Introduction
Antineutrophil cytoplasmic antibody
(ANCA)-associated vasculitis (AAV) is a group of autoimmune
diseases characterized by necrotizing vasculitis of small vessels,
including microscopic polyangiitis, granulomatosis with
polyangiitis and eosinophilic granulomatosis with polyangiitis; for
these diseases, ANCAs targeting proteinase-3 (PR3) or
myeloperoxidase are important diagnostic markers (1,2).
ANCA can also be detected in some patients with
infectious diseases such as subacute infective endocarditis, human
immunodeficiency virus (HIV) infection, chronic hepatitis B virus
infection and tuberculosis (3-6).
It is crucial to distinguish infections from AAV, as they have
completely different treatments and immunosuppressive drugs for
primary vasculitis can worsen infectious diseases (6).
The present study reports a patient who presented
with rapidly progressive glomerulonephritis (RPGN) due to PR3-AAV.
The patient experienced two relapses of acute kidney injury and was
ultimately diagnosed with subacute infective endocarditis after 1
year. Valve replacement surgery and antibiotic treatment achieved
complete remission of both kidney and cardiac function.
With the current case, the present study aimed to
emphasize the importance of identifying the causes of secondary AAV
to improve the understanding of the disease by clinicians,
preventing misdiagnosis and missed diagnosis, and providing
experience for its clinical diagnosis and treatment.
Case report
A 58-year-old male patient who had a history of
hypertension for 6 years was admitted to Peking University First
Hospital (Beijing, China) in December 2017 due to hematuria,
proteinuria and kidney dysfunction for 1 year and dyspnea for 2
weeks.
The patient presented with fatigue and anorexia 1
year beforehand (August 2016). Urinalysis revealed 50-70 deformed
red blood cells (normal range, 0-3) per high-power field. Urinary
protein excretion was 0.51 (normal range, <0.15) g/day and the
serum albumin concentration was 33.2 (normal range, 40-55) g/l. The
serum creatinine (SCr) concentration was 209 (normal range, 44-133)
µmol/l. The white blood cell count of the patient was 6.2 (normal
range, 3.5-9.5) x109/l and the neutrophil count was 3.6
(normal range, 1.8-6.3) x109/l. C-reactive protein (CRP)
was 13.4 (normal range, 0-8) mg/l, and the erythrocyte
sedimentation rate (ESR) was 60 (normal range, 0-15) mm/h. The
serum c-ANCA concentration was 1:32 (serum:diluent; normal range,
negative), and the anti-PR3 antibody concentration was >200
(normal range, <20) RU/ml. The rheumatoid factor (RF)
concentration was 155 (normal range, <30) IU/ml, and the
circulating immune complex (CIC) concentration was 147.1 (normal
range, <20) RU/ml. The IgG concentration was 35.1 (normal range,
7.2-16.8) g/l, and the complement C3 concentration was 0.507
(normal range, 0.6-1.5) g/l. Antinuclear antibody (IIF) and
anti-glomerular basement membrane (GBM) antibody were negative.
Hepatitis B, hepatitis C, syphilis and HIV screening were negative.
Kidney pathology revealed focal proliferative necrotizing
glomerulonephritis accompanied by crescent formation and immune
complex-mediated glomerulonephritis (Fig. 1A). Immunofluorescence staining of
frozen tissues was performed to detect IgG (Fig. 1Aa) and C3 (Fig. 1Ab), and was evaluated under a
fluorescence microscope as described previously (7). Kidney biopsy specimens were fixed in
4% buffered formaldehyde at 4˚C for at least 4 h for light
microscopy as described previously (8). Paraffin-embedded kidney sections (3
µm) were used for histologic staining, including periodic
acid-silver methenamine and Masson trichrome staining (Fig. 1Ac) and periodic acid-Schiff
staining (Fig. 1Ad) as described
previously (7). Approximately 1
cm3 of cortical renal tissue was taken, fixed with 3%
glutaraldehyde, postfixed in 1% osmium acid, dehydrated with a
graded acetone series and then embedded in epoxy resin. Tissues
were then sliced into 80-nm ultrathin sections and analyzed using
the JEM-1400 transmission electron microscope (Fig. 1Ae and Af) as described previously (9). Echocardiography revealed no
vegetation (Fig. 2A). The patient
was treated with a methylprednisolone pulse (500 mg/d x 3 days) two
times, followed by prednisone 50 mg/d for ~1 month, combined with
intravenous cyclophosphamide 0.6 g/month six times. After 1 month,
the SCr concentration decreased to 136 µmol/l, anti-PR3 antibody
level decreased to 154 RU/ml and the general condition of the
patient improved, with a Barthel activities of daily living (ADL)
index score of 100(10).
Prednisone was gradually reduced to 20 mg/d and cyclophosphamide
accumulated 3.6 g.
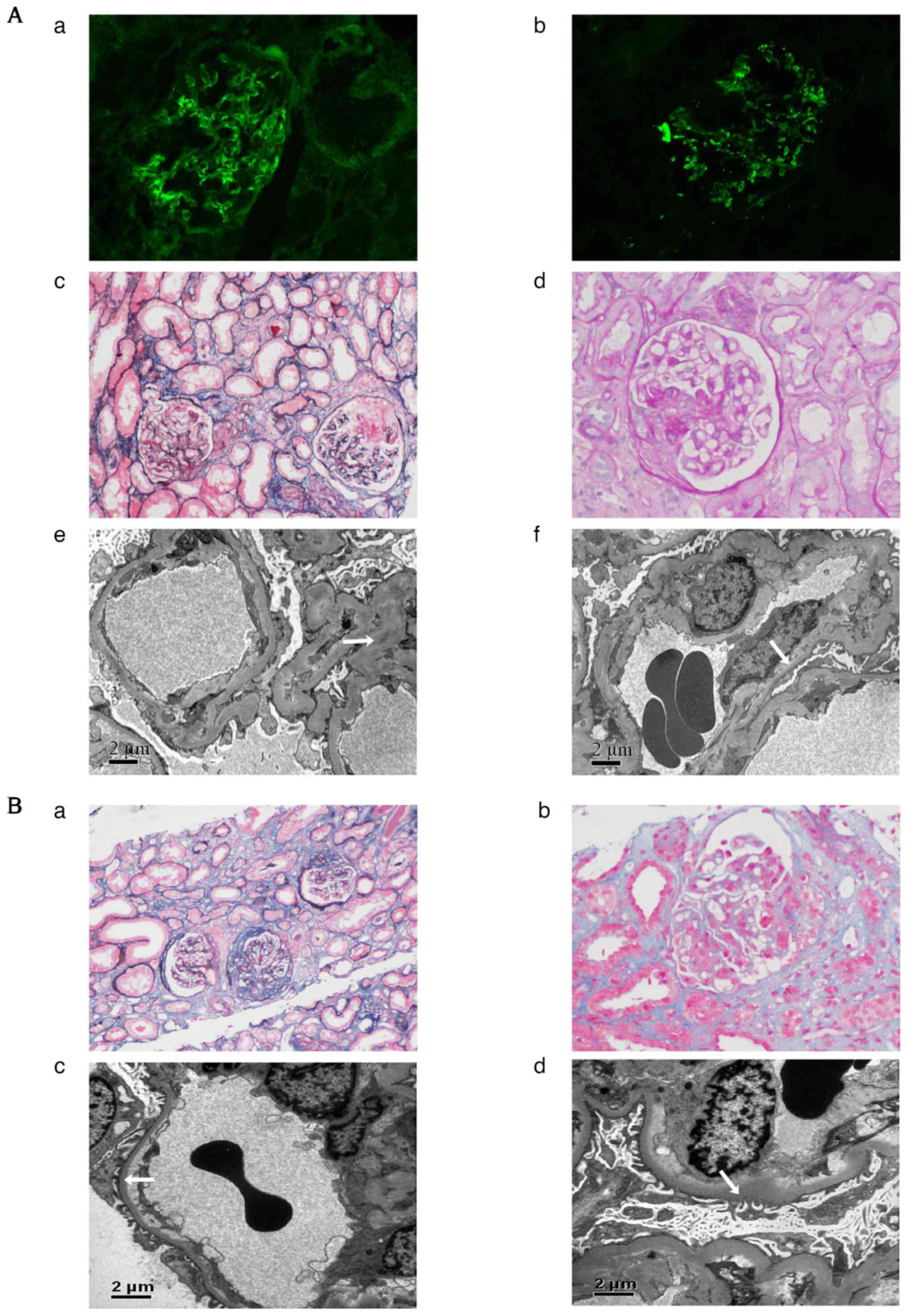 | Figure 1Kidney pathology of the patient. (A)
At the first kidney pathology, immunofluorescence showed granular
deposits of (Aa) IgG++ (magnification, x200) and (Ab)
C3+++ (magnification, x200) in the mesangial area and
along capillary walls. Light microscopy at a magnification of (Ac)
x100 (periodic acid-silver methenamine and Masson trichrome
staining) and (Ad) x200 (periodic acid-Schiff staining) showed
serious damage to glomerular capillary loops, including four
segmental fibrinoid necroses with small cellular crescents, two
cellular crescents, 11 fibrocellular crescents, one fibrous
crescent, four small fibrocellular crescents and six small
fibrocellular crescents in a total of 59 glomeruli. The remaining
31 glomeruli showed mild segmental mesangial hypercellularity. The
tubules presented with epithelial cell vacuolation, focal brush
margin shedding and most of the erythrocytes were cast. Focal
lymphoid and monocyte infiltration and a small amount of neutrophil
infiltration were observed in the interstitium. Transmission
electron microscopy (magnification, x8,000) showed (Ae) segmental
endothelial cell proliferation, massive electron dense deposits in
the mesangial area (arrow), (Af) segmental loose layer widening in
the basement membrane and segmental fusion of the epithelial foot
processes (arrow). (B) At the second kidney pathology,
immunofluorescence showed granular deposits of IgM++ and
C3+++ in the mesangial area. Light microscopy at a
magnification of (Ba) x100 (periodic acid-silver methenamine and
Masson trichrome staining) and (Bb) x200 (Masson trichrome
staining) showed 24 glomeruli, including three fibrous crescents
with sclerosis, one fibrocellular crescent and five small
fibrocellular crescents. The remaining glomeruli showed slight
diffuse hyperplasia of the mesangial cells and matrix, with focal
segmental aggravation and fuchsinophilic deposition. Segmental
endothelial cell proliferation. The tubules presented with
epithelial cell vacuolation and granular degeneration, focal brush
margin shedding and red blood cell and protein casts. The
interstitium was focally infiltrated with lymphocytes and
mononuclear cells. Transmission electron microscopy (magnification,
x6,700) showed (Bc) no electronic dense deposits, (Bd) segmental
loose layer widening in the basement membrane, and extensive fusion
of the epithelial foot processes (arrow). |
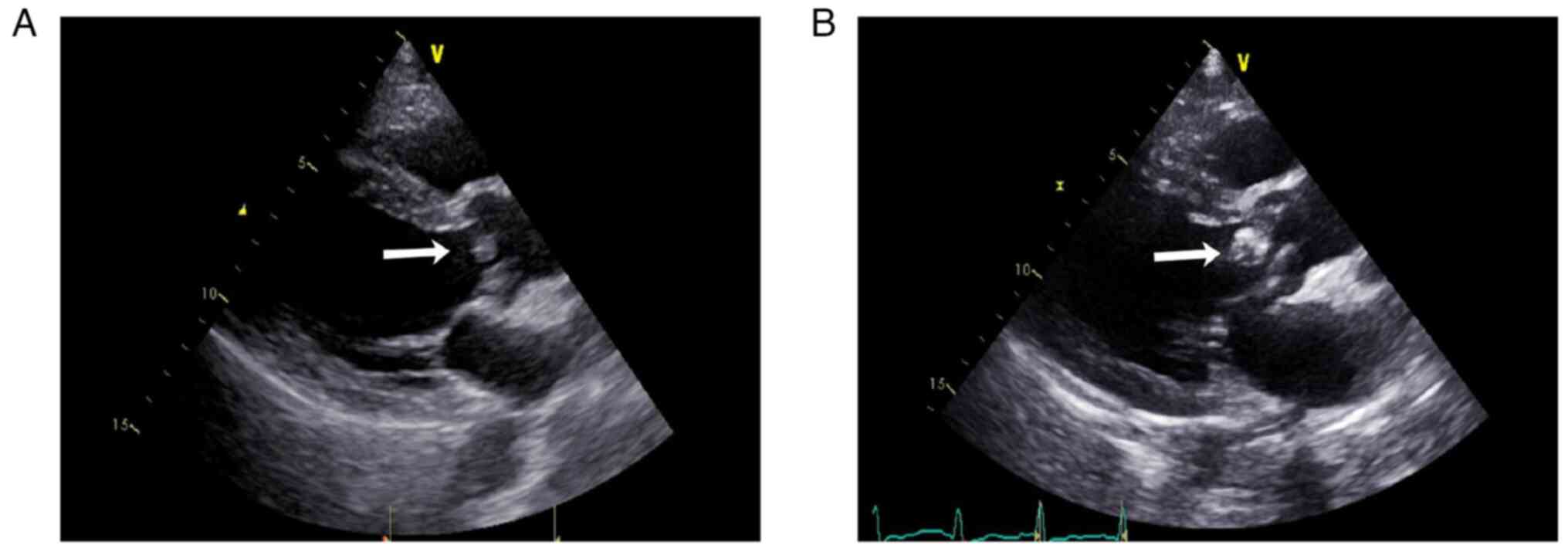 | Figure 2Cardiac ultrasonography of the
patient. (A) At the time of disease onset, cardiac ultrasonography
showed left ventricular symmetrical hypertrophy, moderate aortic
regurgitation, aortic valve sclerosis/calcification (no aortic
valve vegetation, arrow), mild aortic stenosis, mild mitral and
tricuspid regurgitation and a normal left ventricular ejection
fraction. (B) Approximately 1 year later, cardiac ultrasonography
revealed aortic and mitral valve disorders, aortic valve vegetation
(arrow), moderate to severe aortic regurgitation, mild aortic
stenosis, severe mitral regurgitation, left atrium and left
ventricular enlargement, left ventricular symmetrical hypertrophy,
a normal left ventricular ejection fraction (62.3%), mild tricuspid
regurgitation and elevated pulmonary systolic pressure. |
In March 2017, the patient had experienced recurrent
fatigue, anorexia and gross hematuria. Urinary protein excretion
was 2.4 g/d. SCr increased from 141 to 328 µmol/l. The hemoglobin
level of the patient decreased from 104 to 82 (normal range,
130-175) g/l, CRP level rose from 3 to 12 mg/l and ESR was 65 mm/h.
The white blood cell count was 6.3x109/l, and the
neutrophil count was 5.1x109/l. The anti-PR3 antibody
concentration was 156 RU/ml. The patient was positive for
cryoglobulin, monoclonal IgM λ and polyclonal IgG. RF was 50 IU/ml.
C3 was 0.141 g/l and C4 was 0.103 (normal range, 0.12-0.36) g/l. No
obvious abnormalities were found in the bone marrow.
Echocardiography also revealed no vegetation. Kidney pathology was
repeated and revealed AAV-associated kidney injury and
proliferative glomerulonephritis (Fig.
1B). Kidney biopsy specimens were fixed in 4% buffered
formaldehyde at 4˚C for at least 4 h for light microscopy as
described previously (8).
Paraffin-embedded kidney sections (3 µm) were used for histologic
staining, including periodic acid-silver methenamine and Masson
trichrome staining (Fig. 1Ba) and
Masson trichrome staining (Fig.
1Bb) as described previously (7). Approximately 1 cm3 of
cortical renal tissue was taken, fixed with 3% glutaraldehyde,
postfixed in 1% osmium acid, dehydrated with a graded acetone
series and then embedded in epoxy resin. Tissues were then sliced
into 80-nm ultrathin sections and analyzed using the JEM-1400
transmission electron microscope (Fig.
1Bc and Bd) as described
previously (9). The patient
received two rounds of methylprednisolone pulse therapy, 3 L of
plasma exchange every other day for six cycles, and two rounds of
intravenous rituximab for a total of 600 mg (one 100 mg and another
500 mg 2 weeks later). Subsequently, the SCr concentration of the
patient decreased to 170 µmol/l in May 2017 and then to 99 µmol/l
in August 2017. Cryoglobulin testing became negative. RF was 25
IU/ml. C3 was 0.678 g/l, and C4 was 0.211 g/l. The patient's
hemoglobin rose to 114 g/l. The anti-PR3 antibody test was
negative. The number of total B cells was <5/µl. The patient's
general condition improved, the Barthel ADL index score of the
patient was 100 and prednisone was decreased to 7.5 mg/d.
At 2 weeks prior to this admission in December 2017,
the patient developed dyspnea, orthopnea, nausea, anorexia and
gross hematuria. Urinary protein excretion was 0.27 g/d (urine
volume, 150 ml). Urinalysis revealed 100 red blood cells and 80
white blood cells (normal range, 0-5) per high-power field. The
patient's white blood cell count was 23.4x109/l, the
neutrophil count was 19.8x109/l, the hemoglobin level
was 108 g/l and the platelet count was 184 (normal range, 125-350)
x109/l. CRP was 20 mg/l. The procalcitonin (PCT) level
of the patient was 4.03 (normal range, <0.05) ng/ml. Alanine
aminotransferase was 645 (normal range, 9-50) U/l, aspartate
aminotransferase was 705 (normal range, 15-40) U/l, albumin was
28.2 g/l and SCr was 191 µmol/l, lactate dehydrogenase was 2,220
(normal range, 100-240) IU/l, cardiac troponin (CTnI) was 0.76
(normal range, 0-0.03) ng/ml and brain natriuretic peptide was
2,988 (normal range, <100) pg/ml. The patient was treated with
40 mg/d methylprednisolone and admitted to Peking University First
Hospital (Beijing, China) in December 2017.
On admission, the body temperature of the patient
was 36.5˚C, his blood pressure was 90/60 mmHg and heart rate was
110 beats/min. The patient's body weight was 55 kg and BMI was 19
kg/m2. The conjunctiva was pale. There was no distension
in the jugular vein. The breathing sounds in both lower lungs were
weak with wet rales. The left cardiac boundary was enlarged, and S3
and III/6 systolic murmurs were heard in the apical area. The liver
was palpable 3 cm under the right rib. There was mild tenderness
under the xiphoid process, without rebound pain, and shifting
dullness was present. There was no edema in either lower limb.
The patient was positive for type II cryoglobulin.
C3 was <0.058 g/l, C4 was <0.032 g/l and C1q was 116.5
(normal range, 159-223) mg/l. ANCA, antinuclear antibody (IIF),
anti-β2-glycoprotein 1 antibody (ELISA), anticardiolipin antibody
(ELISA) and anti-GBM antibody were negative. The IgG, IgA and IgM
levels were within the normal range. The ESR was 21 mm/h. Infection
screening, which included bacteria, fungi and viruses and was
performed on serology and specimens, including sputum, stool and
urine, was negative. Due to the long-term use of steroids and
immunosuppressants and the high leukocyte, neutrophil counts and
PCT, the patient received 1.5 g of intravenous
cefoperazone-sulbactam every 12 h. Blood culture (after intravenous
cefoperazone-sulbactam at 1.5 g every 12 h for one week) was
negative three times. Echocardiography (Fig. 2B) revealed vegetation on the aortic
valve. The patient denied a history of intravenous illicit drug
use. After careful questioning it was revealed that the patient had
a history of tooth extraction without antibiotics 1 year prior.
The patient was diagnosed with PR3-AAV secondary to
subacute infective endocarditis, accompanied by cryoglobulinemia.
The patient received hemodialysis and was treated with intravenous
cefoperazone-sulbactam at 1.5 g every 12 h for 1 month before
surgery. The glucocorticoid was reduced to 10 mg/d. Mitral valve
and aortic valve replacement and coronary artery bypass grafting
were performed. Pathology revealed fibrous hyperplasia with
calcification and tissue degeneration in the valve tissue, which
was locally covered with endothelial cells. The vegetation culture
test was also negative. Postoperatively, the patient continued to
receive intravenous antibiotic treatment for 1 month, which
included cefoperazone-sulbactam at 1.5 g every 12 h for 1 week,
biapenem at 0.3 g every 12 h for 2 weeks and piperacillin-sulbactam
at 2.5 g every 12 h for 1 week. At 1 week after surgery,
echocardiography showed a normal left ventricular ejection fraction
(55.5%), mild tricuspid regurgitation and mild pulmonary
hypertension. After 3 weeks, the SCr level was normal. The
patient's cardiac function recovered to Grade I (New York Heart
Association grade) (11).
Glucocorticoid and immunosuppressants were all stopped.
Discussion
The present study is a case of refractory PR3-AAV in
which the patient experienced two relapses within 1 year under
intensive glucocorticoid therapy, immunosuppressive therapy and
plasma exchange. The features of cryoglobulinemia,
hypocomplementemia and obvious cardiac insufficiency suggested
secondary reasons for AAV, and echocardiography resulted in the
diagnosis of aortic valve vegetation. Antibiotic treatment and
valve replacement surgery (according to the guidelines of European
Society of Cardiology and Chinese Society of Cardiology) achieved
complete remission of kidney and cardiac dysfunction in the absence
of glucocorticoid and immunosuppressants. The present case
demonstrated that identifying AAV driven by infections is of
importance for preventing the exacerbation of infections caused by
immunosuppressive drugs.
The differential diagnosis of secondary AAV from
primary AAV can be challenging, especially for subacute infective
endocarditis in which ~25% of the patients with RPGN are initially
considered to have primary vasculitis (12). However, distinguishing AAV
secondary to infection is highly valuable because of the entirely
different therapies and outcomes used (12-14).
In the present study, the patient was first diagnosed with primary
PR3-AAV according to the 2012 Revised International Chapel Hill
Consensus Conference Nomenclature of Vasculitides (1), based on crescent formation in the
glomeruli, PR3-ANCA in the circulation and negative results from
echocardiography and other screenings for malignancies or drugs.
The patient received intensive treatment comprising steroids,
immunosuppressants and plasma exchange, but experienced two
relapses within 1 year.
There were several hints in the present case of
PR3-AAV secondary to subacute infective endocarditis. i)
Hypocomplementemia is not common in primary AAV (15). Although complement activation has
been demonstrated to participate in the AAV mechanism (16) and therapies targeting C5 have
achieved effectiveness in clinical trials (17), the circulating levels of C3 and C4
are mostly within the normal range in primary AAV (15). The reductions in C3 and C4,
together with the increase in the circulating immune complex and in
the amount of immune complex deposits in the glomeruli, prompted
possible infections in the current patient. ii) Cryoglobulinemia is
rare in primary AAV. Type II cryoglobulin was detected in the
current patient, which is often due to chronic infections or
malignancies (18). The bone
marrow and lymph nodes were screened, and the results were
negative. Hepatitis, syphilis, HIV and tuberculosis were also
negative. Thus, subacute infective endocarditis was suspected to be
the cause of the patient's cryoglobulinemia. iii) Pericarditis,
myocarditis and abnormal conduction have been reported in patients
with cardiac involvement of primary AAV, but valve lesions are rare
(19-22).
According to the literature review by Chirinos et al
(15), the most common
echocardiographic findings of endocardial compromise in patients
with AAV are aortic valve thickening and aortic insufficiency with
or without aortic root dilatation. Thus, the presence of aortic
valve vegetation in the present patient was suspected to indicate
subacute infective endocarditis rather than cardiac involvement of
the primary AAV. Blood culture was performed three times, and
vegetation culture was performed after surgery; however, all the
results were negative because of continuous antibiotic treatment
during the disease course.
Kidney damage in patients with infective
endocarditis is characterized by necrotizing and crescentic
glomerulonephritis (53%) or endocapillary proliferative
glomerulonephritis (37%). C3 deposition occurs in almost all
patients, but IgG deposition is less common (<30%). Electron
dense deposits can be observed in most patients via electron
microscopy. In addition, 28% of the patients are ANCA positive.
Most of them are PR3-ANCA and may be depleted after the resolution
of infective endocarditis. A total of 56% of the patients are
complicated with hypocomplementemia and some are also cryoglobulin
positive (15,23-25).
These features were also revealed in the present case.
The pathogenesis of ANCA formation in infective
endocarditis is not clear. Mahr et al (26) reported that among patients with
infective endocarditis, ANCA-positive patients had
echocardiography-documented vegetation more often than
ANCA-negative patients did. The antigenic stimulation by
neutrophilic enzymes released within vegetation and the
non-specific hyperimmune humoral response may be the underlying
mechanisms. Konstantinov et al (27) discussed the proposed mechanisms of
ANCA formation during the course of infections, including
autoantigen complementarity, molecular mimicry between bacteria and
self-antigens, epigenetic modifications, neutrophil extracellular
traps and interactions between bacterial components and Toll-like
receptors. Further investigations are required to clarify these
mechanisms to further develop preventive measures and therapeutic
interventions.
In conclusion, the present study reported a case of
PR3-AAV secondary to infective endocarditis, which highlighted the
necessity of identifying the causes of ANCA formation, including
infections, drugs, malignancies and others, not only at the first
diagnosis but also during the disease course.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by grants from the Natural
Science Foundation of China (grant nos. 81870486, 82070732 and
82090021) and CAMS Innovation Fund for Medical Sciences (grant no.
2019-I2M-5-046).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HL and ZC collected and analyzed the patient data
and wrote the manuscript. XJZ and XNH obtained echocardiography
images. ZC, XJZ, YY, XNH, XHL and FDZ advised on patient treatment.
MHZ advised on patient treatment and gave approval of the
manuscript to be published. HL and ZC confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study followed the Declaration of Helsinki and
was approved by the ethics committee of Peking University First
Hospital [approval no. 2017 (1280)].
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the data and images in this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jennette JC, Falk RJ, Bacon PA, Basu N,
Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen
EC, et al: 2012 revised international chapel hill consensus
conference nomenclature of vasculitides. Arthritis Rheum. 65:1–11.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bosch X, Guilabert A and Font J:
Antineutrophil cytoplasmic antibodies. Lancet. 368:404–418.
2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Uh M, McCormick IA and Kelsall JT:
Positive cytoplasmic antineutrophil cytoplasmic antigen with PR3
specificity glomerulonephritis in a patient with subacute bacterial
endocarditis. J Rheumatol. 38:1527–1528. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mandell BF and Calabrese LH: Infections
and systemic vasculitis. Curr Opin Rheumatol. 10:51–57.
1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fukasawa H, Hayashi M, Kinoshita N,
Ishigaki S, Isobe S, Sakao Y, Kato A, Fujigaki Y and Furuya R:
Rapidly progressive glomerulonephritis associated with PR3-ANCA
positive subacute bacterial endocarditis. Intern Med. 51:2587–2590.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ying CM, Yao DT, Ding HH and Yang CD:
Infective endocarditis with antineutrophil cytoplasmic antibody:
Report of 13 cases and literature review. PLoS One.
9(e89777)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xing GQ, Chen M, Liu G, Wang SX and Zhao
MH: Renal neutrophils infiltration in antineutrophil cytoplasmic
antibodies-negative pauci-immune crescentic glomerulonephritis. Am
J Med Sci. 340:474–480. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yong ZH, Yu XJ, Liu JX, Zhou FD, Wang SX
and Zhao MH: Kidney histopathologic spectrum and clinical
indicators associated with MGRS. Clin J Am Soc Nephrol. 17:527–534.
2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yuan X, Su Q, Wang H, Shi S, Liu L, Lv J,
Wang S, Zhu L and Zhang H: Genetic variants of the COL4A3, COL4A4,
and COL4A5 genes contribute to thinned glomerular basement membrane
lesions in sporadic IgA nephropathy patients. J Am Soc Nephrol.
34:132–144. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu W, Unick J, Galik E and Resnick B:
Barthel Index of activities of daily living: Item response theory
analysis of ratings for long-term care residents. Nurs Res.
64:88–99. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McDonagh TA, Metra M, Adamo M, Gardner RS,
Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et
al: 2021 ESC Guidelines for the diagnosis and treatment of acute
and chronic heart failure. Eur Heart J. 42:3599–3726.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ai S, Liu J, Ma G, Ye W, Hu R, Zhang S,
Fan X, Liu B, Miao Q, Qin Y and Li X: Endocarditis-associated
rapidly progressive glomerulonephritis mimicking vasculitis: A
diagnostic and treatment challenge. Ann Med. 54:754–763.
2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brunet A, Julien G, Cros A, Beaudoux O,
Hittinger-Roux A, Bani-Sadr F, Servettaz A and N'Guyen Y:
Vasculitides and glomerulonephritis associated with
Staphylocococcus aureus infective endocarditis: Cases reports and
mini-review of the literature. Ann Med. 52:265–274. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Satoskar AA, Parikh SV and Nadasdy T:
Epidemiology, pathogenesis, treatment and outcomes of
infection-associated glomerulonephritis. Nat Rev Nephrol. 16:32–50.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chirinos JA, Corrales-Medina VF, Garcia S,
Lichtstein DM, Bisno AL and Chakko S: Endocarditis associated with
antineutrophil cytoplasmic antibodies: A case report and review of
the literature. Clin Rheumatol. 26:590–595. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen M, Jayne DRW and Zhao MH: Complement
in ANCA-associated vasculitis: Mechanisms and implications for
management. Nat Rev Nephrol. 13:359–367. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jayne DRW, Merkel PA, Schall TJ and Bekker
P: ADVOCATE Study Group. Avacopan for the treatment of
ANCA-associated vasculitis. N Engl J Med. 384:599–609.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Silva F, Pinto C, Barbosa A, Borges T,
Dias C and Almeida J: New insights in cryoglobulinemic vasculitis.
J Autoimmun. 105(102313)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hagen EC, Daha MR, Hermans J, Andrassy K,
Csernok E, Gaskin G, Lesavre P, Lüdemann J, Rasmussen N, Sinico RA,
et al: Diagnostic value of standardized assays for anti-neutrophil
cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR
project for ANCA assay standardization. Kidney Int. 53:743–753.
1998.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Goodfield NE, Bhandari S, Plant WD,
Morley-Davies A and Sutherland GR: Cardiac involvement in Wegener's
granulomatosis. Br Heart J. 73:110–115. 1995.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bourgarit A, Toumelin PL, Pagnoux C, Cohen
P, Mahr A, Guern VL, Mouthon L and Guillevin L: French Vasculitis
Study Group. Deaths occurring during the first year after treatment
onset for polyarteritis nodosa, microscopic polyangiitis, and
Churg-Strauss syndrome: A retrospective analysis of causes and
factors predictive of mortality based on 595 patients. Medicine
(Baltimore). 84:323–330. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lacoste C, Mansencal N, Ben M'rad M,
Goulon-Goeau C, Cohen P, Guillevin L and Hanslik T: Valvular
involvement in ANCA-associated systemic vasculitis: A case report
and literature review. BMC Musculoskelet Disord.
12(50)2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Choi HK, Lamprecht P, Niles JL, Gross WL
and Merkel PA: Subacute bacterial endocarditis with positive
cytoplasmic antineutrophil cytoplasmic antibodies and
anti-proteinase 3 antibodies. Arthritis Rheum. 43:226–231.
2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bonaci-Nikolic B, Andrejevic S, Pavlovic
M, Dimcic Z, Ivanovic B and Nikolic M: Prolonged infections
associated with antineutrophil cytoplasmic antibodies specific to
proteinase 3 and myeloperoxidase: Diagnostic and therapeutic
challenge. Clin Rheumatol. 29:893–904. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Boils CL, Nasr SH, Walker PD, Couser WG
and Larsen CP: Update on endocarditis-associated
glomerulonephritis. Kidney Int. 87:1241–1249. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mahr A, Batteux F, Tubiana S, Goulvestre
C, Wolff M, Papo T, Vrtovsnik F, Klein I, Iung B and Duval X: IMAGE
Study Group. Brief report: Prevalence of antineutrophil cytoplasmic
antibodies in infective endocarditis. Arthritis Rheumatol.
66:1672–1677. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Konstantinov KN, Ulff-Møller CJ and
Tzamaloukas AH: Infections and antineutrophil cytoplasmic
antibodies: Triggering mechanisms. Autoimmun Rev. 14:201–203.
2015.PubMed/NCBI View Article : Google Scholar
|
















