Introduction
Adenomyosis is a benign uterine disorder
characterized by the presence of abnormal endometrial glands and
stroma in the myometrium (1).
Women with adenomyosis may suffer from heavy menstrual bleeding,
dysmenorrhea, reproductive failure and infertility (2). Hysterectomy is the most effective
treatment for adenomyosis (3). To
increase desired fertility, preserve sexual function and attenuate
stress in younger patients, uterus-sparing therapy tends to be a
popular treatment for adenomyosis (3). Therefore, it is important to explore
the mechanism underlying adenomyosis and develop novel treatments.
Some studies (4-6)
have reported that inflammatory molecules, sex steroid hormone
receptors, growth factors and extracellular matrix enzymes serve
important roles in the pathogenesis of adenomyosis. However, the
underlying etiology remains unclear.
Amino acids are the substrates needed for protein
synthesis, serving critical roles in metabolism as sources of
energy, carbon and nitrogen. Amino acids, including glutamine
(Gln), have been reported to regulate cell proliferation, apoptosis
and metabolism (7). Although Gln
is a non-essential amino acid, it is of great importance in
intermediary metabolism (8). Gln
is a substrate for protein and nucleotide synthesis (9). Furthermore, Gln can be a nitrogen and
carbon donor that provides energy for cell proliferation (10). Gln transporters are the main
regulators of the intracellular Gln level (11). The solute carrier (SLC) superfamily
constitutes the largest group of transporters for transporting
large numbers of substances, including drugs and ions (11,12).
SLC dysfunction can induce a diverse range of diseases, such as
heart disease, Alzheimer's disease, diabetes and cancer (13-15).
SLC family 38 member a2 (SLC38A2) belongs to the system A (alanine
preferential) transporters and contributes to a high Gln
concentration (10). Given the
critical function of SLC38A2 in amino acid transport, SLC38A2
serves an important role in numerous signaling pathways and medical
conditions, including diabetes, neurological diseases and cancer
(10). SLC38A2, a Gln transporter,
is a major regulator of intracellular Gln levels and can lead to
excessive Gln concentrations (10). In immune disorders and other
chronic inflammatory diseases, such as endometriosis, activation of
the immune system consumes a large amount of energy, of which
glucose and Gln are the main sources (10). An increased level of Gln has been
observed in patients with endometriosis (16,17).
As adenomyosis is considered internal endometriosis (16) and as elevated SLC38A2 leads to the
increased uptake of Gln (17), it
is reasonable to hypothesize that SLC38A2 serves a role in
adenomyosis. However, to the best of our knowledge, the Gln content
and the function of SLC38A2 in adenomyosis remain largely unclear.
The purpose of the present study was to explore the function of
SLC38A2 and determine whether SLC38A2 increases the cell
proliferation rate by increasing mitochondrial respiratory function
in adenomyosis.
Materials and methods
Samples
Between January 2023 and February 2023, 10 paired
eutopic endometrial (EU) and ectopic endometrial (EC) tissues were
derived from 10 adenomyotic patients who received a partial
resection of adenomyotic tissues at Shanghai Pudong Hospital
(Shanghai, China). The last tissue was collected on February 16,
2023. The preoperative diagnosis of adenomyosis was based on the
typical clinical symptoms of dysmenorrhea and/or menorrhagia and
pelvic pain, physical examination findings and imaging results,
including transvaginal ultrasound and MRI scans. EC tissue,
referring to the adenomyosis nidus formed locally by the invasion
of the endometrium and stroma into the muscle layer, was collected
during laparoscopic surgical procedures, and the paired EU tissue
was collected through scraping during surgery. The age of the
patients included in the present study ranged between 35 and 45
years, with a mean age of 38.96±5.01 years. All women in the
control and adenomyosis groups had regular menstrual cycles (28-35
days). The exclusion criteria were: i) Patients who were diagnosed
with uterine fibroids and subsequently received hormonal therapy
within the previous 6 months; ii) patients who had reproductive
tract infections, immune system disease or endocrine diseases; iii)
patients whose preoperative hysteroscopy excluded the diagnosis of
endometrial lesions; and iv) patients with comorbidities or major
organ dysfunction. Samples were obtained when the women were in the
early proliferative phase of the menstrual cycle. Normal
endometrial (CE) tissues were obtained between January 2023 and
February 2023 from 10 women (age range, 27-41 years; mean age,
34.27±4.62 years) who voluntarily sought placement of an
intrauterine device at Shanghai Pudong Hospital (Shanghai, China)
and who had no diseases and had not used hormone drugs in the
previous 3 months; these patients were considered controls. Written
informed consent was obtained from all participating patients. The
present study was approved by the Ethics Committee of Shanghai
Pudong Hospital (approval no. 2023-WZ-04) and performed in
accordance with the tenets and guidelines of the Declaration of
Helsinki.
Cells
The three types of fresh tissues (CE, EU and EC
tissues) were washed three times in Hank's Balanced Salt Solution
(Gibco; Thermo Fisher Scientific, Inc.) containing 1,000 U/ml
penicillin and 1,000 µg/ml streptomycin. The 10 CE, EU or EC
samples were digested together using 0.4% collagenase and 0.05%
pancreatic enzyme plus 20 µg/ml DNase I at 37˚C with shaking. After
2-3 h, the digestive solution was replaced with DMEM-F12 (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin at 37˚C with 5% CO2. CE, EU and EC cells
were obtained using a method established in a previous study
(18). The primary cells were
passaged at a fusion rate of 80% after digestion using 0.25%
trypsin. The cells were passaged every 3 days. At 8 days after
tissue digestion, CE, EU and EC cells (third passage) were used for
the subsequent experiments.
Lentivirus construction
Lentiviruses (including SLC38A2 overexpression and
knockdown lentiviruses, constructed into the pLVX-Puro plasmid and
pLKO.1 plasmid, respectively) were custom synthesized from Shanghai
GeneChem Co., Ltd. Briefly, the SLC38A2 coding sequence was cloned
into the pLVX-Puro vector (Clontech; Takara Bio USA, Inc.), and the
SLC38A2 short hairpin RNA (shRNA/sh) was cloned into the pLKO.1
vector (Clontech; Takara Bio USA, Inc.). The 293T cells (The Cell
Bank of Type Culture Collection of The Chinese Academy of Science)
were transfected with the constructed vector, lentiviral packaging
and envelope plasmids (psPAX2 and pMD2G; at the ratio of 10:9:1 µg;
Addgene, Inc.) using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.). The 293T cells were cultured with DMEM-F12
containing 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin
at 37˚C with 5% CO2 for 48 h. The lentiviral
supernatants were then harvested by centrifugation at 3,000 x g at
4˚C for 10 min. and filtered using a 0.45-µm filter. A MOI of 10
was used to infect cells. The EU/EC cells were infected with
lentiviral supernatant, incubated at 37˚C overnight using DMEM-F12
containing 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin
at 37˚C with 5% CO2, selected with 1.5 µg/ml puromycin
(Merck KGaA) for 14 days and maintained with 0.625 µg/ml puromycin
for the subsequent experiments. The following three knockdown
lentiviruses and a non-targeting control (shNC) were used:
shSLC38A2-1, 5'-GCTTTGTTCTTCCTGTTAA-3'; shSLC38A2-2,
5'-GAAGAAGTATGAAACAGAA-3'; shSLC38A2-3, 5'-GCATCTGGATCAATTACAA-3';
and shNC, 5'-GGACGAGCTGTACAAGTAA-3'. The empty pLVX-Puro vector was
used as the NC for overexpression, and the cells in the control
group were not infected.
Cell treatment
The cell experiment was divided into three sections.
In section 1, we have assessed the effects of Gln exposure on the
cell viability of EU and EC cells. The EU and EC cells were treated
with Gln (1.5 mM; G3126; Merck KGaA) and the cells were cultured
with DMEM-F12 containing 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin at 37˚C with 5% CO2 for 48 h. Finally, cell
viability was assessed using a Cell Counting Kit-8 assay. In
section 2, the effects of SLC38A2 knockdown on EC cells and SLC38A2
overexpression on EU cells were evaluated. The effectiveness of the
vector was detected by RT-qPCR and western blotting. The stable
transgenic EC cells and EU cells were cultured with DMEM-F12
containing 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin
at 37˚C with 5% CO2 for 48 h. The cell viability was
assessed at 0, 12, 24 and 48 h. The cellular oxygen consumption
rate (OCR), invasion, Gln content and relative protein levels were
finally evaluated. In section 3, the SLC38A2-overexpressing EU
cells were treated with 10 µM CB-839 (glutaminase inhibitor; Merck
KGaA) and cultured with DMEM-F12 containing 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin at 37˚C with 5%
CO2 for 48 h. The cell viability was assessed at 0, 12,
24 and 48 h. The Gln content and cell invasion were finally
evaluated. Three independent experiments were performed in
duplicate (six duplications for the OCR assay).
Reverse transcription-quantitative
PCR
Total RNA was isolated from tissues or treated cells
(2x107) using TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.) and reverse transcribed into cDNA using a
High Capacity cDNA RT kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. The
fluorophore used in qPCR was 2x SYBR Green PCR Master mix (Thermo
Fisher Scientific, Inc.), and PCR was performed on an ABI7500
real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling conditions were as follows: Denaturation
95˚C for 5 min, followed by 40 cycles of denaturation at 95˚C for
10 sec, annealing at 60˚C for 20 sec and extension at 72˚C for 20
sec. Relative gene expression was analyzed using the
2-ΔΔCq method with GAPDH as the
internal control (19). The primer
sequences are listed in Table I.
Three independent experiments were performed in duplicate.
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| SLC38A1 |
GCCATTATGGGCAGTGGGAT |
TACGAACTTCCCTGTGGTGC |
| SLC38A2 |
GACCGCAGCCGTAGAAGAAT |
ACAGCCAGACGGACAATGAG |
| SLC38A3 |
AGTCTGAGCTGCCACTTGTC |
AGAGAGAAGCCGCTGGAGTA |
| SLC38A4 |
ATGAACACCATCCCGGAACC |
CCCTCCTTCCTTGGCTGTTT |
| SLC38A5 |
TGTGAGGCCCAGATGTTCAC |
AGGTTGCTGTGAGCCCATAC |
| SLC38A6 |
TGTCCAGCAGCCTGAAGAAG |
CAGGAGAGCAACTGTCAGCA |
| SLC38A7 |
CCCCAGGGAGATTGGTTTCC |
GGTCTTCACTTCAGGCTGCT |
| SLC38A8 |
CTCAGCGAGATCGTCAGCAT |
AAGATGAAGGTGCCGACCAG |
| SLC38A9 |
TGATAATCCTATTTTCATTCGCACA |
CCCTGAGCATTCAAGTCAGC |
| SLC38A10 |
TACGCCGGCCTGGCATTC |
ATGGAGGCCATCATGTTCCG |
| SLC38A11 |
TGTAGCCACGCTTGTGTCAT |
CCATGGGTGCAGTCTTGAGT |
| GAPDH |
AATCCCATCACCATCTTC |
AGGCTGTTGTCATACTTC |
Cell proliferation assay
Cells (2x103 cells per well) were seeded
in a 96-well plate with six replicates per group and cultured at
37˚C overnight. Then, cells in each group were treated as
indicated. At each time point, the culture medium was replaced with
90 µl DMEM-F12 without FBS and 10 µl Cell Counting Kit-8 (Beyotime
Institute of Biotechnology) for 1 h of culture. Finally, cell
proliferation was analyzed by measuring the absorbance value at 450
nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Transwell assay
The Transwell assay was performed to evaluated the
invasion of cells. The Transwell chamber (Corning, Inc.) was
pre-coated with 80 µl Matrigel (Corning, Inc.) at 37˚C for 30 min.
Subsequently, cells (1x105 cells/ml; 200 µl) were added
into the upper chamber of the insert with DMEM-F12 without FBS, and
DMEM-F12 with 10% FBS was added to the lower chamber. After 48 h of
incubation at 37˚C, the cells in the lower chamber were fixed with
4% formaldehyde solution for 10 min at room temperature and stained
with 0.5% crystal violet for 30 min at room temperature. Finally,
unmigrated cells in the upper chamber were removed and the migrated
cells in the lower chamber were imaged using a light microscope
(CX33; Olympus Corporation).
Seahorse assay
The cellular OCR was analyzed using a Seahorse XF24
analyzer (Agilent Technologies, Inc.) as described in a previous
study (20). The OCR experiments
were performed in six parallel replicates.
Gln content
Cells were seeded in a 24-well plate at a density of
1x104 cells/well and treated as indicated. The
concentration of Gln was measured using a Glutamine Assay Kit
(Colorimetric) (cat. no. ab197011; Abcam) according to the
manufacturer's instructions.
Western blotting
The total protein of the cell samples was extracted
using protein extraction reagent (RIPA buffer; cat. no. 89901;
Thermo Fisher Scientific, Inc.) and the protein concentration was
initially measured using the BCA method according to the
instructions of the Pierce Rapid Gold BCA assay kit (cat. no.
A53226; Thermo Fisher Scientific, Inc.). Thereafter, total protein
(25 µg/lane) was fractionated via 10% SDS-PAGE and transferred onto
nitrocellulose membranes (MilliporeSigma), which were then blocked
with 5% nonfat dry milk at room temperature for 1 h, and incubated
with primary antibodies at 4˚C overnight. After washing three times
using TBS with Tween-20 (0.1%), the membranes were incubated with
horseradish peroxidase-labelled secondary antibody anti-IgG (cat.
no. A0208/A0192; 1:1,000; Beyotime Institute of Biotechnology) at
37˚C for 1 h. An enhanced chemiluminescence system (Ranon GIS-2008;
Tanon Science and Technology Co., Ltd.) was used to determine the
protein levels using ECL-PLUS reagent (Thermo Fisher Scientific,
Inc.). The protein bands were analyzed using ImageJ 1.8 (National
Institutes of Health). The primary antibodies were: Anti-SLC38A2
(cat. no. bs-12125R; 1:1,000; BIOSS), anti-NADH-ubiquinone
oxidoreductase subunit B8 (NDUFB8; cat. no. ab192878; 1:1,000;
Abcam), anti-ubiquinol-cytochrome c reductase core protein 2
(UQCRC2; cat. no. ab203832; 1:1,000; Abcam) and anti-β-actin (cat.
no. 81115-1-RR; 1:5,000; Proteintech Group, Inc.). β-actin served
as the loading control.
Statistical analysis
The results are presented as the mean ± standard
deviation of three or more independent experiments performed in
duplicate. GraphPad Prism 7 (Dotmatics) was used for statistical
analysis and graph generation. Statistical comparisons between
groups were performed using an unpaired Student's t-test, or
a one-way ANOVA followed by Tukey's multiple comparisons test, or a
two-way ANOVA with Bonferroni as the post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
SLC38A2 expression is increased in EC
samples
The mRNA expression levels of the SLC38A family,
including SLC38A1-11, were examined in CE, EU and EC tissues. As
shown in Fig. 1A-C, SLC38A2,
SLC38A3, SLC38A4, SLC38A5, SLC38A8, SLC38A10 and SLC38A11
expression was increased in EU and EC tissues. However, only
SLC38A2 expression was significantly upregulated in EC samples
compared with that in CE samples.
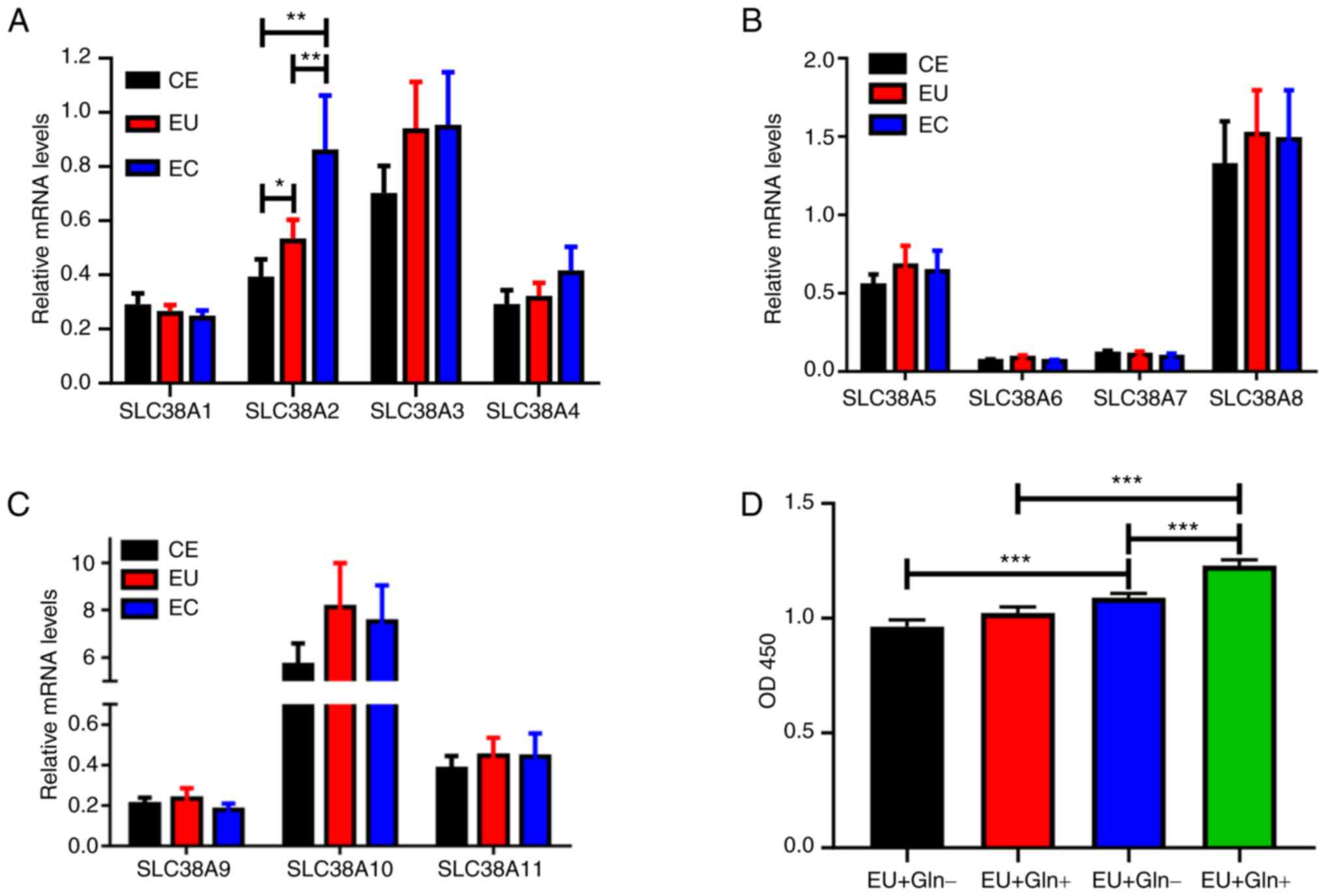 | Figure 1SLC38A2 expression is upregulated in
EC samples. (A) mRNA expression levels of the SLC38A family, (A)
SLC38A1-4, (B) SLC38A5-8 and (C) SLC38A9-11, were detected using
reverse transcription-quantitative PCR in CE, EU and EC tissues.
(D) Cell proliferation was measured in EU and EC cells treated with
or without Gln. *P<0.05, **P<0.01 and
***P<0.001. CE, normal endometrial; EC, ectopic
endometrial; EU, eutopic endometrial; Gln, glutamine; OD, optical
density; SLC38A, solute carrier family 38 member a. |
EC cells exhibit more dependence on
Gln
Gln was applied to the cells derived from EU and EC
tissues. A cell proliferation assay demonstrated that Gln
significantly promoted EC cell proliferation at 48 h after
treatment (Fig. 1D). However, no
significant increase was observed in EU cells with or without Gln
treatment. EC cells with or without Gln treatment exhibited
enhanced viability compared with EU cells, indicating that only EC
cell proliferation was dependent on Gln.
Silencing of SLC38A2 inhibits cell
proliferation, invasion, Gln content and the OCR in EC cells
To explore the function of SLC38A2, lentiviruses
targeting SLC38A2 were constructed to knock down SLC38A2
expression. As shown in Fig. 2A,
both the protein and mRNA levels of SLC38A2 were markedly decreased
following transfection with shSLC38A2 lentiviruses. Cell
proliferation was examined and the results showed that SLC38A2
knockdown decreased the proliferation of EC cells at 48 h (Fig. 2B). Similarly, the number of
invading cells was significantly decreased in the SLC38A2 knockdown
groups compared with that in the NC group, indicating that
silencing of SLC38A2 significantly inhibited the invasion of EC
cells (Fig. 2C). Furthermore,
SLC38A2 knockdown decreased the Gln content in EC cells (Fig. 2D). SLC38A2 knockdown reduced the
OCR, and downregulated the protein levels of NDUFB8 and UQCRC2,
which are markers of mitochondrial respiration (21) (Fig.
2E-G).
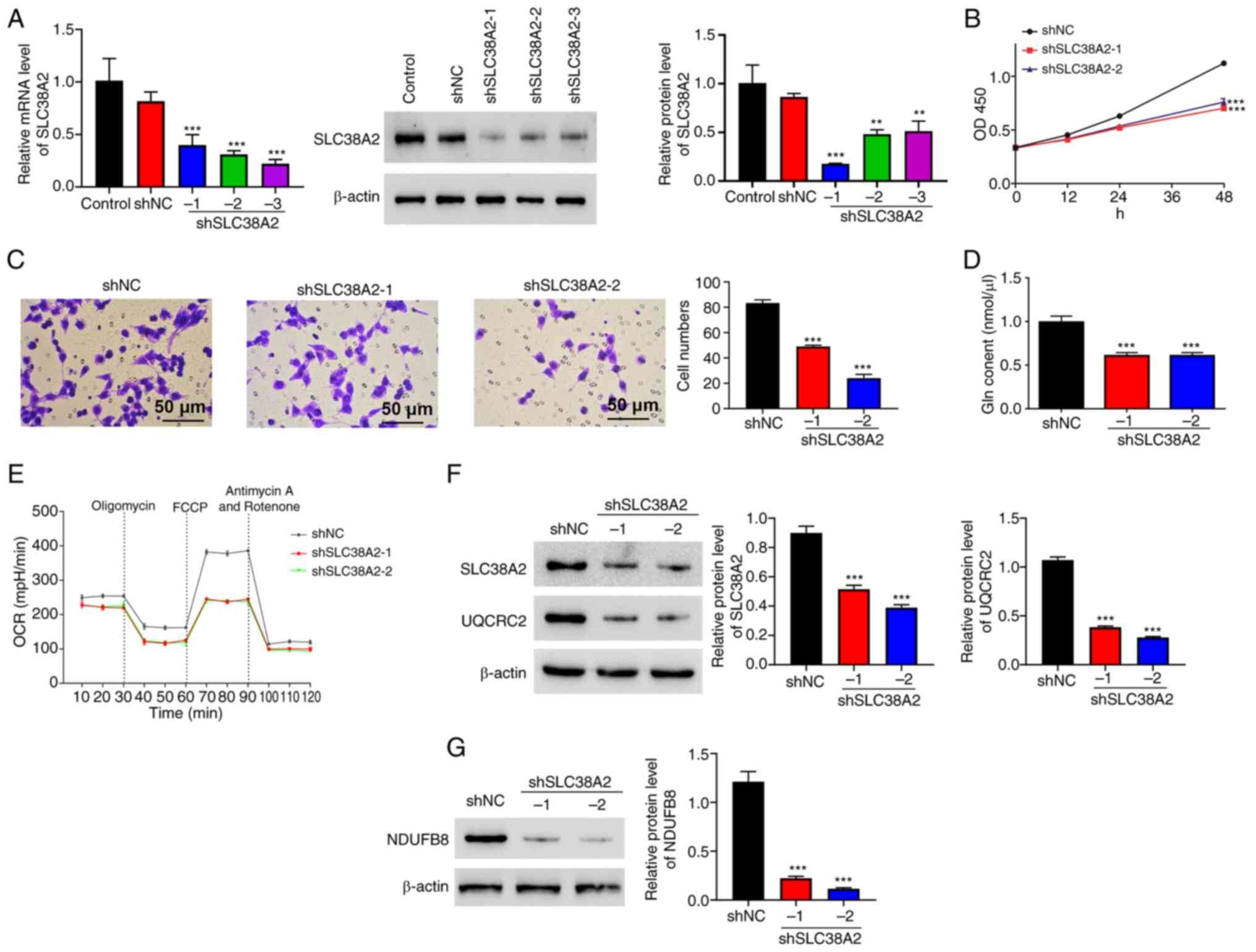 | Figure 2Silencing of SLC38A2 inhibits cell
proliferation, invasion, Gln content and the OCR in EC cells.
SLC38A2 knockdown lentiviruses were applied to EC cells. (A) mRNA
and protein expression levels of SLC38A2 were detected using
reverse transcription-quantitative PCR and western blotting. (B)
Cell proliferation, (C) invasion (scale bar, 50 µm), (D) Gln
content and (E) OCR level, and protein levels of (F) UQCRC2 and (G)
NDUFB8 were examined. **P<0.01 and
***P<0.001 vs. shNC. EC, ectopic endometrial; FCCP,
carbonylcyanide-p-trifluoromethoxyphenylhydrazone; Gln, glutamine;
NC, negative control; NDUFB8, NADH-ubiquinone oxidoreductase
subunit B8; OCR, oxygen consumption rate; OD, optical density; sh,
short hairpin RNA; SLC38A2, solute carrier family 38 member a2;
UQCRC2, ubiquinol-cytochrome c reductase core protein 2. |
SLC38A2 overexpression increases cell
proliferation and invasion rates, Gln content, and the OCR in EU
cells
SLC38A2 was overexpressed in EU cells following
transfection with SLC38A2 overexpression lentivirus (Fig. 3A). Cell proliferation and invasion
were analyzed after SLC38A2 overexpression. As shown in Fig. 3B and C, SLC38A2 overexpression significantly
increased the proliferation and invasion of EU cells at 48 h. In
addition, SLC38A2 overexpression increased the Gln content in EU
cells (Fig. 3D), implying that the
amount of Gln was increased. SLC38A2 overexpression increased the
OCR, and increased the protein levels of NDUFB8 and UQCRC2
(Fig. 3E-G).
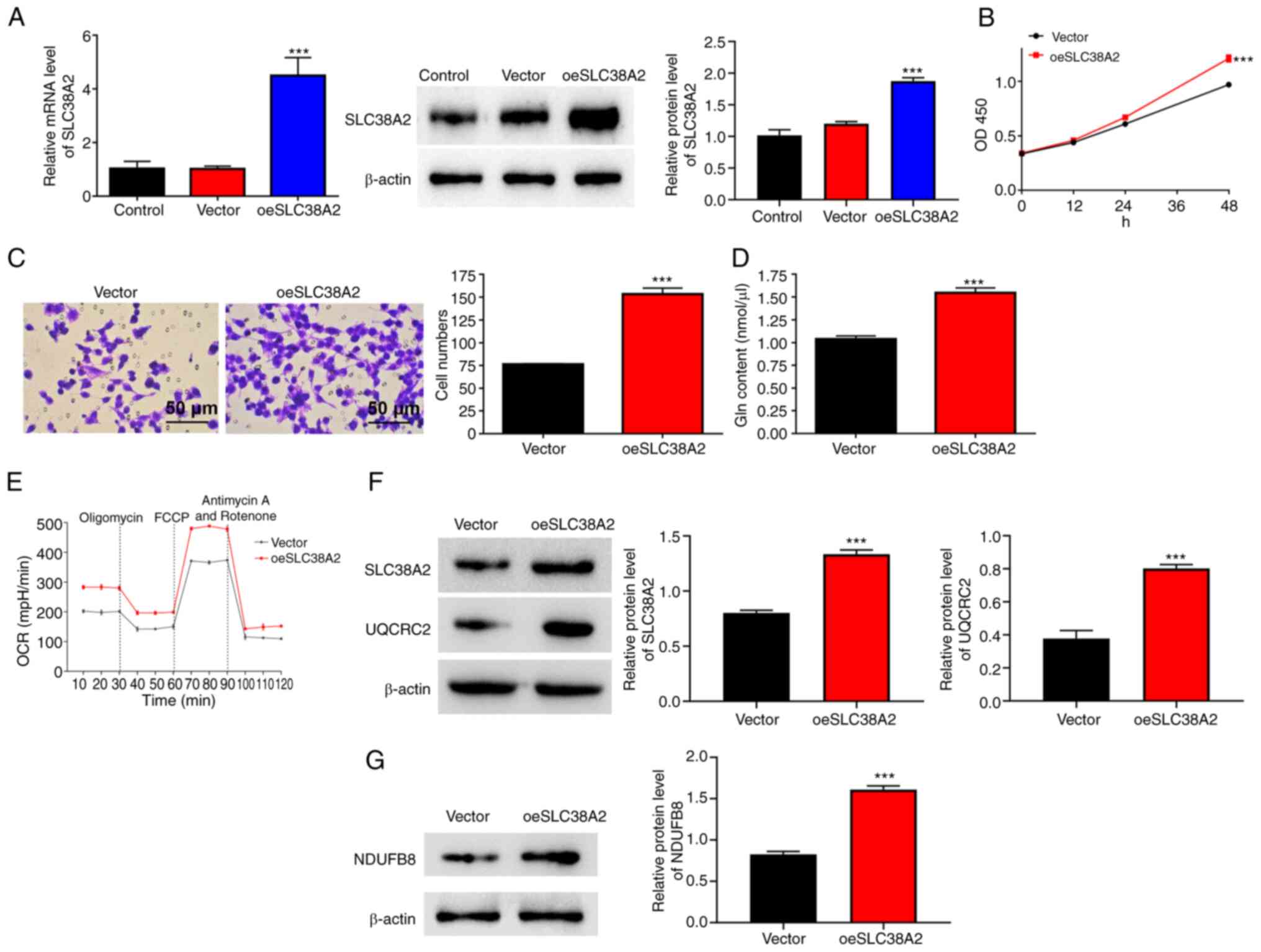 | Figure 3SLC38A2 overexpression increases cell
proliferation, invasion, Gln content and the OCR in EU cells.
SLC38A2 overexpression lentivirus was applied to EU cells. (A) mRNA
and protein expression levels of SLC38A2 were detected using
reverse transcription-quantitative PCR and western blotting. (B)
Cell proliferation, (C) invasion (scale bar, 50 µm), (D) Gln
content and (E) OCR level, and protein levels of (F) UQCRC2 and (G)
NDUFB8 were examined. ***P<0.001 vs. vector. EU,
eutopic endometrial; FCCP,
carbonylcyanide-p-trifluoromethoxyphenylhydrazone; Gln, glutamine;
NDUFB8, NADH-ubiquinone oxidoreductase subunit B8; OCR, oxygen
consumption rate; OD, optical density; SLC38A2, solute carrier
family 38 member a2; oeSLC38A2, SLC38A2 overexpression; UQCRC2,
ubiquinol-cytochrome c reductase core protein 2. |
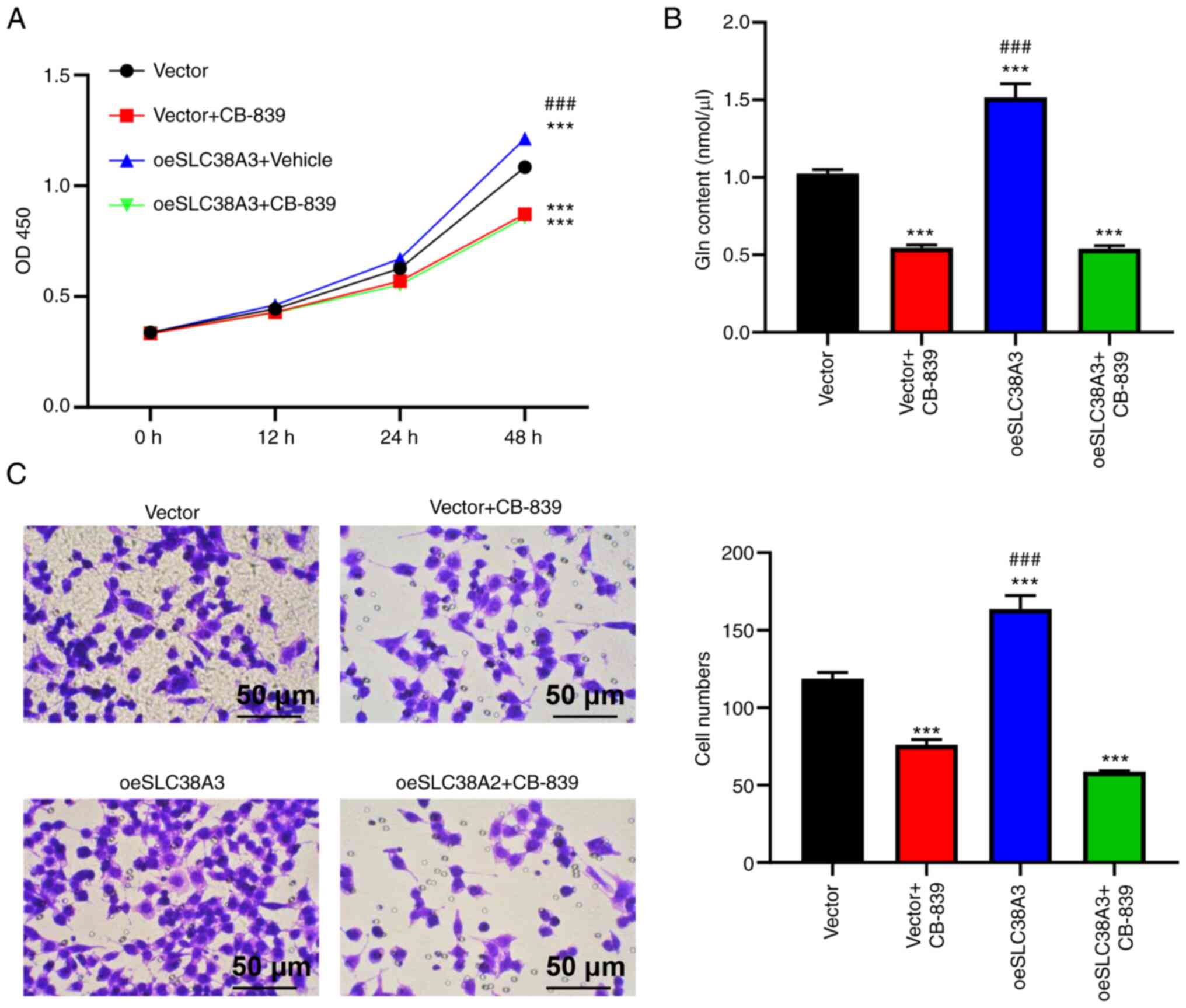
Effects of SLC38A2 overexpression are
attenuated by a glutaminase inhibitor
The present study then explored whether the function
of SLC38A2 was dependent on the Gln content. Accordingly, 10 µM
CB-839, a glutaminase inhibitor, was used to treat EU cells after
SLC38A2 was overexpressed. The increase in cell proliferation
induced by SLC38A2 overexpression was inhibited by CB-839 at 48 h
(Fig. 4A). A higher Gln content
was observed in the SLC38A2 overexpression group compared with the
vector group. Compared with that in the SLC38A2 overexpression
group, the Gln content in the group treated with SLC38A2
overexpression lentivirus and CB-839 was significantly decreased
(Fig. 4B). As shown in Fig. 4C, the number of invading cells was
increased by SLC38A2 overexpression but decreased by CB-839
treatment compared with the vector group, and CB-839 abolished the
effect of SLC38A2 overexpression, indicating that the promoting
effect of SLC38A2 overexpression on cell invasion was blocked by
CB-839. Additionally, CB-839 treatment alone decreased the cell
proliferation at 48 h and invasion rates, and Gln content in EU
cells (Fig. 4A-C). Taken together,
these results indicated that SLC38A2 functioned by regulating Gln
metabolism.
Discussion
Adenomyosis has been extensively studied; however,
to the best of our knowledge, how metabolic pathways are affected
in this disease remains largely unknown. Bourdon et al
(22) reported a higher level of
valine in patients with diffuse inner adenomyosis compared with
that in control individuals. Valine is an essential amino acid and
a precursor of the tricarboxylic acid cycle intermediate
succinyl-coenzyme A (22).
However, whether and how other amino acids, such as Gln, regulate
adenomyosis remains to be elucidated.
To the best of our knowledge, the present study was
the first to use CE, EU and EC tissues and primary cells from these
tissue sources to explore the roles of Gln in adenomyosis. The
results demonstrated that SLC38A2 was upregulated in EC tissues
compared with paired EU tissues and CE tissues. Furthermore,
SLC38A2 knockdown has been demonstrated to inhibit cell
proliferation, decrease Gln content and induce reactive oxygen
species production in Gln-sensitive breast cancer cell lines
(23). Genetic ablation of SLC38A2
decreased the proliferation of skeletal stem and progenitor cells,
thereby reducing the number of osteoblasts and bone-forming
activity (24). The present study
demonstrated that manipulation of the SLC38A2 level interfered with
cell phenotype acquisition (cell proliferation, invasion and Gln
content) in EU and EC cells. The SLC38A2 level was positively
associated with the malignant phenotype of adenomyosis in
vitro.
Gln is the most abundant amino acid in the
circulatory system, serving an essential role in energy generation,
especially in cancer cells (25).
Alterations in Gln and glutamate levels are observed early in the
course of endometriosis in patients, indicating that Gln serves a
role in endometriosis (17).
Altered Gln and glutamate levels have been found to be associated
with endometriosis-associated pelvic pain (26). Additionally, some studies showed
that SLC1A5 knockdown decreased Gln content (27) and an anti-SLC1A5 monoclonal
antibody inhibited tumor growth (28). In the present study, silencing of
SLC38A2 decreased Gln content and inhibited proliferation in EC
cells. Silencing of SLC38A2 was shown to decrease the OCR and the
protein levels of NDUFB8 and UQCRC2, indicating that mitochondrial
respiration was reduced. Furthermore, SLC38A2 knockdown decreased
cell proliferation, probably by mediating mitochondrial
impairment.
SLC38A2 is a Gln transporter that serves a key role
in the rapid division of T cells by mediating net Gln uptake
(29). In immune disorders and
other chronic inflammatory diseases, such as endometriosis,
activation of the immune system consumes large amounts of energy,
with glucose and Gln being the main sources of abundant energy
(30). Elevated Gln levels have
been observed in endometriosis. Elevated SLC38A2 expression leads
to increased Gln uptake (31).
Therefore, it was hypothesized that SLC38A2 serves an important
role in the pathogenesis of endometriosis/adenomyosis. A previous
study has confirmed that both glutamate glutathione and oxidized
glutathione are increased in the myometrium of adenomyosis
(32). The present study examined
the metabolic function of Gln in adenomyosis and found that SLC38A2
promoted adenomyosis in a Gln-dependent manner. However, how Gln
promotes the acquisition of specific cell phenotypes remains
unclear and more studies are needed to explore the mechanism
underlying the effect of SLC38A2 on mitochondrial respiration in
adenomyosis. The limitation of the present study was that the role
of cell death was not further investigated. The role of cell death
may be further investigated in future studies. In addition to in
vitro studies, transgenic mouse models are also needed to
further study the role of SLC38A2 in the pathogenesis of
adenomyosis. SLC38A2 may be a useful target for adenomyosis. In
conclusion, the present study provided evidence to support future
investigation of SLC38A2 as a potential target for adenomyosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key Specialty
Construction Project of Pudong Health and Family Planning
Commission of Shanghai (grant no. PWZzk2022-21) and Talents
Training Program of Pudong Hospital Affiliated to Fudan University
(grant no. PJ201902).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LC and HHK confirm the authenticity of all the raw
data. LC and HHK conceived and designed the study. JCH and YCD
performed the experiments. KW and WG analyzed and interpreted the
data. KW and HKK performed the statistical analysis. KW and WG
drafted the manuscript. HHK revised the manuscript for important
intellectual content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shanghai Pudong Hospital (approval no. 2023-WZ-04;
Shanghai, China) and all patients participating in the study
provided written informed consent prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhai J, Vannuccini S, Petraglia F and
Giudice LC: Adenomyosis: Mechanisms and pathogenesis. Semin Reprod
Med. 38:129–143. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vannuccini S and Petraglia F: Recent
advances in understanding and managing adenomyosis. F1000Res.
8(F1000 Faculty Rev-283)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jiang L, Han Y, Song Z and Li Y: Pregnancy
outcomes after uterus-sparing operative treatment for adenomyosis:
A systematic review and meta-analysis. J Minim Invasive Gynecol.
30:543–554. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Carrarelli P, Yen CF, Funghi L, Arcuri F,
Tosti C, Bifulco G, Luddi A, Lee CL and Petraglia F: Expression of
inflammatory and neurogenic mediators in adenomyosis. Reprod Sci.
24:369–375. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Carrarelli P, Yen CF, Arcuri F, Funghi L,
Tosti C, Wang TH, Huang JS and Petraglia F: Myostatin, follistatin
and activin type II receptors are highly expressed in adenomyosis.
Fertil Steril. 104:744–752.e1. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vannuccini S, Tosti C, Carmona F, Huang
SJ, Chapron C, Guo SW and Petraglia F: Pathogenesis of adenomyosis:
An update on molecular mechanisms. Reprod Biomed Online.
35:592–601. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu G: Amino acids: Metabolism, functions,
and nutrition. Amino Acids. 37:1–17. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang Y, Chen Q, Fan S, Lu Y, Huang Q, Liu
X and Peng X: Glutamine sustains energy metabolism and alleviates
liver injury in burn sepsis by promoting the assembly of
mitochondrial HSP60-HSP10 complex via SIRT4 dependent protein
deacetylation. Redox Rep. 29(2312320)2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang D, Hua Z and Li Z: The role of
glutamate and glutamine metabolism and related transporters in
nerve cells. CNS Neurosci Ther. 30(e14617)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Menchini RJ and Chaudhry FA: Multifaceted
regulation of the system A transporter Slc38a2 suggests nanoscale
regulation of amino acid metabolism and cellular signaling.
Neuropharmacology. 161(107789)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Garibsingh RA and Schlessinger A: Advances
and challenges in rational drug design for SLCs. Trends Pharmacol
Sci. 40:790–800. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Colas C, Ung PM and Schlessinger A: SLC
transporters: Structure, function, and drug discovery. Medchemcomm.
7:1069–1081. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li YY, Lu XZ, Wang H, Yang XX, Geng HY,
Gong G, Zhan YY, Kim HJ and Yang ZJ: Solute carrier family 30
member 8 gene 807C/T polymorphism and type 2 diabetes mellitus in
the Chinese population: A meta-analysis including 6,942 subjects.
Front Endocrinol (Lausanne). 9(263)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li L, He M, Zhou L, Miao X, Wu F, Huang S,
Dai X, Wang T and Wu T: A solute carrier family 22 member 3 variant
rs3088442 G→A associated with coronary heart disease inhibits
lipopolysaccharide-induced inflammatory response. J Biol Chem.
290:5328–5340. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sun T, Bi F, Liu Z and Yang Q: SLC7A2
serves as a potential biomarker and therapeutic target for ovarian
cancer. Aging (Albany NY). 12:13281–13296. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Murgia F, Angioni S, D'Alterio MN, Pirarba
S, Noto A, Santoru ML, Tronci L, Fanos V, Atzori L and Congiu F:
Metabolic profile of patients with severe endometriosis: A
prospective experimental study. Reprod Sci. 28:728–735.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dutta M, Singh B, Joshi M, Das D,
Subramani E, Maan M, Jana SK, Sharma U, Das S, Dasgupta S, et al:
Metabolomics reveals perturbations in endometrium and serum of
minimal and mild endometriosis. Sci Rep. 8(6466)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen L, Li C, Guo J, Luo N, Qu X, Kang L,
Liu M and Cheng Z: Eutopic/ectopic endometrial apoptosis initiated
by bilateral uterine artery occlusion: A new therapeutic mechanism
for uterus-sparing surgery in adenomyosis. PLoS One.
12(e0175511)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee DE, Yoo JE, Kim J, Kim S, Kim S, Lee H
and Cheong H: NEDD4L downregulates autophagy and cell growth by
modulating ULK1 and a glutamine transporter. Cell Death Dis.
11(38)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Burska D, Stiburek L, Krizova J, Vanisova
M, Martinek V, Sladkova J, Zamecnik J, Honzik T, Zeman J, Hansikova
H and Tesarova M: Homozygous missense mutation in UQCRC2 associated
with severe encephalomyopathy, mitochondrial complex III assembly
defect and activation of mitochondrial protein quality control.
Biochim Biophys Acta Mol Basis Dis. 1867(166147)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bourdon M, Santulli P, Kateb F,
Pocate-Cheriet K, Batteux F, Maignien C, Chouzenoux S, Bordonne C,
Marcellin L, Bertho G and Chapron C: Adenomyosis is associated with
specific proton nuclear magnetic resonance (1H-NMR)
serum metabolic profiles. Fertil Steril. 116:243–254.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Morotti M, Zois CE, El-Ansari R, Craze ML,
Rakha EA, Fan SJ, Valli A, Haider S, Goberdhan DCI, Green AR and
Harris AL: Increased expression of glutamine transporter
SNAT2/SLC38A2 promotes glutamine dependence and oxidative stress
resistance, and is associated with worse prognosis in
triple-negative breast cancer. Br J Cancer. 124:494–505.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shen L, Yu Y and Karner CM: SLC38A2
provides proline and alanine to regulate postnatal bone mass
accrual in mice. Front Physiol. 13(992679)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mayers JR and Vander Heiden MG: Famine
versus feast: Understanding the metabolism of tumors in vivo.
Trends Biochem Sci. 40:130–140. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
As-Sanie S, Kim J, Schmidt-Wilcke T,
Sundgren PC, Clauw DJ, Napadow V and Harris RE: Functional
connectivity is associated with altered brain chemistry in women
with endometriosis-associated chronic pelvic pain. J Pain. 17:1–13.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao X, Petrashen AP, Sanders JA, Peterson
AL and Sedivy JM: SLC1A5 glutamine transporter is a target of MYC
and mediates reduced mTORC1 signaling and increased fatty acid
oxidation in long-lived Myc hypomorphic mice. Aging Cell.
18(e12947)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Osanai-Sasakawa A, Hosomi K, Sumitomo Y,
Takizawa T, Tomura-Suruki S, Imaizumi M, Kasai N, Poh TW, Yamano K
and Yong WP: , et al: An anti-ASCT2 monoclonal antibody
suppresses gastric cancer growth by inducing oxidative stress and
antibody dependent cellular toxicity in preclinical models. Am J
Cancer Res. 8:1499–1513. 2018.PubMed/NCBI
|
|
29
|
Carr EL, Kelman A, Wu GS, Gopaul R,
Senkevitch E, Aghvanyan A, Turay AM and Frauwirth KA: Glutamine
uptake and metabolism are coordinately regulated by ERK/MAPK during
T lymphocyte activation. J Immunol. 185:1037–1044. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wik JA, Chowdhury A, Kolan S, Bastani NE,
Li G, Alam K, Grimolizzi F and Skålhegg BS: Endogenous glutamine is
rate-limiting for anti-CD3 and anti-CD28 induced CD4+ T-cell
proliferation and glycolytic activity under hypoxia and normoxia.
Biochem J. 479:1221–1235. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bau DT, Hsieh YY, Wan L, Wang RF, Liao CC,
Lee CC, Lin CC, Tsai CH and Tsai FJ: Polymorphism of XRCC1 codon
arg 399 Gln is associated with higher susceptibility to
endometriosis. Chin J Physiol. 50:326–329. 2007.PubMed/NCBI
|
|
32
|
Song W, Zhang Z, Jiang Y, Cao Y, Zhang B,
Wang Y, Shi H and Zhu L: Integrative metabolomic profiling reveals
aberrations in myometrium associated with adenomyosis: A pilot
study. Reprod Biol Endocrinol. 20(49)2022.PubMed/NCBI View Article : Google Scholar
|