Introduction
Ischemic stroke is the leading cause of mortality
and acquired physical disability worldwide. Ischemic stroke leads
to the ischemic, anoxic necrosis of brain tissue due to
insufficient cerebral blood supply, followed by the rapid
occurrence of defects in the functions of corresponding nerves
(1). It is characterized by a high
incidence, disability, recurrence and a high mortality rate,
accounting for 44 million physical disabilities, and 5.5 million
related deaths worldwide annually (2). The current treatment of ischemic
stroke focuses on rapid reperfusion with intravenous thrombolysis
and endovascular thrombectomy (3);
however, due to the limitations of a strict time window, damage to
brain cells is irreversible, and some patients will have long-term
disability after suffering a stroke (4).
Stem cell therapies have emerged as a potential
therapeutic strategy for stroke in recent decades. Stem cells are
initial and unspecialized cells that have the ability to
self-renew, with high proliferative and multidirectional
differentiation abilities; these cells include embryonic,
mesenchymal and hematopoietic stem cells (5). The research focusing on stem cell
therapy in ischemic stroke in animal models or clinical studies is
rapidly progressing. There is evidence to suggest that stem cell
therapies can repair damaged brain tissue and improve neurological
function through multiple mechanisms, which includes the modulation
of the immune response promotion of endogenous neural cells,
angiogenesis and neuro-regeneration (6). However, stem cell therapies have also
been found to be associated with certain risks, such as allogeneic
cell transplantation, the development of infection and tumor
formation (7).
A logical extension of stem cell therapies is the
direct employment of solely extracellular vesicles as a treatment
modality (8). The therapeutic
benefit of stem cell-derived extracellular vesicles has been
previously analyzed in ischemic stroke (9). Extracellular vesicles, including
exosomes, microvesicles and apoptotic bodies, are membrane vesicles
that are between 40-1,000 nm in diameter and are widely present in
various bodily fluids. They can carry a variety of active
molecules, such as proteins, lipids, messenger RNAs, microRNAs
(miRNAs or miRs) and non-coding RNAs (10). Studies have shown that the content
of exosomes, and the variety of active molecules they carry, can
impact the post transcriptional regulation of numerous genes.
Hence, extracellular vesicles play a critical role in
communications between cells. As natural delivery vehicles, they
may be used for delivery across natural barriers, such as the
blood-brain barrier. In addition, they avoid endogenous
tumorigenicity due to less or no immunogenicity and tumorigenicity
(9). Therefore, extracellular
vesicles are expected to become a promising treatment strategy for
ischemic stroke (10).
A plethora of preclinical studies on ischemic stroke
models using extracellular vesicles have been conducted to explore
their beneficial effects (9).
However, there are only a limited number of trials using
extracellular vesicles for the treatment of ischemic stroke in the
database of clinical trials (6).
To provide pre-clinical evidence for further and larger scale
studies on extracellular vesicles in the treatment of ischemic
stroke and promote the clinical applications of such vesicles in
ischemic stroke, the present study performed a meta-analysis of
animal studies.
Materials and methods
Search strategy
A search for relevant literature was performed using
the following databases: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com), Medline (https://www.nlm.nih.gov/medline/medline_home.html),
Web of Science (https://www.webofscience.com) and the Cochrane Library
(https://www.cochranelibrary.com/). The
full search strategy was based on the following search terms:
(‘extracellular vesicles’ or ‘EVs’) and (‘cerebral ischemia’ or
‘ischemia stroke’ or ‘brain infarct’ or ‘cerebral infarct’). All
publications were required to be published in English until May
2023. The reference list of the selected articles was independently
be screened to identify additional studies excluded in the initial
search. The present meta-analysis was registered in the PROSPERO
database (https://www.crd.york.ac.uk/prospero/) under the
registration number CRD42023442677.
Inclusion and exclusion criteria
The inclusion criteria for the study were as
follows: i) Pre-clinical middle cerebral artery occlusion (MCAO)
animal models, including mice or rats of any age or sex; ii)
intervention: Cell-derived extracellular vesicles without
modifications and transfection; iii) Comparisons: Vehicle, saline,
phosphate-buffered saline, or no treatment; iv) Study designs:
Randomized controlled experiments; v) outcome measures: Inclusion
of neurological function score, apoptotic rate, infarct volumes,
tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6; and
vi) original full research articles. The exclusion criteria were
the following: i) The study was not an animal study with ischemia
stroke; ii) modified or transfected extracellular vesicles; iii)
the intervention was a combination of extracellular vesicles and
other therapy; iv) the study included in vitro experiments;
v) the main information and evaluation indicators of the experiment
were not reported; vi) the study was a review, editorial, case
report, expert opinion, or letter; and vii) duplicate
literature.
Data extraction
Of note, two authors independently extracted
relevant data from the text and graphs. The data extraction sheet
contained the following details from the included studies: i) Name
of the first author and the year of publication; ii) number of
animals per group for comparison; iii) intervention of experimental
groups, control groups; iv) animal species, sex and weight; v)
animal models: Method of induction of ischemia, type of anesthetic
used, duration of reperfusion; vi) intervention of treatment: Time
of administration, administration method, treatment dose, source of
extracellular vesicles; vii) primary outcome measures: Neurological
function score, infarct volumes and apoptotic rate; viii) secondary
outcome measures: TNF-α, IL-1β and IL-6. Data were extracted data
from charts with unavailable numerical values using GetData Graph
Digitizer (version 2.26, https://apps.auto-meris.io/wpd/index.zh_CN.html)
software (11).
Quality assessment
Two investigators independently assessed the quality
of each eligible included study using the CAMARADES checklist for
study quality. The CAMARADES checklist consists of 10 evaluation
indicators, and the evaluation results are represented as ‘Y’, ‘N’
and ‘U’. Any disagreements were resolved by discussion with a third
author (12).
Statistical analysis
All statistical calculations and graphing were
performed using Review Manager5.3 (The Cochrane Collaboration). The
summaries of outcome measures were calculated using the
standardized mean difference with 95% confidence intervals for
continuous outcomes. A value of P<0.05 was considered to
indicate a statistically significant difference. Statistical
heterogeneity was evaluated using the I-square test and Q test. The
analysis was combined using random effects model in the present
study. In addition, subgroup analyses were conducted to explore the
sources of between-study heterogeneity.
Results
Literature search results
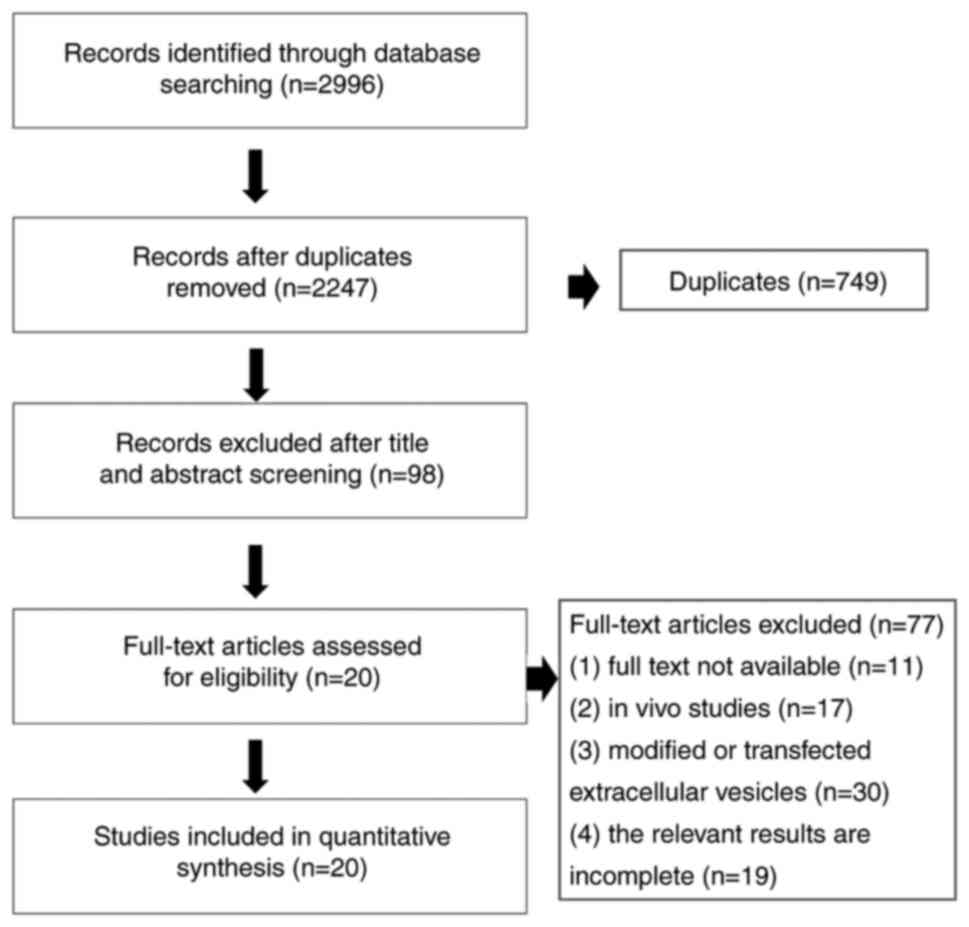
A total of 2,996 potential studies were retrieved
through the primary retrieval from the database, of which 749
articles were excluded due to reduplication. A total of 98 articles
were included in the scope of review based on screening the titles
and abstracts. Among these, 77 articles were excluded, due to the
following reasons: i) The full text was not available; ii) in
vitro studies; iii) modified or transfected extracellular
vesicles; iv) the relevant results were incomplete. Ultimately, 20
studies were selected for the present meta-analysis. The specific
flow chart for the literature search is presented in Fig. 1.
Characteristics of included
studies
There were 20 studies were included in the
meta-analysis (13-32).
All articles were published between 2015 and 2023. Among the
included studies, seven studies were performed using rats, and 13
studies were conducted on mice. The MCAO animal model to induce
ischemic stroke was used in all the studies. The
ischemia/reperfusion (I/R) model was established with filament
insertion, thread loop blockage or microbipolar coagulation. Of the
20 preclinical animal studies, 10 of these used extracellular
vesicles originating from bone marrow-derived mesenchymal stem
cells, three studies used M2 microglial cell-derived extracellular
vesicles, two studies used adipose stem cell-derived extracellular
vesicles, and one study used extracellular vesicles derived from
human umbilical cord perivascular cells, human placental
mesenchymal cells, dental pulp stem cells, neural progenitor cells
and astrocyte cells.
The route of extracellular vesicle administration
was via tail vein injection in 11 studies, lateral ventricle
injection in three studies, intravenous administration in five
studies and nasal injection in one study. A variety of miRNA types
have been researched in these studies, including miR-26a, miR-132,
miR-124, miR-206, miR-135a-5p and miR-23a-3p. The characteristics
of the included articles are presented in Table SI.
Quality assessment
The quality score across the 20 studies ranged from
3 to 10. The most suitable criteria included peer-reviewed
journals, appropriate animal models, statements of potential
conflicts of interests and statements of compliance with ethics or
animal welfare regulations. Moreover, the random allocation of
animals and temperature control were reported in the majority of
the included studies. However, only a small number of studies had
reported the blinding methods of model establishing and outcome
assessment. The further score details of the study quality are
presented in Table I.
 | Table IQuality evaluation of included
studies. |
Table I
Quality evaluation of included
studies.
| ID | References | A | B | C | D | E | F | G | H | I | J |
|---|
| 1 | Seifali et
al (13) | Y | Y | U | U | U | Y | U | U | Y | U |
| 2 | Wang et al
(14) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 3 | Hu et al
(15) | Y | Y | U | Y | U | Y | U | U | Y | Y |
| 4 | Gregorius et
al (16) | Y | Y | Y | Y | U | Y | Y | Y | Y | Y |
| 5 | Dumbrava et
al (17) | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| 6 | Hu et al
(18) | Y | U | U | Y | U | Y | U | Y | Y | Y |
| 7 | Li et al
(19) | Y | Y | Y | Y | U | Y | U | Y | Y | Y |
| 8 | Tian et al
(20) | Y | U | U | U | U | Y | U | U | Y | Y |
| 9 | Han et al
(21) | Y | Y | U | Y | U | Y | Y | Y | Y | Y |
| 10 | Houa et al
(22) | Y | Y | Y | U | U | Y | U | U | Y | Y |
| 11 | Doeppner et
al (23) | Y | U | U | Y | U | Y | Y | Y | Y | Y |
| 12 | Heras-Romero et
al (24) | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| 13 | Li et al
(25) | Y | Y | U | Y | U | Y | U | Y | Y | Y |
| 14 | Barzegarara et
al (26) | Y | Y | U | U | U | Y | U | Y | Y | Y |
| 15 | Feng et al
(27) | Y | Y | Y | U | U | Y | U | Y | Y | Y |
| 16 | Song et al
(28) | Y | Y | U | Y | U | Y | U | Y | Y | Y |
| 17 | Liu et al
(29) | Y | Y | U | U | U | Y | U | U | Y | Y |
| 18 | Dong et al
(30) | Y | Y | Y | Y | U | Y | U | U | Y | Y |
| 19 | Liu et al
(31) | Y | Y | Y | U | U | Y | U | Y | Y | Y |
| 20 | Xie et al
(32) | Y | Y | U | U | U | Y | U | U | Y | Y |
Neurological function score
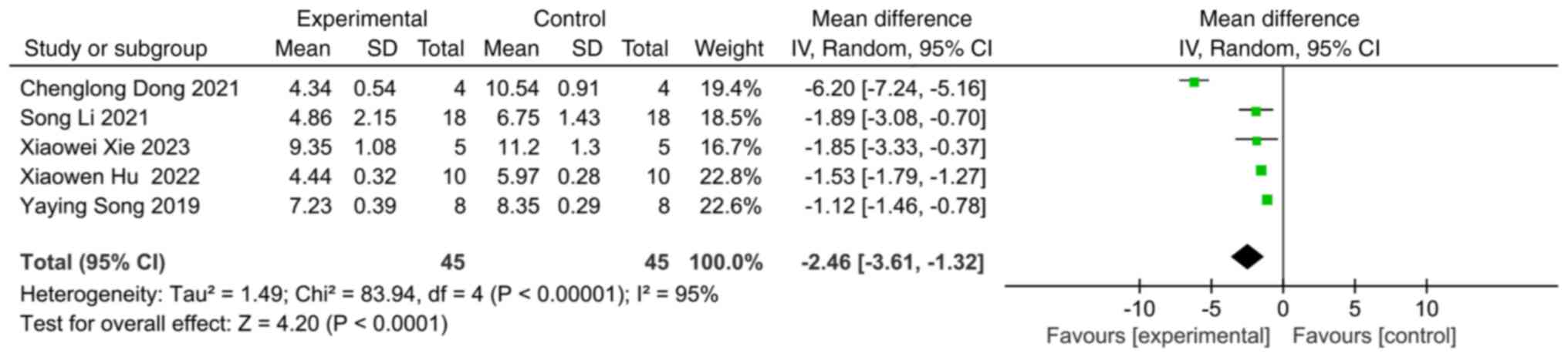
A total of 17 studies investigated neurological
function following ischemic stroke, of which five studies used the
modified neurological severity score (mNSS) to assess neurological
function. Compared with the control group, the experimental group
with extracellular vesicles was found to exhibit a significant
improvement in the neurological function score (SMD: -2.46; 95% CI:
-3.61 to -1.32; heterogeneity: P<0.00001; I2=95%)
(Fig. 2).
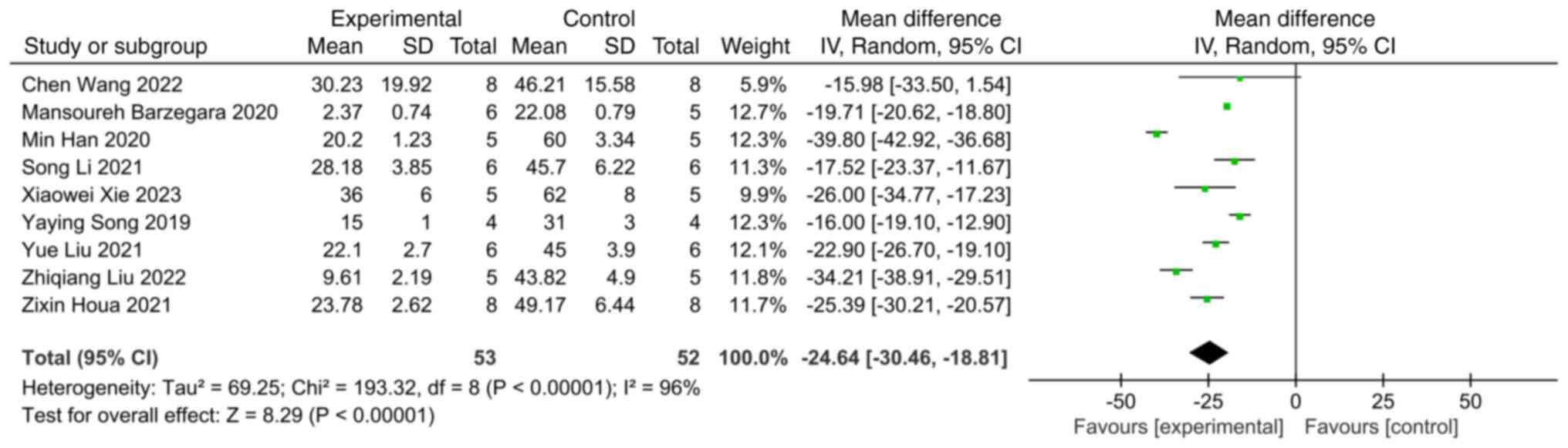
Infarct volume
A total of 9 studies involving infarct volume were
comprehensively analyzed. Compared with the control group,
extracellular vesicles were shown to significantly reduce the
infarct volume following ischemic stroke (SMD: -24.64; 95% CI:
-30.46 to -18.81; heterogeneity: P<0.00001; I2=96%)
(Fig. 3).
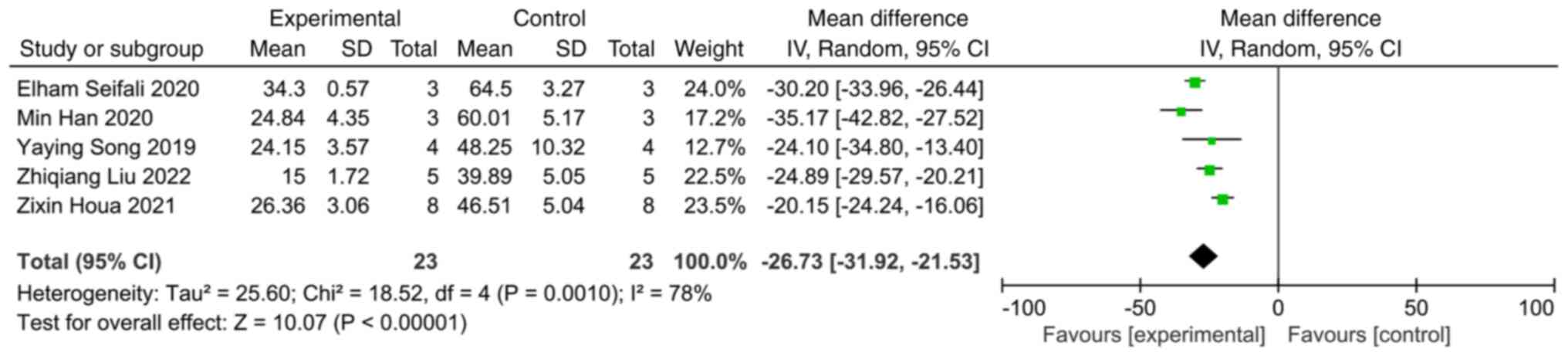
Apoptotic rate
A total of five studies reported the apoptotic rate
in the MCAO animal model. A comprehensive analysis revealed that
extracellular vesicles significantly decreased the apoptotic rate
compared with the control group (SMD: -26.73; 95% CI: -31.92 to
-21.53; heterogeneity: P=0.001; I2=78%) (Fig. 4).
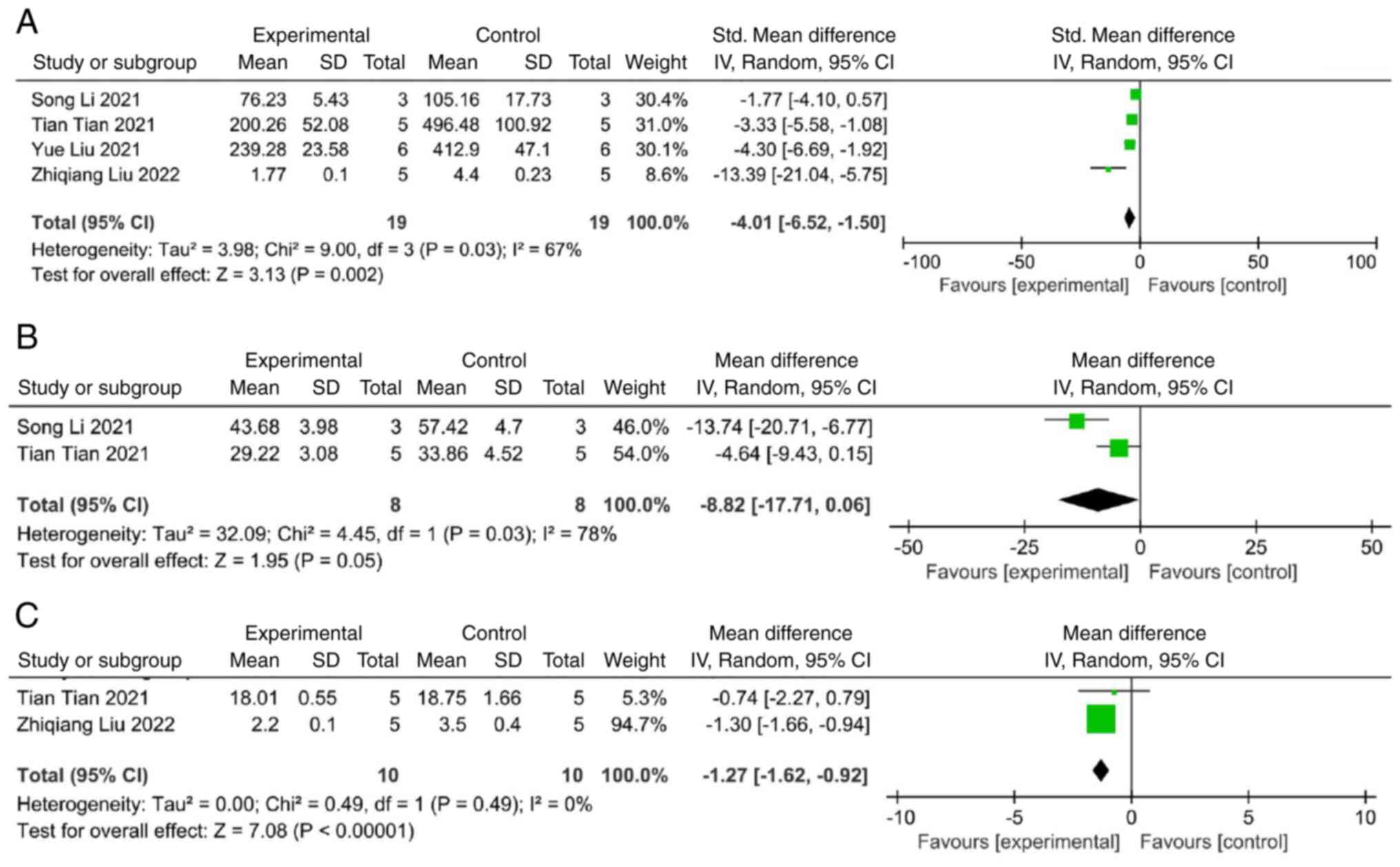
Secondary outcome
Some of the included studies evaluated the
anti-inflammatory effects of external vesicles by measuring the
levels and expression of inflammatory factors. A meta-analysis of
four studies demonstrated that extracellular vesicles significantly
decreased the level of IL-1β compared with the control group (SMD:
-4.01; 95% CI: -6.52 to -1.50; heterogeneity: P=0.03;
I2=67%) (Fig. 5A). A
comprehensive analysis of two studies revealed significant
suppressive effects of extracellular vesicles on the level of IL-6
(SMD: -8.82; 95% CI: -17.71 to 0.06; heterogeneity: P=0.03;
I2=78%) (Fig. 5B).
Additionally, a meta-analysis of two studies demonstrated that the
level of TNF-α exhibited a significant decrease in the
extracellular vesicle group (SMD: -1.27; 95% CI: -1.62 to -0.92;
heterogeneity: P=0.49; I2=0%) (Fig. 5C).
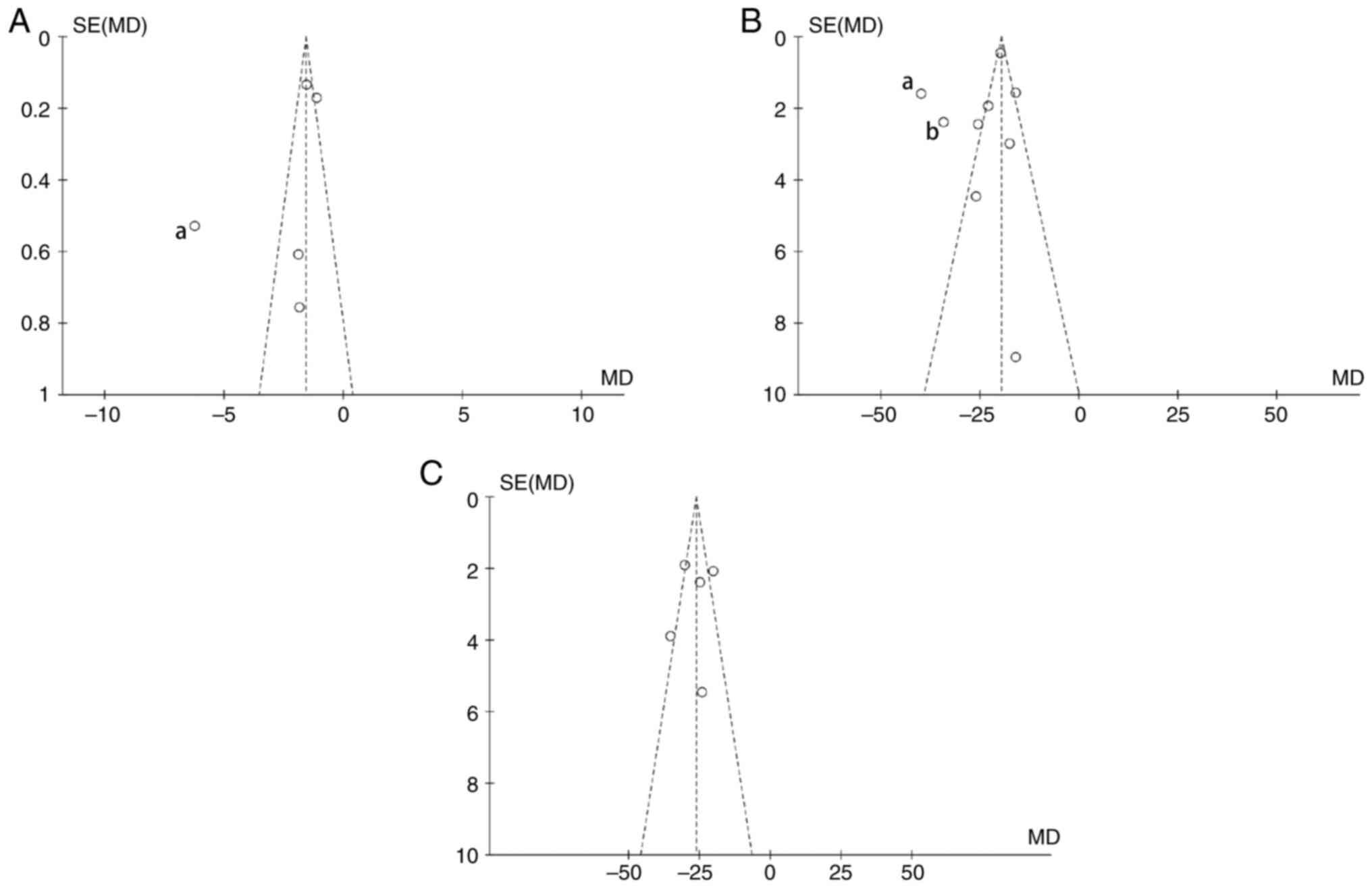
Publication bias analysis
The publication bias of the primary outcomes was
examined using the funnel plot. The funnel plot for infarct volume
and mNSS revealed significant asymmetry, suggesting a certain
publication bias (Fig. 6). It was
hypothesized that selection bias, implementation bias, measurement
bias and reporting bias may all contribute to this asymmetry. Among
the analysis research of neural function scores, the study from
Dong et al (30) in 2021
lying outside of the funnels represent studies that have the
highest publication bias. After reviewing the research process, it
was found that the aforementioned study lacks a detailed
description of the measurement process and analysis of the results
of mNSS score. Possible differences in measurement time and method
in the studies were considered to cause measurement bias. The
studies, including Han et al (21) in 2020 and Liu et al
(31) in 2022, have the highest
publication bias in the analysis of studies on infarct volume. In
the present analysis comparison, it was found that in the study of
Han et al (21) and Liu
et al (31), different
software and methods were used to measure and calculate the infarct
rates, and it was considered whether this would cause some
measurement bias. Meanwhile, it was found that the specific
measurement time of infarction volume was not reported in the study
of Liu et al (31), and the
possible difference in measurement time was also a major possible
factor causing the bias. In addition, subgroup analysis was
conducted from three aspects of the source of extracellular
vesicles, the injection time or the injection method used, to
identify the possible sources of publication bias.
Subgroup analyses
In the present study, subgroup analyses were
conducted to explore the sources of heterogeneity based on
different categories, including animal species, injection method
and the sources of the extracellular vesicles. The results of the
analyses demonstrated that the sources of the extracellular
vesicles did not exhibit significant differences, and the animal
species and injection method were the possible source of
heterogeneity in the present study. All cell-derived extracellular
vesicles improved the nerve function score (P=0.13;
I2=47.7%) (Fig. S1),
decreasing the apoptotic rate (P=0.004; I2=77.4%)
(Fig. S2), and reducing the
infarct volume (P=0.04; I2=64%) (Fig. S3). In terms of neurological
function score and apoptotic rate, the treatment effect of
extracellular vesicles on mice compared with rats was as follows:
mNSS, P<0.00001; I2=98.7% (Fig. S4); apoptotic rate: P=0.006;
I2=86.6%) (Fig. S5);
however, there was no significant difference in the therapeutic
effect of animal species infarct volume (P<0.00001;
I2=96.7%) (Fig. S6).
In addition, the effect size for tail vein injection was
significantly larger than lateral ventricle injection in terms of
neurological function score and apoptotic rate (mNSS: P<0.00001;
I2=98.7% (Fig. S7);
apoptotic rate: P=0.66; I2=0%) (Fig. S8). And the effect size of tail
vein injection and lateral ventricle injection were larger than
that of intravenous injection in infarct volume (P=0.05;
I2=66.4%) (Fig. S9).
The detailed results of subgroup analyses are illustrated in
Fig. S1, Fig. S2, Fig. S3, Fig. S4, Fig. S5, Fig. S6, Fig. S7, Fig. S8 and Fig. S9.
Discussion
The present meta-analysis of 20 studies with a total
of 28 comparisons explored the overall effect of extracellular
vesicles in the treatment of ischemic stroke. The evidence from
included studies indicated that cell-derived extracellular vesicles
significantly improved neurological function, decreased the infarct
volume and inhibited apoptosis in the MACO model. Moreover,
extracellular vesicles were also found to play a neuroprotective
role by reducing neuroinflammation, confirmed by some studies. The
present study used the CAMARADES checklist to evaluate the study
quality, with a median of 7 and range from 3 to 10. Furthermore,
attention needs to be paid to the calculation of sample size and
the blind established model. The publication bias was found drawing
the funnel plot. In summary, extracellular vesicle therapy is a
promising research direction for ischemic stroke.
Previous research has demonstrated the positive
efficacy of extracellular vesicles on infarct volume and
neurological score in stroke models (33). Recently, cell-derived extracellular
vesicles have been widely used in preclinical trials of ischemic
stroke. There are studies that have focused on therapeutic efficacy
and the mechanisms of extracellular vesicles and providing new
evidence for further clinical trials. Hence, an updated
meta-analysis is essential. The majority of previous meta-analyses
on extracellular vesicles have examined infarct volume and neural
function scores. By contrast, the present meta-analysis further
evaluated the effects of extracellular vesicles on apoptosis and
neuroinflammation.
In research investigating the application of
extracellular vesicles in the treatment of ischemic stroke, the
administration route of extracellular vesicles is still widely
being explored. At present, the drug administration methods in
extracellular vesicles in in vivo experiments are mainly
divided into systemic administration and local administration; tail
vein injection is widely used in the majority of preclinical trials
(34). In the present
meta-analysis, 11 included studies applied the tail vein injection,
whereas three studies used lateral ventricle injection. In the
subgroup analyses, the difference of effect size between tail vein
injection and lateral ventricle injection was statistically
significant.
Developing neuroprotective strategies remains a
promising area of research in the treatment of stroke. There is
evidence to demonstrate the neuroprotective potential of
extracellular vesicles in preclinical ischemic stroke models
(35). Extracellular vesicles can
play a neuroprotective role to promote the recovery of nerve
function and improve brain injury in ischemic stroke, as confirmed
by the studies included in the present meta-analysis. For example,
Han et al (21)
demonstrated that mesenchymal stem cell-derived extracellular
vesicles exerted neuroprotective effects, alleviating brain damage
and reducing apoptosis during cerebral ischemia-reperfusion injury,
which may be achieved by the regulation of the AMPK and
JAK2/STAT3/NF-κB signaling pathways (21).
Cerebral vascular oxidative stress and apoptosis
constitute a crucial pathological basis for ischemic stroke
(4). There is increasing evidence
to indicate that apoptosis has become a key target in the treatment
of ischemic stroke. Han et al (21) found that mesenchymal stem
cell-derived extracellular vesicles significantly reduced the
number of apoptotic cells compared with the control group in MCAO
(21). Furthermore, Feng et
al (27) also demonstrated
that miR-132-containing mesenchymal stem cell-derived extracellular
vesicles decreased neuronal injury by inhibiting Acvr2b expression
and the p-Smad2/c-Jun signaling pathway. Seifali et al
(13) reached the conclusion that
extracellular vesicles have the potential to regulate apoptosis and
improve neuronal recovery. Extracellular vesicle therapy following
MCAO decreased neuronal apoptosis, enhanced neuronal density,
reduced dark neurons and improved sensorimotor function (13). Cumulative evidence suggests that
extracellular vesicles exert a positive effect by diminishing
neuronal pathological injury and cell apoptosis following ischemic
stroke.
Inflammation is widely involved in the pathogenesis
of ischemic stroke. Following a stroke, dead cells release
damage-associated molecular patterns, then recruit leukocytes to
the brain and release inflammatory cytokines and chemokines. These
stimulate an inflammatory response in microglia and astrocytes,
which leads to secondary injury to the brain (36). It has been demonstrated that
mesenchymal stem cell-derived exosomes inhibit microglial
inflammation and ischemic cerebral injury by regulating
anti-inflammatory molecules (IL-4 and IL-10) and pro-inflammatory
cytokines (IL-6, TNF-α and IL-1β) (37). Despite the controversy on the
microglia phenotypic classification, the majority of the current
preclinical experiments have opted to explore the treatment
strategy of microglial polarization with a broad concept of the M1
and M2 phenotypes (15). M2
microglia release anti-inflammatory mediators, whereas M1 microglia
secrete pro-inflammatory cytokines to destroy adjacent neurons and
oligodendrocytes. Some experimental results have indicated that in
M2 microglia-derived extracellular vesicles, downregulation of
thioredoxin interacting protein mediates NLRP3 inflammasome
expression via miR-135a-5p, which has been further verified to
function as a novel therapeutic target for repressed neuronal
autophagy and alleviate ischemic brain injury (29). Hu et al (15) demonstrated that adipose stem
cell-derived extracellular vesicles regulated microglial
polarization, and the possible underlying mechanism may be related
to the inhibition of the expression of STAT1 and PTEN. Moreover, Li
et al (19) first reported
that dental pulp stem cell-derived exosomes exerted a
neuroprotective effect against neuro-inflammation in mice with
cerebral I/R by inhibiting the high mobility group box 1
(HMGB1)/Toll-like receptor (TLR)4/MyD88/NF-κB signaling pathway.
Liu et al (31)
innovatively found that exosomes from bone marrow-derived
mesenchymal stem cells inhibited the inflammatory response and
improved neurological function by activating TGR5 to affect cAMP
and NF-κB signaling and reduce the production of inflammatory
factors in the MCAO model. The pooled analyses demonstrated that
anti-inflammation remains key to the treatment of ischemic injury,
and targeting extracellular vesicles related to it is a promising
therapeutic direction.
With new insight into the nature of extracellular
vesicle-mediated intercellular communications, miRNAs have become
the most extensively studied molecules in extracellular vesicles
(38). miRNAs as single stranded
non-coding RNAs, play crucial roles in mediating a range of
biological functions and regulating post-transcriptional protein
expression (8). To date, there are
studies that suggest that miRNAs may be candidates for innovative
gene therapy, playing multiple roles in promoting neurogenesis,
angiogenesis and neuroplasticity. The delivery system for miRNAs
has been developed to improve the biologic efficiency (39). In the studies included in the
present meta-analysis, six related miRNAs were involved, namely
miR-124 (25,28), miR-26a (22), miR-132(27), miR-206(32), miR-23a-3p (30), miR-135a-5p (29). Li et al (25) demonstrated that M2-derived
extracellular vesicles reduced glial scar formation and inhibited
astrocyte inflammation in mice by decreasing the expression of
STAT3 and p-STAT3. Moreover, Song et al (28) reported that miR-124 in M2
microglia-derived exosomes promoted neuronal survival, and the
mechanism involved may be related to miR-124 and its downstream
target, ubiquitin specific peptidase 14 (USP14). These findings
demonstrated that extracellular vesicles play a neuroprotective
role by regulating miR-124 and decreasing the expression of its
downstream target, STAT3/p-STAT3/USP14. In summary, these results
illustrated that miRNAs exert a significant neuroprotective effect
and promote neuro-recovery, thus providing more possibilities for
the treatment of ischemic stroke.
To date, there is increasing evidence to indicate
that extracellular vesicle therapy is the safer and more effective
treatment strategy with which to combat cerebral ischemic injury.
An increasing number of studies are being conducted to expand the
possibilities of the therapeutic mechanisms and modes of
extracellular vesicles. However, the experimental analysis and
clinical application of extracellular vesicles are still faced with
numerous challenges. First, the large-scale production, isolation
and purification of extracellular vesicles continue to be urgent
technical issues that need to be resolved in clinical applications.
Second, the specific treatment plan of extracellular vesicle
therapy and the measurement indices of efficacy evaluation still
need to be more standardized. For example, in the studies included
in the present meta-analysis, there was no uniform measurement
standard for the evaluation of neurobehavioral function following
I/R in mice. Finally, several limitations in the present
meta-analysis itself should be mentioned, such as: i) The present
study was limited to literature published in English before June
2023 and did not include unpublished articles; ii) the majority of
included studies used healthy mice/rats to create I/R models.
However, in clinical practice, a number of basic diseases are all
high-risk factors for ischemic stroke, such as hypertension,
diabetes and hyperlipidemia; and iii) the present study used
WebPlotDigitizer (version 2.26, https://apps.auto-meris.io/wpd/index.zh_CN.html)
software to extract part of the data from graphics, which may alter
the original data to a certain extent and may thus have caused
errors in the results.
In conclusion, extracellular vesicles can
effectively improve nerve function and reduce infarct volume
following ischemic stroke. Additionally, the present study
identified that stem cell-derived extracellular vesicles can reduce
the expression of IL-6, IL-1β and TNF-α and inhibit the
neuroinflammatory response by inhibiting the activation of
MAPK/cAMP/HMGB1/TLR4/MyD88/NF-κB signaling pathway. miRNA may can
impact protein translational suppression and activation of numerous
gene targets. In the current study, extracellular vesicles were
found to promote neuronal survival and exert neuroprotective
effects via six related miRNAs, namely miR-124, miR-26a, miR-132,
miR-206, miR-23a-3p and miR-135a-5p; and targeting and
upregulating/downregulating the expression of STAT1, PTEN, USP14
and TXNZP, the potential downstream targets of miRNAs. Although
some factors, such as publication bias may have influenced the
heterogeneity of results, it is considered that the present
meta-analysis provides an important basis for clinical trials on
extracellular vesicles. At the same time, it also suggests that the
experimental design should be optimized when conducting preclinical
experiments in an aim to minimize bias.
Supplementary Material
Subgroup analysis of the source of
extracellular vesicle for modified neurological severity score.
BMSCs, bone marrow mesenchymal stem cells; DPSCs, dental pulp stem
cells; ADSCs, adipose-derived stem cells; SD, standardized
difference; CI, confidence intervals; df, degrees of freedom; IV,
inverse variance.
Subgroup analysis of the source of
extracellular vesicle for apoptotic rate. BMSCs, bone marrow
mesenchymal stem cells; ADSCs, adipose-derived stem cells; HUCPVCs,
human umbilical cord perivascular cells; SD, standardized
difference; CI, confidence intervals; df, degrees of freedom; IV,
inverse variance.
Subgroup analysis of the source of
extracellular vesicle for infarction volume. BMSCs, bone marrow
mesenchymal stem cells; DPSCs, dental pulp stem cells; ADSCs,
adipose-derived stem cells; hPMSCs, human placental MSCs; SD,
standardized difference; CI, confidence intervals; df, degrees of
freedom; IV, inverse variance.
Subgroup analysis of animal species
for modified neurological severity score. SD, standardized
difference; CI, confidence intervals; df, degrees of freedom; IV,
inverse variance.
Subgroup analysis of animal species
for apoptotic rate. SD, standardized difference; CI, confidence
intervals; df, degrees of freedom; IV, inverse variance.
Subgroup analysis of animal species
for infarction volume. SD, standardized difference; CI, confidence
intervals; df, degrees of freedom; IV, inverse variance.
Subgroup analysis of injection method
for modified neurological severity score. SD, standardized
difference; CI, confidence intervals; df, degrees of freedom; IV,
inverse variance.
Subgroup analysis of injection method
for apoptotic rate. SD, standardized difference; CI, confidence
intervals; df, degrees of freedom; IV, inverse variance.
Subgroup analysis of injection method
for infarction volume. SD, standardized difference; CI, confidence
intervals; df, degrees of freedom; IV, inverse variance.
Characteristics of studies included in
the present meta-analysis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81904173), the Natural
Science Foundation of Hunan (grant no. 2023JJ30362) and the Open
Project of Key Laboratory of Prevention and treatment of
cardiovascular and cerebrovascular diseases of Educational Ministry
in Gannan Medical University (grant no. XN202011).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL contributed to conception and the design of the
study. YuX and XH conducted the literature search and data
extraction. JM and DZh assisted in the analysis and interpretation
of the data. YuX performed the data analysis and wrote the draft.
YL participated in writing and revising the manuscript. TD, LX and
YaX inspected and proofed this systematic review and the final
manuscript, YuX and YL confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang M, Hong Z, Xiao C, Li L, Chen L,
Cheng S, Lei T and Zheng H: Effects of exosomes on neurological
function recovery for ischemic stroke in pre-clinical studies: A
meta-analysis. Front Cell Neurosci. 14(593130)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhao J, Deng H, Xun C, Chen C, Hu Z, Ge L
and Jiang Z: Therapeutic potential of stem cell extracellular
vesicles for ischemic stroke in preclinical rodent models: A
meta-analysis. Stem Cell Res Ther. 14(62)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Campbell BCV, De Silva DA, Macleod MR,
Coutts SB, Schwamm LH, Davis SM and Donnan GA: Ischaemic stroke.
Nat Rev Dis Primers. 5(70)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang ZG and Chopp M: Exosomes in stroke
pathogenesis and therapy. J Clin Invest. 126:1190–1197.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Zakrzewski W, Dobrzyński M, Szymonowicz M
and Rybak Z: Stem cells: Past, present, and future. Stem Cell Res
Ther. 10(68)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nistor-Cseppentö DC, Jurcău MC, Jurcău A,
Andronie-Cioară FL and Marcu F: Stem cell- and cell-based therapies
for ischemic stroke. Bioengineering (Basel). 9(717)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li Y, Hu G and Cheng Q: Implantation of
human umbilical cord mesenchymal stem cells for ischemic stroke:
Perspectives and challenges. Front Med. 9:20–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chopp M and Zhang ZG: Emerging potential
of exosomes and noncoding microRNAs for the treatment of
neurological injury/diseases. Expert Opin Emerg Drugs. 20:523–526.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Doeppner TR, Bähr M, Hermann DM and Giebel
B: Concise review: Extracellular vesicles overcoming limitations of
cell therapies in ischemic stroke. Stem Cells Transl Med.
6:2044–2052. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rao D, Sang C, Lai Z, Zhong J and Tang Z:
Roles of extracellular vesicles in cerebral protection of ischemic
stroke. Neuro Endocrinol Lett. 42:160–170. 2021.PubMed/NCBI
|
|
11
|
Burda BU, O'Connor EA, Webber EM, Redmond
N and Perdue LA: Estimating data from figures with a web-based
program: Considerations for a systematic review. Res Synth Methods.
8:258–262. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bahr-Hosseini M, Bikson M, Iacoboni M,
Liebeskind DS, Hinman JD, Carmichael ST and Saver JL: PRIMED2
Preclinical evidence scoring tool to assess readiness for
translation of neuroprotection therapies. Transl Stroke Res.
13:222–227. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Seifali E, Hassanzadeh G, Mahdavipour M,
Mortezaee K, Moini A, Satarian L, Shekari F, Nazari A, Movassaghi S
and Akbari M: Extracellular vesicles derived from human umbilical
cord perivascular cells improve functional recovery in brain
ischemic rat via the inhibition of apoptosis. Iran Biomed J.
24:347–360. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang C, Börger V, Mohamud Yusuf A, Tertel
T, Stambouli O, Murke F, Freund N, Kleinschnitz C, Herz J, Gunzer
M, et al: Postischemic neuroprotection associated with
anti-inflammatory effects by mesenchymal stromal cell-derived small
extracellular vesicles in aged mice. Stroke. 53:e14–e18.
2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hu X, Pan J, Li Y, Jiang Y, Zheng H, Shi
R, Zhang Q, Liu C, Tian H, Zhang Z, et al: Extracellular vesicles
from adipose-derived stem cells promote microglia M2 polarization
and neurological recovery in a mouse model of transient middle
cerebral artery occlusion. Stem Cell Res Ther.
13(21)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gregorius J, Wang C, Stambouli O, Hussner
T, Qi Y, Tertel T, Börger V, Mohamud Yusuf A, Hagemann N, Yin D, et
al: Small extracellular vesicles obtained from hypoxic mesenchymal
stromal cells have unique characteristics that promote cerebral
angiogenesis, brain remodeling and neurological recovery after
focal cerebral ischemia in mice. Basic Res Cardiol.
116(40)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dumbrava DA, Surugiu R, Börger V, Ruscu M,
Tertel T, Giebel B, Hermann DM and Popa-Wagner A: Mesenchymal
stromal cell-derived small extracellular vesicles promote
neurological recovery and brain remodeling after distal middle
cerebral artery occlusion in aged rats. GeroScience. 44:293–310.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hu B, Chen S, Zou M, He Z, Shao S and Liu
B: Effect of extracellular vesicles on neural functional recovery
and immunologic suppression after rat cerebral apoplexy. Cell
Physiol Biochem. 40:155–162. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li S, Luo L, He Y, Li R, Xiang Y, Xing Z,
Li Y, Albashari AA, Liao X, Zhang K, et al: Dental pulp stem
cell-derived exosomes alleviate cerebral ischaemia-reperfusion
injury through suppressing inflammatory response. Cell Prolif.
54(e13093)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tian T, Cao L, He C, Ye Q, Liang R, You W,
Zhang H, Wu J, Ye J, Tannous BA and Gao J: Targeted delivery of
neural progenitor cell-derived extracellular vesicles for
anti-inflammation after cerebral ischemia. Theranostics.
11:6507–6521. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Han M, Cao Y, Xue H, Chu X, Li T, Xin D,
Yuan L, Ke H, Li G and Wang Z: Neuroprotective effect of
mesenchymal stromal cell-derived extracellular vesicles against
cerebral ischemia-reperfusion-induced neural functional injury: A
pivotal role for AMPK and JAK2/STAT3/NF-κB signaling pathway
modulation. Drug Des Devel Ther. 14:2865–2876. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hou Z, Chen J, Yang H, Hu X and Yang F:
microRNA-26a shuttled by extracellular vesicles secreted from
adipose-derived mesenchymal stem cells reduce neuronal damage
through KLF9-mediated regulation of TRAF2/KLF2 axis. Adipocyte.
10:378–393. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Doeppner TR, Herz J, Görgens A, Schlechter
J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B and
Hermann DM: Extracellular vesicles improve post-stroke
neuroregeneration and prevent postischemic immunosuppression. Stem
Cells Transl Med. 4:1131–1143. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Heras-Romero Y, Morales-Guadarrama A,
Santana-Martínez R, Ponce I, Rincón-Heredia R, Poot-Hernández AC,
Martínez-Moreno A, Urrieta E, Bernal-Vicente BN, Campero-Romero AN,
et al: Improved post-stroke spontaneous recovery by astrocytic
extracellular vesicles. Mol Ther. 30:798–815. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li Z, Song Y, He T, Wen R, Li Y, Chen T,
Huang S, Wang Y, Tang Y, Shen F, et al: M2 microglial small
extracellular vesicles reduce glial scar formation via the
miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics.
11:1232–1248. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Barzegar M, Wang Y, Eshaq RS, Yun JW,
Boyer CJ, Cananzi SG, White LA, Chernyshev O, Kelley RE, Minagar A,
et al: Human placental mesenchymal stem cells improve stroke
outcomes via extracellular vesicles-mediated preservation of
cerebral blood flow. EBioMedicine. 63(103161)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Feng B, Meng L, Luan L, Fang Z, Zhao P and
Zhao G: Upregulation of extracellular vesicles-encapsulated mir-132
released from mesenchymal stem cells attenuates ischemic neuronal
injury by inhibiting Smad2/c-jun pathway via Acvr2b
suppression. Front Cell Dev Biol. 8(568304)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Song Y, Li Z, He T, Qu M, Jiang L, Li W,
Shi X, Pan J, Zhang L, Wang Y, et al: M2 microglia-derived exosomes
protect the mouse brain from ischemia-reperfusion injury via
exosomal miR-124. Theranostics. 9:2910–2923. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu Y, Li YP, Xiao LM, Chen LK, Zheng SY,
Zeng EM and Xu CH: Extracellular vesicles derived from M2 microglia
reduce ischemic brain injury through microRNA-135a-5p/TXNIP/NLRP3
axis. Lab Invest. 101:837–850. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dong C, Chen M, Cai B, Zhang C, Xiao G and
Luo W: Mesenchymal stem cell-derived exosomes improved cerebral
infarction via transferring miR-23a-3p to activate microglia.
Neuromolecular Med. 24:290–298. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu Z, Li X, Ye Z and Lin H:
Neuroprotective effect of exosomes derived from bone marrow
mesenchymal stem cells via activating TGR5 and suppressing
apoptosis. Biochem Biophys Res Commun. 593:13–19. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xie X, Cao Y, Dai L and Zhou D: Bone
marrow mesenchymal stem cell-derived exosomal lncRNA KLF3-AS1
stabilizes Sirt1 protein to improve cerebral ischemia/reperfusion
injury via miR-206/USP22 axis. Mol Med. 29(3)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Thomas JM, Cunningham CJ, Lawrence CB,
Pinteaux E and Allan SM: Therapeutic potential of extracellular
vesicles in preclinical stroke models: A systematic review and
meta-analysis. BMJ Open Sci. 44(e100047)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gupta D, Zickler AM and El Andaloussi S:
Dosing extracellular vesicles. Adv Drug Deliv Rev.
178(113961)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Haupt M, Gerner ST, Bähr M and Doeppner
TR: Neuroprotective strategies for ischemic stroke-future
perspectives. Int J Mol Sci. 24(4334)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
DeLong JH, Ohashi SN, O'Connor KC and
Sansing LH: Inflammatory responses after ischemic stroke. Semin
Immunopathol. 44:625–648. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jiang L, Chen W, Ye J and Wang Y:
Potential role of exosomes in ischemic stroke treatment.
Biomolecules. 12(115)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu T, Zhang Q, Zhang J, Li C, Miao YR,
Lei Q, Li Q and Guo AY: EVmiRNA: A database of miRNA profiling in
extracellular vesicles. Nucleic Acids Res. 47:D89–D93.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li F, Kang X, Xin W and Li X: The emerging
role of extracellular vesicle derived from neurons/neurogliocytes
in central nervous system diseases: Novel insights into ischemic
stroke. Front Pharmacol. 13(890698)2022.PubMed/NCBI View Article : Google Scholar
|