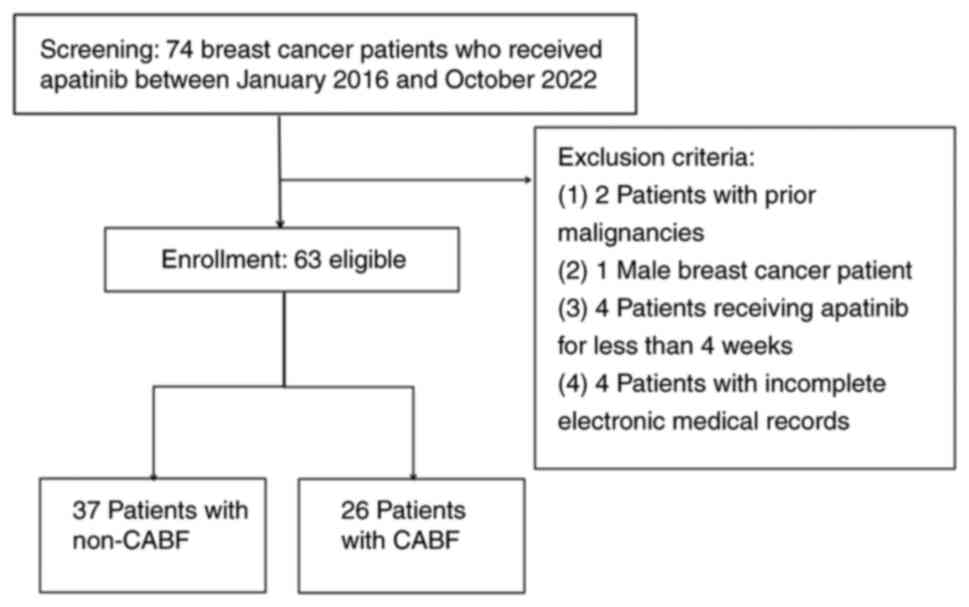
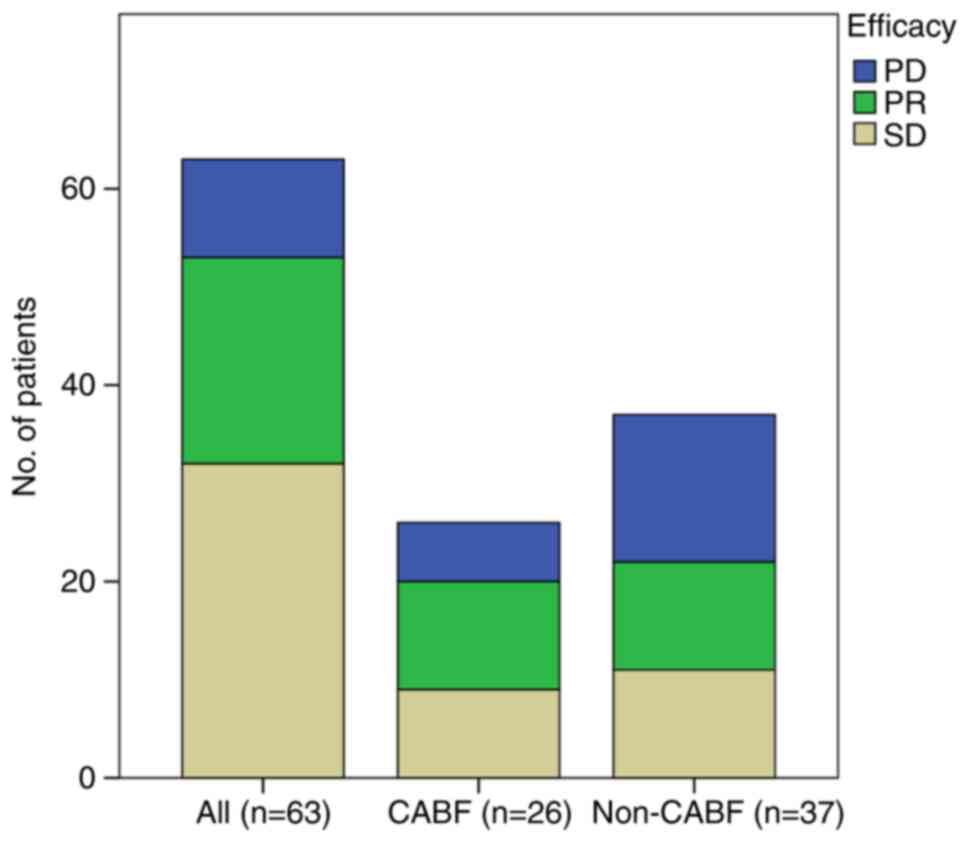
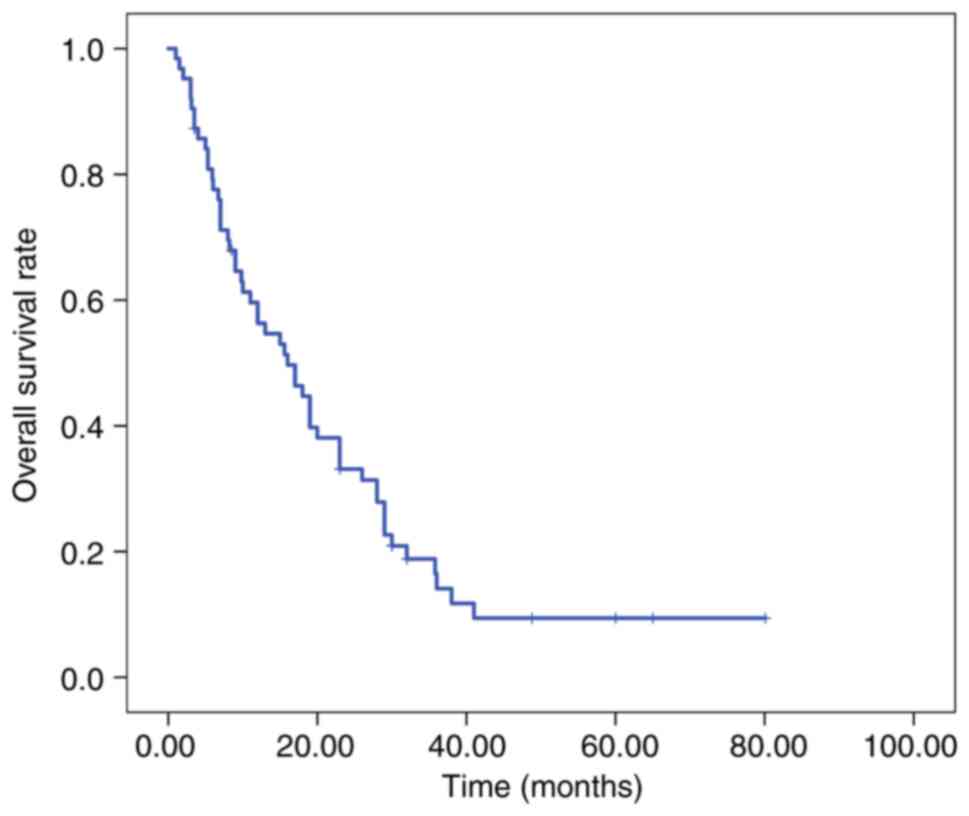
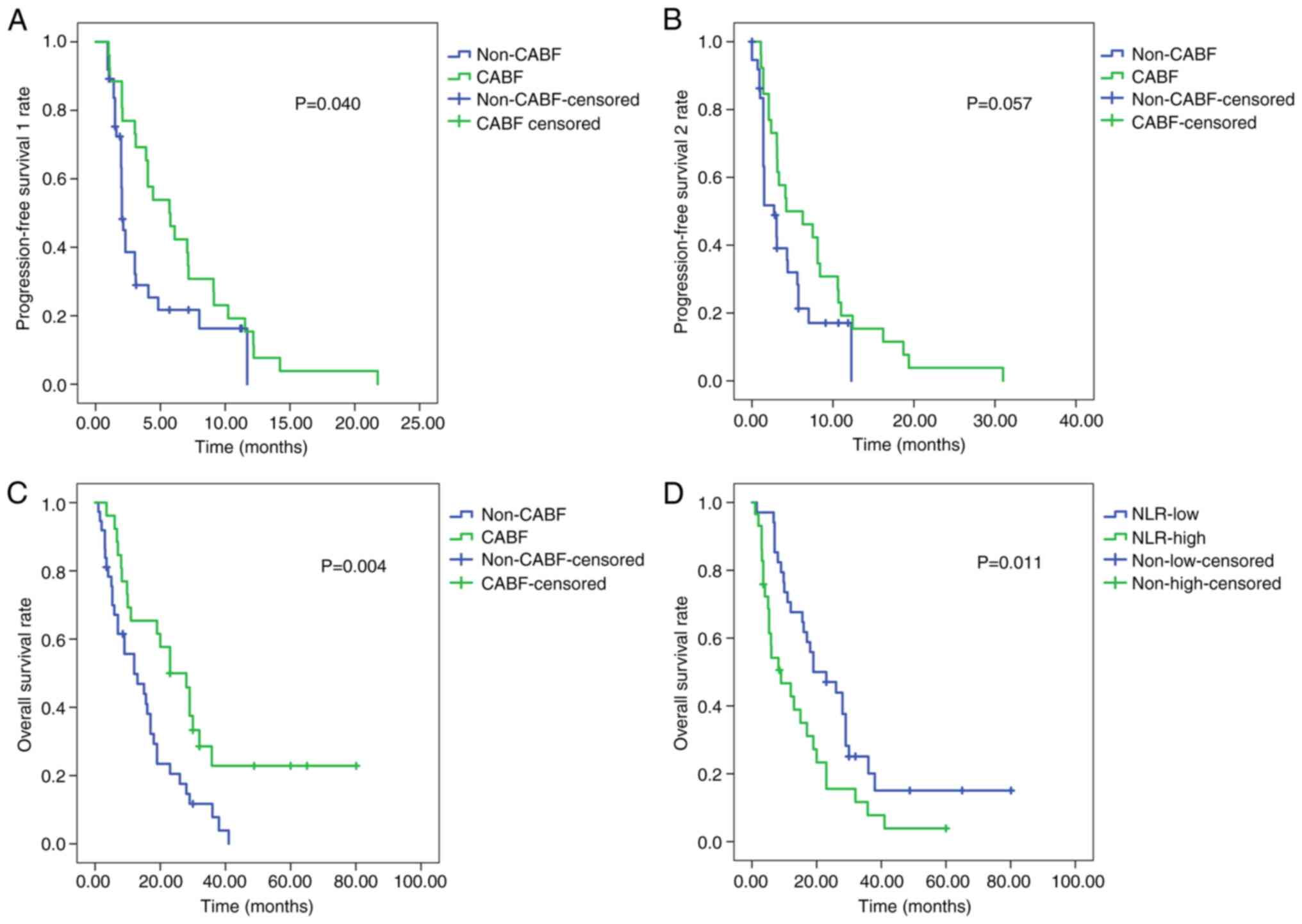
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jiménez-Valerio G and Casanovas O:
Angiogenesis and metabolism: Entwined for therapy resistance.
Trends Cancer. 3:10–18. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roda N, Blandano G and Pelicci PG: Blood
vessels and peripheral nerves as key players in cancer progression
and therapy resistance. Cancers (Basel). 13(4471)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Laborda-Illanes A, Sánchez-Alcoholado L,
Castellano-Castillo D, Boutriq S, Plaza-Andrades I, Aranega-Martín
L, Peralta-Linero J, Alba E, González-González A and Queipo-Ortuño
MI: Development of in vitro and in vivo tools to evaluate the
antiangiogenic potential of melatonin to neutralize the angiogenic
effects of VEGF and breast cancer cells: CAM assay and 3D
endothelial cell spheroids. Biomed Pharmacother.
157(114041)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hervé MA, Buteau-Lozano H, Vassy R, Bieche
I, Velasco G, Pla M, Perret G, Mourah S and Perrot-Applanat M:
Overexpression of vascular endothelial growth factor 189 in breast
cancer cells leads to delayed tumor uptake with dilated
intratumoral vessels. Am J Pathol. 172:167–178. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang L, Wei Y, Shen S, Shi Q, Bai J, Li
J, Qin S, Yu H and Chen F: Therapeutic effect of apatinib on
overall survival is mediated by prolonged progression-free survival
in advanced gastric cancer patients. Oncotarget. 8:29346–29354.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jain RK: Antiangiogenesis strategies
revisited: From starving tumors to alleviating hypoxia. Cancer
Cell. 26:605–622. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu Z, Shan J, Yu Q, Wang X, Song X, Wang
F, Li C, Yu Z and Yu J: Real-World data on apatinib
efficacy-results of a retrospective study in metastatic breast
cancer patients pretreated with multiline treatment. Front Oncol.
11(643654)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cao M, Lu H, Yan S, Pang H, Sun L, Li C,
Chen X, Liu W, Hu J, Huang J, et al: Apatinib plus etoposide in
pretreated patients with advanced triple-negative breast cancer: A
phase II trial. BMC Cancer. 23(463)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu J, He M, Ou K, Wang X, Wang Y, Qi L,
Chai Y, Jiang M, Ma F, Luo Y, et al: Efficacy and safety of
apatinib combined with dose-dense paclitaxel and carboplatin in
neoadjuvant therapy for locally advanced triple-negative breast
cancer: A prospective cohort study with propensity-matched
analysis. Int J Cancer. 154:133–144. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mastri M, Tracz A, Lee CR, Dolan M,
Attwood K, Christensen JG, Liu S and Ebos J: A transient
pseudosenescent secretome promotes tumor growth after
antiangiogenic therapy withdrawal. Cell Rep. 25:3706–3720.e8.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: NCCN guidelines insights breast cancer, version
1.2016. J Natl Compr Canc Netw. 13:1475–1485. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miller TP, Fisher BT, Getz KD, Sack L,
Razzaghi H, Seif AE, Bagatell R, Adamson PC and Aplenc R:
Unintended consequences of evolution of the Common Terminology
Criteria for Adverse Events. Pediatr Blood Cancer.
66(e27747)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Harry JA and Ormiston ML: Novel pathways
for targeting tumor angiogenesis in metastatic breast cancer. Front
Oncol. 11(772305)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Miles D, Cameron D, Bondarenko I, Manzyuk
L, Alcedo JC, Lopez RI, Im SA, Canon JL, Shparyk Y, Yardley DA, et
al: Bevacizumab plus paclitaxel versus placebo plus paclitaxel as
first-line therapy for HER2-negative metastatic breast cancer
(MERiDiAN): A double-blind placebo-controlled randomised phase III
trial with prospective biomarker evaluation. Eur J Cancer.
70:146–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zielinski C, Láng I, Inbar M, Kahán Z,
Greil R, Beslija S, Stemmer SM, Zvirbule Z, Steger GG, Melichar B,
et al: Bevacizumab plus paclitaxel versus bevacizumab plus
capecitabine as first-line treatment for HER2-negative metastatic
breast cancer (TURANDOT): Primary endpoint results of a randomised,
open-label, non-inferiority, phase 3 trial. Lancet Oncol.
17:1230–1239. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Masuda N, Takahashi M, Nakagami K, Okumura
Y, Nakayama T, Sato N, Kanatani K, Tajima K and Kashiwaba M:
First-line bevacizumab plus paclitaxel in Japanese patients with
HER2-negative metastatic breast cancer: Subgroup results from the
randomized Phase III MERiDiAN trial. Jpn J Clin Oncol. 47:385–392.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rossari JR, Metzger-Filho O, Paesmans M,
Saini KS, Gennari A, de Azambuja E and Piccart-Gebhart M:
Bevacizumab and Breast Cancer: A meta-analysis of first-line phase
III studies and a critical reappraisal of available evidence. J
Oncol. 2012(417673)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li Z, Guo F, Han Y, Wang J and Xu B:
Efficacy and safety of bevacizumab in pretreated metastatic breast
cancer: A systematic review and meta-analysis. Oncol Res Treat.
45:608–617. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang N, Chen S, Liu D, Guo J, Sun Y, Zhang
J, Kong Q and He L: Therapeutic effect of small molecule targeting
drug apatinib on gastric cancer and its role in prognosis and
anti-infection mechanism. Saudi J Biol Sci. 27:606–610.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zeng T, Sun C, Liang Y, Yang F, Yan X, Bao
S, Zhang Y, Huang X, Fu Z, Li W and Yin Y: A real-world multicentre
retrospective study of low-dose apatinib for human epidermal growth
factor receptor 2-negative metastatic breast cancer. Cancers
(Basel). 14(4084)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li DD, Tao ZH, Wang BY, Wang LP, Cao J, Hu
XC and Zhang J: Apatinib plus vinorelbine versus vinorelbine for
metastatic triple-negative breast cancer who failed
first/second-line treatment: The NAN trial. NPJ Breast Cancer.
8(110)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ebos JM and Pili R: Mind the gap:
Potential for rebounds during antiangiogenic treatment breaks. Clin
Cancer Res. 18:3719–3721. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mastri M and Ebos JML: Tumor growth fueled
by spurious senescence phenotypes. Mol Cell Oncol.
6(1575707)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kuczynski EA, Sargent DJ, Grothey A and
Kerbel RS: Drug rechallenge and treatment beyond
progression-implications for drug resistance. Nat Rev Clin Oncol.
10:571–587. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ciruelos E, Pérez-García JM, Gavilá J,
Rodríguez A and de la Haba-Rodriguez J: Maintenance therapy in
HER2-Negative metastatic breast cancer: A new approach for an old
concept. Clin Drug Investig. 39:595–606. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vrdoljak E, Marschner N, Zielinski C,
Gligorov J, Cortes J, Puglisi F, Aapro M, Fallowfield L, Fontana A,
Inbar M, et al: Final results of the TANIA randomised phase III
trial of bevacizumab after progression on first-line bevacizumab
therapy for HER2-negative locally recurrent/metastatic breast
cancer. Ann Oncol. 27:2046–2052. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yi M, Jiao D, Qin S, Chu Q, Wu K and Li A:
Synergistic effect of immune checkpoint blockade and
anti-angiogenesis in cancer treatment. Mol Cancer.
18(60)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Noonan DM, De Lerma Barbaro A, Vannini N,
Mortara L and Albini A: Inflammation, inflammatory cells and
angiogenesis: Decisions and indecisions. Cancer Metastasis Rev.
27:31–40. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shaul ME and Fridlender ZG:
Tumour-associated neutrophils in patients with cancer. Nat Rev Clin
Oncol. 16:601–620. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xiang Y, Zhang N, Lei H, Wu J, Wang W,
Zhang H and Zeng X: Neutrophil-to-lymphocyte ratio is a negative
prognostic biomarker for luminal A breast cancer. Gland Surg.
12:415–425. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ethier JL, Desautels D, Templeton A, Shah
PS and Amir E: Prognostic role of neutrophil-to-lymphocyte ratio in
breast cancer: A systematic review and meta-analysis. Breast Cancer
Res. 19(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Guo W, Lu X, Liu Q, Zhang T, Li P, Qiao W
and Deng M: Prognostic value of neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio for breast cancer patients: An updated
meta-analysis of 17079 individuals. Cancer Med. 8:4135–4148.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang H, Wang Z, Hou Z, Yang X, Zhu K, Cao
M, Zhu X, Li H and Zhang T: The Neutrophil-to-Lymphocyte Ratio
(NLR) predicts the prognosis of unresectable intermediate and
advanced hepatocellular carcinoma treated with apatinib. Cancer
Manag Res. 13:6989–6998. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao
C, Jia Y, Shi J, Zhang L, Liu X, et al: Low-Dose apatinib optimizes
tumor microenvironment and potentiates antitumor effect of
PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res. 7:630–643.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kourea HP, Dimitrakopoulos FI, Koliou GA,
Batistatou A, Papadopoulou K, Bobos M, Asimaki-Vlachopoulou A,
Chrisafi S, Pavlakis K, Chatzopoulos K, et al: Clinical
significance of major angiogenesis-related effectors in patients
with metastatic breast cancer treated with trastuzumab-based
regimens. Cancer Res Treat. 54:1053–1064. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
He L, Shen X, Liu Y, Gao L, Wu J, Yu C, Li
G, Wang X and Shao X: The reversal of anti-HER2 resistance in
advanced HER2-positive breast cancer using apatinib: Two cases
reports and literature review. Transl Cancer Res. 11:4206–4217.
2022.PubMed/NCBI View Article : Google Scholar
|