Introduction
Sepsis is a systemic inflammatory response syndrome
(SIRS) that develops in the host against microorganisms. This
response develops away from the primary infection area and results
in end-organ damage (1). The
response that occurs during infection in healthy individuals
continues with pathogen recognition, control and rapid tissue
repair (2,3). Upon activation of the cell-mediated
immune response, anti-/pro-inflammatory mediators are released
(4,5). Overactivation by powerful pathogens
leads to endothelial damage, tissue hypoperfusion, disseminated
intravascular coagulation, treatment-resistant shock, multiple
organ damage and death (6).
Although a number of treatment methods have been developed such as
antibiotics, corticosteroids, fluid and adjunctive therapies; SIRS
and sepsis have high mortality and morbidity in intensive care
units (7). In 2017, 48.9 million
cases of sepsis were reported worldwide, of which 11 million
resulted in death (8). Similar
proportions of mortality and incidence have been reported in
European countries (9-14).
The current clinical approach to treatment starts with early
diagnosis, identification of the source of infection and early
antibiotic treatment, with corticosteroids also playing an
important role (1). However,
although there are studies showing that steroid treatment reduces
mortality in sepsis, its effects on long-term mortality are
controversial (15,16). Therefore, the effectiveness of
novel drugs is being investigated in experimental and clinical
studies (17-21).
Milk thistle (Silybum marianum) is a
historical medicinal plant and its well-known flavonoid silymarin
is an agent that has promising therapeutic efficacy in different
clinical studies (22-24).
S. marianum is a herbal product used in Ancient Greek
medicine to treat gallbladder disorder and protect the liver from
toxic agents (25). Furthermore,
silymarin preparations have been used to treat liver and other
gastrointestinal diseases due to hepatoprotectivity,
neuroprotectivity, anti-fungal and anti-cancer activity (23,26,27).
The anti-inflammatory activity of silymarin may underlie the
positive effects of the agent (26,28).
Several studies have demonstrated the anti-inflammatory activities
of silymarin, which inhibits interferon-g, IL-4 and IL-10 in a
dose-dependent manner (29-31).
Silymarin suppresses NF-κB binding transporter gene transcription
in a rat model of sepsis (32). In
addition to its cell-protective effects via antioxidative and
radical scavenging activity, silymarin also acts via specific
receptor interactions such as P-glycoproteins and estrogen and
nuclear receptors (29).
Derivatives of silymarin could provide new avenues for therapeutic
applications. However, although certain researchers have reported
silymarin to be well-tolerated and safe clinically, there are also
conflicting results (24,33-35).
While gastrointestinal and neurological side effects were reported
in the study by Schrieber et al (33); there are also studies in the
literature, in which no adverse events were observed despite using
similar or higher doses (34,35).
Therefore, it is crucial that this agent be studied experimentally
in organs and tissues before use in clinical practice. Furthermore,
the origin of the milk thistle plant, from which silymarin is
obtained, is along the Mediterranean coast of Europe and therefore,
the fact that this herbal flavonoid is quite common in Anatolia
(36) was also effective in its
selection in the present study as it is possible to obtain pure raw
materials from this plant in Turkey, where the present study was
performed.
The activation of adrenergic α2 receptors causes
hypotension, bradycardia, sedation, arterial and venous
vasoconstriction, decreased presynaptic transmitter release,
thrombus stabilization, hypothermia, decreased gastric acid
secretion and motility and inhibition of lipolysis and pancreatic
insulin release (37,38). A number of studies has shown that
sepsis is associated with sympathetic overactivation, which may
contribute to end-organ damage (39,40).
In septic shock, increased endogenous sympathetic outflow plays a
major role in maintaining vascular tone and tissue perfusion
(41). Despite elevated
concentrations of endogenous vasoconstrictors, such as
noradrenaline, downregulation of adrenergic receptors and
post-receptor signaling pathways leads to significant decline in
vascular response (40,41). To prevent the negative consequences
of excessive sympathetic flow, researchers have investigated the
use of sympathetic blockade in the treatment of sepsis (42,43).
According to Pichot et al, inhibiting sympathetic activity
with an α2 agonist corrects vascular reactivity by upregulating α1
receptors in septic shock, thereby decreasing the need for
vasopressors (44). Similarly,
response to norepinephrine decreases following application of
lipopolysaccharide and the administration of α2 agonists increases
this response in rats (45).
Dexmedetomidine (DEX), is one of the most commonly used sedation
agents in intensive care (46-48).
As a highly selective α2-adrenoreceptor agonist, DEX serves as an
adjunctive therapy through pro-inflammatory downregulation and
control of the anti-inflammatory response in patients with sepsis
(49). DEX suppresses the release
of TNF-α, IL-6, IL-8 and high mobility group box-1 (HMGB-1) in
human whole blood cultured with lipopolysaccharide (50). The suppressive effect of DEX on
proinflammatory mediator production occurs via α2 adrenergic
receptors (49). There are
numerous experimental and retrospective observational studies on
the benefits of this agent in sepsis, which is the most common
cause of mortality in intensive care units (8,51).
To the best of our knowledge, however, there are still insufficient
data on the specific protective benefits of this agent on tissue
and organs. Various studies have shown that DEX, similar to
silymarin, has potential benefits by inducing antioxidant pathways
in different clinical situations such as ischemia-reperfusion,
cancer and sepsis (52-55).
Therefore, it was hypothesized these two agents together may show
strong antioxidant activity and decrease tissue and organ
damage.
There are three current approaches frequently used
to construct sepsis models: Lipopolysaccharide administration,
intravascular or intraperitoneal administration of live bacteria
and the cecal ligation and puncture (CLP) method (56). The CLP method provides the closest
results to sepsis in humans (57).
Although the efficacy of experimental sepsis models in animals and
their adaptability to human studies have been discussed for some
time (58), the cecal ligation and
puncture method still remains valid (59).
The aim of the present study was to investigate the
protective and therapeutic effects of silymarin and DEX in
CLP-induced sepsis in rat lung and kidney tissues.
Materials and methods
Animal studies
The present study was conducted at the Gazi
University Animal Experiments Laboratory (Ankara, Turkey) in July
2021 in accordance with the ARRIVE guidelines (60). The present study was approved by
The Local Ethics Committee of Gazi University Animal Experiments
(approval no. G.Ü.E.T-20.022; Ankara, Turkey). Animal studies were
performed in accordance with The Guide for the Care and Use of
Laboratory Animals by the National Institutes of Health (61). A total 62 male Wistar Albino rats
(Gazi University Animal Experiments Laboratory, Ankara, Turkey)
weighing 225-300 g were used. Rats were kept in a
temperature-controlled (21±1˚C) and humidity-controlled (45-55%)
room and were maintained under a 12-h light/dark cycle. The animals
were fed a standard pellet diet and allowed to drink water ad
libitum. Rats were randomly divided into eight groups as
follows: i) Control (n=6); ii) cecal perforation (CLP; n=8); iii)
silymarin + CLP (n=8; S + CLP; silymarin administered 1 h before
CPL); iv) CLP + S (n=8; silymarin administered 1 h after CLP); v)
DEX + CLP (n=8; D + CLP; DEX administered 1 h before CLP); vi) CLP
+ D (n=8; DEX administered 1 h after CLP); vii) SD + CLP (n=8;
silymarin and DEX administered 1 h before CLP) and viii) CLP + SD
(n=8; silymarin and DEX administered 1 h after CLP).
Rats were anesthetized by 50 mg/kg intramuscular
ketamine hydrochloride (Ketalar® vial; Parke-Davis;
Pfizer, Inc.) and 10 mg/kg xylazine hydrochloride (Alfazyne; 2%;
EGE VET) and placed on a heating pad to maintain their body
temperature. Midline laparotomy was performed in rats whose skin
was aseptically prepared. The intestines were removed using wet
gauze. In the control group, the cecum was manipulated. However,
drilling and ligation were not performed.
After the cecum filled with stool, it was tied with
3/0 silk under the ileocecal valve and the anterior surface of the
cecum was punctured twice using an 18-gauge needle. No treatment
(e.g., dexmedetomidine or slymarin) was applied to the sham or CLP
group. Saline was applied to the peritoneal space to minimize heat
and fluid loss. A total of 100 mg/kg silymarin (Sigma-Aldrich;
Merck KGaA; cat. no. SO292-50G) and 100 µg/kg DEX (Sedodamid; 100
µg/2 ml; Koçak Farma®) was administered
intraperitoneally to the treatment groups. All the rats were
sacrificed 24 h after the operation; rats were anesthetized with
ketamine (50 mg/kg) and xylazine (10 µg/kg) and sacrificed by
collecting blood (5-10 ml) from the abdominal aorta. After
heartbeat and respiration ceased, rats were monitored for a further
2 min to confirm death. Tissue samples were stored at -70˚C for
biochemical analysis and immersed in 10% neutral buffered formalin
for histopathological assessment.
In the present study, two rats were lost in the CLP
and S + CLP groups and one rat in the CLP + S group. No losses were
observed in any of the other groups. In the first 24 h, mortality
rates in the CLP and S groups were similar to those reported by
Kang et al (32), Al-Kadi
et al (62) and Canikli
Adıgüzel et al (63).
Histopathological evaluation
Lung and kidney tissue specimens were fixed in 10%
neutral-buffered formalin for 48 h at room temperature and embedded
in paraffin after routine tissue processing. Tissue specimens were
dehydrated through an increasing-grade series of ethanol.
Dehydrated specimens were cleared in xylene, infiltrated in liquid
paraffin at 60˚C, and embedded in paraffin. Thereafter, 5 µm-thick
tissue sections were cut from paraffin blocks using a microtome
(Leica SM 2000; Leica Microsystems GmbH) and stained with
hematoxylin and eosin (H&E) to analyze histopathological
changes. Lung and kidney sections were incubated with hematoxylin
and eosin stain solutions for 12 min each, at room temperature. The
stained sections were assessed under a light microscope (Leica DM
4000 B; Leica Microsystems GmbH) equipped with a computer, and
micrographs were captured using Leica LAS V4.9 software (Leica
Microsystems GmbH).
H&E-stained kidney sections were examined under
x400 magnification and renal injury was evaluated
semi-quantitatively. Histopathological parameters, including
interstitial edema, peritubular capillary dilatation,
vacuolization, ablation of tubular epithelium from the basement
membrane, loss of brush border in the proximal tubule epithelium,
cell swelling and nuclear defragmentation, were scored 0-3 (0,
none; 1, mild; 2, moderate; 3, severe) and the mean score was
determined for each parameter in each group (64).
H&E-stained lung samples were examined under
200x and 400x magnification and lung injury was assessed
semi-quantitatively. Alveolar wall thickening, capillary
congestion, intra-alveolar hemorrhage and interstitial and
intra-alveolar neutrophil infiltration were scored 0-3 (0, none; 1,
mild; 2, moderate; 3, severe), and the mean score was determined
for each parameter (65).
Biochemical determination
Total antioxidant status (TAS) and total oxidative
status (TOS) were analyzed in blood samples. TAS and TOS were
measured using test kits according to the manufacturer's
instructions (Rel Assay Diagnostics®). TAS levels were
calculated as follows: TAS=[(ΔAbsorbance (Abs) H2O-ΔAbs
sample)/(ΔAbs H2O-ΔAbs standard)], and the results were
expressed in mmol Trolox Eq/l. TOS levels were calculated as
follows: TOS=(ΔAbs sample/ΔAbs standard) x standard concentration
(10 µmol/l), and the results were expressed in µmol
H2O2 Eq/l.
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD) or standard error of mean (SEM). The experiments was
performed once. All statistical analyses were performed using SPSS
(version 26.0; IBM Corp.). The distribution of data was analyzed
using the Shapiro-Wilk test. Comparisons of >2 groups were
performed using Kruskal-Wallis test followed by Dunn's post hoc
test or one-way ANOVA followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant difference.
The intention to treat analysis method was used (66-68).
Results
Kidney tissue histopathological
results
The mean scores for histopathological changes in
kidney specimens are summarized in Table I. The severity of interstitial
edema in kidney was significantly different between the groups
(P=0.003); it was more severe in the CLP, S + CLP, SD + CLP and CLP
+ SD groups than in the control group (P=0.008, P=0.001, P=0.016
and P=0.004, respectively). Interstitial edema was decreased in the
D + CLP group compared with that in the CLP group (P=0.013). The
interstitial edema score was significantly lower in the D + CLP and
CLP + D groups than in the S + CLP group (P=0.001 and P=0.013,
respectively). Peritubular capillary dilatation mean scores were
also different (P=0.034), with a significantly higher score in the
CLP, S + CLP and CLP + S groups than in the control group (P=0.047,
P=0.012 and P=0.012, respectively), whereas it was lower in the CLP
+ D and CLP + SD groups than in both the S + CLP (P=0.020 and
P=0.020, respectively) and CLP + S groups (P=0.020 and P=0.020,
respectively; Table I; Fig. 1). Focal cystic formations along the
more prominent tubular dilatation were observed in the cortex and
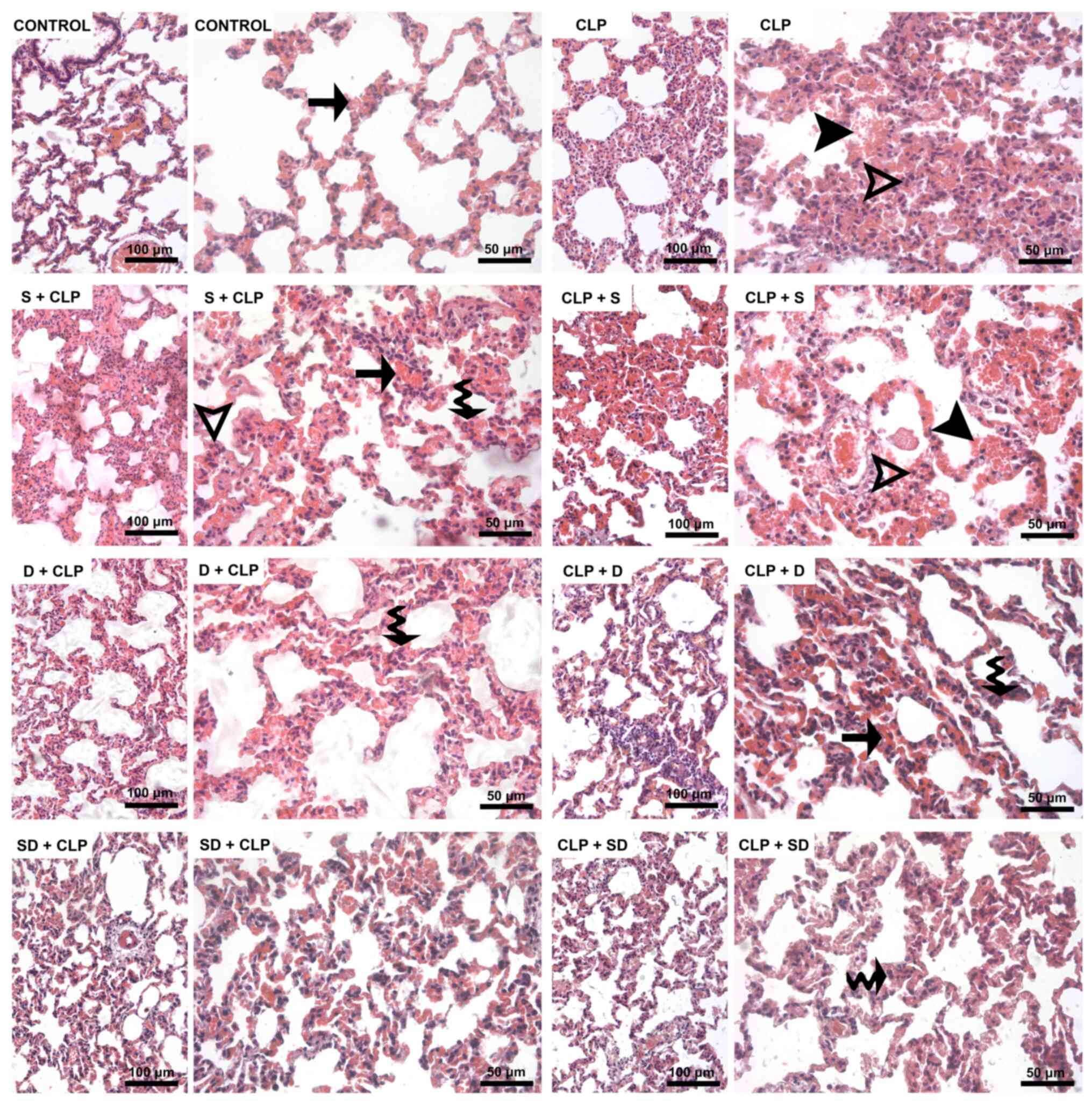
medulla of the kidney from S + CLP and CLP + S groups (Fig. 1).
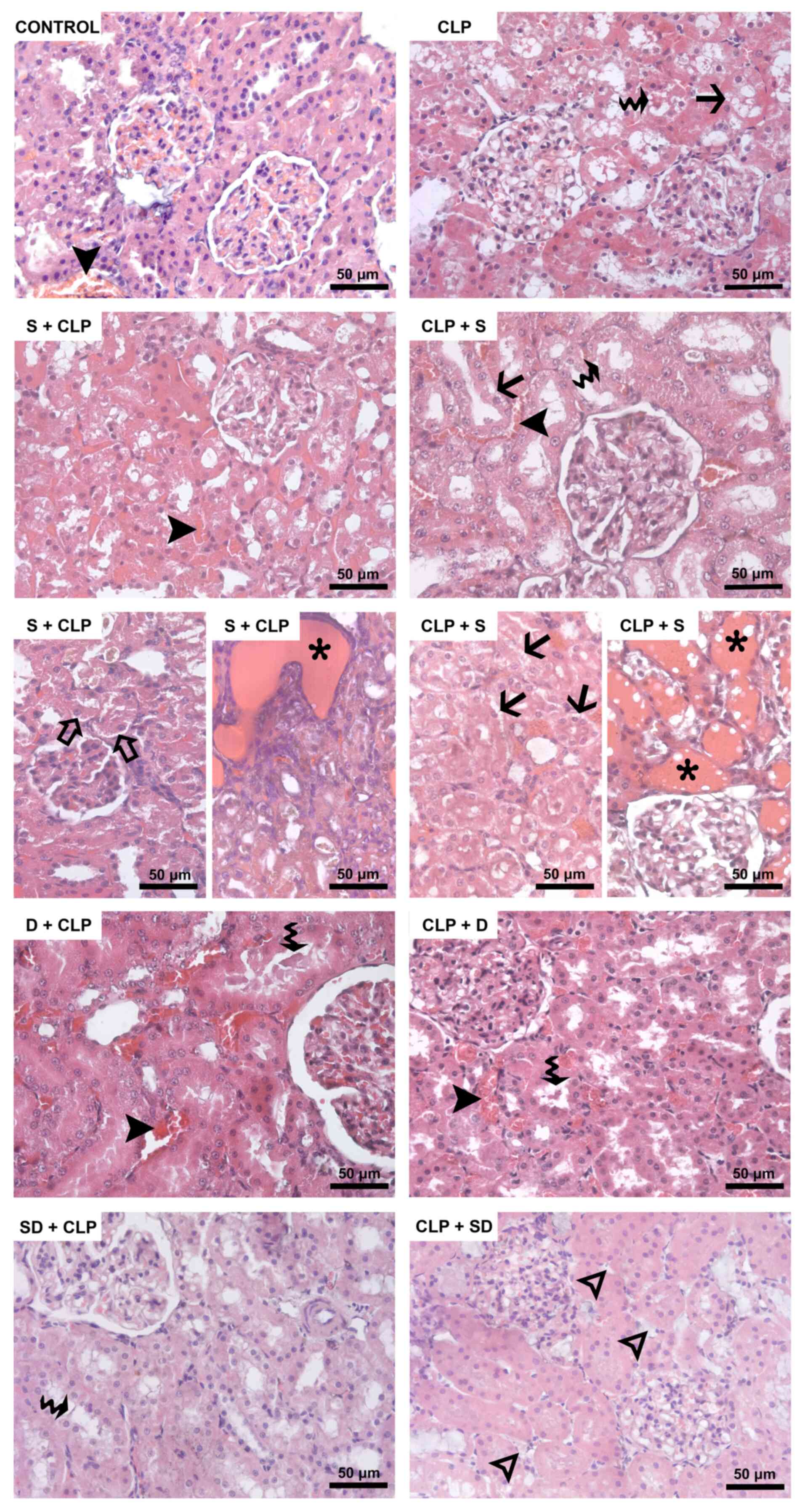 | Figure 1Hematoxylin and eosin-stained kidney
sections. Black arrowhead, dilatation of peritubular capillaries.
Waved arrow, loss of brush border in proximal tubule epithelium.
Black arrow, vacuolization in tubular epithelial cells. Hollow
arrowhead, interstitial edema. Hollow arrow, ablation of tubular
epithelium from the basement membrane. Asterisk, focal cysts in
both the cortex and medulla. CLP, cecal ligation and puncture; S,
silymarin; D, dexmedetomidine; SD, S + D. |
 | Table IHistopathological findings in kidney
tissue (mean ± SEM). |
Table I
Histopathological findings in kidney
tissue (mean ± SEM).
| Histopathological
finding | Control, n=6 | CLP, n=8 | S + CLP, n=8 | CLP + S, n=8 | D + CLP, n=8 | CLP + D, n=8 | SD + CLP, n=8 | CLP + SD, n=8 | Kruskal Wallis test
P-value |
|---|
| Interstitial
edema | 0.33±0.21 |
1.63±0.26a |
2.00±0.50a | 1.25±0.17 |
0.50±0.19a,b,c |
0.88±0.30a,b |
1.50±0.19a |
1.75±0.25a | 0.003 |
| Dilatation of
peritubular capillaries | 0.83±0.31 |
1.75±0.45a |
2.00±0.33a |
2.00±0.27a | 1.63±0.33 |
1.00±0.19c,d | 1.25±0.16 |
1.00±0.27c,d | 0.034 |
| Vacuolization | 0.17±0.17 |
1.75±0.45a |
2.50±0.27a |
2.25±0.49a |
0.38±0.18b,c,d |
0.63±0.26*a,c,d |
0.38±0.18b,c,d |
1.00±0.00*b,d | <0.001 |
| Ablation of tubular
epithelium from the basement membrane | 0.50±0.22 | 1.00±0.50 |
1.88±0.40a |
2.75±0.17a,b |
1.13±0.44d |
0.50±0.19c,d |
0.38±0.19c,d |
0.50±0.19c,d | <0.001 |
| Loss of brush
border in proximal tubule epithelium | 1.16±0.31 |
2.88±0.13a |
2.38±0.32a |
2.75±0.16a |
1.75±0.25b,d |
1.75±0.16b,d |
2.12±0.30a,b |
1.88±0.23b,d | <0.001 |
| Cell swelling and
nuclear defragmentation | 1.33±0.21 |
2.63±0.25a |
2.50±0.27a |
1.75±0.31b |
1.50±0.19b,c |
1.38±0.19b,c |
1.88±0.30b |
1.75±0.31b | 0.004 |
Lung tissue histopathological
results
The histopathological changes in the lung samples
are summarized in Table II.
Alveolar wall thickening in lung scores were significantly
different between the groups (P<0.0001). Alveolar wall
thickening in the CLP, S + CLP, CLP + S, D + CLP, CLP + D and CLP +
SD groups was greater than that in the control group (P<0.0001,
P<0.0001, P=0.004, P=0.012, P=0.004 and P=0.012, respectively).
However, it was significantly reduced in S + CLP, CLP + S, D + CLP,
CLP + D, SD + CLP and CLP + SD groups compared with the CLP group
(P=0.002, P<0.0001, P<0.0001, P<0.0001 and P<0.0001,
respectively). Furthermore, this decrease was more prominent in the
SD + CLP group than that of the S + CLP group (P=0.007). The
difference in severity of interstitial neutrophil infiltration
between the groups was also significant (P=0.015). It was
significantly more severe in the CLP than in the control group
(P<0.0001), whereas it was improved in the S + CLP, CLP + S, D +
CLP, CLP + D, SD + CLP and CLP + SD groups compared with that in
the CLP group (P=0.024, P=0.007, P=0.024, P=0.007, P<0.0001 and
P=0.007, respectively). By contrast, intra-alveolar neutrophil
infiltration scores of all the groups were similar (P=0.158;
Table II; Fig. 2).
 | Table IIHistopathological findings of lung
tissue (mean ± SEM). |
Table II
Histopathological findings of lung
tissue (mean ± SEM).
| Histopathological
finding | Control, n=6 | CLP, n=8 | S + CLP, n=8 | CLP + S, n=8 | D + CLP, n=8 | CLP + D, n=8 | SD + CLP, n=8 | CLP + SD, n=8 | Kruskal Wallis test
P-value |
|---|
| Thickening in the
alveolar wall | 0.50±0.34 |
2.88±0.13a |
1.88±0.30a,b |
1.50±0.27a,b |
1.38±0.27a,b,c |
1.50±0.19a,b,c |
1.00±0.00b,c |
1.38±0.18a,b | <0.001 |
| Capillary
congestion | 1.00±0.52 |
2.63±0.18a |
2.00±0.19a |
1.75±0.32b |
1.25±0.16b,c |
1.50±0.16b |
1.38±0.26b |
1.38±0.18b | 0.001 |
| Intra-alveolar
hemorrhage | 0.00±0.00 |
1.38±0.32a |
1.00±0.36a |
0.88±0.35a |
0.38±0.26b |
0.13±0.13b,c |
0.38±0.26b |
0.00±0.00b,c,d | 0.006 |
| Interstitial
neutrophil infiltration | 1.17±0.17 |
2.25±0.16a |
1.62±0.18b |
1.50±0.19b |
1.63±0.18b |
1.50±0.19b |
1.25±0.25b |
1.50±0.19b | 0.015 |
| Intra-alveolar
neutrophil infiltration | 0.17±0.17 | 0.50±0.19 | 0.25±0.16 | 0.50±0.19 | 0.38±0.26 | 0.00±0.00 | 0.13±0.13 | 0.00±0.00 | 0.158 |
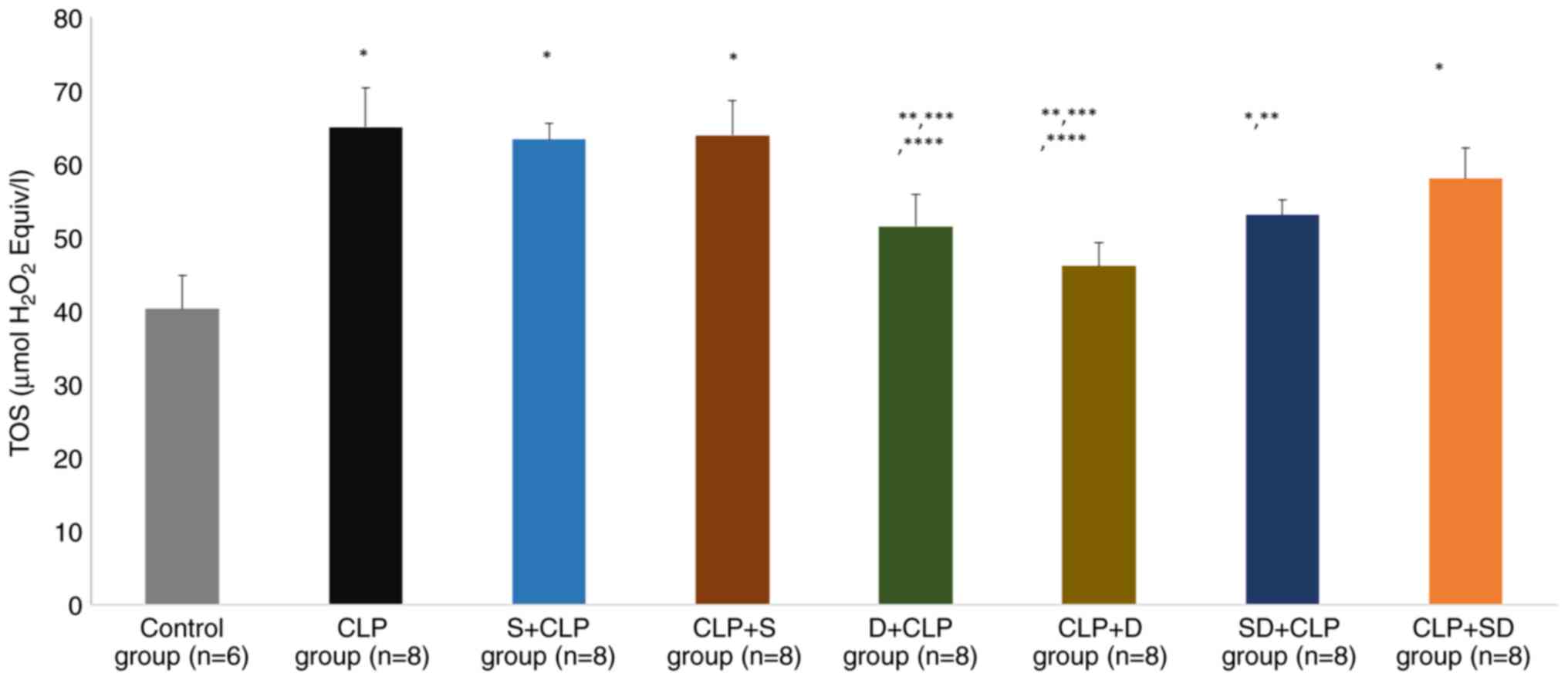
Lung tissue biochemical results
There was a significant difference in lung TOS and
TAS levels (P=0.001 and P=0.001, respectively). The TOS levels were
significantly higher in the CLP, S + CLP, CLP + S, SD + CLP and CLP
+ SD groups than in the control group (P<0.0001, P<0.0001,
P<0.0001, P=0.044 and P=0.005, respectively). TOS levels were
significantly lower in the D + CLP, CLP + D and SD + CLP groups
than in the CLP group (P=0.032, P=0.002 and P=0.043, respectively).
TOS levels were significantly lower in the D + CLP and CLP + D
groups than in the S + CLP group (P=0.041 and P=0.006,
respectively; Fig. 3). Similarly,
TOS levels were significantly lower in the D + CLP and CLP + D
groups than in the CLP + S group (P=0.036 and P=0.003,
respectively; Fig. 3).
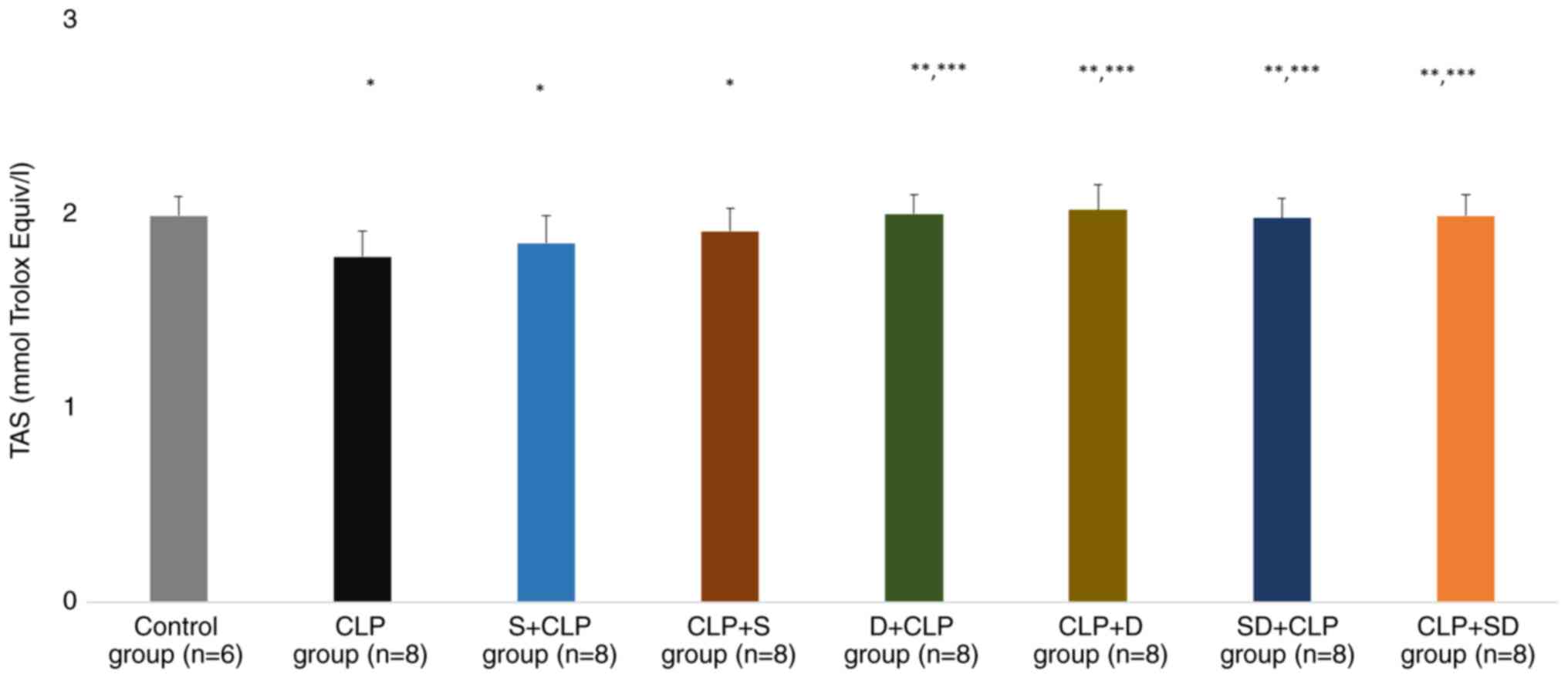
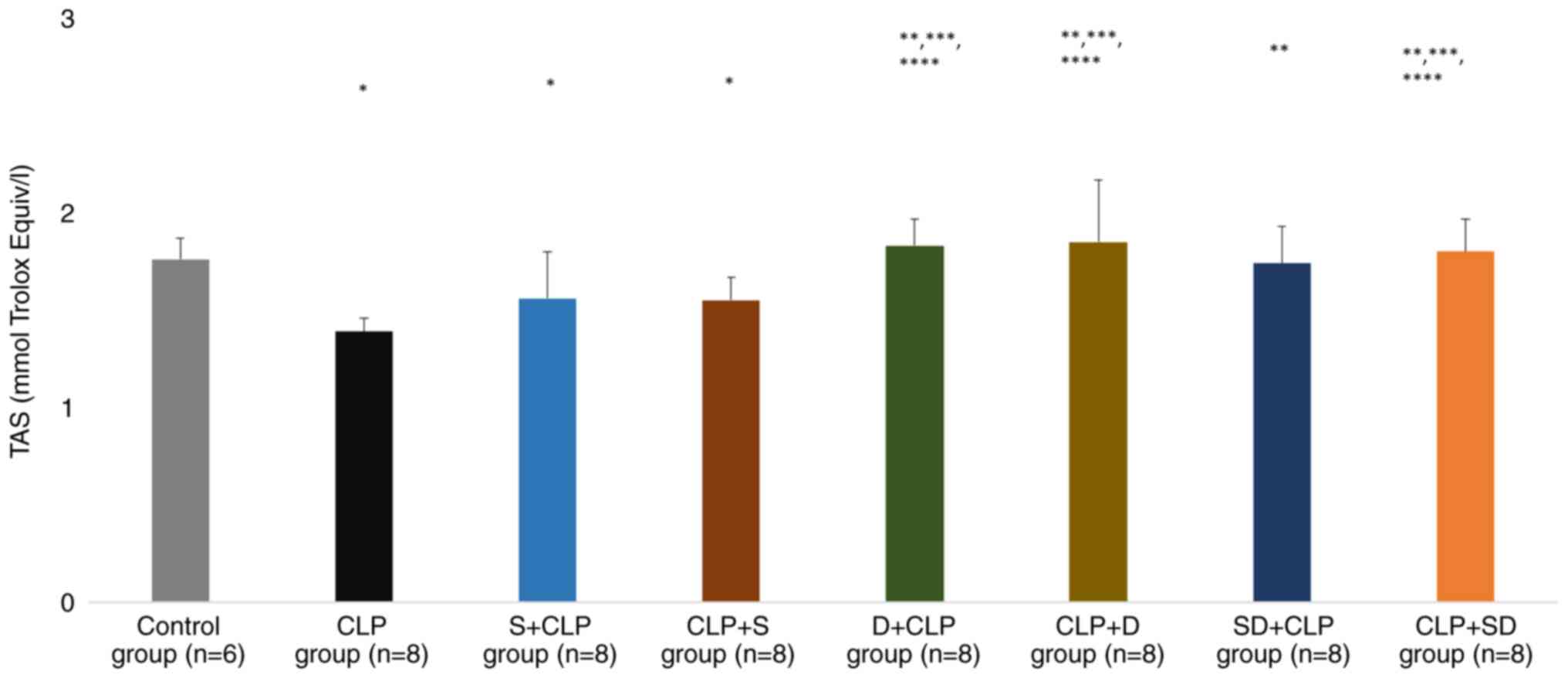
TAS levels were significantly lower in the CLP, S +
CLP and CLP + S groups than in the control group (P=0.002, P=0.039
and P=0.047, respectively). TAS levels were significantly higher in
the D + CLP, CLP + D, SD + CLP and CLP + SD groups than in the CLP
group (P<0.0001, P<0.0001, P=0.001 and P<0.0001,
respectively). Similarly, TAS levels were significantly higher in
the D + CLP, CLP + D, SD + CLP and CLP + SD groups than in the S +
CLP group (P=0.014, P=0.008, P=0.035 and P=0.021, respectively;
Fig. 4).
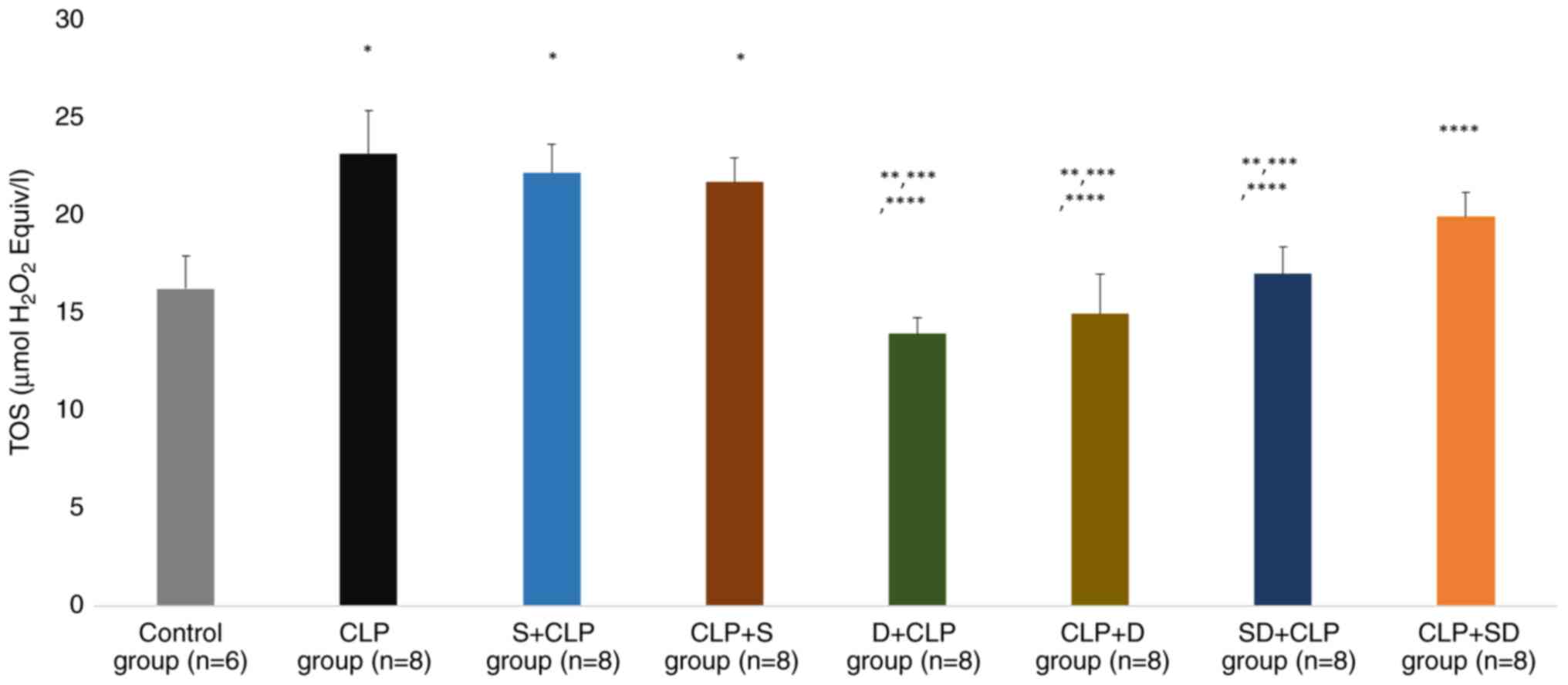
Kidney tissue biochemical results
There was a significant difference in kidney TOS and
TAS levels (P<0.0001 and P<0.0001, respectively). TOS levels
were significantly higher in the CLP, S + CLP and CLP + S groups
than in the control group (P=0.004, P=0.010 and P=0.027,
respectively). TOS levels were significantly lower in the D + CLP,
CLP + D and SD + CLP groups than in the CLP group (P<0.0001,
P<0.0001 and P=0.006, respectively). TOS levels were
significantly lower in the D + CLP, CLP + D and SD + CLP groups
than in the S + CLP group (P<0.0001, P=0.001 and P=0.015,
respectively). Similarly, TOS levels were significantly lower in
the D+ CLP, CLP + D, SD + CLP and CLP+ SD groups than in the CLP +
S group (P=0.001, P=0.003, P=0.035 and P=0.042, respectively;
Fig. 5).
TAS levels were significantly lower in the CLP, S +
CLP and CLP + S groups than in the control group (P<0.0001,
P=0.044 and P=0.035, respectively). TAS levels were significantly
higher in the D + CLP, CLP + D, SD + CLP and CLP + SD groups than
in the CLP group (all P<0.0001). The TAS levels were
significantly higher in the D + CLP, CLP + D and CLP + SD groups
than in the CLP group (P=0.005, P=0.003 and P=0.012, respectively).
Similarly, TAS levels were significantly higher in the D + CLP, CLP
+ D, and CLP + SD groups than in the S + CLP group (P=0.044,
P=0.002, and P=0.009, respectively; Fig. 6).
Discussion
In the clinical use of agents, prophylactic efficacy
is as important as therapeutic efficacy. Therefore, the present
study aimed to observe both the therapeutic and preventive effects
of dexmedetomidine and silymarin. The present study observed
differences following application of agents both before and after
sepsis modeling.
Silymarin and DEX have been used in different doses
in different studies and a definite effective dose has not been
determined yet (24,69). In the present study, dose selection
was based on similar studies (70-75).
Treatment time was also determined based on previous studies, but
since both preventive and therapeutic effects were investigated in
the clinical sepsis model, separate groups were created for
application times (62,76).
Since the polymicrobial peritonitis table created by
the CLP model is termed sepsis in studies in the literature
(77-84),
it was assumed that the clinical picture created by the CLP method
in the present study constitutes a sepsis model. CLP, which is an
experimental technique, may not mimic sepsis in exactly the same
way. Of course, due to the dynamic and developing nature of
science, it may be possible to perform more accurate sepsis
modeling in the coming years if different techniques are
discovered.
In the present study, histopathological damage in
lung and kidney tissue following sepsis modeling was observed.
However, this damage was accompanied by decreased TAS and increased
TOS. Tissue oxidant-antioxidant balance may result in organ damage,
which is in line with the literature (85,86).
Sepsis is a common clinical problem and silymarin and DEX have
shown promising results in recent experimental studies (87-90).
Sepsis is the most common cause of mortality in
intensive care units (91). Lung
and kidney involvement is relatively common in sepsis, and
dysfunction of these organs is associated with poor survival
outcome (92,93). Therefore, it has become
increasingly important to identify agents that have therapeutic or
protective effects on the lungs and kidney during sepsis.
Silymarin is a herbal flavonoid obtained from the
seeds or fruits of S. marianum (thistle) (25). Flavonoids, a class of secondary
metabolites of plants and fungi, have both prooxidant and
antioxidant activity due to their polyphenolic structure (94,95).
These effects vary depending on the dose and cell or tissue types
(72). For example, Malekinejad
et al (96) determined that
silymarin applied at the same dose and time had a protective effect
on the liver, while increasing damage in the brain. Numerous
studies have examined the curative and protective effects of
silymarin on kidney and lung tissue through various mechanisms
(94,95,97).
Al-Kadi et al showed that 1 h after CLP
induction, 100 mg/kg silymarin has a protective effect on kidney
tissue (62). Toklu et al
(94) studied serum and plasma
oxidation markers in lung tissue and concluded that 50 mg/kg per
oral silymarin has potential therapeutic efficacy in a similar
sepsis model and they found that silymarin may reduce
sepsis-induced oxidative organ injury and that this can be
attributed to its ability to balance oxidant-antioxidant status. By
contrast with previous studies (32,88,94)
in the present study, silymarin was administered 1 h before and
after sepsis induction. In our study, a decrease in organ damage
was observed in the kidney and lung tissues examined in
histopathological samples, but no statistically significant
difference was detected between the groups. In addition, TAS and
TOS measurements did not improve in the silymarin-treated groups (S
+ CLP, CLP + S). This may indicate that the biochemical improvement
reported in the literature (98,99)
does not significantly contribute to tissue damage observed in
sepsis.
Silymarin improves kidney tissue damage (62,71,95),
however, this was not observed in the present study. This may be
due to differences in the mechanisms that cause damage (ischemia
reperfusion, sepsis, toxicity, malignancy) or changes in the
selection of drug doses. Flavonoids have also been shown to have
pro-oxidant activity and these pro-oxidant mechanisms are thought
to provide anticarcinogenic activity by triggering cell death in
malignant cells (100).
Therefore, silymarin has different effects on different tissues at
different doses (72,96,100). Although the antioxidant activity
of silymarin is well-known (22,100,101), further studies are required to
understand its protective effects against sepsis and associated
organ damage. In the review of Soleimani et al (24), the side effects and doses used in
the studies conducted with silymarin were examined and it was seen
that it can be used safely at a number of different doses. However,
present study suggested that it may have a prooxidant effect on the
lung and kidney at the dose used in the experimental sepsis model
(100 mg/kg, intraperitoneal). The continued use of silymarin, one
of the oldest known plant-derived medicinal agents, in experimental
studies may be due to novel effects, as demonstrated in the present
study.
Although silymarin has demonstrated promising
results in numerous clinical situations (31,35,72),
it needs larger studies with different doses and drug combinations
before it can be used clinically for its therapeutic or
prophylactic effects. The present study evaluated both preventive
and therapeutic efficacy, performed with the one of the highest
intraperitoneal doses found in the literature (102) and also including interaction with
a different agent. It was hypothesized that the dose of silymarin
used had a pro-oxidant effect, as in other studies (72,96,100), and that this is why the animal
losses occurred. Using the two drugs together had a greater
therapeutic effect than silymarin.
DEX is a α2-adrenergic receptor agonist
that exerts sympatholytic effects such as anxiolysis, sedation and
analgesia in certain regions of the brain (103). Owing to the absence of side
effects such as respiratory depression, it is a frequently
preferred agent for sedation in intensive care units (104). The positive effects of DEX on
in vitro experimental sepsis models have been reported in
literature (105,106). For example, Koca et al
(107) applied 50 µg/kg DEX to
rats and observed improvements in both histomorphological and
immunohistochemical findings in a sepsis model using CLP technique.
Different hypotheses have been proposed for the similar
organ-protective effects of DEX and positive results have been
obtained. Li et al (108)
suggested that the lung protective effect of dexmedetomidine in
septic rats was achieved through increasing vagal tone; Wu et
al (109), in the same
experimental model, argued that the protective effect occurs
through the TLR4/NF-κB pathway. Qiu et al (76) observed that DEX decreased acute
renal failure and increased survival in a sepsis model. They also
suggested that this effect occurred via the NF-KB pathway induced
by lipoxin A4.
In the present study, it was hypothesized that DEX,
which is known to regulate the oxidant-antioxidant balance in
ischemia-reperfusion models (74,75),
may show organ-protective effects in sepsis through
oxidant-antioxidant balance pathway. Statistically significant
positive effects were observed at both the histopathological and
biochemical levels in the DEX treatment groups (D + CLP, CLP + D,
SD + CLP, CLP + SD). DEX improved lung and kidney tissue damage in
the treatment groups. The effects of DEX administration before and
after sepsis were not significantly different.
In the present study, DEX application statistically
significantly increased the total antioxidant score in tissues and
decreased the oxidant score. In a study conducted by Şengel et
al (110), TAS and TOS scores
in kidney tissue after DEX application showed similar changes as in
the present study and a statistically significant improvement in
histopathological damage was observed. These data support the
hypothesis that DEX may exert positive effects on the lung and
kidneys during sepsis. The positive effects of DEX on both TAS and
TOS levels and pathological examinations may be a guide for further
studies on its mechanism of action.
In previous studies, silymarin and dexmedetomidine
have been studied together with different combinations (98,111-116),
but no study has been performed in which these two agents were used
together. Thus, the present study aimed to investigate the effects
of these two agents used together. However, a limitation of the
present study was that the mechanism of action of these agents was
not examined, and only tissue and organ results were studied.
Further studies should study the mechanisms of the effects of these
agents.
Although the present study aimed to observe the
prophylactic effects of the agents before and after sepsis
induction, no differences in the effects were observed when the
application times of the agents were changed; however, it may be
possible to obtain different results using larger sample sizes. The
present study compared both the interactions and preventive and
therapeutic effects of promising agents in an experimental sepsis
model.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MAr and AK designed the study and analyzed and
interpreted the data. ADD, AY, MAl and ZY performed the
experiments. MAl, AİE and ZY confirm the authenticity of all the
raw data. AİE, AK, MArr and MAl critically revised the article for
important intellectual content. AİE and collected samples. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval for the study was obtained from The
Gazi University Experimental Animals Ethics Committee (Ankara,
Turkey; approval no: G.Ü.E.T-20.022).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Evans L, Rhodes A, Alhazzani W, Antonelli
M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M,
Prescott HC, et al: Surviving sepsis campaign: International
guidelines for management of sepsis and septic shock 2021.
Intensive Care Med. 47:1181–1247. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Beisel WR: Metabolic response to
infection. Annu Rev Med. 26:9–20. 1975.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dyck B, Unterberg M, Adamzik M and Koos B:
The impact of pathogens on sepsis prevalence and outcome.
Pathogens. 13(89)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aziz M, Jacob A, Yang WL, Matsuda A and
Wang P: Current trends in inflammatory and immunomodulatory
mediators in sepsis. J Leukoc Biol. 93:329–342. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen XH, Yin YJ and Zhang JX: Sepsis and
immune response. World J Emerg Med. 2:88–92. 2011.PubMed/NCBI
|
|
6
|
Arina P and Singer M: Pathophysiology of
sepsis. Curr Opin Anaesthesiol. 34:77–84. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fleischmann-Struzek C, Mellhammar L, Rose
N, Cassini A, Rudd KE, Schlattmann P, Allegranzi B and Reinhart K:
Incidence and mortality of hospital- and ICU-treated sepsis:
Results from an updated and expanded systematic review and
meta-analysis. Intensive Care Med. 46:1552–1562. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990-2017: Analysis for the global burden of disease
study. Lancet. 395:200–211. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Engel C, Brunkhorst FM, Bone HG,
Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski
U, John S, et al: Epidemiology of sepsis in Germany: Results from a
national prospective multicenter study. Intensive Care Med.
33:606–618. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Karlsson S, Varpula M, Ruokonen E, Pettilä
V, Parviainen I, Ala-Kokko TI, Kolho E and Rintala EM: Incidence,
treatment, and outcome of severe sepsis in ICU-treated adults in
Finland: The Finnsepsis study. Intensive Care Med. 33:435–443.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Blanco J, Muriel-Bombín A, Sagredo V,
Taboada F, Gandía F, Tamayo L, Collado J, García-Labattut A,
Carriedo D, Valledor M, et al: Incidence, organ dysfunction and
mortality in severe sepsis: A Spanish multicentre study. Crit Care.
12(R158)2008.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Sakr Y, Elia C, Mascia L, Barberis B,
Cardellino S, Livigni S, Fiore G, Filippini C and Ranieri VM:
Epidemiology and outcome of sepsis syndromes in Italian ICUs: A
muticentre, observational cohort study in the region of Piedmont.
Minerva Anestesiol. 79:993–1002. 2013.PubMed/NCBI
|
|
13
|
Weng L, Xu Y, Yin P, Wang Y, Chen Y, Liu
W, Li S, Peng JM, Dong R, Hu XY, et al: National incidence and
mortality of hospitalized sepsis in China. Crit Care.
27(84)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rhee C, Dantes R, Epstein L, Murphy DJ,
Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE,
et al: Incidence and trends of sepsis in US hospitals using
clinical vs claims data, 2009-2014. JAMA. 318:1241–1249.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lamontagne F, Rochwerg B, Lytvyn L, Guyatt
GH, Møller MH, Annane D, Kho ME, Adhikari NKJ, Machado F, Vandvik
PO, et al: Corticosteroid therapy for sepsis: A clinical practice
guideline. BMJ. 362(k3284)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rochwerg B, Oczkowski SJ, Siemieniuk RAC,
Agoritsas T, Belley-Cote E, D'Aragon F, Duan E, English S,
Gossack-Keenan K, Alghuroba M, et al: Corticosteroids in sepsis: An
updated systematic review and meta-analysis. Crit Care Med.
46:1411–1420. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vignon P, Laterre PF, Daix T and François
B: New agents in development for sepsis: Any reason for hope?
Drugs. 80:1751–1761. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Üstündağ H, Doğanay S, Kalındemirtaş FD,
Demir Ö, Huyut MT, Kurt N, Özgeriş FB and Akbaba Ö: A new treatment
approach: Melatonin and ascorbic acid synergy shields against
sepsis-induced heart and kidney damage in male rats. Life Sci.
329(121875)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Usmani J, Khan T, Ahmad R and Sharma M:
Potential role of herbal medicines as a novel approach in sepsis
treatment. Biomed Pharmacother. 144(112337)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang W, Jiang H, Wu G, Huang P, Wang H,
An H, Liu S and Zhang W: The pathogenesis and potential therapeutic
targets in sepsis. MedComm (2020). 4(e418)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Holubar M, Meng L, Alegria W and
Deresinski S: Bacteremia due to methicillin-resistant
staphylococcus aureus: An update on new therapeutic approaches.
Infect Dis Clin North Am. 34:849–861. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hadi A, Pourmasoumi M, Mohammadi H,
Symonds M and Miraghajani M: The effects of silymarin
supplementation on metabolic status and oxidative stress in
patients with type 2 diabetes mellitus: A systematic review and
meta-analysis of clinical trials. Complement Ther Med. 41:311–319.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Koltai T and Fliegel L: Role of silymarin
in cancer treatment: Facts, hypotheses, and questions. J Evid Based
Integr Med. 27(2515690X211068826)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Soleimani V, Delghandi PS, Moallem SA and
Karimi G: Safety and toxicity of silymarin, the major constituent
of milk thistle extract: An updated review. Phytother Res.
33:1627–1638. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Abenavoli L, Izzo AA, Milić N, Cicala C,
Santini A and Capasso R: Milk thistle (Silybum marianum): A
concise overview on its chemistry, pharmacological, and
nutraceutical uses in liver diseases. Phytother Res. 32:2202–2213.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Camini FC and Costa DC: Silymarin: Not
just another antioxidant. J Basic Clin Physiol Pharmacol.
31(20190206)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tighe SP, Akhtar D, Iqbal U and Ahmed A:
Chronic liver disease and silymarin: A biochemical and clinical
review. J Clin Transl Hepatol. 8:454–458. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aghazadeh S, Amini R, Yazdanparast R and
Ghaffari SH: Anti-apoptotic and anti-inflammatory effects of
Silybum marianum in treatment of experimental
steatohepatitis. Exp Toxicol Pathol. 63:569–574. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Saller R, Melzer J, Reichling J, Brignoli
R and Meier R: An updated systematic review of the pharmacology of
silymarin. Forsch Komplementmed. 14:70–80. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Surai A and Surai PF: Chapter 10 Silymarin
and inflammation: From understanding molecular mechanisms to
practical applications. In: Silymarin Puzzle. Wageningen Academic,
pp287-317, 2023.
|
|
31
|
Sharma S, Kumar P, Ashawat MS, Pandit V,
Verma CS and Sharma DK: Silymarin: A Phytoconstituent with
Significant Therapeutic Potential-A Narrative Review. Curr Drug
Ther. 18:89–97. 2023.
|
|
32
|
Kang JS, Jeon YJ, Park SK, Yang KH and Kim
HM: Protection against lipopolysaccharide-induced sepsis and
inhibition of interleukin-1beta and prostaglandin E2 synthesis by
silymarin. Biochem Pharmacol. 67:175–181. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Schrieber SJ, Hawke RL, Wen Z, Smith PC,
Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ, Meyers CM, et
al: Differences in the disposition of silymarin between patients
with nonalcoholic fatty liver disease and chronic hepatitis C. Drug
Metab Dispos. 39:2182–2190. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shahbazi F, Sadighi S, Dashti-Khavidaki S,
Shahi F, Mirzania M, Abdollahi A and Ghahremani MH: Effect of
silymarin administration on cisplatin nephrotoxicity: Report from a
pilot, randomized, double-blinded, placebo-controlled clinical
trial. Phytother Res. 29:1046–1053. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fried MW, Navarro VJ, Afdhal N, Belle SH,
Wahed AS, Hawke RL, Doo E, Meyers CM and Reddy KR: Silymarin in
NASH and C Hepatitis (SyNCH) Study Group. Effect of silymarin (milk
thistle) on liver disease in patients with chronic hepatitis C
unsuccessfully treated with interferon therapy: A randomized
controlled trial. JAMA. 308:274–282. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Marmouzi I, Bouyahya A, Ezzat SM, El Jemli
M and Kharbach M: The food plant Silybum marianum (L.)
Gaertn.: Phytochemistry, Ethnopharmacology and clinical evidence. J
Ethnopharmacol. 265(113303)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nguyen V, Tiemann D, Park E adz and Salehi
A: Alpha-2 agonists. Anesthesiol Clin. 35:233–245. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Giovannitti JA Jr, Thoms SM and Crawford
JJ: Alpha-2 adrenergic receptor agonists: A review of current
clinical applications. Anesth Prog. 62:31–39. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cioccari L, Luethi N, Bailey M, Shehabi Y,
Howe B, Messmer AS, Proimos HK, Peck L, Young H, Eastwood GM, et
al: The effect of dexmedetomidine on vasopressor requirements in
patients with septic shock: A subgroup analysis of the Sedation
Practice in Intensive care evaluation [SPICE III] trial. Crit Care.
24(441)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ferreira J: The Theory is out there: The
use of ALPHA-2 agonists in treatment of septic shock. Shock.
49:358–363. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Morelli A, Sanfilippo F, Arnemann P,
Hessler M, Kampmeier TG, D'Egidio A, Orecchioni A, Santonocito C,
Frati G, Greco E, et al: The effect of propofol and dexmedetomidine
sedation on norepinephrine requirements in septic shock patients: A
crossover trial. Crit Care Med. 47:e89–e95. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Suzuki T, Suzuki Y, Okuda J, Kurazumi T,
Suhara T, Ueda T, Nagata H and Morisaki H: Sepsis-induced cardiac
dysfunction and β-adrenergic blockade therapy for sepsis. J
Intensive Care. 5(22)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ferreira JA and Bissell BD: Misdirected
sympathy: The role of sympatholysis in sepsis and septic shock. J
Intensive Care Med. 33:74–86. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pichot C, Géloën A, Ghignone M and Quintin
L: Alpha-2 agonists to reduce vasopressor requirements in septic
shock? Med Hypotheses. 75:652–656. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Geloen A, Chapelier K, Cividjian A,
Dantony E, Rabilloud M, May CN and Quintin L: Clonidine and
dexmedetomidine increase the pressor response to norepinephrine in
experimental sepsis: A pilot study. Crit Care Med. 41:e431–e438.
2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Møller MH, Alhazzani W, Lewis K,
Belley-Cote E, Granholm A, Centofanti J, McIntyre WB, Spence J, Al
Duhailib Z, Needham DM, et al: Use of dexmedetomidine for sedation
in mechanically ventilated adult ICU patients: A rapid practice
guideline. Intensive Care Med. 48:801–810. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wiegand A, Behal M, Robbins B, Bissell B,
Pandya K and Mefford B: Niche roles for dexmedetomidine in the
intensive care unit. Ann Pharmacother. 57:1207–1220.
2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Page V and McKenzie C: Sedation in the
intensive care unit. Curr Anesthesiol Rep. 11:92–100.
2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zi SF, Li JH, Liu L, Deng C, Ao X, Chen DD
and Wu SZ: Dexmedetomidine-mediated protection against septic liver
injury depends on TLR4/MyD88/NF-κB signaling downregulation partly
via cholinergic anti-inflammatory mechanisms. Int Immunopharmacol.
76(105898)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chang Y, Huang X, Liu Z, Han G, Huang L,
Xiong YC and Wang Z: Dexmedetomidine inhibits the secretion of high
mobility group box 1 from lipopolysaccharide-activated macrophages
in vitro. J Surg Res. 181:308–314. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang T, Mei Q, Dai S, Liu Y and Zhu H:
Use of dexmedetomidine in patients with sepsis: A systematic review
and meta-analysis of randomized-controlled trials. Ann Intensive
Care. 12(81)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao
X, Huang WQ and Liu KX: Dexmedetomidine administration before, but
not after, ischemia attenuates intestinal injury induced by
intestinal ischemia-reperfusion in rats. Anesthesiology.
116:1035–1046. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kuyrukluyildiz U, Delen LA, Onk D, Yazici
GN, Gulaboglu M and Suleyman H: The effect of dexmedetomidine on
gastric ischemia reperfusion injury in rats. Biochemical and
histopathological evaluation. Acta Cir Bras.
36(e360104)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kotanoğlu MS, Kadioğlu E, Emerce E, Kaymak
C, Özcan A and Başar H: Antioxidant effects of dexmedetomidine
against hydrogen peroxide-induced DNA damage in vitro by alkaline
Comet assay. Turk J Med Sci. 50:1393–1398. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li W, Chen M, Gong Y, Lin F and Sun C:
Effects of dexmedetomidine on oxidative stress, programmed cell
death, liver function, and expression of peripheral immune cells in
patients with primary liver cancer undergoing hepatectomy. Front
Physiol. 14(1159746)2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Poli-de-Figueiredo LF, Garrido AG,
Nakagawa N and Sannomiya P: Experimental models of sepsis and their
clinical relevance. Shock. 30 (Suppl 1):S53–S59. 2008.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sjaastad FV, Jensen IJ, Berton RR,
Badovinac VP and Griffith TS: Inducing experimental polymicrobial
sepsis by cecal ligation and puncture. Curr Protoc Immunol.
131(e110)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Alverdy JC, Keskey R and Thewissen R: Can
the cecal ligation and puncture model be repurposed to better
inform therapy in human sepsis? Infect Immun. 88:e00942–19.
2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Drechsler S and Osuchowski M: Cecal
ligation and puncture. In: Sepsis: Methods and Protocols; Walker WE
(ed). Springer: New York, NY, USA, pp1-8, 2021.
|
|
60
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. J Cereb Blood Flow Metab. 40:1769–1777.
2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Care IoLARCo, Animals UoL: Guide for the
care and use of laboratory animals: US Department of Health and
Human Services, Public Health Service, National Academies Press
(US), 2011.
|
|
62
|
Al-Kadi A, Ahmed AS, El-Tahawy NFG,
Khalifa MMA and El-Daly M: Silymarin protects against
sepsis-induced acute liver and kidney injury via anti-inflammatory
and antioxidant mechanisms in the rat. J Adv Biomed Pharm Sci.
3:190–197. 2020.
|
|
63
|
Canikli Adıgüzel Ş, Pirat A, Türkoğlu S,
Bayraktar N, Özen Ö and Kaya M: A rat model of acute respiratory
distress silymarin's antiinflamatory and antioxidant effect. J Turk
Soc Intens Care. 14:18–27. 2016.
|
|
64
|
Schick MA, Isbary TJ, Schlegel N, Brugger
J, Waschke J, Muellenbach R, Roewer N and Wunder C: The impact of
crystalloid and colloid infusion on the kidney in rodent sepsis.
Intensive Care Med. 36:541–548. 2010.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Li XH, Gong X, Zhang L, Jiang R, Li HZ, Wu
MJ and Wan JY: Protective effects of polydatin on septic lung
injury in mice via upregulation of HO-1. Mediators Inflamm.
2013(354087)2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Abraha I, Cozzolino F, Orso M, Marchesi M,
Germani A, Lombardo G, Eusebi P, De Florio R, Luchetta ML, Iorio A
and Montedori A: A systematic review found that deviations from
intention-to-treat are common in randomized trials and systematic
reviews. J Clin Epidemiol. 84:37–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gupta SK: Intention-to-treat concept: A
review. Perspect Clin Res. 2:109–112. 2011.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Tripepi G, Chesnaye NC, Dekker FW, Zoccali
C and Jager KJ: Intention to treat and per protocol analysis in
clinical trials. Nephrology (Carlton). 25:513–517. 2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Dardalas I, Stamoula E, Rigopoulos P,
Malliou F, Tsaousi G, Aidoni Z, Grosomanidis V, Milonas A,
Papazisis G, Kouvelas D and Pourzitaki C: Dexmedetomidine effects
in different experimental sepsis in vivo models. Eur J Pharmacol.
856(172401)2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ni J, He J, Kang L, Zhong Z, Wang L and
Yin S: Effects of dexmedetomidine pretreatment on rats with
sepsis-induced acute kidney injury and miR-146a expression. Cell
Mol Biol (Noisy-le-grand). 66:93–98. 2020.PubMed/NCBI
|
|
71
|
Ustyol L, Demiroren K, Kandemir I, Erten
R, Bulan K, Kaba S, Demir N and Basunlu MT: Comparative
nephroprotective effects of silymarin, N-acetylcysteine, and
thymoquinone against carbon tetrachloride-induced nephrotoxicity in
rats. Iran Red Crescent Med J. 19(e37746)2017.
|
|
72
|
Guzel S, Sahinogullari ZU, Canacankatan N,
Antmen SE, Kibar D and Coskun Yilmaz B: Potential renoprotective
effects of silymarin against vancomycin-induced nephrotoxicity in
rats. Drug Chem Toxicol. 43:630–636. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Ozdemir A, Topçu A, Mercantepe T, Arpa M,
Karakaş SM, Ozdemir A, Tümkaya L and Mercantepe F: The effects of
dexmedetomidine on early acute kidney injury in severely burned
rats. Eur Rev Med Pharmacol Sci. 27:1311–1321. 2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Gonullu E, Ozkardesler S, Kume T, Duru LS,
Akan M, Guneli ME, Ergur BU, Meseri R and Dora O: Comparison of the
effects of dexmedetomidine administered at two different times on
renal ischemia/reperfusion injury in rats. Braz J Anesthesiol.
64:152–158. 2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Cakir M, Polat A, Tekin S, Vardi N,
Taslidere E, Rumeysa Duran Z and Tanbek K: The effect of
dexmedetomidine against oxidative and tubular damage induced by
renal ischemia reperfusion in rats. Ren Fail. 37:704–708.
2015.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Qiu R, Yao W, Ji H, Yuan D, Gao X, Sha W,
Wang F, Huang P and Hei Z: Dexmedetomidine restores septic renal
function via promoting inflammation resolution in a rat sepsis
model. Life Sci. 204:1–8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Tanaka S, Genève C, Zappella N, Yong-Sang
J, Planesse C, Louedec L, Viranaïcken W, Bringart M, Montravers P,
Denamur E, et al: Reconstituted high-density lipoprotein therapy
improves survival in mouse models of sepsis. Anesthesiology.
132:825–838. 2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Bedet A, Voiriot G, Ternacle J, Marcos E,
Adnot S, Derumeaux G and Mekontso Dessap A: Heart rate control
during experimental sepsis in mice: Comparison of ivabradine and
β-blockers. Anesthesiology. 132:321–329. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Zhong M, Wu W, Wang Y, Mao H, Song J, Chen
S and Zhu D: Inhibition of sphingosine kinase 1 attenuates
sepsis-induced microvascular leakage via inhibiting macrophage
NLRP3 inflammasome activation in mice. Anesthesiology.
132:1503–1515. 2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Park D, Ro M, Lee A-J, Kwak DW, Chung Y
and Kim JH: Contributory role of BLT2 in the production of
proinflammatory cytokines in cecal ligation and puncture-induced
sepsis. Mol Cells. 44:893–899. 2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Abdelnaser M, Alaaeldin R, Attya ME and
Fathy M: Hepatoprotective potential of gabapentin in cecal ligation
and puncture-induced sepsis; targeting oxidative stress, apoptosis,
and NF-kB/MAPK signaling pathways. Life Sci.
320(121562)2023.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kim GO, Kim N, Song GY and Bae JS:
Inhibitory activities of rare ginsenoside Rg4 on cecal ligation and
puncture-induced sepsis. Int J Mol Sci. 23(10836)2022.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Li J, Li M, Li L, Ma J, Yao C and Yao S:
Hydrogen sulfide attenuates ferroptosis and stimulates autophagy by
blocking mTOR signaling in sepsis-induced acute lung injury. Mol
Immunol. 141:318–327. 2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Tripathi AS, Awasthi S, Maurya RK, Yasir
M, Mohapatra L and Srivastav V: Protective effect of vanillin on
the management of cecal ligation and puncture induced sepsis rat
model. Microb Pathog. 165(105493)2022.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Daenen K, Andries A, Mekahli D, Van
Schepdael A, Jouret F and Bammens B: Oxidative stress in chronic
kidney disease. Pediatr Nephrol. 34:975–991. 2019.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Krzemińska J, Wronka M, Młynarska E,
Franczyk B and Rysz J: Arterial hypertension-oxidative stress and
inflammation. Antioxidants (Basel). 11(172)2022.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Zhao X, Wang H, Yang Y, Gou Y, Wang Z,
Yang D and Li C: Protective effects of silymarin against
D-Gal/LPS-induced organ damage and inflammation in mice. Drug Des
Devel Ther. 15:1903–1914. 2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Alikiaii B, Bagherniya M, Askari G,
Johnston TP and Sahebkar A: The role of phytochemicals in sepsis: A
mechanistic and therapeutic perspective. Biofactors. 47:19–40.
2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Mei B, Li J and Zuo Z: Dexmedetomidine
attenuates sepsis-associated inflammation and encephalopathy via
central α2A adrenoceptor. Brain Behav Immun. 91:296–314.
2021.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Hu H, An S, Sha T, Wu F, Jin Y, Li L, Zeng
Z, Wu J and Chen Z: Association between dexmedetomidine
administration and outcomes in critically ill patients with
sepsis-associated acute kidney injury. J Clin Anesth.
83(110960)2022.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Esposito S, De Simone G, Boccia G, De Caro
F and Pagliano P: Sepsis and septic shock: New definitions, new
diagnostic and therapeutic approaches. J Glob Antimicrob Resist.
10:204–212. 2017.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Fujishima S: Organ dysfunction as a new
standard for defining sepsis. Inflamm Regen. 36(24)2016.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Sun S, Chen R, Dou X, Dai M, Long J, Wu Y
and Lin Y: Immunoregulatory mechanism of acute kidney injury in
sepsis: A narrative review. Biomed Pharmacother.
159(114202)2023.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Toklu HZ, Akbay TT, Velioglu-Ogunc A,
Ercan F, Gedik N, Keyer-Uysal M and Sener G: Silymarin, the
antioxidant component of Silybum marianum, prevents
sepsis-induced acute lung and brain injury. J Surg Res.
145:214–222. 2008.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Turgut F, Bayrak O, Catal F, Bayrak R,
Atmaca AF, Koc A, Akbas A, Akcay A and Unal D: Antioxidant and
protective effects of silymarin on ischemia and reperfusion injury
in the kidney tissues of rats. Int Urol Nephrol. 40:453–460.
2008.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Malekinejad H, Rahmani F, Valivande-Azar
S, Taheri-Broujerdi M and Bazargani-Gilani B: Long-term
administration of Silymarin augments proinflammatory mediators in
the hippocampus of rats: Evidence for antioxidant and pro-oxidant
effects. Hum Exp Toxicol. 31:921–930. 2012.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Sajedianfard J, Nazifi S, Izadi A,
Chahardahcherik M and Honarmand M: Effect of various doses of
silymarin on the oxidative stress induced by busulfan
administration in the different organs of rats. Turk J Pharm Sci.
13:233–240. 2016.
|
|
98
|
Yardımcı M, Göz M, Aydın MS, Kankılıç N
and Temiz E: Antioxidant actions of thymoquinone, silymarin, and
curcumin on experimental aortic ischemia-reperfusion model in
wistar albino rats. Braz J Cardiovasc Surg. 37:807–813.
2022.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Azizoğlu M, Arslan S, Gökalp Özkorkmaz E,
Aşır F, Basuguy E, Okur MH, Aydoğdu B, Alagöz Karabel M and Kaplan
I: Protective effects of Silymarin on testicular torsion/detorsion
in rats. Eur Rev Med Pharmacol Sci. 27:10446–10453. 2023.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Surai PF: Silymarin as a natural
antioxidant: An overview of the current evidence and perspectives.
Antioxidants (Basel). 4:204–247. 2015.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Taleb A, Ahmad KA, Ihsan AU, Qu J, Lin N,
Hezam K, Koju N, Hui L and Qilong D: Antioxidant effects and
mechanism of silymarin in oxidative stress induced cardiovascular
diseases. Biomed Pharmacother. 102:689–698. 2018.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Kim MJ, Kim DU, Choi JW, Kim DG, Song HJ,
Bae GS and Park SJ: Silymarin attenuates the severity of
cerulein-induced acute pancreatitis. Pancreas. 49:89–95.
2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Cormack JR, Orme RM and Costello TG: The
role of alpha2-agonists in neurosurgery. J Clin Neurosci.
12:375–378. 2005.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Carollo DS, Nossaman BD and Ramadhyani U:
Dexmedetomidine: A review of clinical applications. Curr Opin
Anaesthesiol. 21:457–461. 2008.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Aidoni Z, Pourzitaki C, Stamoula E,
Kotzampassi K, Tsaousi G, Kazakos G, Foroulis CN, Skourtis C,
Vasilakos DG and Grosomanidis V: Circulatory effects of
dexmedetomidine in early sepsis: A randomised controlled
experimental study. Naunyn Schmiedebergs Arch Pharmacol. 393:89–97.
2020.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Wang C, Yuan W, Hu A, Lin J, Xia Z, Yang
CF, Li Y and Zhang Z: Dexmedetomidine alleviated sepsis-induced
myocardial ferroptosis and septic heart injury. Mol Med Rep.
22:175–184. 2020.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Koca U, Olguner ÇG, Ergür BU, Altekin E,
Taşdöğen A, Duru S, Girgin P, Gündüz K, Cilaker Mıcılı S, Güzeldağ
S and Akkuş M: The effects of dexmedetomidine on secondary acute
lung and kidney injuries in the rat model of intra-abdominal
sepsis. ScientificWorldJournal. 2013(292687)2013.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Li Y, Wu B, Hu C, Hu J, Lian Q, Li J and
Ma D: The role of the vagus nerve on dexmedetomidine promoting
survival and lung protection in a sepsis model in rats. Eur J
Pharmacol. 914(174668)2022.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Wu Y, Liu Y, Huang H, Zhu Y, Zhang Y, Lu F
and Zhou C, Huang L, Li X and Zhou C: Dexmedetomidine inhibits
inflammatory reaction in lung tissues of septic rats by suppressing
TLR4/NF-κB pathway. Mediators Inflamm. 2013(562154)2013.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Şengel N, Köksal Z, Dursun AD, Kurtipek Ö,
Sezen ŞC, Arslan M and Kavutçu M: Effects of dexmedetomidine
administered through different routes on kidney tissue in rats with
spinal cord ischaemia-reperfusion injury. Drug Des Devel Ther.
16:2229–2239. 2022.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Hernández G, Tapia P, Alegría L, Soto D,
Luengo C, Gomez J, Jarufe N, Achurra P, Rebolledo R, Bruhn A, et
al: Effects of dexmedetomidine and esmolol on systemic hemodynamics
and exogenous lactate clearance in early experimental septic shock.
Crit Care. 20(234)2016.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Zhao W, Jia L, Yang HJ, Xue X, Xu WX, Cai
JQ, Guo RJ and Cao CC: Taurine enhances the protective effect of
dexmedetomidine on sepsis-induced acute lung injury via balancing
the immunological system. Biomed Pharmacother. 103:1362–1368.
2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Yang CL, Chen CH, Tsai PS, Wang TY and
Huang CJ: Protective effects of dexmedetomidine-ketamine
combination against ventilator-induced lung injury in endotoxemia
rats. J Surg Res. 167:e273–e281. 2011.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Özkan F, Yüksek A, Demirel A and Kantekin
Ç: Effects of dexmetatomidine and midazolam on immunity in
sepsis-induced rats. Med J Bakirkoy. 19:180–185. 2023.
|
|
115
|
Choi MW, Ko DR, Kong T, Choa MH, You JS
and Chung SP: Comparison of silymarin, penicillin, N-acetylcysteine
in patient with amatoxin poisoning: A systematic review. J Korean
Soc Clin Toxicol. 16:33–41. 2018.
|
|
116
|
Abdel Salam OM, Sleem AA, Omara EA and
Hassan NS: Effect of ribavirin alone or combined with silymarin on
carbon tetrachloride induced hepatic damage in rats. Drug Target
Insights. 2:19–27. 2007.PubMed/NCBI
|