients presented at the hospital with upper
abdominal discomfort and pain, while 33% of patients were
asymptomatic and their splenosis was discovered incidentally during
physical examinations. With the exception of one patient without a
history of splenectomy, the remaining patients had undergone
splenectomy due to spleen rupture resulting from trauma, blood
diseases or other conditions. The predominant site of gastrosplenic
implantation was the fundus of the stomach, followed by the body of
the stomach, antrum, gastroesophageal junction and pylorus. Tumor
foci were predominantly solid, round or oval, with a minority
exhibiting irregular or lobulated shapes, and diameters ranging
from 0.4 to 6 cm. During gastroscopy, the lesions typically
manifested as smooth submucosal protuberant lesions, with only one
case displaying multi-strip protuberant lesions resembling varicose
veins (14). EUS revealed that the
lesions generally exhibited low echo, high echo or medium-low echo,
maintaining a uniform echo pattern. Calcifications were observed in
certain cases, predominantly in round, oval or fusiform shapes,
with a small number of specific calcifications exhibiting lobulated
features. The lesions, originating from the intrinsic muscle layer,
displayed well-defined soft tissue density, often demonstrating
enhancement in the arterial phase on CT.
Discussion
Albrecht, a German scholar, first elucidated the
phenomenon of splenic implantation in 1896, while Von Kutner
provided the initial definition of splenosis at autopsy in
1910(1). Shaw and Shafi (2) subsequently reported 6 clinical cases
of splenosis following splenic surgery in 1937. Various forms of
ectopic splenic tissue exist, encompassing splenosis, accessory
spleen, wandering spleen and polysplenism (3). Splenosis, the primary focus of the
present study, denotes the process whereby a segment of the spleen
detaches and integrates into other anatomical sites due to
traumatic rupture or therapeutic resection of the spleen.
Gradually, it establishes a blood circulation to regenerate spleen
tissue, a phenomenon observable after splenic trauma or surgery. On
the other hand, an accessory spleen represents a congenital anomaly
arising on the left side of the dorsal mesentery of the stomach
during embryonic development. The splenic graft is characterized by
an irregular structure, poorly formed capsule and the absence of a
hilum. Lastly, wandering spleen is an uncommon condition where the
spleen deviates from its normal position and the causative factor
is associated with laxity or dysplasia of the splenic ligament
(4).
Patients with splenosis typically remain
asymptomatic, although those experiencing symptoms may present with
diverse clinical manifestations. The majority of patients
incidentally discover mild abdominal discomfort, noting abdominal
occupancy during medical evaluations. Symptomatic patients exhibit
varying symptomatology contingent on the location and size of the
implant, often prompting medical attention due to torsion,
spontaneous rupture, bleeding or cystic formation. Consequently,
the accurate incidence of the disease remains indeterminate. In
recent decades, the escalating incidence of car accidents or spleen
ruptures attributable to improper exercise has emerged as a
significant factor necessitating splenectomy. Furthermore,
advancements in medical examination technology have paralleled an
increase in both the prevalence and detection of this condition.
Statistically, the likelihood of splenosis resulting from splenic
trauma or rupture post-splenectomy is ~67%, with 16-17% of patients
undergoing elective splenectomy for hematological disease (6). The time interval between splenectomy
and the clinical identification of splenosis varies from 5 months
to 36 years, averaging 18.8 years (7). In specific cases, patients may
experience abdominal pain due to the compression of large gastric
splenosis on the sensory nerve of the stomach. In the case of the
present study, gastric splenosis was discovered >2 years after
splenectomy, aligning with descriptions found in the
literature.
Splenosis manifests as the autologous implantation
of splenic tissue following splenic trauma or resection procedures
(5). The disseminated spleen
tissue can be propagated through various means, such as direct
dissemination or via splenic venous blood. Direct dissemination
commonly results in implantation in the splenic hilum, pancreas,
omentum, pelvic organs and the serosal layer of the intestinal
wall. Transsplenic vein blood dissemination may lead to
implantation in the liver, pancreas, stomach, small intestine and,
in rare instances, breast and brain tissue (32-34).
The splenic pulp primarily disseminates in the abdominal cavity or
other organs through the circulation. The vascular network and
lymphoid tissue of the regenerated spleen tissue undergo
adjustments, with undifferentiated reticular cells and fibrous
tissue-forming scaffolds. Subsequent differentiation results in the
formation of endothelial sinuses, capillaries and lymphocytes,
ultimately constituting splenic tissue (35,36).
The blood supply is maintained by several small vessels in the
surrounding tissues rather than the splenic arteries (37). Debris can be implanted in the body
cavity or any other part, gradually establishing blood circulation
supply for the development and regeneration of spleen tissue. In
the 1980s, Chatterjee et al (38) transplanted spleen tissue slices of
rats and rabbits into subcutaneous sites and muscle, achieving a
success rate of >90% in the operation, indicating the strong
regenerative capacity of spleen tissue. Pathological features
typically include a complete fibrous envelope, absence of splenic
hilum, muscle and elastic fiber components, predominantly red pulp,
imperfect white pulp, abnormal vascular structure and the absence
of the ‘portal’ vascular structure of the splenic hilum, which
contains hematin (36,39). The patient reported in the present
study had a history of laparoscopic splenectomy. Given that
laparoscopic splenectomy at times involves the removal of a sample
after the spleen has ruptured in the abdominal cavity, this
procedure may result in implantation in the stomach through direct
or hematogenous dissemination. Pathological examination of the
resected lesion revealed visible red and white pulp, with the
structure of the white pulp being imperfect and the absence of a
splenic hilum, consistent with the diagnosis of splenosis.
Regarding imaging examinations, the density and
enhancement pattern of splenosis observed on CT and MRI were akin
to those of a normal spleen. On a contrast-enhanced CT scan, the
lesion exhibited uniform enhancement in the three stages: Prominent
enhancement in the arterial stage, sustained enhancement in the
portal vein stage and a slight decrease in the delayed stage.
Splenosis can manifest in various organs, such as the lung,
pancreas, liver, kidney and gastrointestinal tract, necessitating
differentiation from the primary tumor organ (40,41).
On contrast-enhanced CT scans, splenosis often lacks the speck
enhancement characteristic of normal splenic tissue in the arterial
stage. This enhancement pattern may be confused with the imaging
features of GIST, leading to potential misdiagnosis (15). Furthermore, careful attention must
be given to distinguishing splenosis from accessory spleen. The key
distinction lies in the fact that splenosis is supplied by the
implant organ without the splenic hilum, whereas accessory spleen
receives its blood supply from the splenic vessel. Occasionally,
splenosis is erroneously diagnosed as a malignant tumor (42). For instance, in a case reported by
Ksiadzyna (43) in 2011, a
54-year-old woman with a history of splenectomy exhibited multiple
abdominal nodules in areas such as the ileum, greater omentum and
uterus, raising suspicions of disseminated malignant tumors.
However, histological examination revealed a typical spleen
structure, leading to the diagnosis of abdominal splenosis. In the
case reported in the present study, the CT plain scan displayed a
uniformly, slightly enhanced round soft tissue density image in the
arterial phase, continued enhancement in the venous phase and
weakened enhancement in the delayed phase. Of note, the patient had
undergone splenectomy, and the contrast with the enhancement
pattern of a normal spleen was absent. The arterial phase imaging
features of gastric splenosis often overlap with those of GISTs,
posing challenges in their differentiation (6).
The presence of splenosis as a tumor may result in
unnecessary surgical intervention, potentially leading to the
oversight of the actual underlying condition due to misdiagnosis.
This may significantly heighten patient anxiety and psychological
stress (33), underscoring the
crucial importance of accurately diagnosing splenosis. The rapid
advancement in medical examination technology has contributed to an
increased occurrence and detection of this disease (44). Spleen imaging using Tc-99m sulphur
colloid scanning and Tc-99m-tagged heat-damaged autologous red
blood cells (99mTc-DRBC) serve as the ‘gold standard’
for diagnosing ectopic splenic tissue (45). The fundamental principles
underlying the noninvasive Tc-99m sulphur colloid scanning and
99mTc-DRBC in the diagnosis of splenosis are outlined as
follows: The spleen has a role in phagocytosing foreign bodies in
the blood and destroying senescent red blood cells. Following the
introduction of the former radioactive colloid into the body,
~5-10% of colloidal particles are engulfed by mononuclear
macrophages in the spleen, inducing spleen development. As for the
latter, upon passing through the spleen, ~90% of
radionuclide-labelled denatured red blood cells are intercepted and
phagocytosed by macrophages in the medullary cord. These cells
selectively remain in the spleen, thus revealing the location,
size, morphology and function of the spleen. Consequently, ectopic
splenic tissue can be more effectively displayed irrespective of
its location, even detecting small splenic nodules that may be
overlooked by CT. This imaging approach is highly specific and
non-invasive (12,13). In addition, a recent study
(40) has constructed a deeply
comprehensive Cancer Serum Protein Atlas to improve the sensitivity
and specificity of multicancer detection and classification based
on mass spectrometry. The plasma metabolic fingerprint of patients
with gastric cancer was obtained by the nanoparticle-enhanced laser
desorption/ionisation mass spectrometry technique and the
diagnostic and prognostic model was established (41). Furthermore, the application of
nanomaterials in assisted metabolic analysis and in vitro
diagnosis was advanced (42).
These new diagnostic methods help to improve the efficiency of
cancer diagnosis and treatment and have a positive impact.
Concerning splenosis treatment, surgical resection
remains the primary therapeutic option for symptomatic or large
lesions, particularly in cases where symptoms such as intestinal
obstruction, bleeding, abdominal pain or hemoptysis are evident. In
such instances, surgical intervention is deemed necessary and
should be promptly performed (37). However, it is well-documented that
ectopic splenic tissue possesses certain compensatory and
proliferative properties, exerting extensive immune functions in
hematopoiesis and red blood cell clearance. This capability may
reduce the incidence of fulminant infection (46). Consequently, asymptomatic splenosis
should, in principle, not be treated, including surgical
intervention (8-10).
Furthermore, certain scholars argue that splenectomy serves as a
therapeutic measure for patients with blood disorders and splenosis
may result in the partial restoration of splenic function,
potentially leading to disease recurrence. The question of whether
surgical resection is necessary for patients with blood diseases
remains controversial (47,48).
In the case of a patient with thalassemia and hypersplenism who
underwent laparoscopic splenectomy, the hemoglobin level was 90 g/l
post-surgery. Two years later, upon hospitalization due to
abdominal pain, the hemoglobin level was found to be 74 g/l.
Following the excision of the gastric splenosis lesion, the
hemoglobin level increased to 91 g/l two months after surgery.
Therefore, it is thought that splenosis may contribute to the
partial recovery of spleen function and potentially lead to disease
recurrence.
In summary, splenic rupture resulting from splenic
trauma frequently occurs during splenectomy, leading to the
autologous implantation of fragmented splenic tissue. This
implantation, when observed elsewhere months or years later, is
often misinterpreted as a tumor. Hence, meticulous attention to the
collection of medical history, particularly the details of splenic
rupture and splenectomy, is imperative for the accurate diagnosis
of ectopic spleen. When identification proves challenging, the
application of Tc-99m tagged heat-damaged autologous red blood
cells (99mTc-DRBC) and Tc-99m sulphur colloidal
noninvasive nuclear medicine technology can enhance the
visualization of ectopic spleen tissue, thereby improving the
diagnostic accuracy for gastric splenosis diseases. For patients
with confirmed splenosis, particularly those exhibiting symptoms
and a history of blood disorders, individualized treatment is not
only feasible but also necessary.
Supplementary Material
Flow chart of literature
screening.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the National Natural Science
Foundation of the P.R. China (grant no. 82260353).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XH and GH conceived and designed the study. XL
acquired the data and performed the literature review. JC and PW
analyzed and interpreted the data and critically revised the
manuscript for intellectual content. BZ acquired the scanning
images and managed the patient. XH and XL checked and confirmed the
authenticity of all the raw data. All authors contributed to the
article and have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from both
parents of the patient to publish this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kadyrova A, Baudinov I, Mamytova E,
Ibraimov K, Arzykulov T, Mamatov S, Vityala Y and Tagaev T: A very
rare case of splenosis and acquired chronic non-cirrhotic portal
vein thrombosis with cavernous transformation. Clin Case Rep.
10(e05799)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bernard Shaw A and Shafi A: Traumatic
autoplastic transplantation of splenic tissue in man with
observations on the late results of splenectomy in six cases. J
Pathol Bacteriol. 45:215–235. 1937.
|
|
3
|
Tandon YK, Coppa CP and Purysko AS:
Splenosis: A great mimicker of neoplastic disease. Abdominal
Radiol. 43:3054–3059. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Okano K, Inoue K and Suzuki Y:
Gastrointestinal: Solitary splenosis in the gastric fundus
mimicking gastrointestinal stromal tumor. J Gastroenterol Hepatol.
36:2033. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ma X, Gao J, Li Y, Xie J, Feng Z, Jia X
and Chen W: Transplantation of splenic tissue after splenectomy: A
case report. Exp Ther Med. 24(612)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang K, Chen X, Liu J, Wu B, Chen X and Hu
J: Splenosis in gastric wall mimicking gastrointestinal stromal
tumor. Endoscopy. 45:E82–E83. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Buttar SN and Ravn J: Intrathoracic
splenosis without clinical evidence of diaphragmatic rupture. Ann
Thor Surg. 108:e221–e222. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nardo-Marino A, Braunstein TH, Petersen J,
Brewin JN, Mottelson MN, Williams TN, Kurtzhals JAL, Rees DC and
Glenthøj A: Automating pitted red blood cell counts using deep
neural network analysis: A new method for measuring splenic
function in sickle cell anaemia. Front Physiol.
13(859906)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gunes O, Bag YM, Turgut E, Gunes A, Sumer
F and Kayaalp C: Splenic surgery: A ten years experience of a
tertiary center in Turkey. Ann Ital Chir. 92:59–64. 2022.PubMed/NCBI
|
|
10
|
Beuran M, Venter MD, Venter DP, Oprescu C,
Vâlcea S and Tănase TG: Intraomental Splenic implant-an attempt of
reassessment. Chirurgia (Buchar). 116:756–768. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lin XY, Han Q, Zhang Y and Wang EH:
Intrahepatic splenosis clinically diagnosed as a Carcinoma: A case
report. Int J Clin Exp Med. 9:4888–4891. 2016.
|
|
12
|
Ksiadzyna D and Peña AS: Abdominal
splenosis. Rev Esp Enferm Dig. 103:421–426. 2011.PubMed/NCBI(In English, Spanish).
|
|
13
|
Maggialetti N, Ciaccia M, Rubini D, Pisani
AR, Santo G, Lucarelli NM and Ianora AAS: Multimodal study of
pelvic splenosis: A rare cause of abdominal pain. Radiol Case Rep.
17:3601–3606. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang W, Yusufu Y and Wang C:
Gastrointestinal: A rare case of upper gastrointestinal bleeding:
Splenosis mimicking gastric varices. J Gastroenterol Hepatol.
37:1651. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li B, Huang Y, Chao B, Zhao Q, Hao J, Qin
C and Xu H: Splenosis in gastric fundus mimicking gastrointestinal
stromal tumor: A report of two cases and review of the literature.
Int J Clin Exp Pathol. 8:6566–6570. 2015.PubMed/NCBI
|
|
16
|
Fujita A, Nakahara K, Matsuda K, Ozawa SI
and Itoh F: Splenosis diagnosed by EUS-guided FNA. Gastrointest
Endosc. 92:1129–1130. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jing L, Cao Q and Li J: Gastric fundus
splenosis. J Gastrointest Surg. 26:2239–2240. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kanagalingam G, Vyas V, Sostre V and Arif
MO: Gastric splenosis mimicking gastrointestinal stromal tumor.
Cureus. 13(e12816)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rompou VA, Korkolis D, Skafida E, Tsamis D
and Plastiras A: Splenosis mimicking gastric obstructive tumor:
Diagnostic workup and surgical excision. Clin Case Rep.
9(e05225)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Guan B, Li XH, Wang L, Zhou M, Dong ZW,
Luo GJ, Meng LP, Hu J and Jin WY: Gastric fundus splenosis with
hemangioma masquerading as a gastrointestinal stromal tumor in a
patient with schistosomiasis and cirrhosis who underwent
splenectomy: A case report and literature review. Medicine
(Baltimore). 97(e11461)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Isopi C, Vitali G, Pieri F, Solaini L and
Ercolani G: Gastric splenosis mimicking a gastrointestinal stromal
tumor: A case report. World J Gastrointest Surg. 12:435–441.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zheng LF, Li DZ and Wang W: Endoscopic
full-thickness resection of gastric ectopic splenic nodules. BMC
Gastroenterol. 20(388)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang WJ, Li WQ, Sun YL, Zhao YL, Zhu RT,
Li J and Zhang H: Intra-gastric ectopic splenic tissue. J
Gastrointest Surg. 20:218–220. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Deng Y, Jin Y, Li F and Zhou Y: Splenosis
mimicking an extramural duodenal mass: A case report. Oncol Lett.
8:2811–2813. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Barbuscio I, Fantin A, Ghisa M, Savarino
EV, Mescoli C and Farinati F: Gastric fundal splenosis presenting
as a stromal tumor and diagnosed by endoscopic ultrasound-guided
SharkCore biopsy. Endoscopy. 51:E160–E161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang JB and Liu DL: Gastric fundus
splenosis mimicking stromal tumor. Revista Española de Enfermedades
Digestivas. 107:392–393. 2015.PubMed/NCBI
|
|
27
|
Falk GA, Means JR and Pryor AD: A case of
ventral hernia mesh migration with splenosis mimicking a gastric
mass. BMJ Case Rep. 2009(bcr0620092033)2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Agha FP: Regenerated splenosis
masquerading as gastric fundic mass. Am J Gastroenterol.
79:576–578. 1984.PubMed/NCBI
|
|
29
|
Carrara S, Rahal D and Repici A: A Case of
gastric splenosis mimicking a stromal tumor. Clin Gastroenterol
Hepatol. 14:e50–e51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Deutsch JC, Sandhu IS and Lawrence SP:
Splenosis presenting as an ulcerated gastric mass: Endoscopic and
endoscopic ultrasonographic imaging. J Clin Gastroenterol.
28:266–267. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Arroja B, Almeida N, Macedo CR, Moreira
AP, Oliveira P, Tomé L, Gouveia H and Sofia C: Gastric splenosis: A
rare cause of digestive bleeding. Rev Esp Enferm Dig. 103:377–378.
2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu Y, Dai Y, Xiao F, Liu S, Wu Y and Ran
E: Rare case of upper gastrointestinal hemorrhage due to accessory
spleen: A case report. Medicine (Baltimore).
10(e29636)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zang G, Dong B, Zhu G, Qiu X and Zhao Y:
Accessory spleen after splenectomy mimicking adrenal tumor: A case
report. Transl Cancer Res. 9:5679–5683. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen G, Nie L and Zhang T: An extremely
rare case of a gastric accessory spleen: Case report and review of
the literature. BMC Gastroenterol. 21(275)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Elchaninov A, Vishnyakova P, Sukhikh G and
Fatkhudinov T: Spleen: Reparative regeneration and influence on
liver. Life (Basel). 12(626)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wong A, Fung KFK, Wong WC, Ng KK, Kung BT
and Kan YLE: Multimodality imaging of developmental splenic
anomalies: Tips and pitfalls. Clin Radiol. 77:319–325.
2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Abeywardana K and de Mel VPRS: Shahini
Winson Gnanathayalan. Yalinie R, Gunasinghe ADKA and Arasalingam A:
Acute lung infection severity score (ALISS)-A scoring scale for
chest-X-RAy (CXR) findings in lung infection. Jaffna Medical
Association, 2020.
|
|
38
|
Chatterjee SN, Eckles D and Gershwin ME:
Preferential B-cell reconstitution following splenic
autotransplantation in mice. Transplant Proc. 11:1458–1459.
1979.PubMed/NCBI
|
|
39
|
Liu W, Chen S, Chen J, Jiang T, Quan L and
Xie S: Application of multimodal imaging in the diagnosis of
intrahepatic splenosis: Two case reports and a literature review.
BJR Case Rep. 8(20210170)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu Z, Huang Y, Hu C, Du L, Du YA, Zhang Y,
Qin J, Liu W, Wang R, Yang S, et al: Efficient plasma metabolic
fingerprinting as a novel tool for diagnosis and prognosis of
gastric cancer: A large-scale, multicentre study. Gut.
72:2051–2067. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang J, Huang L and Qian K:
Nanomaterials-assisted metabolic analysis toward in vitro
diagnostics. Exploration (Beijing). 2(20210222)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang J, Hu A, Chen X, Shen F, Zhang L,
Lin Y and Shen H: Pan-targeted quantification of deep and
comprehensive cancer serum proteome improves cancer detection.
View. 4(20220039)2023.
|
|
43
|
Ksiadzyna D: A case report of abdominal
Splenosis-a practical mini-review for a gastroenterologist. J
Gastrointestin Liver Dis. 20:321–324. 2011.PubMed/NCBI
|
|
44
|
Vernuccio F, Dimarco M, Porrello G,
Cannella R, Cusmà S, Midiri M and Brancatelli G: Abdominal
splenosis and its differential diagnoses: What the radiologist
needs to know. Curr Probl Diagn Radiol. 50:229–235. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Grande M, Lapecorella M, Ianora AAS, Longo
S and Rubini G: Intrahepatic and widely distributed intraabdominal
splenosis: Multidetector CT, US and scintigraphic findings. Intern
Emerg Med. 3:265–267. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Harfouche MN, Dhillon NK and Feliciano DV:
Update on Nonoperative Management of the Injured Spleen. Am Surg.
88:2649–2655. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu Y, Jin S, Xu R, Ding C, Pang W, Li Y
and Chen Y: Hereditary spherocytosis before and after splenectomy
and risk of hospitalization for infection. Pediatr Res.
93:1336–1341. 2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Parrinello NL, Triolo A, Toro A, Schembari
E, Palermo F, Rizzo G, Corsale G, Romano A, Di Raimondo F and Di
Carlo I: PB2261 Autologous transplantation of spleen tissue can
recover splenic function after traumatic splenectomy. HemaSphere.
3:1011–1012. 2019.
|