Introduction
Surgery is the standard treatment for uterine
sarcoma, as chemotherapy has a limited effect; therefore, novel
treatment strategies are required. Given the low frequency of
sarcoma in Japan compared with other malignant tumors, there is a
delay in basic and clinical research, despite ongoing efforts to
analyze various genetic abnormalities as potential factors involved
in the development of sarcoma (1).
Our previous study revealed that resveratrol
exhibits a concentration-dependent inhibitory effect on the
proliferation of uterine sarcoma cells, suggesting its potential as
a novel therapeutic agent for uterine sarcoma. Furthermore, our
previous study demonstrated that the mechanism of resveratrol
action was mediated by the Wnt signaling pathway (2). Additionally, resveratrol has been
shown to exert anticancer effects on various types of carcinoma
such as colon cancer and breast cancer, and it has been reported to
be involved in the Wnt signaling pathway (3-5).
The present study focused on secreted
frizzled-related proteins (SFRPs), which are proteins that affect
the Wnt signaling pathway and are implicated in the development of
various types of cancer (6,7). The
inactivation of SFRPs could hypothesized to enhance the Wnt
signaling pathway (6), promoting
cancer growth; therefore, Wnt and SFRPs might be novel molecular
targets for cancer treatment.
Benign uterine leiomyomas do not typically progress
to uterine sarcoma, rendering treatment unnecessary as they tend to
shrink after menopause (8).
Nevertheless, considering cases where leiomyoma may have progressed
to sarcoma, it is important to evaluate the progression of uterine
fibroids to sarcoma. By elucidating the impact of SFRPs on sarcoma
and the dynamics of the Wnt signaling pathway, SFRPs could emerge
as a new therapeutic strategy for sarcoma.
Although there are few reports on the role of the
Wnt signaling pathway in the proliferation and metastasis of
uterine sarcoma cells (9,10), there are no reports, to the best of
our knowledge, on treatment strategies for uterine sarcoma focusing
on SFRPs. Therefore, the present study examined the differences in
SFRP4 expression between normal uterine smooth muscle cells and
uterine leiomyosarcoma. In addition, the inhibitory effect of SFRP4
on uterine sarcoma cells was assessed, as well as its effect on
cell migration and adhesion.
Materials and methods
Cell line and culture
The SKN human uterine leiomyosarcoma cell line
(European Collection of Authenticated Cell Cultures) was cultured
in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS, Thermo Fisher Scientific, Inc.), penicillin (100 U/ml) and
streptomycin (100 µg/ml) in an incubator containing 5%
CO2 at 37˚C.
Immunohistochemistry
SFRP4 was purchased from PeproTech, Inc. (cat. no.
120-50). SFRP4 cells were homogenized and filter-sterilized through
a 0.2 µm filter, then frozen at -20˚C. Immunostaining of SFRP4 was
performed on four cases of normal uterine smooth muscle, uterine
fibroids and uterine leiomyosarcoma. The tissues were sectioned at
a thickness of 5 µm. To block endogenous peroxidase and phosphatase
activity, tissues were incubated with 3% hydrogen peroxide for 10
min at room temperature. Blocking was performed using 5% goat serum
(Chemicon International; Thermo Fisher Scientific, Inc.) for 30 min
at room temperature. Rabbit monoclonal antibodies against SFRP4
(dilution, 1:900; cat. no. 154167; Abcam) were added to tissues and
incubated for 1 h at 37˚C. The tissues were incubated with the
HRP-conjugated secondary antibody (1:1,000, cat. no. ab6721, Abcam)
for 1 h at 37˚C. For chromogen detection, the Pierce DAB Substrate
kit (cat. no. 34002; Thermo Fisher Scientific, Inc.) was used. As a
positive control, immunohistochemical staining of SFRP4 in human
colonic mucosa using SFRP4 polyclonal antibodies (1:400, cat. no.
15328-1-AP, Proteintech Group, Inc.) was performed. In the positive
control tissues, the antigen-antibody reaction could be
sufficiently visualized using the primary antibodies. Tissues were
incubated for 1.5 h at 37˚C.
The intensity score (0-3) and proportion score (0-3)
were used as evaluation methods (Table
I). The human tissue samples used in the present study were
obtained from patients who underwent surgery for uterine fibroids
and uterine sarcoma at the Obstetrics and Gynecology Department of
Tokushima University Hospital (Tokushima, Japan) between January
2000 and December 2020. There were a total of 19 patients, ranging
in age from 46-69 years, with the mean age of 57 years. Normal
uterine smooth muscle tissue was collected from 8 patients, aged
46-57 years, with a mean age of 52 years, who underwent surgery for
uterine fibroids. The colon mucosal tissue used as a positive
control was a portion of the sample from a patient who underwent
surgery at Tokushima University Hospital that was scheduled to be
discarded, but was kept in a way that could not identify the
individual. There were no exclusion criteria for the present study.
For using sample tissues, an opt-out method was used, where an
information disclosure document was posted on the Tokushima
University Hospital web page, thereby omitting the need to obtain
consent from individual research subjects.
Discontinuation/withdrawal from the study occurred when a research
subject (or their representative consenter) expressed a refusal to
continue participation, or when the research director or researcher
deemed it appropriate to discontinue the research. The present
study was approved by the Ethics Committee of Tokushima University
Hospital (Tokushima, Japan; approval no. 4159).
 | Table IIntensity and proportion scores for
normal uterine smooth muscle tissue, uterine fibroid tissue and
uterine leiomyosarcoma tissue. |
Table I
Intensity and proportion scores for
normal uterine smooth muscle tissue, uterine fibroid tissue and
uterine leiomyosarcoma tissue.
| Score | 0 | 1 | 2 | 3 |
|---|
| Intensity | Not stained at
all | Slightly stained with
strong to medium enlargement | Stained with low to
medium enlargement | Stained well with
weak enlargement |
| Proportion | <1% | 1-10% | 10-50% | >50% |
In order to confirm the expression of SFRP4 by
immunostaining, in four cases of normal uterine smooth muscle and
three cases of uterine leiomyosarcoma, the number of stained cells
was measured using a BZ-800 Analyzer (fluorescence, Keyence
Corporation), and the ratio of the area was measured
(magnification, x20).
Cell treatment and viability
assay
SKN uterine leiomyosarcoma cells were seeded on a
96-well microplate at a density of 5x102 cells/well and
were incubated at 37˚C for 24 h prior to treatment. SFRP4 was added
to cells at concentrations of 1.25 and 2.5 µg/ml based on the
results of a previous study (11),
and, after 48 h at 37˚C, cell viability was assessed at an
absorbance of 450 nm using the WST-1 assay (Roche Diagnostics)
according to the manufacturer's instructions.
Cell migration and adhesion
assays
To assess changes in the migration of uterine
sarcoma cells following SFRP4 administration, the CytoSelect™
24-well Cell Migration Assay Kit (cat. CBA-100; Cell Biolabs, Inc.)
was used. The CytoSelect Cell Migration Assay Kit contains
polycarbonate membrane inserts (pore size, 8 µm) in a 24-well
plate. The membrane serves as a barrier for differentiating
migratory cells from non-migratory cells. Migratory cells can
extend their protrusions towards chemoattractants, such as FBS (via
actin cytoskeleton reorganization), and ultimately pass through the
pores of the polycarbonate membrane (12). The migrating cells that passed
through the membrane were collected and used for staining and
semi-quantification.
Briefly, uterine leiomyosarcoma cells were seeded at
a density of 5x102 cells/well in 100 µl culture in the
CytoSelect 24-well Cell Migration Assay plate and divided into a
group treated with SFRP4 at a concentration of 2.5 µg/ml at 37˚C
for 24 h and a control group that received no treatment. WST-1
reagent (10%) was added to each well and the cells were incubated
at 37˚C for 1 h. The samples were washed and the absorbance was
measured at 450 nm using a microplate reader (Thermo Scientific
Varioskan Flash Multimode reader; Thermo Fisher Scientific,
Inc.).
Similarly, to assess changes in cell adhesion,
uterine leiomyosarcoma cells were seeded at a density of
5x102 cells/well in a CytoSelect 48-well Cell Adhesion
Assay plate (cat. no. CBA-070; Cell Biolabs, Inc.) and divided into
a group treated with SFRP4 at a concentration of 2.5 µg/ml at 37˚C
for 24 h and a control group that received no treatment. After
adherence for 24 h to fibronectin, collagen I, collagen IV, laminin
I, fibrinogen and BSA, the samples were washed twice with cold PBS
and the absorbance was read at 450 nm using a microplate reader.
BSA was used as a control and was included in the aforementioned
kit.
Statistical analysis
Statistical analyses were performed on triplicate
repeats using the Ekuseru-Toukei 2012 software (version 1.16;
Social Survey Research Information Co., Ltd.). Data were expressed
as the mean ± standard error of the mean. The statistical
significance of the differences between the experimental and
control groups was assessed using one-way ANOVA followed by
Dunnett's test. Comparisons between two groups were evaluated using
the unpaired Student's t-test. Immunohistochemistry scores were
statistically analyzed using Kruskal-Wallis test followed by Dunn's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
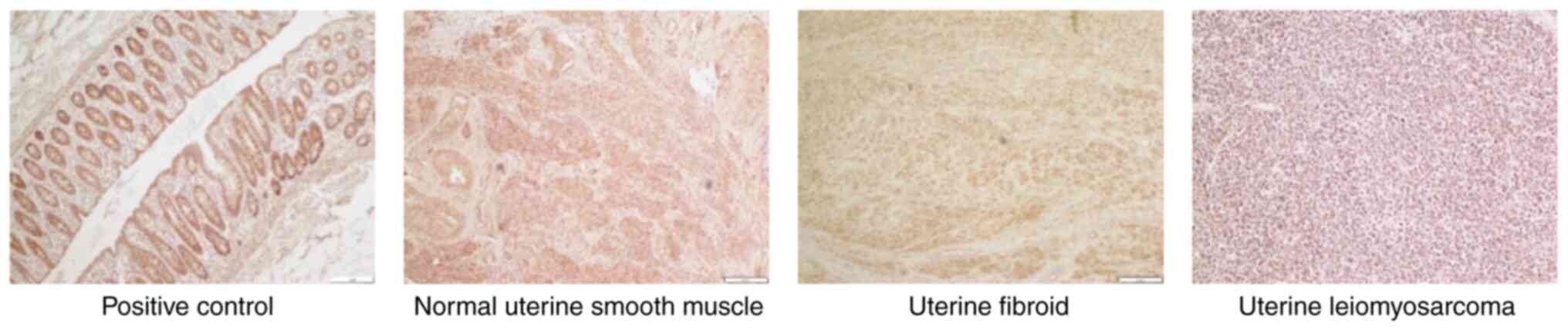
The median intensities of SFRP4 expression in
uterine smooth muscle, fibroids and leiomyosarcomas were 2.5, 3.0
and 1.0, respectively, and the median proportions were 3.0, 3.0 and
1.5, respectively. The expression levels of SFRP4 were
significantly lower in uterine leiomyosarcoma tissues than those in
normal smooth muscle and uterine fibroid tissues (P=0.01; Table II; Fig. 1).
 | Table IIExpression of SFRP4 in different
tissues. |
Table II
Expression of SFRP4 in different
tissues.
| A, Expression of
SFRP4 in normal uterine smooth muscle tissue |
|---|
| Tissue | Intensity | Proportion |
|---|
| Normal uterine smooth
muscle 1 | 3 | 3 |
| Normal uterine smooth
muscle 2 | 1 | 2 |
| Normal uterine smooth
muscle 3 | 3 | 3 |
| Normal uterine smooth
muscle 4 | 2 | 3 |
| B, Expression of
SFRP4 in uterine fibroid tissue |
| Tissue | Intensity | Proportion |
| Uterine fibroid | 3 | 3 |
| Uterine fibroid
2 | 3 | 3 |
| Uterine fibroid
3 | 2 | 3 |
| Uterine fibroid
4 | 3 | 3 |
| C, Expression of
SFRP4 in uterine leiomyosarcoma tissue |
| Tissue | Intensity | Proportion |
| Uterine
leiomyosarcoma 1 | 1 | 1 |
| Uterine
leiomyosarcoma 2 | 1 | 2 |
| Uterine
leiomyosarcoma 3 | 1 | 1 |
| Uterine
leiomyosarcoma 4 | 2 | 3 |
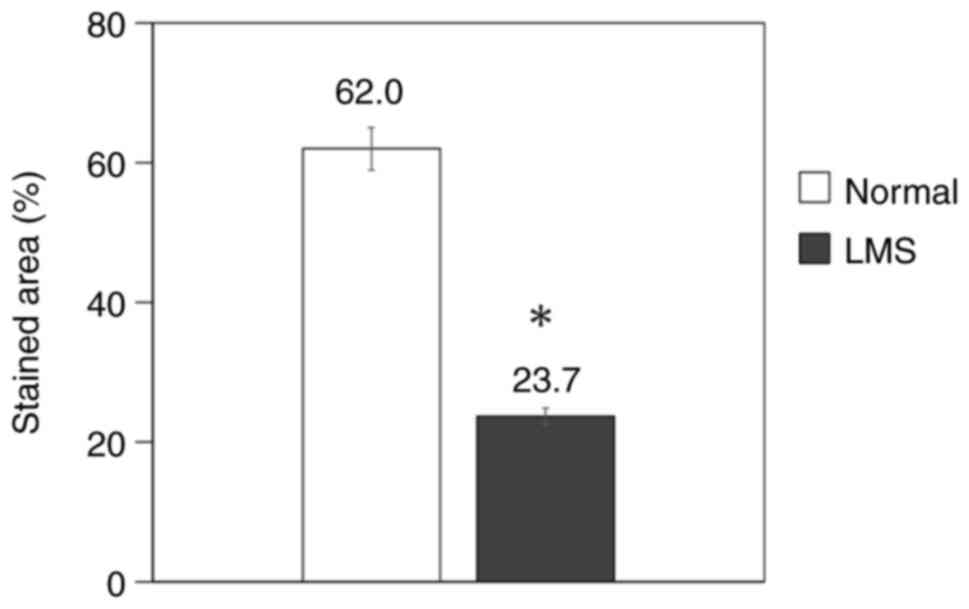
The number of stained cells was measured and the
area ratio was calculated in normal uterine smooth muscle and
uterine leiomyosarcoma. Normal smooth muscle showed an area ratio
of 62.0%, whereas it was 23.7% in leiomyosarcoma, which was
significantly lower than that in normal smooth muscle (P=0.03;
Table III; Fig. 2).
 | Table IIISFRP4 stained cell area ratio in
normal uterine smooth muscle and uterine leiomyosarcoma
tissues. |
Table III
SFRP4 stained cell area ratio in
normal uterine smooth muscle and uterine leiomyosarcoma
tissues.
| Tissue | Total area,
µm2 | Stained area,
µm2 | Ratio, % |
|---|
| Normal uterine smooth
muscle 1 | 579,907 | 329,900 | 57 |
| Normal uterine smooth
muscle 2 | 288,917 | 235,857 | 82 |
| Normal uterine smooth
muscle 3 | 1,062,726 | 569,027 | 54 |
| Normal uterine smooth
muscle 4 | 634,039 | 350,073 | 55 |
| Uterine
leiomyosarcoma 1 | 2,675,916 | 1,282,439 | 48 |
| Uterine
leiomyosarcoma 2 | 2,328,849 | 2,273,09 | 10 |
| Uterine
leiomyosarcoma 3 | 2,156,167 | 290,801 | 13 |
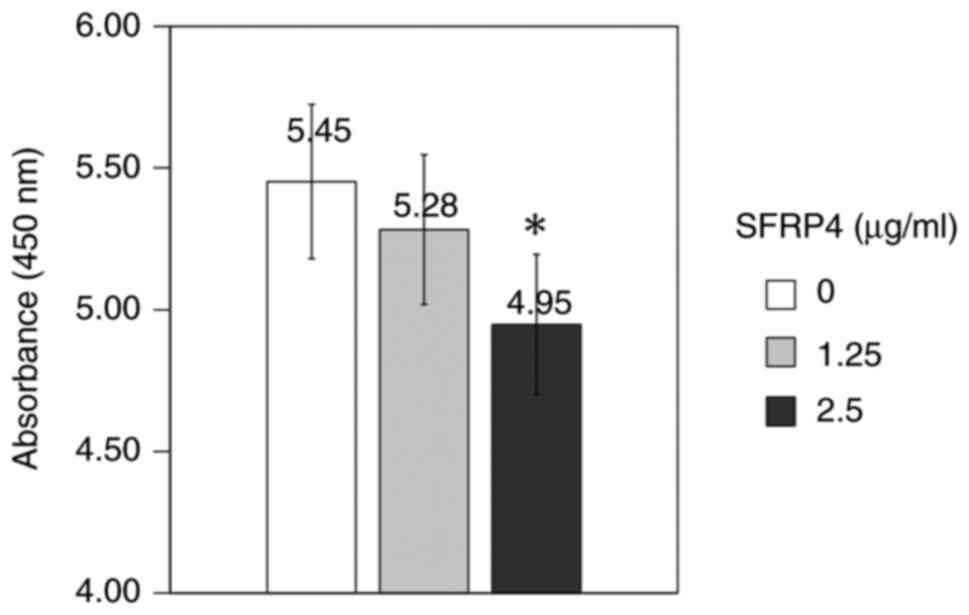
SFRP4 was added to SKN uterine leiomyosarcoma cells
at a concentration of 1.25 and 2.5 µg/ml and the absorbance was
measured to examine the cell viability. The control group showed an
absorbance value of 5.45, the SFRP4 1.25 µg/ml group showed an
absorbance value of 5.28, whereas the SFRP4 2.5 µg/ml group showed
an absorbance value of 4.95. Cell viability was significantly
suppressed in response to a concentration of 2.5 µg/ml SFRP4
compared with that in the control group (P=0.001; Fig. 3).
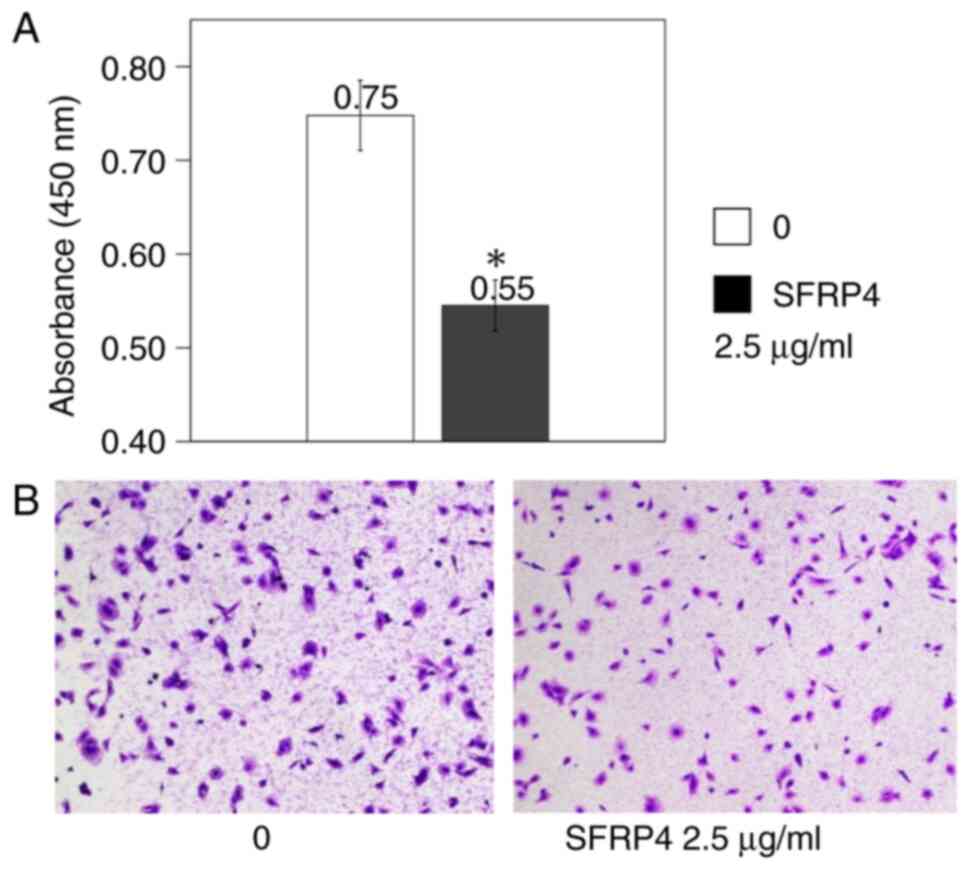
SKN uterine leiomyosarcoma cells were cultured in
the CytoSelect 24-well Cell Migration Assay plates, SFRP4 was added
at a concentration of 2.5 µg/ml, and the absorbance was measured.
The control group showed an absorbance value of 0.75, whereas the
absorbance value was 0.55 in the SFRP4 group, showing a significant
decrease in cell migration (P=0.007; Fig. 4).
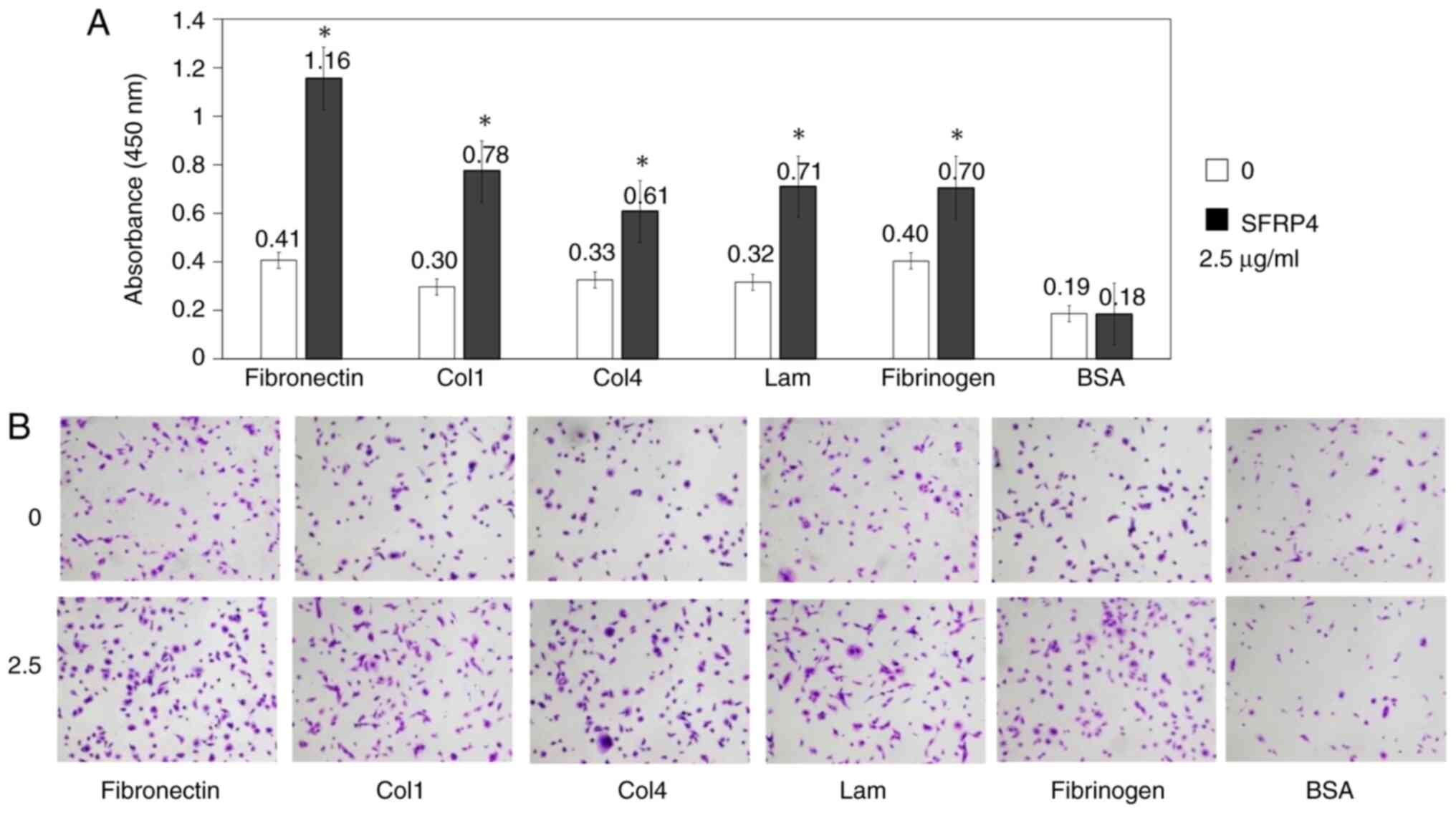
SKN uterine leiomyosarcoma cells were cultured in
the CytoSelect 48-well Cell Adhesion Assay plates, SFRP4 was added
at a concentration of 2.5 µg/ml, and the absorbance was measured.
For each cell adhesion molecule (fibronectin, collagen-1,
collagen-4, laminin fibrinogen and BSA), the values were 0.41,
0.30, 0.33, 0.32 and 0.40 in the control group and 1.16, 0.78,
0.61, 0.71 and 0.70 in the SFRP4 group, respectively. The adhesion
ability of leiomyosarcoma cells to each cell adhesion molecule was
significantly increased in response to SFRP4 compared with that in
the control cells (P=0.0004, 0.002, 0.002, 0.001 and 0.007,
respectively; Fig. 5).
Discussion
Our previous study has shown that resveratrol
suppresses the proliferation of uterine sarcoma cells via the Wnt
signaling pathway (1). Therefore,
the present study focused on SFRPs, which have been indicated to be
involved in the Wnt signaling pathway, along with resveratrol. Wnt
is an extracellular protein with a molecular weight of ~40,000
g/mol. When Wnt binds to Wnt receptor Frizzled in the cytoplasm,
three types of intracellular signal transduction pathways are
activated, namely the β-catenin pathway, the planar cell polarity
pathway and the Ca2+ pathway (13).
The β-catenin pathway regulates cell proliferation
and differentiation through gene expression by regulating the
intracellular levels of β-catenin. Numerous genetic abnormalities
within constituents of the β-catenin pathway have been identified
in various types of human carcinoma, leading to abnormal
accumulation of β-catenin in the cytoplasm and nucleus (13). Furthermore, abnormal accumulation
of β-catenin is known to cause overexpression of cancer-related
genes, such as c-myc, ultimately causing abnormal cell
proliferation (13).
Fang et al (14) revealed that the Wnt signaling
pathway is activated in osteosarcoma cells. Zou et al
(3) reported that resveratrol
suppresses the Wnt signaling pathway in osteosarcoma.
All members of the SFRP gene family are small
proteins, ~30 kDa in size, belonging to five gene families,
SFRP1-5, which are widely conserved across species. Comparison of
amino acid sequence homology among families suggests that SFRP1, 2
and 5 and SFRP3 and 4 form separate subfamilies, and there are
differences in their expression levels in each organ. SFRPs
suppress the Wnt signaling pathway by directly inhibiting the
binding of Wnt to its receptor, Frizzled, via extracellular
secretion. The enhancement of Wnt signaling by inactivating SFRP
may serve an important role in activation of the Wnt signaling
pathway in cancer (15). Thus, Wnt
and SFRP are considered novel molecular targets for cancer
treatment.
Several studies have reported on the relationship
between SFRP levels and several types of cancer. SFRP4 inhibits the
Wnt signaling pathway in ovarian cancer (11). Furthermore, multiple SFRP genes are
frequently inactivated in colorectal cancer (16). Decreased SFRP1 expression, and
induction of apoptosis due to its overexpression, have been
confirmed in cervical cancer (17). In addition, SFRP1 and SFRP2
suppress cell proliferation via the Wnt signaling pathway in
cervical cancer (18). SFRP5 has
been shown to inhibit cervical tumorigenesis by interfering with
the Wnt pathway in vitro (19). In addition, the roles of the Wnt
signaling pathway and SFRP4 in ovarian cancer have been reported
(20).
To the best of our knowledge, there have been no
reports on treatment strategies for sarcoma focusing on SFRPs. By
elucidating the expression of SFRPs in uterine sarcoma and their
effects on inhibitory action against sarcoma cell proliferation,
migration and adhesion of sarcoma cells, SFRPs may be considered as
a new therapeutic agent for uterine sarcoma.
In the present study, immunostaining revealed that
uterine leiomyosarcoma cells expressed significantly less SFRP4
than normal uterine smooth muscle cells and uterine fibroids. The
present study hypothesized that enhancement of Wnt signaling by the
inactivation of SFRP may be involved in cancer growth, thus
suggesting that SFRP inactivation may be involved in the growth of
uterine sarcoma. In addition, cell viability was suppressed by the
treatment of uterine sarcoma cells with SFRP4. Previous studies
have reported that SFRP suppresses the Wnt signaling pathway, and
it is possible that SFRP suppresses cell proliferation via this
pathway.
In previous years, cell adhesion has been elucidated
at the molecular level, and it has become clear that various cell
adhesion molecules are involved in tumor metastasis through complex
processes. Cell migration is a multistep process that serves an
important role not only in tumor infiltration and metastasis, but
also in wound healing, cell differentiation and embryonic
development. Cell infiltration involves cell migration, wherein the
cells migrate beyond the extracellular matrix and settle at new
sites (21). Notably, attempts
have been made to suppress tumor metastasis by controlling cell
adhesion (22,23). In the present study, the treatment
of uterine sarcoma cells with SFRP4 decreased their migratory
ability and increased their adhesive ability; thus suggesting that
SFRP4 may be involved in the infiltration and metastasis of uterine
sarcoma cells.
In conclusion, the present study demonstrated that
uterine leiomyosarcoma expresses less SFRP4 than normal uterine
smooth muscle tissues, and that SFRP4 suppresses the proliferation
of uterine leiomyosarcoma cells, reduces their migration and
increases their adhesion.
These results should be interpreted with caution
because of the small sample size of human samples used in the
present study, and it is important to consider a number of
limitations. A small sample size may skew statistical tests when
identifying relationships and connections within the resulting data
set. The role of SFRP4 could be better understood by increasing the
number of samples. In addition, since SFRP4 was shown to inhibit
the proliferation of SKN leiomyosarcoma cells, complementary
experiments investigating apoptosis would provide evidence to
support the results of the present study. Although we did not
examine the Wnt signaling pathway in detail in this study, further
analysis of the effects of SFRP on sarcoma, and changes in the
factors involved in the Wnt signaling pathway may verify the use of
SFRPs as a novel treatment option for uterine sarcoma.
Acknowledgements
For the present study, the laboratory and
experimental equipment at the Support Center for Advanced Medical
Sciences at the Tokushima University Graduate School of Biomedical
Sciences (Tokushima, Japan) were used to perform cell culture and
the absorbance measurements.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ToK and AM analyzed and interpreted data regarding
the effect of SFRP on uterine sarcoma. ToK and AM confirm the
authenticity of all the raw data. ToK, AM, TN, AS, RA, HI, HN, AY,
RK, YY, KY, TaK, MN and TI made substantial contributions to
conception and design, or acquisition of data or analysis and
interpretation of data. MN, KY and TI wrote and revised the
manuscript. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
When using sample tissues in the present study, an
opt-out method was used, where an information disclosure document
was posted on the Tokushima University Hospital web page, thereby
omitting the need to obtain consent from individual research
subjects. The present study was approved by the Ethics Committee of
Tokushima University Hospital (approval no. 4159).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nakata E, Fujiwara T, Kunisada T, Ito T,
Takihira S and Ozaki T: Immunotherapy for sarcomas. Jpn J Clin
Oncol. 51:523–537. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mineda A, Nishimura M, Kagawa T, Takiguchi
E, Kawakita T, Abe A and Irahara M: Resveratrol suppresses
proliferation and induces apotosis of uterine sarcoma cells by
inhibiting the Wnt signaling pathway. Exp Ther Med. 17:2242–2246.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zou Y, Yang J and Jiang D: Resveratrol
inhibits canonical Wnt signaling in human MG-63 osteosarcoma cells.
Mol Med Rep. 12:7221–7226. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220.
1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lavergne E, Hendaoui I, Coulouarn C,
Ribault C, Leseur J, Eliat PA, Mebarki S, Corlu A, Clément B and
Musso O: Blocking Wnt signaling by SFRP-like molecules inhibits in
vivo cell proliferation and tumor growth in cells carrying active
β-catenin. Oncogene. 30:423–433. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu Q, Xu C, Zeng X, Zhang Z, Yang B and
Rao Z: Tumor suppressor role of sFRP-4 in hepatocellular carcinoma
via the Wnt/β-catenin signaling pathway. Mol Med Rep.
23(336)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
De La Cruz MS and Buchanan EM: Uterine
fibroids: Diagnosis and treatment. Am Fam Physician. 95:100–107.
2017.PubMed/NCBI
|
|
9
|
Pridgeon MG, Grohar PJ, Steensma MR and
Williams BO: Wnt signaling in ewing sarcoma, osteosarcoma, and
malignant peripheral nerve sheath tumors. Curr Osteoporos Rep.
15:239–246. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Singla A, Wang J, Yang R, Geller DS, Loeb
DM and Hoang BH: Wnt signaling in osteosarcoma. Adv Exp Med Biol.
1258:125–139. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ford CE, Jary E, Ma SSQ, Nixdorf S,
Heinzelmann-Schwarz VA and Ward RL: The Wnt gatekeeper SFRP4
modulates EMT, cell migration and downstream Wnt signalling in
serous ovarian cancer cells. PLoS One. 8(e54362)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: Integrating signals from front to back. Science.
302:1704–1709. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kikuchi A, Yamamoto H and Kishida S:
Multiple of the interactions of Wnt proteins and their receptors.
Cell Signal. 19:659–671. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fang F, VanCleave A, Helmuth R, Torres H,
Rickel K, Wollenzien H, Sun H, Zeng E, Zhao J and Tao J: Targeting
the Wnt/β-catenin pathway in human osteosarcoma cells. Oncotarget.
9:36780–36792. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rattner A, Hsieh JC, Smallwood PM, Gilbert
DJ, Copeland NG, Jenkins NA and Nathans J: A family of secreted
proteins contains homology to the cysteine-rich ligand-binding
domain of frizzled receptors. Proc Natl Acad Sci USA. 94:2859–2863.
1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Suzuki H, Watkins DN, Jair KW, Schuebel
KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van
Engeland M, et al: Epigenetic inactivation of SFRP genes allows
constitutive WNT signaling in colorectal cancer. Nat Genet.
36:417–422. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Ko J, Ryu KS, Lee YH, Na DS, Kim YS, Oh
YM, Kim IS and Kim JW: Human secreted frizzled-related protein is
down-regulated and induces apoptosis in human cervical cancer. Exp
Cell Res. 280:280–287. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chung MT, Lai HC, Sytwu HK, Yan MD, Shih
YL, Chang CC, Yu MH, Liu HS, Chu DW and Lin YW: SFRP1 and SFRP2
suppress the transformation and invasion abilities of cervical
cancer cells through Wnt signal pathway. Gynecol Oncol.
112:646–653. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lin YW, Chung MT, Lai HC, Yan MD, Shih YL,
Chang CC and Yu MH: Methylation analysis of SFRP genes family in
cervical adenocarcinoma. J Cancer Res Clin Oncol. 135:1665–1674.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jacob F, Ukegjini K, Nixdorf S, Ford CE,
Olivier J, Caduff R, Scurry JP, Guertler R, Hornung D, Mueller R,
et al: Loss of secreted frizzled-related protein 4 correlates with
an aggressive phenotype and predicts poor outcome in ovarian cancer
patients. PLoS One. 7(e31885)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kawauchi T: Cell adhesion and its
endocytic regulation in cell migration during neural development
and cancer metastasis. Int J Mol Sci. 13:4564–4590. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mei J, Jin LP, Ding D, Li MQ, Li DJ and
Zhu XY: Inhibition of IDO1 suppresses cyclooxygenase-2 and matrix
metalloproteinase-9 expression and decreases proliferation,
adhesion and invasion of endometrial stromal cells. Mol Hum Reprod.
18:467–476. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tsukamoto H, Kondo S, Mukudai Y, Nagumo T,
Yasuda A, Kurihara Y, Kamatani T and Shintani S: Evaluation of
anticancer activities of benzo[c]phenanthridine alkaloid
sanguinarine in oral squamous cell carcinoma cell line. Anticancer
Res. 31:2841–2846. 2011.PubMed/NCBI
|