Introduction
Radiation modification technology is an eco-friendly
chemical technique that can be applied to transform the chemical
structures of biological materials, thereby contributing to an
enhancement of their biological efficacy (1,2).
Several research groups have previously reported the use of
ionizing radiation for the semi-synthesis of novel compounds and
the development of anti-obesity materials from natural products,
such as rotenone, curcumin and rosmarinic acid using ionizing
irradiation (3,4). It has been widely suggested that γ
irradiation can enhance the anti-inflammatory effect of natural
products (5,6). Furthermore, we have previously
demonstrated that γ irradiation combined with the anti-depressant
nomifensine (Merital) can contribute to the suppression of the
proliferation of breast cancer cells (7). Given its diverse properties, ionizing
radiation may thus represent an innovative technique for the
development of incrementally modified drugs. To the best of the
authors' knowledge, no previous studies have evaluated the
structural and immunity enhancement effects of γ irradiation on
hydrazine-based compounds.
With the growing awareness of companion animal
health, there has been an increasing necessity for methods that can
be used to discover novel drugs for these animals. In this regard,
DH82 cells, a canine macrophage cell line, can serve as a valuable
tool for evaluating the anti-inflammatory activity of candidate
drugs for companion animals (8,9). Due
to their striking resemblance to in vivo macrophages, these
cells are excellent for screening natural extracts, chemical
compounds, or other functional materials with potential therapeutic
benefits for canine and feline inflammatory diseases. Studies
assessing anti-inflammatory activity in DH82 canine macrophages
cells for screening functional companion animal materials are
rapidly emerging. Inflammation is a localized response to injury or
infection that involves the accumulation of body fluids, plasma
proteins and white blood cells that can trigger the excessive
production of inflammatory mediators such as pro-inflammatory
nitric oxide (NO), prostaglandin E2 (PGE2),
TNF-α and IL-6 (10,11). The formation of inflammatory
mediators, such as prostaglandins, plays a key role in
inflammation. These mediators result from the interaction of
arachidonic acid with cyclooxygenase (COX)-2(12) and the NO production is known to be
regulated by the enzyme inducible NO synthase (iNOS) (11). Excessive inflammation can
contribute to the development of a range of chronic
inflammation-related diseases and disorders, including diabetes,
cancer, arthritis and cardiovascular disease, both in humans and
companion animals.
Nialamide, a non-selective, irreversible monoamine
oxidase inhibitor, has in the past been used clinically to treat
depression in both humans and animals (13,14).
However, it was withdrawn as an antidepressant in the 1970s owing
to the adverse hepatotoxic effects (15). However, despite this detrimental
activity, scientific interest in nialamide as a novel drug
candidates has persisted and several studies in this regard have
been reported (16). For examples,
Lougheed et al (17)
demonstrated that nialamide is a potent anti-tuberculosis agent
that can effectively inhibits Mycobacterium tuberculosis at
low concentrations. As part of an ongoing search for new drugs for
humans and companion animals, the present study assessed the
γ-ray-induced modification of nialamide and examined the
enhancement of its biological properties. The present study
detailed the isolation and characterization of the novel cyclized
compound 2 and degradation compounds 3-7 from irradiated nialamide.
These compounds, when compared with the nialamide parent (compound
1) exhibited significantly enhanced anti-inflammatory properties in
lipopolysaccharide (LPS)-stimulated both RAW 264.7 and DH82
macrophages.
Materials and methods
Materials
Nialamide (purity >99%), acetonitrile, methanol,
formic acid [high-performance liquid chromatography (HPLC) grade],
dimethyl sulfoxide (DMSO)-d6, LPS and Griess
reagent were purchased from MilliporeSigma. All other chemicals
used were of analytical grade. Compound analyses were conducted
using an Agilent HPLC 1200 system (Agilent Technologies, Inc.)
equipped with a photodiode array detector (1200 Infinity series;
Agilent Technologies, Inc.) and a series of YMC-Pack ODS A-302
columns (4.6 mm i.d.x150 mm, particle size 5 µm; YMC Co., Ltd.)
were used to analysis the compounds. HPLC was performed using an
ODS column (YMC-Pack A-302; YMC Co., Ltd.) using an elution
gradient of 5-100% MeCN in 0.1% HCOOH (detection: 280 nm; flow
rate: 1.0 ml/min; oven temperature: 40˚C). 1H and
13C nuclear magnetic resonance (NMR) spectra were
measured using an Avance NEO-600 instrument (Bruker Corporation)
operated at 600 and 150 MHz, respectively. Chemical shifts are
reported as δ (parts per million) values relative to those of the
solvent DMSO-d6 (δH 2.50;
δC 39.5) on a tetramethylsilane scale. High-resolution
electrospray ionization (ESI) mass spectra were obtained using a
Vanquish UPLS System (Thermo Fisher Scientific, Inc.) and an
Infinite F200 microplate reader (Tecan Austria GmbH) was used to
measure absorbances.
γ-irradiation of nialamide
Nialamide irradiation was performed at the Advanced
Radiation Technology Institute (ARTI; Jeongeup, Republic of Korea)
using a cobalt-60 irradiator (point source AELC; IR-79; MDS Nordion
International Co. Ltd.) with a source strength of ~320 kCi and a
dose of 10 kGy/h. Pure nialamide (1 g) was initially dissolved in
MeOH (1:l), placed in chapped glass bottles and then directly
irradiated with a dose of 50 kGy. After irradiation, the sample
solution was promptly concentrated using a rotary vacuum evaporator
to eliminate the methanol and was subsequently lyophilized.
Isolation of the newly modified
products
The irradiated reactant (965.6 mg) irradiated at a
dose of 50 kGy was promptly subjected to open column chromatography
on a YMC gel ODS AQ HG (2.5 cm i.d.x40 cm) column (YMC Co., Ltd.)
with aqueous MeOH, to obtain fractions N1-N6. Subfraction N4 (225.4
mg), which was flowed with 50% MeOH, was isolated using preparative
HPLC (YMC-prep column, 20 mm i.d.x250 mm; flow rate: 9 ml/min;
solvent systems 78:22=H2O:MeOH; YMC Co., Ltd.) to
produce compounds 3 (2.7 mg, tR 10.6 min), 5 (6.7
mg, tR 10.0 min) and 7 (56.7 mg,
tR 15.5 min). Fraction N5 (67.0 mg) was separated
using preparative-HPLC (YMC-prep column, 20 mm i.d.x250 mm;
solvent, flow rate: 9 ml/min; solvent systems
75:25=H2O:MeOH; YMC Co., Ltd.) to yield compounds 2 (2.0
mg, tR 21.6 min), 4 (2.8 mg, tR
21.6 min) and 6 (1.4 mg, tR 21.6 min). Fractions
N1-N3 were subjected to recrystallization and identified as HBPA
(compound 5; 500 mg).
Cell culture
Canine DH82 macrophages were purchased from the
American Type Culture Collection and murine RAW 264.7 macrophages
were purchased from the Korean Cell Line Bank. The DH82 and RAW
264.7 macrophages cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum (FBS) and 1% (v/v)
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and
were incubated at 37˚C in an incubator with a humidified atmosphere
of 5% CO2.
Cell viability
Cell viability was measured using the conventional
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Macrophage cells were seeded in 24-well plates at a density
of 1x105 cells/well, cultured for 24 h and treated with
various concentrations of nialamide and isolated compounds 2-7 for
an additional 24 h. Thereafter, the MTT reagent was added to each
well. The formazan crystals thus produced were dissolved in DMSO
and the absorbance of the well contents was measured at 570 nm
using a scanning a microplate reader. The cell viability was
determined from the proportions of viable and dead cells (18).
Measurement of NO, PGE2 and cytokine
productions
RAW 264.7 and DH82 macrophage cells were cultured in
24-well plates at a density of 1x105 cells/well for 24
h. Following this initial incubation, the cells were pre-treated
with nialamide and derived compounds 2-7 for 1 h and subsequently
treated with LPS (RAW 264.7 cells: 0.1 µg/ml; DH82 cells: 2 µg/ml)
for an additional 24 h. NO production in the macrophage culture
medium was assessed using the Griess reagent method with a standard
curve for quantification (19). In
addition, the macrophage supernatant was used to assess for levels
of PGE2 and cytokines using the respective enzyme-linked
immunosorbent assay (ELISA) kits (BD Biosciences), according to the
manufacturer's instructions.
Western blot analysis
RAW 264.7 and DH82 cells were cultured in six-well
plates at a density of 2x105 cells/well for 24 h.
Following incubation at 37˚C, the cells were pre-treated with
compounds 1 and 5 for 1 h and were thereafter treated with LPS (RAW
264.7 cell: 0.1 µg/ml; DH82 cell: 2 µg/ml) for an additional 24 h.
Total proteins were extracted using RIPA buffer (Rockland
Immunochemicals, Inc.) and the concentrations of the isolated
proteins were determined using a bicinchoninic acid protein assay
kit. Proteins (40 µg) were separated by sodium
dodecyl-sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10%)
and subsequently transferred to polyvinylidene difluoride membranes
(Merck KGaA). The membranes were then blocked with 5% non-fat milk
in Tris-buffered saline containing 10% Tween-20 (TBST) for 1 h at
room temperature and subsequently probed overnight at 4˚C with the
following primary antibodies at dilution of 1:1,000: Anti-COX-2
(cat. no. 4842), anti-iNOS (cat. no. 2977), anti-p65 (cat. no.
8242), anti-p-p65 (cat. no. 3033), anti-IκB (cat. no. 4812),
anti-p-IκB (cat. no. 2859) and anti-GAPDH (cat. no. 2118; all
obtained from Cell Signaling Technology, Inc.). For canine
macrophages, the following primary antibodies were used at a
dilution of 1:1,000: anti-COX-2 (cat. no. PA1-9032), anti-iNOS
(cat. no. PA1-036) and anti-GAPDH (cat. no. PA1-987; Invitrogen;
Thermo Fisher Scientific, Inc.). Following incubation with the
primary antibodies, the membranes were incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies (1:3,000; cat. no.
7074; Cell Signaling Technology, Inc.) for 2 h at room temperature.
Protein bands were detected using a chemiluminescence (ECL) reagent
(Thermo Fisher Scientific, Inc.) and exposure to an X-ray film. The
blots were scanned and densitometric analysis was performed using
ImageJ software (version 1.51k; National Institutes of Health).
Measurement of reactive oxygen species
(ROS)
The production of intracellular ROS was measured
using ROS detection reagents. In brief, RAW 264.7 macrophage cells
were seeded in 96-well plates at a density of 1x104
cells/well and treated with 0.1 µg/ml LPS in combination with
varying concentrations of compound 5 (50 to 200 µM) at 37˚C for 2
h. On day 1, the cells were washed with phosphate-buffered saline
(PBS) and incubated with 7'-dichlorofluorescein diacetate (DCFH-DA)
at 37˚C for 45 min. DCF fluorescence was measured at 0, 10 and 30
min using a microplate reader at excitation and emission
wavelengths of 492 and 520 nm, respectively (20).
The scavenging activity of
1,1-diphenyl-2-picrylhydrazyl (DPPH) radical of the derived
compounds was assessed using a modified Blois method (21). Briefly, a solution of DPPH (200 µM)
was prepared in ethanol (EtOH), 60 µl of which was mixed with 120
µl of each of the assessed compounds at concentrations from 50 to
200 µM in 70% EtOH. After incubation at 30˚C in the dark for 15
min, the reduction in absorbance at 517 nm was recorded using an
ELISA reader. For the determination of
3-ethylbenzothiazoline-6-sulphonic acid (ABTS+) radical
scavenging activity, an ABTS+) solution was prepared by
mixing 5 ml of a 7 mM ABTS+ solution in ethanol with 5
ml of a 2.4 mM potassium persulfate solution and incubating the
mixture in the dark at 25˚C for 24 h prior to use. The
ABTS+ solution (100 µl) was thereafter added to the
wells of 96-well plates containing different concentrations of
compound 5 and mixed for 30 sec, followed by incubation for 30 min
at 25˚C (22). The DPPH and
ABTS+ radical-scavenging activities were calculated
using the following formula:
radical scavenging activity
(%)=[1-(A2/A1)]x100
,
where A1 is the absorbance of reaction
mixtures without samples and A2 is the absorbance of
reaction mixtures containing the assessed compounds.
Statistical analysis
Each experiment was performed at least three times
and the results were expressed as the mean ± standard deviation.
For multiple comparisons, one-way analysis of variance (ANOVA) was
used followed by Tukey's multiple comparisons test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Structural determination of the
nialamide degradation products
Nialamide irradiated at a 50 kGy dose was
established to be characterized by the most significantly enhanced
inhibitory effects with respect to the production of NO and
PGE2 in LPS-stimulated RAW 264.7 cells, with
IC50 values of 98.7±0.9 and 88.2±0.9 µg/ml,
respectively. Comparatively, an IC50 value >200 µg/ml
was obtained for the parent compound, nialamide. DH82 canine
macrophages treated with irradiated nialamide displayed notable
inhibitions in both NO and PGE2 production (Table I). The molecular changes in
irradiated nialamide were assessed using reverse-phase HPLC. Newly
generated peaks in the HPLC chromatograms corresponding to the
modified products of nialamide were accordingly detected (Fig. S1). Successive chromatographic
separation of this sample resulted in the purification of a new
cyclized product, designated compound 2, along with five known
degradation products (compounds 3-7).
 | Table IInhibitory activities of modification
products 2-7 on LPS-stimulated NO and PGE2 production in
macrophages1. |
Table I
Inhibitory activities of modification
products 2-7 on LPS-stimulated NO and PGE2 production in
macrophages1.
| | RAW 264.7
cells | DH82 cells |
|---|
| Compounds | Cell viability (%)
2 | IC50 of
NO, µM | IC50 of
PGE2, µM | Cell viability,
%2 | IC50 of
NO, µM | IC50 of
PGE2, µM |
|---|
| Nialamide (1) | 102.3±2.3 |
>200a |
>200a | 99.9±1.2 |
>200a |
>200a |
| irradiated
nialamide3 | 101.0±1.3 | 98.5±0.9 | 88.2±0.9 | 99.9±1.0 | 89.0±1.0 | 67.9±0.8 |
| 2 | 99.5±1.9 |
186.2±2.1a |
150.7±2.0b | 98.3±1.3 |
101.6±1.5b |
98.5±1.0b |
| 3 | 101.5±2.0 |
170.5±1.9a |
155.1±1.9b | 99.7±1.2 |
158.2±1.7b |
122.2±1.3b |
| 4 | 102.0±2.2 | 177.6±2.0
a |
160.9±2.0a | 97.6±1.1 |
171.1±2.1a |
125.5±1.3b |
| 5 | 99.9±2.2 |
63.5±0.8c |
55.4±0.7c | 97.2±1.0 |
57.5±0.6c |
49.4±0.4c |
| 6 | 100.7±1.8 |
>200a |
>200a | 98.6±0.9 |
194.4±2.3a |
176.4±1.7a |
| 7 | 100.8±1.9 |
>200a |
>200a | 98.4±1.2 |
>200a |
>200a |
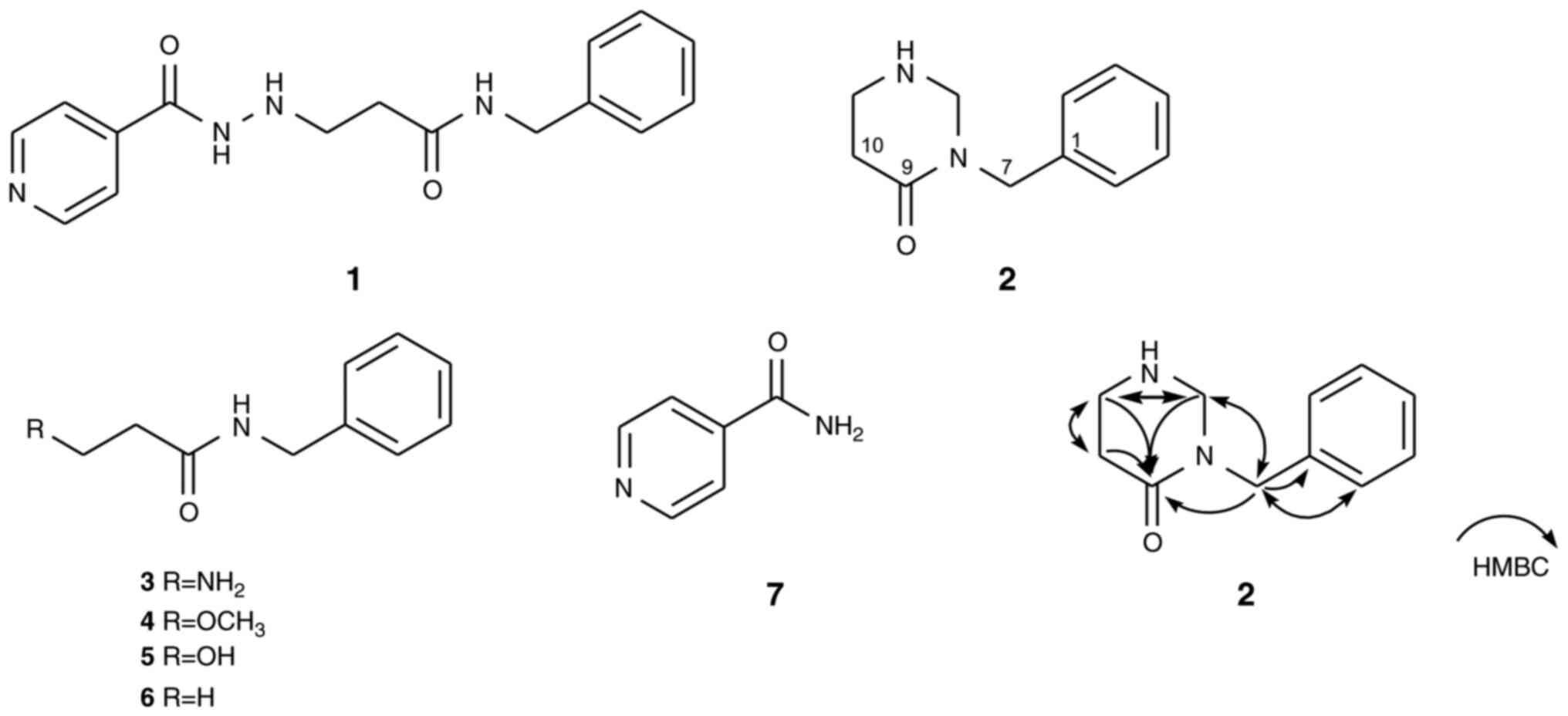
Compound 2 was obtained as a colorless oil. The
pseudomolecular ion in the positive high-resolution electrospray
ion mass spectrum (HRESIMS) at m/z 191.1176
(M+H)+ had the molecular formula
C12H15N2O. The 1H NMR
spectrum of compound 2 (Fig. S2)
showed characteristic resonances of a benzene ring system at
δH 7.34 (2H; t; J=1.8 Hz; H-3; 5), 7.26 (2H; dd;
J=8.4; 1.8 Hz; H-2; 6) and 7.24 (1H; dd; J=8.4; 1.8
Hz; H-4) and a nitrogenated methylene proton at δH 4.44
(2H; s; H-7), indicating the presence of a benzylamide moiety in
the molecule of compound 2. Additionally, the low-field region of
the 1H NMR spectrum displayed signals for doubly
nitrogenated methylene protons at δH 4.03 (2H; d;
J=6.0 Hz; H-13) and two cyclized methylene protons at
δH 2.92 (2H; br d; J=6.0 Hz; H-11) and 2.25 (2H;
t; J=6.0 Hz; H-10), which is consistent with a
tetrahydropyrimidione skeleton (23).
In addition, 13C NMR combined with
heteronuclear single quantum coherence (HSQC) analysis of compound
2 (Figs. S3 and S4) revealed 11 carbon signals,
attributable to one carbonyl carbon signal at δC 167.5
(C-9), five benzylamide signals at δC 137.9 (C-1), 128.6
(C-3; 5), 127.6 (C-2; 6), 127.1 (C-4) and 46.3 (C-7) and three
methylene carbons at δC 63.0 (C-13), 42.0 (C-11) and
32.9 (C-10). Collectively, the aforementioned informative results
provide evidence to indicate that compound 2 is a
tetrahydropyrimidione-substituted benzylamide. A detailed
interpretation of the combination of 1H-1H
correlated spectroscopy, heteronuclear multiple bond correlation
(HMBC) and nuclear Overhauser effect spectroscopy data for compound
2 further indicated that it features a characteristic cyclized
product (Fig. S5, Fig. S6 and Fig. S7). The proposed linkage between
the benzylamide and tetrahydropyrimidione moieties is suggested to
occur at the C-7 position, as supported by the HMBC correlations
(Fig. 1). On the basis of the
aforementioned analyses, the planar structure of compound 2 was
assigned to nialamionsin, a novel benzylamide analog with a
tetrahydropyrimidione unit (Fig.
1).
Nialaminosin (compound 2): Colorless oil: UV
λmax (MeOH): 207 (3.14), 220 (sh) and 249 (2.26) nm;
1H NMR (DMSO-d6; 600 MHz): δ 7.34 (2H;
t; J=1.8 Hz; H-3; 5), 7.26 (2H; dd; J=8.4; 1.8 Hz;
H-2; 6), 7.24 (1H; dd; J=8.4; 1.8 Hz; H-4), 4.44 (2H; s;
H-7), 4.03 (2H; d; J=6.0 Hz; H-13), 2.92 (2H; br d;
J=6.0 Hz; H-11), 2.25 (2H; t; J=6.0 Hz; H-10);
13C NMR (DMSO-d6; 150 MHz): δ 167.5
(C-9), 137.9 (C-1), 128.6 (C-3; 5), 127.6 (C-2; 6), 127.1 (C-4),
63.0 (C-13), 46.3 (C-7), 42.0 (C-11), 32.9 (C-10); ESIMS m/z
191 (M+H)+; HRESIMS m/z 191.1176
(M+H)+ (calculated for
C11H15N2O, 191.1178; Fig. S8).
The five known isolated compounds were characterized
as 3-amino-N-benzylpropanamide (compound 3; ABPA),
3-methoxy-N-benzylpropanamide (compound 4; MBPA),
3-hydroxy-N-benzylpropanamide (compound 5; HBPA),
N-benzylpropanamide (compound 6; BPA) and isonicotinamide
(compound 7; INA), based on comparisons of the respective
spectroscopic data (NMR and MS) with reports from the literature
(24-28).
ABPA (compound 3): Colorless oil: 1H NMR
(DMSO-d6; 600 MHz): δ 7.33 (2H; t; J=1.2
Hz; H-3; 5), 7.27 (2H; dd; J=7.8; 1.2 Hz; H-2; 6), 7.23 (1H;
dd; J=7.8; 1.2 Hz; H-4), 4.45 (2H; s; H-7), 2.93 (2H; br d;
J=6.0 Hz; H-11), 2.26 (2H; t; J=6.0 Hz; H-10),
13C NMR (DMSO-d6; 150 MHz): δ 167.7
(C-9), 137.0 (C-1), 127.2 (C-3; 5), 126.4 (C-2; 6), 126.0 (C-4),
46.2 (C-7), 42.2 (C-11), 32.3 (C-10); ESIMS m/z 179
(M+H)+ (24,25).
3-Methoxy-N-benzylpropanamide (compound 4):
Colorless oil: 1H NMR (DMSO-d6, 600
MHz): δ 7.33 (2H; t; J=1.8 Hz; H-3; 5), 7.24 (2H; dd;
J=7.8; 1.8 Hz; H-2; 6), 7.22 (1H; dd; J=7.8; 1.8 Hz;
H-4), 4.26 (2H; d; J=6.0 Hz; H-7), 3.55 (2H; t; J=6.0
Hz; H-11), 3.22 (3H; s; 11-OCH3), 2.38 (2H; t;
J=6.0 Hz; H-10); 13C NMR
(DMSO-d6; 150 MHz): δ 170.2 (C-9), 139.6 (C-1),
128.4 (C-3; 5), 127.3 (C-2; 6), 125.8 (C-4), 65.5 (C-11), 58.0
(11-OCH3), 42.1 (C-7), 35.1 (C-10), ESIMS m/z 194
(M+H)+ (26).
3-Hydroxy-N-benzylpropanamide (compound 5):
Colorless oil: 1H NMR (DMSO-d6; 600
MHz): δ 7.32 (2H; t; J=1.8 Hz; H-3; 5), 7.26 (2H; dd;
J=8.4; 1.8 Hz; H-2; 6), 7.22 (1H; dd; J=8.4; 1.8 Hz;
H-4), 4.27 (2H; d; J=6.0 Hz; H-7), 3.65 (2H; t; J=6.0
Hz; H-11), 2.31 (2H; t; J=6.0 Hz; H-10); 13C NMR
(DMSO-d6; 150 MHz): δ 170.9 (C-9), 139.9 (C-1),
128.5 (C-3; 5), 127.5 (C-2; 6), 126.9 (C-4), 57.9 (C-11), 42.2
(C-7), 39.0 (C-10); ESIMS m/z 180 (M+H)+
(25).
N-Benzylpropanamide (compound 6): Colorless
oil: 1H NMR (DMSO-d6; 600 MHz): δ 7.32
(2H; d; J=7.8 Hz; H-2; 6), 7.26 (2H; t; J=1.8 Hz;
H-3; 5), 7.23 (1H; dd; J=8.4; 1.8 Hz; H-4), 4.27 (2H; d;
J=6.0 Hz; H-7), 2.15 (2H; q; J=7.8 Hz; H-10), 1.03
(3H; t; J=7.8 Hz; H-11); 13C NMR
(DMSO-d6; 150 MHz): δ 173.3 (C-9), 140.2 (C-1),
128.7 (C-2; 6), 127.6 (C-3; 5), 127.1 (C-4), 42.4 (C-7), 18.9
(C-10), 10.4 (C-11); ESIMS m/z 164 (M+H)+
(27).
Isonicotinamide (compound 7): White amorphous
powder; 1H NMR (DMSO-d6; 600 MHz): δ
8.73 (2H; d; J=8.4 Hz; H-3; 5), 7.72 (2H; d; J=8.4
Hz; H-2; 6); ESIMS m/z 123 (M+H)+ (28).
Effects of the nialamide degradation
products on NO and PGE2 production
Inflammatory responses promote the excessive
production of pro-inflammatory mediators, such as NO and
PGE2 (11), the
abnormal overproduction of which is implicated in the development
of a diverse range of disorders, including circulatory shock,
cancer and atherosclerosis (29,30).
Consequently, regulating the production of pro-inflammatory agents
is a desirable pharmacological property when seeking to develop new
drugs. To investigate the anti-inflammatory activities of isolated
products 2-7, the present study treated both RAW 264.7 and DH82
macrophages with different concentrations of these compounds. Using
the MTT assay, the cytotoxicity of these compounds was initially
assessed at concentrations ranging from 10-200 µM and it was
accordingly established that all assessed compounds showed
negligible cytotoxicity toward these two cell types at
concentrations of ≥200 µM (Table
I).
In the current study, compounds were evaluated for
their inhibitory activity on the production of NO and
PGE2 in LPS-stimulated macrophages. Among these
compounds, compared with the parent nialamide (1) the novel cyclized product nialaminosin
(2) showed enhanced inhibitory
effects against the production of NO and PGE2 in
LPS-stimulated RAW 264.7 cells with respective IC50
values of 86.2±1.1 and 80.7±1.0 µM (Table I). Similarly, enhanced
anti-inflammatory effects were detected for benzylpropanamide
derivatives 3-6, with IC50 values ranging from
63.5-194.4 µM for NO production and from 55.4-176.4 µM for
PGE2 production (Table
I). Among these derivatives, it was found that hydroxylation at
the C-11 position of HBPA conferred the most potent inhibitory
activity against the activation of pro-inflammatory mediators
(Table I). Comparatively, the
simple pyridine amide isonicotinamide (7) was characterized by relatively lower
inhibitory activity against the production of both NO and
PGE2 in RAW 264.7 macrophages (Table I). Consistently, in canine DH82
macrophages, the hydroxyl-substituted benzylpropanamide derivative
was established have the most potent inhibitory activity against
the production of NO and PGE2, with IC50
values of 57.5±0.6 and 49.4±0.4 µM, respectively (Table I). On the basis of these
observations, the potent compound 5 was accordingly selected for
further evaluation of its inhibitory effects on the release of NO
and PGE2 using western blot analysis.
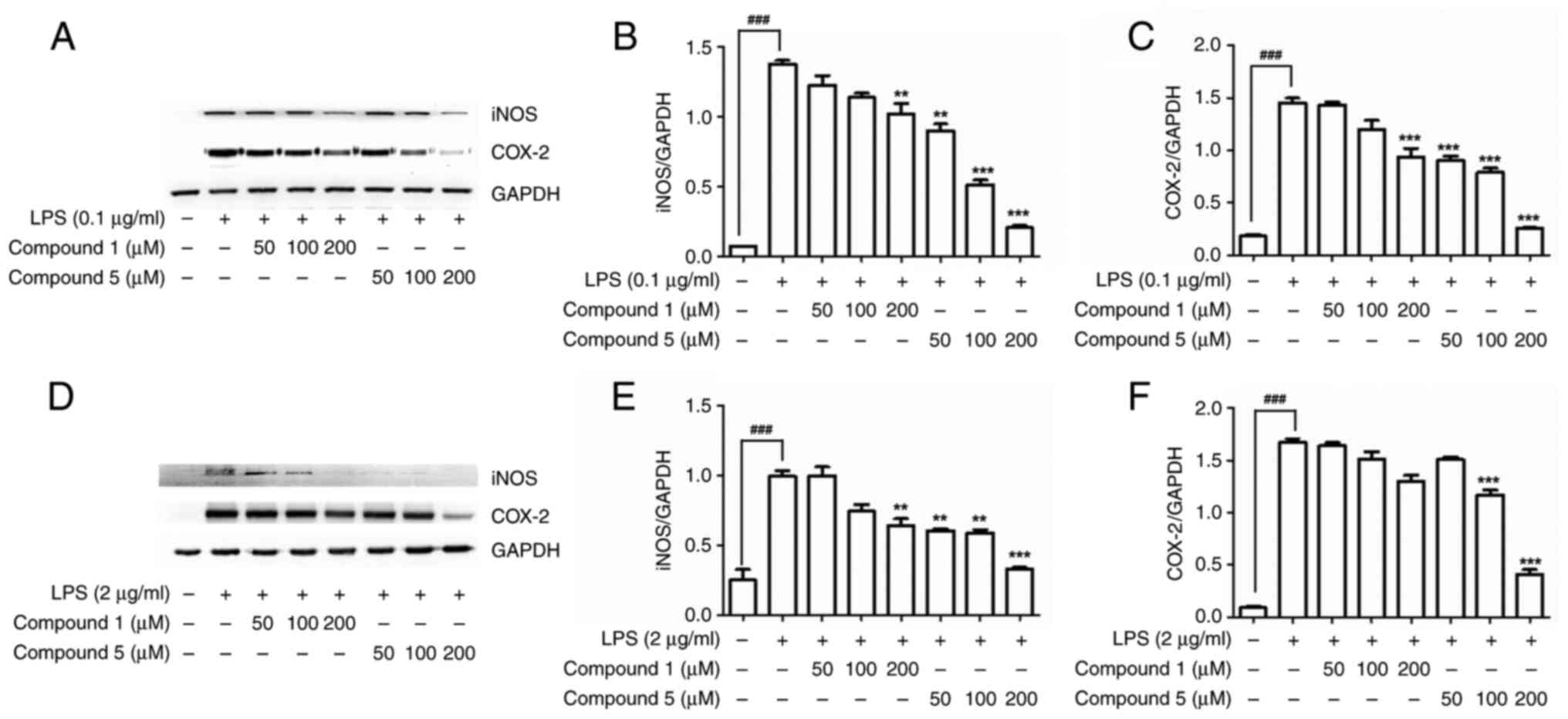
Effects of compound 5 on iNOS and
COX-2 protein expression
Within cells, iNOS and COX-2 are induced in
response to a range of stimuli, including pro-inflammatory
mediators, growth factors, oncogenes, carcinogens and tumor
promoters, thereby indicating that these two proteins play roles in
both inflammation and the control of cell growth (31,32).
Accordingly, for the development of new anti-inflammatory drugs, it
would be desirable to identify compounds that can inhibit both the
activity and expression of iNOS and COX-2 (33,34).
The present study found that, compared with nialamide (1), compound 5 (identified in this study
as the most potent inhibitor of NO and PGE2 production)
promoted a significant dose-dependent suppression of the expression
of iNOS and COX-2 in both LPS-stimulated RAW 264. (Fig. 2A-C) and canine DH82 (Fig. 2D-F) cells, thereby resulting in an
inhibition of the pro-inflammatory mediators NO and PGE2
(Fig. S9).
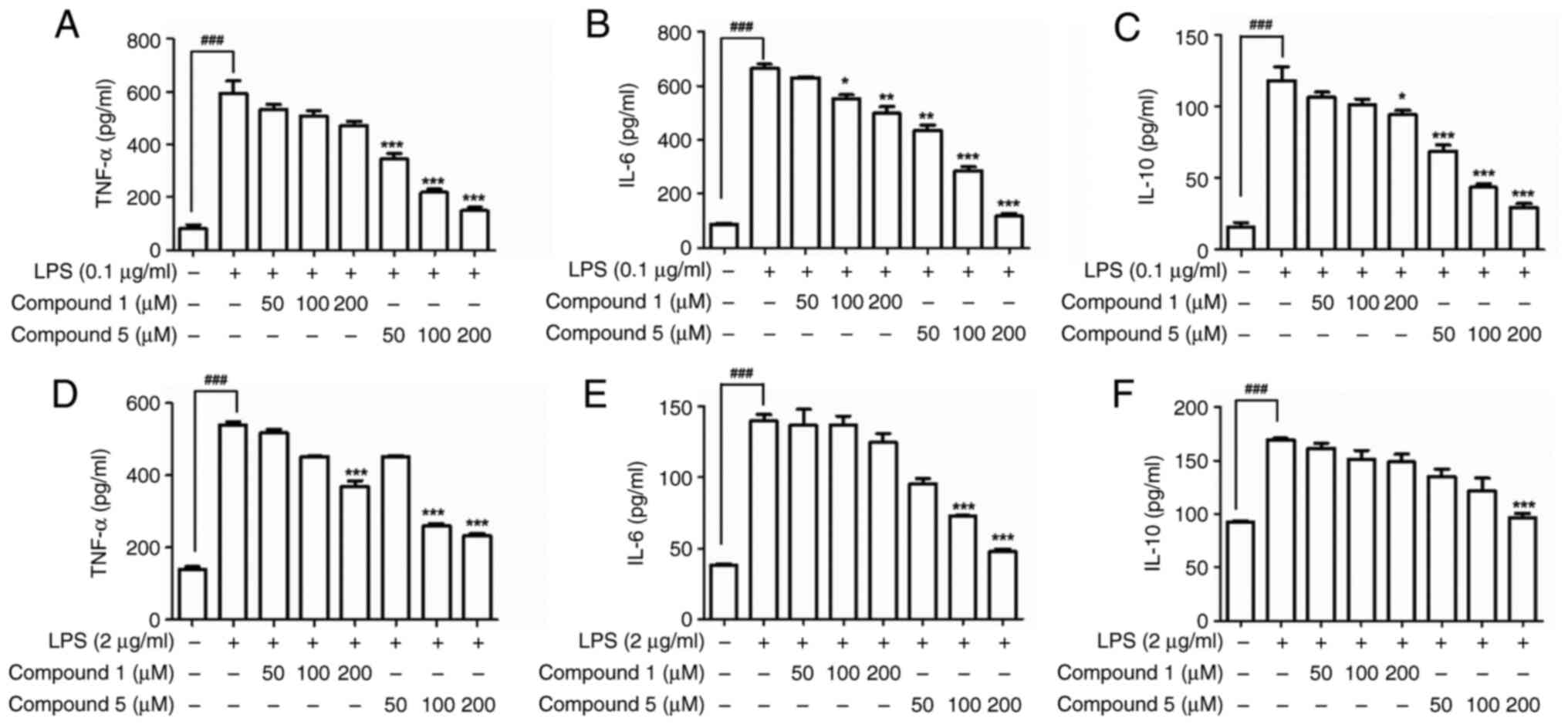
Effects of compound 5 on cytokine
production
Pro-inflammatory cytokines serve as key signaling
molecules in the immune system and thereby play important roles in
inflammatory responses (35).
However, an excessive release of pro-inflammatory cytokines such as
TNF-α and IL-6 can lead to damage to the epithelial barrier,
initiate epithelial cell apoptosis and induce inflammation
(36,37). Several studies have reported that
the anti-inflammatory activities of benzylamide derivatives are
associated with the control of the levels of diverse
pro-inflammatory cytokines (35,38).
In macrophages, exposure to LPS can induce the upregulation of
IL-10, a well-established anti-inflammatory cytokine, by promoting
the activation of the p38, JNK and ERK1/2 MAPK signaling pathways,
which typically lead to the phosphorylation and activation of the
Sp1 transcription factor and, in turn, expression of the IL-10
gene. The analysis in the present study of the inhibitory effects
of compound 5 on the production of the cytokines TNF-α, IL-6 and
IL-10 in LPS-induced macrophages revealed that, compared with the
original nialamide, product 5 was characterized by a potent
dose-dependent suppression of all three of these cytokines in
LPS-stimulated RAW 264.7 cells, with similar effects being detected
in LPS-stimulated DH82 macrophages (Fig. 3D-F).
Radical scavenging activity
ROS are key regulators of inflammatory responses,
particularly the activation of the transcription factor NF-κB and
its downstream signals (39).
However, given their high reactivity, when generated in excess, ROS
can have detrimental effects, particularly those associated with
peroxidation and oxidative damage to normal DNA and proteins
(40). Several studies have
nevertheless reported that modified drugs can contribute to
reductions in ROS production and enhance antioxidant activity
(41,42). To investigate the inhibitory
effects of major product 5 on ROS generation, LPS-activated RAW
264.7 macrophages were stained with DCFH-DA and fluorescence
microscopy observations performed. Treatment with compound 5 was
found to effectively suppress ROS production in a dose-dependent
manner (Fig. 4A and B). Previous study have demonstrated that
LPS-stimulated ROS generation is associated with NF-κB activation
(43) and, consistently, in the
present study, significant increases in the phosphorylation levels
of NF-κB and IκB in the treated RAW 264.7 cells were detected
(Fig. S10). By contrast,
treatment with compound 5 was found to promote a dose-dependent
inhibition of the LPS-stimulated phosphorylation of NF-κB (Fig. 4C and D). Moreover, compared with cells treated
with the parent compound nialamide, an enhancement of DPPH radical
and ABTS+ scavenging activities were detected in those
cells treated with compound 5 (Fig.
4E and F). Collectively, these
findings thus provide evidence to indicate that the γ
irradiation-mediated modification of nialamide contributes to
enhancing the anti-inflammatory properties of this drug.
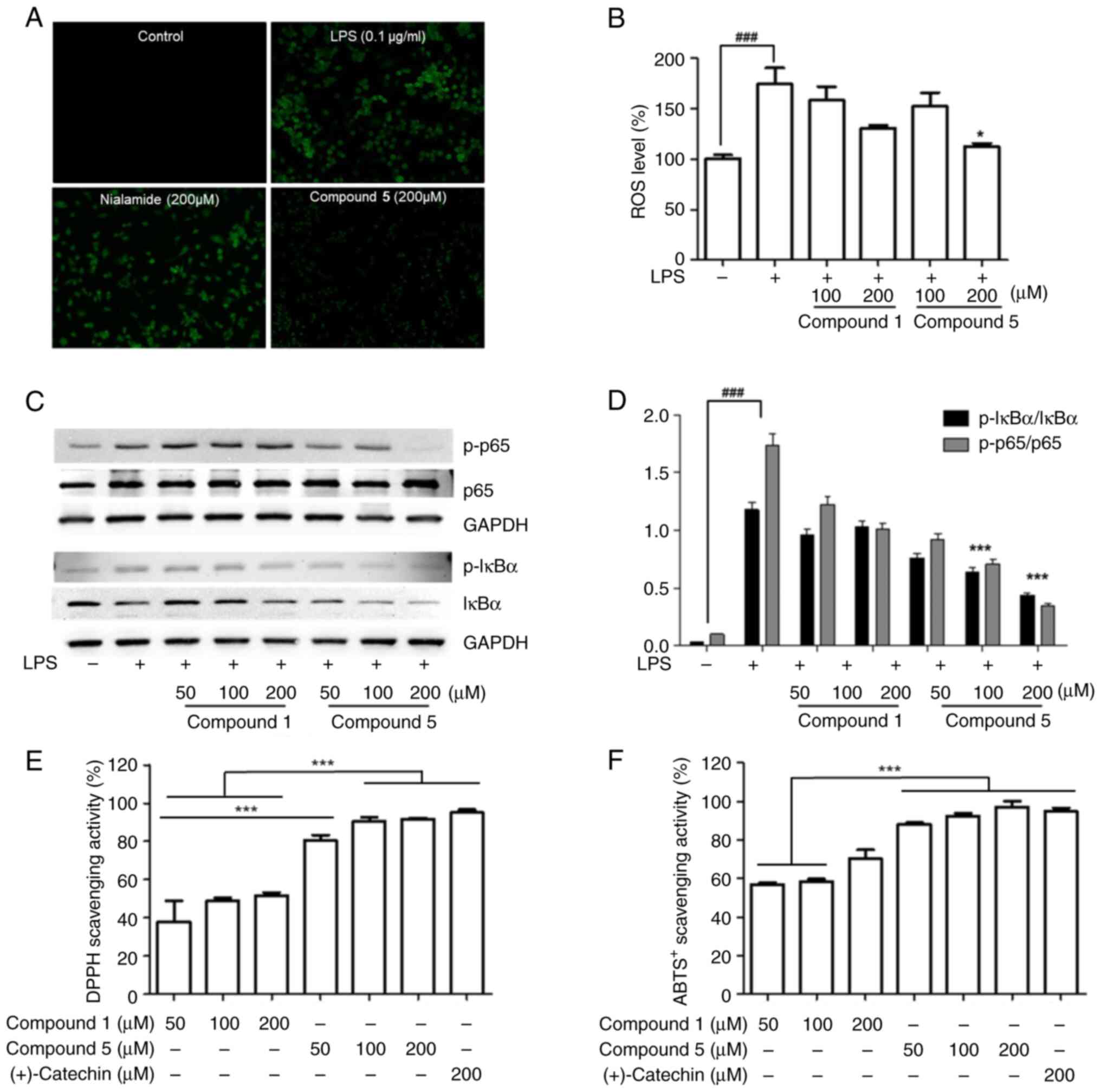 | Figure 4Effects of major product 5 on the
radical scavenging activity and ROS production of LPS-activated RAW
264.7 cells. RAW 264. cells were pretreated with compounds for 1 h
prior to incubation with LPS (0.1 µg/ml) for 24 h. (A) Cells were
incubated for 24 h with 50 µM DCFH-DA, washed twice with PBS and
incubated with LPS in the presence or absence of compound 5
(magnification, x10). (B) Cells were incubated with DCFH-DA for 45
min and the DCF fluorescence was measured at 492 nm (excitation)
and 520 nm (emission) for 30 min. (C) The protein levels of p-IκBα,
IκBα, p-p65 and p65 in cell lysates were analyzed by western
blotting. GAPDH was used as a loading control. (D) Western blotting
data are shown as representative values of three independent
experiments. (E) DPPH and (F) ABTS+ radical scavenging
activities of compound 5. The results are expressed as the
mean±standard deviation (n=3). ###P<0.001 vs. the
control group. *P<0.05 and ***P<0.001
vs. the LPS-induced group. ROS, reactive oxygen species; LPS,
lipopolysaccharide; DCFH-DA, 7'-dichlorofluorescein diacetate; PBS,
phosphate-buffered saline; p-, phosphorylated; DPPH,
1,1-diphenyl-2-picrylhydrazyl; ABTS+,
3-ethylbenzothiazoline-6-sulphonic acid; 1, nialamide;
5,3-hydroxy-N-benzylpropanamide. |
Discussion
Exposure to ionizing radiation, which generates a
range of free radicals including hydroxymethyl
(•CH2OH), hydroxyl (•OH), methoxy
(CH3O•) and peroxyl (HOO•)
radicals, is an effective approach for the incremental modification
of drugs that is more rapid and safer than conventional synthetic
methods and can be used to obtain higher product yields (44). For example, studies have reported
the enhanced anti-inflammatory capacities of natural flavonoids,
both in vitro and in vivo, following modification
using ionizing radiation, along with the isolation and
characterization of the corresponding modified products (45,46).
Similarly, radiolytic modification of hydrazine-based nomifensine
has been demonstrated to enhance the anticancer effects of this
drug in treated breast cancer cells (7). Radiolytic radicals are powerful
modifying agents that can initiate a range of modification
reactions in lead compounds during irradiation (47). The findings in the present study
indicate that under methanolic conditions, γ irradiation-induced
ROS and free radicals can contribute to the degradation,
deamination, methoxylation and hydroxylation of nialamide,
resulting in the generation of lower molecular weight amide
derivatives, such as nialaminosin (2), 3-amino-N-benzylpropanamide (3), 3-methoxy-N-benzylpropanamide
(4), HBPA (5), N-benzylpropanamide (6) and isonicotinamide (7). Compared with the parent nialamide
(1), the major
irradiation-modified product HBPA was found to be characterized by
enhanced anti-inflammatory effects in LPS-stimulated RAW 264.7 and
DH82 macrophages, involving marked suppression of the expression of
NO, PGE2, iNOS, COX-2 and the pro-inflammatory cytokines
TNF-α, IL-6 and IL-10, with negligible cytotoxicity toward either
of these cell lines. Treating RAW 264.7 macrophages with LPS
induced ROS production, which was effectively inhibited by the
γ-irradiated derivative of compound 5. This reduction in ROS
probably contributed to the significant downregulation of NF-κB and
IκB phosphorylation observed, suggesting newly generated product 5
from irradiated nialamide potential anti-inflammatory activity
through suppressing the NF-κB-mediated inflammatory signaling
pathway. To further assess its antioxidant properties, the present
study evaluated its DPPH and ABTS+ radical scavenging
abilities, as scavenging these radicals mimics the removal of
harmful free radicals found in vivo. While no control group
was included for compound 5, (+)-catechin, a known radical
scavenger, was utilized as a positive control to ensure accurate
measurement. Notably, (+)-catechin was excluded from the ROS and
NF-κB assays to avoid potential interference. Additionally,
compound 5 markedly suppressed the expression of NF-κB, the key
regulator of pro-inflammatory mediators such as iNOS, COX-2, TNF-α
and IL-6, in stimulated mouse macrophages. These synergistic
anti-inflammatory effects suggested that compound 5 holds promising
application potential not only in companion animal drugs but also
in broader therapeutic development. Further research is needed to
explore its full range of potential uses and optimize its
production process.
Positive controls were not included in all cell
experiments due to the focus on comparing the relative activity of
modified compounds. The present study compared nialamide and
modified products to both untreated and LPS-treated control groups.
This internal comparison strategy provided sufficient information
for assessing the effectiveness of the modifications without
introducing the potential variability associated with an
imperfectly matched positive control. While this approach does not
provide absolute activity values, it offers a robust and relevant
perspective within the context of our research goals. Additionally,
in research on improving the functionality of natural products or
abandoned pharmaceuticals, a number of researchers choose not to
use positive controls, instead focusing on comparing the activity
of the modified compound with the parent compound (6,7,44,45).
The present study focused on unlocking the hidden potential of
nialamide, a drug withdrawn from the antidepressant market. By
delving into its unexplored anti-inflammatory capabilities, it is
hoped to develop novel therapeutic options for inflammatory
diseases. This could potentially lead to safer and more effective
treatments with established safety profiles. However, despite the
recent developments in research on the application of γ irradiation
technology for repurposing withdrawn pharmaceuticals, concerns
still remain regarding the potential changes in toxicity and side
effects of irradiation-modified products and data pertaining to the
original drugs may not be strictly applicable to the modified
versions. Accordingly, it is plan to conduct comprehensive animal
studies to assess the safety profiles of modified drugs generated
using γ radiation.
In conclusion, given its adverse side effects, the
antidepressant drug nialamide has been withdrawn from the
pharmaceutical industry. The present study investigated the
structural modification of nialamide using ionizing irradiation and
characterized the anti-inflammatory properties of the
irradiation-modified products. Nialamide can be readily modified to
yield the new compounds nialaminosin,
3-amino-N-benzylpropanamide,
3-methoxy-N-benzylpropanamide, HBPA,
N-benzylpropanamide, and isonicotinamide, the chemical
structures of which were determined using one- and two-dimensional
NMR analysis, as well as HRESIMS spectral data interpretation.
Among these compounds, it was found that, compared with the parent
nialamide, the hydroxyl-substituted benzylamide derivative was
characterized by a more pronounced anti-inflammatory activity
against the production of NO and PGE2 in LPS-stimulated
macrophages. Moreover, this compound was found to reduce the
expression of iNOS and COX-2 proteins, suppress cytokine production
and exhibit potent antioxidant activity. Collectively, the findings
in the present study indicated that the modification of
hydrazine-based benzylamide drugs using ionizing radiation could
provide a unique approach to the semi-synthesis of hydroxylated
benzylpropanamide compounds with enhanced anti-inflammatory
capacity in both humans and companion animals. It was considered
that by enhancing drug bioactivity and safety, the application of
radiation technology for the modification of withdrawn drugs could
provide a valuable novel approach for future drug discovery and
development.
Supplementary Material
HPLC chromatograms of the isolated
compounds 2-6 and nialamide (1).
HPLC, high-performance liquid chromatography; 1 nialamide; 2,
nialamionsin; 3,3-amino-N-benzylpropanamide;
4,3-methoxy-N-benzylpropanamide;
5,3-hydroxy-N-benzylpropanamide; 6,
N-benzylpropanamide.
1H NMR spectrum of compound
2 in DMSO-d6. NMR, nuclear magnetic resonance; 2,
nialamionsin; ppm, parts per million.
13C NMR spectrum of compound 2
in DMSO-d6. NMR, nuclear magnetic resonance; 2,
nialamionsin; ppm, parts per million.
1H-1H COSY spectrum of
compound 2 in DMSO-d6. COSY, correlated
spectroscopy; 2, nialamionsin; ppm, parts per million.
HSQC spectrum of compound 2 in
DMSO.d6. HSQC, heteronuclear single quantum coherence; 2,
nialamionsin; ppm, parts per million.
HMBC spectrum of compound 2 in
DMSO-d6. HMBC, heteronuclear multiple bond
correlation; 2, nialamionsin; ppm, parts per million.
NOESY spectrum of compound 2 in
DMSO-d6. NOESY, nuclear Overhauser effect
spectroscopy; 2, nialamionsin; ppm, parts per million.
HRESIMS spectrum of compound 2.
HRESIMS, high-resolution electrospray ion mass spectrum; 2,
nialamionsin; ppm, parts per million.
Full-length western blotting for iNOS
and COX-2 of compounds 1 and 5 in LPS-stimulated RAW264.7 and DH82
cells iNOS, inducible NO synthase; COX, cyclooxygenase; LPS,
lipopolysaccharide; 1, nialamide; 5,
3-hydroxy-N-benzylpropanamide.
Full-length western blotting for
p-NF-κB and p-IκB of compounds 1 and 5 in LPS-stimulated RAW264.7
cell. p-, phosphorylated; 1, nialamide; 5,
3-hydroxy-N-benzylpropanamide; LPS, lipopolysaccharide.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Convergence Research
Group project (grant no. CRC21022-300) of the National Research
Council of Science and Technology, Republic of Korea.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HL, SYW, and HJC were involved in conceptualization
and design of the methodology. GHJ and BYC performed the
experiments. HL, KBL and HWB contributed to data curation. HL, GHJ
and KBL analyzed the data. HL wrote the original draft. GHJ, KBL
and HWB reviewed and edited the manuscript. HL and GHJ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bak DH, Kang SH, Park CH, Chung BY and Bai
HW: A novel radiolytic rotenone derivative, rotenoisin A, displays
potent anticarcinogenic activity in breast cancer cells. J Radiat
Res. 62:249–258. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lee EH, Park CH, Choi HJ, Kawala RA, Bai
HW and Chung BY: Dexamethasone modified by gamma-irradiation as a
novel anticancer drug in human non-small cell lung cancer. PLoS
One. 13(e0194341)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim TH, Kim JK, Ito H and Jo C:
Enhancement of pancreatic lipase inhibitory activity of curcumin by
radiolytic transformation. Bioorg Med Chem Lett. 21:1512–1514.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Han Jeong G, Cho JH, Jo C, Lee S, Sik Lee
S, Bai HW, Chung BY and Hoon Kim T: Gamma irradiation-assisted
degradation of rosmarinic acid and evaluation of structures and
anti-adipogenic properties. Food Chem. 258:181–188. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu X, Jeong SM, Lee JE, Kang WS, Ryu SH,
Kim K, Byun EH, Cho YJ and Ahn DH: Characterization of undaria
pinnatifida root enzymatic extracts using crude enzyme from
shewanella oneidensis PKA 1008 and its anti-inflammatory
effect. J Microbiol Biotechnol. 30:79–84. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Byun EB, Sung NY, Park JN, Yang MS, Park
SH and Byun EH: Gamma-irradiated resveratrol negatively regulates
LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages.
Int Immunopharmacol. 25:249–259. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kang SH, Bak DH, Yeoup Chung B and Bai HW:
Transformation of nomifensine using ionizing radiation and
exploration of its anticancer effects in MCF-7 cells. Exp Ther Med.
23(306)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Association AVM. US pet ownership and
demographics sourcebook, 2012.
|
|
9
|
Nieforth LO and O'Haire ME: The role of
pets in managing uncertainty from COVID-19. Psychol Trauma.
12:S245–S246. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Forrester SJ, Kikuchi DS, Hernandes MS, Xu
Q and Griendling KK: Reactive oxygen species in metabolic and
inflammatory signaling. Circ Res. 122:877–902. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sharma JN, Al-Omran A and Parvathy SS:
Role of nitric oxide in inflammatory diseases.
Inflammopharmacology. 15:252–259. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ji JD, Lee YH and Song GG: Prostaglandin
E2 (PGE2): Roles in immune responses and
inflammation. J Korean Rheum Assoc. 11:307–316. 2004.(In
Korean).
|
|
13
|
Ostadkarampour M and Putnins EE: Monoamine
oxidase inhibitors: A review of their anti-inflammatory therapeutic
potential and mechanisms of action. Front Pharmacol.
12(676239)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bortolato M and Shih JC: Behavioral
outcomes of monoamine oxidase deficiency: Preclinical and clinical
evidence. Int Rev Neurobiol. 100:13–42. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wimbiscus M, Kostenko O and Malone D: MAO
inhibitors: Risks, benefits and lore. Cleve Clin J Med. 77:859–882.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu Y, Feng S, Subedi K and Wang H:
Attenuation of ischemic stroke-caused brain injury by a monoamine
oxidase inhibitor involves improved proteostasis and reduced
neuroinflammation. Mol Neurobiol. 57:937–948. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lougheed KE, Taylor DL, Osborne SA, Bryans
JS and Buxton RS: New anti-tuberculosis agents amongst known drugs.
Tuberculosis (Edinb). 89:364–370. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stockert JC, Blázquez-Castro A, Cañete M,
Horobin RW and Villanueva Á: MTT assay for cell viability:
Intracellular localization of the formazan product is in lipid
droplets. Acta Histochemica. 114:785–796. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun J, Zhang X, Broderick M and Fein H:
Measurement of nitric oxide production in biological systems by
using griess reaction assay. Sensors. 3:276–284. 2003.
|
|
20
|
Rosenkranz AR, Schmaldienst S, Stuhlmeier
KM, Chen W, Knapp W and Zlabinger GJ: A microplate assay for the
detection of oxidative products using
2',7'-dichlorofluorescin-diacetate. J Immunol Methods. 156:39–45.
1992.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Blois MS: Antioxidant determinations by
the use of a stable free radical. Nature. 181:1199–1200. 1958.
|
|
22
|
Re R, Pellegrini N, Proteggente A, Pannala
A, Yang M and Rice-Evans C: Antioxidant activity applying an
improved ABTS radical cation decolorization assay. Free Radic Biol
Med. 26:1231–1237. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pratt JA, Sutherland IO and Newton RF:
Macrocyclic and macropolycyclic compounds based upon 1,
3-disubstituted propane units. J Chem Soc Perkin. 1:13–22.
1988.
|
|
24
|
Zhao R, Zeng BL, Jia WQ, Zhao HY, Shen LY,
Wang XJ and Pan XD: LiCl-promoted amination of β-methoxy amides
(γ-lactones). RSC Adv. 10:34938–34942. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vaidyanathan G and Wilson JW: Reaction of
cyclopropanamines with hypochlorite. J Org Chem. 54:1815–1820.
1989.
|
|
26
|
Johnson DC II and Widlanski TS: A
reversible safety-catch method for the hydrogenolysis of N-benzyl
moieties. Tetrahedron Lett. 45:8483–8487. 2004.
|
|
27
|
Liu Y, Zhou C, Jiang M and Arndtsen BA:
Versatile palladium-catalyzed approach to acyl fluorides and
carbonylations by combining visible light-and ligand-driven
operations. J Am Chem Soc. 144:9413–9420. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dhore J, Pethe GB, Wagh SP and Thorat G:
Synthesis, characterization and biological studies of some
triazolyl isonicotinamide. Arch Appl Sci Res. 3:407–414. 2011.
|
|
29
|
Gomez I, Foudi N, Longrois D and Norel X:
The role of prostaglandin E2 in human vascular inflammation.
Prostaglandins Leukot Essent Fatty Acids. 89:55–63. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tripathi P, Tripathi P, Kashyap L and
Singh V: The role of nitric oxide in inflammatory reactions. FEMS
Immunol Med Microbiol. 51:443–452. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Minghetti L: Cyclooxygenase-2 (COX-2) in
inflammatory and degenerative brain diseases. J Neuropathol Exp
Neurol. 63:901–910. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ahmad N, Ansari MY and Haqqi TM: Role of
iNOS in osteoarthritis: Pathological and therapeutic aspects. J
Cell Physiol. 235:6366–6376. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sacco RE, Waters WR, Rudolph KM and Drew
ML: Comparative nitric oxide production by LPS-stimulated
monocyte-derived macrophages from ovis canadensis and ovis aries.
Comp Immunol Microbiol Infect Dis. 29:1–11. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kim HS, Ye SK, Cho IH, Jung JE, Kim DH,
Choi S, Kim YS, Park CG, Kim TY, Lee JW and Chung MH:
8-hydroxydeoxyguanosine suppresses NO production and COX-2 activity
via Rac1/STATs signaling in LPS-induced brain microglia. Free Radic
Biol Med. 41:1392–1403. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Watkins LR, Maier SF and Goehler LE:
Immune activation: The role of pro-inflammatory cytokines in
inflammation, illness responses and pathological pain states. Pain.
63:289–302. 1995.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Allen MJ, Myer BJ, Khokher AM, Rushton N
and Cox TM: Pro-inflammatory cytokines and the pathogenesis of
Gaucher's disease: Increased release of interleukin-6 and
interleukin-10. QJM. 90:19–25. 1997.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Holm S, Mackiewicz Z, Holm AK, Konttinen
YT, Kouri VP, Indahl A and Salo J: Pro-inflammatory, pleiotropic
and anti-inflammatory TNF-alpha, IL-6, and IL-10 in experimental
porcine intervertebral disk degeneration. Vet Pathol. 46:1292–1300.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Aroonrerk N, Niyomtham N and
Yingyoungnarongkul BE: Anti-inflammation of
N-benzyl-4-bromobenzamide in lipopolysaccharide-induced human
gingival fibroblasts. Med Princ Pract. 25:130–136. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Di Meo S, Reed TT, Venditti P and Victor
VM: Role of ROS and RNS sources in physiological and pathological
conditions. Oxid Med Cell Longev. 2016(1245049)2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Marnett LJ: Oxyradicals and DNA damage.
Carcinogenesis. 21:361–370. 2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xu D, Hu MJ, Wang YQ and Cui YL:
Antioxidant activities of quercetin and its complexes for medicinal
application. Molecules. 24(1123)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen F, Huang G, Yang Z and Hou Y:
Antioxidant activity of momordica charantia polysaccharide and its
derivatives. Int J Biol Macromol. 138:673–80. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bubici C, Papa S, Pham CG, Zazzeroni F and
Franzoso G: The NF-kappaB-mediated control of ROS and JNK
signaling. Histol Histopathol. 21:69–80. 2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Byun EB, Jang BS, Byun EH and Sung NY:
Effect of γ irradiation on the change of solubility and
anti-inflammation activity of chrysin in macrophage cells and
LPS-injected endotoxemic mice. Radiat Phys Chem. 127:276–285.
2016.
|
|
45
|
Byun EB, Jang BS, Kim HM, Yang MS, Sung NY
and Byun EH: Gamma irradiation enhanced tollip-mediated
anti-inflammatory action through structural modification of
quercetin in lipopolysaccharide-stimulated macrophages. Int
Immunopharmacol. 42:157–167. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee SS, Lee EM, An BC, Kim TH, Lee KS, Cho
JY, Yoo SH, Bae JS and Chung BY: Effects of irradiation on
decolourisation and biological activity in Schizandra
chinensis extracts. Food Chem. 125:214–220. 2011.
|
|
47
|
An T, Gao Y, Li G, Kamat PV, Peller J and
Joyce MV: Kinetics and mechanism of (•)OH mediated degradation of
dimethyl phthalate in aqueous solution: Experimental and
theoretical studies. Environ Sci Technol. 48:641–648.
2014.PubMed/NCBI View Article : Google Scholar
|