Introduction
Acute myocardial infarction (AMI) is increasing in
incidence of worldwide, and has gradually become an important cause
of mortality (1). With the
continuous development of interventional techniques, the treatment
of coronary heart disease and AMI is becoming more advanced.
However, ischemia/reperfusion (I/R) injury may occur as treatment
progresses (2), which is an urgent
clinical issue (3). Myocardial I/R
injury can offset the benefits of reperfusion therapy and even
worsen the prognosis of AMI (4,5). I/R
injury is defined as reduced cardiac systolic function, reperfusion
arrhythmia, dysfunctional myocardial metabolic capacity, oxidative
damage, inflammation, cardiac dysfunction and other injuries
following the recovery of the blood supply to ischemic myocardial
tissues (6). Myocardial ischemia
causes numerous cellular changes, including changes in cell
membrane potential and intracellular ion distribution, such as
increased Ca2+ and Na+ influx, cell swelling
and rupture, and cell acidosis (7,8). It
is widely considered that the upregulated release of reactive
oxygen species (ROS) is a major cause of early I/R injury (9). Under normal physiological conditions,
ROS are continuously produced in the myocardium, but can be removed
via a cellular clearance mechanism. However, in the pathological
state of hypoxia/reoxygenation, the disordered myocardial
metabolism produces a large quantity of ROS that cannot be cleared
away, which may lead to pathological changes such as DNA injury and
protease degeneration (10). Other
mechanisms of myocardial I/R injury are known, including
intracellular Na+ and Ca2+ accumulation, pH
reduction, mitochondrial dysfunction, increased free radical
formation and nitric oxide metabolism disorder (11-13).
The occurrence of autophagy also plays an important role (14).
Autophagy is a key metabolic pathway by which
eukaryotic cells maintain homeostasis when stimulated by various
physicochemical factors, such as inflammation, starvation, anoxia
and reoxygenation injury. It is also a multi-step intracellular
catabolic process involved in the pathophysiological processes of
various diseases. During autophagy, damaged organelles and
macromolecules are enclosed in specialized vesicles called
autophagosomes. The resulting autophagosomes are eventually
transferred to lysosomes for fusion and degradation of their
contents (15). Autophagy has been
shown to be involved in the process of myocardial I/R injury
(16). Mitochondrial autophagy
involves the mitochondria in hypoxic cardiomyocytes being
surrounded by autophagosomes and undergoing phagocytosis (17). Therefore, autophagy and oxidative
stress are important factors to be considered in the treatment of
myocardial I/R injury.
Nicotinamide riboside (NR) is a precursor of
nicotinamide adenine dinucleotide (NAD+) that has
potential health benefits due to the production of NAD+
in the body. NAD+ plays a key role in cellular
oxidation-reduction reactions, including catabolic and anabolic
reactions such as glycolysis and the tricarboxylic acid cycle
(18-20).
NAD+-depleting enzymes are mediators of aging that are
mainly induced by stressors such as DNA damage, oxidative stress
and inflammation. These include sirtuins, which are
NAD+-dependent deacetylases/deacylases that deplete
NAD+ when removing acetyl or other acyl groups from
proteins (21). Sirtuin 1 (sirt 1)
is a member of the sirtuin family, which has been shown to
participate in myocardial I/R injury via the regulation of
autophagy (22).
NR can participate in the regulation of autophagy
and oxidative stress (21).
However, the effect of NR on myocardial I/R injury is not clear.
Therefore, the aim of the present study was to investigate the role
of NR in hypoxia/reoxygenation (H/R)-induced myocardial cell injury
to evaluate the therapeutic potential of NR as a treatment for I/R
injury.
Materials and methods
Animals
A total of 24 wild-type C57BL/6 male mice (8-10
weeks old; body weight, 24.5±3.7 g) were provided by the Shanghai
Model Organisms Center, Inc. All protocols were approved by the
Animal Care Ethics Committee of Zhonghong Boyuan Biotechnology Co.,
Ltd. (K-2023-0513-1; Nanchang, China) and implemented according to
the Guide for the Care and Use of Experimental Animals of the
National Institutes of Health. The validity of the approval was
verified by the Ethics Committee of Nanchang University (Nanchang,
China). The mice were placed in a constant temperature of 20-26˚C
and humidity of 40-70% environment with a 12-h light/dark cycle and
5 g daily food and 6-7 milliliters of water. The NR treatment was
administered as a once daily dose of oral NR (Shanghai Macklin
Biochemical Co., Ltd.) via gavage, which was guaranteed to provide
a 450 mg/kg dose. The treatment was administered for 14 days.
According to a previous study (23), the administration of NR to mice at
a dose of 450 mg/kg and duration of ≥14 days significantly
increases the body content of NAD+. The mice were
randomly divided into four groups (n=6/group): Control group, no
treatment; sham group, opening of the chest without ligation of the
left anterior descending (LAD) coronary artery; I/R group, 30-min
LAD ligation followed by 24-h reperfusion; and I/R + NR group,
pre-treatment with NR followed by I/R as described for the I/R
group.
Myocardial I/R injury
A mouse myocardial I/R injury model was established
using the aforementioned mice. During modeling, anesthesia was
induced using 2.5% isoflurane and maintained with 1% isoflurane
inhalation. After fixing the mice in a stable position, the chest
cavity was opened, the LAD arterial branch was ligated with a 6-0
silk thread, and then the chest cavity was closed following release
of the ligature. Following 30 min of ligation, the ligature was
released to allow reperfusion for 24 h, after which the I/R model
was constructed (24). The mice
were then euthanized using CO2 at a flow rate of 60%
volume displacement/min followed by cervical dislocation. Serum was
collected from the mice for determination of CK-MB, and their
hearts were excised and some were frozen for later use.
Echocardiography detection
To evaluate the effect of NR supplementation on I/R
injury in the mice, echocardiography was performed to detect the
structure and function of the heart and basic data were obtained
after 24-h perfusion, namely the left ventricular ejection fraction
(EF) and left ventricular shortening fraction (SF).
Evan's blue/triphenyl tetrazolium
chloride (TTC) and hematoxylin and eosin (H&E) staining
Infarction was measured using the TTC staining
method. Following euthanasia, the hearts were immediately extracted
from the mice and the coronary arteries were perfused with PBS. The
blood was washed away with normal saline and the left anterior
coronary artery was re-occluded. Evan's blue dye (~1 ml, 2%) was
injected into the heart via the aorta until the heart turned blue.
The heart was then cut into 5 or 6 transverse sections and
incubated in 1% TTC solution at 37˚C for 20 min. The non-ischemic
area was stained blue, the white tissue was the infarcted area, and
the white/red tissue was the area at risk (AAR). ImageJ software
was used to calculate the AAR.
With regard to H&E staining, the heart was first
embedded in paraffin, fixed with 12% formalin at room temperature
for 12 h and sliced into 5-µm sections. The sections were then
dewaxed and stained at room temperature for 15 min with hematoxylin
solution. After this, the sections were rinsed with running water
for 1 h, dehydrated with ethanol and stained with eosin solution
for 5 min at room temperature. The stained tissues were dehydrated
again and soaked in xylene. Finally, the slices were covered with a
glass coverslip and observed at x200 magnification using an optical
microscope and bright field illumination at room temperature.
Cell culture and H/R treatments
H9c2 cells (cat. no. AW-CELLS-R0006) were obtained
from Anwei Biotechnology Co., Ltd. (Shanghai, China) and cultured
in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) in 37˚C. Prior to the
simulation of I/R injury, the cytogenic medium was discarded, the
cells were rinsed with PBS, DMEM without FBS was added, and the
cells were cultured for 24 h. Firstly, the effect of treatment with
different concentrations of NR alone on the survival of the H9C2
cells was investigated. The cells were pretreated with different
concentrations of NR and their survival status was determined using
a Cell Counting Kit-8 (CCK-8) assay. Secondly, the minimum
effective concentration of NR for protecting against H/R injury was
determined. The cells were pretreated with different concentrations
of NR followed by H/R treatment, and cell survival status was
detected using the CCK-8 assay. In these assays, various dilutions
of NR (0, 0.01, 0.1, 1, 5 and 10 mM) were established using DMEM.
The cells were seeded in six-well plates, and the different
dilutions of NR were added. The NR pre-treatment of the cells was
performed for 24 h in 37˚C (25).
The cells were then placed in a three-gas anoxic incubator (1%
O2 + 94% N2 + 5% CO2) for 3 h.
After this hypoxic treatment, the cell culture medium was replaced
with DMEM containing 10% FBS and the plate was placed in a normal
37˚C incubator with 95% air and 5% CO2 and cultured for
another 3 h. Then the cell culture supernatant was collected and
transferred to an Eppendorf tube, which was placed in a -80˚C
freezer for later use. Control group, normal cell culture without
any treatment; The H/R group was cultured at 37˚C for 3 h in an
anaerobic incubator, while the H/R+NR group was pretreated with NR
for 24 h before undergoing hypoxia reoxygenation. The H/R+NR+EX527
and NR+EX527 groups were pretreated for 24 h before undergoing
hypoxia reoxygenation. According to the instructions provided for
EX527 (HY-15452, MedChemExpress, USA) by the manufacturer, a
concentration of 10 µM was used.
NAD+ measurements
Previous studies (23,26)
have shown that the oral administration of NR increases the content
of NAD+ in mice. To determine whether NR also has this
effect in vitro, the NAD+ content of the cells
was measured after NR pretreatment and H/R culture. The
NAD+ content was determined using an enzyme-linked
immunosorbent assay (ELISA) kit (AAT-B15258; Amplite®;
AAT Bioquest, Inc.), according to the manufacturer's
instructions.
Lactate dehydrogenase (LDH) and
creatine kinase myocardial band (CK-MB) determination by ELISA
The cells were tested using an LDH Assay Kit (AK141;
BIOSS) and the cells and mouse cardiac tissue were tested using an
CK-MB ELISA Kit (H191-1-1; Nanjing Jiancheng Bioengineering
Institute) according to the manufacturers' instructions. Finally,
the absorbance was measured at a wavelength of 450 nm using a
microplate reader (Mindray Medical International, Ltd.) (27).
ROS measurements
An ROS detection kit (E-004-1-1; Nanjing Jiancheng
Bioengineering Institute) was used to analyze the H9c2 cells and
mouse cardiac tissue according to the manufacturer's instructions.
Blank and sample holes were set up in the enzyme assay plate.
Following the addition of enzyme labeling reagent and color
rendering, the absorbance of each hole was measured using a
microplate reader at a wavelength of 450 nm.
Malondialdehyde (MDA) and superoxide
dismutase (SOD) measurement by ELISA
The MDA and SOD contents of the H9c2 cells were
analyzed according to the instructions provided by the
manufacturers of the MDA Assay Kit (cat. no. AK289; BIOSS) and SOD
Assay Kit (AK061; BIOSS). The absorbance for MDA at 532 and 600 nm
and for SOD in 550 nm were measured using a microplate reader.
Cell viability measurement by CCK-8
assay
Cell viability was measured in 96-well plates
according to the instructions of the CCK-8 assay kit (Beyotime
Institute of Biotechnology). In brief, 10 µl CCK-8 reagent was
added to each well of the plate and the cells were cultured at 37˚C
for 3 h in the absence of light. Finally, the absorbance of each
well at 450 nm was measured using a microplate reader.
Trypan blue assay
Following digestion of the H9c2 cells with trypsin
solution (Beijing Solarbio Science & Technology Co., Ltd.),
Trypan blue reagent (Beijing Solarbio Science & Technology Co.,
Ltd.) was added to the cell mixture. After 3 min at room
temperature, 20 µl cell suspension was put on a cell counter plate
and inserted into a cell counter to analyze the survival status of
the cells.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
After treatment, RNA was extracted from the cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and the RNA was reverse transcribed into cDNA
using an iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, Inc.)
in 70˚C for 5 min. Then an SYBR Green PCR kit (Bio-Rad
Laboratories, Inc.) was used for qPCR with the following
thermocycling conditions: 95˚C for 5 min, 95˚C for 15 sec and 60˚C
for 45 sec, for 40 cycles. The relative expression of the target
gene was calculated by the 2-ΔΔCq method with GADPH
normalization (24). The primers
used were GAPDH forward: 5'-GGGTGTGAACCACGAGAAAT-3' and reverse:
5'-ACTGTGGTCATGAGCCCTTC-3'; sirt 1 forward:
5'-CCAGACCTCCCAGACCCTCAAG-3' and reverse:
5'-GTGACACAGAGACGGCTGGAAC-3'.
Western blot analysis
The cells were digested with trypsin, collected and
then lysed at low temperature or on ice with a mixture of
radioimmunoprecipitation assay buffer and phenylmethylsulfonyl
fluoride (both from Beyotime Institute of Biotechnology). After
lysis for 30 min, the supernatant was obtained by centrifugation at
4˚C and 12,000 g for 15 min. The protein lysate (30 µg
protein/lane) was subjected to 10-15% SDS-PAGE, and the gel was
transferred to a polyvinylidene fluoride membrane after
electrophoresis. The membranes were blocked in
QuickBlock™ Blocking Buffer for Western Blot (Beyotime
Institute of Biotechnology) for 10 min at room temperature, and
then incubated overnight at 4˚C with primary antibodies targeting
P62 (18420-1-Ag; 1:5,000; Proteintech Group, Inc.), Beclin 1
(11306-1-Ap; 1:1,000; Proteintech Group, Inc.), GADPH (60004-1-Ig;
1:50,000; Proteintech Group, Inc.), microtubule-associated protein
1A/1B-light chain 3 (LC3; cat. no. 206019; 1:1,000; Zen-Bio, inc.)
and sirt 1 (ab189494; 1:1,000; Abcam). Following primary antibody
incubation, clean the membrane three times with TBST for 10 min
each time. Subsequently, the membrane was incubated with the
secondary rabbit antibody (ab205718; 1:10,000; Abcam) at room
temperature for 1 h. After incubation, the membrane was washed
three times with Tris-buffered saline containing 0.01% Tween-20 for
10 min each time. Finally, a GelView 6000M System (BoLuTeng, China)
was used to capture images of the membrane. ImageJ software v1.8.0
(National Institutes of Health) was used to densitometrically
analyze the blots (27).
Statistical analysis
Each group of experiments was repeated three times,
and all data are presented as the mean ± standard deviation. The
data were analyzed using GraphPad Prism version 9.0 (Dotmatics).
One-way ANOVA followed by Tukey's post hoc test was used to compare
data among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
NR alleviates the effects of
myocardial I/R injury in mice
To explore the potential of NR supplementation in
the treatment of I/R, an I/R model with NR pre-treatment was
established in mice. Following euthanasia of the mice, Evan's
blue/TTC staining and pathological staining were performed to
observe the area of myocardial infarction and the histopathological
changes in the mice. The Evan's blue/TTC staining results in
Fig. 1A show that treatment with
NR significantly reduced the AAR following myocardial I/R injury in
mice. In addition, H&E staining revealed that the NR treatment
ameliorated the pathological changes induced by I/R injury in the
mouse heart (Fig. 1B). Under an
optical microscope, it was observed that the structure of the heart
tissue in the control and sham groups was normal, with orderly and
uninterrupted arrangement of the myocardial fibers, no inflammatory
cell infiltration and no evident pathological changes. However, the
hearts of the I/R group had a large number of broken myocardial
fibers that had a loose and disorderly appearance, with an
increased distance between myocardial fibers compared with those in
the control and sham groups, accompanied by bleeding and
inflammatory cell infiltration. The heart tissue structure of the
I/R + NR group was almost normal, with more orderly myocardial
fibers, mild edema, and only a small amount of bleeding and
inflammatory cell infiltration. Measurement of the ROS and CK-MB
levels in the mice revealed that treatment with NR prior to I/R
significantly reduced the levels of ROS and CK-MB compared with
those in the I/R group (Fig. 1C).
In addition, echocardiography demonstrated that the pretreatment of
the mice with NR significantly increased the EF and FS compared
with those in the I/R group (Fig.
1D). These results indicate that NR has a protective effect
against myocardial I/R injury.
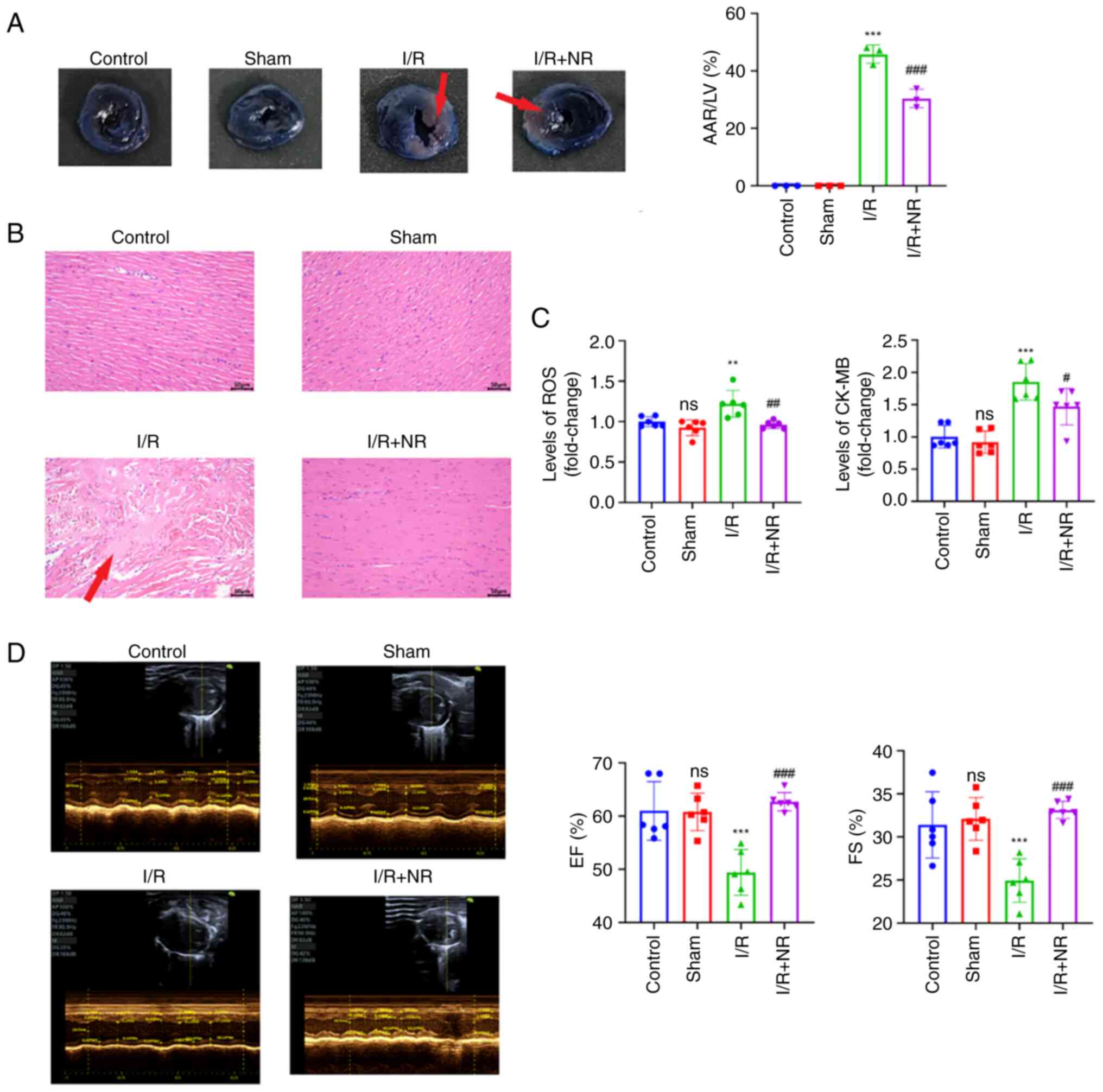 | Figure 1NR treatment ameliorates I/R injury
in mice. (A) Representative images of Evan's blue and triphenyl
tetrazolium chloride staining, and the ratio of the AAR to the LV
area in each group. The red arrows indicate the infarcted area. (B)
Representative images of hematoxylin and eosin staining (scale bar,
50 µm). The red arrow indicates the lesion area. (C) NR treatment
reduces the levels of ROS and CK-MB in mice after I/R injury. (D)
Representative echocardiographic images of each group and the
changes in EF and FS in each group. **P<0.01 and
***P<0.001 vs. Sham group. #P<0.05,
##P<0.01 and ###P<0.001 vs. the I/R
group. NR, nicotinamide riboside; I/R, ischemia/reperfusion; AAR,
area at risk; LV, left ventricular; ROS, reactive oxygen species;
CK-MB, creatine kinase myocardial band; EF, left ventricular
ejection fraction; FS, fractional shortening; ns, not
significant. |
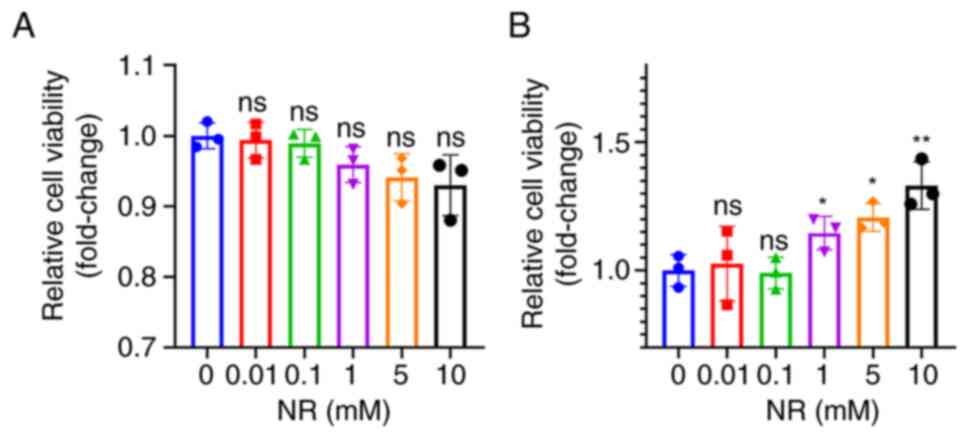
Cytotoxicity of NR to H9c2 cells and
its protective effect against H/R injury
To explore the optimal concentration of NR for H9c2
cells, the cells were first treated with different concentrations
of NR, and cell viability was detected by CCK-8 assay. As shown in
Fig. 2A, all concentrations of NR
had no significant effect on cell viability. Secondly, the same
concentrations were used to explore the optimal concentration of NR
for protection against H/R injury. As Fig. 2B shows, concentrations from 1 to 10
mM had a significant protective effect, which increased as the
concentration of NR increased. A concentration of 10 mM was
selected for use in subsequent assays
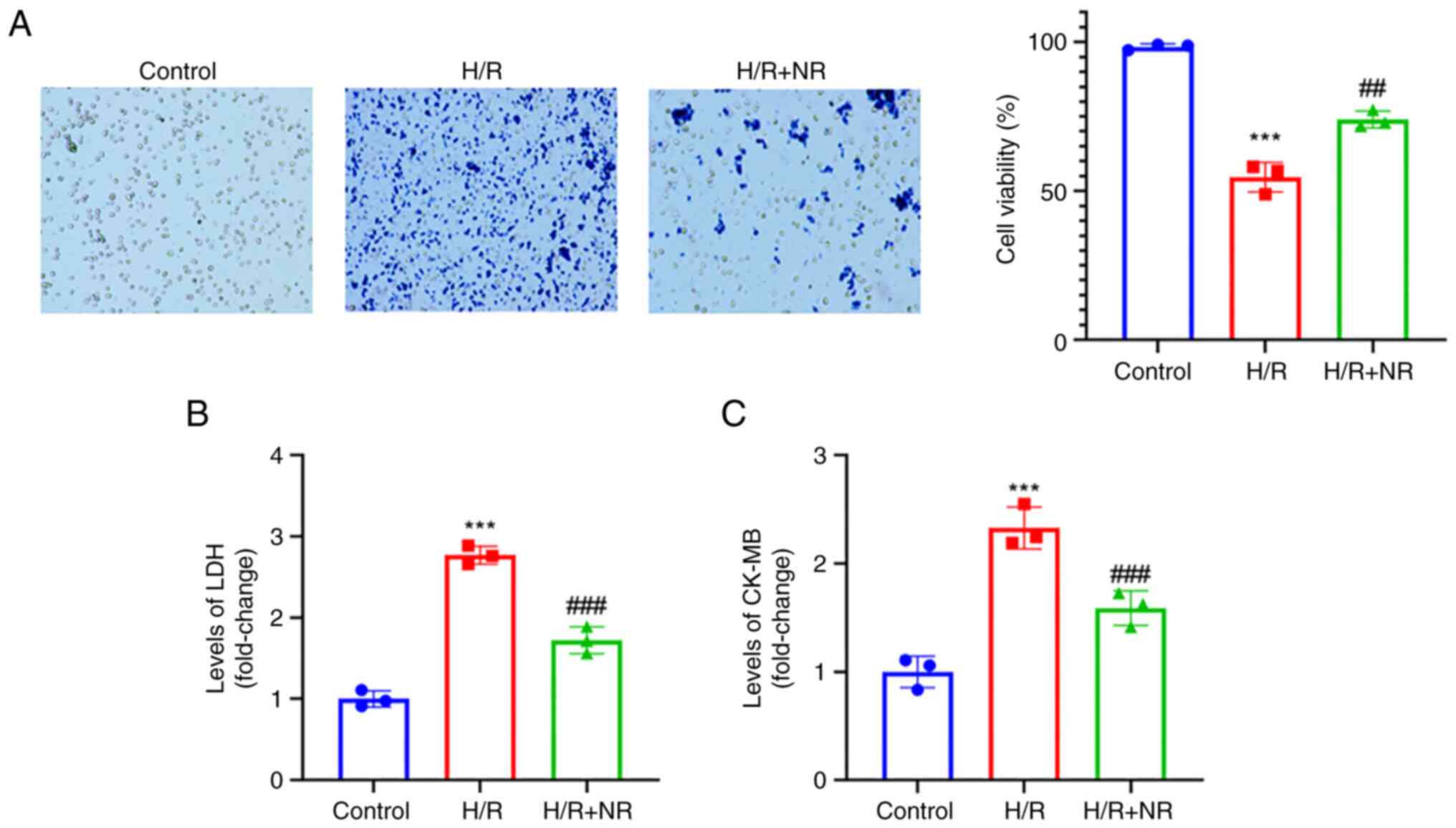
NR prevents the H/R-induced injury of
H9c2 cardiomyocytes
H/R is recognized to simulate the pathological state
of myocardial cell I/R injury (27). A blank control group was
established and cells were exposed to H/R in a medium with or
without NR for 24 h. The results of Trypan blue staining indicated
that cell viability was significantly decreased under H/R
conditions compared with those in the control group, while the
viability of cells treated with NR and exposed to H/R was
significantly higher than that of the cells exposed to H/R without
NR treatment, as shown in Fig. 3A.
In addition, the level of LDH in the H/R group was significantly
higher than that in the control group, while the level of LCH in
the H/R + NR group was significantly reduced compared with that in
the H/R group (Fig. 3B). An
increase in CK-MB can be regarded as a marker of myocardial damage
(16). Subsequently, an ELISA was
used to detect the CK-MB level of the cells. The CK-MB level was
significantly increased under H/R conditions compared with that in
the control group, while the H/R-induced increase in the level of
CK-MB was reversed by NR treatment (Fig. 3C). These findings indicate that NR
treatment protects against the H/R injury of H9c2
cardiomyocytes.
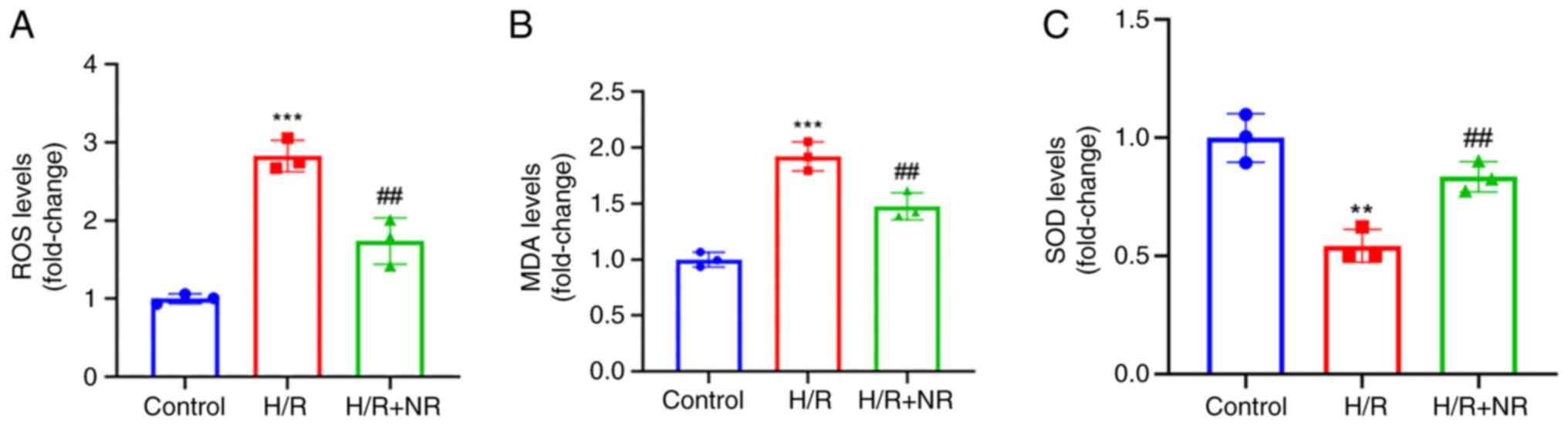
NR alleviates oxidative stress in H9c2
cardiomyocytes
Myocardial oxidative stress is regarded as an early
marker of myocardial I/R injury (24). Therefore, ROS, MDA and SOD levels
were detected using ELISAs to evaluate the oxidative stress level
of the cells. It was found that the levels of ROS and MDA in the
H/R group were significantly increased compared with those in the
control group, while the levels of all three variables were
significantly decreased in the H/R + NR group compared with those
in the H/R group, the level of SOD is contrary (Fig. 4). These results demonstrate the
ability of NR to alleviate the oxidative stress caused by I/R
injury in H9c2 myocardial cells.
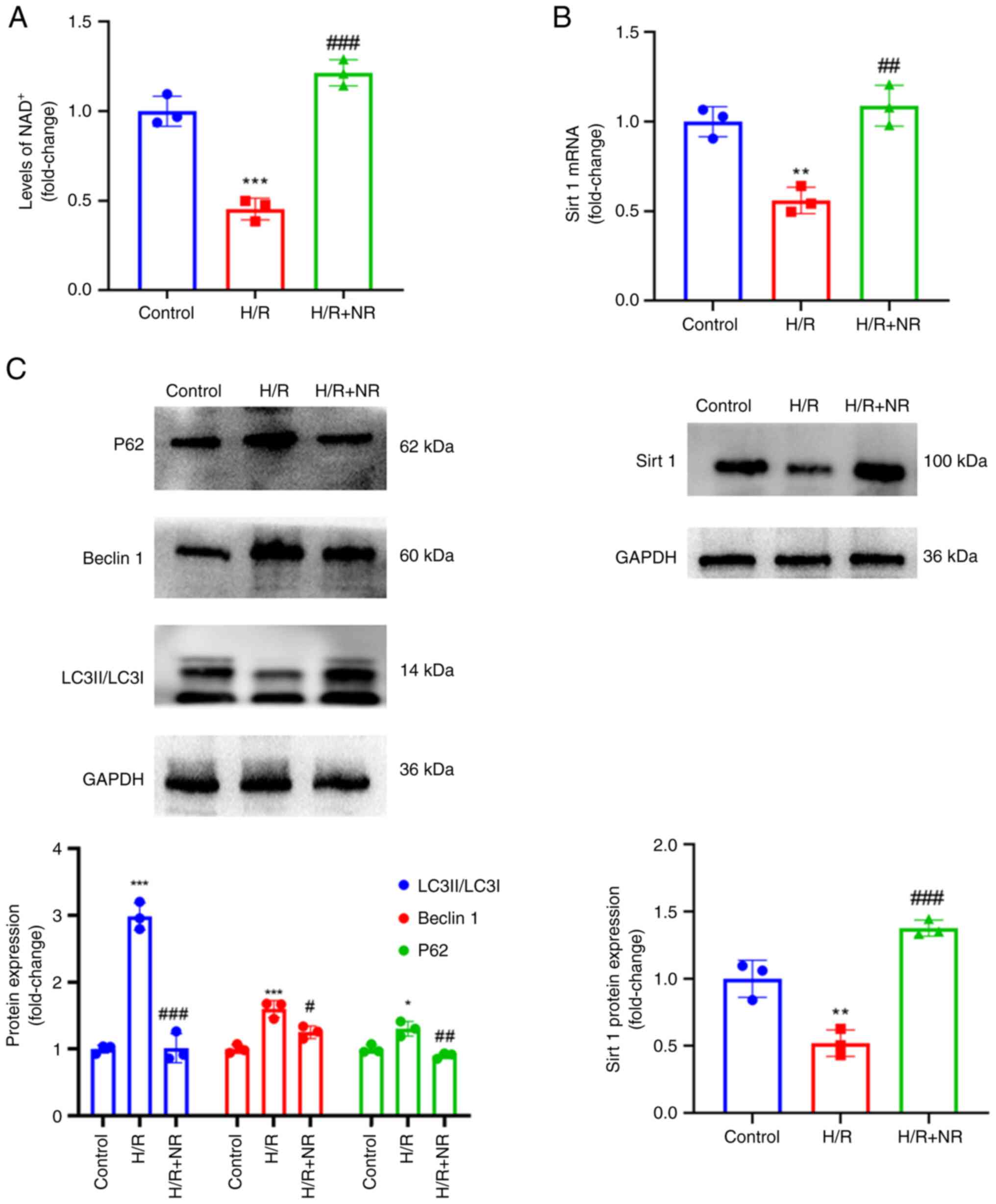
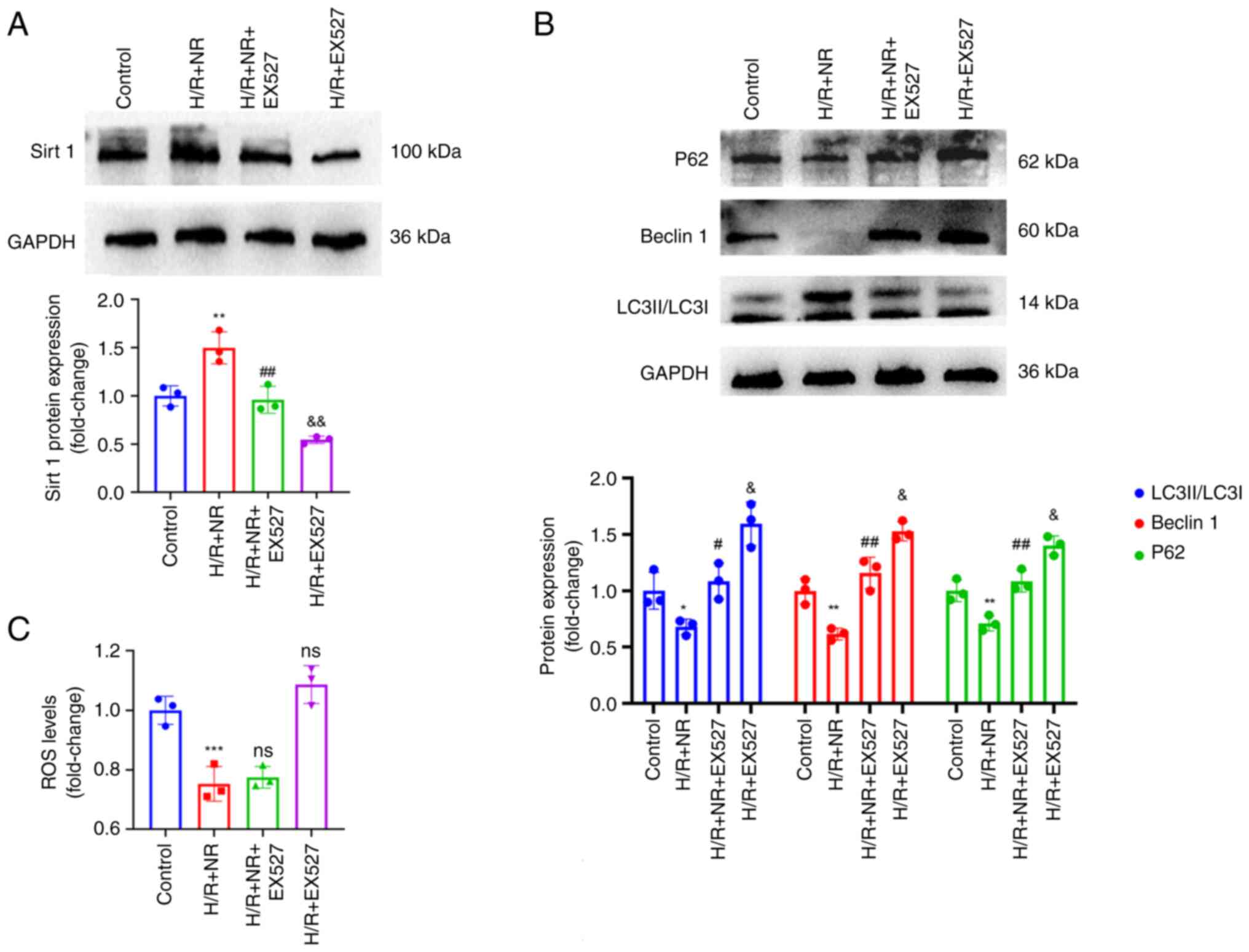
NR increases sirt 1 expression and
regulates autophagy
Sirt 1 is a deacetylase that relies on
NAD+ activation (21).
Therefore, the NAD+ content of the H9c2 myocardial cells
was first detected and then the expression of sirt 1 was detected
using RT-qPCR and western blotting to determine whether NR
increases sirt 1 expression. The results of an NAD+
ELISA (Fig. 5A) showed that
pretreatment with NR increased the content of NAD+ in
the H/R exposed cells, and RT-qPCR and western blotting results
showed a significant concurrent increase in sirt 1 expression
(Fig. 5B). Secondly, through the
preliminary detection of autophagy-associated protein expression,
it was found that supplementation of the culture medium with NR
regulated autophagy. The protein levels of P62, Beclin 1 and
LC3II/LC3I in the H/R group were increased compared with those in
the control group, and decreased in the H/R + NR group compared
with those in the H/R group (Fig.
5C). These results showing that NR treatment reduces the
protein levels of P62, beclin 1 and LC3II indicate that NR has the
ability to regulate autophagy.
NR upregulates sirt 1 to improve the
protective effect of autophagy on H/R injury in H9c2
cardiomyocytes
Sirt 1 is an NAD+-dependent deacetylase,
and NAD+ is its exclusive substrate (28,29).
As aforementioned, Sirt 1 has been shown to regulate autophagy.
However, it is unclear whether NR regulates autophagy through sirt
1. Therefore, a sirt 1 inhibitor (EX527) was used to investigate
the underlying mechanism. Western blotting showed that NR
significantly increased expression of sirt 1 in the H9c2 cells
under H/R conditions, and the use of EX527 with H/R and NR
significantly reduced the content of sirt 1 in the cells (Fig. 6A). In addition, as shown in
Fig. 6B, NR significantly reduced
the protein levels of beclin 1, P62 and LC3II/LC3I in H9c2
cardiomyocytes challenged with H/R, while the addition of EX527
significantly attenuated the NR-induced reduction in the expression
levels of all proteins under these conditions. However, the use of
EX527 did not significantly change the levels of ROS in the NR
treated cells under H/R conditions (Fig. 6C). Therefore, it may be concluded
that NR protects H9c2 cardiomyocytes from H/R injury by the
upregulation of sirt 1, but the upregulation of sirt 1 does not
ameliorate oxidative stress.
Discussion
Myocardial I/R injury is commonly associated with
cardiac surgery, coronary artery bypass grafting, coronary artery
thrombolysis and coronary artery occlusive stent recanalization
(30,31). As the incidence rate of myocardial
infarction has increased and coronary interventions have matured,
myocardial I/R has gradually become a frequent pathophysiological
phenomenon in clinical practice. Therefore, it has become urgently
necessary to develop methods for the reduction of I/R injury.
Currently, only one study has investigated the role
of NR in myocardial I/R injury. The analysis of combinations of
promising cardioprotective compounds with various routinely used
drugs, it was concluded that NR may have a protective effect on
myocardial I/R injury (32);
however, there has not yet been any basic research on the
application of NR in I/R injury.
NR is an NAD+ intermediate and a
precursor of NADH (33). NR has
been shown to play an important role in insulin sensitivity and
liver health (34,35). In addition, the supplementation of
NR has been shown to prolong the lifespan of mice (23). The effect of NR on heart function
has also been studied, and it was found that the supplementation of
NR preserved the cardiac function of mice with dilated heart
disease (26). Sirt 1 is an
NAD+-dependent enzyme that has been shown to serve a key
role in autophagy (36). Autophagy
is a protective reaction that occurs when cells are damaged. It
removes denatured proteins and rupture aged organelles to maintain
homeostasis within cells (37).
However, it has been reported that during myocardial I/R,
myocardial cells undergo excessive autophagy, resulting in the
abnormal accumulation of autophagic material and anomalous
degradation of proteins and organelles (38). In the present study, it was found
that when H9c2 cardiomyocytes underwent H/R, the protein levels of
P62, LC3II and Beclin 1 in the cells increased, indicating that
autophagy occurred in the cardiomyocytes during H/R injury and the
autophagic flux increased. When NR was supplemented prior to H/R,
the protein levels of P62, LC3II and beclin 1 decreased. Moreover,
the present study has demonstrated that NR has a protective effect
against H/R injury in H9c2 cardiomyocytes. This suggests that when
cardiomyocytes undergo H/R injury, excessive autophagy causes cell
damage, and NR plays a protective role via the inhibition of
excessive autophagy. In addition, previous studies have shown that
while autophagy is protective in myocardial I/R injury, excessive
autophagy can exacerbate I/R injury, leading to an increase in
cardiomyocyte apoptosis and greater cardiac dysfunction (39,40).
The mitochondrial ROS produced by the interaction
between the dysfunctional respiratory chain and oxygen during I/R
are nonspecific products (41).
During tissue ischemia, the excessive production of mitochondrial
ROS may be associated with inflammation and hypoxia, which lead to
cell injury (42). One of the main
mechanisms by which oxidative stress regulates cell damage is
considered to be the opening of mitochondrial permeability
transition pores (43). In the
present study, it was found that under H/R conditions, cell
viability was impaired and apoptosis occurred with elevated levels
of LDH and CK-MB, accompanied by oxidative stress. A significant
increase in ROS levels was observed following H/R, which is
consistent with previous research results (44). NR, as a precursor of
NAD+, can directly participate in oxidative reduction,
thereby reducing the level of oxidative stress in cardiomyocytes.
In the present study, it was observed that after treatment with NR,
the reduction in cell viability caused by H/R and the average
reduction in inflammatory exudation were alleviated. Therefore, it
may be concluded that NR serves a protective role in H/R injury via
the alleviation of oxidative stress in myocardial cells.
Myocardial I/R injury comprises myocardial ischemia
injury and reperfusion injury, and the latter may sometimes offset
the benefits of treatment, or even aggravate the original
myocardial injury. The mechanism of myocardial I/R injury is
unclear, but oxidative stress is often considered an important
factor (45). In the present
study, NR was demonstrated to alleviate the damage induced by
oxidative stress in H9c2 cardiomyocytes, with mitigation of damage
via the regulation of autophagy through the regulation of sirt 1.
EX527 treatment offset the protective effect of NR against H/R
injury, indicating that the protective role of NR is mediated via
the regulation of sirt 1. However, the lack of sirt 1 inhibition
had no significant effect on oxidative stress. It is prudent to
consider carefully whether sirt 1 has no regulatory impact on
oxidative stress, or whether the inhibitory effects of sirt 1
offset those on NAD+ in NR treated cells without
significant effect; further studies are required to investigate
this.
One limitation of this study is that knockout mice
were not used, which would have enhanced the comprehensiveness of
the study. Secondly, sirt 1 levels were not assessed in cells
treated with sirt 1 inhibitor-alone. In summary, despite these
limitations, the present study demonstrates the potential of NR in
providing protection against myocardial I/R injury.
In summary, the present research suggests that
treatment with NR improved the cardiac function of mice with
myocardial I/R injury, and reduced the generation of myocardial
injury- and oxidative stress-associated biomarkers. NR may protect
against autophagy by increasing the NAD+ content in the
body via the Sirt 1 pathway; however, the sirt 1 pathway does not
appear to affect oxidative stress. These findings provide a novel
potential approach for the clinical treatment of myocardial I/R
injury.
Acknowledgements
The authors would like to thank Mr Xiyuan Jiang and
Miss Lili Rao of the East China Institute of Digital Medical
Engineering for technical assistance with the experiments.
Funding
Funding: The present study was supported by the Jiangxi
Provincial Natural Science Foundation Fund Project (grant no.
20224BA206004) and the Jiangxi Provincial Key Research and
Development Plan General Project (grant no. 20202BBGL73070).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CY was responsible for the conception, design and
quality control of the study, and reviewed and edited the
manuscript. CY, HY and WL performed literature search, data
extraction and statistical analyses, were major contributors to
writing the manuscript . JY and YT participated in revising the
manuscript and statistical analyses. CY and HY confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All protocols were approved by the Animal Care
Ethics Committee of Jiangxi Zhonghong Boyuan Biotechnology Co.,
Ltd. (approval no. K-2023-0513-1). The validity of this approval
was verified by the Ethics Committee of Nanchang University
(Nanchang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Writing Group Members. Mozaffarian D,
Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de
Ferranti S, Després JP, et al: Heart disease and stroke
statistics-2016 update a report from the American heart
association. Circulation. 133:e38–e360. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Buja LM: Myocardial ischemia and
reperfusion injury. Cardiovasc Pathol. 14:170–175. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mokhtari-Zaer A, Marefati N, Atkin SL,
Butler AE and Sahebkar A: The protective role of curcumin in
myocardial ischemia-reperfusion injury. J Cell Physiol.
234:214–222. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Yellon DM and Baxter GF: Protecting the
ischaemic and reperfused myocardium in acute myocardial infarction:
A distant dream or near reality? Heart. 83:381–387. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goldhaber JI and Weiss JN: Oxygen free
radicals and cardiac reperfusion abnormalities. Hypertension.
20:118–127. 1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Collard CD and Gelman S: Pathophysiology,
clinical manifestations, and prevention of ischemia-reperfusion
injury. Anesthesiology. 94:1133–1138. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Burke AP and Virmani R: Pathophysiology of
acute myocardial infarction. Med Clin North Am. 91:553–572.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Becker LB: New concepts in reactive oxygen
species and cardiovascular reperfusion physiology. Cardiovasc Res.
61:461–470. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Martinet W, Knaapen MW, Kockx MM and De
Meyer GR: Autophagy in cardiovascular disease. Trends Mol Med.
13:482–491. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ferrari R, Balla C, Malagù M, Guardigli G,
Morciano G, Bertini M, Biscaglia S and Campo G: Reperfusion
damage-a story of success, failure, and hope. Circ J. 81:131–141.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moens AL, Claeys MJ, Timmermans JP and
Vrints CJ: Myocardial ischemia/reperfusion-injury, a clinical view
on a complex pathophysiological process. Int J Cardiol.
100:179–190. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Du J, Li Y and Zhao W: Autophagy and
myocardial ischemia. Adv Exp Med Biol. 1207:217–222.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yorimitsu T and Klionsky DJ: Autophagy:
Molecular machinery for self-eating. Cell Death Differ. 12 (Suppl
2):1542–1552. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hu S, Cao S, Tong Z and Liu J: FGF21
protects myocardial ischemia-reperfusion injury through reduction
of miR-145-mediated autophagy. Am J Transl Res. 10:3677–3688.
2018.PubMed/NCBI
|
|
17
|
Zhang J, Liu J, Huang Y, Chang JY, Liu L,
McKeehan WL, Martin JF and Wang F: FRS2α-mediated FGF signals
suppress premature differentiation of cardiac stem cells through
regulating autophagy activity. Circ Res. 110:e29–e39.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chambon P, Weill JD and Mandel P:
Nicotinamide mononucleotide activation of new DNA-dependent
polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res
Commun. 11:39–43. 1963.PubMed/NCBI View Article : Google Scholar
|
|
19
|
De Flora A, Zocchi E, Guida L, Franco L
and Bruzzone S: Autocrine and paracrine calcium signaling by the
CD38/NAD+/cyclic ADP-ribose system. Ann N Y Acad Sci. 1028:176–191.
2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Imai S, Armstrong CM, Kaeberlein M and
Guarente L: Transcriptional silencing and longevity protein Sir2 is
an NAD+-dependent histone deacetylase. Nature. 403:795–800.
2000.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Mehmel M, Jovanović N and Spitz U:
Nicotinamide riboside-the current state of research and therapeutic
uses. Nutrients. 12(1616)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Luo G, Jian Z, Zhu Y, Zhu Y, Chen B, Ma R,
Tang F and Xiao Y: Sirt1 promotes autophagy and inhibits apoptosis
to protect cardiomyocytes from hypoxic stress. Int J Mol Med.
43:2033–2043. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang H, Ryu D, Wu Y, Gariani K, Wang X,
Luan P, D'Amico D, Ropelle ER, Lutolf MP, Aebersold R, et al:
NAD+ repletion improves mitochondrial and stem cell
function and enhances life span in mice. Science. 352:1436–1443.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin JH, Yang KT, Ting PC, Luo YP, Lin DJ,
Wang YS and Chang JC: Gossypol acetic acid attenuates cardiac
ischemia/reperfusion injury in rats via an antiferroptotic
mechanism. Biomolecules. 11(1667)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hou Y, Wei Y, Lautrup S, Yang B, Wang Y,
Cordonnier S, Mattson MP, Croteau DL and Bohr VA: NAD+
supplementation reduces neuroinflammation and cell senescence in a
transgenic mouse model of Alzheimer's disease via cGAS-STING. Proc
Natl Acad Sci USA. 118(e2011226118)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Diguet N, Trammell SAJ, Tannous C, Deloux
R, Piquereau J, Mougenot N, Gouge A, Gressette M, Manoury B, Blanc
J, et al: Nicotinamide riboside preserves cardiac function in a
mouse model of dilated cardiomyopathy. Circulation. 137:2256–2273.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sun L, Wang H, Xu D, Yu S, Zhang L and Li
X: Lapatinib induces mitochondrial dysfunction to enhance oxidative
stress and ferroptosis in doxorubicin-induced cardiomyocytes via
inhibition of PI3K/AKT signaling pathway. Bioengineered. 13:48–60.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lu ZY, Liu ZY and Fang B: Propofol
protects cardiomyocytes from doxorubicin-induced toxic injury by
activating the nuclear factor erythroid 2-related factor
2/glutathione peroxidase 4 signaling pathways. Bioengineered.
13:9145–9155. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Donato AJ, Walker AE, Magerko KA, Bramwell
RC, Black AD, Henson GD, Lawson BR, Lesniewski LA and Seals DR:
Life-long caloric restriction reduces oxidative stress and
preserves nitric oxide bioavailability and function in arteries of
old mice. Aging Cell. 12:772–783. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Donato AJ, Magerko KA, Lawson BR, Durrant
JR, Lesniewski LA and Seals DR: SIRT-1 and vascular endothelial
dysfunction with ageing in mice and humans. J Physiol.
589:4545–4554. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ding S, Liu D, Wang L, Wang G and Zhu Y:
Inhibiting microRNA-29a protects myocardial ischemia-reperfusion
injury by targeting SIRT1 and suppressing oxidative stress and
NLRP3-mediated pyroptosis pathway. J Pharmacol Exp Ther.
372:128–135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xiao Y, Phelp P, Wang Q, Bakker D,
Nederlof R, Hollmann MW and Zuurbier CJ: Cardioprotecive properties
of known agents in rat ischemia-reperfusion model under clinically
relevant conditions: Only the NAD precursor nicotinamide riboside
reduces infarct size in presence of fentanyl, midazolam and
cangrelor, but not propofol. Front Cardiovasc Med.
8(712478)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bogan KL and Brenner C: Nicotinic acid,
nicotinamide, and nicotinamide riboside: A molecular evaluation of
NAD+ precursor vitamins in human nutrition. Annu Rev Nutr.
28:115–130. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Trammell SA, Weidemann BJ, Chadda A, Yorek
MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA and Brenner
C: Nicotinamide riboside opposes type 2 diabetes and neuropathy in
mice. Sci Rep. 6(26933)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Houstis N, Rosen ED and Lander ES:
Reactive oxygen species have a causal role in multiple forms of
insulin resistance. Nature. 440:944–948. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu C, Wang L, Fozouni P, Evjen G, Chandra
V, Jiang J, Lu C, Nicastri M, Bretz C, Winkler JD, et al: SIRT1 is
downregulated by autophagy in senescence and ageing. Nat Cell Biol.
22:1170–1179. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang M, Xi N, Gao M and Yu Y: Sitagliptin
mitigates hypoxia/reoxygenation (H/R)-induced injury in
cardiomyocytes by mediating sirtuin 3 (SIRT3) and autophagy.
Bioengineered. 13:13162–13173. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu CY, Zhang YH, Li RB, Zhou LY, An T,
Zhang RC, Zhai M, Huang Y, Yan KW, Dong YH, et al: LncRNA CAIF
inhibits autophagy and attenuates myocardial infarction by blocking
p53-mediated myocardin transcription. Nat Commun.
9(29)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gao C, Wang R, Li B, Guo Y, Yin T, Xia Y,
Zhang F, Lian K, Liu Y, Wang H, et al: TXNIP/Redd1 signalling and
excessive autophagy: A novel mechanism of myocardial
ischaemia/reperfusion injury in mice. Cardiovasc Res. 116:645–657.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Marek-Iannucci S, Thomas A, Hou J, Crupi
A, Sin J, Taylor DJ, Czer LS, Esmailian F, Mentzer RM Jr, Andres AM
and Gottlieb RA: Myocardial hypothermia increases autophagic flux,
mitochondrial mass and myocardial function after
ischemia-reperfusion injury. Sci Rep. 9(10001)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:4349–4360.
2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chouchani ET, Pell VR, Gaude E,
Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord
ENJ, Smith AC, et al: Ischaemic accumulation of succinate controls
reperfusion injury through mitochondrial ROS. Nature. 515:431–435.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cadenas S: ROS and redox signaling in
myocardial ischemia-reperfusion injury and cardioprotection. Free
Radic Biol Med. 117:76–89. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jin Q, Jiang Y, Fu L, Zheng Y, Ding Y and
Liu Q: Wenxin granule ameliorates hypoxia/reoxygenation-induced
oxidative stress in mitochondria via the PKC-δ/NOX2/ROS
pathway in H9c2 cells. Oxid Med Cell Longev.
2020(3245483)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sinning C, Westermann D and Clemmensen P:
Oxidative stress in ischemia and reperfusion: Current concepts,
novel ideas and future perspectives. Biomark Med. 11:11031–1040.
2017.PubMed/NCBI View Article : Google Scholar
|