Introduction
As a harmful gas, ammonia (NH3) can
combine with nitric acid and sulfuric acid to make up particulate
matter 2.5, causing air pollution and endangering human health
(1). NH3 in livestock
houses reduces the antioxidant capacity, immunity and performance
of dairy cows, and causes inflammation (2). Furthermore, NH3 is also a
ubiquitous by-product of cellular metabolism, which is ejected from
cells (3,4). NH3 can meet the large
demand for amino acid synthesis through the recycling mode in
rapidly dividing cells (3).
Autophagy and apoptosis are involved in various physiological and
pathological processes, such as maintaining the homeostasis of the
intracellular environment, having a defensive role in disease
development and preventing harmful substances from invading cells
(5,6). Substantial documents have reported
that an increase in NH3 concentration in the culture
medium may induce cell apoptosis and oxidative stress, inhibiting
cell growth and affecting metabolism (7-9).
NH3 activates the p53 signal and mitochondrial
apoptosis, as well as increases the number of apoptotic cells,
leading to the apoptosis of mammary epithelial cells in dairy cows
(10). Previous studies by our
group demonstrated that NH3 may induce inflammatory
responses, autophagy and apoptosis, as well as inhibit the
proliferation of bovine mammary epithelial cells (11).
Sirtuin5 (SIRT5) is a member of the mammalian
sirtuin family and belongs to the class III histone deacetylase
enzyme dependent on nicotinamide adenine dinucleotide, which is
mainly located in the mitochondrial matrix, but also exists in the
cytoplasm and nucleus (12,13).
SIRT5 exhibits a special affinity for negatively charged acyl
lysine modification, which indicates demalonylation,
desuccinylation and deglutarylation, along with a less efficient
deacetylase activity (13-16).
SIRT5 regulates several important metabolic processes, such as
fatty acid-oxidation, tricarboxylic acid cycle, amino acid
degradation, nitrogen metabolism, antioxidant defense and apoptosis
(13-19).
Evidence has demonstrated that SIRT5 promotes
autophagy and has a role as a proliferation factor in several
cancer types, including colorectal cancer and gastric cancer
(20-22).
Specfically, SIRT5 exerts an anti-apoptotic effect, up-regulating
Bcl-2 and Bcl-XL expression and downregulating Caspase-3, Caspase-7
and Bax levels (23). Furthermore,
SIRT5 promotes cell proliferation and accelerates the process of
autophagy (24). Of note, SIRT5
can desuccinylate glutaminase (GLS) to control NH3
release and further regulate autophagic activity (25). Of note, SIRT5 has aroused interest
as a potential drug target for treating these diseases. The roles
of SIRT5 in various diseases, including metabolic disorders,
infectious diseases and cancer, need to be studied.
The PI3K/Akt/mTOR signaling pathway is involved in
the regulation of cell proliferation, growth, apoptosis,
differentiation, autophagy and metabolism (26,27).
SIRT5 was observed to inhibit the growth, invasion and migration of
tumor cells, and to modulate the inherent and acquired immunity via
the PI3K/Akt pathway (28). The
signal of mechanistic target of rapamycin kinase (mTOR) has an
important role in regulating autophagy (29). The mTOR signal promotes glutamine
uptake through GLS and accelerates the release of NH3
(30,31). NH3 can rely on the PI3K
signaling pathway to regulate mTOR signals, thereby possibly
affecting mammary epithelial cell apoptosis and autophagy in dairy
cows (11). Considering that SIRT5
has a significant role in NH3-induced autophagy in tumor
cells (21), the present study is
based on the speculation that SIRT5 regulates
NH3-induced mammary epithelial cell autophagy and
apoptosis in dairy cows. Therefore, in this research, SIRT5
overexpression or knockdown cell lines were constructed using
bovine mammary epithelial cells, in addition to treating bovine
mammary epithelial cells with SIRT5 inhibitors. The effect of SIRT5
on NH3 release was evaluated by detecting the level of
NH3, GLS activity and glutamate content in the cells.
The apoptosis and autophagy of MAC-T cells were evaluated using
reverse transcription-quantitative (RT-q)PCR, western blot, flow
cytometry with Annexin V FITC/PI staining and transmission electron
microscopy (TEM) methods. Furthermore, SIRT5 overexpression or
knockdown cell lines or mammary epithelial cells incubated by SIRT5
inhibitor were treated with ammonium chloride (NH4Cl),
combined with PI3K or mTOR inhibitor treatment. Finally, the effect
of SIRT5 on the apoptosis and autophagy in NH3-treated
bovine mammary epithelial cells was clarified. The present results
showed that SIRT5 inhibited NH3 production from the
metabolism of glutamine and inhibited mammary epithelial cell
apoptosis and autophagy in dairy cows. Of note, the index of cell
energy balance, the ratio of ADP/ATP, was changed in relation to
NH3 release regulated by SIRT5. Furthermore, SIRT5
regulated apoptosis and autophagy in NH3-treated bovine
mammary epithelial cells through the PI3K/Akt/mTOR signaling
pathway. This research will provide guidance for improving the
health and production performance of dairy cows.
Materials and methods
Generation of SIRT5-overexpressing and
-silenced stable cell lines
Bovine mammary epithelial cells (MAC-T cells,
preserved in the laboratory, originally from ATCC) were grown in
Dulbecco's Modified Eagle Medium (DMEM) with high glucose (HyClone;
Cytiva), supplemented with 10% fetal bovine serum (ExCell
Biotechnology Co., Ltd.) and 1% penicillin-streptomycin at 37˚C
with 5% CO2 in an incubator. An expression vector for
bovine SIRT5 was constructed using the plasmid expressing enhanced
green fluorescence protein (pEGFP-N1) (Beijing Huayueyang
Biotechnology Co., Ltd.) (32),
and stably transfected into MAC-T cells for implementing SIRT5
overexpression. In brief, according to the CDS region of the bovine
SIRT5 gene (NM_001034295), the cDNA encoding SIRT5 was inserted
into the pEGFP-N1 vector to construct pEGFP-SIRT5 vector.
Subsequently, according to the instructions for the Lipofectamine
3000 reagent, the pEGFP-N1 vector and the pEGFP-SIRT5 vector were
respectively transfected into MAC-T cells. After 24 h, G418 was
used to select SIRT5-overexpressing MAC-T cells
(SIRT5+/+). Subsequently, the fluorescence intensity in
MAC-T cells was observed under a fluorescence microscope to confirm
successful transfection. The cells were divided into 2 groups:
Empty vector (pEGFP, transfected with pEGFP-N1 vector) and SIRT5
overexpression (pEGFP-SIRT5, transfected with pEGFP-SIRT5
vector).
The stable SIRT5 knockdown cells
(SIRT5-/-) were generated with CRISPR-CAS9 gene editing
systems to target the SIRT5 gene in MAC-T cells. Three 20-bp guide
sequences targeting bovine SIRT5 gene (NM_001034295) were as
follows: Single guide (sg)RNA1, 5'-GTTCTACCACTACCGGCGGG-3'; sgRNA2,
5'-GGGAGTTCTACCACCGG-3'; and sgRNA3, 5'-GGAGTTCTACCACTACCGGC-3'.
sgRNAs were synthesized by Sangon Biotech Co., Ltd. pX330 (Beijing
Huayueyang Biotechnology Co., Ltd.) was selected as the knockdown
vector. The synthesized RNA interference sequences were cloned into
the pX330 vector at the NheI and BamHI sites using
restriction enzymes (Thermo Fisher Scientific Inc.). After enzyme
digestion identification, the vectors were named as
pX330-sgRNA-SIRT5. MAC-T cells were resuscitated and supplemented
with DMEM containing 10% fetal bovine serum at 37˚C in an incubator
with 5% CO2. After the cells had reached 70-80%
confluency, pX330 vector or pX330-sgRNA-SIRT5 vector was
transfected with Lipofectamine 3000 reagent according to the
manufacturer's protocol. After 24 h, puromycin was used to screen
for SIRT5-/- cells. The cells were divided into 2
groups: Empty vector (pX330, transfected with pX330 vector) and
SIRT5 knockdown (sgRNA-1, sgRNA-2 and sgRNA-3, transfected with
pX330-sgRNA-SIRT5 vectors).
Overexpression and knockdown efficacy was validated
by RT-qPCR and western blot. The plasmid kits and transfection
reagent Lipofectamine 3000 were purchased from Thermo Fisher
Scientific, Inc. RNAiso Plus, T4 DNA ligase, SYBR®
PremixExTaq™ II (TliRNaseHPlus), LATaq enzyme and DL2000
Marker were from Takara Biotechnology Co., Ltd and were used
according to the manufacturer's instructions.
Cell treatment
NH4Cl, LY294002 (LY; PI3K inhibitor) and
Rapamycin (RA; mTOR inhibitor) were used to treat the cells and had
been obtained from Sigma-Aldrich (Merck KGaA). MC3482 (SIRT5
inhibitor) was from MedChemExpress. The MAC-T cells and
SIRT5-/-, SIRT5+/+ cell lines were washed
twice with PBS and then cultured in medium supplemented with the
indicated agents. The MAC-T cells were randomized into the four
experimental groups: i) Control (CT); ii) NH4Cl (NC);
iii) MC3482 (MC); and iv) MC3482 + NH4Cl (MCN). The
SIRT5+/+ cells were randomized into four experimental
groups: i) SIRT5+/+ (SO); ii) SIRT5+/+ +
NH4Cl (SON); iii) SIRT5+/+ + NH4Cl
+ LY (SNL); and iv) SIRT5+/+ + NH4Cl + RA
(SNR). The SIRT5-/- cells were randomized into two
experimental groups: i) SIRT5-/- (SD); and ii)
SIRT5-/- + NH4Cl (SDN). Each of the above
groups contained three independent repeats. In the CT group, the
MAC-T cells were cultured with basal medium for 12 h. In the SO and
SD groups, the SIRT5+/+ and SIRT5-/- cells
were cultured with basal medium for 12 h, respectively. In the NC,
SON and SDN groups, the MAC-T, SIRT5+/+ and
SIRT5-/- cells were exposed to 4 mM NH4Cl for
12 h. In the MC group, MAC-T cells were incubated with 20 µM MC3482
for 12 h. In the MCN group, MAC-T cells were incubated with MC3482
(20 µM) for 30 min and then the cells were treated with 4 mM
NH4Cl for 12 h. In the SNL and SNR groups,
SIRT5+/+ cells were respectively incubated with LY294002
(20 mM) or Rapamycin (10 µM) for 30 min, and then the cells were
treated with 4 mM NH4Cl for 12 h.
RNA extraction and quantitative
real-time PCR (qPCR)
Total RNA was extracted from cells with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
protocol provided by the manufacturer. The integrity of RNA was
analyzed by agarose gel electrophoresis. cDNA was synthesized using
the RT kit (cat. no. 6110A; Takara Biotechnology Co., Ltd.)
following to the manufacturer's instructions. qPCR was performed
using SYBR Premix Ex Taq (cat. no. RR820A; Takara Biotechnology
Co., Ltd.) following the manufacturer's instructions. PCR
conditions were as follows: 95˚C for 5 min, followed by 30 cycles
at 95˚C for 30 sec, 58˚C for 20 sec and 72˚C for 20 sec. All
experiments were performed thrice. GADPH was used as a housekeeping
gene. Gene expression was quantified relative to GAPDH expression
amplified in the same sample, and gene expression was analyzed
using the 2-ΔΔCq method (11). Primers used for the real-time qPCR
assay are listed in Table I.
 | Table IPrimer sequences used in the
study. |
Table I
Primer sequences used in the
study.
| Target gene | Sequence
(5'-3') | Accession no. |
|---|
| Bax | | XM_015458140 |
|
Forward |
CTTTTGCTTCAGGGTTTCA | |
|
Reverse |
GCTCAGCTTCTTGGTGGAT | |
| Caspase 3 | | XM_010820245 |
|
Forward |
CCGAGGAGGAGACAGGATGC | |
|
Reverse |
CAGGCCATGCCAGTATTTTCG | |
| Bcl-2 | | NM_001166486.1 |
|
Forward |
CATGTGTGTGGAGAGCGTCA | |
|
Reverse |
TACAGCTCCACAAAGGCGTC | |
| LC3B | | NM-001001169.1 |
|
Forward |
CCGACTTATCCGAGAGCAGC | |
|
Reverse |
TGAGCTGTAAGCGCCTTCTT | |
| p62 | | NM-176641.1 |
|
Forward |
GGGAACTTCAGCCCCTTCAA | |
|
Reverse |
ATGGTGTGGTGGTTGTTGGT | |
| Beclin1 | | NM_001033627.2 |
|
Forward |
TGGACACGAGCTTCAAGATTCTGG | |
|
Reverse |
CCTCCTGGGTCTCTCCTGGTTTC | |
| SIRT5 | | NM_001034295.2 |
|
Forward |
GATTTGCCTAACAATGGCTC | |
|
Reverse |
GGTTTGGAGAAAACCTGGA | |
| GAPDH | | NM_001034034.2 |
|
Forward |
GATGGTGAAGGTCGGAGTGAAC | |
|
Reverse |
GTCATTGATGGCGACGATGT | |
Western blot analysis
Total protein was extracted from cells in each cell
group using RIPA lysis buffer (Thermo Fisher Scientific, Inc.). The
protein concentration was determined using a BCA kit (cat. no.
BCA01; Beijing Dingguo Changsheng Biotechnology Co., Ltd.). Next,
5X SDS loading buffer (Beijing Dingguo Changsheng Biotechnology
Co., Ltd.) was added to the extracted proteins, which were then
denatured at 99˚C for 10 min. The proteins (24 µg/lane) were
separated by on 10% gels using SDS-PAGE and finally transferred to
polyvinylidene fluoride (PVDF) membranes (EMD Millipore). The
membranes were then blocked with Tris-buffered saline containing
Tween-20 (TBST) with 5% skimmed milk for 1 h and incubated with the
corresponding antibodies overnight at 4˚C. The PVDF membranes were
washed with TBST for 30 min and incubated with horseradish
peroxidase (HRP)-conjugated anti-IgG antibodies for 2 h at room
temperature. Finally, the visualized protein bands were detected
under a developer using enhanced chemiluminescence reagent (EMD
Millipore), and ImageJ software 1.4.3.67 (National Institutes of
Health) was used to analyze the grayscale values of the protein
bands and calculate the relative expression of proteins in each
group. Mouse anti-Bcl-2 antibody (cat. no. bsm-33047M; 1:500
dilution), rabbit anti-Bax antibody (cat. no. bs-0127R; 1:500),
rabbit anti-β-actin antibody (cat. no. bs-33036M; 1:1,000), rabbit
anti-Caspase-3 antibody (cat. no. bs-0081R; 1:500) were purchased
from Beijing Bioss Technology Co., Ltd. Rabbit anti-light chain 3β
(LC3B) antibody (cat. no. ab48394; 1:1,000 dilution),
rabbit-anti-p62 antibody (cat. no. ab101266; 1:1,000) were
purchased from Abcam. Rabbit anti-Beclin1 antibody (cat. no.
AP0769; 1:500) and rabbit anti-SIRT5 antibody (cat. no. BS91247;
1:500) were purchased from Bioworld Technology, Inc. Goat
anti-mouse IgG (H+L) (cat. no. RGAM001; 1:5,000) and goat
anti-rabbit IgG (H+L) (cat. no. SA00001-2; 1:5,000) were purchased
from Proteintech Group, Inc.
TEM
Regarding the analysis of autophagic vesicle
formation, TEM is the gold standard for evaluating autophagic
activity (11). First, the cells
were digested with trypsin and isolated. Next, the cells were fixed
with electron microscope fixative and then fixed with 1% ozonated
acid for 2 h, at room tempreture. After dehydration,
permeabilisation and embedding, ultrathin sections were observed
and analyzed in representative areas by TEM (HT7700; Hitachi).
Flow cytometric analysis
Flow cytometry was performed to analyze the
apoptotic ratio. In brief, cells were cultured under the
aforementioned conditions. Subsequently, they were double-stained
with Annexin V FITC/PI, which had been obtained from Yisheng
Biotechnology Co., Ltd. Next, cells were analyzed using a flow
cytometer according to the manufacturer's instructions. All
experiments were performed at least in triplicate. The apoptosis
ratio was detected by flow cytometry (CytoFLEX; Beckman Coulter)
and calculated with the help of the CytExpert software (11).
Biochemical assays
The cells were lysed and centrifuged. Subsequently,
the supernatant was collected. The protein concentration was
determined using the bicinchoninic acid protein assay kit (Abcam;
cat. no. ab102536). The NH3 content in the cell culture
medium was determined using an NH3 assay kit
(Sigma-Aldrich; Merck KGaA; cat. no. AA0100) following the
manufacturer's protocol. The content of glutamate, GLS activity and
the ADP/ATP ratio in the cells were determined using relevant kits,
including glutamate measurement kit (Nanjing Jiancheng
Bioengineering Institute; cat. no. A074-1-1), GLS test kit (Nanjing
Jiancheng Bioengineering Institute; cat. no. A124-1-1) and ADP/ATP
ratio assay kit (Sigma-Aldrich; Merck KGaA; cat. no. MAK135). The
above kits were used according to the manufacturers'
specifications. All assays were performed in triplicates.
Statistical analysis
All experiments were performed at least in
triplicates and quantitative data were presented as the mean ±
standard deviation. GraphPad Prism software (version 6.01;
GraphPad; Dotmatics) was used for statistical analysis. Differences
among multiple groups were calculated using one-way ANOVA followed
by post-hoc Bonferroni's correction. P<0.05 was considered to
indicate a statistically significant difference.
Results
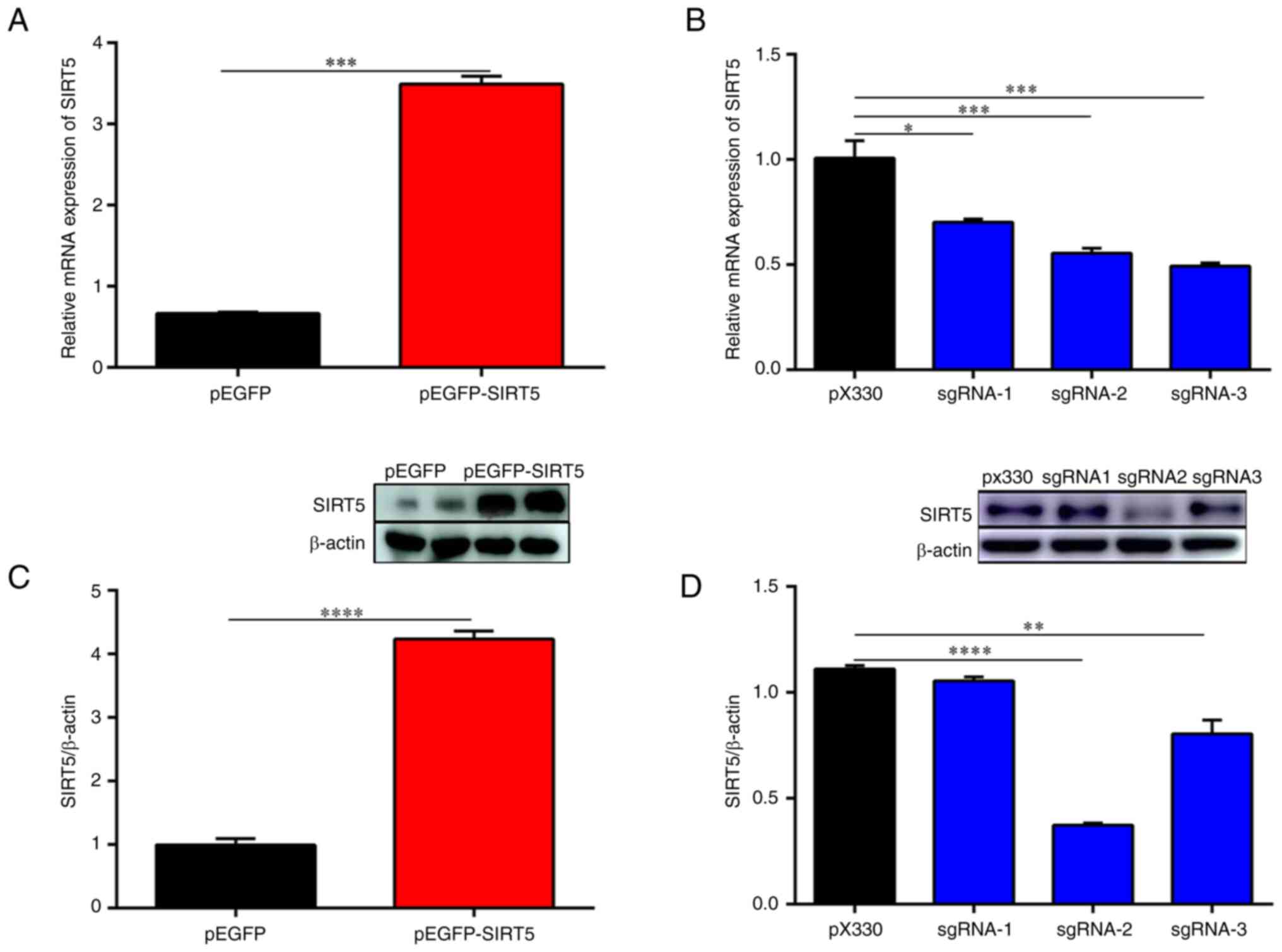
SIRT5 expression in stably transfected
cell lines
The fluorescence intensity in MAC-T cells was
observed under a fluorescence microscope to confirm successful
transfection. As indicated in Fig.
S1A, no fluorescence was present in non-transfected MAC-T
cells. However, fluorescence was observed in cells transfected with
the pEGFP-N1 vector and the pEGFP-SIRT5 vector (Fig. S1B and C). Furthermore, in Fig. 1, the expression of SIRT5 mRNA and
protein in SIRT5+/+ cells was increased compared with
that in MAC-T cells (Fig. 1A and
C), while the expression of SIRT5
mRNA and protein in SIRT5-/- cells was significantly
decreased (Fig. 1B and D). Specifically, the mRNA and protein
expression levels of SIRT5 in the pX330-sgRNA-SIRT5-transfected
cells were significantly reduced, indicating that pX330-sgRNA-SIRT5
vectors efficiently knocked down SIRT5 in MAC-T cells (Fig. 1B and D). Furthermore, the knockdown efficiency
of pX330-sgRNA-2-SIRT5 vector was high and the expression of SIRT5
decreased obviously (P<0.05). Therefore, in the subsequent
experiments, the pX330-sgRNA-2-SIRT5 vector was used to transfect
MAC-T cells to obtain SIRT5-/- cells. In addition,
MC3482 was adopted as a specific inhibitor of SIRT5 to treat MAC-T
cells. As indicated in Fig. 2,
MC3482 at 20 mM inhibited the expression of SIRT5 (Fig. 2D, H and K).
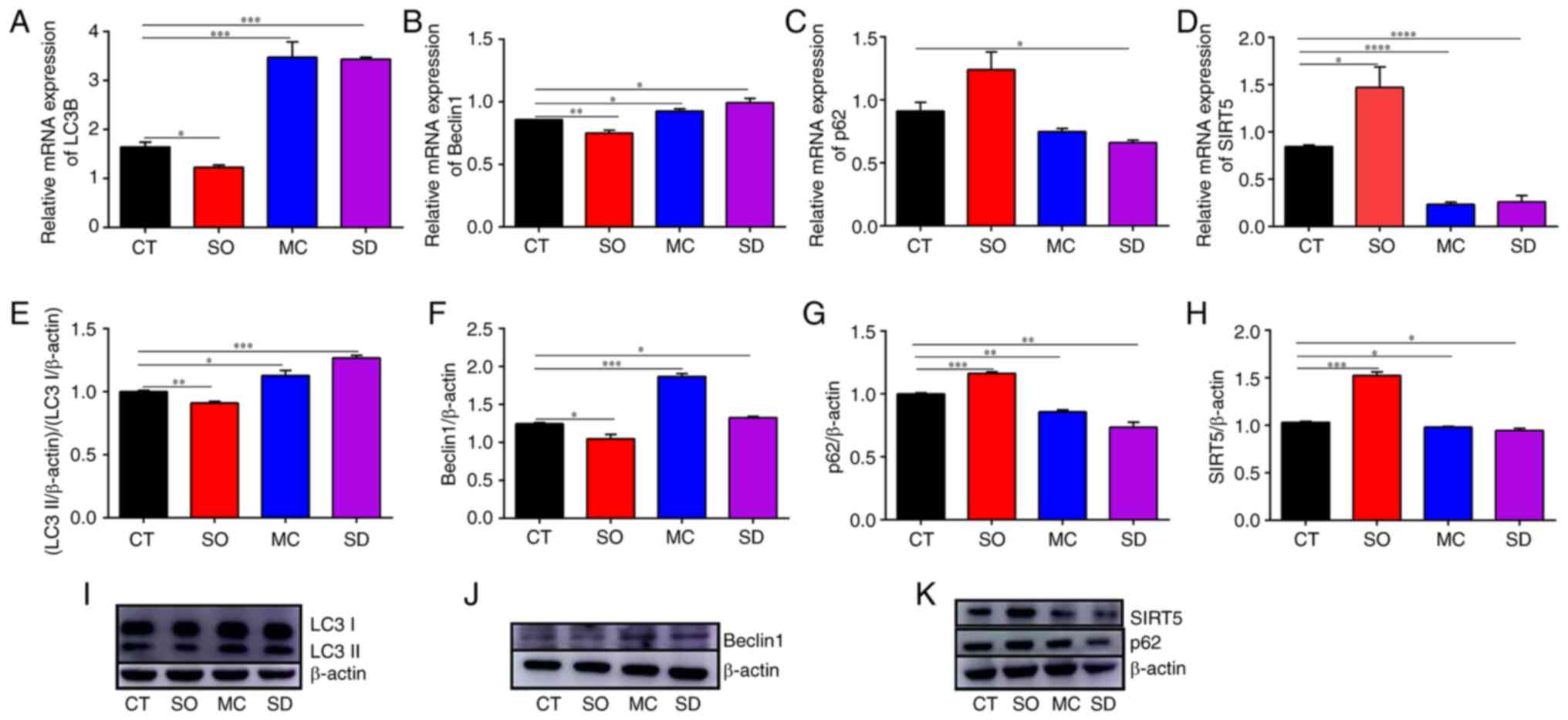 | Figure 2Autophagic markers and SIRT5
expression in cells of different groups. Reverse
transcription-quantitative PCR results: (A) LC3B, (B) Beclin1, (C)
p62 and (D) SIRT5. Western blotting results: (E and I) LC3II/I, (F
and J) Beclin1, (G and K) p62, and (H and K) SIRT5. In the CT
group, the MAC-T cells were cultured with basal medium for 12 h. In
the SO group, the SIRT5+/+ cells were cultured with
basal medium for 12 h. In the SD group, the SIRT5-/-
cells were cultured with basal medium for 12 h. In the MC group,
MAC-T cells were incubated with 20 µM MC3482 for 12 h.
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 vs. CT group.
LC3B, light chain 3β; SIRT5, sirtuin 5. |
SIRT5 inhibits autophagy and apoptosis
in MAC-T cells
There are numerous similarities between autophagy
and apoptosis, e.g. both of them can cause cell death. Furthermore,
a variety of autophagy-related proteins are involved in cell
apoptosis (33). Substantial
evidence demonstrated that the SIRT family participates in cell
autophagy and apoptosis (34-36).
Accumulating studies illustrated that SIRT5 regulates autophagy and
apoptosis in cancer cells (20,21).
Autophagy and mitophagy increased in SIRT5-silenced cells and
similar results were also observed in MDA-MB-231 and C2C12 cells
treated with MC3482. Of note, autophagy and mitophagy decreased in
SIRT5-overexpressing cells (25).
Furthermore, SIRT5 promoted autophagy and maintained the balance of
autophagy and apoptosis (21).
However, the effects of SIRT5 on autophagy and apoptosis in bovine
mammary epithelial cells have remained elusive. Thus, in the
present study, the effect of SIRT5 on MAC-T-cell autophagy and
apoptosis was examined.
TEM observation was adopted to display the autophagy
process initially. As shown in Fig.
3, an accumulation of autophagosomes and autolysosomes was
present in SIRT5-silenced cells (SD group) and in MC3482-treated
MAC-T cells (MC group), compared with cells in the CT and SO groups
(SIRT5+/+ cells). Next, the levels of the autophagic
markers LC3 and Beclin1 and the autophagy flux marker SQSTM1/p62
were also determined. The levels of LC3B and Beclin1 increased and
those of SQSTM1/p62 decreased in SIRT5-/- cells (SD
group) as compared with the CT group (Fig. 2). Again, a similar increase in LC3
and Beclin1 and decrease in SQSTM1/p62 were present in MAC-T cells
treated with MC3482 (Fig. 2).
However, SIRT5 overexpression decreased the levels of LC3 and
Beclin1, while increasing the levels of SQSTM1/p62 in the SO group
(Fig. 2). All of these results
preliminarily proved that SIRT5 inhibits autophagy in MAC-T
cells.
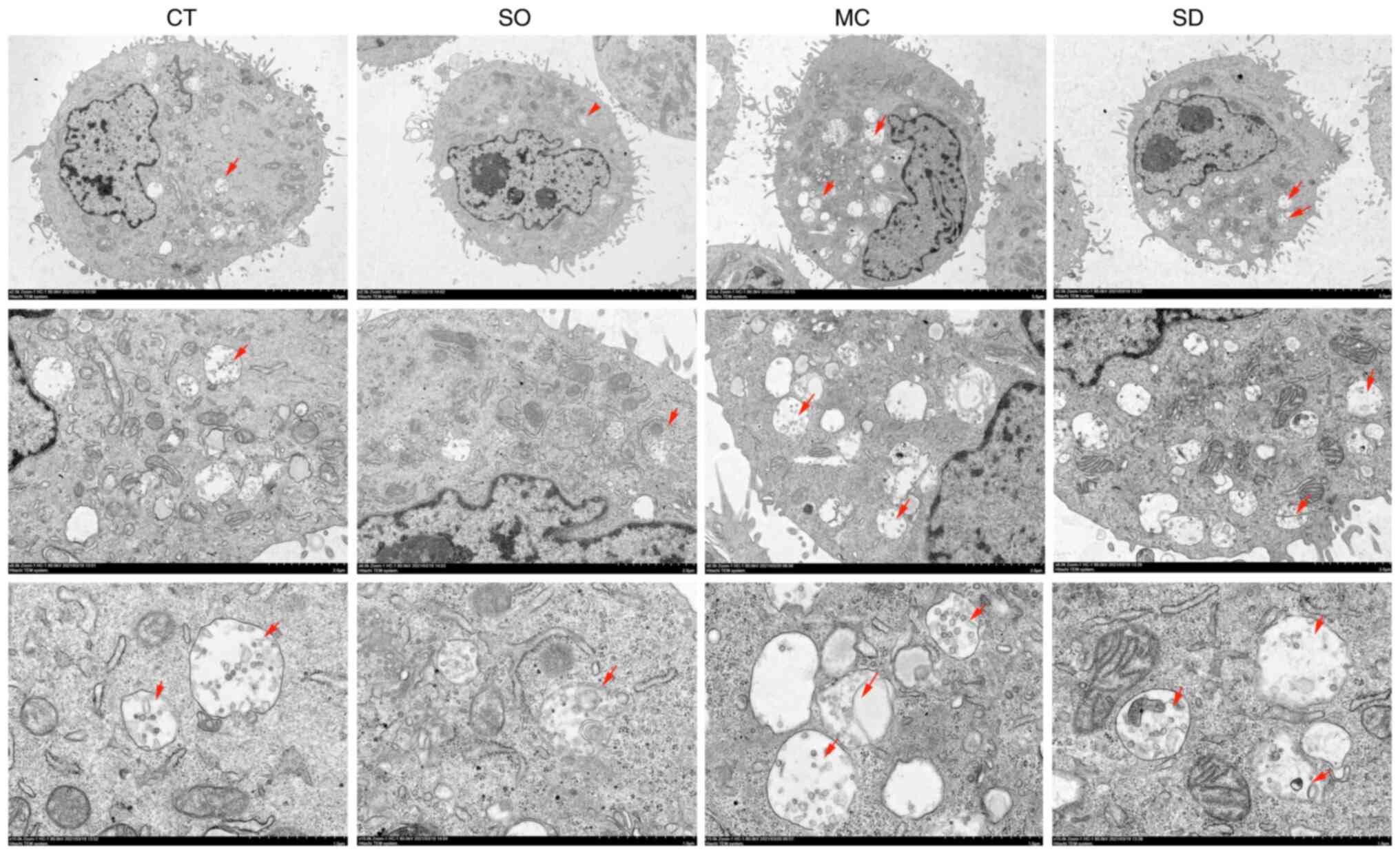 | Figure 3Effect of SIRT5 on autophagy of MAC-T
cells by transmission electron microscopy observation. The CT group
shows some autolysosomes. Compared with the CT group, the SO group
shows a small number of autolysosomes, the MC and SD groups show
more autolysosomes [magnification, x1,200 (upper row), x5,000
(middle row) and x12,000 (bottom row); the red arrows point at the
autolysosomes]. In the CT group, the MAC-T cells were cultured with
basal medium for 12 h. In the SO group, the SIRT5+/+
cells were cultured with basal medium for 12 h. In the SD group,
the SIRT5-/- cells were cultured with basal medium for
12 h. In the MC group, MAC-T cells were incubated with 20 µM MC3482
for 12 h. SIRT5, sirtuin 5. |
In sequence, the apoptotic role of SIRT5 in MAC-T
cells was evaluated. The flow cytometry results revealed that the
number of cells with positive fluorescence staining increased in
SIRT5-/- cells and MAC-T cells treated with MC3482,
indicating a higher rate of apoptosis than that in the CT and SO
groups (Fig. 4). At the same time,
the expression levels of the proapoptotic regulatory factor Bax,
the apoptosis executive factor Caspase-3, as well as the
anti-apoptotic factor Bcl-2 were also determined. Compared with
cells in the CT group and SO group, the mRNA and protein expression
levels of Bax and caspase-3 in SIRT5-/- cells and MAC-T
cells treated with MC3482 increased significantly, while the mRNA
and protein levels of Bcl-2 decreased significantly (Fig. 5). However, compared with those in
the CT group, the mRNA and protein expression levels of Bax and
caspase-3 in the SIRT5+/+ cells was significantly
decreased, and the mRNA and protein levels of Bcl-2 was
significantly increased (Fig. 5).
Hence, the aforementioned results suggested that SIRT5 inhibited
the apoptotic occurrence in MAC-T cells.
Ammonia levels decline in
SIRT5-overexpressing and increase in SIRT5-silenced cells
Previous studies have found that the addition of
exogenous NH4Cl-induced MAC-T-cell apoptosis and
autophagy (11). NH3 in
cells was mainly from glutamine catabolism. Research pointed out
that SIRT5 regulates glutamine metabolism (25). However, SIRT5 was ubiquitously
expressed (37). Therefore, in the
present study, it was examined whether SIRT5 also participates in
regulating NH3 levels in MAC-T cells, and ultimately
affects apoptosis and autophagy of MAC-T cells induced by
NH3. It was found that SIRT5 overexpression
significantly reduced the NH3 concentration in the
culture medium (P<0.01; Fig.
6A). On the contrary, SIRT5 knockdown obviously promoted
NH3 accumulation compared to MAC-T cells (Fig. 6A). Furthermore, an elevation in
NH3 levels was obtained when MAC-T cells were treated
with MC3482, an inhibitor of SIRT5 (Fig. 6A). NH3 levels were
further examined in SIRT5+/+, SIRT5-/- or
MAC-T cells treated with MC3482, and combined with NH4Cl
treatment as the donor of NH3. In Fig. 7, a decline in the content of
NH3 appeared in SIRT5+/+ cells treated with
NH4Cl, and a significant increase was present in
SIRT5-/- cells treated with NH4Cl and MAC-T
cells co-treated with MC3482 and NH4Cl (Fig. 7A). These results indicated that
SIRT5 may reduce the generation of NH3 in MAC-T
cells.
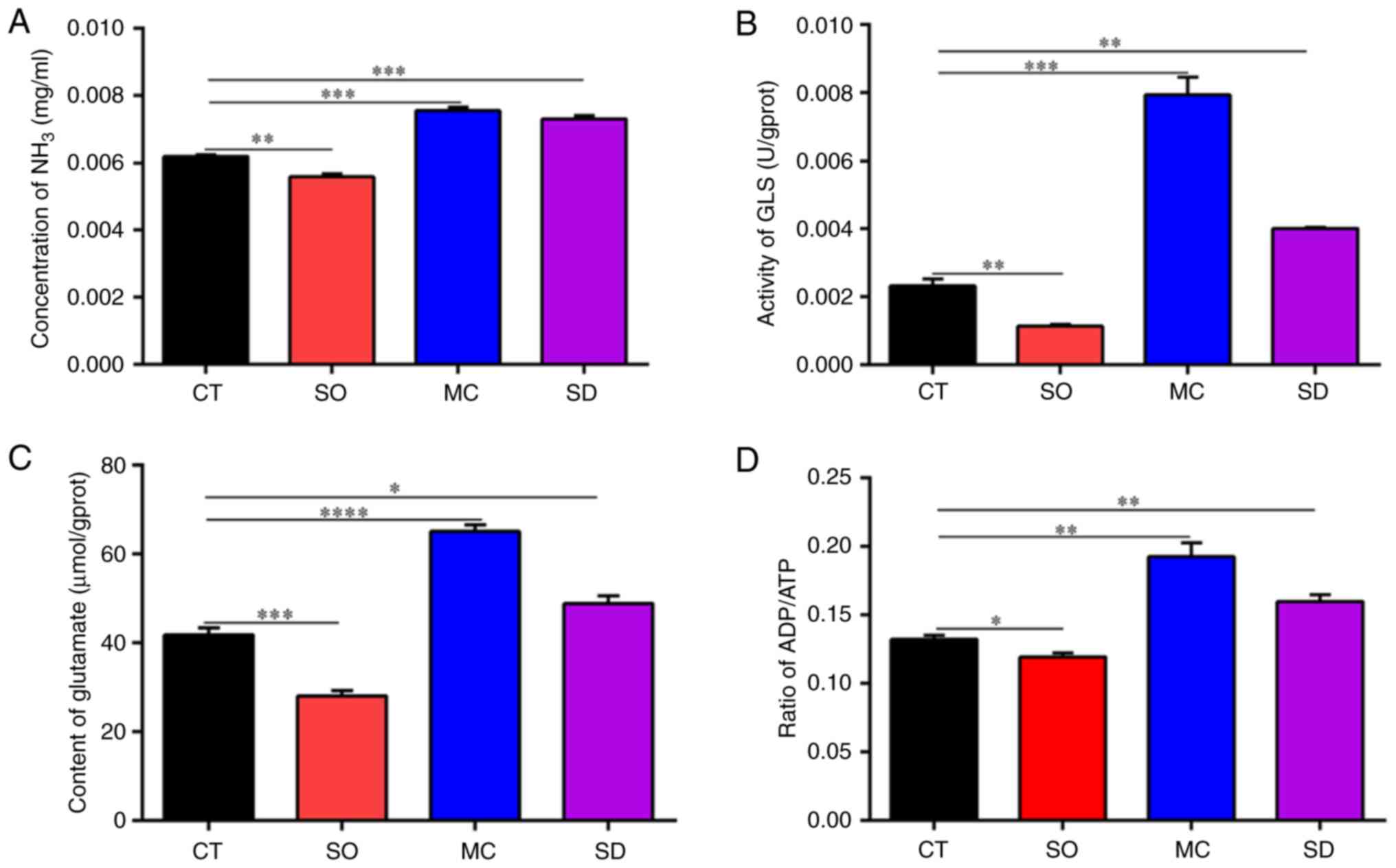 | Figure 6Effect of SIRT5 on ammonia release in
MAC-T cells. (A) Concentration of NH3 in cells, (B) activity of GLS
in cells, (C) content of glutamate in cells and (D) ratio of
ADP/ATP. In the CT group, the MAC-T cells were cultured with basal
medium for 12 h. In the SO group, the SIRT5+/+ cells
were cultured with basal medium for 12 h. In the SD group, the
SIRT5-/- cells were cultured with basal medium for 12 h.
In the MC group, MAC-T cells were incubated with 20 µM MC3482 for
12 h. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 vs. CT group.
SIRT5, sirtuin 5; GLS, glutaminase. |
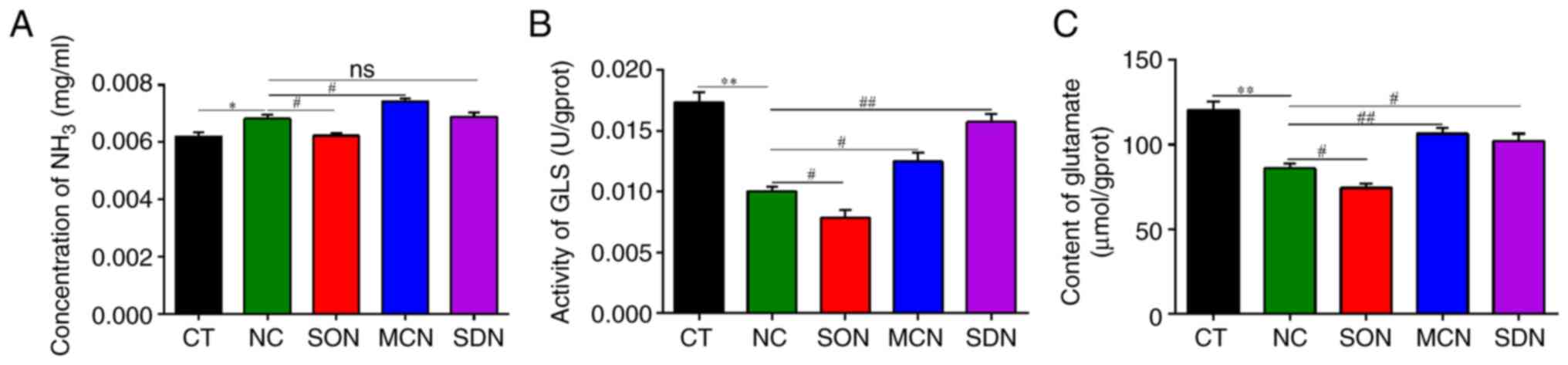 | Figure 7Effect of SIRT5 on ammonia release in
MAC-T cells treated with NH4Cl. (A) Concentration of NH3 in cells,
(B) activity of GLS in cells and (C) content of glutamate in cells.
In the CT group, the MAC-T cells were cultured with basal medium
for 12 h. In the NC, SON and SDN groups, the MAC-T,
SIRT5+/+ and SIRT5-/- cells were exposed to 4
mM NH4Cl for 12 h, respectively. In the MCN group, MAC-T cells were
incubated with MC3482 (20 µM) for 30 min, and then the cells were
treated with 4 mM NH4Cl for 12 h. *P<0.05,
**P<0.01 vs. CT group, #P<0.05,
##P<0.01 vs. NC group. ns, not significant; SIRT5,
sirtuin 5; GLS, glutaminase. |
SIRT5 regulates the activity of
GLS
Glutamine is a non-essential amino acid, which acts
as a precursor for glutamate and NH3, and has a critical
role in various tissues (38,39).
Glutamine is transformed into glutamate and NH3 by the
enzyme GLS in the mitochondria (40,41).
Of note, a previous study reported that SIRT5 can desuccinylate GLS
to regulate NH3 production in MDA-MB-231 and C2C12 cell
lines, and further found that SIRT5 co-immunoprecipitates with GLS
(25). The enzyme GLS has a
mitochondrial localization and its functionally relevant structural
domain was oriented towards the mitochondrial matrix, while SIRT5
was also located in the mitochondrial matrix (40). Hence, in the present study, the
activity of GLS in MAC-T cells was measured to investigate whether
SIRT5 regulates the activity of GLS to control NH3
production. To verify this point, MAC-T cells were either treated
or untreated with MC3482, and the activity of GLS was then
detected. Similarly, an increase in GLS levels in MC3482-treated
MAC-T cells was observed (Fig.
6B). To demonstrate that SIRT5 may regulate GLS activity, the
glutamate content in MAC-T cells was detected (Fig. 6B). Fig. 6C shows that glutamate levels were
declined in SIRT5+/+ cells and enhanced in
SIRT5-/- and MC3482-treated MAC-T cells. The above
results indicated that SIRT5 inhibited the metabolism of glutamine
in MAC-T cells, further reducing NH3 release in cells.
It was found that a decrease in the content of NH3 and
glutamate, as well as GLS activity, were present in
SIRT5+/+ treated with NH4Cl, while a
significant increase was observed in SIRT5-/- treated
with NH4Cl (Fig. 7).
Furthermore, the content of glutamate and GLS activity also
increased significantly in MAC-T cells co-treated with MC3482 and
NH4Cl (Fig. 7). This
indicated that SIRT5 inhibited the metabolism of glutamine in MAC-T
cells. In addition, the ratio of ADP/ATP was similar in
SIRT5-/- and SIRT5 inhibitor-treated cells, and
alleviation of ATP levels was more obvious in SIRT5-/-
and SIRT5 inhibitor-treated cells (Fig. 6D). By contrast, ATP levels in
SIRT5+/+ were enhanced (Fig. 6D). This indicated that the changes
of cell energy may be involved in the NH3 production
regulated by SIRT5.
SIRT5 regulates NH3-induced
apoptosis and autophagy
Previous research by our group indicated that
NH3 regulates the apoptotic and autophagic activity in
bovine mammary epithelial cells via PI3K/Akt/mTOR signaling
(11). As confirmed by the results
of the present study, SIRT5 regulates the activity of GLS, and
NH3 originated from glutaminolysis enhanced apoptosis
and autophagy (11,42). It was further explored whether
NH3 production in SIRT5+/+,
SIRT5-/- or MC3482-treated MAC-T cells, and combined
with NH4Cl treatment as the donor of NH3, is
able to induce apoptosis and autophagy. NH3-stimulated
autophagy is characteristic of MC3482-treated or
SIRT5-/- cells with NH4Cl co-treatment, since
LC3 and Beclin1 expression were significantly elevated, while in
SIRT5+/+ cells, the opposite result was obtained, as LC3
and Beclin1 levels were significantly decreased with
NH4Cl treatment in Fig.
8 (A, B, D, E and M). The expression of SQSTM1/p62 as a marker
of autophagosomal cargo lysosomal degradation was also assessed.
Degradation of SQSTM1/p62 was hindered in SIRT5-overexpressing
cells, whereas it was not impaired in MC3482-treated or
SIRT5-/- cells with NH4Cl co-treatment
(Fig. 8C, F and M).
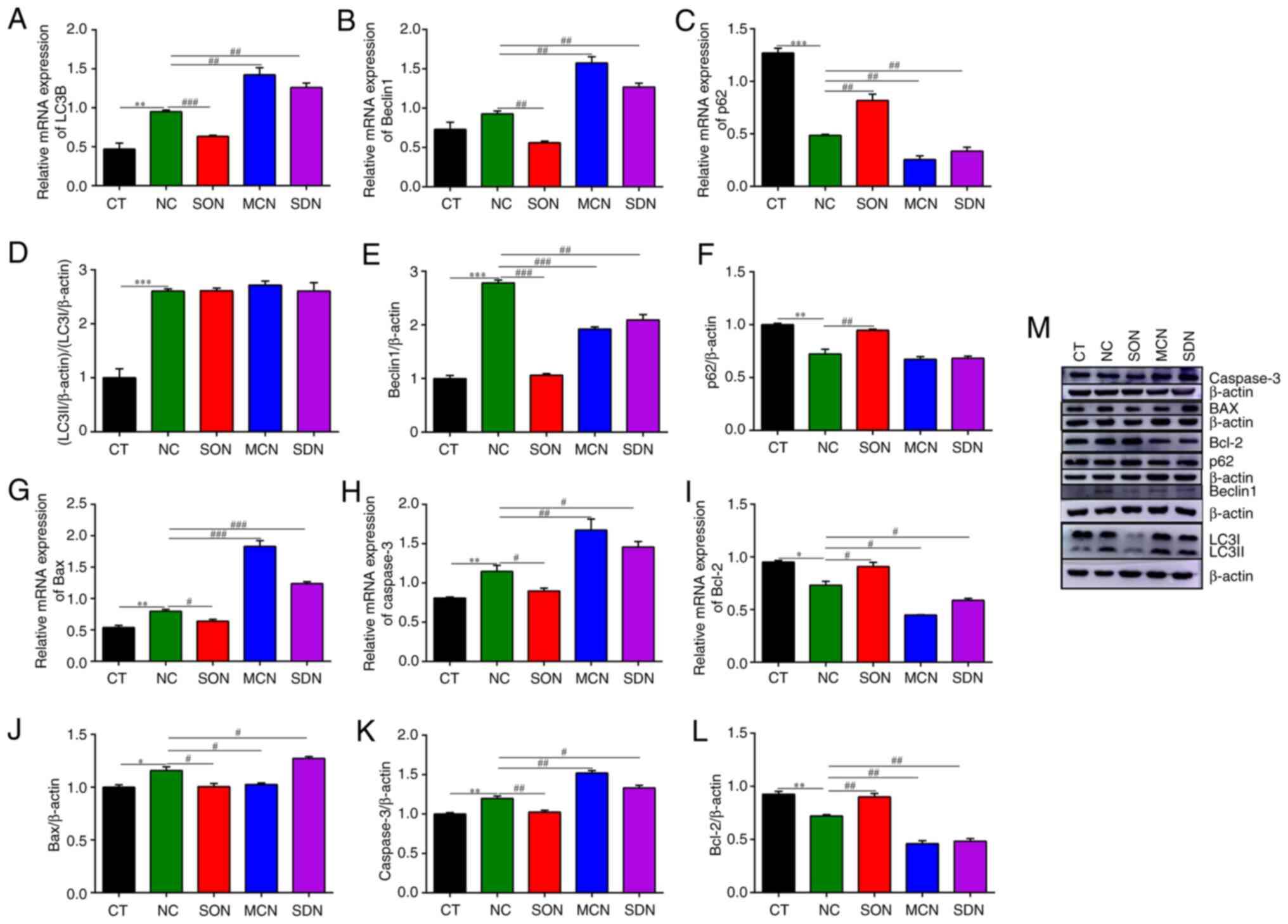 | Figure 8Effect of SIRT5 on autophagic and
apoptotic marker expression in MAC-T cells induced by NH4Cl.
Reverse transcription-quantitative PCR results: (A) LC3B, (B)
Beclin1, (C) p62, (G) Bax, (H) Caspase-3 and (I) Bcl-2. Western
blot results: (D and M) LC3II/I, (E and M) Beclin1, (F and M) p62,
(J and M) Bax, (K and M) Caspase-3 and (L and M) Bcl-2. In the CT
group, the MAC-T cells were cultured with basal medium for 12 h. In
the NC, SON and SDN groups, the MAC-T, SIRT5+/+ and
SIRT5-/- cells were exposed to 4 mM NH4Cl for 12 h,
respectively. In the MCN group, MAC-T cells were incubated with
MC3482 (20 µM) for 30 min, and then the cells were treated with 4
mM NH4Cl for 12 h. *P<0.05, **P<0.01,
***P<0.001 vs. CT group, #P<0.05,
##P<0.01, ###P<0.0001 vs. NC group.
LC3B, light chain 3β; SIRT5, sirtuin 5. |
In MAC-T cells, the number of cells with positive
fluorescence staining in the SIRT5+/+ and
NH4Cl co-treatment group (SON group) was reduced, which
significantly reduced the number of cells with positive
fluorescence staining in the MC3482 and NH4Cl
co-treatment group (MCN group), while the SIRT5-/- and
NH4Cl co-treatment group (SDN group) exhibited a
significant enhancement of the apoptosis rate compared with the
NH4Cl group (Fig. 9).
Subsequently, apoptotic marker expression was also determined.
Compared with the NH4Cl group, in the cells of the
SIRT5+/+ and NH4Cl co-treatment group (SON
group), the mRNA and protein expression of Bax and Caspase-3
decreased obviously (Fig. 8G,
H, J, K and
M), while the levels of Bcl-2 mRNA
and protein increased significantly in the SON group (Fig. 8I, L and M).
Of note, the protein levels of Bax and Caspase-3 in the MC3482 and
NH4Cl co-treatment group (MCN group) and
SIRT5-/- and NH4Cl co-treatment group (SDN
group) were significantly increased, while Bcl-2 expression was
significantly decreased (Fig.
8G-M). The expression of apoptosis-related factors also
confirmed that SIRT5 inhibited the occurrence of MAC-T-cell
apoptosis induced by NH4Cl.
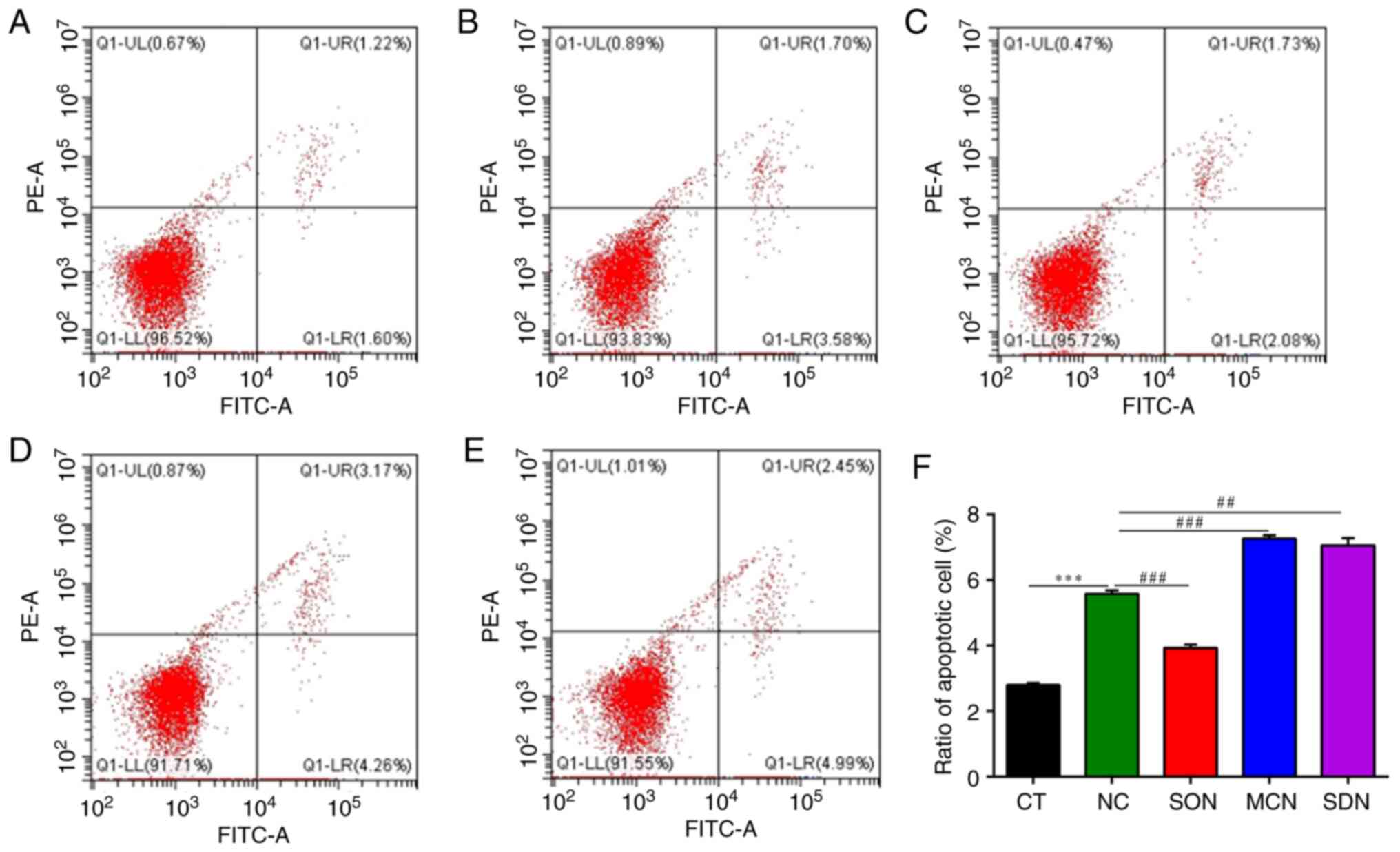 | Figure 9Flow cytometric detection of the
apoptotic ratio of MAC-T cells induced by NH4Cl. (A-E) Flow
cytometry dot plots of (A) CT group, (B) NC group, (C) SON group,
(D) MCN group and (E) SDN group). (F) Quantified ratio of apoptotic
cells (%). ***P<0.001 vs. CT group,
##P<0.01, ###P<0.0001 vs. NC group. In
the CT group, the MAC-T cells were cultured with basal medium for
12 h. In the NC, SON and SDN groups, the MAC-T, SIRT5+/+
and SIRT5-/- cells were exposed to 4 mM NH4Cl
for 12 h, respectively. In the MCN group, MAC-T cells were
incubated with MC3482 (20 µM) for 30 min, and then the cells were
treated with 4 mM NH4Cl for 12 h. SIRT5, sirtuin 5. |
PI3K/Akt/mTOR signaling is involved in
SIRT5-regulated autophagy and apoptosis induced by
NH3
PI3K/Akt/mTOR signaling participates in controlling
cell proliferation, growth, differentiation, apoptosis, autophagy
and metabolism (26,27). SIRT5 reportedly inhibited cancer
cell growth, invasion and migration, as well as modulated inherent
and acquired immunity via the PI3K/Akt pathway (28). The mTOR signal plays a critical
role in regulating autophagy (29). The mTOR signal promotes glutamine
uptake through GLS and accelerates the release of NH3
(30,31). A study demonstrated that
NH3 relies on PI3K signaling to regulate mTOR activity,
further affecting apoptosis and autophagy in mammary epithelial
cells (11). In the present study,
LY294002 (PI3K inhibitor) or Rapamycin (mTOR inhibitor) were used
to treat SIRT5+/+ cells, and the apoptotic and
autophagic markers were detected to clarify the mechanism of SIRT5
regulating apoptosis and autophagy induced by NH3 in
MAC-T cells. As indicated in Fig.
9, the number of cells with positive fluorescence staining in
the SIRT5+/+ and NH4Cl co-treatment group
(SON group) was obviously lower than that in the NH4Cl
group (NC group), showing that SIRT5 expression significantly
decreased the rate of apoptosis. Of note, the addition of LY294002
or Rapamycin further decreased cell apoptosis, and the number of
cells with positive fluorescence staining of apoptotic cells
markedly declined and the apoptosis rate was notably reduced
(Fig. 10). At the same time,
LY294002 or Rapamycin treatment alleviated the increase in the
expression of Bax and Caspase-3 caused by the co-treatment of
SIRT5+/+ and NH4Cl, and enhanced the level of
Bcl-2 mRNA and protein (Fig. 11).
Similarly, in comparison with the SIRT5+/+ and
NH4Cl co-treatment group, LY294002 or Rapamycin
treatment led to a significant decline in the mRNA and protein
expression of autophagy factor Beclin1, and p62 expression was
obviously enhanced (Fig. 11).
Unexpectedly, blocking the PI3K signal led to a significant
increase in LC3 mRNA and protein expression, while blocking the
mTOR signal led to the opposite effect on LC3 mRNA and protein
expression (Fig. 11). These
results indicated that PI3K/Akt/mTOR signaling has a certain role
in SIRT5 regulating autophagy and apoptosis stimulated by
NH3.
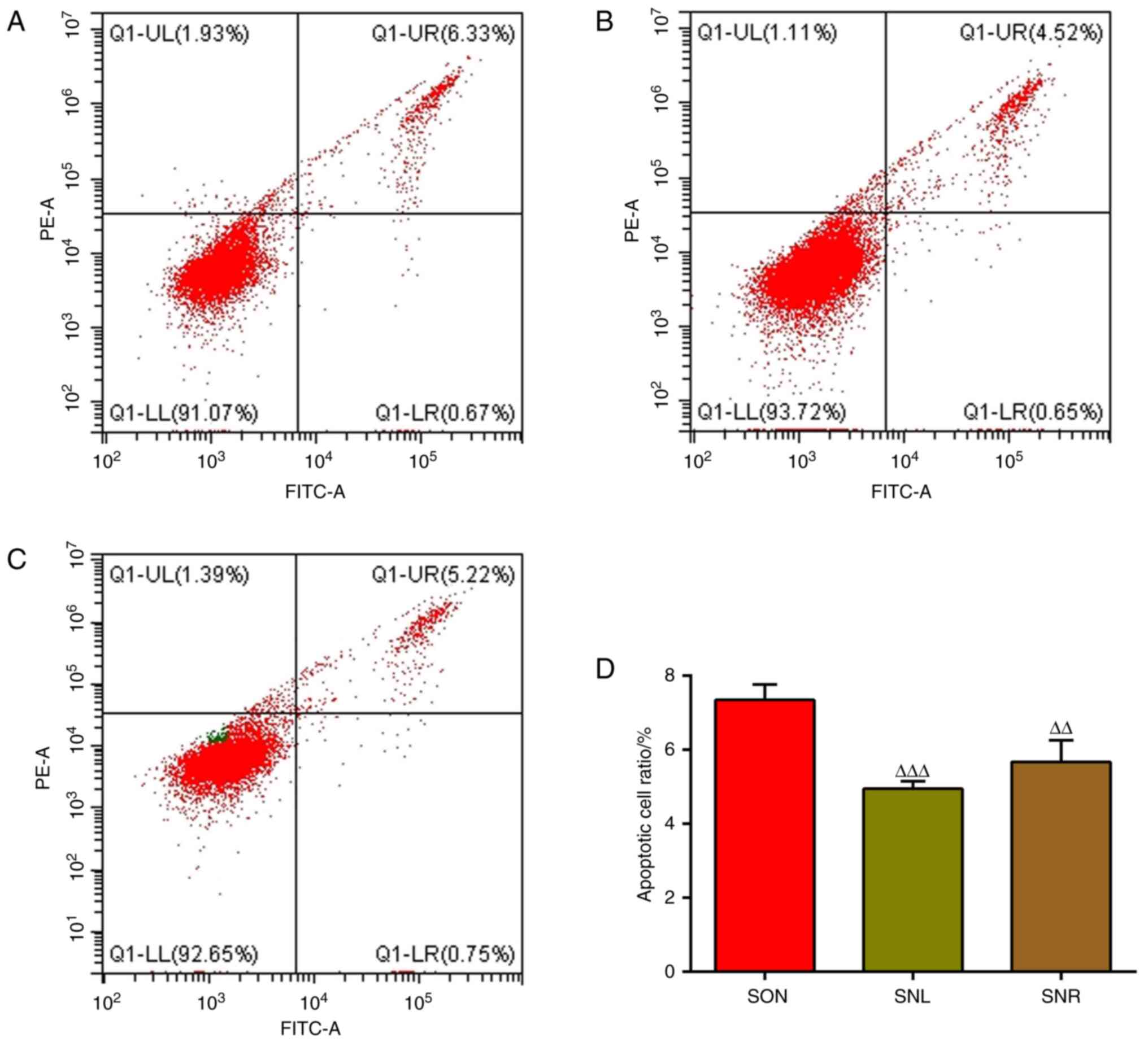
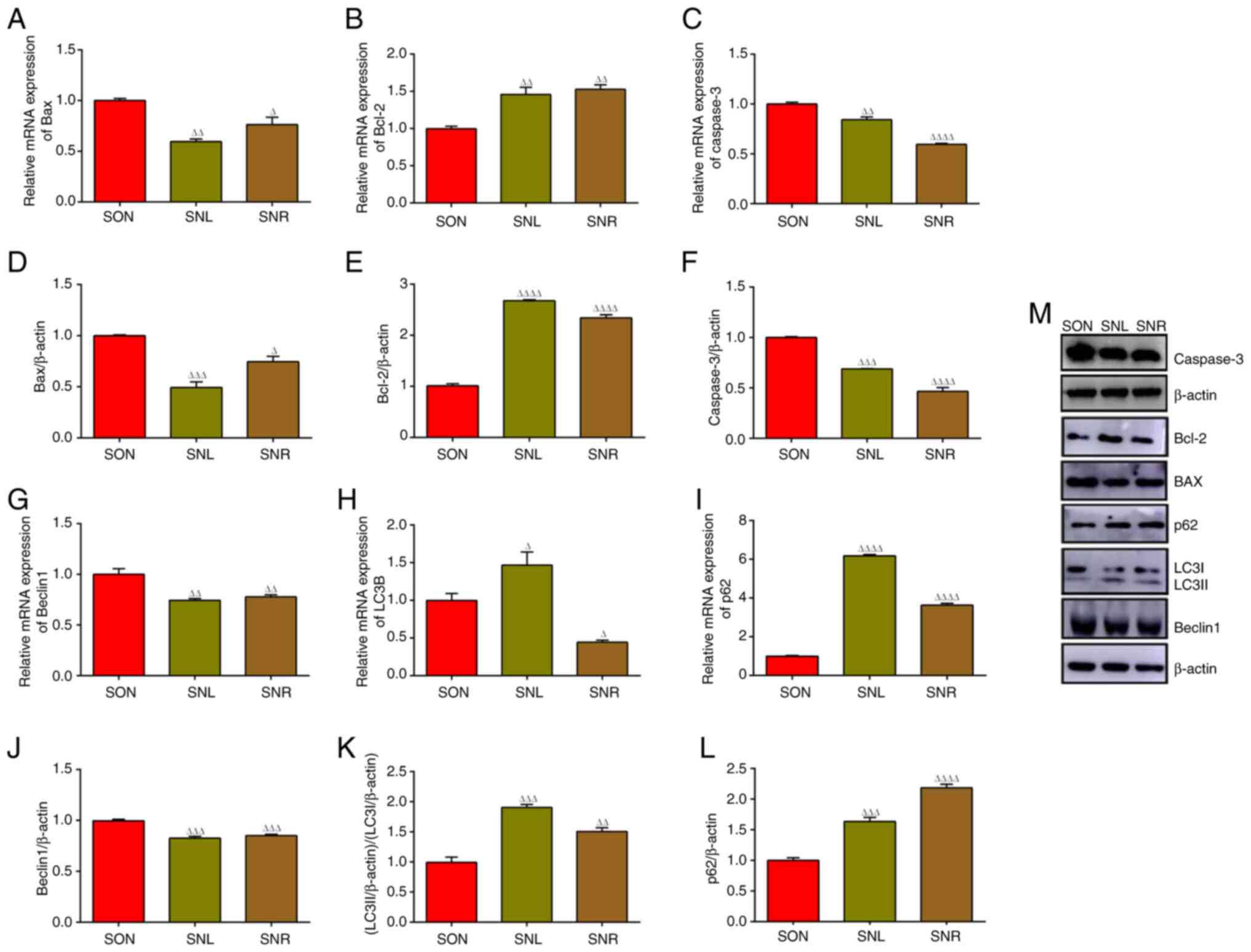 | Figure 11Apoptotic and autophagic marker
expression in SIRT5+/+ cells treated with PI3K or mTOR
inhibitor and NH4Cl. Reverse transcription-quantitative PCR
results: (A) Bax, (B) Bcl-2, (C) Caspase-3, (G) Beclin1, (H) LC3B
and (I) p62. Western blotting results: (D and M) Bax, (E and M)
Bcl-2, (F and M) Caspase-3, (J and M) Beclin1, (K and M) LC3II/I
and (L and M) p62. In the SON group, the SIRT5+/+ cells
were exposed to 4 mM NH4Cl for 12 h. In the SNL and SNR groups,
SIRT5+/+ cells were incubated with LY294002 (20 mM) or
Rapamycin (10 µM) for 30 min, respectively, and then the cells were
treated with 4 mM NH4Cl for 12 h. ∆P<0.05,
∆∆P<0.01, ∆∆∆P<0.001,
∆∆∆∆P<0.0001 vs. SON group. SIRT5, sirtuin 5; LC3B,
light chain 3β. |
Discussion
NH3, originating mainly from the
deamination of amino acids and glutamine, is one of the important
toxic components in blood and tissues, and may reduce the
performance, immunity and antioxidant capacity, and affect bovine
health (2,43). The NH3 released into the
environment may damage sensitive ecosystems, causing acidification
and decreasing species diversity. Numerous experiments have shown
that elevated NH3 levels in vivo or in culture
media induce cell apoptosis and oxidative stress (10,43).
According to a previous report, 2.5 mM NH3 can induce
autophagy in mouse skeletal muscle cells (44). Of note, NH3 originated
from glutamine catalyzed by GLS also strongly induces autophagy in
cancer cells (45,46). In a previous study, it was found
that NH3 stimulated autophagy and apoptosis in bovine
mammary epithelial cells (11).
SIRT5 has multiple enzymatic activities and is
involved in various processes, such as apoptosis, autophagy and
metabolism. Furthermore, SIRT5 regulates the production of
NH3 in human breast cancer cells and mouse myoblast
cells through mediating glutamine metabolism (25). Furthermore, SIRT5 can regulate GLS
activity in HepG2 cells, which further controls glutamine
metabolism to produce NH3 (47). In the present study, the
NH3 and glutamate content, as well as GLS activity were
detected in MAC-T cells with SIRT5 overexpression or knockdown, or
treated by MC3482, to estimate the role of SIRT5 in NH3
production of bovine mammary epithelial cells. The results showed
that SIRT5 was able to reduce NH3 production in MAC-T
cells, decrease the content of glutamate and the activity of GLS,
indicating that SIRT5 inhibited the metabolism of glutamine, and
further reduced the release of NH3 in MAC-T cells. Of
note, the expression of SIRT5 in the heart and cardiomyocytes of
energy-deficient mice and ATP production in mitochondria decreased,
while the AMP/ATP ratio increased, suggesting that SIRT5 has a
critical role in maintaining energy balance (48). Compared to MAC-T cells, the ratio
of ADP/ATP in SIRT5+/+ cells decreased, while the ratio
of ADP/ATP increased in SIRT5-/- cells and
MC3482-treated MAC-T cells. These results suggested that
NH3 release regulated by SIRT5 was related to a change
in energy balance.
Mitochondria are an important site of energy
metabolism in the body. The extracellular protein Bcl-2 family
death repressor, pro-apoptotic member Bax is important in the
regulation of apoptosis (49). The
Bcl-2/Bax ratio gradually decreases in cell apoptosis (50). In addition, cytochrome C, which
initiates the mitochondrial apoptotic pathway, was excreted from
mitochondria, further activating Caspase-3(51). Studies have shown that a high
Bax/Bcl-2 ratio was related to mitochondrial damage (52). Furthermore, NH3 may
stimulate mitochondrial damage and apoptosis in MAC-T cells, as
evidenced by the elevated Bax/Bcl-2 ratio in the
NH3-treated group (53). Overexpression of SIRT5 can repair
mitochondrial dysfunction, reduce Bcl-2 accumulation and slow down
carcinogenic cell growth (54). In
particular, SIRT5 inhibits the level of Caspase-3 lysis and the
level of cytochrome C, further reducing cell apoptosis and
mitochondrial damage (55).
Furthermore, SIRT5 upregulates the level of Bcl-2 and Bcl-XL, and
downregulates the content of Caspase-3, Caspase-7 and Bax during
fatty acid-induced pancreatic β-cell apoptosis, exerting an
anti-apoptotic effect (23). In
the present experiments, anti-apoptotic Bcl-2 levels increased in
SIRT5+/+ cells, while expression of the pro-apoptotic
proteins Bax and Caspase-3 declined. At the same time,
SIRT5-/- or inhibitor MC3482 treatment had the opposite
effects. The above results verified that SIRT5 alleviated apoptosis
occurrence in MAC-T cells. Furthermore, fluorescence observation of
cell apoptosis again confirmed this point. In addition, it was also
found that SIRT5 overexpression notably reduced the apoptosis
factors Bax and Caspase-3 induced by NH4Cl at the mRNA
and protein level, and enhanced Bcl-2 levels. The observations in
the SIRT5 inhibitor and knockdown groups were just the opposite.
The same results were also observed by fluorescence analysis of
cell apoptosis. Taken together, the results demonstrated that SIRT5
reduced the apoptosis occurrence of MAC-T cells induced by
NH4Cl.
Autophagy is important for maintaining the basic
functions in cells, since it cleans up redundant protein materials
and accumulated harmful material, which are aggregated in
autophagosomes and degraded in lysosomes (56,57).
Numerous documents have clarified that SIRTs have key roles in
cancer and metastases processes by mediating inflammation,
angiogenesis and epithelial-to-mesenchymal transition progression
(58-60).
SIRT5 mainly induces late autophagy and promotes cell proliferation
(55). The NH3
production by glutamine deamination supports both basic autophagy
and cell proliferation through autophagy or paracrine modes of
action as an autophagy flux medium (42). LC3 participates in autophagosome
formation and is often used as a marker factor for autophagy
(61). p62 is also called SQSTM1,
which is an ubiquitinated protein that binds to LC3II in
autophagosomes. When autophagosomes and lysosomes are combined,
SQSTM1 is continuously degraded under the enzymatic hydrolysis of
lysosomes. Therefore, the level of autophagy activity is opposite
to the expression of p62 (62,63).
Beclin1 is an important member of the bilayer membrane structure of
autophagosomes after the occurrence of autophagy. Hence, an
increase in Beclin1 is the key initial stage in the development of
autophagy (64). In the present
study, it was found that SIRT5+/+ promoted the
expression of p62 and reduced the expression of LC3B and Beclin1,
while SIRT5-/- or inhibitor treatment produced the
opposite result. By observing autophagic vesicles, there were far
fewer autophagic vesicles in the SO group (SIRT5+/+
cells). More autophagic vesicles were found in the SD
(SIRT5-/- cells) and the MC group (inhibitor treatment).
The above results indicated that SIRT5 was able to inhibit
autophagy in MAC-T cells. It was also identified that SIRT5
overexpression significantly reduced the expression of autophagy
factors LC3B and Beclin1 induced by NH4Cl at the mRNA
and protein levels, and upregulated the expression of p62. Adding
inhibitors and SIRT5 knockdown had the opposite results. These
findings indicated that SIRT5 inhibited the autophagy activity
induced by NH4Cl in MAC-T cells.
The PI3K/Akt/mTOR signaling pathway was reported to
be closely related to autophagy caused by poisoning (65). Of note, NH3 can depend
on PI3K signaling to regulate mTOR activity (66). NH3 induces cardiac
toxicity in pigs and activates autophagy via PI3K/Akt/mTOR
signaling (67).
NH3-mediated nephrotoxicity in pigs inhibited the
PI3K/Akt/mTOR pathway and enhanced the secretion of inflammatory
cytokines to induce autophagy and inflammation (68). Excess NH3 caused energy
metabolism disorder to induce mitochondrial and autophagic damage
through the AMPK/mTOR/unc-51 like autophagy activating kinase
1-Beclin1 pathway in chicken livers (69). NH3 exposure inhibited
the Akt/mTOR pathway to accelerate autophagy through oxidative
stress-mediated inflammation in the porcine hypothalamus (70). Other reports have demonstrated that
PI3K/Akt/mTOR signaling was involved in breast tumor cell apoptosis
and autophagy induced by the anticancer agent (71). A previous study by our group
demonstrated that LY294002 or Rapamycin co-treatment with
NH4Cl effectively inhibited apoptosis (11). Similarly, the results of the
present study indicated that LY294002 or Rapamycin and
NH4Cl produced an antagonistic effect, reducing cell
autophagy. Taken together, NH4Cl and LY294002 or
Rapamycin have a synergistic antagonistic effect, inhibiting the
autophagy reaction of MAC-T cells. Furthermore, it has been
demonstrated that NH3 may regulate the apoptosis and
autophagic response of bovine mammary epithelial cells through the
PI3K/Akt/mTOR signaling pathway (11). It is established that SIRT5 can
promote autophagy in gastric cancer cells via AMPK/mTOR signaling
pathway (72). However, the
signaling pathways by which SIRT5 exerts its functions remain to be
elucidated.
For the sake of clarifying the mechanism of SIRT5
regulating the apoptosis and autophagy induced by NH3 in
MAC-T cells, NH4Cl was used to treat cells, combined
with treatment by PI3K inhibitor LY294002 or mTOR inhibitor
Rapamycin, respectively. The Annexin V FITC/PI results revealed
that NH4Cl and SIRT5 knockdown or MC3482 co-treatment
significantly increased the apoptotic activity in MAC-T cells, as a
decrease in the apoptotic response was observed in the SON group,
compared with that in the NC group. RT-qPCR and western blot
detection of related apoptotic factors indicated that after the
addition of inhibitors, SIRT5 overexpression in MAC-T cells led to
a decrease in the expression of NH4Cl-induced apoptosis
factors Bax and Caspase-3 and autophagy factors LC3B and Beclin1,
and an increase in the expression of apoptosis factor Bcl-2 and
autophagy factor p62. Of note, with the addition of LY294002 or
Rapamycin, the number of cells with positive fluorescence staining
markedly declined and the apoptosis rate, as well as Bax and
Caspase-3 expression, were notably reduced, while the mRNA and
protein levels of Bcl-2 were enhanced. Similarly, LY294002 or
Rapamycin treatment led to a significant reduction of the mRNA and
protein expression of autophagy factor Beclin1 and obviously
enhanced p62 expression. These results suggested that the
PI3K/Akt/mTOR signals have a certain role in SIRT5 regulating the
autophagy and apoptosis stimulated by NH3. However, when
blocking PI3K or the mTOR signal, LC3B mRNA expression and the
LC3II/I ratio were inconsistent with the change of Beclin1.
Unexpectedly, blocking the PI3K signal led to a significant
increase in LC3 mRNA and protein expression, while blocking the
mTOR signal led to the opposite effect on LC3 mRNA and protein
expression. This raises a new question to further explore.
In addition, SIRT5 may regulate metabolism,
autophagy, apoptosis and energy balance. SIRT5 can desuccinylate
GLS to control NH3 release and further regulate
autophagy activity (25). The
above results suggested that the PI3K/Akt/mTOR signals may mediate
the effect of SIRT5 on the apoptosis and autophagy induced by
NH3 in MAC-T cells. Hence, a model of SIRT5 regulating
the apoptosis and autophagy of MAC-T cells induced by
NH3 was proposed, as shown in Fig. 12. The mTOR signal promotes GLS
activity to increase glutamine uptake and accelerates the release
of NH3 (30,31). NH3 relies on PI3K
signaling to regulate mTOR activity, further affecting apoptosis
and autophagy in mammary epithelial cells (11). In the present study, SIRT5
inhibited GLS activity, reduced NH3 release and further
alleviated the apoptosis and autophagy induced by NH3 in
MAC-T cells. Furthermore, SIRT5 regulated the apoptosis and
autophagy induced by NH3 in MAC-T cells, depending on
the PI3K/Akt/mTOR signals.
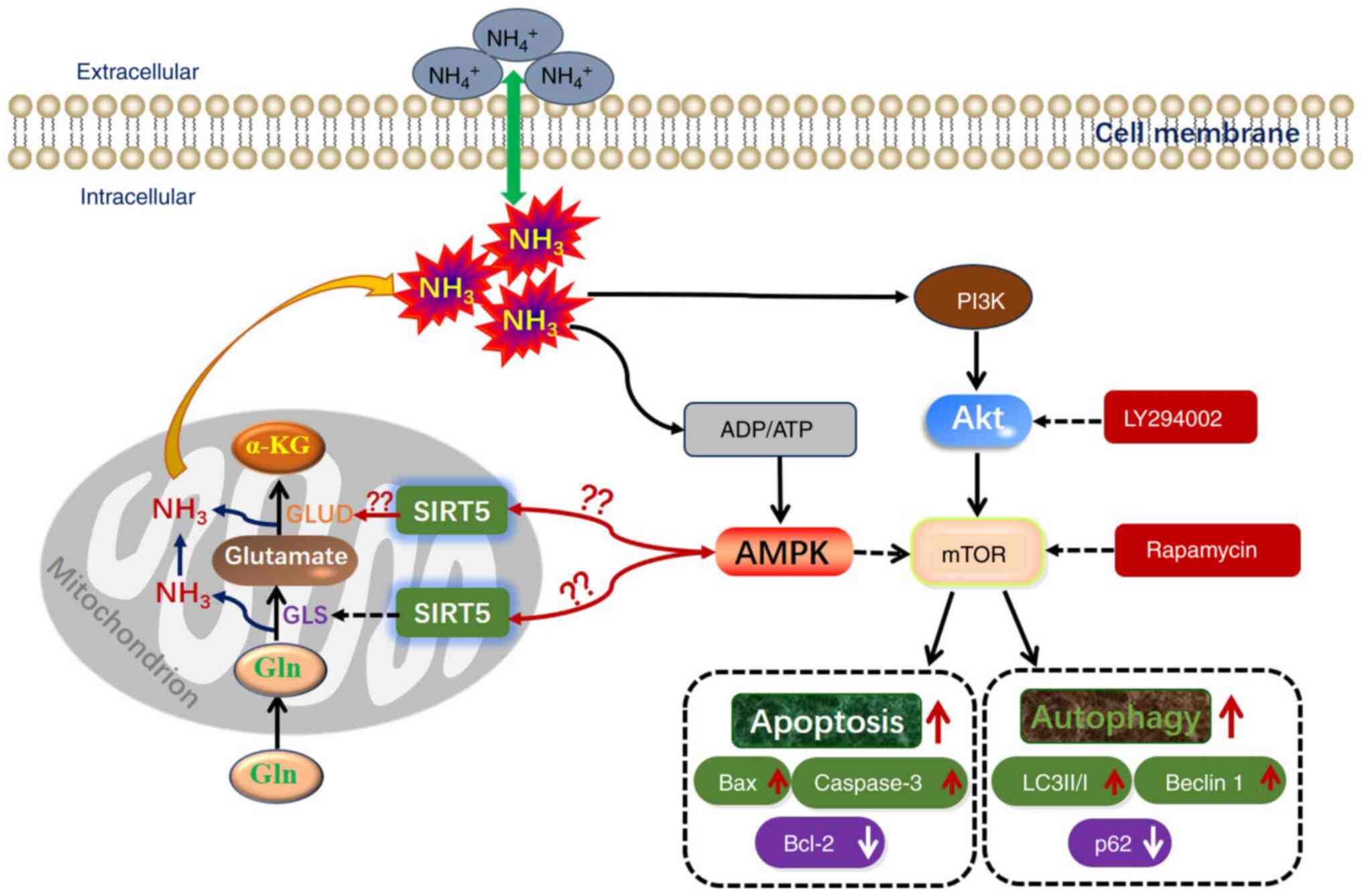 | Figure 12A model of SIRT5 regulating the
apoptosis and autophagy of MAC-T cells treated with NH4Cl. The mTOR
signal promotes GLS activity to increase glutamine uptake and
accelerates the release of NH3 (30,31).
Ammonia relies on the PI3K signaling to regulate mTOR activity,
further affecting the apoptosis and autophagy in mammary epithelial
cells (11). In the present study,
SIRT5 inhibited GLS activity, reduced ammonia release and further
alleviated the apoptosis and autophagy induced by ammonia in MAC-T
cells. Furthermore, SIRT5 regulated the apoptosis and autophagy
induced by ammonia in MAC-T cell, depending on PI3K/Akt/mTOR
signaling. SIRT5, sirtuin 5; LC3B, light chain 3β; GLS,
glutaminase; KG, ketoglutarate; Gln, glutamin; GLUD, glutamate
dehydrogenase. |
AMPK has a key role in the upregulation of
catabolism and inactivation of anabolism, as an energy sensor in
cells. Under various physiological and pathological conditions,
AMPK can be phosphorylated by an upstream kinase and bind to AMP or
ADP rather than ATP, leading to its activation. Activated AMPK can
inhibit mTOR activity to further enhance autophagy (73). Of note, the present study indicated
that SIRT5 was able to reduce the ADP/ATP ratio in cells. The above
results provide us with new ideas: i) Whether SIRT5 alters ADP/ATP
ratio to regulate NH3 release, and further to regulate
cell apoptosis and autophagy via the AMPK/mTOR signaling pathway;
ii) whether SIRT5 regulates GLS activity to participate in
NH3 release through post-translational modifications;
iii) whether SIRT5 regulates glutamate dehydrogenase activity to
affect intracellular NH3 production; and iv) whether
SIRT5 regulates glutamine metabolism based on the NH3
concentration to balance cell apoptosis and autophagic activity.
The effect of ammonia on the physiological functions in dairy cows
will be further explored thoroughly.
Supplementary Material
Fluorescence observation in MAC-T
cells. (A) Non-transfected MAC-T cells, (B) MAC-T cells transfected
with pEGFP-N1 vector and (C) MAC-T cells transfected with
pEGFP-SIRT5 vector. Magnification, x40. pEGFP, plasmid expressing
enhanced green fluorescence protein (control); SIRT5, sirtuin
5.
Acknowledgements
The authors would like to thank Dr Sun Ning, from
the Core Facility and Technical Support, Electron Microscope Center
of Henan University of Traditional Chinese Medicine (Zhengzhou,
China) for providing help with TEM.
Funding
Funding: This work was supported by the National Natural Science
Fund (grant no. 32172809). The funding agency had no role in the
study design, data collection, interpretation or the decision to
submit the work for publication.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HPL and YYW conceived and designed the experiments.
JHH, LPF, HLY and SKG performed the experiments. JRD and GYL
cultured the cells. XYZ and LYL treated the cells. KZ and SG drew
the figures and designed the primers. GMZ and LQH performed data
analyses. JHH, LPF, HPL and YYW interpreted the data and wrote the
manuscript. All authors have read and approved the final
manuscript. JHH and YYW confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Meng Z, Lin W, Zhang R, Han Z and Jia X:
Summertime ambient ammonia and its effects on ammonium aerosol in
urban Beijing, China. Sci Total Environ. 579:1521–1530.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Phillips CJ, Pines MK, Latter M, Muller T,
Petherick JC, Norman ST and Gaughan JB: The physiological and
behavioral responses of steers to gaseous ammonia in simulated
long-distance transport by ship. J Anim Sci. 88:3579–3589.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Spinelli JB, Yoon H, Ringel AE, Jeanfavre
S, Clish CB and Haigis MC: Metabolic recycling of ammonia via
glutamate dehydrogenase supports breast cancer biomass. Science.
358:941–946. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kappler M, Pabst U, Rot S, Taubert H,
Wichmann H, Schubert J, Bache M, Weinholdt C, Immel UD, Grosse I,
et al: Normoxic accumulation of HIF1α is associated with
glutaminolysis. Clin Oral Investig. 21:211–224. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Murrow L and Debnath J: Autophagy as a
stress-response and quality-control mechanism: Implications for
cell injury and human disease. Annu Rev Pathol. 8:105–137.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nixon RA: The role of autophagy in
neurodegenerative disease. Nat Med. 19:983–997. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cruz HJ, Freitas CM, Alves PM, Moreira JL
and Carrondo MJ: Effects of ammonia and lactate on growth,
metabolism, and productivity of BHK cells. Enzyme Microb Technol.
27:43–52. 2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen XY, Shao JZ, Xiang LX and Liu XM:
Involvement of apoptosis in malathion-induced cytotoxicity in a
grass carp (Ctenopharyngodon idellus) cell line. Comp Biochem
Physiol C Toxicol Pharmacol. 142:36–45. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gottlieb E, Armour SM, Harris MH and
Thompson CB: Mitochondrial membrane potential regulates matrix
configuration and cytochrome c release during apoptosis. Cell Death
Differ. 10:709–717. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang F, Chen S, Jiang Y, Zhao Y, Sun L,
Zheng B, Chen L, Liu Z, Zheng X, Yi K, et al: Effects of ammonia on
apoptosis and oxidative stress in bovine mammary epithelial cells.
Mutagenesis. 33:291–299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Feng L, Liao H, Liu J, Xu C, Zhong K, Zhu
H, Guo S, Guo Y, Han L, Li H and Wang Y: Inhibition of
PI3K/Akt/mTOR pathway by ammonium chloride induced apoptosis and
autophagy in MAC-T cell. Res Vet Sci. 136:622–630. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu S, Dong Z, Ke X, Hou J, Zhao E, Zhang
K, Wang F, Yang L, Xiang Z and Cui H: The roles of sirtuins family
in cell metabolism during tumor development. Semin Cancer Biol.
57:59–71. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nishida Y, Rardin MJ, Carrico C, He W,
Sahu AK, Gut P, Najjar R, Fitch M, Hellerstein M, Gibson BW and
Verdin E: SIRT5 regulates both cytosolic and mitochondrial protein
malonylation with glycolysis as a major target. Mol Cell.
59:321–332. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Park J, Chen Y, Tishkoff DX, Peng C, Tan
M, Dai L, Xie Z, Zhang Y, Zwaans BM, Skinner ME, et al:
SIRT5-mediated lysine desuccinylation impacts diverse metabolic
pathways. Mol Cell. 50:919–930. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tan M, Peng C, Anderson KA, Chhoy P, Xie
Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, et al: Lysine
glutarylation is a protein posttranslational modification regulated
by SIRT5. Cell Metab. 19:605–617. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kumar S and Lombard DB: Functions of the
sirtuin deacylase SIRT5 in normal physiology and pathobiology. Crit
Rev Biochem Mol Biol. 53:311–334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fabbrizi E, Fiorentino F, Carafa V,
Altucci L, Mai A and Rotili D: Emerging roles of SIRT5 in
metabolism, cancer, and SARS-CoV-2 infection. Cells.
12(852)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rardin MJ, He W, Nishida Y, Newman JC,
Carrico C, Danielson SR, Guo A, Gut P, Sahu AK, Li B, et al: SIRT5
regulates the mitochondrial lysine succinylome and metabolic
networks. Cell Metab. 18:920–933. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lin ZF, Xu HB, Wang JY, Lin Q, Ruan Z, Liu
FB, Jin W, Huang HH and Chen X: SIRT5 desuccinylates and activates
SOD1 to eliminate ROS. Biochem Biophys Res Commun. 441:191–195.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shi L, Yan H, An S, Shen M, Jia W, Zhang
R, Zhao L, Huang G and Liu J: SIRT5-mediated deacetylation of LDHB
promotes autophagy and tumorigenesis in colorectal cancer. Mol
Oncol. 13:358–375. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gu W, Qian Q, Xu Y, Xu X, Zhang L, He S
and Li D: SIRT5 regulates autophagy and apoptosis in gastric cancer
cells. J Int Med Res. 49(300060520986355)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Garva R, Thepmalee C, Yasamut U, Sudsaward
S, Guazzelli A, Rajendran R, Tongmuang N, Khunchai S, Meysami P,
Limjindaporn T, et al: Sirtuin family members selectively regulate
autophagy in osteosarcoma and mesothelioma cells in response to
cellular stress. Front Oncol. 9(949)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Y, Liu Q, Huan Y, Li R, Li C, Sun S,
Guo N, Yang M, Liu S and Shen Z: Sirtuin 5 overexpression
attenuates glucolipotoxicity-induced pancreatic β cells apoptosis
and dysfunction. Exp Cell Res. 371:205–213. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cantó C, Jiang LQ, Deshmukh AS, Mataki C,
Coste A, Lagouge M, Zierath JR and Auwerx J: Interdependence of
AMPK and SIRT1 for metabolic adaptation to fasting and exercise in
skeletal muscle. Cell Metab. 11:213–219. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Polletta L, Vernucci E, Carnevale I,
Arcangeli T, Rotili D, Palmerio S, Steegborn C, Nowak T,
Schutkowski M, Pellegrini L, et al: SIRT5 regulation of
ammonia-induced autophagy and mitophagy. Autophagy. 11:253–270.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang C, He X, Sheng Y, Xu J, Yang C,
Zheng S, Liu J, Li H, Ge J, Yang M, et al: Allicin regulates energy
homeostasis through brown adipose tissue. iScience.
23(101113)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fiorentino F, Mautone N, Menna M, D'Acunzo
F, Mai A and Rotili D: Sirtuin modulators: past, present, and
future perspectives. Future Med Chem. 14:915–939. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Caccamo A, Majumder S, Richardson A,
Strong R and Oddo S: Molecular interplay between mammalian target
of rapamycin (mTOR), amyloid-beta, and Tau: Effects on cognitive
impairments. J Biol Chem. 285:13107–13120. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Csibi A, Fendt SM, Li C, Poulogiannis G,
Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T,
et al: The mTORC1 pathway stimulates glutamine metabolism and cell
proliferation by repressing SIRT4. Cell. 153:840–854.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Csibi A, Lee G, Yoon SO, Tong H, Ilter D,
Elia I, Fendt SM, Roberts TM and Blenis J: The mTORC1/S6K1 pathway
regulates glutamine metabolism through the eIF4B-dependent control
of c-Myc translation. Curr Biol. 24:2274–2280. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cheng A, Zhang Y, Mei H, Fang S, Ji P,
Yang J, Yu L and Guo W: Construction of recombinant pEGFP-N1-hPer2
plasmid and its expression in osteosarcoma cells. Oncol Lett.
11:2768–2772. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Djavaheri-Mergny M, Maiuri MC and Kroemer
G: Cross talk between apoptosis and autophagy by caspase-mediated
cleavage of Beclin 1. Oncogene. 29:1717–1719. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gu X, Han D, Chen W, Zhang L, Lin Q, Gao
J, Fanning S and Han B: SIRT1-mediated FoxOs pathways protect
against apoptosis by promoting autophagy in osteoblast-like
MC3T3-E1 cells exposed to sodium fluoride. Oncotarget.
7:65218–65230. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang S, Jiang S, Wang H, Di W, Deng C,
Jin Z, Yi W, Xiao X, Nie Y and Yang Y: SIRT6 protects against
hepatic ischemia/reperfusion injury by inhibiting apoptosis and
autophagy related cell death. Free Radic Biol Med. 115:18–30.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu D, Jiang X, He H, Liu D, Yang L, Chen
H, Wu L, Geng G and Li Q: SIRT2 functions in aging, autophagy, and
apoptosis in post-maturation bovine oocytes. Life Sci.
232(116639)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nakagawa T, Lomb DJ, Haigis MC and
Guarente L: SIRT5 deacetylates carbamoyl phosphate synthetase 1 and
regulates the urea cycle. Cell. 137:560–570. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rama Rao KV and Norenberg MD: Glutamine in
the pathogenesis of hepatic encephalopathy: The trojan horse
hypothesis revisited. Neurochem Res. 39:593–598. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wilkinson DJ, Smeeton NJ and Watt PW:
Ammonia metabolism, the brain and fatigue; revisiting the link.
Prog Neurobiol. 91:200–219. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Aledo JC, de Pedro E, Gómez-Fabre PM,
Núñez de Castro I and Márquez J: Submitochondrial localization and
membrane topography of Ehrlich ascitic tumour cell glutaminase.
Biochim Biophys Acta. 1323:173–184. 1997.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Katt WP and Cerione RA: Glutaminase
regulation in cancer cells: A druggable chain of events. Drug
Discov Today. 19:450–457. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Eng CH, Yu K, Lucas J, White E and Abraham
RT: Ammonia derived from glutaminolysis is a diffusible regulator
of autophagy. Sci Signal. 3(ra31)2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Visek WJ: Ammonia: Its effects on
biological systems, metabolic hormones, and reproduction. J Dairy
Sci. 67:481–498. 1984.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Qiu J, Tsien C, Thapalaya S, Narayanan A,
Weihl CC, Ching JK, Eghtesad B, Singh K, Fu X, Dubyak G, et al:
Hyperammonemia-mediated autophagy in skeletal muscle contributes to
sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab.
303:E983–E993. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tooze SA and Dikic I: Autophagy captures
the nobel prize. Cell. 167:1433–1435. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Anding AL and Baehrecke EH: Cleaning
house: Selective autophagy of organelles. Dev Cell. 41:10–22.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Greene KS, Lukey MJ, Wang X, Blank B,
Druso JE, Lin MJ, Stalnecker CA, Zhang C, Negrón Abril Y, Erickson
JW, et al: SIRT5 stabilizes mitochondrial glutaminase and supports
breast cancer tumorigenesis. Proc Natl Acad Sci USA.
116:26625–26632. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li Z, Ji X, Wang W, Liu J, Liang X, Wu H,
Liu J, Eggert US, Liu Q and Zhang X: Ammonia induces autophagy
through dopamine receptor D3 and mTOR. PLoS One.
11(e0153526)2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Motyl T, Gajkowska B, Zarzyńska J,
Gajewska M and Lamparska-Przybysz M: Apoptosis and autophagy in
mammary gland remodeling and breast cancer chemotherapy. J Physiol
Pharmacol. 57 (Suppl 7):S17–S32. 2006.PubMed/NCBI
|
|
50
|
Gao CF, Ren S, Zhang L, Nakajima T,
Ichinose S, Hara T, Koike K and Tsuchida N: Caspase-dependent
cytosolic release of cytochrome c and membrane translocation of Bax
in p53-induced apoptosis. Exp Cell Res. 265:145–151.
2001.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ding HF and Fisher DE: p53, caspase 8, and
regulation of apoptosis after ionizing radiation. J Pediatr Hematol
Oncol. 23:185–188. 2001.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
53
|
Wang F, Zhao Y, Chen S, Chen L, Sun L, Cao
M, Li C and Zhou X: Astragaloside IV alleviates ammonia-induced
apoptosis and oxidative stress in bovine mammary epithelial cells.
Int J Mol Sci. 20(600)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhang M, Wu J, Sun R, Tao X, Wang X, Kang
Q, Wang H, Zhang L, Liu P, Zhang J, et al: SIRT5 deficiency
suppresses mitochondrial ATP production and promotes AMPK
activation in response to energy stress. PLoS One.
14(e0211796)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li W, Yang Y, Li Y, Zhao Y and Jiang H:
Sirt5 attenuates cisplatin-induced acute kidney injury through
regulation of Nrf2/HO-1 and Bcl-2. Biomed Res Int.
2019(4745132)2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Madrigal-Matute J and Cuervo AM:
Regulation of liver metabolism by autophagy. Gastroenterology.
150:328–339. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Menzies FM, Fleming A and Rubinsztein DC:
Compromised autophagy and neurodegenerative diseases. Nat Rev
Neurosci. 16:345–357. 2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Yeung F, Hoberg JE, Ramsey CS, Keller MD,
Jones DR, Frye RA and Mayo MW: Modulation of NF-kappaB-dependent
transcription and cell survival by the SIRT1 deacetylase. EMBO J.
23:2369–2380. 2004.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Guarani V, Deflorian G, Franco CA, Krüger
M, Phng LK, Bentley K, Toussaint L, Dequiedt F, Mostoslavsky R,
Schmidt MHH, et al: Acetylation-dependent regulation of endothelial
Notch signalling by the SIRT1 deacetylase. Nature. 473:234–238.
2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Malik S, Villanova L, Tanaka S, Aonuma M,
Roy N, Berber E, Pollack JR, Michishita-Kioi E and Chua KF: SIRT7
inactivation reverses metastatic phenotypes in epithelial and
mesenchymal tumors. Sci Re. 5(9841)2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Kihara A, Kabeya Y, Ohsumi Y and Yoshimori
T: Beclin-phosphatidylinositol 3-kinase complex functions at the
trans-Golgi network. EMBO Rep. 2:330–335. 2001.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Pankiv S, Clausen TH, Lamark T, Brech A,
Bruun JA, Outzen H, Øvervatn A, Bjørkøy G and Johansen T:
p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lamark T, Svenning S and Johansen T:
Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays
Biochem. 61:609–624. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zhong Y, Wang QJ and Yue Z: Atg14L and
rubicon: Yin and yang of Beclin 1-mediated autophagy control.
Autophagy. 5:890–891. 2009.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Huang W, Cao Z, Zhang J, Ji Q and Li Y:
Aflatoxin B1 promotes autophagy associated with
oxidative stress-related PI3K/AKT/mTOR signaling pathway in mice
testis. Environ Pollut. 255(113317)2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Merhi A, Delrée P and Marini AM: The
metabolic waste ammonium regulates mTORC2 and mTORC1 signaling. Sci
Rep. 7(44602)2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Cheng Z, Shu Y, Li X, Li Y, Zhou S and Liu
H: Evaluation of potential cardiotoxicity of ammonia:
L-selenomethionine inhibits ammonia-induced cardiac autophagy by
activating the PI3K/AKT/mTOR signaling pathway. Ecotoxicol Environ
Saf. 233(113304)2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Han Q, Wang A, Fu Q, Zhou S, Bao J and
Xing H: Protective role of selenium on ammonia-mediated
nephrotoxicity via PI3K/AKT/mTOR pathway: Crosstalk between
autophagy and cytokine release. Ecotoxicol Environ Saf.
242(113918)2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Li Z, Miao Z, Ding L, Teng X and Bao J:
Energy metabolism disorder mediated ammonia gas-induced autophagy
via AMPK/mTOR/ULK1-Beclin1 pathway in chicken livers. Ecotoxicol
Environ Saf. 217(112219)2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Wang J, Wang J, Li Y, Han Q, Wang Y, Liu H
and Bao J: Organic selenium alleviates ammonia-mediated abnormal
autophagy by regulating inflammatory pathways and the Keap1/Nrf2
axis in the hypothalamus of finishing pigs. Biol Trace Elem Res.
201:3812–3824. 2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wei T, Xiaojun X and Peilong C:
Magnoflorine improves sensitivity to doxorubicin (DOX) of breast
cancer cells via inducing apoptosis and autophagy through AKT/mTOR
and p38 signaling pathways. Biomed Pharmacother.
121(109139)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Hong J, Mei C, Raza SHA, Khan R, Cheng G
and Zan L: SIRT5 inhibits bovine preadipocyte differentiation and
lipid deposition by activating AMPK and repressing MAPK signal
pathways. Genomics. 112:1065–1076. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Li Y and Chen Y: AMPK and autophagy. Adv
Exp Med Biol. 1206:85–108. 2019.PubMed/NCBI View Article : Google Scholar
|