Introduction
Glioma is the most prevalent type of primary tumor
in the brain, accounting for 81% of central nervous system
malignancies, and is one of the leading causes of mortality
worldwide (1). Glioma is a highly
heterogeneous group of tumors, including astrocytomas [World Health
Organization (WHO) grade I-IV], ependymomas (WHO grade II-III) and
oligodendrogliomas (2). Despite
major efforts to optimize early diagnosis and treatment,
conventional therapies including surgical resection, radiotherapy
and chemotherapy, possess limited improvements in the prognosis of
patients with glioma, and the median overall survival time of
patients with grade IV glioma is ~15 months, along with a poor
5-year survival rate of <10% (3). Hence, it is of great importance and
urgency to explore the molecular mechanisms underlying the
progression of glioma.
Vasculogenic mimicry (VM), initially described in
uveal melanomas by Maniotis et al (4) in 1999, is considered a novel form of
blood supply independent of blood vessels, attributed to its
formation of microvascular channels composed of tumor cells,
distinguishing it from the traditional angiogenetic process
involving vascular endothelium (5). VM occurs in numerous malignancies,
including prostate cancer, breast cancer, ovarian cancer and glioma
(6-9).
It has been reported that VM formation promotes tumor cell
proliferation and invasion, and typically predicts a poor prognosis
in patients with glioma (10,11).
Therefore, VM is regarded as a novel target for glioma therapy.
The frizzled family proteins (FZDs, including
FZD1-10) are 10-transmembrane receptors for Wnt ligands, and are
not only involved in embryogenesis and development but also in
cancer progression (12). Of note,
FZD2 is a highly conserved signaling molecule that also belongs to
the G protein-coupled receptor family. Accumulating evidence has
revealed the aberrant expression of FZD2 in various malignancies,
including tongue squamous cell carcinoma, breast cancer and
hepatocellular carcinoma, in which FZD2 acts as an oncogene
(13-15).
By contrast, FZD2 serves as a tumor-suppressor gene in salivary
adenoid cystic carcinoma (16),
demonstrating that FZD2 has a dual role in different types of
tumors. A recent report based on RNA-sequencing data from clinical
glioma samples revealed that FZD1/2/5/7/8 was significantly highly
expressed in tumor tissues. Furthermore, the FZD2 expression level
increased as the progression of the glioma increased from grade II
to grade IV, and FZD2 was suggested to be a novel independent
predictor of unfavorable prognosis in glioma (17). Notably, FZD2 has been discovered to
promote the VM phenotype in hepatocellular carcinoma, indicating a
potential association between FZD2 and VM formation (13). Nevertheless, the specific role of
FZD2 in glioma progression has not been completely understood.
Therefore the present study aimed to explore the
molecular function of FZD2 in glioma progression, its regulatory
effect on VM formation during glioma progression and its potential
regulatory mechanism. The present study may therefore provide novel
ideas for developing therapeutic strategies for glioma
treatment.
Materials and methods
Cell culture and treatment
Human astrocyte HEB cells (4th passage) were
obtained from Jennio Biotech Co., Ltd. The human glioma cell lines,
A172 (cat. no. iCell-h002), T98G (cat. no. iCell-h210) and LN229
(cat. no. iCell-h124), and the U87MG glioblastoma cell line of
unknown origin (cat. no. iCell-h224) were obtained from iCell
Bioscience Inc. and authenticated by STR analysis. All cells were
cultured in Dulbecco's Modified Eagle Medium (Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin
mixture (Invitrogen; Thermo Fisher Scientific, Inc.) in a
humidified incubator with 5% CO2 at 37˚C.
In addition, to explore the relevant mechanisms of
action, the Notch agonist Jagged-1 (JAG) peptide (50 µg/ml; R&D
Systems, Inc.) was used to treat U87MG glioblastoma cells at 37˚C
for 48 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells utilizing TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in-line with
the manufacturer's guidelines, followed by RT to cDNA using
SuperScript Reverse Transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols.
Subsequently, qPCR was conducted using FastStart Universal Probe
Master (Roche Diagnostics) and a Bio-Rad CFX96 Real-Time PCR System
(Bio-Rad Laboratories, Inc.). The thermocycling conditions were as
follows: Preheating at 90˚C for 10 min, followed by 30 cycles of
95˚C for 30 sec, 55˚C for 30 sec and 72˚C for 60 sec, with a final
elongation step at 72˚C for 10 min and 4˚C on hold. The primer
sequences used in the present study were as follows: FZD2 forward,
5'-AGTTCTATCCGCTGGTGAAGGT-3' and reverse,
5'-GCCCAGAAACTTGTAGCTGAGA-3'; Nanog forward,
5'-GTGATTTGTGGGCCTGAAGA-3' and reverse, 5'-ACACAGCTGGGTGGAAGAGA-3';
Sox2 forward, 5'-ACACCAATCCCATCCACACT-3' and reverse,
5'-GCAAACTTCCTGCAAAGCTC-3'; Oct4 forward,
5'-AGCCCTCATTTCACCAGGCC-3' and reverse, 5'-CCCCCACAGAACTCATACGG-3';
GAPDH forward, 5'-CAGGAGGCATTGCTGATGAT-3' and reverse,
5'-GAAGGCTGGGGCTCATTT-3'. Target gene expression was calculated
using the 2-∆∆Cq method (18), normalized to GAPDH.
Western blotting
Total proteins were extracted from cells utilizing
RIPA buffer (Beyotime Institute of Biotechnology), followed by
quantification using a BCA Protein Assay Kit (Beyotime Institute of
Biotechnology) to determine the protein concentration. The proteins
(30 µg/lane) were fractionated by electrophoresis using a 10%
SDS-polyacrylamide gel, transferred to polyvinylidene difluoride
membranes (MilliporeSigma), blocked with 5% non-fat milk at room
temperature for 1 h and probed using primary antibodies against
FZD2 (cat. no. 24272-1-AP; 1:500; Proteintech Group, Inc.), Nanog
(cat. no. ab109250; 1:1,000; Abcam), Sox2 (cat. no. ab92494;
1:1,000; Abcam), Oct4 (cat. no. ab200834; 1:10,000; Abcam),
E-cadherin (cat. no. ab40772; 1:1,000; Abcam), N-cadherin (cat. no.
ab76011; 1:5,000; Abcam), Vimentin (cat. no. ab92547; 1:1,000;
Abcam), Snail (cat. no. ab216347; 1:1,000; Abcam), N1ICD (cat. no.
ab52301; 1:1,000; Abcam), Hes1 (cat. no. ab71559; 1:1,000; Abcam),
phosphorylated (p-)NF-κB p65 (cat. no. ab239882; 1:1,000; Abcam),
NF-κB p65 (cat. no. ab207297; 1:1,000; Abcam) and GAPDH (cat. no.
ab9485; 1:2,500; Abcam) at 4˚C overnight. On the following day,
membranes were incubated with a horseradish peroxidase-conjugated
secondary antibody (cat. no. ab6721; 1:3,000; Abcam) at room
temperature for 2 h. Immunoreactivity was developed using Western
Chemiluminescent HRP substrate (MilliporeSigma) and semi-quantified
using ImageJ software 1.52 (National Institutes of Health).
Cell transfection
Short hairpin (sh)RNAs targeting FZD2, including
sh-FZD2-1 (5'-CATCCTATCTCAGCTACAA-3') and sh-FZD2-2
(5'-CCGACTTCACGGTCTACAT-3'), were synthesized by Shanghai
GenePharma Co., Ltd., and the empty shRNA plasmid (pGPU6/Neo)
served as the negative control (sh-NC; Shanghai GenePharma, Co.,
Ltd.). The shRNAs (500 ng/µl) were transfected into U87MG
glioblastoma cells using Lipofectamine 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) in-line with the manufacturer's
guidelines. The cells in the control group were those that did not
receive transfection. After 48 h, the transfection efficiency was
determined by RT-qPCR and western blotting.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 kit (Dojindo Laboratories, Inc.) was
utilized to determine the cell proliferation ability. In brief,
U87MG glioblastoma cells were inoculated into 96-well plates and
incubated at 37˚C under 5% CO2 for 24, 48 and 72 h.
Then, 10 µl CCK-8 solution was added to each well and the cells
were incubated for another 2 h. Finally, the absorbance of each
well at 450 nm was detected using a microplate reader (BioTek;
Agilent Technologies, Inc.).
5-Ethynyl-2'-deoxyuridine (EdU)
staining assay
U87MG cells were cultured in 96-well plates and
treated with 100 µl EdU (50 µM; Abcam). After incubation at 37˚C
under 5% CO2 for 2 h, the cells were fixed in 4%
paraformaldehyde for 15 min at room temperature and permeabilized
with 0.5% Triton X-100 for 15 min at room temperature.
Subsequently, cells were incubated with 100 µl EdU reaction
cocktail (cat. no. ab219801; Abcam) for 20 min at room temperature,
and 4,6-diamino-2-phenylindole (DAPI) was used to counterstain the
nuclei for 15 min at room temperature. Finally, images were
captured using a fluorescence microscope (Olympus Corporation).
Colony formation assay
U87MG cells (1x103) were inoculated into
6-well plates and cultured at 37˚C under 5% CO2 for 10
days. During this period, the culture medium was refreshed every
2-3 days. Finally, the colonies consisting of >50 cells were
fixed with 4% paraformaldehyde for 20 min at room temperature and
then stained with 0.1% crystal violet for 20 min at room
temperature for visualization and counting using ImageJ software
1.52 (National Institutes of Health).
Wound healing assay
U87MG cells were seeded into 6-well plates and
incubated at 37˚C under 5% CO2. Once 100% confluency was
reached, a straight scratch was generated using a 200-µl pipette
tip. The cells were then washed with PBS and incubated in
serum-free medium at 37˚C under 5% CO2 for 48 h. Images
at 0 and 48 h were captured using a bright-field microscope
(Olympus Corporation). The migration rate was determined according
to the width of the wounds measured using ImageJ software 1.52
(National Institutes of Health).
Transwell assay
The cell invasion potential was assessed using
Matrigel-coated (37˚C for 30 min) Transwell assay inserts with a
8-µm pore size (Corning, Inc.). 1x105 U87MG cells were
resuspended in serum-free medium and inoculated into the upper
chamber of a Transwell plate. Then, 500 µl complete medium
containing 10% FBS was added to the lower chamber. After incubation
for 48 h at 37˚C, the invaded cells were fixed with 4%
paraformaldehyde for 20 min at room temperature then stained with
0.1% crystal violet for 10 min at room temperature. The invaded
cells were observed under a bright-field microscope (Olympus
Corporation).
Sphere formation assay
U87MG cells (1x103) were resuspended in
cancer stemness medium (cat. no. 12400-024; Gibco; Thermo Fisher
Scientific, Inc.) containing B27, 20 ng/ml basic fibroblast growth
factor and 20 ng/ml epidermal growth factor, then seeded into
24-well ultra-low attachment plates (Corning, Inc.) and incubated
at 37˚C under 5% CO2. Following a 10-day incubation,
cell spheres with a diameter >75 µm were observed using a
bright-field microscope (Olympus Corporation).
3D culturing
The in vitro VM formation potential of U87MG
cells was evaluated by 3D culturing as previously described
(11). In brief, 96-well plates
were pre-coated with Matrigel (BD Biosciences) at 37˚C for 30 min.
U87MG cells (1x105) were resuspended in serum-free
medium and seeded on the Matrigel. After incubation at 37˚C under
5% CO2 for 8 h, images were captured using a
bright-field microscope (Olympus Corporation), and tube formation
was assessed manually.
Immunofluorescence assay
U87MG cells were fixed in 4% paraformaldehyde for 15
min at room temperature and permeabilized with 0.5% Triton X-100
for 15 min at room temperature. Then, after blocking with 10%
normal goat serum (Beijing Solarbio Science & Technology Co.,
Ltd.) at 37˚C for 30 min, the cells were probed with
anti-VE-cadherin antibody (cat. no. ab313632; 1:50; Abcam) at 4˚C
overnight. On the following day, the cells were incubated with Goat
Anti-Rabbit IgG (Alexa Fluor® 488) preadsorbed antibody
(cat. no. ab150081; 1:1,000; Abcam) at 37˚C for 1 h in the dark.
DAPI was used to counterstain the nuclei for 15 min at room
temperature. Finally, images were captured using a fluorescence
microscope (Olympus Corporation).
Bioinformatic and statistical
analysis
Quantitative data are presented as the mean ±
standard deviation. All statistical analyses were conducted using
GraphPad Prism 8 (Dotmatics). Comparisons were conducted using
one-way ANOVA followed by Tukey's post hoc test. To assess the role
of FZD2 in glioma/glioblastoma, the expression of FZD2 in
glioblastoma was explored using the Gene Expression Profiling
Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn/) (19) and UALCAN (https://ualcan.path.uab.edu/index.html) databases
(20). The unpaired Student's
t-test was applied for difference comparisons. In addition, the
Chinese Glioma Genome Atlas (CGGA; http://www.cgga.org.cn/) (21) was applied to assess the survival
probability in patients with grade I-III glioma (cut-off value of
50% to determine high and low FZD2 status) P<0.05 was considered
to indicate a statistically significant difference.
Results
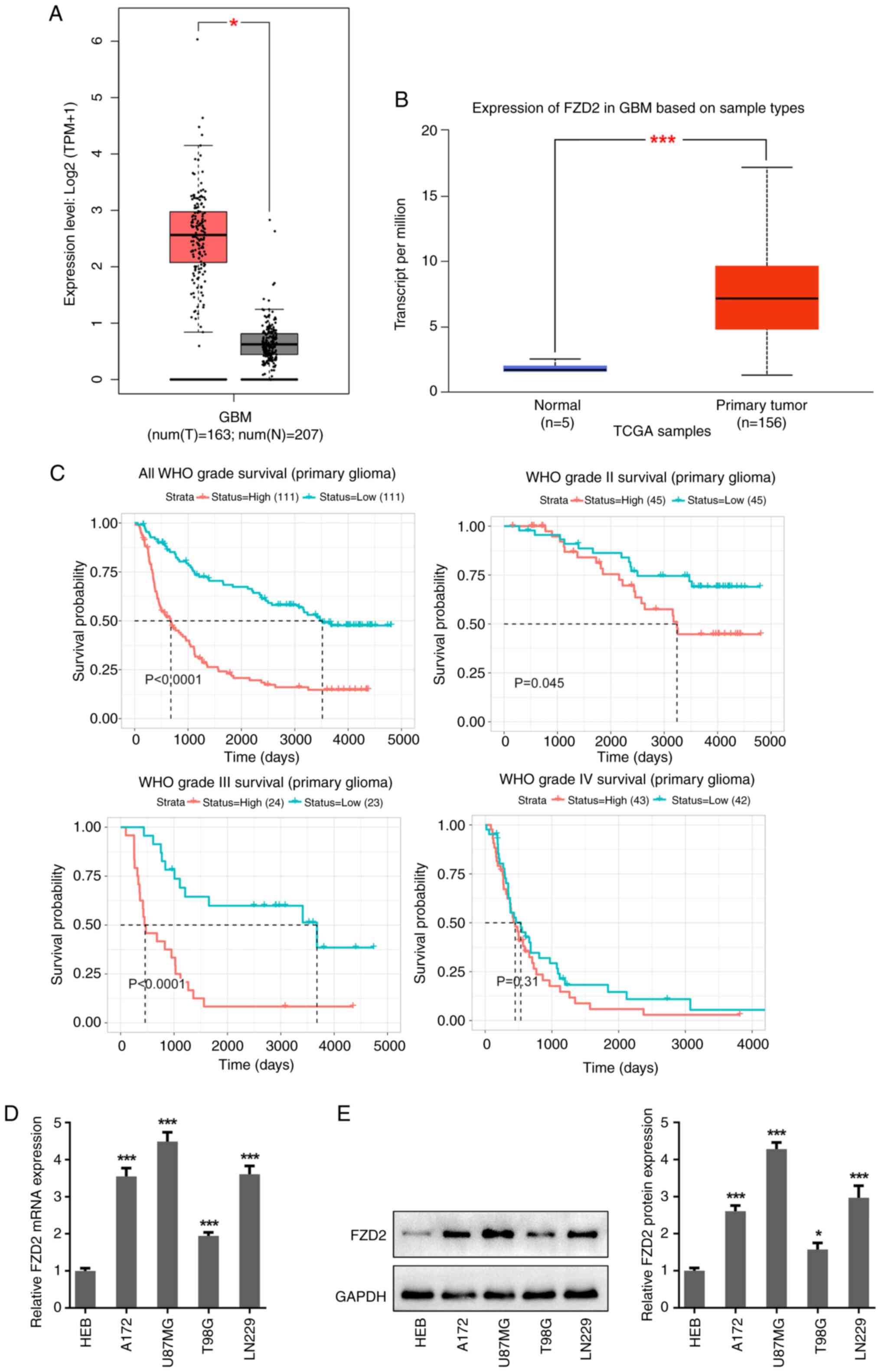
FZD2 is upregulated in glioma tissues
and cells
To uncover the role of FZD2 in glioma/glioblastoma,
the expression of FZD2 in glioma was first explored using the GAPIA
and UALCAN databases. According to these databases, the FZD2
expression level in the tumor tissues of patients with glioma was
higher than that in the normal tissues (Fig. 1A and B). In addition, based on data from the
CGGA, it was found that high expression of FZD2 was positively
associated with poor survival probability in patients with grade
I-III glioma (Fig. 1C). To confirm
the aberrant high level of FZD2 in glioma, the expression of FZD2
was also detected in multiple glioma/glioblastoma cell lines and
astrocyte HEB cells. As shown in Fig.
1D and E, both the mRNA and
protein expression levels of FZD2 were significantly higher in
glioma/glioblastoma cell lines (A172, U87MG, T98G and LN229)
compared with HEB cells, and the highest levels were observed in
U87MG glioblastoma cells.
Interference of FZD2 restricts the
proliferation and stemness of U87MG cells
To clarify the regulatory role of FZD2 in glioma,
shRNA cell transfections were conducted using U87MG cells. Compared
with the sh-NC group, the expression level of FZD2 in the sh-FZD2-1
and sh-FZD2-2 groups was significantly reduced (Fig. 2A and B). sh-FZD2-1 was adopted in the
subsequent experiments due to the more optimized transfection
efficacy. Thereafter, a series of in vitro experiments were
conducted to assess the impacts of FZD2 on cellular biological
activities. According to the results from the CCK-8, EdU staining
and colony formation assays (Fig.
2C-E), the relative cell viability, the number of
EdU+ cells and the number of colonies in the sh-FZD2
group were significantly decreased compared with the sh-NC group,
indicating that interference with FZD2 expression greatly
restricted the proliferation ability of U87MG cells. In addition,
it was observed that significantly smaller spheres were formed by
FZD2-knockdown U87MG cells compared with the control cells
(Fig. 2F), meaning that FZD2
knockdown lowered the sphere formation ability, thereby alleviating
cell stemness in glioma. Furthermore, it was also discovered that
FZD2 knockdown significantly lowered both the mRNA and protein
expression levels of Nanog, Sox2 and Oct4 (Fig. 2G and H), the critical factors of cell stemness.
Taken together, these results demonstrated that FZD2 knockdown may
lower glioma cell proliferation ability and stemness.
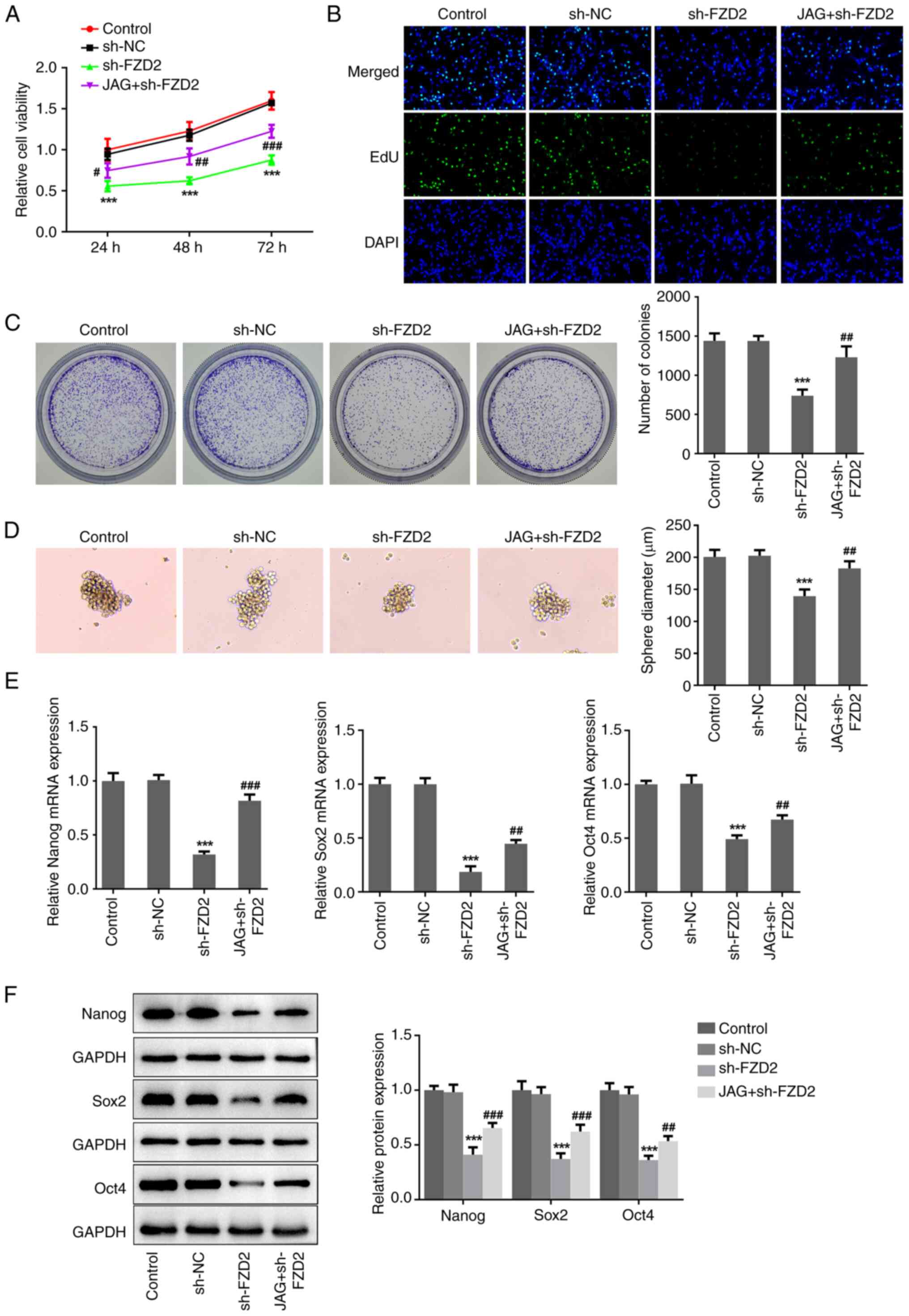
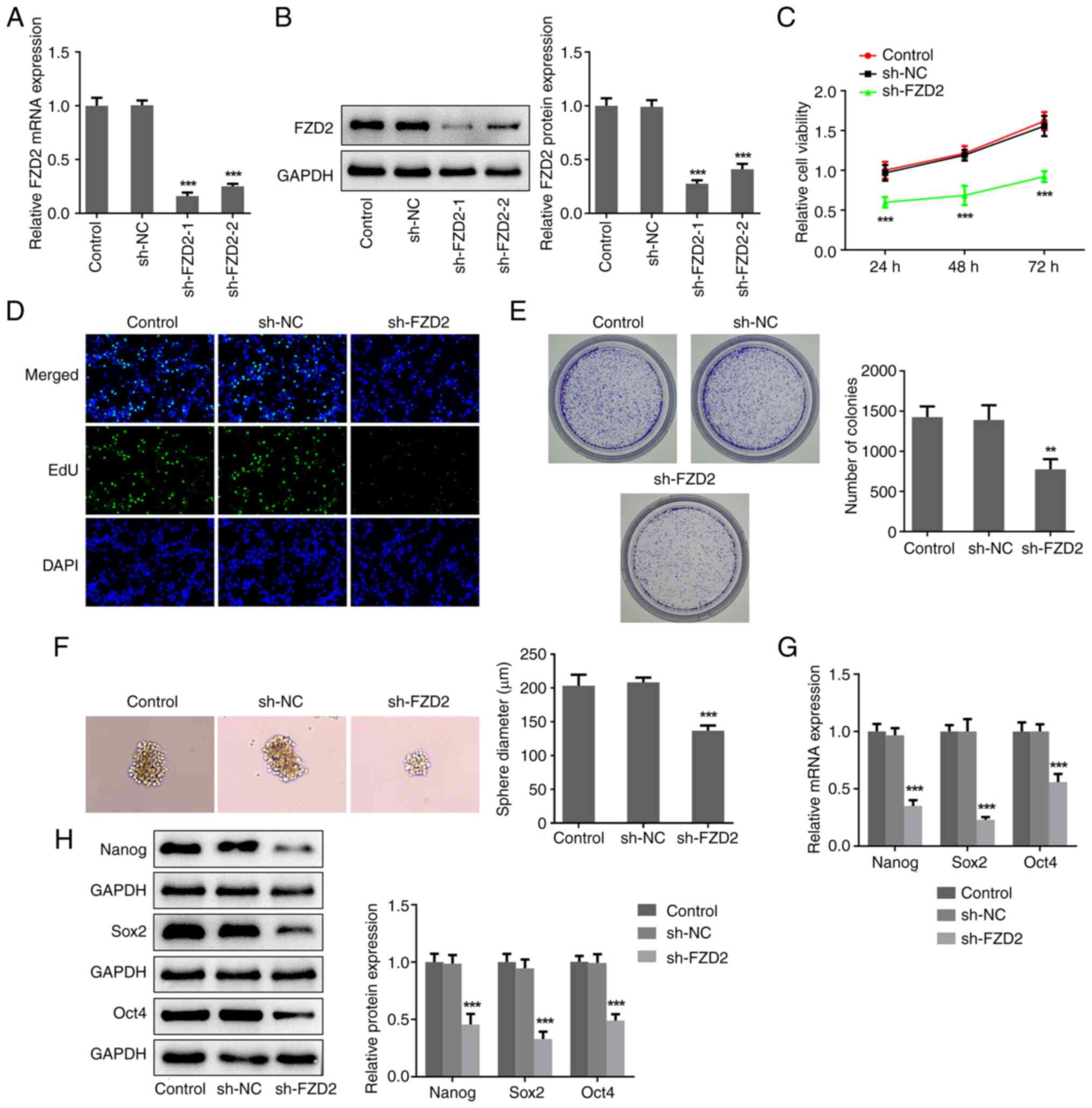 | Figure 2Interference of FZD2 restricts the
proliferation and stemness of U87MG cells. U87MG cells were
transfected with sh-NC and sh-FZD2-1/2, and the (A) mRNA and (B)
protein expression levels of FZD2 were detected by RT-qPCR and
western blotting, respectively. (C) Cell Counting Kit-8, (D) EdU
staining (magnification, x200) and (E) colony formation assays were
performed to assess cell proliferation. (F) Sphere formation assay
was conducted to assess cell stemness (magnification, x100). (G)
mRNA and (H) protein expression levels of Nanog, Sox2 and Oct4 were
detected by RT-qPCR and western blot, respectively.
**P<0.01, ***P<0.001 vs. control. EdU,
5-Ethynyl-2'-deoxyuridine; sh, short hairpin; NC, negative control;
FZD2, frizzled family protein 2; DAPI,
4,6-diamino-2-phenylindole. |
Interference of FZD2 represses the
migration, invasion and VM formation capabilities of U87MG
cells
In addition, the impacts of FZD2 on cell migration,
invasion and VM formation in glioma was also investigated. As shown
in Fig. 3A-C, compared with the
sh-NC group, the wound closure and cell invasion rates of the
sh-FZD2 group were significantly lower, suggesting that FZD2
knockdown inhibited the migration and invasion abilities of U87MG
cells. Meanwhile, FZD2 knockdown significantly increased the
protein expression level of E-cadherin but reduced the protein
expression levels of N-cadherin, Vimentin and Snail (Fig. 3D), reflecting an inhibitory effect
of FZD2 knockdown on epithelial-mesenchymal transition (EMT) in
glioma cells. In addition, U87MG cells formed typical channels and
tube-like structures in the in vitro 3D culturing model,
while the number of formed tubes were significantly reduced when
FZD2 expression was knocked down (Fig.
3E). Meanwhile, the significantly reduced VE-cadherin level
following FZD2 knockdown further confirmed that interference of
FZD2 expression restricted the VM formation ability of glioma cells
(Fig. 3F).
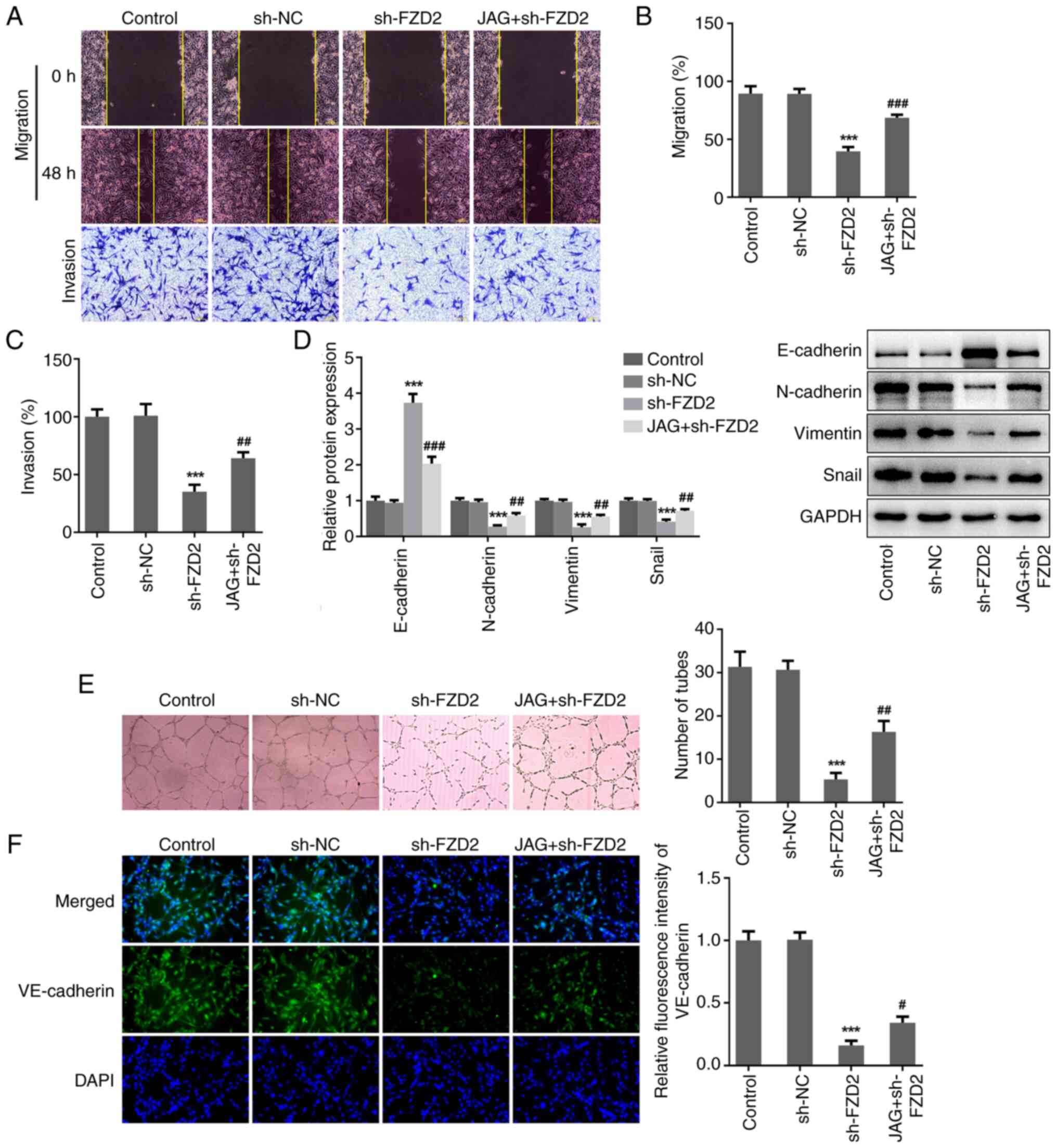
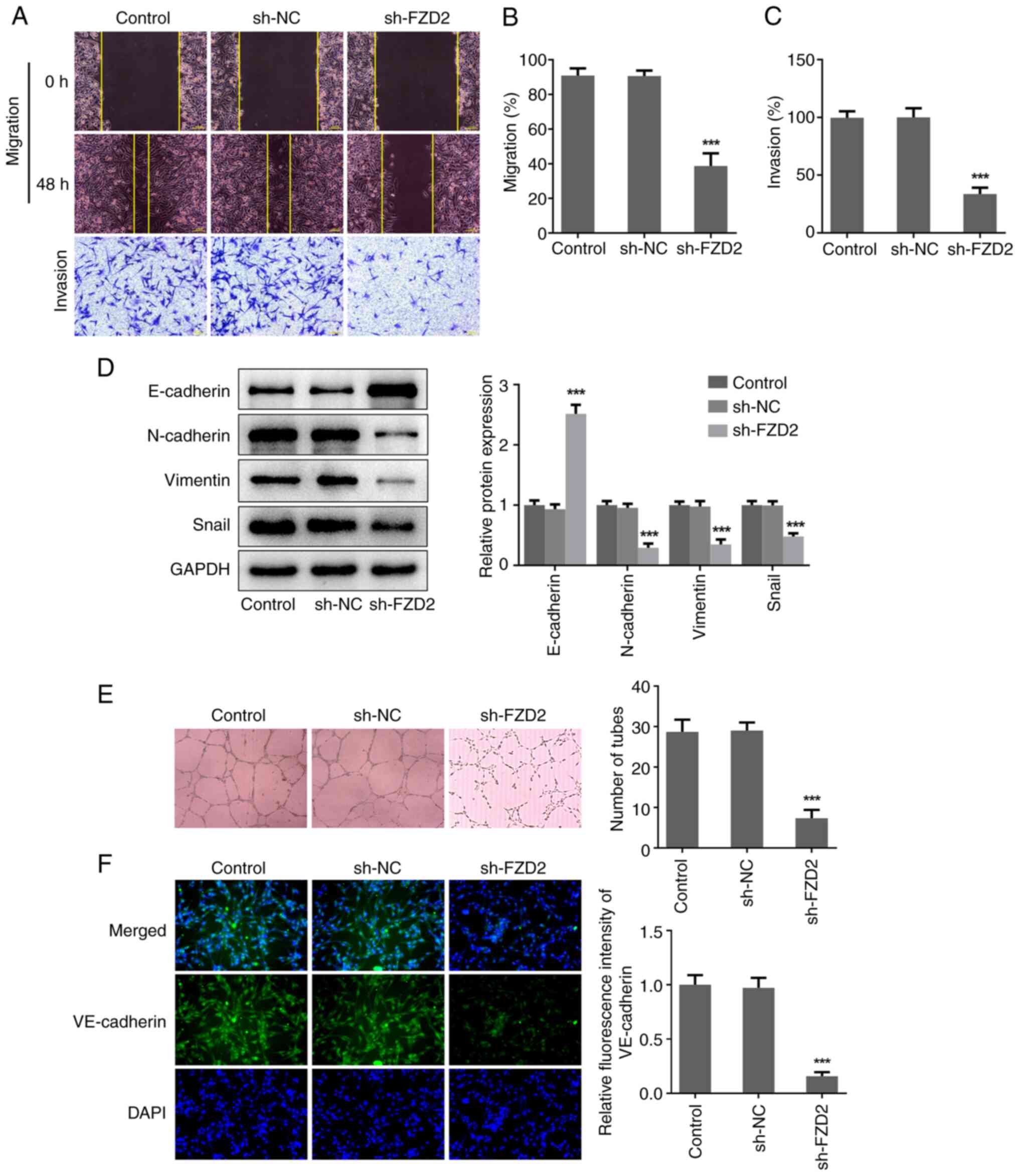 | Figure 3Interference of FZD2 represses the
migration, invasion and VM formation capabilities of U87MG cells.
(A) U87MG cells were transfected with sh-NC and sh-FZD2,
wound-healing and Transwell assays were conducted to assess cell
migration and invasion, respectively (magnification, x100). (B) The
migration rate and (C) invasion rate were quantified. (D) Protein
expression levels of E-cadherin, N-cadherin, Vimentin and Snail
were examined by western blotting. (E) In vitro 3D culturing
model was constructed to assess VM formation ability
(magnification, x100). (F) Cell immunofluorescence assay was
performed to detect VE-cadherin expression (magnification, x200).
***P<0.001. VM, vasculogenic mimicry; sh, short
hairpin; NC, negative control; FZD2, frizzled family protein 2;
DAPI, 4,6-diamino-2-phenylindole. |
Interference of FZD2 blocks the
Notch/NF-κB signaling pathway in U87MG cells
Next, an attempt was made to elucidate the
underlying molecular basis behind the regulatory role of FZD2 in
glioma. Since FZD2 can induce Notch signaling and Notch signaling
has a critical role in regulating the malignant metastasis of
glioma (22,23), the impact of FZD2 on Notch
signaling in glioma was also explored. As shown in Fig. 4A, FZD2 knockdown significantly
inhibited the protein expression levels of intracellular domain of
NOTCH1 receptor (N1ICD), Hes1 and p-NF-κB p65, revealing that FZD2
knockdown restricted the activation of Notch/NF-κB signaling in
glioma cells. To confirm the importance of Notch signaling
underlying FZD2-mediated glioma progression, the Notch agonist JAG
peptide was used to treat sh-FZD2-transfected U87MG cells. The
western blotting results revealed that the inhibitory effect of
FZD2 knockdown on the N1ICD, Hes1 and p-NF-κB p65 protein
expression levels was partially weakened by JAG treatment (Fig. 4B), further proving the FZD2
regulation of the Notch/NF-κB signaling pathway in glioma.
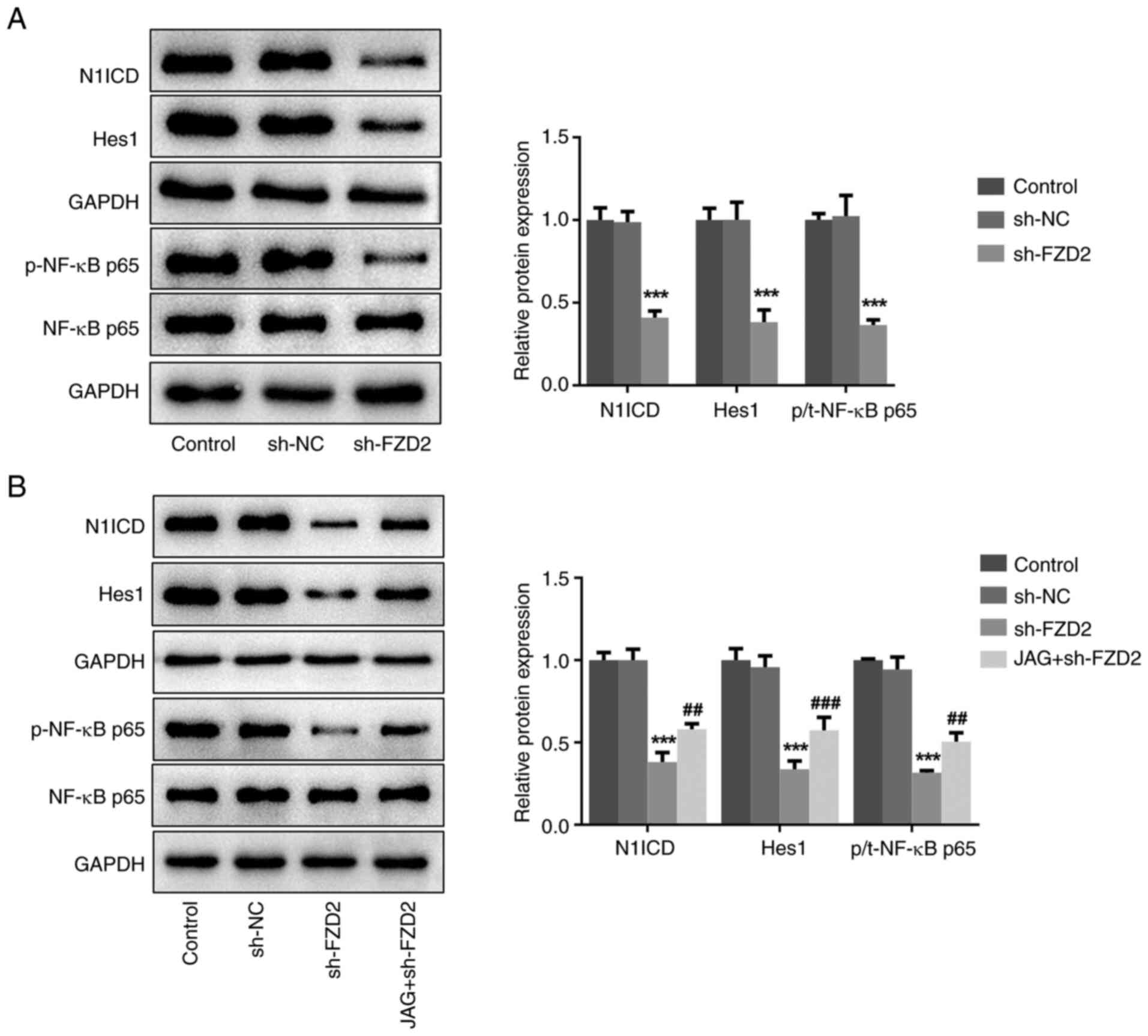 | Figure 4Interference of FZD2 blocks the
Notch/NF-κB signaling pathway in U87MG cells. (A) U87MG cells were
transfected with sh-NC or sh-FZD2 and the expression levels of
Notch/NF-κB signaling-associated proteins were measured using
western blotting. (B) U87MG cells were transfected with sh-FZD2
with or without treatment with the Notch agonist, JAG, and the
expression levels of Notch/NF-κB signaling-associated proteins were
measured using western blotting. ***P<0.001 vs sh-NC;
##P<0.01, ###P<0.001 vs sh-FZD2. sh,
short hairpin; NC, negative control; FZD2, frizzled family protein
2; JAG, Jagged-1; p-, phosphorylated; t-, total; N1ICD, Notch 1
intracellular domain. |
Activation of Notch abolishes the FZD2
knockdown-mediated antioncogenic effects in U87MG cells
Finally, to highlight the importance of the
Notch/NF-κB signaling pathway underlying FZD2-mediated glioma,
cellular biological behaviors were again examined but in the
presence of JAG. As shown in Fig.
5A-C, activation of Notch by JAG significantly weakened the
antiproliferation property of FZD2 knockdown in glioma, as
evidenced by the elevated cell viability, the number of
EdU+ cells and the number of colonies in the JAG+sh-FZD2
group compared with the sh-FZD2 group. Meanwhile, JAG treatment
partially abolished the suppressive effects of FZD2 knockdown on
sphere formation ability and the expression of Nanog, Sox2 and Oct4
(Fig. 5D-F), indicating that the
FZD2 knockdown-associated lowered cell stemness in glioma was
hindered by the activation of Notch. Additionally, JAG treatment
also significantly facilitated wound closure, elevated the rate of
cell invasion and upregulated N-cadherin, Vimentin and Snail
protein expression and downregulated E-cadherin protein expression
in sh-FZD2-transfeted U87MG cells (Fig. 6A-D), reflecting that JAG partially
hindered the regulatory function of FZD2 knockdown on cell
migration, invasion and EMT in glioma cells. Furthermore, compared
with the sh-FZD2 group, the elevated number of tubes and
VE-cadherin expression level in the JAG + sh-FZD2 group confirmed
that JAG also abolished the inhibitory effect of FZD2 knockdown on
VM formation in glioma cells (Fig.
6E and F).
 | Figure 5Activation of Notch abolishes the
inhibitory effects of FZD2 knockdown on cell proliferation and
stemness in U87MG cells. U87MG cells were transfected with sh-FZD2
with or without treatment of the Notch agonist, JAG. (A) Cell
Counting Kit-8, (B) EdU staining (magnification, x200) and (C)
colony formation assays were performed to assess cell proliferation
ability. (D) Sphere formation assay was conducted to assess cell
stemness (magnification, x100). (E) mRNA and (F) protein expression
levels of Nanog, Sox2 and Oct4 were detected by reverse
transcription- quantitative PCR and western blotting, respectively.
***P<0.001 vs sh-NC; #P<0.05,
##P<0.01, ###P<0.001 vs sh-FZD2. sh,
short hairpin; NC, negative control; FZD2, frizzled family protein
2; JAG, Jagged-1. |
 | Figure 6Activation of Notch abolishes the
inhibitory effects of FZD2 knockdown on cell migration, invasion
and VM formation in U87MG cells. (A) U87MG cells were transfected
with sh-FZD2 with or without treatment with the Notch agonist, JAG.
Wound healing and Transwell assays were conducted to assess cell
migration and invasion, respectively (magnification, x100). (B) The
migration rate and (C) invasion rate were quantified. (D) Protein
expression levels of E-cadherin, N-cadherin, Vimentin and Snail
were examined by western blotting. (E) In vitro 3D culturing
model was constructed to assess VM formation ability
(magnification, x100). (F) Cell immunofluorescence assay was
performed to detect VE-cadherin expression. (magnification, x200).
***P<0.001 vs sh-NC; #P<0.05,
##P<0.01, ###P<0.001 vs sh-FZD2. VM,
vasculogenic mimicry; sh, short hairpin; NC, negative control;
FZD2, frizzled family protein 2; JAG, Jagged-1; DAPI,
4,6-diamino-2-phenylindole. |
Discussion
In the present study, FZD2 was shown to be highly
expressed in glioma cells. Functionally, interference of FZD2
expression led to inhibition of the malignant biological properties
of glioma cells including proliferation, migration, invasion, EMT,
VM formation and stemness. More notably, the importance of the
Notch/NF-κB signaling pathway in the oncogenic role of FZD2 in
glioma was confirmed.
Vascularization is crucial for the growth and
metastasis of tumors. Antiangiogenic therapy is a new treatment
method for glioma; however, the current antiangiogenic drugs,
including irinotecan and bevacizumab, are far from satisfactory due
to the existence of VM (24,25).
Hence, investigating the drugs/targets that restrict VM formation
may be a new avenue in the treatment of glioma, which has attracted
attention in recent years. For instance, Zhu et al (26) attributed the activity of celastrol,
a potential antitumor drug against glioma, to the inhibition of VM
formation and angiogenesis in glioma. Pan et al (27) indicated a potential role for
migration-inducing gene-7 (Mig-7) as a target in the treatment of
glioma as silencing Mig-7 inhibited cell invasion and VM formation
in glioma cells. In terms of FZD2, a study reported by Ou et
al (13) revealed that FZD2
could promote the clinically relevant VM phenotype while FZD2
knockdown suppressed the VM phenotype in hepatocellular carcinoma,
which was partially responsible for the oncogenic action of FZD2 in
hepatocellular carcinoma cells, preliminarily demonstrating the
impact of FZD2 on VM. Accordingly, in the present study, the
results also revealed that FZD2 knockdown significantly repressed
the VM formation of glioma cells. Meanwhile, FZD2 knockdown also
significantly restricted the malignant activities of glioma cells
by inhibiting the proliferation, migration and invasion
capabilities of glioma cells. Therefore, the oncogenic role of FZD2
in glioma may be associated with VM. Additionally, a small number
of cancer cells possessing stem cell-like properties are the
strongest angiogenic cells in tumors, accounting for the
development and recurrence of tumors (28). In particular, it has been observed
that glioma stem-like cells may act as progenitors for VM
formation, highlighting the association between VM and stemness in
glioma (29). Furthermore, a
previous study reported that FZD2-mediated malignant behaviors in
hepatocellular carcinoma were associated with VM and stemness
(13). In addition, FZD2 knockdown
disturbed migration, invasion and the mesenchymal-like phenotype of
breast cancer cells, confirming the oncogenic role of FZD2 in
breast cancer via the promotion of cell mesenchymal-like stemness
(15). In agreement with these
existing findings, the present study also demonstrated the
inhibitory effects of FZD2 knockdown on the stemness phenotype of
glioma cells. Taken together, the oncogenic role of FZD2 in glioma
may be associated with VM and stemness phenotypes, and the
inhibitory effects of FZD2 knockdown on the malignant behaviors of
glioma cells, including proliferation, migration, invasion and EMT,
may be partially associated with the restriction of these VM and
stemness phenotypes.
Notch signaling is a highly conserved pathway which
has a critical role in maintaining embryonic development and adult
tissue homeostasis and is involved in regulating multiple cellular
processes, including cell proliferation and differentiation, stem
cell maintenance and cell fate decisions (30,31).
Disrupted Notch signaling has been implicated in various
pathological diseases, including cancer. It has been proposed that
Notch is activated in the classical and proneural subtypes of
glioma, and that Notch signaling cross-talk with NF-κB p65
contributes to glioma growth (32,33).
The Notch signaling pathway can promote migration, invasion, growth
and the self-renewal of glioma cells, and inhibition of Notch is
suggested to be a promising target for restricting tumor growth
during glioma development (32-34).
For instance, NFIX circular RNA can promote glioma progression
through upregulating Notch signaling (35). Notably, it has also been
demonstrated that Notch signaling can exert a dual role in glioma,
serving as an oncogene or a tumor suppressor depending on the
intratumoral (stem) cell heterogeneity, disease stage and crosstalk
with other signaling pathways (36). In the present study, it was found
that FZD2 knockdown, which exerted antioncogenic activity in glioma
cells, significantly inhibited activation of the Notch signaling
pathway. Furthermore, JAG treatment partially inhibited the
antioncogenic activity of FZD2 knockdown in glioma cells,
confirming that inactivation of Notch signaling may be beneficial
to inhibit glioma development, and that Notch might be partially
responsible for the regulatory functions of FZD2 in the malignant
behaviors of glioma.
However, there were some limitations in the present
study. First, this study was only conducted using in vitro
cellular experiments to demonstrate the regulatory role of FZD2 in
glioma, and clinical validation and in vivo verification
will be beneficial to prove the significance of FZD2 in glioma.
Secondly, this study only demonstrated that the regulatory role of
FZD2 was dependent on the Notch/NF-κB signaling pathway, while
there might be multiple factors and pathways responsible for this
oncogenic role in glioma. Thus, more potential regulatory
mechanisms should be explored in further research. Finally, how
FZD2 affected the Notch/NF-κB pathway remains unclear. According to
the String (https://cn.string-db.org/) and
GENEMANIA (http://genemania.org/) websites,
multiple proteins were found to interact with FZD2, and of note,
FZD2 was closely related to Wnt pathway-related factors, such as
Wnt5a. The Wnt pathway is one of the important pathways affecting
Notch signaling output and the regulatory role of Wnt-Notch
signaling in cancer progression has been widely reported (37-39).
The Wnt pathway might act as a mediator between FZD2 and the
Notch/NF-κB pathway, but this hypothesis requires future
exploration.
In conclusion, taken together, the findings of the
present study may deepen the understanding of FZD2 in glioma. An
oncogenic role of FZD2 in glioma was identified and FZD2 knockdown
suppressed the proliferation, migration, invasion, VM formation and
stemness of glioma cells. In addition, FZD2 may contribute to the
malignant biological behaviors of glioma cells through activating
the Notch/NF-κB signaling pathway. The present study therefore may
provide novel ideas for developing therapeutic strategies for the
treatment of glioma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CL designed the experiments; YR, SH, DG and XC
collected and analyzed the data; YR and SH interpreted the data and
drafted the manuscript. CL revised the manuscript. CL and YR
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu S, Tang L, Li X, Fan F and Liu Z:
Immunotherapy for glioma: Current management and future
application: Cancer. Lett. 476:1–12. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo
Y, Ren D, Hua Y, Yu B, Zhou Y, et al: Mechanisms of vasculogenic
mimicry in hypoxic tumor microenvironments. Mol Cancer.
20(7)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lim D, Do Y, Kwon BS, Chang W, Lee MS, Kim
J and Cho JG: Angiogenesis and vasculogenic mimicry as therapeutic
targets in ovarian cancer. BMB Rep. 53:291–298. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li H, Wang D, Yi B, Cai H, Wang Y, Lou X,
Xi Z and Li Z: SUMOylation of IGF2BP2 promotes vasculogenic mimicry
of glioma via regulating OIP5-AS1/miR-495-3p axis. Int J Biol Sci.
17:2912–2930. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Morales-Guadarrama G, García-Becerra R,
Méndez-Pérez EA, García-Quiroz J, Avila E and Díaz L: Vasculogenic
mimicry in breast cancer: Clinical relevance and drivers. Cells.
10(1758)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Luo Y, Yang Z, Yu Y and Zhang P: HIF1α
lactylation enhances KIAA1199 transcription to promote angiogenesis
and vasculogenic mimicry in prostate cancer. Int J Biol Macromol.
222:2225–2243. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu Y, Li F, Yang YT, Xu XD, Chen JS, Chen
TL, Chen HJ, Zhu YB, Lin JY, Li Y, et al: IGFBP2 promotes
vasculogenic mimicry formation via regulating CD144 and MMP2
expression in glioma. Oncogene. 38:1815–1831. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cai HP, Wang J, Xi SY, Ni XR, Chen YS, Yu
YJ, Cen ZW, Yu ZH, Chen FR, Guo CC, et al: Tenascin-cmediated
vasculogenic mimicry formation via regulation of MMP2/MMP9 in
glioma. Cell Death Dis. 10(879)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Y, Liu Z and Zhang Y: Expression and
prognostic impact of FZDs in pancreatic adenocarcinoma. BMC
Gastroenterol. 21(79)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ou H, Chen Z, Xiang L, Fang Y, Xu Y, Liu
Q, Hu Z, Li X, Huang Y and Yang D: Frizzled 2-induced
epithelial-mesenchymal transition correlates with vasculogenic
mimicry, stemness, and Hippo signaling in hepatocellular carcinoma.
Cancer Sci. 110:1169–1182. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang L, Luo EL, Xie J, Gan RH, Ding LC,
Su BH, Zhao Y, Lin LS, Zheng DL and Lu YG: FZD2 regulates cell
proliferation and invasion in tongue squamous cell carcinoma. Int J
Biol Sci. 15:2330–2339. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yin P, Wang W, Gao J, Bai Y, Wang Z, Na L,
Sun Y and Zhao C: Fzd2 Contributes to breast cancer cell
mesenchymal-like stemness and drug resistance. Oncol Res.
28:273–284. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ding LC, Huang XY, Zheng FF, Xie J, She L,
Feng Y, Su BH, Zheng DL and Lu YG: FZD2 inhibits the cell growth
and migration of salivary adenoid cystic carcinomas. Oncol Rep.
35:1006–1012. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang K, Xu H, Han L, Xu R, Xu Z and Xie
Y: Identification of therapeutic targets and prognostic biomarkers
among frizzled family genes in glioma. Front Mol Biosci.
9(1054614)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li C, Tang Z, Zhang W, Ye Z and Liu F:
GEPIA2021: Integrating multiple deconvolution-based analysis into
GEPIA. Nucleic Acids Res. 49:W242–W246. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chandrashekar DS, Karthikeyan SK, Korla
PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne
U, et al: UALCAN: An update to the integrated cancer data analysis
platform. Neoplasia. 25:18–27. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao Z, Zhang KN, Wang Q, Li G, Zeng F,
Zhang Y, Wu F, Chai R, Wang Z, Zhang C, et al: Chinese glioma
genome atlas (CGGA): A comprehensive resource with functional
genomic data from Chinese glioma patients. Genomics Proteomics
Bioinformatics. 19:1–12. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tuluhong D, Chen T, Wang J, Zeng H, Li H,
Dunzhu W, Li Q and Wang S: FZD2 promotes TGF-β-induced
epithelial-to-mesenchymal transition in breast cancer via
activating notch signaling pathway. Cancer Cell Int.
21(199)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li Q, Wang J, Ma X, Wang M and Zhou L:
POFUT1 acts as a tumor promoter in glioblastoma by enhancing the
activation of notch signaling. J Bioenerg Biomembr. 53:621–632.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu S, Ruan X, Liu X, Zhang F, Wang D, Liu
Y, Yang C, Shao L, Liu Q, Zhu L, et al: HNRNPD interacts with ZHX2
regulating the vasculogenic mimicry formation of glioma cells via
linc00707/miR-651-3p/SP2 axis. Cell Death Dis.
12(153)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vredenburgh JJ, Desjardins A, Herndon JE
II, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S,
Gururangan S, Wagner M, et al: Phase II trial of bevacizumab and
irinotecan in recurrent malignant glioma. Clin Cancer Res.
13:1253–1259. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu Y, Liu X, Zhao P, Zhao H, Gao W and
Wang L: Celastrol suppresses glioma vasculogenic mimicry formation
and angiogenesis by blocking the PI3K/Akt/mTOR signaling pathway.
Front Pharmacol. 11(25)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pan Z, Zhu Q, You W, Shen C, Hu W and Chen
X: Silencing of Mig-7 expression inhibits in-vitro invasiveness and
vasculogenic mimicry of human glioma U87 cells. Neuroreport.
30:1135–1142. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Medina MA, Muñoz-Chápuli R and Quesada AR:
Challenges of antiangiogenic cancer therapy: Trials and errors, and
renewed hope. J Cell Mol Med. 11:374–382. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hori K, Sen A and Artavanis-Tsakonas S:
Notch signaling at a glance. J Cell Sci. 126:2135–2140.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Siebel C and Lendahl U: Notch signaling in
development, tissue homeostasis, and disease. Physiol Rev.
97:1235–1294. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hai L, Zhang C, Li T, Zhou X, Liu B, Li S,
Zhu M, Lin Y, Yu S, Zhang K, et al: Notch1 is a prognostic factor
that is distinctly activated in the classical and proneural subtype
of glioblastoma and that promotes glioma cell survival via the
NF-κB(p65) pathway. Cell Death Dis. 9(158)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang X, Chen T, Zhang J, Mao Q, Li S,
Xiong W, Qiu Y, Xie Q and Ge J: Notch1 promotes glioma cell
migration and invasion by stimulating β-catenin and NF-κB signaling
via AKT activation. Cancer Sci. 103:181–190. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yi L, Zhou X, Li T, Liu P, Hai L, Tong L,
Ma H, Tao Z, Xie Y, Zhang C, et al: Notch1 signaling pathway
promotes invasion, self-renewal and growth of glioma initiating
cells via modulating chemokine system CXCL12/CXCR4. J Exp Clin
Cancer Res. 38(339)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu H, Zhang Y, Qi L, Ding L, Jiang H and
Yu H: NFIX circular RNA promotes glioma progression by regulating
miR-34a-5p via notch signaling pathway. Front Mol Neurosci.
11(225)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Parmigiani E, Taylor V and Giachino C:
Oncogenic and tumor-suppressive functions of NOTCH signaling in
glioma. Cells. 9(2304)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gao J, Fan L, Zhao L and Su Y: The
interaction of notch and Wnt signaling pathways in vertebrate
regeneration. Cell Regen. 10(11)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Borggrefe T, Lauth M, Zwijsen A,
Huylebroeck D, Oswald F and Giaimo BD: The Notch intracellular
domain integrates signals from Wnt, hedgehog, TGFβ/BMP and hypoxia
pathways. Biochim Biophys Acta. 1863:303–313. 2016.PubMed/NCBI View Article : Google Scholar
|