Introduction
Breast cancer is the most common malignant tumor and
the fifth leading cause of cancer-associated mortality worldwide
(1). Although it is commonly
treated based on the molecular type according to various
guidelines, advanced breast cancer (ABC), including recurrent and
metastatic breast cancer, is incurable. Angiogenesis significantly
impacts the occurrence and development of cancer owing to the
ability of new blood vessels to deliver oxygen and nutrients to
tumor cells, promoting tumor growth, metastasis and invasion
(2,3). Therefore, inhibition of angiogenesis
can limit the growth and spread of tumors by preventing the
delivery of nutrients, thus starving the tumors (4). Various mouse models have demonstrated
that vascular endothelial growth factor (VEGF) induces breast
cancer cell proliferation (5).
VEGF and VEGF receptor 2 (VEGFR-2) are core participants in
pathological angiogenesis, and key targets for the development of
drugs against angiogenesis (6,7).
Apatinib, as a VEGFR2 inhibitor, has shown moderate efficacy in the
treatment of metastatic breast cancer, and is currently used as an
option for maintenance salvage therapy following failure of
treatment with multiple lines of treatment (8,9).
Apatinib combined with dose-dense paclitaxel and carboplatin
neoadjuvant therapy was previously shown to significantly improve
the pathological complete response rate to 60.9% in patients with
triple-negative breast cancer who underwent surgery (10). However, rebound tumor growth
occurred when VEGFR tyrosine kinase inhibitor (TKI) treatment was
discontinued after the acquisition of resistance, and this growth
was ultimately reversed after long-term anti-angiogenic treatment
withdrawal (11). Given the poor
prognosis of patients with ABC and the difficulty in the selection
of therapeutic regimens following failure of routine treatments,
the present study retrospectively analyzed the efficacy of
sustained apatinib treatment in patients with ABC in a real-world
setting to address the importance of anti-angiogenic treatment and
the feature of rebound tumor growth mediated by anti-angiogenic
treatment withdrawal.
Materials and methods
Patients and methods
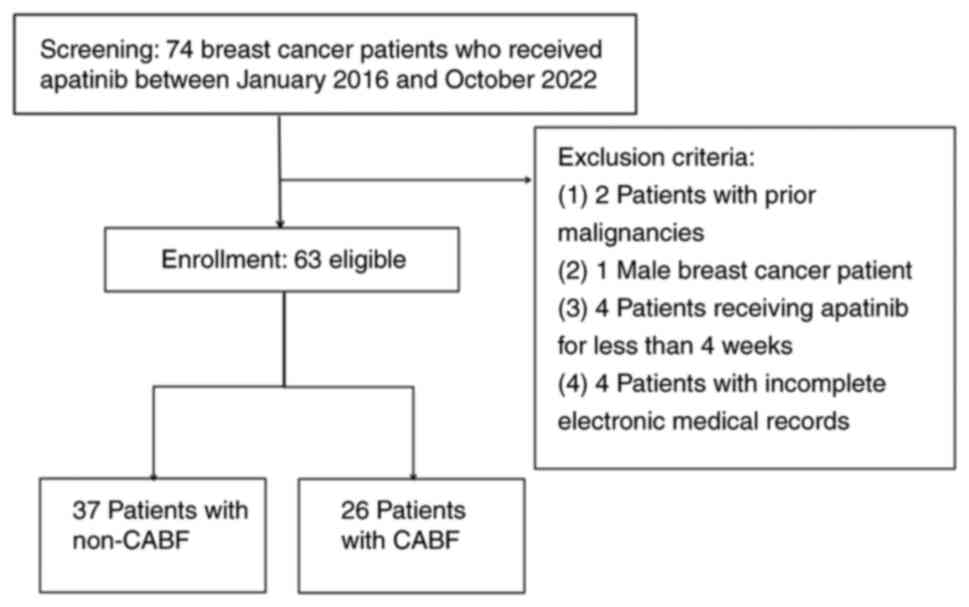
Between January 2016 and October 2022, 74 patients
with ABC who were treated with apatinib at Tangshan People's
Hospital (Tangshan, China) were screened for inclusion. All
patients received at least three lines of standard treatment
according to the National Comprehensive Cancer Network (NCCN)
guidelines (12). All patients
were administered oral apatinib combined with other therapies, such
as chemotherapeutic agents, endocrine therapy or targeted therapy.
The patients were divided into neutrophil-to-lymphocyte ratio
(NLR)-high and -low group, taking the median value of the NLR as
the cut off. All patients met the following inclusion criteria: i)
Pathologically diagnosed as having breast cancer; ii) underwent
multiple lines of line therapy (≥4 lines) according to the NCCN
guidelines; iv) had an Eastern Cooperative Oncology Group score of
1-3; v) were of clinical stage IV: vi) (13) possessed a measurable target lesion
based on the Response Evaluation Criteria in Solid Tumors (RECIST)
Version 1.1 criteria; vii) (14)
were treated with oral apatinib for >4 weeks; and viii) had
complete routine blood data available for at least 1 week before
apatinib treatment. The exclusion criteria were as follows: i)
Possessed another malignant tumor; and ii) had incomplete clinical
pathological or follow-up data.
Treatment and follow-up
Eligible patients who had received multiple lines of
treatment that had failed according to the NCCN guidelines received
apatinib orally at a dose of 250 mg daily (one cycle every 4
weeks). Symptomatic treatment was scheduled if a patient exhibited
grade II-III adverse events or above until the adverse events
remitted to grade 1 or less. Patients received oral apatinib as a
monotherapy or combination therapy, including chemotherapy,
endocrine therapy, targeted therapy. Certain patients were
continued on apatinib, but the combination of antitumor drugs was
changed upon first PD, while other patients were switched from the
apatinib and combined drug treatment to a different antitumor drug
regimen upon first PD; this choice was determined by the physicians
based on the patient's toxicity tolerance and the treatment's
short-term efficacy.
The primary endpoint was overall survival (OS),
calculated from the time of taking apatinib to either death from
any cause or the last follow-up. The progression-free survival
(PFS1), defined as the time from the beginning of apatinib to first
PD. Patients were continued on apatinib combined with other drugs
post-PD, such as etoposide, capecitabine, vinorelbine, gemcitabine,
albumin paclitaxel, targeted therapy and ulvestrant endocrine
therapy, with the aim of addressing persistent anti-angiogenic
effects. PFS2 was defined as the time between the first PD to the
second PD in next-line therapy or death from any cause. Patients
who were continued on apatinib combined with other drugs as a
next-line treatment post-first PD were defined as the CABF group,
while those who discontinued apatinib were defined as the non-CABF
group (Fig. 1).
Follow-up was performed by visits to the clinic,
hospital admissions and telephone contact as of December 2022.
Efficacy evaluation
The first endpoint was overall survival (OS), and
the secondary endpoints included PFS1, PFS2, PFS, objective
response rate (ORR) and safety. Treatment responses were determined
according to the RECIST 1.1 criteria. Computed tomography and
ultrasound were performed every 4-6 weeks after oral apatinib until
PD or patient withdrawal. Adverse events were assessed based on the
Common Terminology Criteria for Adverse Events Version 4.0(15) and graded as 0-4.
Ethics statement
This study was conducted in line with the
Declaration of Helsinki (as revised in 2013) and was approved by
the Ethics Committee of Tangshan People's Hospital (approval no.
RMYY-LLKS-2019-1224).
Statistical analysis
SPSS version 23.0 software (IBM Corp.) was used to
conduct all statistical analyses. In the descriptive analysis,
quantitative variables are described as the mean and range, while
qualitative variables are described as quantity and percentage.
Comparisons of the variables were performed using a χ2
or Fisher's test as appropriate. The normal distribution
quantitative variables were described as mean ± standard deviation,
and independent t-test was used for inter-group comparison.
Survival analysis was conducted using Kaplan-Meier curves.
Univariate analysis and log-rank test were performed, and the
resulting significant variables were included in further
multivariate Cox regression analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics
A total of 63 patients were enrolled in the present
study. The median age of the patients was 53 years (range: 29-78).
The research population was entirely female. At first, 55 patients
received a combination of apatinib and chemotherapeutic drugs,
including capecitabine (20 patients), etoposide (20 patients),
vinorelbine (7 patients), gemcitabine (4 patients) and albumin
paclitaxel (4 patients). In addition, 4 patients received apatinib
monotherapy, 2 patients received apatinib combined with pyrotinib
targeting therapy and 2 patients received apatinib combined with
endocrine therapy. After the first PD, apatinib was continued in 26
patients (26/63, 41.27%) who were defined as the CABF group. Among
them, 6 patients (6/26, 23.08%) received combined therapy with
etoposide, 4 patients (4/26, 15.38%) were treated with
capecitabine, 2 patients (2/26, 7.69%) were treated with
vinorelbine, 3 patients (3/26, 11.54%) were treated with
gemcitabine, 4 patients (4/26, 15.38%) were treated with albumin
paclitaxel, 6 patients (6/26, 23.08%) were treated with targeted
therapy and 1 patient (1/26, 3.85%) received a combination with
fulvestrant endocrine therapy. Based on the progression, the other
37 patients were allocated to the non-CABF group; that is, they
were switched from apatinib-containing treatment to a new antitumor
drug regimen. The detailed clinical differences in the baseline
characteristics between the non-CABF and CABF groups of the 63
patients are shown in Table I.
 | Table IBaseline characteristics of the
non-CABF (n=37) and CABF (n=26) groups. |
Table I
Baseline characteristics of the
non-CABF (n=37) and CABF (n=26) groups.
| Clinical
characteristics | Non-CABF | CABF | P-value |
|---|
| Age,
yearsb | 54.4 (35-78) | 51.9 (29-77) | 0.801 |
| ECOG, n | | | 0.502 |
|
1-2 | 29 | 23 | |
|
3 | 8 | 3 | |
| Molecular
subtype | | | 0.645 |
|
Luminal
(HER2-negative) | 13 | 12 | |
|
HER2-positive | 9 | 6 | |
|
Triple-negative | 15 | 8 | |
| Menstrual
status | | | 0.271 |
|
Premenopause | 9 | 9 | |
|
Postmenopause | 28 | 17 | |
| Lung
metastasis | | | 0.198 |
|
Yes | 19 | 17 | |
|
No | 18 | 9 | |
| Liver metastasis, n
(%) | | | 0.143 |
|
Yes | 11 | 12 | |
|
No | 26 | 14 | |
| Brain, n (%) | | | 0.495 |
|
Yes | 7 | 4 | |
|
No | 30 | 22 | |
| Bone, n (%) | | | 0.254 |
|
Yes | 17 | 15 | |
|
No | 20 | 11 | |
| Serous cavity, n
(%) | | | 0.257 |
|
Yes | 5 | 6 | |
|
No | 32 | 20 | |
| Lymph node, n
(%) | | | 0.155 |
|
Yes | 11 | 4 | |
|
No | 26 | 22 | |
| ≥3 organ
metastases, n (%) | | | 0.341 |
|
Yes | 24 | 19 | |
|
No | 13 | 7 | |
| Previous number of
treatment lines, n (%) | | | 0.018a |
|
4 | 16 | 4 | |
|
>4 | 21 | 22 | |
| Combined therapy, n
(%) | | | 0.566 |
|
Chemotherapy | 31 | 24 | |
|
Endocrine
therapy | 1 | 1 | |
|
Monotherapy | 3 | 1 | |
|
Targeted
therapy | 2 | 0 | |
The complete routine blood data of the total 63
patients within 1 week before apatinib treatment are shown in
Table II. There was no
significant difference between the non-CABF and CABF groups.
 | Table IISerum characteristics of the patients
with ABC before apatinib treatment. |
Table II
Serum characteristics of the patients
with ABC before apatinib treatment.
| Variables | Median of
total | Non-CABF | CABF | P-value |
|---|
| Mean WBC count
(x109/l) | 4.52 | 5.35±2.25 | 4.83±1.63 | 0.321 |
| Neutrophils
(x109/l) | 2.84 | 3.47±1.73 | 3.01±1.33 | 0.254 |
| Lymphocytes
(x109/l) | 1.34 | 1.44±0.69 | 1.40±0.54 | 0.780 |
| NLR | 2.1 | 2.69±1.37 | 2.35±1.29 | 0.329 |
| HGB
(x109/l) | 115 | 112.76±18.39 | 119.04±16.40 | 0.168 |
| PLT count
(x109/l) | 235 | 229.32±84.38 | 222.23±80.08 | 0.738 |
Short-term efficacy
As of the cutoff date of December 2022, the median
follow-up time was 15.6 months (range, 1-80 months). In the first
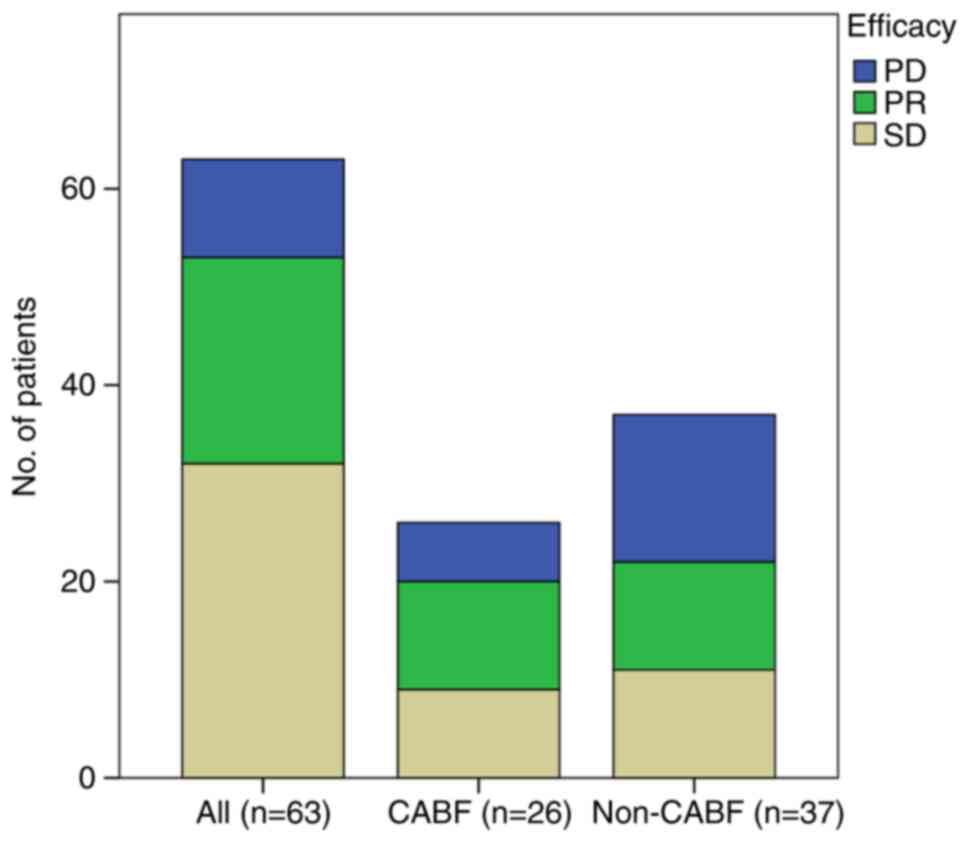
efficacy evaluation, the therapeutic outcomes were PD in 10
patients (10/63, 15.87%), stable disease (SD) in 32 patients
(32/63, 50.79%) and partial response (PR) in 21 patients (21/63,
33.33%). The ORR and the disease control rate were 33.3 and 84.1%,
respectively. Among the 26 patients who continued oral apatinib
treatment, PD was observed in 6 patients (6/26, 23.08%), SD in 9
patients (9/26, 34.62%) and PR in 11 patients (11/26, 42.31%). Of
the 37 patients who refused continued apatinib treatment after
progression, 40.54% experienced PD (15/37), 29.7% experienced SD
(11/37) and 29.7% exhibited a PR (11/37). The best tumor response
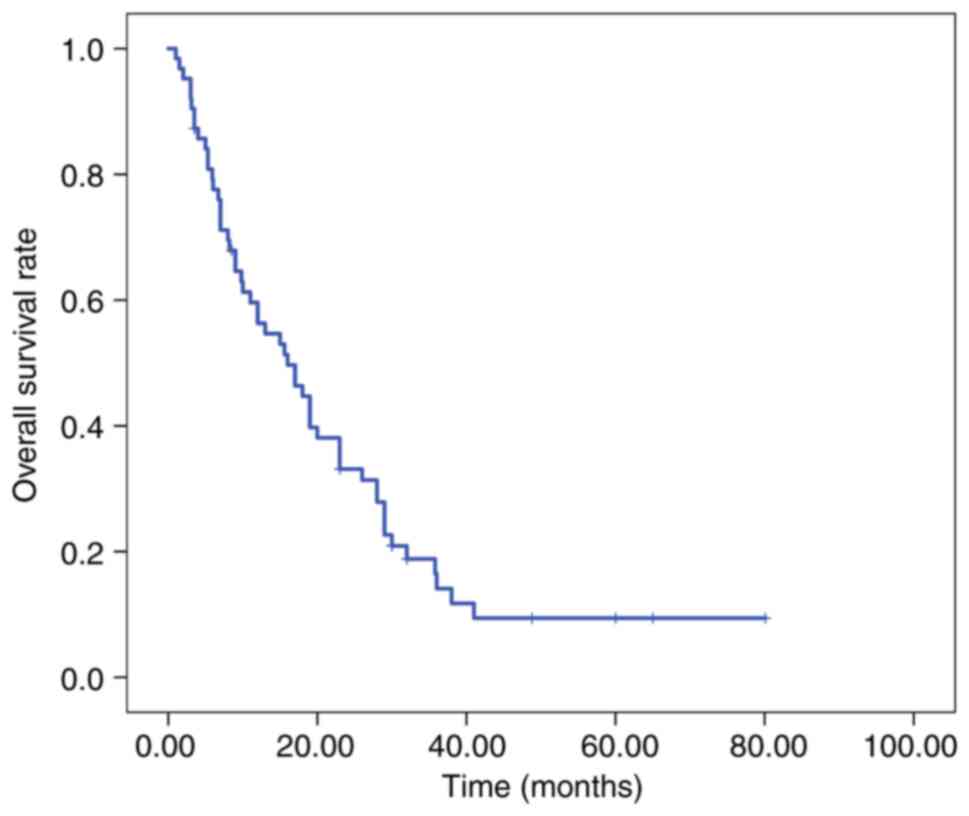
before and post-first progression is shown in Fig. 2. The overall population median OS
time was 16.0 months (95% CI, 9.52-22.48; Fig. 3).
Log-rank univariate analysis for
OS
Table III shows
results of the univariate analysis of the OS with various clinical
parameters in patients with ABC treated with apatinib. The results
of the univariate analysis of OS showed statistically significant
differences between the three groups in terms of molecular subtype,
namely, luminal [human epidermal growth factor receptor 2
(HER2)-negative] (28 months; 95% CI, 16.62-39.38), HER2-positive (6
months; 95% CI, 0.00-12.06) and triple-negative (15 months; 95% CI,
7.89-22.11) (P=0.014). Statistically significant differences were
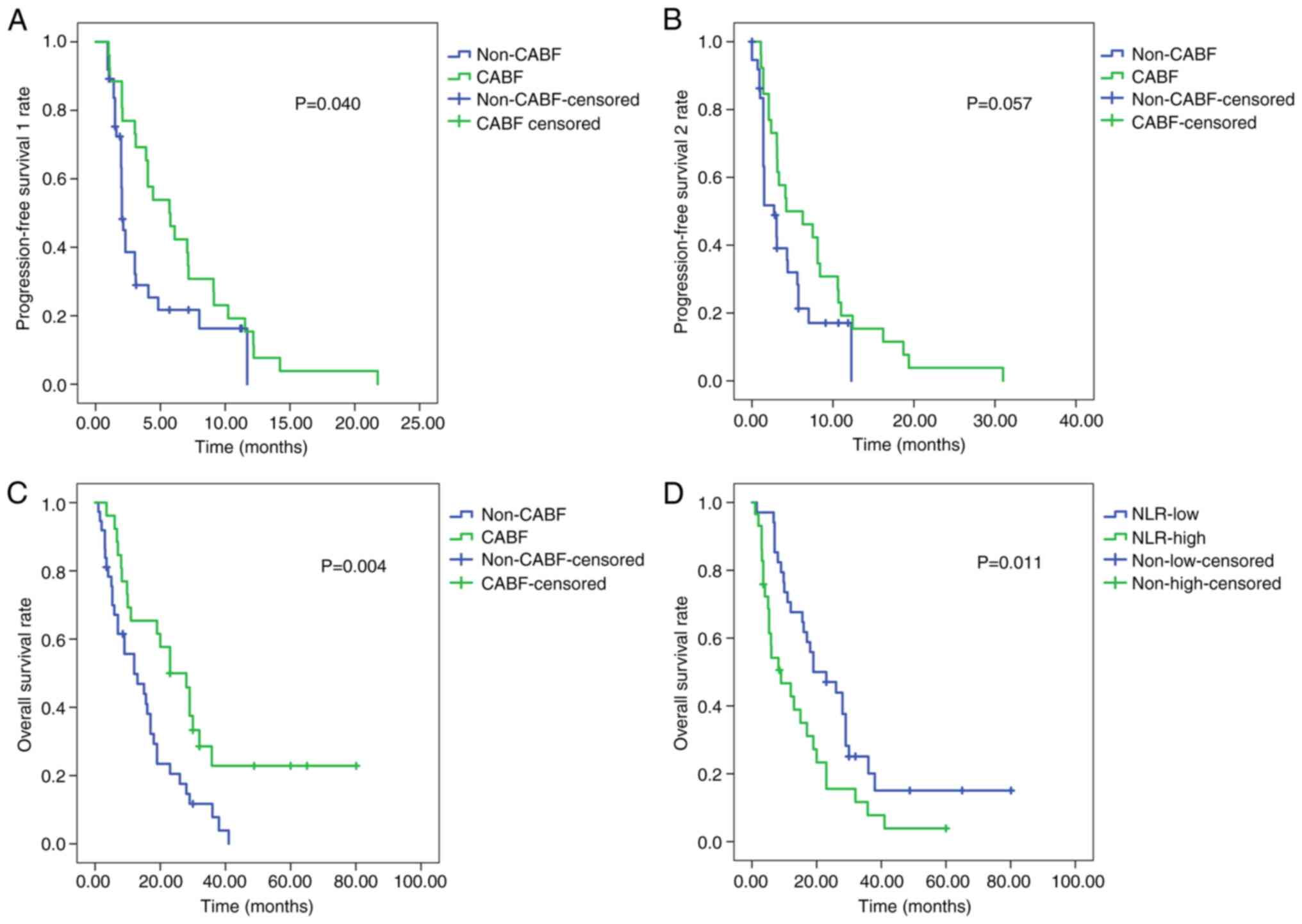
observed in PFS1, PFS2 and OS between the patients in the CABF and
non-CABF groups (P<0.05; Fig.
4A-C). The median OS time of the patients in NLR-low group (19
months; 95% CI, 7.76-30.24) was significantly longer than that of
the patients in the NLR-high group (9 months; 95% CI, 1.45-16.55)
(P=0.011; Fig. 4D).
 | Table IIIUnivariate analysis of OS with
clinical parameters in patients with ABC treated with apatinib. |
Table III
Univariate analysis of OS with
clinical parameters in patients with ABC treated with apatinib.
| | PFS | OS |
|---|
| Clinical
parameter | mPFS1 time,
months | χ2
value | P-value | mOS time,
months | χ2
value | P-value |
|---|
| ECOG PS score | | 14.031 | <0.001 | | 2.153 | 0.142 |
|
1-2 | 5.96 | | | 22.09 | | |
|
3 | 2.06 | | | 15.79 | | |
| Molecular
subtype | | 11.595 | 0.003 | | 8.541 | 0.014 |
|
Luminal
(HER2-negative) | 7.17 | | | 28.00 | | |
|
HER2-positive | 2.03 | | | 6.00 | | |
|
Triple-negative | 3.10 | | | 15.00 | | |
| Number of treatment
cycles | | 0.049 | 0.824 | | 0.352 | 0.553 |
|
4 | 2.07 | | | 19.00 | | |
|
>4 | 3.10 | | | 12.00 | | |
| ≥3 organ
metastases | | 4.282 | 0.039 | | 1.359 | 0.244 |
|
Yes | 3.03 | | | 13.00 | | |
|
No | 4.07 | | | 19.00 | | |
| Brain
metastasis | | 1.314 | 0.252 | | 2.216 | 0.137 |
|
Yes | 4.06 | | | 14.21 | | |
|
No | 5.56 | | | 23.79 | | |
| Apatinib sustained
post-first PD | | 4.233 | 0.040 | | 8.446 | 0.004 |
|
CABF | 5.70 | | | 23.00 | | |
|
Non-CABF | 2.03 | | | 12.00 | | |
| NLR | | 6.703 | 0.010 | | 6.491 |
0.011a |
|
Low | 4.43 | | | 19.00 | | |
|
High | 2.00 | | | 9.00 | | |
| Hemoglobin | | 4.812 | 0.028 | | 2.024 | 0.155 |
|
Low | 2.13 | | | 12.00 | | |
|
High | 4.83 | | | 19.00 | | |
| Platelet count | | 0.330 | 0.566 | | 0.125 | 0.723 |
|
Low | 3.10 | | | 15.60 | | |
|
High | 4.03 | | | 17.00 | | |
| Secondary
hypertension | | 0.455 | 0.500 | | 0.651 | 0.420 |
|
Yes | 4.83 | | | 19.00 | | |
|
No | 3.03 | | | 12.00 | | |
| Proteinuria | | 9.954 | 0.002 | | 2.126 | 0.145 |
|
Yes | 12.2 | | | 19.00 | | |
|
No | 3.03 | | | 15.60 | | |
Multivariate Cox regression
analysis
The results of the multivariate logistic regression
analysis showed that CABF and NLR were independent predictive
factors for OS (Table IV).
 | Table IVMultivariable analysis of overall
survival with significant clinical parameters in patients with
advanced breast cancer treated with apatinib. |
Table IV
Multivariable analysis of overall
survival with significant clinical parameters in patients with
advanced breast cancer treated with apatinib.
| Clinical
parameters | B | SE | Wald | df | P-value | Exp(B) |
|---|
| Molecular
subtype | 0.157 | 0.155 | 1.028 | 1 | 0.311 | 1.170 |
| CABF | -0.712 | 0.299 | 5.656 | 1 | 0.017 | 0.491 |
| NLR | 0.581 | 0.284 | 4.169 | 1 | 0.041 | 1.787 |
Adverse events
The overall incidence of grade ≥3 adverse events was
low, with no cases of death caused by adverse events. Adverse
events primarily included secondary hypertension, hand-foot
syndrome, oral cavity mucositis, secondary proteinuria, fatigue and
diarrhea, with no cases of gastrointestinal bleeding. Grade 2 or
above adverse events were dominant in all patients and could be
alleviated after symptomatic treatment or dose adjustment of
apatinib (Table V).
 | Table VIncidence of the main adverse events
related to apatinib. |
Table V
Incidence of the main adverse events
related to apatinib.
| Adverse
effects | Grade I-II, n
(%) | Grade III-IV, n
(%) |
|---|
| Hematology | | |
|
Neutropenia | 22 (34.9) | 4 (6.3) |
|
Thrombocytopenia | 12 (19.0) | 1 (1.6) |
| Non-hematology | | |
|
Secondary
hypertension | 19 (30.2) | 3 (4.8) |
|
Hand-foot
syndrome | 17 (27.0) | 1 (1.6) |
|
Fatigue | 21 (33.3) | 2 (3.2) |
|
Secondary
proteinuria | 7 (11.1) | 0 (0.0) |
|
Oral cavity
mucositis | 8 (12.7) | 0 (0.0) |
|
Diarrhea | 11 (17.5) | 0 (0.0) |
Discussion
Angiogenesis, the process by which new blood vessels
are formed from pre-existing vasculature, has been implicated in
the growth, progression and metastasis of cancer, and tumor
angiogenesis has been explored as a key therapeutic target for
decades (16). Several trials have
shown that adding bevacizumab to paclitaxel significantly improves
the PFS time of patients with HER2-negative metastatic breast
cancer (mBC) (17-19).
Moreover, a meta-analysis of the E2100, AVADO and RIBBON-1 studies
suggested that bevacizumab combined with chemotherapy as a
first-line treatment for mBC significantly improved the ORR and PFS
time, but did not improve OS time in patients with increased grade
3-4 adverse effects (20).
However, the results of another meta-analysis suggested that use of
chemotherapy with bevacizumab as an adjuvant, considering its
favorable effects on clinical outcomes, was a preferred therapeutic
option for patients with MBC, for whom the disease must be rapidly
treated (21).
Apatinib, a anti-angiogenic TKI with moderate
adverse events, can suppress angiogenesis, tumor growth and
metastasis by inhibiting the phosphorylation of VEGFR2 and blocking
downstream signaling pathways (22). Apatinib has been clinically used in
patients with refractory ABC and is considered an efficient
treatment for patients with mild adverse effects (23,24).
In the present real-world study, a PR was observed in 21 patients
(21/63, 33.33%) and SD was observed in 32 patients (32/63, 50.79%),
with mild adverse effects (grades 1-2), such as secondary
hypertension, hand-foot syndrome, oral cavity mucositis, secondary
proteinuria, fatigue and diarrhea, with no cases of
gastrointestinal bleeding. In addition, the enrolled patients had
been heavily treated according to NCCN guidelines, and it was
difficult to formulate a standard therapeutic schedule for them.
Apatinib-containing treatment may be a promising therapeutic
strategy for patients with ABC who have developed multidrug
resistance to traditional chemotherapeutic agents.
However, tumor vascular rebound or increased growth
has been reported following treatment discontinuation (25). These withdrawal-mediated tumor
growth rebounds were found to decrease following long-term periods
of discontinuation (11,26). Evidence suggests that in certain
circumstances, continuing therapy beyond disease progression can
result in antitumor activity (27,28).
After first-line treatment with bevacizumab with chemotherapy,
maintenance of bevacizumab treatment until disease progression or
unacceptable levels of toxicity is a reasonable strategy to improve
and maintain the clinical response, increase the time to
progression, extend OS time, relieve tumor-related symptoms and
delay the use of aggressive therapies, without compromising a
patient's quality of life (28).
The TANIA trial demonstrated that maintaining bevacizumab after the
first and second PD led to improved second-line PFS times, which
may be associated with increased redundancy of angiogenic pathways
in the later stages in patients with ABC who were pretreated with
bevacizumab (29). Low-dose
apatinib has been shown to have a moderate effect in patients with
ABC (9,23,24).
However, to the best of our knowledge, no previous study has
investigated apatinib rechallenge in patients with ABC. In the
present study, of the 26 patients who continued to take apatinib
after the first PD, 9 patients achieved SD and 11 achieved a PR.
Univariate analysis revealed that apatinib sustained after the
first PD was significantly associated with PFS1 and PFS2, and in
some cases, even OS. Additionally, CABF was also an independent
risk factor for OS, as shown by the results of the multivariate Cox
regression analysis. This demonstrated that there was a rebound in
tumor growth during the interval of anti-angiogenic therapy.
Low-dose apatinib offers improved survival in patients with ABC and
retains its antitumor activity even beyond disease progression.
Anti-angiogenic therapy not only reduces the
formation of new blood vessels, which are essential for cancer
growth and metastasis, but also reprograms the immune
microenvironment of the tumor (30). Neutrophils produce various
angiogenic molecules and an equally wide range of anti-angiogenic
molecules (31). Neutrophils
accumulate in the peripheral blood of patients with cancer,
especially in those with advanced-stage disease, and a high
circulating NLR is a robust biomarker of poor clinical outcomes in
various types of cancer (32). As
an easily accessible prognostic marker, a high NLR has been
reported to be associated with a poor prognosis in breast cancer in
several studies (33-35).
Apatinib, as a moderate anti-angiogenic TKI, has been used in
patients with refractory ABC in the clinic, but to the best of our
knowledge, no association with NLR has been reported. Moreover,
patients with hepatocellular carcinoma treated with apatinib with a
low pretreatment NLR have been reported to have significantly
longer OS and PFS times than those with a high pretreatment NLR
(36). In the present study, using
the median NLR as a cutoff, the patients in the NLR-low group
showed a longer median OS time than those in the NLR-high group (19
vs. 9 months). This was likely due to low-dose apatinib alleviating
hypoxia and remodeling the immunosuppressive tumor microenvironment
to make it more permissive for antitumor immunity (37). In this manner, the drugs can be
efficiently delivered to promote the release of new tumor antigens,
increase the immune response and eventually improve the therapeutic
outcome.
The results of the univariate analysis showed that
the mean OS times of patients with luminal (HER2-negative),
triple-negative, and HER2-positive molecular subtypes were 28, 15
and 6 months, respectively, but the results were not statistically
significant. This may be since more HER2-positive patients in the
CABF group had additional HER2 targeting therapy; nevertheless,
only 2 patients (2/15) were treated with HER2 targeting therapy
during first-line treatment with apatinib. Furthermore, 27.9% of
the HER2-positive patients showed high VEGFR2 expression (38). According to a recent case report
(39), two patients with
multi-line anti-HER2 treatment failure who underwent apatinib and
anti-HER2 combination treatment still had PFS times of 8.4 and 10.6
months; this suggests that apatinib can restore the HER2-targeting
sensitivity and improve survival, but does not suggest abandoning
the use of HER2-targeted therapy.
The present study was an observational trial in the
real world and thus has associated limitations, such as a small
sample size, a lack of diversity in the patient population and a
lack of randomized design. However, a real-world study is more
complicated and closer to clinical reality than a prospective
study. Future studies with a larger cohort of patients are needed
to verify these findings.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Health Planning
Projects in Hebei Province, China (grant no. 20191614).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JW contributed to the design of the study, the
analysis and interpretation of data, and the first draft of the
manuscript. JJ, XY and JL performed the data collection and
analysis, and revised the manuscript. ZY contributed to the design
of the study, analysis and interpretation of data, and revised the
manuscript. All authors have read and approved the final
manuscript. JW, JJ, JL, XY and ZY confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
This study was conducted in line with the
Declaration of Helsinki (as revised in 2013). The Ethics Committee
of Tangshan People's Hospital (Tangshan, China) approved the study
and waived the requirement for written informed consent (approval
no. MYY-LLKS-2019-1224).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jiménez-Valerio G and Casanovas O:
Angiogenesis and metabolism: Entwined for therapy resistance.
Trends Cancer. 3:10–18. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roda N, Blandano G and Pelicci PG: Blood
vessels and peripheral nerves as key players in cancer progression
and therapy resistance. Cancers (Basel). 13(4471)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Laborda-Illanes A, Sánchez-Alcoholado L,
Castellano-Castillo D, Boutriq S, Plaza-Andrades I, Aranega-Martín
L, Peralta-Linero J, Alba E, González-González A and Queipo-Ortuño
MI: Development of in vitro and in vivo tools to evaluate the
antiangiogenic potential of melatonin to neutralize the angiogenic
effects of VEGF and breast cancer cells: CAM assay and 3D
endothelial cell spheroids. Biomed Pharmacother.
157(114041)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hervé MA, Buteau-Lozano H, Vassy R, Bieche
I, Velasco G, Pla M, Perret G, Mourah S and Perrot-Applanat M:
Overexpression of vascular endothelial growth factor 189 in breast
cancer cells leads to delayed tumor uptake with dilated
intratumoral vessels. Am J Pathol. 172:167–178. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang L, Wei Y, Shen S, Shi Q, Bai J, Li
J, Qin S, Yu H and Chen F: Therapeutic effect of apatinib on
overall survival is mediated by prolonged progression-free survival
in advanced gastric cancer patients. Oncotarget. 8:29346–29354.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jain RK: Antiangiogenesis strategies
revisited: From starving tumors to alleviating hypoxia. Cancer
Cell. 26:605–622. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu Z, Shan J, Yu Q, Wang X, Song X, Wang
F, Li C, Yu Z and Yu J: Real-World data on apatinib
efficacy-results of a retrospective study in metastatic breast
cancer patients pretreated with multiline treatment. Front Oncol.
11(643654)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cao M, Lu H, Yan S, Pang H, Sun L, Li C,
Chen X, Liu W, Hu J, Huang J, et al: Apatinib plus etoposide in
pretreated patients with advanced triple-negative breast cancer: A
phase II trial. BMC Cancer. 23(463)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu J, He M, Ou K, Wang X, Wang Y, Qi L,
Chai Y, Jiang M, Ma F, Luo Y, et al: Efficacy and safety of
apatinib combined with dose-dense paclitaxel and carboplatin in
neoadjuvant therapy for locally advanced triple-negative breast
cancer: A prospective cohort study with propensity-matched
analysis. Int J Cancer. 154:133–144. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mastri M, Tracz A, Lee CR, Dolan M,
Attwood K, Christensen JG, Liu S and Ebos J: A transient
pseudosenescent secretome promotes tumor growth after
antiangiogenic therapy withdrawal. Cell Rep. 25:3706–3720.e8.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: NCCN guidelines insights breast cancer, version
1.2016. J Natl Compr Canc Netw. 13:1475–1485. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miller TP, Fisher BT, Getz KD, Sack L,
Razzaghi H, Seif AE, Bagatell R, Adamson PC and Aplenc R:
Unintended consequences of evolution of the Common Terminology
Criteria for Adverse Events. Pediatr Blood Cancer.
66(e27747)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Harry JA and Ormiston ML: Novel pathways
for targeting tumor angiogenesis in metastatic breast cancer. Front
Oncol. 11(772305)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Miles D, Cameron D, Bondarenko I, Manzyuk
L, Alcedo JC, Lopez RI, Im SA, Canon JL, Shparyk Y, Yardley DA, et
al: Bevacizumab plus paclitaxel versus placebo plus paclitaxel as
first-line therapy for HER2-negative metastatic breast cancer
(MERiDiAN): A double-blind placebo-controlled randomised phase III
trial with prospective biomarker evaluation. Eur J Cancer.
70:146–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zielinski C, Láng I, Inbar M, Kahán Z,
Greil R, Beslija S, Stemmer SM, Zvirbule Z, Steger GG, Melichar B,
et al: Bevacizumab plus paclitaxel versus bevacizumab plus
capecitabine as first-line treatment for HER2-negative metastatic
breast cancer (TURANDOT): Primary endpoint results of a randomised,
open-label, non-inferiority, phase 3 trial. Lancet Oncol.
17:1230–1239. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Masuda N, Takahashi M, Nakagami K, Okumura
Y, Nakayama T, Sato N, Kanatani K, Tajima K and Kashiwaba M:
First-line bevacizumab plus paclitaxel in Japanese patients with
HER2-negative metastatic breast cancer: Subgroup results from the
randomized Phase III MERiDiAN trial. Jpn J Clin Oncol. 47:385–392.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rossari JR, Metzger-Filho O, Paesmans M,
Saini KS, Gennari A, de Azambuja E and Piccart-Gebhart M:
Bevacizumab and Breast Cancer: A meta-analysis of first-line phase
III studies and a critical reappraisal of available evidence. J
Oncol. 2012(417673)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li Z, Guo F, Han Y, Wang J and Xu B:
Efficacy and safety of bevacizumab in pretreated metastatic breast
cancer: A systematic review and meta-analysis. Oncol Res Treat.
45:608–617. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang N, Chen S, Liu D, Guo J, Sun Y, Zhang
J, Kong Q and He L: Therapeutic effect of small molecule targeting
drug apatinib on gastric cancer and its role in prognosis and
anti-infection mechanism. Saudi J Biol Sci. 27:606–610.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zeng T, Sun C, Liang Y, Yang F, Yan X, Bao
S, Zhang Y, Huang X, Fu Z, Li W and Yin Y: A real-world multicentre
retrospective study of low-dose apatinib for human epidermal growth
factor receptor 2-negative metastatic breast cancer. Cancers
(Basel). 14(4084)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li DD, Tao ZH, Wang BY, Wang LP, Cao J, Hu
XC and Zhang J: Apatinib plus vinorelbine versus vinorelbine for
metastatic triple-negative breast cancer who failed
first/second-line treatment: The NAN trial. NPJ Breast Cancer.
8(110)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ebos JM and Pili R: Mind the gap:
Potential for rebounds during antiangiogenic treatment breaks. Clin
Cancer Res. 18:3719–3721. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mastri M and Ebos JML: Tumor growth fueled
by spurious senescence phenotypes. Mol Cell Oncol.
6(1575707)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kuczynski EA, Sargent DJ, Grothey A and
Kerbel RS: Drug rechallenge and treatment beyond
progression-implications for drug resistance. Nat Rev Clin Oncol.
10:571–587. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ciruelos E, Pérez-García JM, Gavilá J,
Rodríguez A and de la Haba-Rodriguez J: Maintenance therapy in
HER2-Negative metastatic breast cancer: A new approach for an old
concept. Clin Drug Investig. 39:595–606. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vrdoljak E, Marschner N, Zielinski C,
Gligorov J, Cortes J, Puglisi F, Aapro M, Fallowfield L, Fontana A,
Inbar M, et al: Final results of the TANIA randomised phase III
trial of bevacizumab after progression on first-line bevacizumab
therapy for HER2-negative locally recurrent/metastatic breast
cancer. Ann Oncol. 27:2046–2052. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yi M, Jiao D, Qin S, Chu Q, Wu K and Li A:
Synergistic effect of immune checkpoint blockade and
anti-angiogenesis in cancer treatment. Mol Cancer.
18(60)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Noonan DM, De Lerma Barbaro A, Vannini N,
Mortara L and Albini A: Inflammation, inflammatory cells and
angiogenesis: Decisions and indecisions. Cancer Metastasis Rev.
27:31–40. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shaul ME and Fridlender ZG:
Tumour-associated neutrophils in patients with cancer. Nat Rev Clin
Oncol. 16:601–620. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xiang Y, Zhang N, Lei H, Wu J, Wang W,
Zhang H and Zeng X: Neutrophil-to-lymphocyte ratio is a negative
prognostic biomarker for luminal A breast cancer. Gland Surg.
12:415–425. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ethier JL, Desautels D, Templeton A, Shah
PS and Amir E: Prognostic role of neutrophil-to-lymphocyte ratio in
breast cancer: A systematic review and meta-analysis. Breast Cancer
Res. 19(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Guo W, Lu X, Liu Q, Zhang T, Li P, Qiao W
and Deng M: Prognostic value of neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio for breast cancer patients: An updated
meta-analysis of 17079 individuals. Cancer Med. 8:4135–4148.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang H, Wang Z, Hou Z, Yang X, Zhu K, Cao
M, Zhu X, Li H and Zhang T: The Neutrophil-to-Lymphocyte Ratio
(NLR) predicts the prognosis of unresectable intermediate and
advanced hepatocellular carcinoma treated with apatinib. Cancer
Manag Res. 13:6989–6998. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao
C, Jia Y, Shi J, Zhang L, Liu X, et al: Low-Dose apatinib optimizes
tumor microenvironment and potentiates antitumor effect of
PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res. 7:630–643.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kourea HP, Dimitrakopoulos FI, Koliou GA,
Batistatou A, Papadopoulou K, Bobos M, Asimaki-Vlachopoulou A,
Chrisafi S, Pavlakis K, Chatzopoulos K, et al: Clinical
significance of major angiogenesis-related effectors in patients
with metastatic breast cancer treated with trastuzumab-based
regimens. Cancer Res Treat. 54:1053–1064. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
He L, Shen X, Liu Y, Gao L, Wu J, Yu C, Li
G, Wang X and Shao X: The reversal of anti-HER2 resistance in
advanced HER2-positive breast cancer using apatinib: Two cases
reports and literature review. Transl Cancer Res. 11:4206–4217.
2022.PubMed/NCBI View Article : Google Scholar
|