1. Introduction
Psoriasis is a chronic and refractory skin-related
autoimmune disease, which is characterized by abnormal
differentiation and proliferation of keratinocytes (KCs) and
inflammatory cell infiltration. Although the pathogenesis of
psoriasis remains to be elucidated, emerging evidence suggests that
the aberrant adjustment of immune cells in the skin, especially
T-cells, plays a crucial part in the development of psoriasis
(1). Studies have mainly focused
on the functions of Th17 cells and their related secreted
cytokines, namely interleukin (IL)-17 and IL-23, in the pathogenic
pathway of psoriasis (2,3). It has been reported that the
activation and upregulation of IL-17 create a ‘feed forward’
inflammatory reaction in KCs, thus resulting in the abnormal
proliferation and differentiation of KCs as well as in the
recruitment of this subset of leukocytes in the skin, eventually
promoting the formation of psoriasis plaques (4).
T-cell activation and expansion require a
co-signaling mechanism. One signal can be provided by the antigen
peptide/major histocompatibility complex (MHC) on the surface of
antigen-presenting cells (APCs) recognized by T-cell receptor
(TCR). The other signal can be initialized by the interaction of
co-signaling molecules on the surface of T-cells and APCs. To avoid
an immune system overreaction, co-inhibitory molecules commonly
send feedback inhibitory signals to activated T-cells (5).
The effects of co-inhibitory molecules have been
fully studied in patients with types of cancer (6,7).
Studies have also led to an awareness of co-inhibitory molecules in
autoimmune diseases, including autoimmune glomerulonephritis,
multiple sclerosis, atopic dermatitis and systemic lupus
erythematosus (8-11).
Enthusiasm in the field of co-inhibitory molecules in psoriasis has
been aroused by the encouraging results obtained by the application
of immunotherapy with co-inhibitory molecules for treating cancer
and other immune-related diseases.
The current treatment approaches for psoriasis
mainly include topical agents, phototherapy, traditional systemic
drugs and biological agents (12).
Compared with traditional therapy, biologic agents have become a
mainstay in the treatment of psoriasis. However, there is a tiny
subset of individuals who have no response to current biologics or
for whom therapeutic efficacy diminishes over time. Furthermore,
some patients may still experience notable side effects from
presently accessible biologics. Severe adverse events included
serious infections, reactivation of hepatitis B and C,
tuberculosis, drug-induced lupus and demyelinating central nervous
system disorders, with common side effects such as nasopharyngitis,
upper respiratory tract infection, headache and fatigue (13). Therefore, in order to develop
innovative therapeutics, studies investigating the immunological
etiology of psoriasis continue (14).
The current review article mainly focused on the
potential roles of co-inhibitory molecules in the pathogenesis of
psoriasis and their preclinical studies and clinical applications
in the treatment of psoriasis, hoping to provide new potential
options for psoriasis treatment (Fig.
1; Table I).
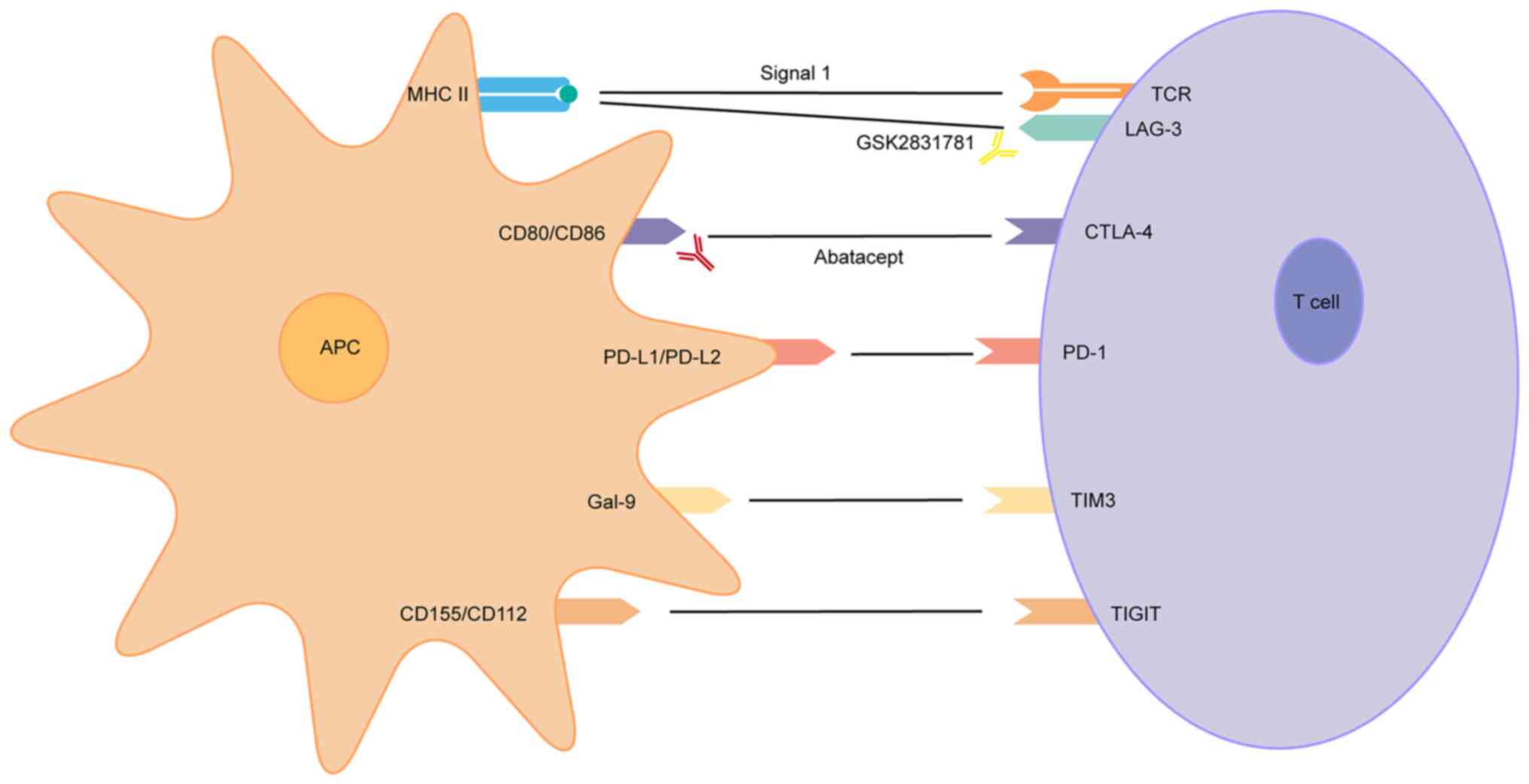 | Figure 1Co-inhibitory molecular targets for
the treatment of psoriasis. APC, antigen-presenting cell; MHC,
major histocompatibility complex; TCR, T-cell receptor; LAG-3,
lymphocyte-activation gene 3; CD, cluster of differentiation;
CTLA-4, cytotoxic T lymphocyte associated protein 4; PD-L1/PD-L2,
programmed death ligand 1/2; PD-1, programmed death 1; Gal-9,
galectin 9; TIM-3, T-cell immunoglobulin and mucin
domain-containing protein 3; TIGIT, T-cell immunoreceptor with
immunoglobulin and immunoreceptor tyrosine-based inhibitory motif
domains. |
 | Table IBiologics targeting co-inhibitory
molecules for psoriasis treatment. |
Table I
Biologics targeting co-inhibitory
molecules for psoriasis treatment.
| Author, year | Name | Type | Targeting | Status | Efficacy | Adverse event | (Refs.) |
|---|
| Abrams et
al, 1999 Mease et al, 2017 Mease et al, 2011
Strand et al, 2018 | Abatacept | Fusion protein | CD80/CD86 | Phase III | Modest impact on
psoriasis lesions while beneficial trends overall in PsA | Increased risk of
infection, transient headache | (23,27,62,63) |
| Kim et al,
2016 Peng et al, 2020 Imai et al, 2015 | PD-L1-Fc | Fusion protein | PD-1 | Preclinical | Alleviated
psoriatic inflammation and exhibit additive effects with or without
other biologics | / | (43,45,64) |
| Ellis et al,
2021 | GSK2831781 | mAb | LAG-3 | Phase I | LAG-3+
and CD3+ T-cell counts reduced in peripheral blood and
biopsies, reduced pro-inflammatory genes expression, disease
activity improved up | Headache,
nasopharyngitis, back pain | (56) |
| Niwa et al,
2009 | sGal-9 | Stable form of
galectin-9 | TIM-3 | Preclinical | Alleviated
epidermal thickness and skin inflammation, inhibited STAT3
expression | / | (61) |
2. Cytotoxic T lymphocyte antigen-4
(CTLA-4)
CTLA-4 is a homologous dimmer of CD28 that binds to
B7 molecules. Although CTLA-4 is only expressed on activated
T-cells, it has a 20-fold stronger affinity for B7 than has CD28.
CTLA-4 has an immunoreceptor tyrosine inhibitory motif in its
cytoplasmic domain that activates protein tyrosine phosphatase and
inhibits T-cell activation signal transduction and is thereby
negatively associated with T-cell activation (15).
Role of CTLA-4 in psoriasis
A previous study revealed that KCs and particular
dermal cells in skin with psoriatic lesions expressed CTLA-4 on
their surface, while those in skin without lesions expressed very
little or no CTLA-4(16). Liu
et al (17) indicated that
the severity of psoriasis was inversely related to the levels of
membrane CTLA-4 (mCTLA-4). In imiquimod-induced mice models,
mCTLA-4 alleviated epidermal hyperplasia and inflammation. However,
blocking mCTLA-4 resulted in a worsening of psoriasis,
demonstrating that mCTLA-4 depletion could aggravate psoriasis.
The relationship between the presence of the CTLA-4
gene variants and the development of different autoimmune diseases
has been also studied (18). The
-318C>T polymorphism in the CTLA-4 gene works as a powerful
promoter to alter the transcription of gene (19). Another study demonstrated that the
+49A>G polymorphism in the leader sequence of CTLA-4, could
serve a critical role in the binding of CTLA-4 molecule with
B7-1(20). Furthermore, the
CT60A>G polymorphism may influence the alternative splicing and
the generation of soluble CTLA-4(21). Dursun et al (22) explored the effects of the above
three single-nucleotide polymorphisms in the CTLA-4 gene in
psoriasis vulgaris patients and healthy volunteers. The results
showed that the +49A>G and CT60A>G polymorphisms could be
considered as risk factors for the formation of psoriasis vulgaris.
In addition, the CGG and CAG haplotypes may play a promoting role
in disease progression, while the CAA haplotype displays a
protective role.
CTLA-4 in the treatment of psoriasis.
Abatacept, a CTLA4Ig, which block the co-stimulation of T-cells via
inhibiting the B7-CD28/CTLA4 pathway, is a completely human
recombinant soluble fusion protein composed of a human CTLA-4
extracellular domain and an IgG1 Fc fragment. A 26-week phase I,
open-label, dose-escalation research proved the efficacy of
abatacept in treating patients with psoriasis vulgaris, in which
19/41 (46%) patients receiving medication had a ≥50% improvement in
the disease activity index compared with the baseline. The side
effects were acceptable and comparatively minor, which mainly
included upper respiratory tract infection and temporary headache,
each occurring in 16% (23). The
efficacy of abatacept was linked with attenuated T-cells
activation, KCs and dendritic cells (DCs) in lesions (24).
In the study by Altmeyer et al (25), two patients with intractable
psoriasis and psoriatic arthritis (PsA) were treated with
abatacept. The patients received an initial dose of 10 mg/kg
abatacept after failing to respond to conventional therapy and
biologic agents such as etanercept, adalimumab and efalizumab. They
all experienced a reduction in skin lesions as well as in joint
pain and swelling, but eventually both of the patients' responses
were not sustained. Because of the severity and drug resistance of
the two patients reported in this case report, they may not
represent the majority of moderate to severe psoriasis patients who
may be benefit from this treatment. No adverse events were reported
in either patient.
Recently, abatacept was approved by the American
College of Rheumatology/National Psoriasis Foundation to treat PsA
(26). In a randomized,
double-blind, placebo-controlled phase III study, 20% improvement
in American College of Rheumatology score was attained in 39.4% of
PsA patients in the abatacept group (n=213) and only 22.3% in the
placebo group (n=211) at week 24. Of all participants, ~60% had
previously received tumor necrosis factor inhibitor (TNFi) agents,
while abatacept showed a maximal effect on TNFi-naïve patients. All
participants treated with abatacept tolerated it well and
demonstrated favorable outcomes in musculoskeletal manifestations.
However, the effect of abatacept on psoriasis lesions was limited
(27). In another case study, a
47-years-old Caucasian male with intractable psoriasis and PsA was
injected with 125 mg/week s.c abatacept and 25 mg/week s.c.
methotrexate. The combined regimen showed a superior effectiveness
on musculoskeletal manifestations compared with skin endpoints due
to their different sensitivities to abatacept (28). Combination therapy of CTLA-4
molecular agents can be applied for improving the symptoms of
psoriasis patients, particularly for those who have peripheral
joint involvement, are TNFi-naïve and have limited skin
involvement. However, this treatment strategy lacks rigorous
experimental support and therefore further studies are needed. A
double-blind, randomized clinical trial involving 108 patients with
moderate to severe plaque psoriasis demonstrated that abatacept
failed to prevent recurrence of psoriasis and was unable to sustain
the inhibition of psoriasis-related inflammation factor IL-23
molecule in lesions following ustekinumab withdrawal, which may be
attributed to the compensatory mechanism of residuary T-cell
activation in lesions (29).
3. Programmed death 1 (PD-1)-PD-ligand
(PD-L)1/2
PD-1, a type I transmembrane protein, is mainly
expressed on activated T-cells and binds to PD-L1 and PD-L2 to
prevent T-cells from being overactivated and to inhibit T-cell
proliferation, differentiation and cytokine production.
Additionally, PD-1 serves a significant role in immune regulation,
homeostasis and tolerance (30).
Role of PD-1-PD-L1/2 in psoriasis
Khatery et al (31) reported that PD-1 was increased in
the serum of patients with psoriasis and in skin lesions and
peripheral lesions. In line with these findings, another study
found that increased PD-1 expression was related to the severity of
chronic plaque psoriasis (32). In
addition, a significantly thicker epidermis, more obvious vascular
dilation, higher psoriasis area and severity index (PASI) scores
and a longer disease course were observed in the PD-1 high
expression group compared with the PD-1 low expression group. These
results could be attributed to the compensatory upregulation of
PD-1 to overcome the Th17 and Th22 pathways (32).
Controversially, a study confirmed that PD-L1 was
downregulated in the epidermis of patients with psoriasis in mRNA,
protein levels and immunohistochemical staining (33). This study also suggested that PD-L1
expressed on the surface of KCs, rather than PD-L2, is important in
the pathogenesis of psoriasis. This finding was in line with the
prior conclusion that PD-L1 on the surface of T-cells is the major
ligand for PD-1 compared with PD-L2(34). Tanaka et al (35) further confirmed that PD-L1 could
exert dominating roles in Th1- and Th17-mediated immunity, whereas
PD-L2 was mainly involved in Th2-mediated immunity. Therefore, PD-1
and PD-L1 could interact to inhibit Th17 cell differentiation,
while blocking this interaction could induce Th17 cell
differentiation. Notably, it has been detected that anti-PD-1/PD-L1
immune checkpoint antibodies might induce or aggravate psoriatic
lesions during the clinical treatment of tumors and autoimmune
diseases (36-40).
Preclinical study of PD-1-PD-L1/2 in
treating psoriasis
By interacting with the p40 subunit of IL-23 and
IL-12, anti-p40 therapy could prevent IL-23 from inducing the
production of Th17 cytokines. It is an approved agent for the
clinical treatment of psoriasis and PsA (41,42).
However, several patients still have residual lesions following
anti-p40 treatment, thus indicating that further treatment
strategies are needed for the management of non-IL-23-related
psoriasis inflammation. Kim et al (43) discovered that PD-L1-Fc could
inhibit anti-CD3-induced IL-17A production in CD27-Vγ1-Vγ4-γδ
T-cells in imiquimod-induced mice. In addition, combining PD-L1-Fc
with anti-p40 therapy was shown to have a cumulative efficacy on
psoriatic inflammation in mice, which may be ascribed to the effect
of the above two drugs on targeting different IL-17A-secreting
γδT-cell populations. Similarly, anti-TNF-α has good efficacy in
treating patients with psoriasis and has been licensed for clinical
application (44). Peng et
al (45) indicated that
PD-L1-Fc could reduce psoriatic inflammation and show potential
synergistic effects with anti-TNF-α treatment in imiquimod-treated
mice.
A cell-free carrier called PD-L1 overexpressed
mesenchymal stem cell (MSC)-derived extracellular vesicles
(MSC-sEVs-PD-L1) has been developed to treat autoimmune diseases.
Therefore, MSC-sEVs-PD-L1 could target and repair tissue damage via
inhibiting immunoinflammatory cells through the PD-1-PD-L1 pathway.
Due to its simplicity of preparation, cheap cost, practicality and
biosafety, the MSC-sEVs-PD-L1 technology may have strong clinical
application potential (46).
The aforementioned studies indicated that PD-L1-Fc
alone or in combination could be considered as a therapeutic
approach for treating psoriasis. However, no research has proved
the efficacy of PD-L1-Fc in treating human psoriasis. Since the
T-cell subsets generating IL-17 in psoriasis animal models and
human patients differ, future studies are needed to determine
whether this variation could affect the efficiency of PD-L1 protein
on inhibiting psoriasis-related inflammation. In any case, PD-1 or
PD-L1 targeted treatment for psoriasis remains worthy of
exploration.
4. Lymphocyte-activation gene 3 (LAG-3)
LAG-3 (CD223) belongs to the superfamily of
immunoglobulins and negatively regulates the proliferation,
activation and homeostasis of T lymphocytes (47). To date, MHC-II, galectin-3, liver
sinusoidal endothelial cell C-type lectin (LSECtin), a-synuclein
and fibrinogen-like protein 1 (FGL1) have been identified to
interact with LAG-3(48). It has
been reported that galectin-3 and LSECtin are involved in T-cell
regulation (49,50), while a-synuclein is involved in the
neurological function of LAG3s (51). FGL1 is a key LAG-3 immune
inhibitory ligand (52).
Role of LAG-3 in psoriasis
A previous study revealed an inverse association
between the PASI score and the level of CD4+CD49b+LAG-3+Type 1
Tregs in the blood of patients with psoriasis (53). Reduced LAG-3 levels were also found
in patients with PsA (54). For
psoriasis, the production of interferon (IFN)a by plasmacytoid DCs
is the initial event in the innate cascade to pathologic
inflammation. The anti-LAG-3 mAb can activate LAG-3-mediated
signaling in pDCs in psoriatic lesions, thus attenuating IFNa
production and hindering the activation of dermal DCs and the onset
of pathogenic Th1 responses. Additionally, cytotoxic anti-LAG-3 mAb
could also decrease the number of autoreactive LAG-3+
T-cells (55).
LAG-3 in the treatment of
psoriasis
GSK2831781, a humanized IgG1 monoclonal antibody,
has a strong affinity with Fc receptors and LAG-3 and can therefore
deplete LAG-3 expressing cells. A phase I/Ib, double-blind,
placebo-controlled clinical study assessed the safety, tolerability
and therapeutic effect of GSK2831781 on patients with psoriasis.
The results showed that there were no safety or tolerability
concerns associated with GSK2831781. In addition, the treatment of
patients with GSK2831781 reduced the number of LAG-3+
and CD3+ T-cells in peripheral blood and psoriasis
plaque biopsies. Furthermore, a 5 mg/kg dosage of GSK2831781
decreased the expression of pro-inflammatory genes, such as those
of IL-17A, IL-17F, IFN and S100A12, and enhanced those associated
with epidermal barrier function, including cadherin-related family
member 1. All GSK2831781 dosages (0.5, 1.5 and 5 mg/kg) improved
the activity of psoriasis compared with a placebo group up to day
43(56). To the best of our
knowledge this was the first time that LAG-3 antibodies were used
in clinical practice to treat psoriasis. Single doses of >5
mg/kg were well tolerated and could reduce the number of
LAG-3+ T-cells in the blood and psoriatic lesions in a
dose-dependent manner. GSK2831781 was also related with reduced
disease activity in patients with mild-to-moderate, possibly due to
its downstream effects on pro-inflammatory and epithelial integrity
genes in psoriatic plaques.
Nevertheless, there are few studies on the
relationship between LAG-3 and psoriatic immunity, the molecular
mechanism and the compensation mechanism of its synergistic action
with other immune checkpoints. Therefore, further research is
urgently needed.
5. T-cell immunoglobulin and mucin
domain-containing protein 3 (TIM-3)
TIM-3 belongs to the Tim family and is mainly
expressed on Th1 and Th17 cells. It has been reported that TIM-3 is
involved in mediating cell apoptosis or inhibiting cell
differentiation, while it suppresses the IFN-γ- and IL-17-triggered
immune responses via adversely regulating their expression. The
currently known TIM-3 ligands include galectin 9 (Gal-9),
phosphatidylserine, high mobility group protein B1 and
carcinoembryonic antigen cell adhesion molecule 1(57). The immune regulation mediated by
TIM-3 and Gal-9 has been widely explored in various
immunological-related diseases.
Role of TIM-3 in psoriasis
Kanai et al (58) determined the expression levels of
TIM-3 in peripheral blood IL-17- or INF-γ-secreting T-cells through
flow cytometry to evaluate if T-cells have functional disorders in
psoriasis patients. In psoriasis, IL-17- or INF-γ-secreting T-cells
seem to have defective TIM-3 expression, which causes Th17/Th1
immunity to act more actively since Tim-3 could not provide braking
signals. Additionally, a markedly high expression of Gal-9 was
determined in the dermal fibroblasts isolated from patients with
psoriasis vulgaris. This finding was also verified in in
vitro experiments, indicating that IFN-γ could also stimulate
fibroblasts form the human dermis, thus successfully inducing Gal-9
expression (59).
Preclinical study of TIM-3 in the
treatment of psoriasis
Nishi et al (60) constructed a stable type of Gal-9
(sGal-9) by selectively deleting the linker peptide, which was
extremely resistant to proteolysis and kept its biological
activity. In a IL-23-induced psoriatic mouse model, sGal-9
administration ameliorated epidermal thickness and dermal cell
infiltration. At the same time, the levels of IL-17, IL-22, IL-6
and TNF-α in psoriatic lesions were reduced. Moreover, sGal-9
inhibited the expression of activated phosphorylated STAT3 in
epidermal KCs. Therefore, inhibiting the proteolysis of Gal-9 could
be considered as a potential therapy for Th1 or Th17 cell-mediated
autoimmune diseases, including psoriasis (61). However, no clinical trials or case
reports have been published on the effect of TIM-3 checkpoint
agents in the treatment of psoriasis at present.
6. Summary and scope
Psoriasis, a common but challenging disease in
dermatology, is a long-lasting autoimmune disease characterized by
abnormal skin patches. Co-inhibitory molecules are natural targets
for the immunotherapy of autoimmune diseases. Over the last few
decades, great progress has been made in the identification of
alternative targets and development of innovative targeted
medicines for the treatment of autoimmune disorders. In particular,
the successful clinical application of CTLA-4, PD-1 and PD-L1
targeting therapies has produced interest in identifying novel
therapeutic targets. When traditional therapy and biologics are
unresponsive or adverse reactions are intolerable, targeting
co-inhibitory molecules agents can be considered. In order to
maximize the efficacy of immunotherapy in treating psoriasis, there
are some noteworthy problems that still need to be solved. First, a
more in-depth and detailed understanding of these areas is required
to create and improve therapeutic approaches to these novel
targets. For example, TIM-3 has multiple ligands whose functions
have not been fully elucidated. Therefore, increased understanding
of the mechanisms of co-inhibitory molecules and their ligands in
psoriasis may contribute to their clinical application. Second, how
to transform the research on immune targets into clinical fields is
still a large challenge. It is hoped that more drug candidates can
be translated into clinical applications through rigorous
evidence-based studies, thus bringing new options for the treatment
of patients with psoriasis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LW and GZ contributed to the study conception and
design. Material preparation, data collection and analysis were
performed by YY, LZ, XH and JZ. The draft of the manuscript was
written by YY and all authors commented on previous versions of the
manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cai Y, Fleming C and Yan J: New insights
of T cells in the pathogenesis of psoriasis. Cell Mol Immunol.
9:302–309. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kim J and Krueger JG: Highly effective new
treatments for psoriasis target the IL-23/Type 17 T cell autoimmune
axis. Annu Rev Med. 68:255–269. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lowes MA, Russell CB, Martin DA, Towne JE
and Krueger JG: The IL-23/T17 pathogenic axis in psoriasis is
amplified by keratinocyte responses. Trends Immunol. 34:174–181.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hawkes JE, Chan TC and Krueger JG:
Psoriasis pathogenesis and the development of novel targeted immune
therapies. J Allergy Clin Immunol. 140:645–653. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang Q and Vignali DA: Co-stimulatory and
co-inhibitory pathways in autoimmunity. Immunity. 44:1034–1051.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mahoney KM, Rennert PD and Freeman GJ:
Combination cancer immunotherapy and new immunomodulatory targets.
Nat Rev Drug Discov. 14:561–584. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Schnell A, Bod L, Madi A and Kuchroo VK:
The yin and yang of co-inhibitory receptors: Toward anti-tumor
immunity without autoimmunity. Cell Res. 30:285–299.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reynolds J, Sando GS, Marsh OB, Salama AD,
Evans DJ, Cook HT and Pusey CD: Stimulation of the PD-1/PDL-1
T-cell co-inhibitory pathway is effective in treatment of
experimental autoimmune glomerulonephritis. Nephrol Dial
Transplant. 27:1343–1350. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ibañez-Vega J, Vilchez C, Jimenez K,
Guevara C, Burgos PI and Naves R: Cellular and molecular regulation
of the programmed death-1/programmed death ligand system and its
role in multiple sclerosis and other autoimmune diseases. J
Autoimmun. 123(102702)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kurita M, Yoshihara Y, Ishiuji Y, Chihara
M, Ishiji T, Asahina A and Yanaba K: Expression of T-cell
immunoglobulin and immunoreceptor tyrosine-based inhibitory motif
domain on CD4(+) T cells in patients with atopic dermatitis. J
Dermatol. 46:37–42. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Du Y, Nie L, Xu L, Wu X, Zhang S and Xue
J: Serum levels of soluble programmed death-1 (sPD-1) and soluble
programmed death ligand 1(sPD-L1) in systemic lupus erythematosus:
Association with activity and severity. Scand J Immunol.
92(e12884)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Greb JE, Goldminz AM, Elder JT, Lebwohl
MG, Gladman DD, Wu JJ, Mehta NN, Finlay AY and Gottlieb AB:
Psoriasis. Nat Rev Dis Primers. 2(16082)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee HJ and Kim M: Challenges and future
trends in the treatment of psoriasis. Int J Mol Sci.
24(13313)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lin X and Huang T: Co-signaling molecules
in psoriasis pathogenesis: Implications for targeted therapy. Hum
Immunol. 76:95–101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Salama AK and Hodi FS: Cytotoxic
T-lymphocyte-associated antigen-4. Clin Cancer Res. 17:4622–4628.
2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Suárez-Fariñas M, Li K, Fuentes-Duculan J,
Hayden K, Brodmerkel C and Krueger JG: Expanding the psoriasis
disease profile: Interrogation of the skin and serum of patients
with moderate-to-severe psoriasis. J Invest Dermatol.
132:2552–2564. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu P, He Y, Wang H, Kuang Y, Chen W, Li
J, Chen M, Zhang J, Su J, Zhao S, et al: The expression of mCTLA-4
in skin lesion inversely correlates with the severity of psoriasis.
J Dermatol Sci. 89:233–240. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang K, Zhu Q, Lu Y, Lu H, Zhang F, Wang X
and Fan Y: CTLA-4 +49 G/A polymorphism confers autoimmune disease
risk: An updated meta-analysis. Genet Test Mol Biomarkers.
21:222–227. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang XB, Zhao X, Giscombe R and Lefvert
AK: A CTLA-4 gene polymorphism at position -318 in the promoter
region affects the expression of protein. Genes Immun. 3:233–234.
2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ueda H, Howson JM, Esposito L, Heward J,
Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova
G, et al: Association of the T-cell regulatory gene CTLA4 with
susceptibility to autoimmune disease. Nature. 423:506–511.
2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pérez-García A, Osca G, Bosch-Vizcaya A,
Kelleher N, Santos NY, Rodríguez R, González Y, Roncero JM, Coll R,
Serrando M, et al: Kinetics of the CTLA-4 isoforms expression after
T-lymphocyte activation and role of the promoter polymorphisms on
CTLA-4 gene transcription. Hum Immunol. 74:1219–1224.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dursun HG, Yılmaz HO, Dursun R and
Kulaksızoğlu S: Association of Cytotoxic T Lymphocyte Antigen-4
gene polymorphisms with psoriasis vulgaris: A case-control study in
turkish population. J Immunol Res. 2018(1643906)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy
BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ,
et al: CTLA4Ig-mediated blockade of T-cell costimulation in
patients with psoriasis vulgaris. J Clin Invest. 103:1243–1252.
1999.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Abrams JR, Kelley SL, Hayes E, Kikuchi T,
Brown MJ, Kang S, Lebwohl MG, Guzzo CA, Jegasothy BV, Linsley PS
and Krueger JG: Blockade of T lymphocyte costimulation with
cytotoxic T lymphocyte-associated antigen 4-immunoglobulin
(CTLA4Ig) reverses the cellular pathology of psoriatic plaques,
including the activation of keratinocytes, dendritic cells, and
endothelial cells. J Exp Med. 192:681–694. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Altmeyer MD, Kerisit KG and Boh EE:
Therapeutic hotline. Abatacept: Our experience of use in two
patients with refractory psoriasis and psoriatic arthritis.
Dermatol Ther. 24:287–290. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Singh JA, Guyatt G, Ogdie A, Gladman DD,
Deal C, Deodhar A, Dubreuil M, Dunham J, Husni ME, Kenny S, et al:
Special Article: 2018 American College of Rheumatology/National
psoriasis foundation guideline for the treatment of psoriatic
arthritis. Arthritis Rheumatol. 71:5–32. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mease PJ, Gottlieb AB, van der Heijde D,
FitzGerald O, Johnsen A, Nys M, Banerjee S and Gladman DD: Efficacy
and safety of abatacept, a T-cell modulator, in a randomised,
double-blind, placebo-controlled, phase III study in psoriatic
arthritis. Ann Rheum Dis. 76:1550–1558. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu S, Xu J and Wu J: The role of
co-signaling molecules in psoriasis and their implications for
targeted treatment. Front Pharmacol. 12(717042)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Harris KM, Smilek DE, Byron M, Lim N,
Barry WT, McNamara J, Garcet S, Konrad RJ, Stengelin M, Bathala P,
et al: Effect of costimulatory blockade with abatacept after
ustekinumab withdrawal in patients with moderate to severe plaque
psoriasis: The PAUSE Randomized clinical trial. JAMA Dermatol.
157:1306–1315. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sharpe AH and Pauken KE: The diverse
functions of the PD1 inhibitory pathway. Nat Rev Immunol.
18:153–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Khatery BH, Shaker OG, El-Tahlawi S,
Abd-Elrahim TA, Fawzi M, Ali EM and Mohammed MH: Are programmed
cell death protein-1 and Angiopoietins-2 effective biomarkers for
detection the severity of psoriatic patients? J Cosmet Dermatol.
21:5208–5214. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jung CJ, Yang HJ, Bang SH, Lee WJ, Won CH,
Lee MW, Song Y and Chang SE: Clinicoprognostic and
histopathological features of guttate and plaque psoriasis based on
PD-1 expression. J Clin Med. 10(5200)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim DS, Je JH, Kim SH, Shin D, Kim TG, Kim
DY, Kim SM and Lee MG: Programmed death-ligand 1, 2 expressions are
decreased in the psoriatic epidermis. Arch Dermatol Res.
307:531–538. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tanaka R, Ichimura Y, Kubota N, Saito A,
Nakamura Y, Ishitsuka Y, Watanabe R, Fujisawa Y, Mizuno S,
Takahashi S, et al: Differential Involvement of Programmed Cell
Death Ligands in Skin Immune Responses. J Invest Dermatol.
142:145–154.e8. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bonigen J, Raynaud-Donzel C, Hureaux J,
Kramkimel N, Blom A, Jeudy G, Breton AL, Hubiche T, Bedane C,
Legoupil D, et al: Anti-PD1-induced psoriasis: A study of 21
patients. J Eur Acad Dermatol Venereol. 31:e254–e257.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sibaud V, Meyer N, Lamant L, Vigarios E,
Mazieres J and Delord JP: Dermatologic complications of
anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol.
28:254–263. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Voudouri D, Nikolaou V, Laschos K,
Charpidou A, Soupos N, Triantafyllopoulou I, Panoutsopoulou I,
Aravantinos G, Syrigos K and Stratigos A: Anti-PD1/PDL1 induced
psoriasis. Curr Probl Cancer. 41:407–412. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Murata S, Kaneko S, Harada Y, Aoi N and
Morita E: Case of de novo psoriasis possibly triggered by
nivolumab. J Dermatol. 44:99–100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sanlorenzo M, Vujic I, Daud A, Algazi A,
Gubens M, Luna SA, Lin K, Quaglino P, Rappersberger K and
Ortiz-Urda S: Pembrolizumab cutaneous adverse events and their
association with disease progression. JAMA Dermatol. 151:1206–1212.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lebwohl M, Strober B, Menter A, Gordon K,
Weglowska J, Puig L, Papp K, Spelman L, Toth D, Kerdel F, et al:
Phase 3 studies comparing brodalumab with ustekinumab in psoriasis.
N Engl J Med. 373:1318–1328. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
McInnes IB, Kavanaugh A, Gottlieb AB, Puig
L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM,
et al: Efficacy and safety of ustekinumab in patients with active
psoriatic arthritis: 1 year results of the phase 3, multicentre,
double-blind, placebo-controlled PSUMMIT 1 trial. Lancet.
382:780–789. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kim JH, Choi YJ, Lee BH, Song MY, Ban CY,
Kim J, Park J, Kim SE, Kim TG, Park SH, et al: Programmed cell
death ligand 1 alleviates psoriatic inflammation by suppressing
IL-17A production from programmed cell death 1-high T cells. J
Allergy Clin Immunol. 137:1466–1476.e3. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Leonardi CL, Powers JL, Matheson RT, Goffe
BS, Zitnik R, Wang A and Gottlieb AB: Etanercept Psoriasis Study
Group. Etanercept as monotherapy in patients with psoriasis. N Engl
J Med. 349:2014–2022. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Peng S, Cao M, Sun X, Zhou Y, Chen CY, Ma
T, Li H, Li B, Zhu B and Li X: Recombinant programmed cell death 1
inhibits psoriatic inflammation in imiquimod-treated mice. Int J
Mol Med. 46:869–879. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Xu F, Fei Z, Dai H, Xu J, Fan Q, Shen S,
Zhang Y, Ma Q, Chu J, Peng F, et al: Mesenchymal stem cell-derived
extracellular vesicles with high PD-L1 expression for autoimmune
diseases treatment. Adv Mater. 34(e2106265)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Anderson AC, Joller N and Kuchroo VK:
Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized
functions in immune regulation. Immunity. 44:989–1004.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hemon P, Jean-Louis F, Ramgolam K,
Brignone C, Viguier M, Bachelez H, Triebel F, Charron D, Aoudjit F,
Al-Daccak R and Michel L: MHC class II engagement by its ligand
LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J
Immunol. 186:5173–5183. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kouo T, Huang L, Pucsek AB, Cao M, Solt S,
Armstrong T and Jaffee E: Galectin-3 Shapes antitumor immune
responses by suppressing CD8+ T Cells via LAG-3 and inhibiting
expansion of plasmacytoid dendritic cells. Cancer Immunol Res.
3:412–423. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Xu F, Liu J, Liu D, Liu B, Wang M, Hu Z,
Du X, Tang L and He F: LSECtin expressed on melanoma cells promotes
tumor progression by inhibiting antitumor T-cell responses. Cancer
Res. 74:3418–3428. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Mao X, Ou MT, Karuppagounder SS, Kam TI,
Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, et al:
Pathological α-synuclein transmission initiated by binding
lymphocyte-activation gene 3. Science. 353(aah3374)2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang J, Sanmamed MF, Datar I, Su TT, Ji L,
Sun J, Chen L, Chen Y, Zhu G, Yin W, et al: Fibrinogen-like Protein
1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell.
176:334–347.e12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kim J, Lee J, Gonzalez J, Fuentes-Duculan
J, Garcet S and Krueger JG: Proportion of CD4(+)CD49b(+)LAG-3(+)
type 1 regulatory T cells in the blood of psoriasis patients
inversely correlates with psoriasis area and severity index. J
Invest Dermatol. 138:2669–2672. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gertel S, Polachek A, Furer V, Levartovsky
D and Elkayam O: CD4(+) LAG-3(+) T cells are decreased in active
psoriatic arthritis patients and their restoration in vitro is
mediated by TNF inhibitors. Clin Exp Immunol. 206:173–183.
2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Castelli C, Triebel F, Rivoltini L and
Camisaschi C: Lymphocyte activation gene-3 (LAG-3, CD223) in
plasmacytoid dendritic cells (pDCs): A molecular target for the
restoration of active antitumor immunity. Oncoimmunology.
3(e967146)2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ellis J, J B Marks D, Srinivasan N,
Barrett C, Hopkins TG, Richards A, Fuhr R, Albayaty M, Coenen M,
Liefaard L, et al: Depletion of LAG-3(+) T cells translated to
pharmacology and improvement in psoriasis disease activity: A phase
I randomized study of mAb GSK2831781. Clin Pharmacol Ther.
109:1293–1303. 2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wolf Y, Anderson AC and Kuchroo VK: TIM3
comes of age as an inhibitory receptor. Nat Rev Immunol.
20:173–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kanai Y, Satoh T, Igawa K and Yokozeki H:
Impaired expression of Tim-3 on Th17 and Th1 cells in psoriasis.
Acta Derm Venereol. 92:367–371. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Igawa K, Satoh T, Hirashima M and Yokozeki
H: Regulatory mechanisms of galectin-9 and eotaxin-3 synthesis in
epidermal keratinocytes: Possible involvement of galectin-9 in
dermal eosinophilia of Th1-polarized skin inflammation. Allergy.
61:1385–1391. 2006.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Nishi N, Itoh A, Fujiyama A, Yoshida N,
Araya S, Hirashima M, Shoji H and Nakamura T: Development of highly
stable galectins: Truncation of the linker peptide confers
protease-resistance on tandem-repeat type galectins. FEBS Lett.
579:2058–2064. 2005.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Niwa H, Satoh T, Matsushima Y, Hosoya K,
Saeki K, Niki T, Hirashima M and Yokozeki H: Stable form of
galectin-9, a Tim-3 ligand, inhibits contact hypersensitivity and
psoriatic reactions: A potent therapeutic tool for Th1- and/or
Th17-mediated skin inflammation. Clin Immunol. 132:184–194.
2009.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Mease P, Genovese MC, Gladstein G, Kivitz
AJ, Ritchlin C, Tak PP, Wollenhaupt J, Bahary O, Becker JC, Kelly
S, et al: Abatacept in the treatment of patients with psoriatic
arthritis: Results of a six-month, multicenter, randomized,
double-blind, placebo-controlled, phase II trial. Arthritis Rheum.
63:939–948. 2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Strand V, Alemao E, Lehman T, Johnsen A,
Banerjee S, Ahmad HA and Mease PJ: Improved patient-reported
outcomes in patients with psoriatic arthritis treated with
abatacept: Results from a phase 3 trial. Arthritis Res Ther.
20(269)2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Imai Y, Ayithan N, Wu X, Yuan Y, Wang L
and Hwang ST: Cutting Edge: PD-1 regulates imiquimod-induced
psoriasiform dermatitis through inhibition of IL-17A expression by
innate γδ-Low T Cells. J Immunol. 195:421–425. 2015.PubMed/NCBI View Article : Google Scholar
|















