Introduction
Cervical spine disease is very common in modern
times and can seriously affect people's lives. In severe cases, it
may cause weakness or numbness in the limbs. Surgery is often
required in patients who do not respond to conservative treatment
and have progressively worsening symptoms. Anterior cervical
discectomy with fusion (ACDF) has been recognized as one of the
most effective treatments for cervical spine disorders (1). ACDF can directly relieve compression
in the front of the spinal cord, including the herniated
intervertebral discs, hyperplastic osteophytes behind the vertebral
body and thickened posterior longitudinal ligaments. It can
maintain the physiological curvature of the cervical spine, restore
the height of the intervertebral space and improve cervical spine
stability. Decompression, bone grafting and fusion are the key
aspects of ACDF. After discectomy, fusion cages need to be
implanted to restore spinal stability. The fusion effect is
directly affected by the composition of the fusion cage. The
commonly used iliac bone autograft transplantation often causes
pain, numbness and other discomfort, which hinders postoperative
recovery (2). It has therefore
gradually been replaced by various grafts and fusion devices. The
commonly used polyether ether ketone (PEEK) fusion device overcomes
the disadvantages of difficult access to autologous iliac bone
grafts and localized pain. However, because of its biological
inertia, bone grafting is required to promote fusion. There are
also shortcomings, such as cage sinking, large elastic modulus and
stress shielding (3,4). The emergence of 3-dimensional
(3D)-printed intervertebral fusion cages has introduced new
possibilities. The 3D-printed porous titanium interbody fusion cage
uses 3D printing technology to print the titanium alloy into a
microporous structure similar to the trabecular bone structure,
providing space conditions for the growth of osteocytes. The fusion
cage is more suitable for the upper and lower vertebrae and can
regulate the elastic modulus, which is conducive to the growth of
osteocytes and has high biocompatibility (5). To date, titanium alloy 3D printing
technology has been successfully applied in numerous aspects,
including artificial joint replacement, 3D printing of an
artificial vertebral body after vertebral resection, bone
reconstruction after tumor resection (6-9).
Studies have shown that 3D-printed cages can achieve good stability
and bone fusion rates (10). The
purpose of this meta-analysis was to compare and evaluate the
efficacy of 3D-printed porous titanium cages and PEEK cages in ACDF
and to explore the efficacy of 3D-printed porous titanium
cages.
Materials and methods
Guideline and registration
This meta-analysis adheres to the reporting
guidelines outlined in the Preferred Reporting Items for Systematic
reviews and Meta-Analyses (11),
which does not require patient agreement and ethical reviews, given
that all studies included in the analysis were derived from
published research data. The protocol for this meta-analysis is
available from the International Prospective Register of Systematic
Reviews (https://www.crd.york.ac.uk/PROSPERO/; no.
CRD42023461773).
Search strategy
The PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science
(https://www.webofscience.com/), Embase
(https://www.embase.com/), China National
Knowledge Infrastructure (https://www.cnki.net/), Wanfang (https://www.wanfangdata.com.cn/), and VIP
databases (http://qikan.cqvip.com/) were
searched to identify relevant studies. The effectiveness and safety
of 3D-printed porous titanium and PEEK interbody fusion cages in
ACDF were collected from the establishment of the database to
September 2023. The search keywords used were 3D printing, porous
material, titanium, internal fixators, cervical vertebrae,
discectomy, decompression surgery, spinal fusion and ACDF. The
retrieval strategy was as follows: (‘Printing, Three-Dimensional’
OR ‘Porous’ OR ‘Titanium’) AND (‘Internal Fixators’ OR ‘cage’) AND
(‘Cervical Vertebrae’ OR ‘Diskectomy’ OR ‘Decompression, Surgical’
OR ‘Spinal Fusion’ OR ‘ACDF’).
Surgical techniques
The patient was placed in a supine position and a
transverse incision was made in the anterior cervical region to
reveal the anterior part of the vertebral body. Fluoroscopy was
used to locate the diseased cervical vertebrae, fixation nails were
inserted in the upper and lower vertebral bodies of the responsible
interspaces and the intervertebral space was properly expanded by
the distractor. This was followed by removal of the disc tissues,
dealing with the cartilaginous endplates, trying to mold to test
the size of the fusion device, placing a 3D or PEEK cage of the
appropriate height and angle, choosing the appropriate anterior
steel plate for fixation, placing the drains and suturing the
wound.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i)
Clinically controlled studies; ii) studies that included patients
requiring surgery after failure of conservative treatment for
cervical disc herniation and cervical stenosis; iii) the surgical
method was ACDF; iv) studies using 3D and PEEK cages as
interventions; v) studies that reported one of the following in the
literature: Operative time, intraoperative blood loss,
hospitalization days, complications, fusion rate, Cobb's angle,
intervertebral space height, Japanese Orthopaedic Association
Assessment of Treatment (JOA) (12) and visual analog scale (VAS) score
(13). The exclusion criteria were
as follows: i) Comorbid deformities, infections, neoplastic
diseases and traumatic cervical spinal cord injury; ii) history of
cervical spine surgery; iii) review of manuscripts, conferences,
expert opinions, case reports and literature without access to the
full text; iv) animal experiments and biomechanical studies; and v)
non-case-control studies.
Data extraction and literature quality
assessment
The literature was independently screened and
identified for study inclusion by two researchers through the
title, abstract and full text of the article. When there was a
difference in opinion regarding the study, it was discussed and
resolved. When necessary, a third researcher was consulted. The
quality of the literature was determined according to the Cochrane
Risk of Bias Assessment Criteria (14): i) Whether the experimental design
used the principle of randomization; ii) whether the principle of
double-blinding was used for participants, performers and
measurements; iii) whether the experimental data were complete and
trustworthy; iv) whether the allocation concealment method was
used; v) whether the experiments used a selective method of data
reporting; and vi) other biases. The quality of the included
literature was assessed using the Newcastle-Ottawa Scale (NOS)
(15) and categorized as low
(score, <5), moderate (score, 5-7) and high (score, 8 or 9)
quality.
Statistical analysis
Review Manager 5.4 (The Cochrane Collaboration) was
used to process the collected data. Continuous variables were
expressed as mean differences (MD) and 95% confidence intervals
(CI), and dichotomous variables were expressed as odds ratios (OR)
and 95% CI. Since there is always heterogeneity in intervention
effects between multiple studies from different groups and
geographic locations, random-effects models were adopted for the
included studies, regardless of the size of I2. Studies
were removed one by one to perform a sensitivity analysis. When the
number of included studies was ≥10, a funnel plot or Egger's test
was used to determine whether there was publication bias. P<0.05
was considered to indicate statistical significance.
Results
Basic characteristics of the included
studies
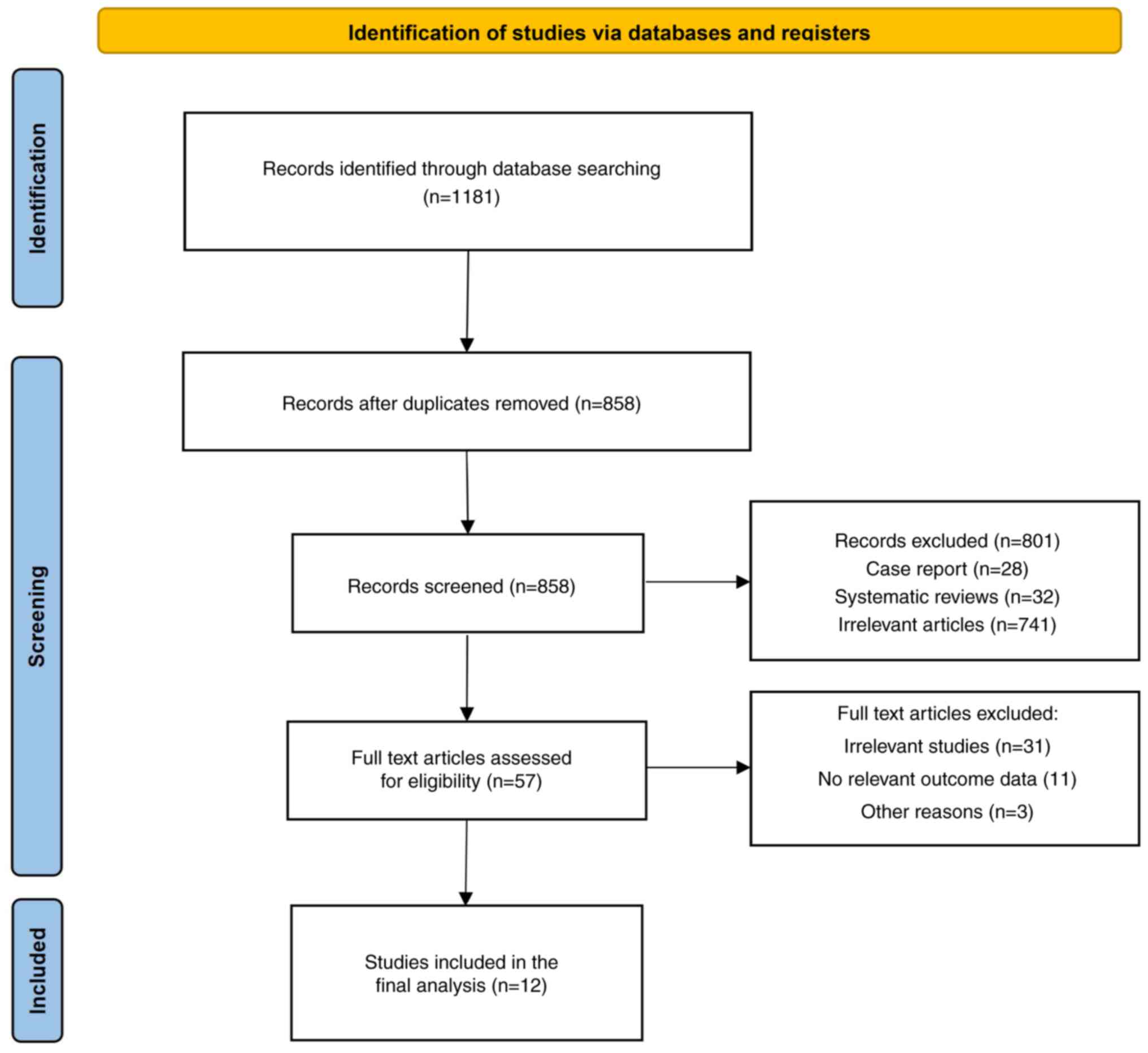
A total of 1,181 related studies were obtained from
the database after closely adhering to the inclusion and exclusion
criteria. After excluding non-case-control studies and other
unrelated studies, 57 relevant studies were initially screened.
Finally, the complete texts were carefully studied and 12 papers
were included (10,16-25, Xu, unpublished data). The literature
screening process and results are shown in Fig. 1 and the basic characteristics of
the included studies are listed in Table I.
 | Table IGeneral characteristics of included
studies. |
Table I
General characteristics of included
studies.
| First author(s),
year | Type of study | Country | Cases of 3D cage
vs. PEEK cage, n | Mean age, years in
3D cage vs. PEEK cage group | Sex, M/F in 3D cage
vs. PEEK cage group | Outcomes | Dominant group | NOS scale | (Refs.) |
|---|
| Arts et al,
2020 | Retrospective | Netherlands | 49 vs. 48 | 50.3 vs. 49.4 | 23/26 vs.
25/23 | ⑤⑨ | 3D cage | 6 | (10) |
| Bai et al,
2023 | Retrospective | China | 30 vs. 26 | 58.0±6.1 | 18/12 vs.
14/12 | ①②③④⑥⑧⑨ | 3D cage | 6 | (16) |
| Jiang et al,
2022 | RCT | China | 45 vs. 45 | 47.25±9.36 vs.
45.87±10.23 | 25/20 vs.
21/24 | ①②③⑤⑥⑦ | 3D cage | 7 | (17) |
| Li, 2020 | Retrospective | China | 30 vs. 28 | 47.56±6.42 vs.
46.83±5.68 | 16/14 vs.
15/13 | ①②③④⑥⑦⑧⑨ | 3D cage | 6 | (18) |
| Li et al,
2021 | RCT | China | 35 vs. 36 | 46.4±4.9 vs.
46.1±4.3 | 26/9 vs. 23/13 | ①②③④⑤⑧ | 3D cage | 7 | (19) |
| Liu et al,
2021 | RCT | China | 31 vs. 32 | 45.72±4.58 vs.
46.23±4.39 | 19/12 vs.
21/11 | ①②③④⑥⑦ | 3D cage | 8 | (20) |
| Wang et al,
2021 | Retrospective | China | 13 vs. 16 | 55 vs. 60 | 10/3 vs. 12/4 | ①②③④⑤⑥⑧⑨ | 3D cage | 8 | (21) |
| Wang et al,
2023a | Retrospective | China | 30 vs. 30 | 47.56±6.42 vs.
46.83±5.68 | 16/14 vs.
15/15 | ①②③④⑥⑦⑧⑨ | 3D cage | 8 | (22) |
| Wang et al,
2023b | RCT | China | 33 vs. 34 | 52.12±10.04 vs.
51.35±10.87 | 23/10 vs.
22/12 | ①②④⑤⑥⑧ | 3D cage | 8 | (23) |
| Xu et al,
2021 | Retrospective | China | 36 vs. 32 | 53.56±6.28 vs.
54.24±7.13 | 19/17 vs.
15/17 | ①②③④⑥⑦⑧⑨ | 3D cage | 7 | (Xu, unpublished
data) |
| Yang et al,
2020 | Retrospective | China | 30 vs. 30 | 56.7±10.5 vs.
57.3±11.0 | 13/17 vs.
16/14 | ①②④⑤⑥⑦⑧⑨ | 3D cage | 8 | (24) |
| Zhang et al,
2021 | Retrospective | China | 18 vs. 21 | 48.8±10.5 vs.
47.4±7.8 | 8/10 vs. 9/12 | ①②③④⑤⑥⑦⑧⑨ | 3D cage | 7 | (25) |
Outcome indicators of included
studies
The outcome indicators included were intraoperative
indicators, perioperative indicators, imaging evaluation indicators
and follow-up indicators. Intraoperative indicators included
operation time and intraoperative blood loss, which compared the
two groups in terms of difficulty and trauma of the operation.
Perioperative indicators included hospitalization days and
complications, which was to compare the two groups in terms of the
speed of postoperative recovery and whether it would affect the
complication rate. Complications included in the study were wound
infection, fusion collapse, dysphagia, screw loosening,
cerebrospinal fluid leakage, neurologic injury, rejection and
intraspinal hematoma. Imaging evaluation indicators included the
fusion rate, Cobb's angle and intervertebral space height, which
were to evaluate the two groups in terms of spinal fusion as well
as correction of spinal curves. Bony fusion was observed on
radiographs as a continuous trabecular passage of bone through the
fusion space. Follow-up indexes included the JOA and VAS score,
which were to compare the two groups in terms of postoperative
functional recovery as well as pain.
Quality assessment of included
articles
A total of 12 articles were included in this study,
comprising four randomized controlled trials and eight
retrospective studies. A total of 758 patients were included; 380
patients received the 3D cage and 378 patients received the PEEK
cage. The NOS score table was used for quality evaluation,
including five articles with 8 points, four articles with 7 points,
and three articles with 6 points, resulting in a total of three
high-quality literature articles and seven medium-quality
literature articles (Table I).
Outcomes. Perioperative
indicators
Perioperative indicators included operation time,
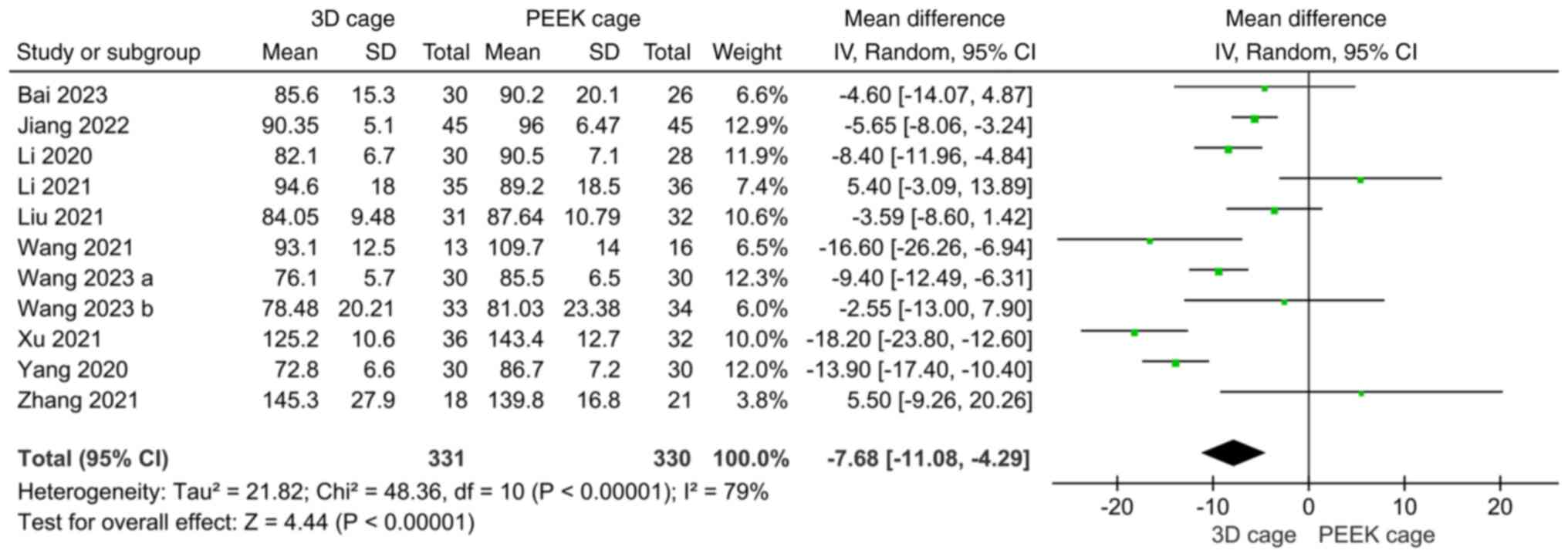
blood loss and length of hospital stay. A total of 11 studies
compared the operation time of 3D-printed porous titanium and PEEK
cages in ACDF. The heterogeneity test showed that the heterogeneity
of each study was significant (I2=79%), and the
random-effects model was used for analysis. The results showed that
the operation time for using 3D cage in ACDF was shorter than that
for using PEEK cage, and the difference was statistically
significant (MD: -7.68; 95%CI: -11.08, -4.29; P<0.00001;
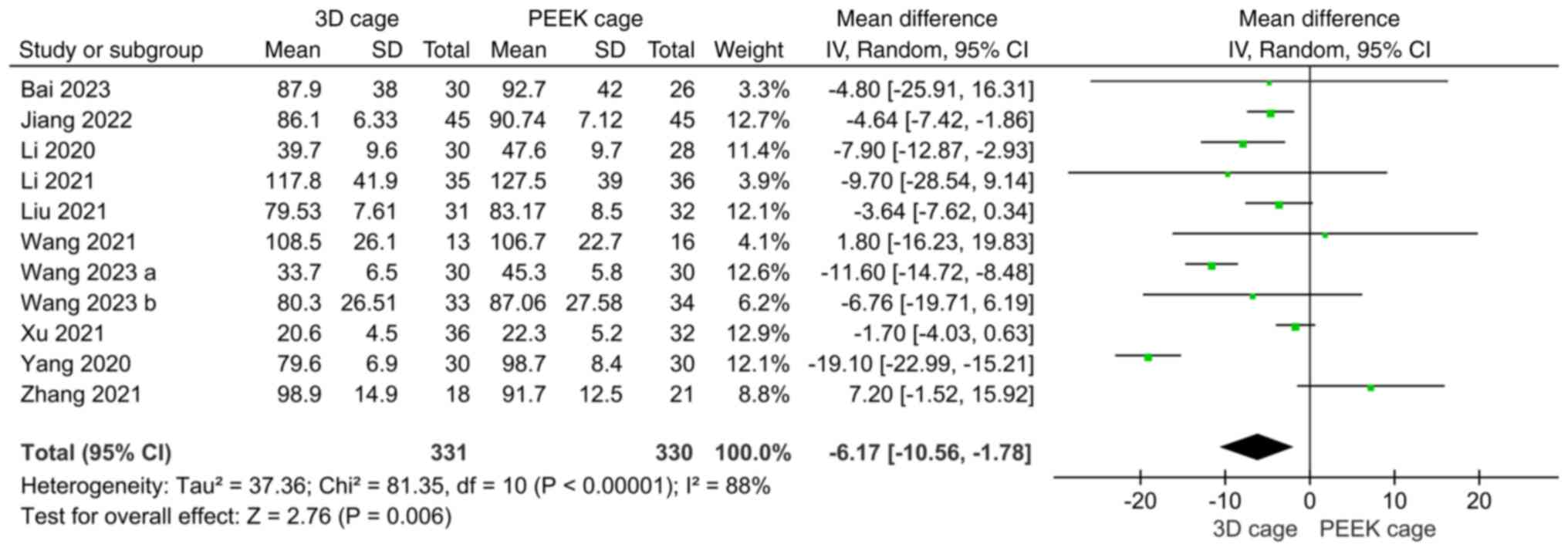
Fig. 2). Furthermore, 11 studies
compared intraoperative blood loss and the heterogeneity test
showed significant heterogeneity (I2=88%); accordingly,
a random-effects model was used. The results showed that
intraoperative bleeding was less in the 3D cage group than in the
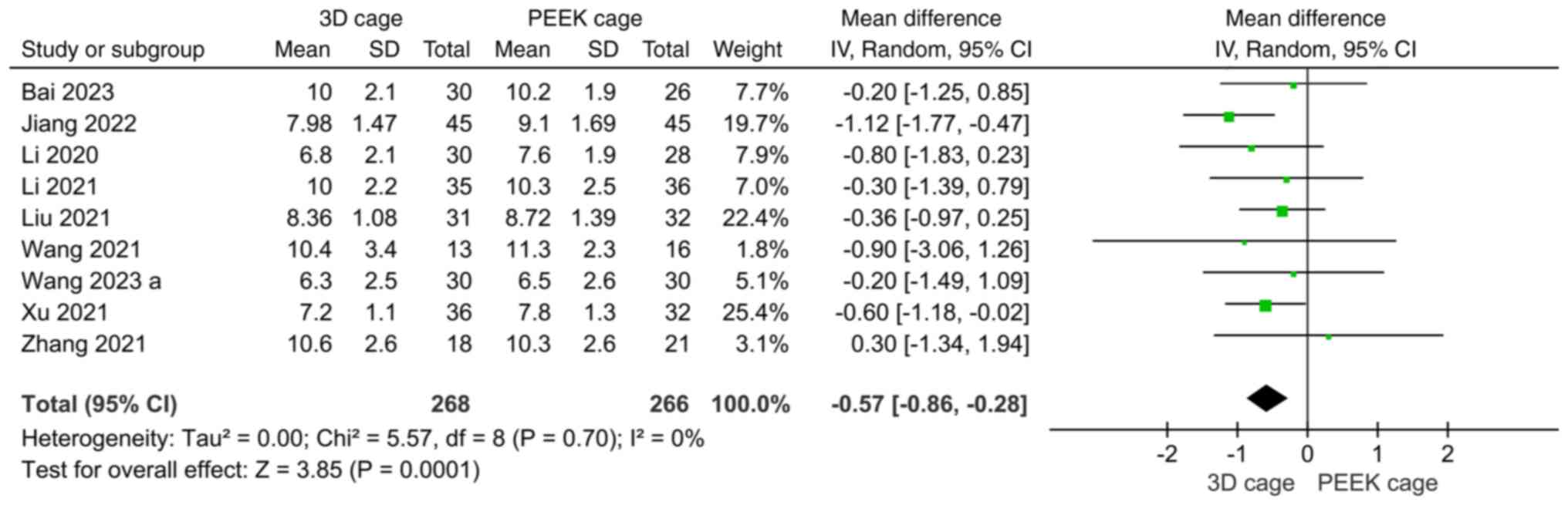
PEEK cage group (MD: -6.17; 95%CI: -10.56, -1.78; P=0.006; Fig. 3). A total of 9 studies compared the
length of stay, with insignificant heterogeneity
(I2=0%). A random-effects model was used for
meta-analysis, which showed that the length of stay in the 3D cage
group was shorter than that in the PEEK cage group, with the
difference being statistically significant (MD: -0.57; 95%CI:
-0.86, -0.28: P=0.0001; Fig.
4).
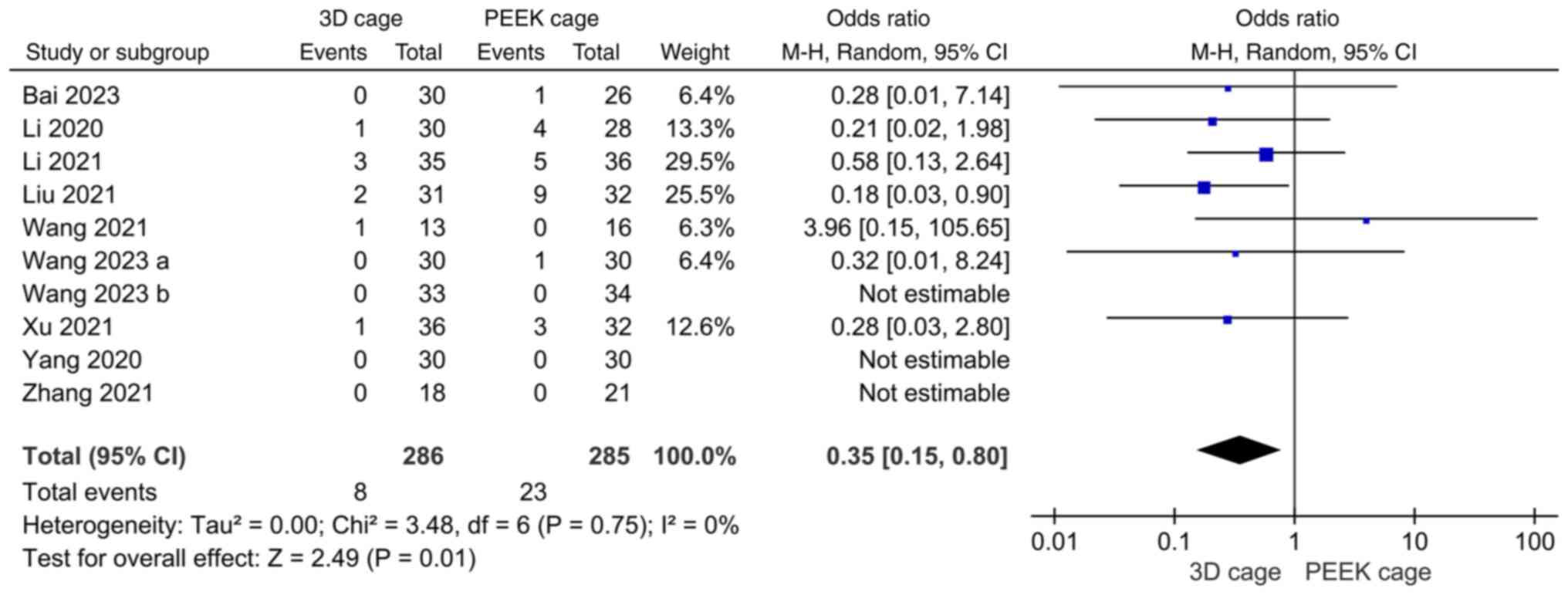
Postoperative complications. Altogether, 10
studies compared 3D-printed porous titanium interbody fusion
devices with PEEK interbody fusion devices for postoperative
complications after ACDF, with low heterogeneity across studies
(I2=0%). Using a random-effects model, the results
demonstrated that application of 3D cages in ACDF was associated
with fewer postoperative complications than the use of PEEK cages.
The difference was statistically significant (OR: 0.35; 95%CI:
0.15, 0.80; P=0.01; Fig. 5).
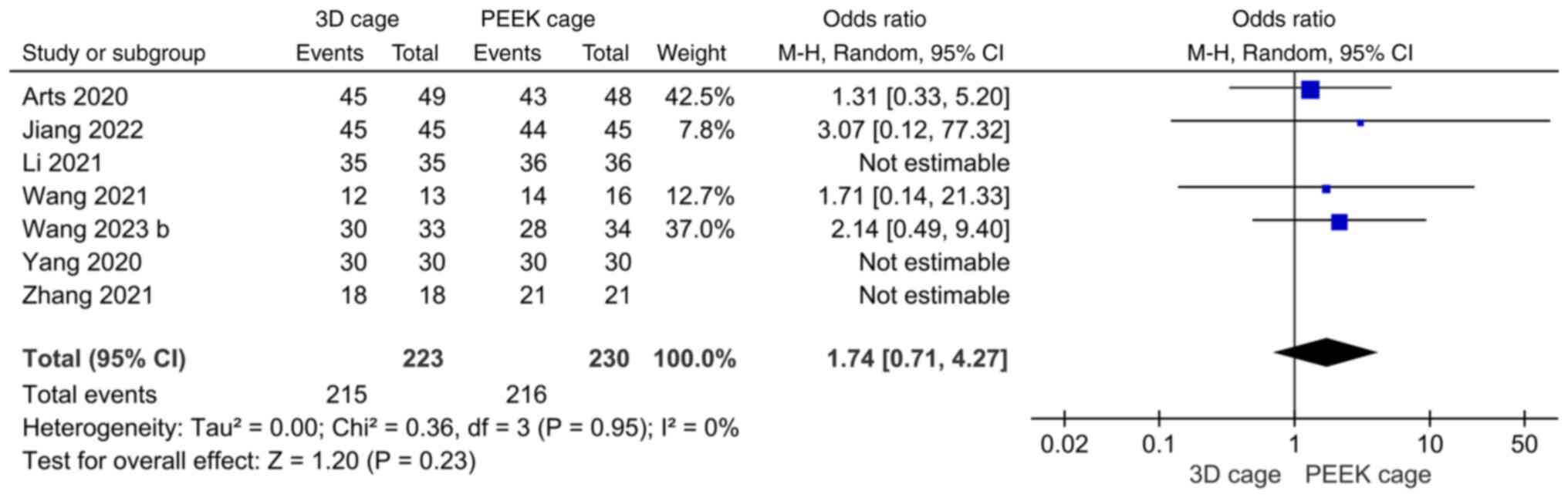
Fusion rate. A total of seven papers reported
on fusion rates, with low heterogeneity across studies
(I2=0%). Meta-analysis with a random-effects model
showed no significant difference in fusion rates after ACDF with a
3D or PEEK cage (OR: 1.74; 95%CI: 0.71, 4.27; P=0.23; Fig. 6).
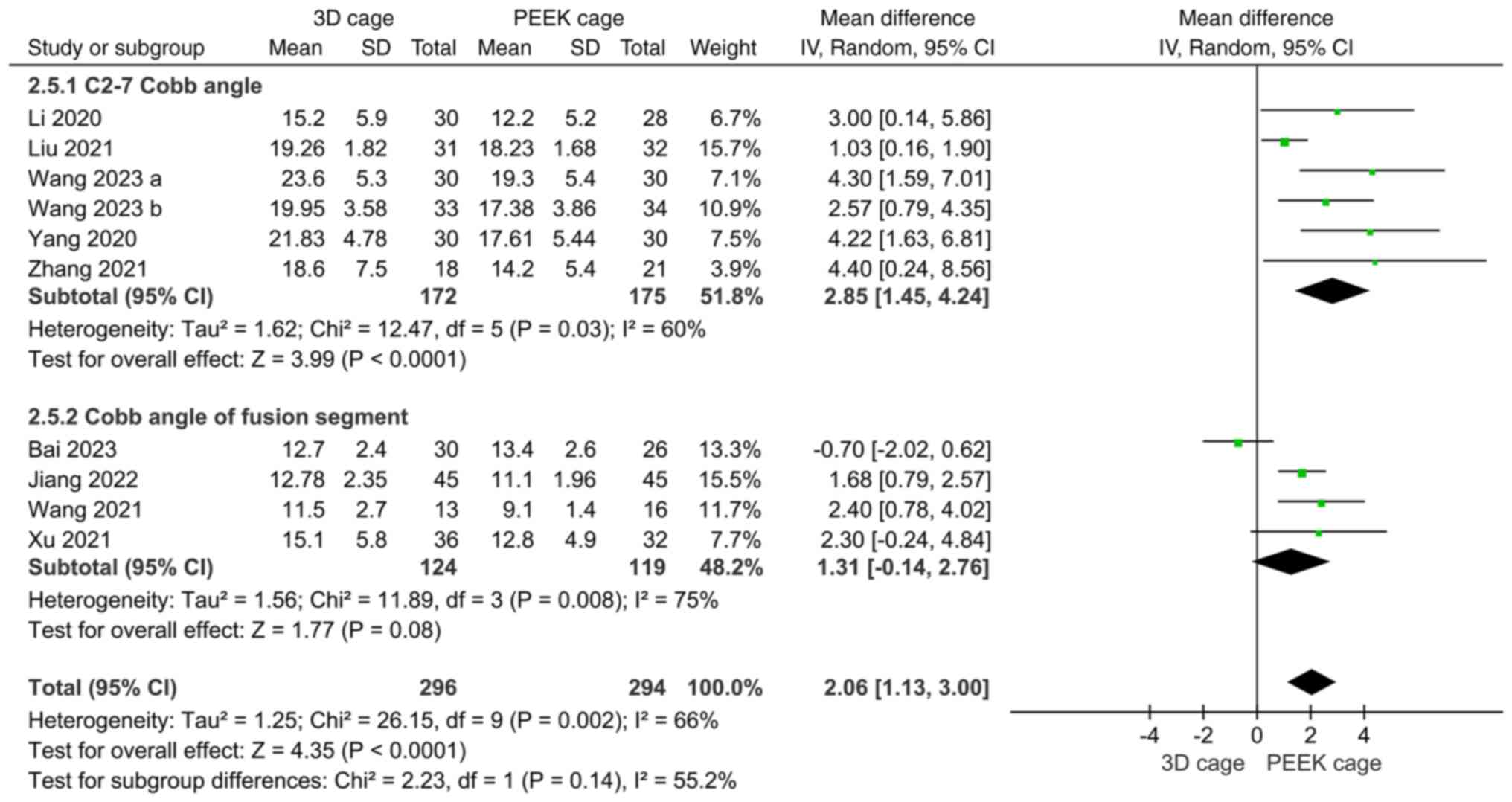
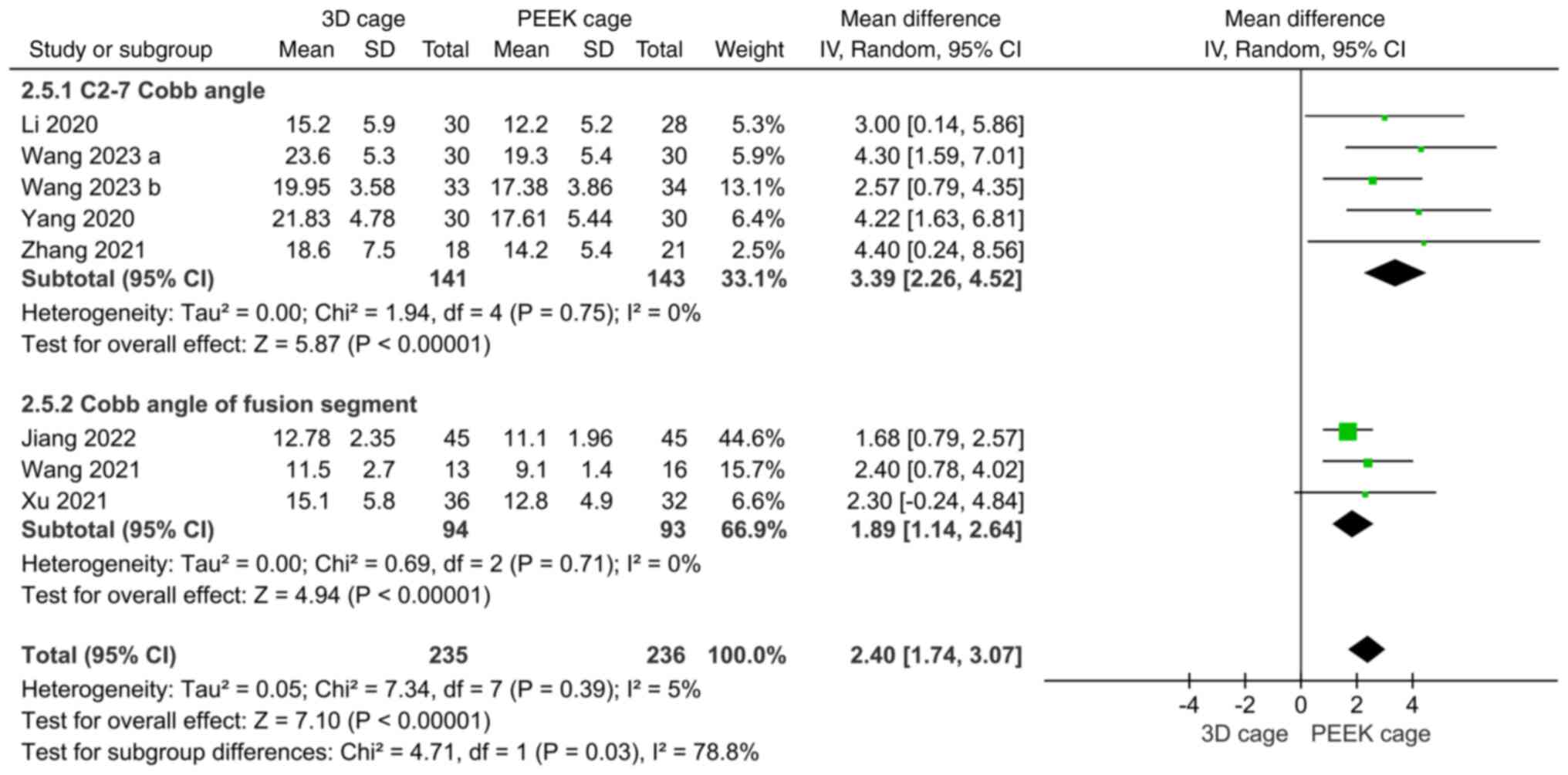
Cobb angle. The Cobb angle was mentioned in
10 papers on fusion rates, of which 6 reported C2-7 Cobb angles and
4 reported fused segmental Cobb angles, with high heterogeneity
(I2=60 and 75%, respectively). Meta-analysis with the
random-effects model indicated that the postoperative C2-7 Cobb
angles in the 3D cage group were greater than those in the PEEK
cage group (MD: 2.85; 95%CI: 1.45, 4.24; P<0.0001; Fig. 7), and the fused segment Cobb angles
were not significantly different (MD: 1.31; 95%CI: -0.14, 2.76;
P=0.08; Fig. 7).
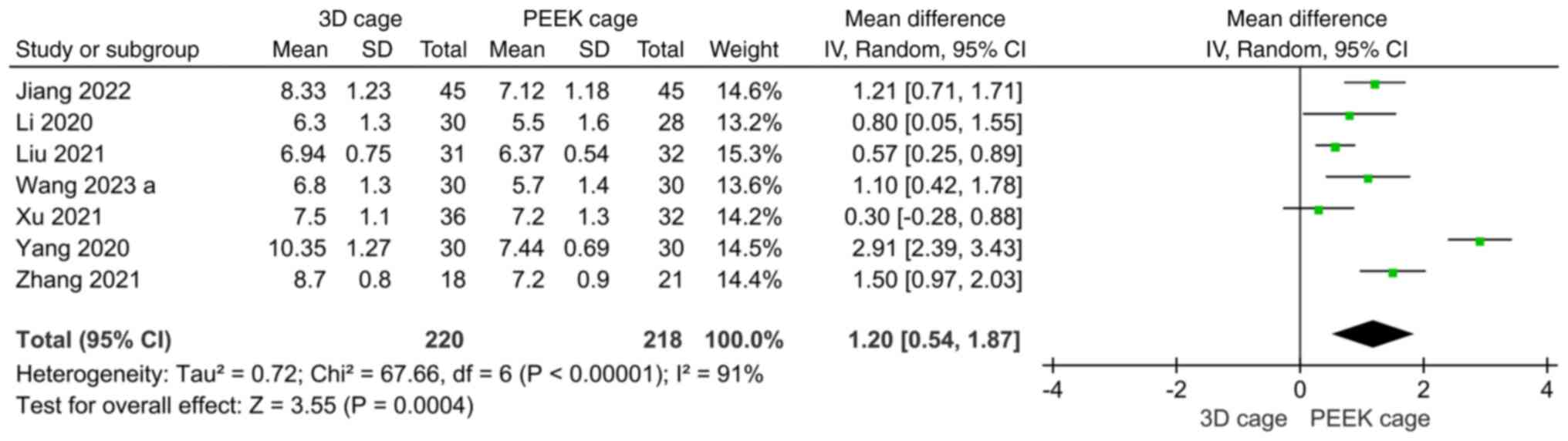
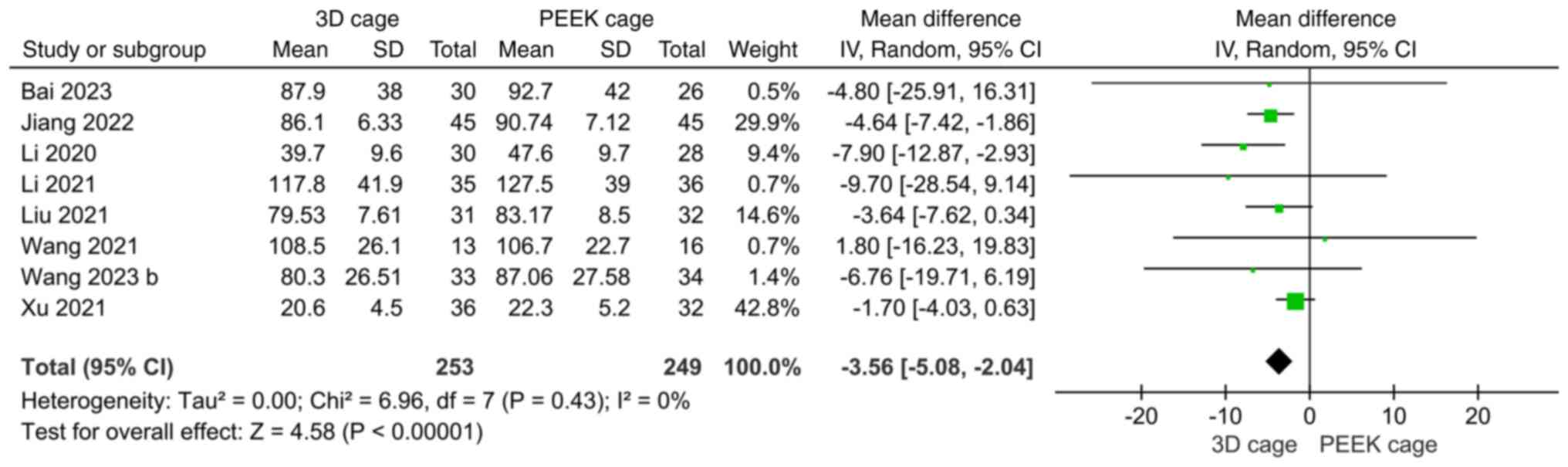
Intervertebral space height. A total of seven
studies reported the height of the intervertebral space. The
heterogeneity was high (I2=91%) and a random-effects
model was used. The results showed that the height of the
intervertebral space in the 3D cage group was greater than that in
the PEEK cage group, and the difference was statistically
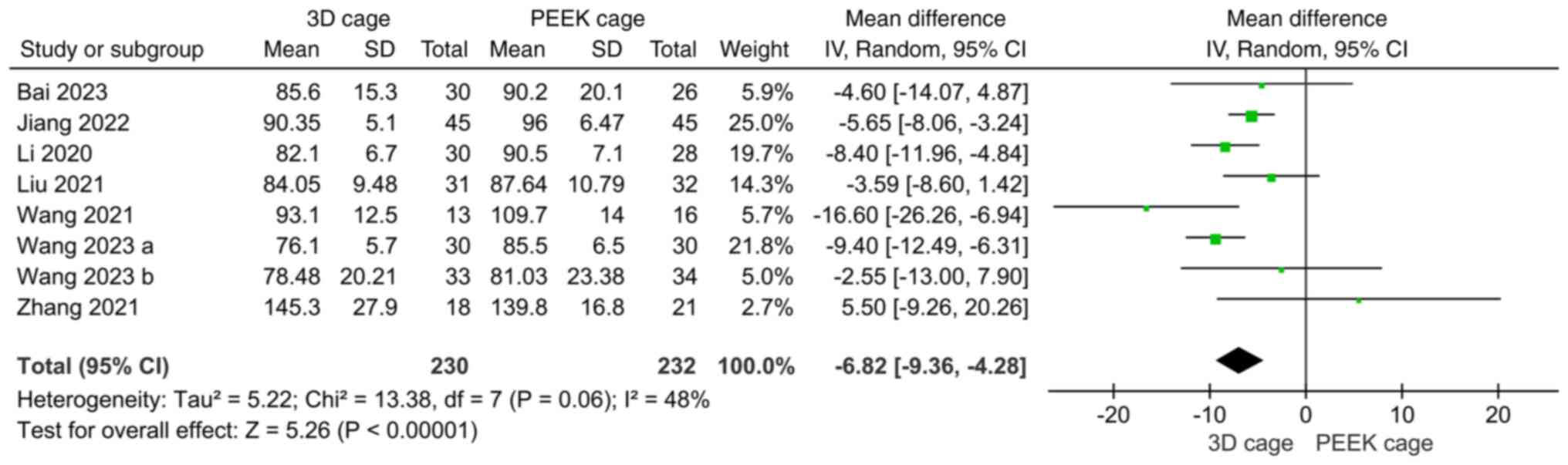
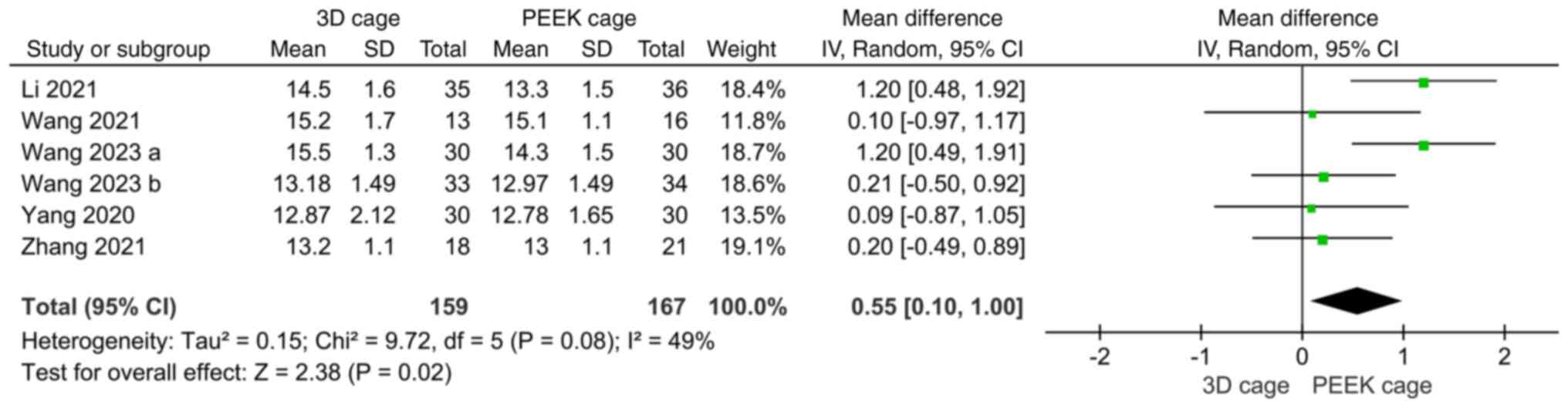
significant (MD: 1.20; 95%CI: 0.54, 1.87; P=0.0004; Fig. 8).
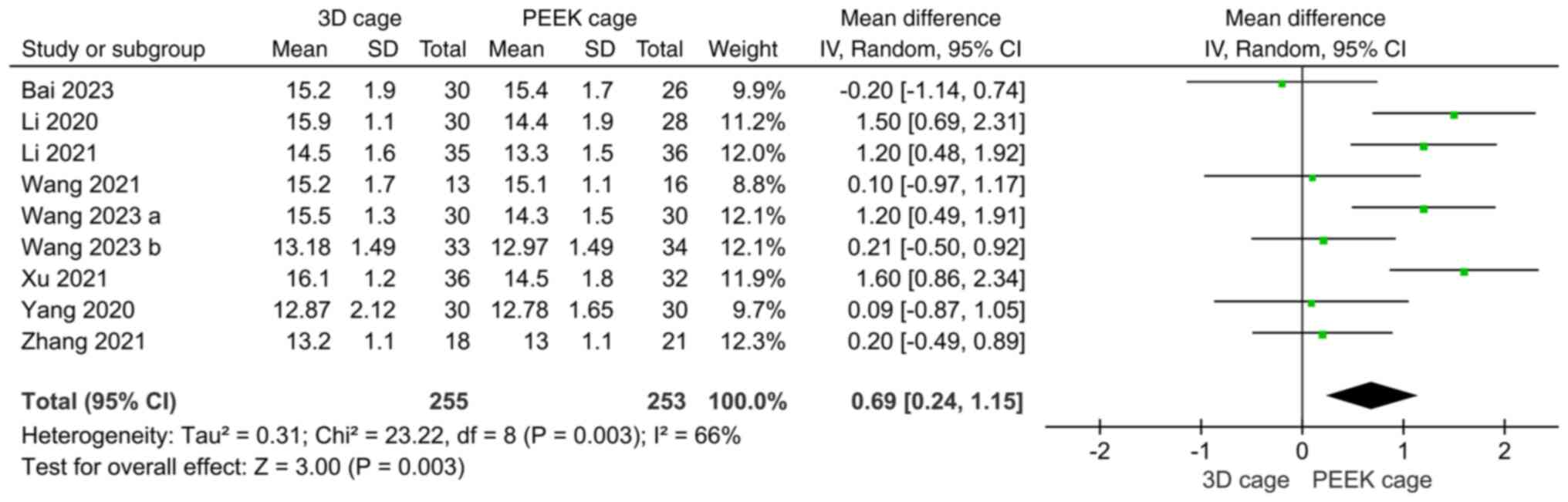
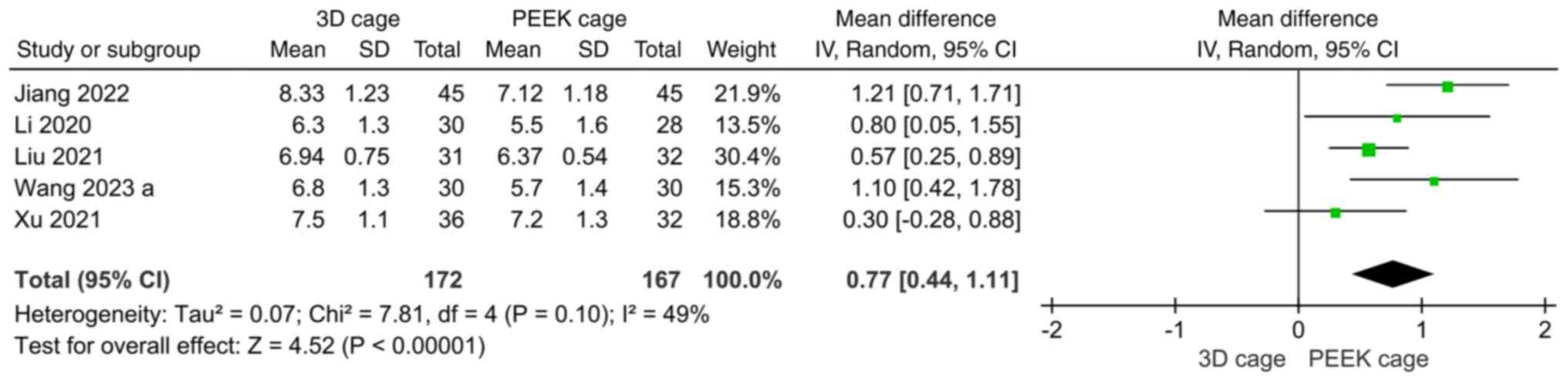
JOA. A total of 9 articles compared the
postoperative JOA score; the overall heterogeneity was high
(I2=66%) and the random-effects model was used. The
results showed that the JOA score of the 3D cage group was higher
than that of the PEEK cage group and the difference was
statistically significant (MD: 0.69; 95%CI: 0.24, 1.15; P=0.003;
Fig. 9).
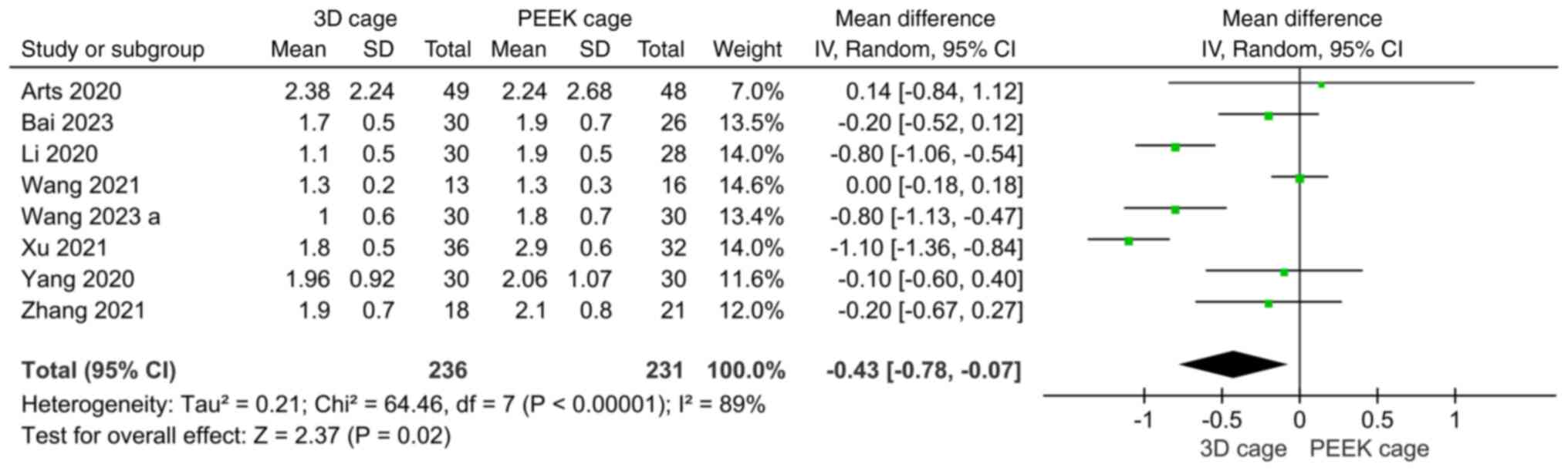
VAS. A total of eight papers reported results
of postoperative VAS scores, with significant heterogeneity across
studies (I2=89%), and a random-effects model was used.
The results showed that the postoperative VAS scores were lower in
the 3D cage group than in the PEEK cage group, with a statistically
significant difference (MD: -0.43; 95%CI: -0.78, -0.07; P=0.02;
Fig. 10).
Publication bias and sensitivity
analysis
Nine outcome indicators of operative time,
intraoperative blood loss, hospital days, complications, fusion
rate, Cobb angle, intervertebral space height, JOA and VAS were
analyzed; utilizing the statistical program Review Manager 5.4,
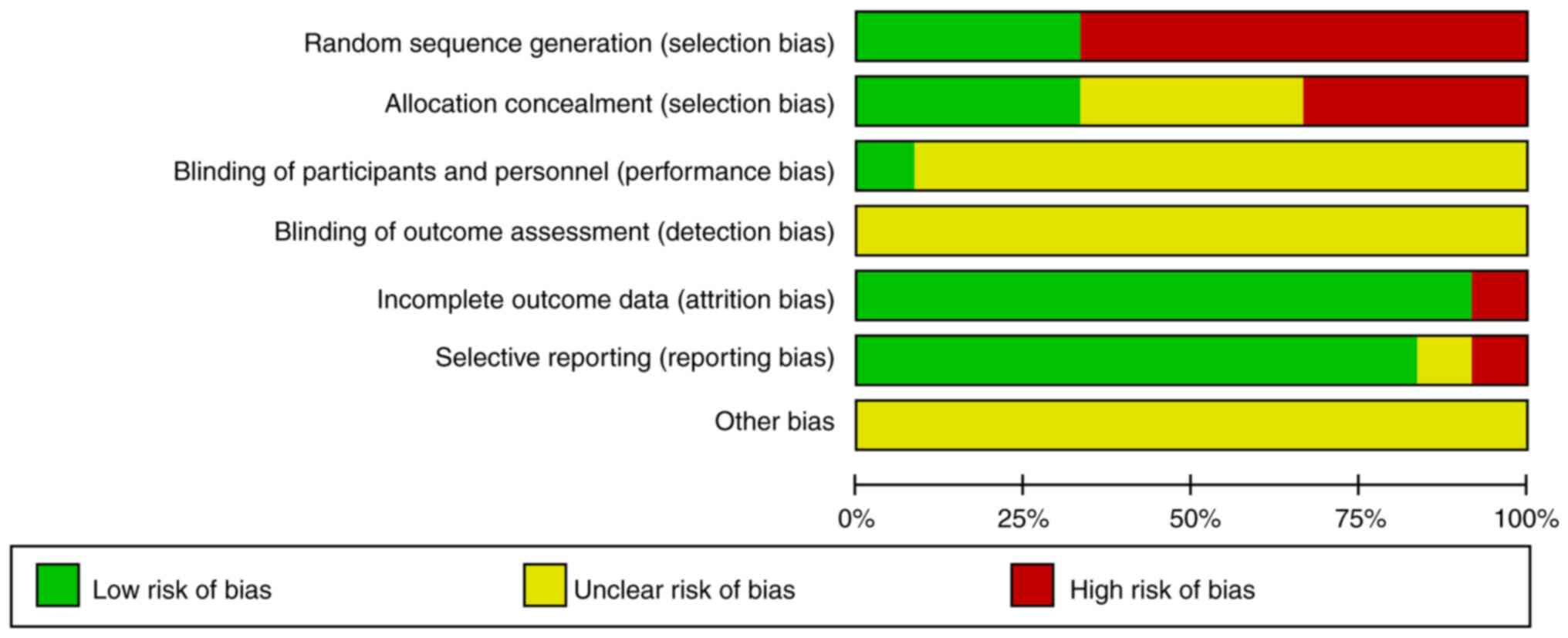
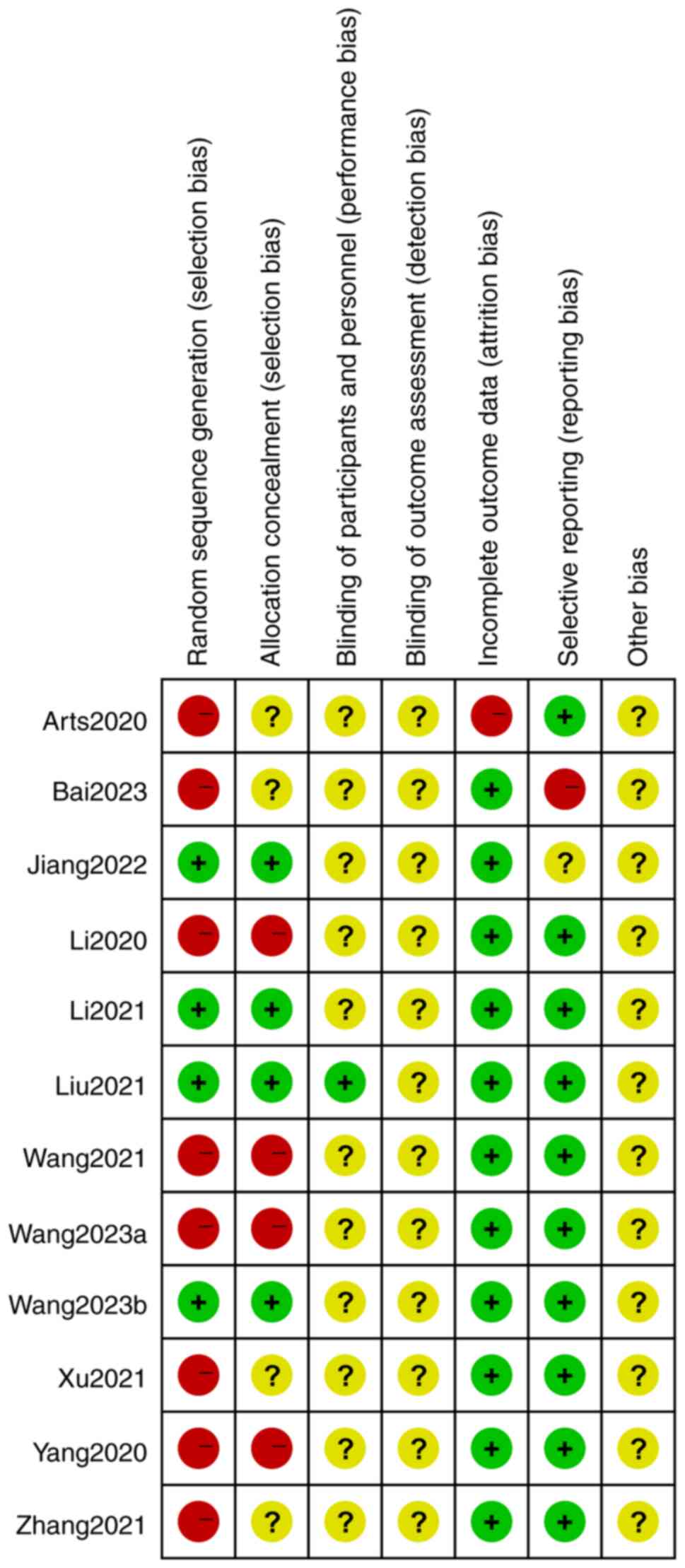
publication bias analysis was carried out (Figs. 11 and 12). A total of 4 studies (17,19,20,23)
were randomized controlled trials using random number sequences
with low-risk of selection bias and no allocation concealment; only
one paper (20) mentioned blinding
of the study, while the others did not mention blinding; 1 paper
(13) had incomplete data on
outcome indicators; one paper (16) had problems with selective reporting
of data; and finally, no other risks of bias were identified. The
findings demonstrated no clear publication bias because the funnel
plots for each item were symmetrical (Fig. 13). Although there were seven
studies related to the fusion rate, only four were included in the
analysis because three of the studies had a 100% fusion rate, and
these data could not be used for meta-analysis; however, there was
low heterogeneity, indicating good stability of the results. While
the heterogeneity of operation time, intraoperative blood loss,
Cobb angle, intervertebral space height, JOA and VAS scores was
high, after sensitivity analysis, the results of the meta-analysis
showed no directional change, indicating that the results of the
studies were relatively stable and reliable. The metrics with high
heterogeneity in this study included operative time, blood loss,
Cobb angle, intervertebral space height, JOA and VAS scores. Among
them, the heterogeneity was significantly reduced after the removal
of surgical time from 3 studies (19,24, Xu, unpublished data),
blood loss from 3 studies (22,24,25),
Cobb angle from 2 studies (16,20),
intervertebral space height from 2 studies (24,25)
and JOA from 3 studies (16,18, Xu, unpublished data), and the
I2 was 48, 0, 0, 49 and 49% (Fig. 14, Fig. 15, Fig. 16, Fig. 17 and Fig. 18, respectively). By contrast, the
decrease in heterogeneity of VAS scores after sensitivity analyses
was not significant.
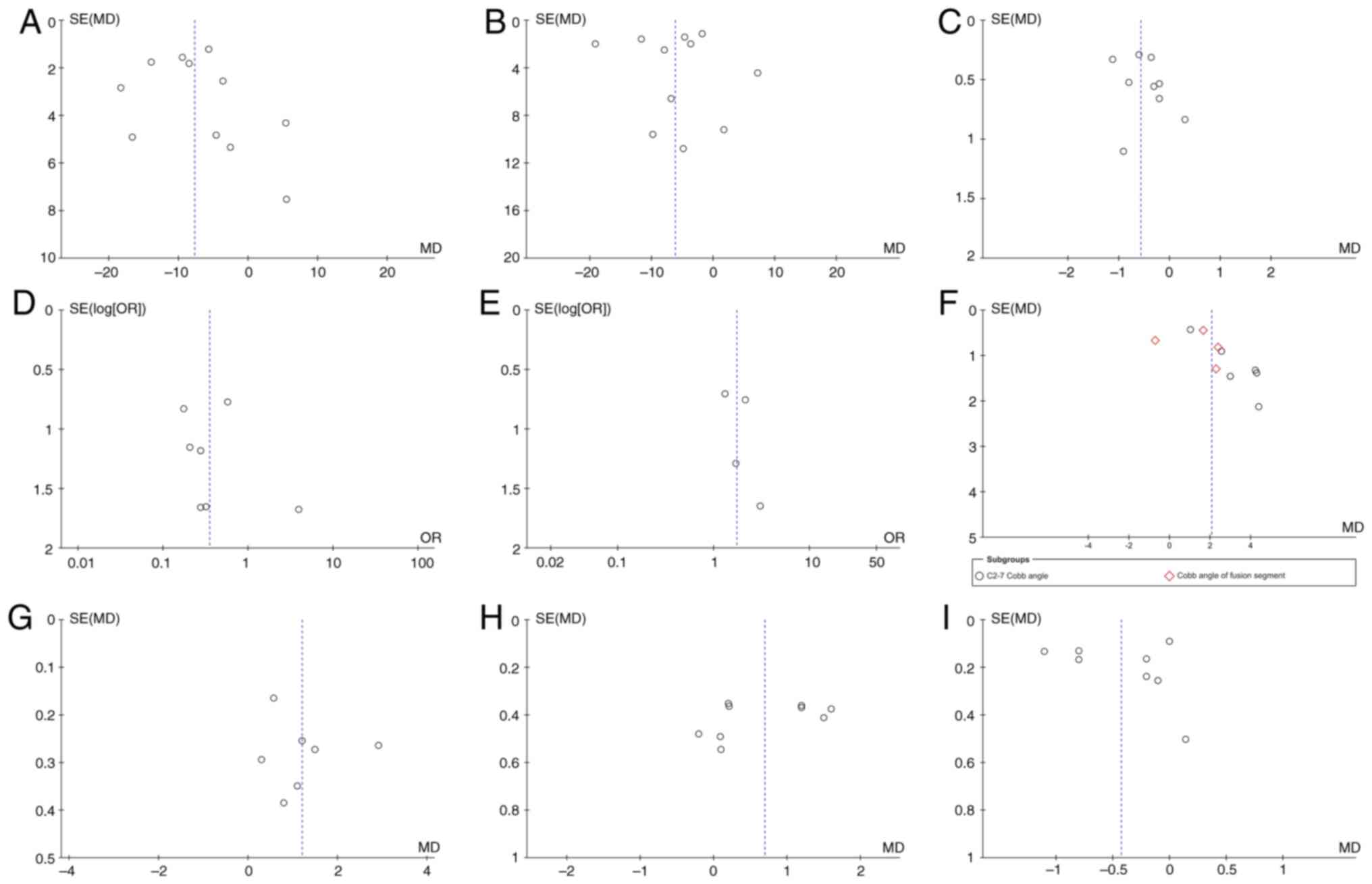 | Figure 13Funnel plots created to assess
publication bias for (A) operation time, (B) intraoperative blood
loss, (C) hospitalization time, (D) postoperative complications,
(E) fusion rate, (F) Cobb angle, (G) intervertebral space height,
(H) Japanese Orthopaedic Association Assessment of Treatment and
(I) visual analogue scale score. SE, standard error; MD, mean
difference; OR, odds ratio. |
Discussion
Cervical spine disorders are becoming increasingly
common in populations with changes in living habits and are
increasingly seen in younger populations. Currently, the main
treatments are rehabilitation, conservative treatment and surgery,
and severe cervical spine disease often requires surgery (26). ACDF is popular in clinical practice
and can directly decompress the posterior compression of the
vertebral body from the cervical incision approach, which can
remove the posterior longitudinal ligament and hyperplastic bone
and rebuild the cervical curvature and stability. There are various
problems in clinical practice, such as autologous or allogeneic
bone transplantation, fusion cages of various materials and
artificial intervertebral discs. The type of fusion device used
during surgery is problematic. The 3D-printed fusion cage has
characteristics that the previous fusion cage does not have; it is
more matched with the anatomical structure of the patient's
vertebral body and the mechanical structure is more stable. The
3D-printed porous titanium interbody fusion cage has an
interconnected microporous structure that simulates the structure
of the trabecular bone, which not only regulates the elastic
modulus but also reduces stress shielding. The microporous
structure and rough surface of the 3D cage also provide better
growth conditions for bone tissue adhesion, proliferation and
differentiation, which are conducive to intervertebral fusion
(27). However, the PEEK cage has
few hydrophilic groups, high biological inertia and a smooth
surface, and can easily be wrapped by fibrous tissue, which cannot
provide conditions for the adhesion of osteoblasts, resulting in
weak osseointegration ability (28). Furthermore, the bone blocks
implanted in PEEK cages are mainly allogeneic. The affinity and
bone conduction ability of allogeneic bone cells are weak, and
osteoblasts have difficulty adhering to and differentiating on
their surfaces (29). At present,
titanium alloy materials are widely used in the field of 3D-printed
implants. It has the advantages of high histocompatibility,
strength, corrosion resistance, reduced postoperative immune
rejection and fusion collapse (30,31).
The high connectivity and porosity of the porous titanium structure
promote the adhesion of osteoblasts, which have a role in bone
growth and tissue differentiation, thus increasing the fusion rate.
The fusion rate of triple-D printed porous titanium fusion devices
is closely related to the porosity, which should be at least
>50%, preferably in the range of 65-80%, and their structure and
elastic modulus are similar to those of human bone trabeculae
(32). A finite element analysis
found that the modulus of elasticity decreased as the aperture size
and porosity increased accordingly and found that the modulus of
elasticity of a fusion with an average aperture size of 0.75
mm/porosity of 72.8% was within the range of the modulus of
elasticity of natural bone and was stable after implantation, which
is beneficial to the recovery of the human lumbar spine (33). The present study compared the
efficacy of 3D-printed porous titanium and PEEK fusions in ACDF to
investigate the safety and efficacy of 3D-printed cages.
In the present meta-analysis, the operation time and
blood loss in the 3D-printed cage group were lower than those in
the PEEK cage group, and the difference was statistically
significant. This was because the PEEK cage group required repeated
mold testing and bone grafting. The 3D-printed cage group underwent
planning for the corresponding model size prior to surgery and did
not require bone grafting, which is easy to operate and saves time
by reducing bleeding. The 3D-printed cage group had a significantly
shorter hospitalization time and fewer postoperative complications
than the PEEK cage group. Intravertebral hematomas accounted for
~1/3 of the complications, while the ratio of intravertebral
hematomas in the 3D-printed and PEEK cage groups was 3:7. The
reason is that the 3D-printed cage has a porous structure, which is
more conducive to the exclusion of hematoma; all three cases of
rejection reaction were in the PEEK cage group. The PEEK cage had
high biological inertia, whereas the titanium alloy had high
histocompatibility, and no rejection reactions occurred. The other
1/3 showed cage collapse, implant movement and screw loosening,
while the ratio of them in the 3D-printed and PEEK cage groups was
2:9,, which was significantly better than that of the PEEK cage
group. The porous structure can effectively reduce the stress
shielding effect of the interface between the cage and vertebral
body, slow the loss of intervertebral height, provide growth
conditions for the proliferation and differentiation of osteocytes,
accelerate intervertebral fusion and prevent vertebral subsidence
(34,35). Animal experiments have also shown
that 3D cages have better osseointegration characteristics and
stability (36,37). The fusion rate is an important
indicator for testing the effects of an operation. There was no
significant difference in the fusion rate between the two groups,
indicating that the porous titanium fusion cage fabricated via 3D
printing in ACDF produced the same fusion results as the
conventional PEEK fusion cage. Theoretically, the fusion effect of
3D-printed cages should be higher than that of the PEEK cage, but
our results are not consistent. It may be considered that the
results of various studies lack long-term efficacy, or as the
fusion effect is affected by numerous factors, the material aspect
should be considered. The postoperative C2-7 Cobb angle and
intervertebral space height in the 3D cage group were greater than
those in the PEEK cage group. The C2-7 Cobb angle is an important
index for evaluating sagittal imbalance of the cervical spine.
Sagittal imbalance of the cervical spine may cause postoperative
pain and decrease cervical spine mobility and function (38). Reconstruction of sagittal balance
of the cervical spine is important. A decrease in the C2-7 Cobb
angle indicates that the cervical curvature becomes straight or
even reversed, which may lead to excessive tension and spasm of the
muscles of the neck, pain and dysfunction of the neck, and even
accelerate the degeneration of the cervical spine, spinal canal
stenosis and spinal cord nerve compression. The loss of the C2-7
Cobb angle and intervertebral height in the 3D cage group were
significantly smaller than those in the PEEK cage group, indicating
that 3D-printed cages had more advantages in maintaining the C2-7
Cobb angle and intervertebral height, which could effectively
reduce the loss of cervical curvature and intervertebral height.
The upper and lower surfaces of the PEEK cage are dentate features
that are in point contact with the upper and lower endplate
surfaces and can cause stress concentration. These dentate surfaces
have the potential to fall into the vertebral body and reduce the
height of the intervertebral gap. The surface of the 3D-printed
fusion cage is fabricated according to the curved surface structure
of the endplate. The contact area is larger and the stability is
better. The complex microstructure of a rough surface is conducive
to bone healing (39). Correct
handling of the end plates, precise placement angle and the depth
of the screws on the plate are key factors in preventing
subsidence. When handling endplates with more severe degeneration,
care should be taken to not exert too much force to avoid damaging
the endplates; furthermore, when placing the interbody fusion cage,
it should be entered along the inclined angle of the intervertebral
space to prevent the cage from damaging the endplates, leading to
postoperative subsidence of the cage (40). However, the studies included in the
present meta-analysis did not consider the porosity of the porous
titanium fusion device, the treatment of the end plate or the angle
of fusion device placement. In terms of postoperative VAS and JOA
scores, the 3D-printed cage group was superior to the PEEK cage
group, indicating that the application of 3D-printed cage can
reduce postoperative pain and accelerate postoperative functional
recovery. The postoperative hospital stay was shorter than that of
the PEEK cage group, which also proved this point. This shows that
the application of 3D-printed cage can reduce the operation time,
intraoperative bleeding and postoperative complications of ACDF,
promote recovery of the cervical curvature, improve stability,
reduce postoperative pain and accelerate postoperative functional
recovery.
The pathogenesis of traumatic cervical spinal cord
injury is different from that of cervical spondylosis. Traumatic
cervical spinal cord injury refers to damage to the cervical spinal
cord caused by an external force that impairs the structural
integrity of the cervical vertebrae, thereby involving the cervical
spinal cord. Cervical spondylosis is a disease based on
degenerative pathological changes in the intervertebral discs.
Traumatic cervical spinal cord injury is a serious injury, which
frequently leads to impaired sensory, motor and reflex functions of
the spinal cord, specifically manifested as quadriplegia and
sensory disorders, as well as urinary and fecal dysfunction. The
surgery is frequently more difficult, the postoperative recovery
process is longer, the recovery of postoperative function is poorer
and there are more complications. This is a source of heterogeneity
that was expected to affect the present analysis, and thus, it was
excluded. Herniated nucleus pulposus (HNP) is one of the common
causes of cervical spondylosis. Different parts of protrusion
compression are divided into cervical spondylotic radiculopathy and
cervical spondylotic myelopathy. Cervical spondylosis caused by HNP
was included in the present study, and both nerve root cervical
spondylosis and spinal cord cervical spondylosis were also included
in this study. The present study focused on cervical spondylosis,
but the condition is divided into various types, including cervical
spondylotic radiculopathy and myelopathy, the scores of which may
vary according to the different etiologic causes of the disease.
Toci et al (41) used the
Short-Form 12 Physical Component and Mental Component scores, VAS
arm score and Neck Disability Index to compare improvements in
outcome measures reported by patients who underwent ACDF for
cervical spondylotic myelopathy or cervical spondylotic
radiculopathy, and found that patients with cervical spondylotic
myelopathy had better baseline functioning but relatively less
postoperative improvement.
The age of the patient is one of the factors that
influence the difficulty of intraoperative maneuvers and
postoperative outcomes. While the average age of patients in the
literature included in the present study ranged from 46-60 years,
although the age span was large, statistical comparisons were made
in each of the studies, and there was no statistically significant
difference between the age of the control group and the
experimental group (P>0.05). A total of eight of the studies
(17-20,22-24, Xu, unpublished data) had P-values of 0.506, 0.649,
0.813, 0.653, 0.26, 0.765, 0.612 and 0.658, and the remaining four
studies (10,16,21,25)
did not provide any P-values but all mentioned P>0.05 in the
text. The effect of patient age on the results needs to be
controlled in clinical controlled trials and in the present
analysis, patient age in the different studies was a confounding
factor, which requires to be controlled. Therefore, it may be
assumed that the age factor did not have a significant effect on
the present results.
In the present study, it was discovered that using
PEEK interbody fusion cages and 3D-printed porous titanium
interbody fusion cages in ACDF can reduce postoperative pain and
increase functional recovery. The operation time, intraoperative
blood loss, postoperative complications and hospitalization time of
the 3D-printed porous titanium interbody fusion cage group were
less than those of the PEEK interbody fusion cage group, and it
also had advantages in maintaining cervical curvature and
intervertebral height. It also reduces pain and accelerates
postoperative rehabilitation. No significant differences were
observed in the rates of intervertebral fusion. Therefore, it is
indicated that 3D-printed porous titanium interbody fusion cages
are superior to PEEK interbody fusion cages when ACDF is
required.
The limitations of the present study were as
follows: i) There was only a small proportion of randomized
controlled studies included in the present analysis. It is well
known that randomized controlled trials are a class of study with
the highest quality; however, only four articles were retrieved in
line with the search strategy and included in the present study.
ii) Currently, research on related issues is focused in China, and
there is a lack of research in other countries and ethnic groups;
the reason for this is elusive and it is not clear whether the
findings are applicable to other countries or ethnic groups. Our
group is planning to conduct a collaborative network analysis of
related issues in the future. Since there is always heterogeneity
in intervention effects among multiple studies from different
groups and geographic regions, a random-effects model was always
used to account for this. iii) No outcome indicators on
sedimentation were reported in the literature. iv) The study
population included patients with cervical spondylosis who
underwent ACDF using a 3D cage or PEEK cage and there was no
differentiation between cervical spondylotic radiculopathy and
cervical spondylotic myelopathy; however, the postoperative scores
of patients with cervical spondylosis of different etiologies who
underwent the same procedure may vary and this is a source of
heterogeneity. v) Differences in the number of operated segments
among the included studies may have also affected the present
results, and different numbers of segments may affect outcome
metrics such as operative time and bleeding. Among the studies
included, there were three papers (17,21, Xu, unpublished data)
with multisegment ACDF, in which the results of the statistical
comparison of the literature regarding the number of surgical
segments were P=0.792, 0.749 and 0.520, which were all >0.05,
and there was thus no significant difference. Therefore, a
sensitivity analysis was performed for each outcome metric and
consistent results were obtained after excluding the studies one by
one, which indicates that the present results are reliable.
However, heterogeneity cannot be excluded due to differences in
study design in the original literature, which is a limitation.
Future research is needed to reduce the influence of confounding
factors and more randomized controlled trials with larger sample
sizes are needed to obtain more reliable results.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Changzhi City
Science and Technology Bureau Fund Project (grant no. 2022sy008)
and the Heping Hospital Affiliated to Changzhi Medical College
Youth Start-up Fund Project.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WJZ and LL were accountable for the design of this
study and both performed the statistical analyses. YHG and SLQ made
significant contributions to the analysis and interpretation of the
data. PFH and YFX drafted the manuscript and critically revised its
intellectual content, and contributed significantly to the
conceptualization and design of the study. PFH and YFX confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang T, Guo N, Gao G, Liu H, Li Y, Gao F,
Zhang Q, Tao X, Yang W and Wang Y: Comparison of outcomes between
Zero-p implant and anterior cervical plate interbody fusion systems
for anterior cervical decompression and fusion: A systematic review
and meta-analysis of randomized controlled trials. J Orthop Surg
Res. 17(47)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schmitz P, Cornelius Neumann C, Neumann C,
Nerlich M and Dendorfer S: Biomechanical analysis of iliac crest
loading following cortico-cancellous bone harvesting. J Orthop Surg
Res. 13(108)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Walsh WR, Pelletier MH, Christou C, He J,
Vizesi F and Boden SD: The in vivo response to a novel Ti coating
compared with polyether ether ketone: Evaluation of the periphery
and inner surfaces of an implant. Spine J. 18:1231–1240.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xu J, He Y, Li Y, Lv GH, Dai YL, Jiang B,
Zheng Z and Wang B: Incidence of subsidence of seven intervertebral
devices in anterior cervical discectomy and fusion: A network
meta-analysis. World Neurosurg. 141:479–489.e4. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Choy WJ, Parr WCH, Phan K, Walsh WR and
Mobbs RJ: 3-dimensional printing for anterior cervical surgery: A
review. J Spine Surg. 4:757–769. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Broekhuis D, Boyle R, Karunaratne S, Chua
A and Stalley P: Custom designed and 3D-printed titanium pelvic
implants for acetabular reconstruction after tumour resection. Hip
Int. 33:905–915. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fang T, Zhang M, Yan J, Zhao J, Pan W,
Wang X and Zhou Q: Comparative analysis of 3D-printed artificial
vertebral body versus titanium mesh cage in repairing bone defects
following single-level anterior cervical corpectomy and fusion. Med
Sci Monit. 27(e928022)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hao L, Zhang Y, Bian W, Song W, Li K, Wang
N, Wen P and Ma T: Standardized 3D-printed trabecular titanium
augment and cup for acetabular bone defects in revision hip
arthroplasty: A mid-term follow-up study. J Orthop Surg Res.
18(521)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shen J, Yang M, Zhong N, Jiao J and Xiao
J: 3D-printed titanium prosthetic reconstruction of unilateral bone
deficiency after surgical resection of tumor lesions in the upper
cervical spine: Clinical outcomes of three consecutive cases and
narrative review. Clin Spine Surg. 36:256–264. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arts M, Torensma B and Wolfs J: Porous
titanium cervical interbody fusion device in the treatment of
degenerative cervical radiculopathy; 1-year results of a
prospective controlled trial. Spine J. 20:1065–1072.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. J Clin Epidemiol.
62:1006–1012. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Furlan JC and Catharine Craven B:
Psychometric analysis and critical appraisal of the original,
revised, and modified versions of the Japanese Orthopaedic
Association score in the assessment of patients with cervical
spondylotic myelopathy. Neurosurg Focus. 40(E6)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
McCormack HM, Horne DJ and Sheather S:
Clinical applications of visual analogue scales: A critical review.
Psychol Med. 18:1007–1019. 1988.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stang A: Critical evaluation of the
newcastle-ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bai C, Zhang Y, Guo J, Liu Q, Li Z and
Song Y: Early clinical study on anterior cervical fusion surgery
with 3D printed interbody fusion cage for treatment of cervical
disk herniation. J Nat Sci. 24:236–239. 2023.
|
|
17
|
Jiang C, Jiang S, Tang Y and Jiang L:
Efficacy of 3D printed microporous titanium fusion device applied
to anterior cervical decompression graft fusion and its effects on
cervical spine anatomy and stress hormones. Chin J Tissue Eng Res.
27:2837–2841. 2023.
|
|
18
|
Li L: Clinical study of 3D printing spine
intervertebral fusion cage in treatment of cervical spondylosis.
Chengde Medical University, 2020.
|
|
19
|
Li Y, Wang H and Cui W: Comparison of the
efficacy of ACDF in the treatment of cervical spondylotic
myelopathy using 3D printed intervertebral fusion cage and
Polyether-ether-ketone (PEEK) intervertebral fusion cage. Chin J
Spine and Spinal Cord. 31:16–24. 2021.(In Chinese).
|
|
20
|
Liu Z, Wang Y, Zhang Y, Ming Y, Sun Z and
Sun H: Application of 3D printed interbody fusion cage for cervical
spondylosis of spinal cord type: Half-year follow-up of recovery of
cervical curvature and intervertebral height. Chin J Tissue Eng
Res. 25:849–853. 2021.(In Chinese).
|
|
21
|
Wang Z, Feng H, Ma X, Chen C, Deng C and
Sun L: Effectiveness of three-dimensional printing artificial
vertebral body and interbody fusion Cage in anterior cervical
surgery. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 35:1147–1154.
2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
22
|
Wang J, Wu D, Sun H, Zhang Y, Xin L and Li
L: Follow-up of the clinical efficacy and sagittal balance of 3D
printing spine intervertebral fusion cage in treatment of cervical
spondylosis. Chin J Clin Anat. 41:342–348. 2023.(In Chinese).
|
|
23
|
Wang Y, Zhou Y, Wang Y, Hao Y and Zhang B:
Application of 3D print porous titanium alloy intervertebral fusion
cage in anterior cervical discectomy and bone graft fusion for
treatment of cervical spondylopathy. Chin J Bone and Joint Inj.
38:1–5. 2023.(In Chinese).
|
|
24
|
Yang X, Zhao X, Qi D, et al: Changes in
cervical sagittal balance after three-dimensional printing ACT
titanium cage in anterior cervical discectomy with fusion. Chin J
Tissue Eng Res. 24:5741–5748. 2020.(In Chinese).
|
|
25
|
Zhang Y, Liu Z, Fang Z, Wu W and Li F:
Clinical efficacy of 3D-printed titanium cage in the treatment of
cervical spondylotic myelopathy and changes in cervical sagittal
parameter. Orthopaedics. 12:97–102. 2021.
|
|
26
|
Bridges KJ, Simpson LN, Bullis CL, Rekito
A, Sayama CM and Than KD: Combined laminoplasty and posterior
fusion for cervical spondylotic myelopathy treatment: A literature
review. Asian Spine J. 12:446–458. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Berger MB, Slosar P, Schwartz Z, Cohen DJ,
Goodman SB, Anderson PA and Boyan BD: A review of biomimetic
topographies and their role in promoting bone formation and
osseointegration: Implications for clinical use. Biomimetics
(Basel). 7(46)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu Y, Cao Z, Peng Y, Hu L, Guney T and
Tang B: Facile surface modification method for synergistically
enhancing the biocompatibility and bioactivity of poly(ether ether
ketone) that induced osteodifferentiation. ACS Appl Mater
Interfaces. 11:27503–27511. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yamamoto N, Hayashi K and Tsuchiya H:
Progress in biological reconstruction and enhanced bone
revitalization for bone defects. J Orthop Sci. 24:387–392.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kaur M and Singh K: Review on titanium and
titanium based alloys as biomaterials for orthopaedic applications.
Mater Sci Eng C Mater Biol Appl. 102:844–862. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tamayo JA, Riascos M, Vargas CA and Baena
LM: Additive manufacturing of Ti6Al4V alloy via electron beam
melting for the development of implants for the biomedical
industry. Heliyon. 7(e06892)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang F, Xu H, Wang H, Geng F, Ma X, Shao
M, Xu S, Lu F and Jiang J: Quantitative analysis of near-implant
magnesium accumulation for a Si-containing coated AZ31 cage from a
goat cervical spine fusion model. BMC Musculoskelet Disord.
19(105)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li SL, Zhao JS and Yang LX: Numerical
simulation analysis of mechanical properties of irregular porous
titanium lumbar fusion apparatus mechanical science and technology
for aerospace engineering: 1-7, 2023.
|
|
34
|
Fogel G, Martin N, Lynch K, Pelletier MH,
Wills D, Wang T, Walsh WR, Williams GM, Malik J, Peng Y and Jekir
M: Subsidence and fusion performance of a 3D-printed porous
interbody cage with stress-optimized body lattice and microporous
endplates-a comprehensive mechanical and biological analysis. Spine
J. 22:1028–1037. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jin YZ, Zhao B, Lu XD, Zhao YB, Zhao XF,
Wang XN, Zhou RT, Qi DT and Wang WX: Mid- and long-term follow-up
efficacy analysis of 3D-printed interbody fusion cages for anterior
cervical discectomy and fusion. Orthop Surg. 13:1969–1978.
2021.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Li X, Wu S, Li Y, Zhang Y and Guo Z:
3D-printed porous titanium cage versus polyetheretherketone cage in
sheep vertebral fusion. Chin J Orthop Trauma. 17:34–39. 2015.(In
Chinese).
|
|
37
|
Wu SH, Li Y, Zhang YQ, Li XK, Yuan CF, Hao
YL, Zhang ZY and Guo Z: Porous titanium-6 aluminum-4 vanadium cage
has better osseointegration and less micromotion than a
poly-ether-ether-ketone cage in sheep vertebral fusion. Artif
Organs. 37:E191–E201. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Obermueller T, Wagner A, Kogler L, Joerger
AK, Lange N, Lehmberg J, Meyer B and Shiban E: Radiographic
measurements of cervical alignment, fusion and subsidence after
ACDF surgery and their impact on clinical outcome. Acta Neurochir
(Wien). 162:89–99. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shah FA, Snis A, Matic A, Thomsen P and
Palmquist A: 3D printed Ti6Al4V implant surface promotes bone
maturation and retains a higher density of less aged osteocytes at
the bone-implant interface. Acta Biomater. 30:357–367.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Luan H, Peng C, Liu K and Song X:
Comparing the efficacy of unilateral biportal endoscopic
transforaminal lumbar interbody fusion and minimally invasive
transforaminal lumbar interbody fusion in lumbar degenerative
diseases: A systematic review and meta-analysis. J Orthop Surg Res.
18(888)2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Toci GR, Lambrechts MJ, Karamian BA,
Canseco JA, Hilibrand AS, Kepler CK, Vaccaro AR and Schroeder GD:
Patients with radiculopathy have worse baseline disability and
greater improvements following anterior cervical discectomy and
fusion compared to patients with myelopathy. Spine J. 23:238–246.
2023.PubMed/NCBI View Article : Google Scholar
|