Introduction
Cancer remains to be a leading cause of mortality globally, with both the incidence and mortality rates of cancer increasing from the 1990s to 2020(1). Ovarian cancer is a malignancy of the female reproductive system and accounts for ~4% of all new cancer diagnoses in women in the United States (2). Ovarian cancer is characterized by lesions located in the pelvic cavity and typically remains asymptomatic during the early stages of development (2). Consequently, a significant proportion of patients are first diagnosed already at advanced stages (Federation International of Gynecology and Obstetrics stage III-IV), which has resulted in an overall 5-year survival rate of <40% (3,4). The increased risk of ovarian cancer is associated with genetic factors such as Lynch Syndrome, reproductive factors such as nulliparity, hormonal factors such as long-term hormone replacement therapy and lifestyle factors such as obesity (5).
The standard treatment method for ovarian cancer is surgery, with aims of reducing the tumor burden, which is typically combined with systemic therapy, including chemotherapy, targeted drugs (such as bevacizumab and olaparib) and hormonal agents (such as tamoxifen and letrozole) (6). Despite advancements in the treatment options, the prognosis for patients with advanced ovarian cancer remains poor. One significant challenge in clinical practice is platinum resistance, which reduces the effectiveness of standard chemotherapy treatments that often include platinum-based drugs (such as cisplatin or carboplatin). Platinum resistance occurs when cancer cells develop the ability to withstand the effects of these drugs, leading to reduced treatment efficacy and limited options for effective therapy (6-8). Platinum compounds disrupt DNA replication and transcription through the formation of platinum-DNA adducts (9). The molecular mechanism underlying this process involves the disruption of DNA structures and the subsequent induction of destructive cellular responses, particularly apoptosis (9-11). These DNA adducts hinder DNA replication and transcription, leading to the activation of the DNA damage response (DDR). The DDR can arrest the cell cycle and initiate repair processes following acute sporadic DNA damage (9-11). However, extensive DNA damage will typically result in apoptosis. The p53 protein serves a crucial role in this process, since it can promote the transcription of genes, such as B-cell lymphoma 2 (bcl-2)-associated X protein, that lead to apoptosis when activated by DNA damage signals (9-11).
Platinum resistance mechanisms that have been reported to be applied by cancers include enhanced DNA repair, alterations in drug uptake and efflux and evasion of apoptosis (9-11). Moreover, a recent study added a layer of complexity to the current understanding of cisplatin resistance in ovarian cancer. Cisplatin-induced exosomes transfer long non-coding RNA-PANDAR and lead to a rapid adaptation of ovarian cancer cell survival through accumulating SRSF9 following cisplatin stress exposure. In other words, the exosomes are involved in the communications between ovarian cancer cells, aiming to develop platinum resistance (12). Enhanced DNA repair mechanisms, particularly through the restoration of homologous recombination, significantly contribute to platinum resistance in ovarian cancer (13). Secondary mutations in breast cancer susceptibility gene (BRCA) that restore the homologous recombination pathway have been shown to confer resistance to platinum-based therapies (13). In addition, alterations in drug uptake and efflux have been reported to promote developing platinum resistance. Overexpression of copper transporters adenosine triphosphatase copper transporting α polypeptide and ATPase copper transporting β polypeptide have been associated with reduced cisplatin accumulation in ovarian cancer cells, thereby diminishing its efficacy (14). Evasion of apoptosis is another documented mechanism of resistance to platinum compounds in ovarian cancer. Overexpression of Bcl-2, an anti-apoptotic protein, has been previously observed to protect cancer cells from cisplatin-induced apoptosis, which was reversed by targeting Bcl-2 using small interfering RNA (14).
Under physiological conditions, hepatocyte growth factor receptor [HGFR (also known as c-Met)] serves a critical role in various cellular responses to stress and damage, including those induced by chemotherapeutic agents (such as cisplatin). Activation of HGFR has been shown to promote cell survival and proliferation, which can mitigate the effects of cisplatin and contribute to drug resistance (15). The interactions between various protein components and cisplatin sensitivity, such as the suppression of HGFR expression through forkhead box protein P2, indicate additional layers of regulation that can impact chemosensitivity in cancer cells, highlighting potential targets for overcoming platinum resistance (15). These aforementioned factors highlight the requirement for overcoming platinum resistance in ovarian cancer treatment (9-11).
Platinum-resistant ovarian cancer can be divided into primary and secondary resistance (16). Primary platinum resistance accounts for 55.3% of recurrent ovarian cancer cases (17). The majority of patients with ovarian cancer will relapse within 3 years following initial platinum-based chemotherapy and subsequently develop resistance (18,19). The efficacy of platinum-based retreatment in such cases is <10%, highlighting a requirement for the development of novel effective treatments for platinum-resistant recurrent ovarian cancer (19,20).
Current treatment recommendations for patients with platinum-resistant recurrent ovarian cancer suggest several options. These include clinical trials, non-platinum chemotherapeutic drugs or supportive care for the treatment of patients with platinum-resistant recurrent ovarian cancer, with combinations of poly ADP-ribose polymerase (PARP) inhibitors, targeted therapy (such as bevacizumab and olaparib), immunotherapy (such as pembrolizumab) and chemotherapy (such as tegiol) all having been previously tested (6,7,21-23). The introduction of anti-angiogenesis treatments has shifted the treatment paradigm for ovarian cancer. In particular, apatinib, a small molecule tyrosine kinase inhibitor that can target VEGFR-2, has been demonstrated to improve survival and quality of life in patients with ovarian cancer (24,25). Apatinib exerts notable therapeutic effects and tolerable adverse reactions in patients with platinum-resistant ovarian cancer (26-28), suggesting potential as a novel treatment regimen for platinum-resistant recurrent ovarian cancer. Previous clinical trials have demonstrated the therapeutic effects of apatinib combined with various chemotherapy regimens in ovarian cancer or thyroid cancer (29,30). Furthermore, results of previous studies demonstrated that combination therapy improved the survival and disease control rates (DCR) in patients with platinum-resistant recurrent ovarian cancer. However, the DCR results differed between studies (28,31,32). Therefore, further investigations into the efficacy and safety of apatinib are required, with focus on adverse reactions, such as bone marrow suppression and hypertension. Notably, results of a previous study demonstrated that tyrosine kinase inhibitor (TKI) treatment was interrupted due to intolerance in a portion of patients, which may impact the therapeutic effect (33). Therefore, further investigations into the overall efficacy and safety of apatinib-based regimens are required.
To address the gap in effective treatment options for platinum-resistant recurrent ovarian cancer, the present study aimed to summarize the results of clinical studies into apatinib, with aims of evaluating the efficacy and safety of apatinib-containing regimens. In addition, the present study aimed to provide a theoretical basis for the effective treatment of patients with platinum-resistant recurrent ovarian cancer.
Materials and methods
Search strategy
PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/) and Wanfang Data (https://wanfangdata.com.cn/) databases were searched to identify studies focused on the treatment of platinum-resistant recurrent ovarian cancer using apatinib, spanning from the inception of these databases to April 2023. The Population, Intervention, Comparison, Outcomes and Study design framework (https://www.cochranelibrary.com) was used to refine the literature search. A combination of key words and free terms were used, including ‘ovarian cancer’, ‘ovarian tumors’, ‘apatinib’, ‘angiogenesis inhibitors’ and ‘anti-angiogenesis drugs’. This approach ensured the broad capture of relevant studies (Table SI).
Data sources
The present study included patients aged ≥18 years who were diagnosed with ovarian cancer of any pathological subtype. The interventions examined were apatinib, administered either alone or in conjunction with chemotherapy, compared with a control group receiving chemotherapy, apatinib alone or placebo. Outcomes of interest included efficacy indicators, such as overall response rate (ORR), DCR, progression-free survival (PFS) and overall survival (OS). Safety indicators were also examined, including drug-related adverse reactions categorized as per the National Cancer Institute Adverse Reaction General Terminology Standard (version 5.0) (34). Adverse events (AEs) were divided into the following grades: i) Grade 1, consisting of mild, asymptomatic or mild, no treatment required; ii) grade 2, which were considered moderate, requiring minor, local or non-invasive treatment; iii) grade 3, which are serious or medically important but not immediately life-threatening; iv) grade 4, which are considered life threatening, necessitating emergency treatment; and v) grade 5, which is mortality associated with AEs (34). Serious AEs were defined as grade ≥3.
Systematic reviews, meta-analyses, clinical guidelines and case reports were excluded from the present analysis. Studies with research content, research objects and interventions that did not meet the requirements of the present study were considered inconsistent and thus excluded. In addition, single-arm studies, animal experiments, in vitro cell experiments, studies with unscientific experimental designs (lack of control group or insufficient data reporting), repeated publications, studies with insufficient data and studies enrolling patients with other types of malignancies were also excluded.
Data extraction
Data extraction was performed by two independent researchers, with discrepancies resolved through consultation with a third researcher. This process gathered detailed information from each study, including demographics, treatment regimens and outcome measures. The extracted content included study title, first author, publication time, trial type [randomized controlled trials (RCTs)], retrospective nature, patient sex, patient age, regimen details in different treatment arms, sample size in each group, survival indicators (PFS and OS), efficacy indicators (ORR and DCR) and adverse reactions (type, grade and number of adverse reaction events in each group).
Literature quality assessment
Quality assessment was performed using the Cochrane risk of bias tool for RCTs (35) and the Newcastle-Ottawa Scale (NOS) (36) for retrospective studies, ensuring a thorough evaluation of study integrity. For RCTs, the Cochrane risk of bias assessment tool was also used to assess the risk of bias for each study. Each assessment included three outcomes, namely low risk, unclear risk of bias and high risk (35). The results of the quality evaluation were organized and the Cochrane risk assessment chart was generated using Review Manager software (version 5.4; The Cochrane Collaboration). For retrospective studies, the NOS was used, which assessed the risk of bias through assessing study selection, comparability and outcome. Studies scoring 7-9 would be considered high quality, those scoring 4-6 would be considered medium quality and studies scoring <4 would be considered low quality (37).
Statistical analysis
R (version 3.4.1; RStudio, Inc.) and GraphPad (version 9.0; Dotmatics) were used to analyze and present the data. Survival data (OS and PFS) were expressed as the mean ± 95% CI. The ORR was calculated using the formula: ORR=[number of patients with complete response (CR) + partial response (PR)]/total number of patients in each group. The DCR was calculated using the formula: DCR=(number of patients with CR + PR + stable disease)/total number of patients in each group. Direct and indirect network combined analysis was performed using odds ratio (OR) values calculated from the different arms. The results were expressed as OR and 95% CI. The number of patients who experienced adverse reactions in each group and the total number of patients in each group was used to calculate the incidence of adverse events. The risk ratio (RR) values calculated from each study were combined for meta-analysis and the results were expressed as RR and 95% CI. R software was used to conduct the network meta-analysis, generate a network diagram of each intervention, calculate the area under the cumulative ranking probability curve (SUCRA) and classify each intervention priority according to the SUCRA values of the ORR and DCR. Probability ranking was then performed, where the larger the SUCRA value, the more optimal the treatment regimen.
The R packages used in the analysis were as follows: GEMTC (38), DEMTAR (39), NETMETA (40) and RJAGS (41). Specifically, GEMTC was used for conducting network meta-analysis within a Bayesian framework, which allowed for the simultaneous comparison of multiple treatment options by integrating direct and indirect evidence. DMETAR facilitated the meta-analysis process by providing tools and functions for data preparation, network plotting, statistical analysis, model fitting, inconsistency checks and result summarization, which simplified the network meta-analysis. NETMETA was used to perform network meta-analysis using frequentist methods, providing an alternative approach to the Bayesian methods used in GEMTC, verifying the robustness of the results using different statistical techniques. RJAGS served as the interface to the JAGS library for Bayesian data analysis and was essential for running the complex Bayesian models built with GEMTC to estimate the treatment effects and their uncertainties, including parameter uncertainty, model uncertainty and sampling uncertainty in the network meta-analysis.
Heterogeneity was assessed using forest plots, Q-tests and I²-tests, with subsequent subgroup analyses used to explore potential sources of variability. P≥0.1 and I2>50% indicated a high risk of heterogeneity before the source of heterogeneity was subsequently investigated (35). If the source of heterogeneity could not be found, a random effects model was used for further analysis. If heterogeneity remained outside of the acceptable range, the quantitative analysis would be discarded and a qualitative descriptive interpretation was performed.
The risk of publication bias was investigated using a funnel plot. When the number of studies included in the analysis was ≥10, a funnel plot was generated to analyze the publication bias of the analysis. The Egger test was performed using STATA software (version 14; StataCorp LLC) to assess publication bias by examining funnel plot asymmetry through regression of the standard normal deviate of the treatment effect against its precision.
Results
Search results
The present systematic analysis yielded 271 articles. Following the removal of duplicates and screening based on the inclusion criteria, 221 documents, including meta-analyses, systematic reviews, guidelines, case reports, content inconsistencies and animal studies, were excluded. This resulted in 31 studies remaining (25-29,31,32,42-65). A review of the full texts led to the exclusion of 14 studies from this list due to the inclusion of reviews, single-arm studies or studies lacking complete trial data. In total, 17 studies (25,28,45,49-53,55-58,61-65) were included in the present analysis (Fig. 1).
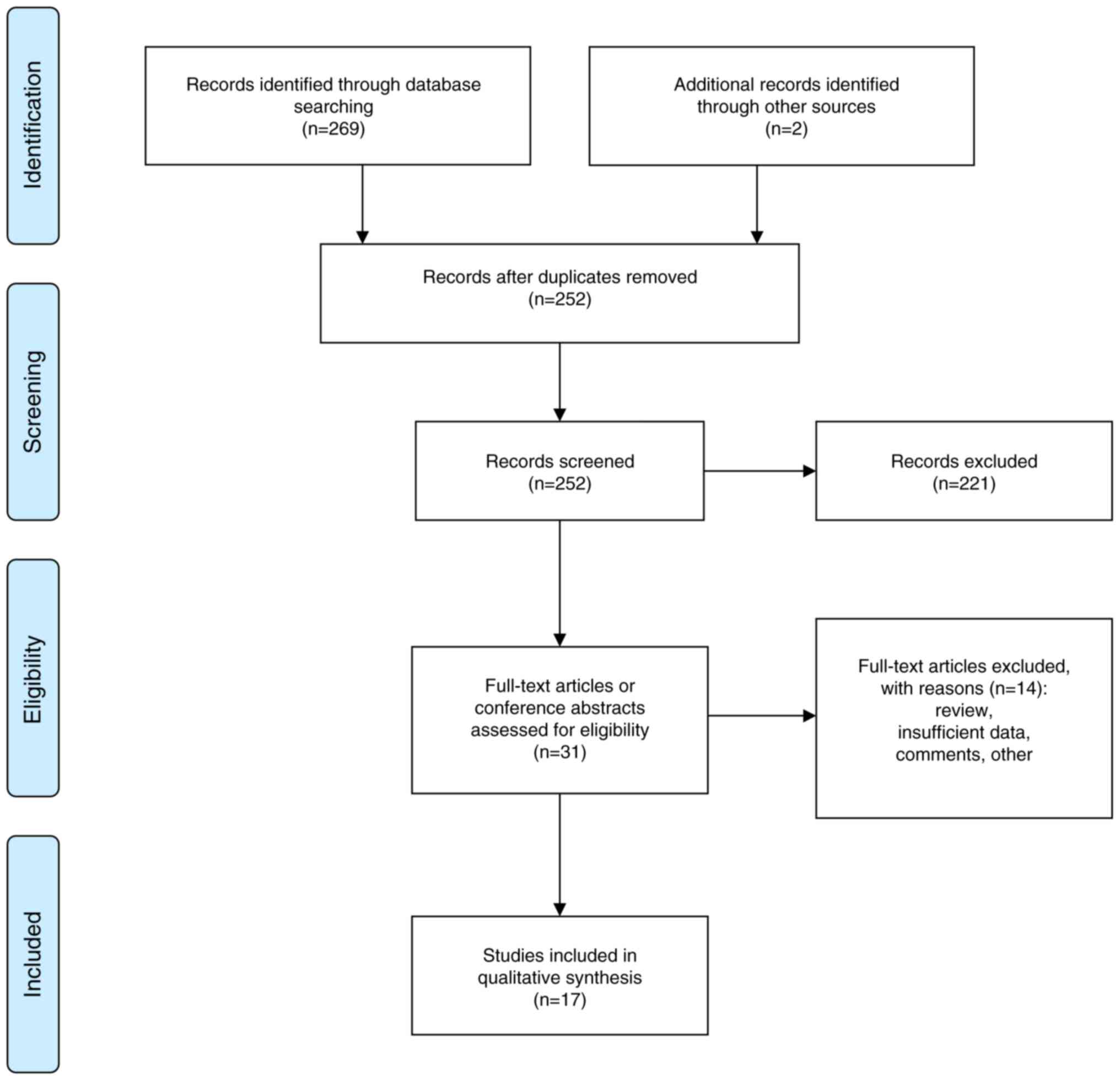 |
Figure 1
Flow chart of the study selection process.
|
Baseline information of the included studies
The final selection comprised seven prospective (25,50,55,58,61-63) and 10 retrospective studies (28,45,49,51-53,56,57,64,65). These included three studies comparing apatinib monotherapy with chemotherapy, seven studies evaluating apatinib combined with chemotherapy compared with apatinib alone and 10 studies assessing apatinib combined with chemotherapy compared with chemotherapy alone in the treatment of platinum-resistant recurrent ovarian cancer (Table I). Follow-up information was reported in 8 studies, with an average follow-up duration of 16 months. The outcomes reported across these studies varied, with 17 studies focusing on ORR, 16 studies focusing on DCR, nine studies focusing on OS, 12 studies focusing on PFS and 12 studies focusing on adverse reaction indicators. The present analysis included nine treatment regimens, involving 816 participants in the experimental groups (both apatinib with chemotherapy and apatinib alone) and 740 participants in the control groups (chemotherapy alone). Specific studies, such as Wu and Chen (62), were divided into multiple arms for analysis, one comparing the combination therapy with apatinib alone (Wu-a 2022) and another comparing combination therapy with chemotherapy alone (Wu-b 2022). Similarly, the study performed by Guo et al (58) was segmented into combination therapy compared with apatinib alone (Guo-a 2022) and combination therapy compared with chemotherapy (Guo-b 2022). Safety indicators analyzed in the present study included hand-foot syndrome, hypertension, serious adverse reactions, neutropenia, vomiting and nausea, proteinuria and oral mucositis.
 |
Table I
Baseline characteristics of the included studies of patients with platinum-resistant ovarian cancer in China.
|
Table I
Baseline characteristics of the included studies of patients with platinum-resistant ovarian cancer in China.
| First author/s, year |
Study type |
Number of patients, n |
Age, yearsa |
Follow-up duration, months |
Treatment strategy |
Outcomes measured |
(Refs.) |
| Chen et al, 2019 |
RCT |
72 |
NA |
9 |
Apatinib vs. CT |
Efficacy, survival, AEs |
(25) |
| Zhang et al, 2019 |
RCT |
60 |
35-70 |
NA |
Apatinib + CT vs. Apatinib + CT |
Efficacy, AEs |
(50) |
| Zhang et al, 2019 |
Retrospective |
28 |
59 |
9.5 |
Apatinib + CT vs. apatinib |
Efficacy, survival, AEs |
(49) |
| Yang et al, 2019 |
Retrospective |
22 |
55.6 |
NA |
Apatinib + CT vs. apatinib |
Efficacy, survival, AEs |
(45) |
| Yuan et al, 2020 |
Prospective |
44 |
NA |
NA |
Apatinib + CT vs. apatinib |
Efficacy, survival |
(55) |
| Li, et al, 2020 |
Retrospective |
64 |
NA |
10.1 |
Apatinib + CT vs. CT |
Efficacy, survival, AEs |
(51) |
| Li, et al, 2020 |
Retrospective |
63 |
NA |
NA |
Apatinib + CT vs. CT |
Efficacy, survival, AEs |
(52) |
| Qiu et al, 2020 |
Retrospective |
90 |
47.39 |
36 |
Apatinib + CT vs. apatinib |
Efficacy |
(53) |
| Song and Wang 2021 |
Retrospective |
60 |
NA |
NA |
Apatinib + CT vs. CT |
Efficacy, AEs |
(56) |
| Wang et al, 2021 |
Retrospective |
41 |
53 |
NA |
Apatinib + CT vs. apatinib |
Efficacy, survival |
(57) |
| Wang et al, 2022 |
Prospective |
146 |
22-76 |
8.7 |
Apatinib + CT vs. CT |
Efficacy, survival, AEs |
(61) |
| Yan and He, 2022 |
Prospective |
74 |
35-80 |
NA |
Apatinib + CT vs. CT |
Efficacy, AEs |
(63) |
| Guo et al, 2022 |
RCT |
66 |
NA |
NA |
Apatinib + CT vs. CT vs. apatinib |
Efficacy, survival, AEs |
(58) |
| Wu and Chen, 2022 |
RCT |
115 |
NA |
4-52 |
Apatinib + CT vs. CT vs. apatinib |
Efficacy, survival, AEs |
(62) |
| Geng et al, 2022 |
Retrospective |
70 |
NA |
11 |
Apatinib + CT vs. CT |
Efficacy, survival, AEs |
(28) |
| Pan et al, 2023 |
Retrospective |
108 |
NA |
28 |
Apatinib + CT vs. CT |
Efficacy, survival |
(64) |
| Yang et al, 2023 |
Retrospective |
105 |
NA |
NA |
Apatinib + CT vs. CT |
Efficacy, survival, AEs |
(65) |
Quality evaluation
The Cochrane quality assessment tool was used to appraise the quality of the RCTs included in the present meta-analysis. Of the studies analyzed, four (25,50,58,62) were identified as RCTs and three (55,61,63) were prospective in design. All RCTs disclosed their methods of randomization, which ensured the validity of group allocations. These trials were conducted as open-label studies, with two trials (25,61) providing detailed accounts of dropout cases along with the reasons (such as withdrawal of consent) for these dropouts. The reported number of dropouts was minimal and insufficient to compromise the comparative balance among the study groups. Overall, these studies were considered high quality according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (35). The bias risk assessment results were analyzed (Figs. S1 and S2). These studies were at low risk of selection bias, detection bias and attrition bias, at moderate risk of selection bias and reporting bias and at high risk of performance bias, as blinding of participants and personnel was often not feasible. Notably, a prospective design was used in four (50,55,58,63) studies, which had higher risk of reporting bias compared with that in the other prospective studies.
Regarding the retrospective cohort studies, evaluation using the NOS indicated that these studies met the essential criteria for selection, comparability and exposure. Notably, limitations included variations in follow-up times and failure to report survival outcomes (Tables I and SII).
A total of nine studies (45,50,52,55-58,63,65) did not report the specific follow-up period and had a higher risk of attrition bias and reporting bias compared with the other eight studies. Of the nine aforementioned studies, five (45,52,56,57,65) had a retrospective design and their NOS scores were ranged from 6 to 7, whilst the other five (28,49,51,53,64) studies from this retrospective group had higher scores, ranging from 7 to 9. The lack of specified follow-up periods in nine of the studies could be due to various reasons, such as the primary focus being on short-term outcomes, variability in patient follow-up times or inadequate reporting of follow-up information. The lack of specified follow-up periods potentially impacted the quality and introduced a risk of bias in several manners. It may have increased the risk of bias in outcome reporting. In addition, it may have increased the difficulty of comparative analysis. The absence of uniform follow-up periods could complicate comparisons among studies, potentially skewing results of pooled data analyses.
Meta-analysis results
The present analysis investigated a total of 17 studies on apatinib in the treatment of platinum-resistant recurrent ovarian cancer that were focused on ORR and DCR as primary outcome measures. Network diagrams were generated using different outcome indicators (Fig. 2).
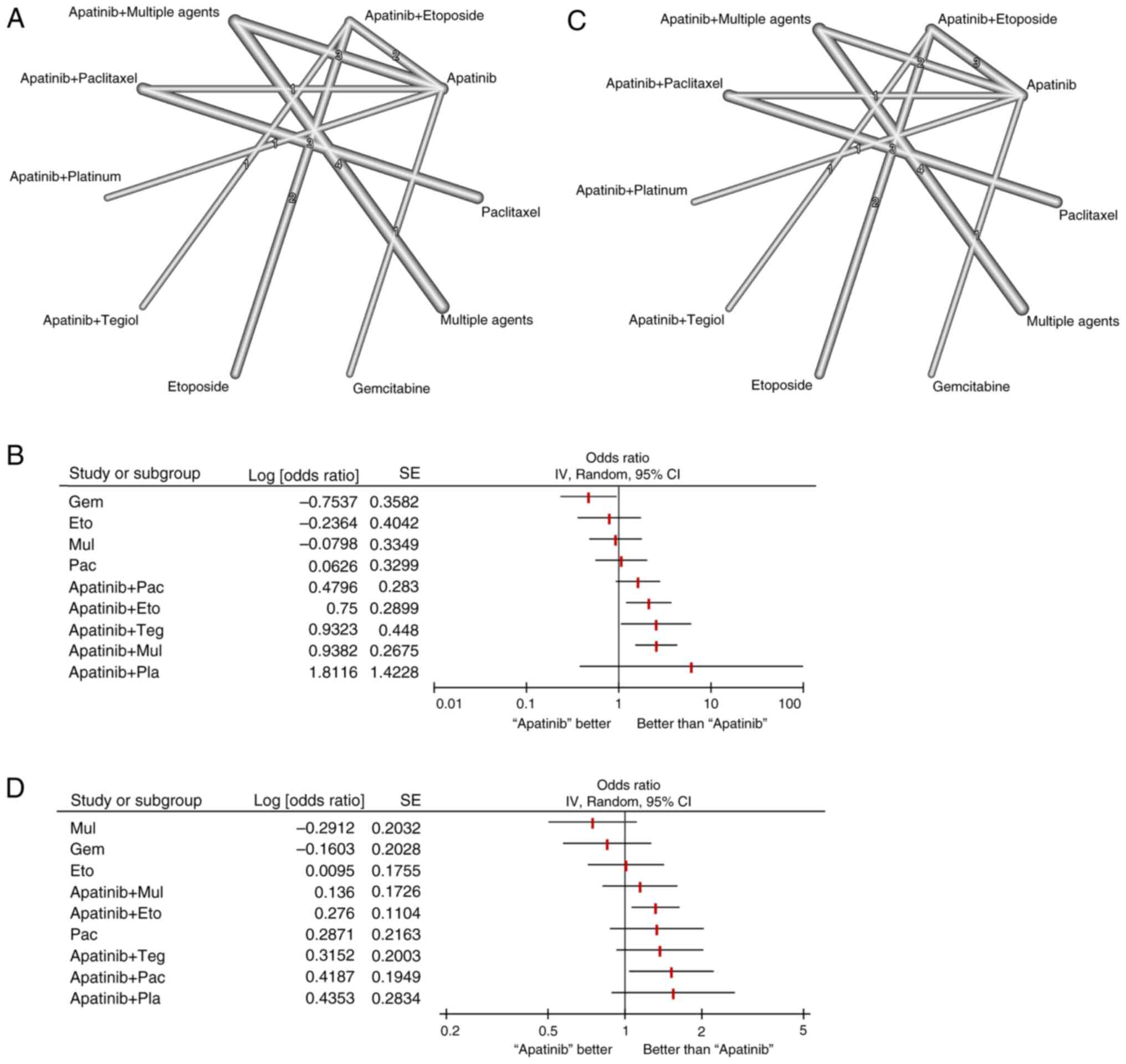 |
Figure 2
Network meta-analysis of efficacy indicators in patients with platinum-resistant ovarian cancer who were treated with an apatinib-based regimen. (A) Network plot of the overall response rate comparing the different treatment arms, (B) Network meta-analysis of the overall response rate, (C) Network plot of the disease control rate comparing the different treatment arms and (D) Network meta-analysis of the disease control rate. Dots represent a specific intervention and a straight line connecting two dots indicates a direct comparison between the two interventions. The thicker the line, the higher the number of studies that directly compared the two interventions. OR, odds ratio; Eto, Etoposide; Pla, Platinum; Pac, Paclitaxel; Gem, Gemcitabine; Dox, Doxorubicin; Mul, Multiple chemotherapy agents; Teg, Tegiol.
|
In total, nine individual treatment arms were compared, including gemcitabine, etoposide, apatinib, paclitaxel, multiple chemotherapeutic agents, apatinib plus multiple chemotherapeutic agents, apatinib plus etoposide, apatinib plus tegiol and apatinib plus paclitaxel nodes (Fig. 2A). In the present analysis, apatinib was used as the reference arm (Table SIII). Results of the present study demonstrated that the conventional chemotherapy drug gemcitabine (OR, 0.47; 95% CI, 0.23-0.95) demonstrated a significantly lower ORR compared with that in the apatinib group (P<0.05), suggesting that compared to gemcitabine, apatinib may significantly improve the ORR of patients with platinum-resistant recurrent ovarian cancer (Fig. 2B). Other regimens containing apatinib, such as apatinib plus etoposide (OR, 2.12; 95% CI, 1.20-3.74), apatinib plus tegiol (OR, 2.54; 95% CI, 1.06-6.11) and apatinib plus multiple chemotherapeutic agents (OR, 2.56; 95% CI, 1.51-4.32) also demonstrated a significantly improved ORR, compared with that in apatinib alone (P<0.05). Apatinib plus paclitaxel markedly improved ORR compared with that in apatinib alone (OR, 1.62; 95% CI, 0.93-2.81; Fig. 2B). In terms of DCR, nine individual treatment arms were compared (Fig. 2C). Apatinib plus paclitaxel exhibited the highest significant OR (OR, 1.52; 95% CI, 1.04-2.23), followed by apatinib plus etoposide (OR, 1.32; 95% CI, 1.06-1.64), compared with that in apatinib alone (P<0.05 for all; Fig. 2D). Similarly, apatinib plus tegiol, apatinib plus multiple chemotherapeutic agents and paclitaxel alone markedly improved DCR compared with that in apatinib alone (Table SIV).
The results of the net rank analysis shows the probabilities (%) of each treatment being ranked from best to worst, based on 1,000 simulations, accounting for parameter uncertainty. Results of the net rank analysis of the ORR demonstrated that apatinib plus tegiol ranked the highest, apatinib plus multiple chemotherapeutic agents ranked second and apatinib plus etoposide ranked third, which was followed by apatinib plus paclitaxel and other regimens (the first bar in each group represents the best, while the last bar represents the worst rank) (Fig. 3A). Notably, these results were consistent with results of the network meta-analysis with regard to the improvemnet of ORR. Results of the net rank analysis of the DCR demonstrated that the apatinib plus paclitaxel treatment group exhibited the highest DCR, with apatinib plus tegiol ranking second and apatinib plus etoposide ranking third (Fig. 3B).
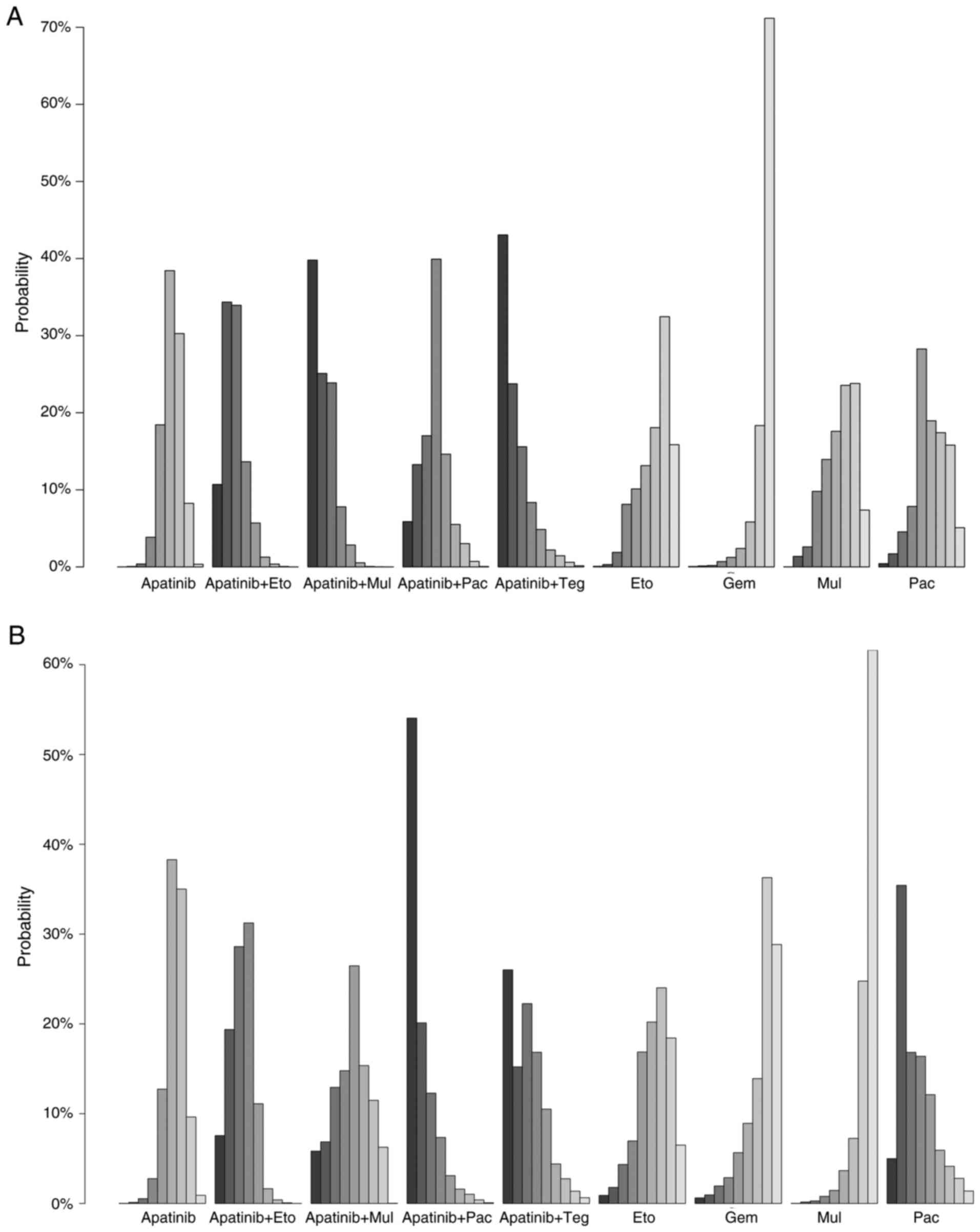 |
Figure 3
Net-rank plots of efficacy indicators in patients with platinum-resistant ovarian cancer treated with apatinib-based regimen. Net-rank plots for (A) overall response rate and (B) disease control rate. The net rank data show rank probabilities where each bar represents the probability of each treatment being at a specific rank. Eto, etoposide; Pla, platinum; Pac, paclitaxel; Gem, gemcitabine; Dox, doxorubicin; Mul, multiple chemotherapy agents; Teg, tegiol.
|
The efficacy of each intervention was next categorized according to the SUCRA value. Results of the present study demonstrated that the ORR of each treatment regimen was as follows: Apatinib plus multiple chemotherapeutic agents, 0.79; apatinib plus tegiol, 0.78; apatinib plus etoposide, 0.72; apatinib plus paclitaxel, 0.60; paclitaxel, 0.35; apatinib, 0.32; multiple chemotherapeutic agents, 0.29; etoposide, 0.24; and gemcitabine, 0.06 (Fig. 4A). The combination of apatinib with multiple chemotherapeutic agents and tegiol demonstrated the highest efficacy in terms of ORR among the treatment regimens evaluated, while gemcitabine showed the lowest efficacy. The DCR of each treatment regimen was as follows: Apatinib plus paclitaxel, 0.84; apatinib plus tegiol, 0.71; apatinib plus etoposide, 0.68; paclitaxel, 0.64; apatinib plus multiple chemotherapeutic agents, 0.50; etoposide, 0.32; apatinib, 0.29; gemcitabine, 0.17; and multiple chemotherapeutic agents, 0.07 (Fig. 4B). The results indicated that the combination of apatinib with paclitaxel had the highest DCR among the treatment regimens evaluated, whereas multiple chemotherapeutic agents alone have the lowest DCR.
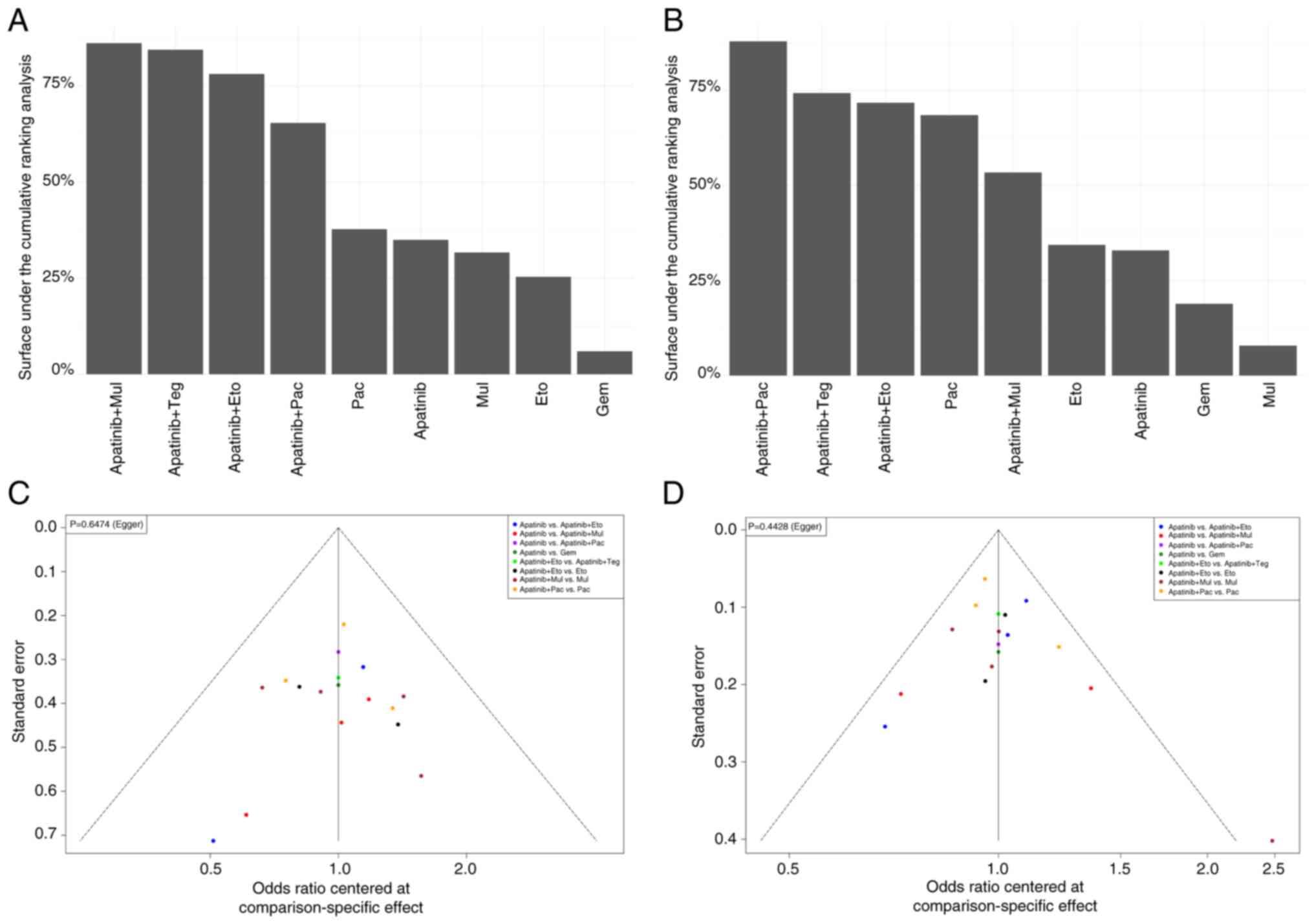 |
Figure 4
SUCRA and publication bias analysis. Surface under the cumulative ranking analysis for (A) overall response rate and (B) disease response rate. Funnel plot for (C) overall response rate and (D) disease control rate. SUCRA, Surface under the cumulative ranking analysis. Eto, etoposide; Pla, platinum; Pac, paclitaxel; Gem, gemcitabine; Dox, doxorubicin; Mul, multiple chemotherapy agents; Teg, tegiol.
|
A funnel plot was used to analyze the ORR and DCR indicators of apatinib combined with chemotherapy, compared with chemotherapy alone or apatinib alone for the treatment of platinum-resistant recurrent ovarian cancer (Fig. 4C and D). The dot on the right of the funnel plot represents a study with higher odds ratio and/or larger sample size. The studies represented by each point fell within each side of the midline and the funnel plot was almost symmetrical, suggesting that the present study may have a low risk of publication bias. Results of the Egger test demonstrated a P-value of 0.65 for ORR and a P-value of 0.44 for DCR.
The present analysis investigated a total of 10 studies on apatinib in the treatment of platinum-resistant recurrent ovarian cancer that were focusing on OS as a secondary outcome measure (Fig. 5A) and 12 studies that were focusing on PFS as a secondary outcome measure (Fig. 5B). Descriptive methods were used and the results demonstrated that the median OS time of patients in the apatinib plus chemotherapy group ranged from 7.9-23.0 months, compared with 6.0-14.8 months for the chemotherapy group and 6.1-11.6 months for the apatinib group. Wang et al (61) reported that patients in the apatinib plus pegylated liposomal doxorubicin group exhibited a median OS time of 23.0 months (Fig. 5A). The combination of apatinib with chemotherapy, particularly with pegylated liposomal doxorubicin, significantly improves median OS in patients compared with chemotherapy or apatinib alone, indicating enhanced efficacy of the combination treatment. The median PFS time of patients in the apatinib plus chemotherapy group was 5.0-9.7 months, compared with 3.3-6.0 months for the chemotherapy group and 3.0-8.0 months for the apatinib group (Fig. 5B). The combination of apatinib with chemotherapy significantly improves median PFS time in patients compared to chemotherapy or apatinib alone, suggesting enhanced efficacy of the combination treatment in delaying disease progression.
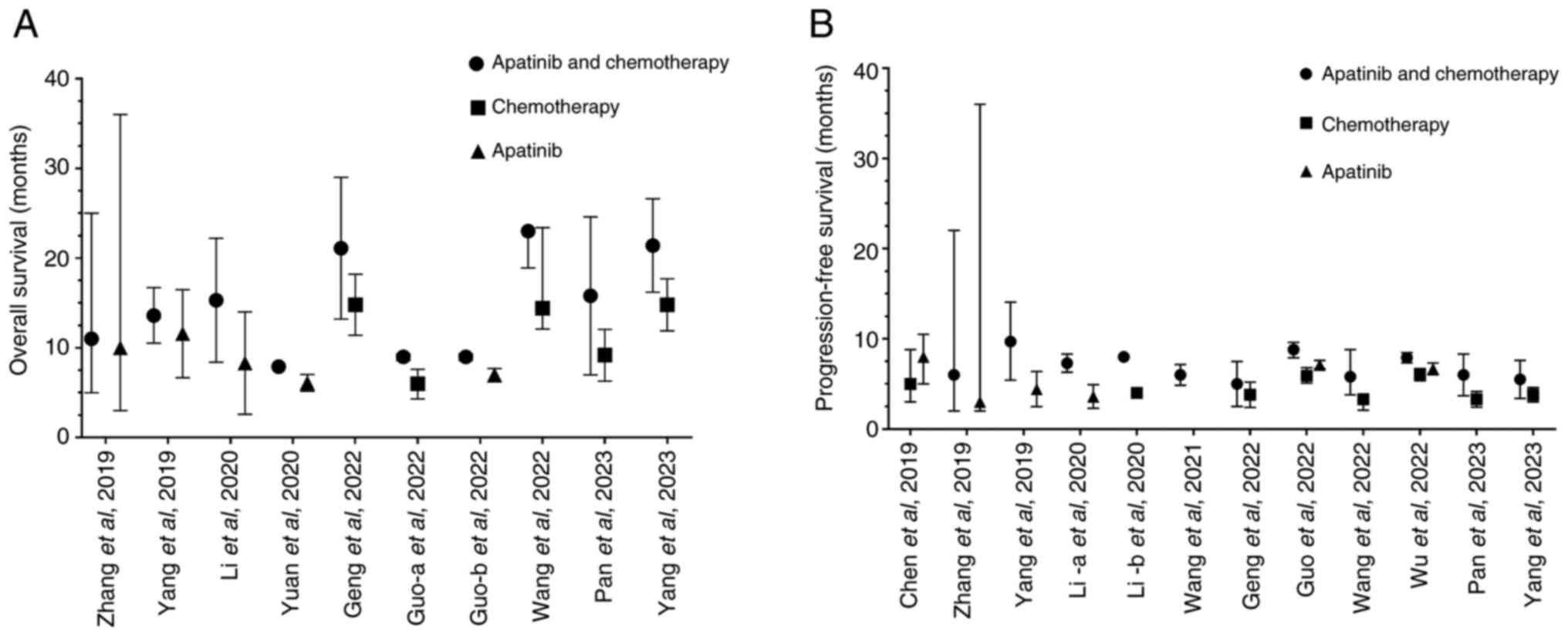 |
Figure 5
Summary of survival outcomes in patients with platinum-resistant ovarian cancer who were treated with an apatinib-based regimen. (A) Overall survival and (B) progression-free survival. Data are presented as the mean and 95% confidence interval.
|
AEs
With regards to AEs, studies were grouped according to treatment and control arms. The first group was defined as apatinib with or without chemotherapy, compared with chemotherapy alone (Fig. 6) and the second group was defined as apatinib with chemotherapy, compared with apatinib alone (Fig. 6). Compared with that in the chemotherapy group, groups treated with apatinib alone exhibited a significantly higher risk of hand and foot syndrome (RR, 4.23; 95% CI, 1.80-9.95) and hypertension (RR, 4.80; 95% CI, 1.53-15.05). However, the risk of nausea and vomiting (RR, 0.65; 95% CI, 0.13-3.29), neutropenia (RR, 1.53; 95% CI, 0.51-4.58), oral mucositis (RR, 0.95; 95% CI, 0.26-3.42) and proteinuria (RR, 1.47; 95% CI, 0.27-7.93) were comparable between the two groups.
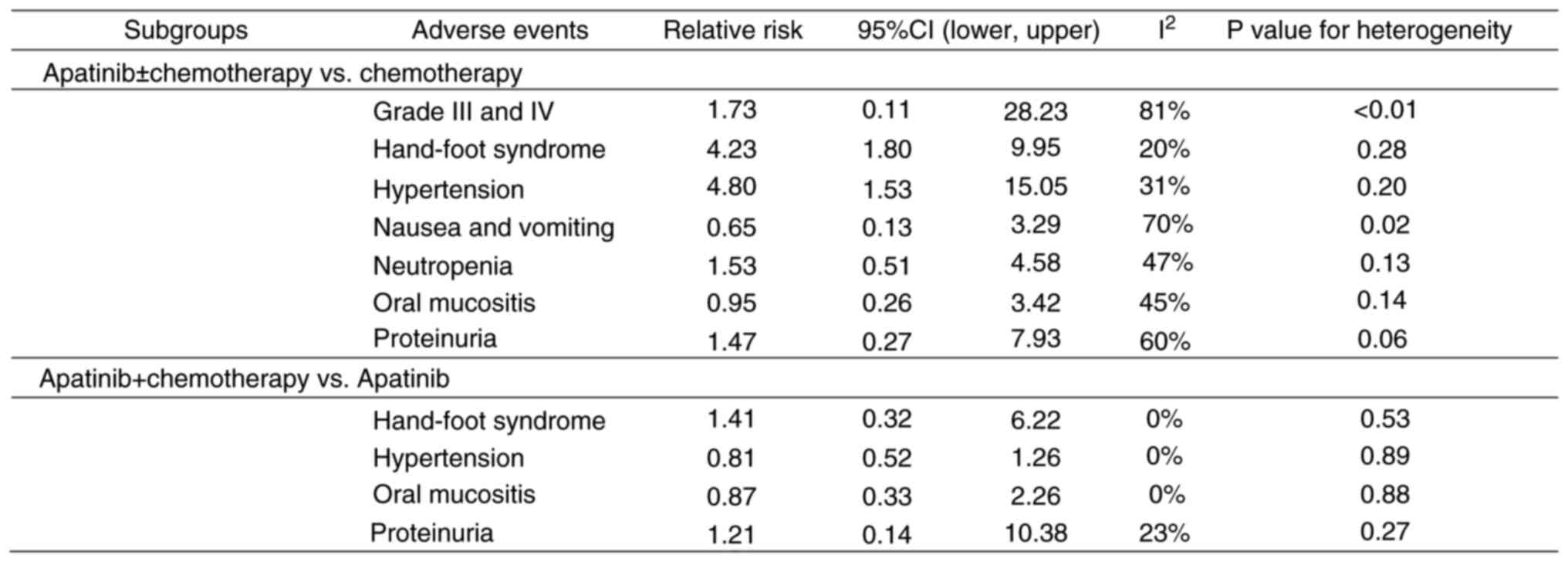 |
Figure 6
Adverse events in patients with platinum-resistant ovarian cancer who were treated with an apatinib-based regimen. CI, confidence interval.
|
Compared with the group treated with apatinib alone, all combination apatinib-based regimens were not significantly associated with increased risks of hand and foot syndrome (RR, 1.41; 95% CI, 0.32-6.22), hypertension (RR, 0.81; 95% CI, 0.52-1.26), oral mucositis (RR, 0.87; 95% CI, 0.33-2.26) and proteinuria (RR, 1.21; 95% CI, 0.14-10.38).
Discussion
Platinum resistance contributes to the mortality of patients with ovarian cancer. The development of platinum resistance in ovarian cancer involves multiple mechanisms, including enhanced DNA repair, reduced drug accumulation, increased detoxification and the evasion of apoptosis. These adaptations allow initially sensitive cancer cells to survive and proliferate despite ongoing platinum-based chemotherapy which may lead to resistance (18,19). Platinum resistance, characterized by disease recurrence within 6 months after achieving a complete or partial response to first-line platinum chemotherapy, leads to challenges in the management of ovarian cancer (18,19). The efficacy of chemotherapy in these patients is notably diminished, highlighting the requirement for novel innovative and more effective therapeutic strategies (22). Apatinib, which is mainly utilized for the treatment of ovarian cancer in China (59,62,66), exhibits potential for the treatment of patients with platinum-resistant recurrent ovarian cancer. The absence of direct head-to-head clinical trials led to the indirect network meta-analysis applied in the present study, which aimed to evaluate the efficacy of apatinib in combination with chemotherapy and analyze the risk of associated adverse reactions.
The present meta-analysis included 17 clinical studies and the results demonstrated a significant improvement in DCR following apatinib monotherapy, particularly when combined with tegiol, compared with that in the gemcitabine chemotherapy group. Notably, four studies (57,58,64,65) also reported that in the population receiving apatinib treatment, the apatinib combined with chemotherapy group exhibited the highest level of efficacy. In addition, apatinib, particularly in conjunction with chemotherapy, displayed superior efficacy in improving both the DCR and ORR, demonstrating the potential of apatinib combined with tegiol and paclitaxel as leading treatment options based on SUCRA value rankings. Therefore, for patients with platinum-resistant recurrent ovarian cancer, apatinib combined with chemotherapy may be considered as a potential treatment strategy.
In addition, the results of multiple studies on the effects of apatinib in patients with platinum-resistant recurrent ovarian cancer suggested that the apatinib treatment group exhibited an improved rate of survival (58,59,62,63,66,67). Specifically, the combined treatment groups demonstrated enhanced OS and PFS compared with the controls (apatinib or CT). Despite the inability to conduct a network meta-analysis on hazard ratios due to data limitations (only a few of the included studies reported these data), these findings emphasized the potential of apatinib in managing patients with platinum-resistant recurrent ovarian cancer.
In the present meta-analysis, studies were divided into multiple subgroups according to adverse reactions, including hand-foot syndrome, hypertension, incidence of serious adverse reactions, nausea and vomiting, neutropenia, oral mucositis and proteinuria. Adverse reaction analysis identified hand-foot syndrome and hypertension as being more prevalent in the apatinib combination therapy group compared with those in the group treated with chemotherapy alone. However, no significant differences were observed in the incidence of the other adverse reactions assessed. Compared with the group treated with apatinib alone, the apatinib combination group did not exhibit a significantly increased risk for the development of hand-foot syndrome, hypertension, oral mucositis or proteinuria. These results suggest that apatinib, particularly in combination with other treatments, is well-tolerated and safe for patients with platinum-resistant recurrent ovarian cancer.
The clinical application of targeted therapeutic drugs, such as apatinib, highlight their potential in combination with other therapies for the treatment of advanced stage diseases, such as thyroid cancer and ovarian cancer (30,68-70). For ovarian cancer patients with advanced stage disease, combination therapies may be used to achieve an improved therapeutic effect. Numerous clinical trials are ongoing to assess the novel treatment strategies for various cancers (71,72). For example, Li et al (71) investigated apatinib antiangiogenic therapy for its predictive adverse events in different cancer types, while Wu and Fang (72) evaluated the efficacy of a combination of paclitaxel, tegiol and apatinib in the conversion therapy for unresectable advanced gastric cancer. The clinical application of apatinib in platinum-resistant recurrent ovarian cancer has demonstrated notable antitumor effects without significantly increasing the risk of adverse reactions in the present study, except hypertension and hand-foot syndrome. In particular, apatinib has exhibited clinical therapeutic benefits in a variety of tumor types. Low-dose apatinib combined with first-line chemotherapy for the conversion treatment of advanced gastric cancer was found to improve anti-tumor efficacy, reduced tumor marker levels, improved R0 resection rate and short-term survival rate whilst exhibiting a good safety profile (73). In addition, apatinib prolonged PFS and OS whilst improving the ORR and DCR in patients with advanced ovarian cancer (49,51). However, apatinib may increase the incidence of hypertension, hand-foot syndrome and proteinuria, compared with conventional chemotherapy (74). In patients with recurrent cervical cancer treated with paclitaxel plus cisplatin chemotherapy, apatinib combined with chemotherapy was found to improve the ORR compared with bevacizumab plus chemotherapy, and exhibited an improved safety profile (75).
Although the included studies in the present meta-analysis did not report on the BRCA status of the participants, it is a critical factor in understanding the physiology and treatment response of patients with ovarian cancer. BRCA testing is crucial in the management of ovarian cancer due to its implications for targeting treatment and preventive strategies (13). Previous studies have underscored the importance of identifying BRCA1 and BRCA2 mutations for cancer treatment, as they can influence treatment choices and familial risk management (13,76). These mutations can predispose individuals to ovarian and breast cancers, meaning that their identification can facilitate tailored therapeutic interventions, particularly with PARP inhibitors, which are effective due to their mechanism of synthetic lethality in BRCA-mutated cancers (13,76). Therefore, current medical guidelines, including those from the National Comprehensive Cancer Network, strongly recommend genetic testing for all women diagnosed with epithelial ovarian cancer (21,77,78). The absence of BRCA status reporting in the included studies did not result from the selective inclusion of studies. Instead, the primary focus of these studies was to evaluate the efficacy and safety of apatinib-containing regimens for the treatment of platinum-resistant ovarian cancer, irrespective of BRCA mutation status. There may be several reasons for this condition. The study design and objectives may be a contributor. A number of the studies may have been designed to focus on the treatment outcomes that were considered to be independent of genetic factors. In addition, the reporting of BRCA status may have been omitted if the authors considered it to not associate with the primary endpoints of response to apatinib. Another factor could be patient population diversity. Depending on the geographic and demographic diversity in the study populations, BRCA testing practices may vary, where some may not routinely include this as part of the initial cancer evaluation, impacting the availability of this data. Given the relevance of BRCA status, it is important for future studies to include this information to more effectively stratify patient responses to treatments and tailor therapeutic approaches accordingly. Including BRCA status may also enhance understanding into the efficacy of new treatments, such as apatinib, in patients with platinum-resistant ovarian cancer.
The present study has a number of limitations. Multiple studies with small sample sizes were included. The lack of large-scale, multi-center trials may have introduced heterogeneity and selection bias. In addition, only studies conducted in China were included in the present study, which may have limited the applicability of findings to other populations. Discrepancies in study design, patient baseline characteristics and apatinib dosages highlight the requirement for more comprehensive clinical trials to further support the use of apatinib for the treatment of platinum-resistant recurrent ovarian cancer. A number of studies also did not explain the randomization and blinding process, which may have resulted in selection bias. In the studies included in the present analysis, heterogeneity in OS and PFS were observed, which may be attributed to the inclusion of retrospective studies. Of note, nine of the included studies monitored the patients after the treatment has been applied. However, they did not specify the exact mean follow-up duration in their published results. These studies assessed the efficacy, survival and safety profiles of apatinib-containing regimens without providing the detailed follow-up data. The lack of specified follow-up periods could be due to various reasons, such as the primary focus being on short-term outcomes, variability in patient follow-up times or inadequate reporting of follow-up information. Therefore, large-scale clinical trials are required to address this.
In conclusion, the results from the present meta-analysis suggest that apatinib, in combination with chemotherapy, may exhibit potential as an effective treatment strategy for platinum-resistant recurrent ovarian cancer in the future. However, further large-scale RCTs are required to confirm the efficacy and safety profile of this treatment regime.
Supplementary Material
Risk of bias summary of the included randomized controlled trials. The green ‘+’ signs and green circles indicate a low risk of bias. The red ‘-’ signs and red circles indicate a high risk of bias.
Risk of bias graph of the included randomized controlled trials.
PubMed search strategya.
Newcastle-Ottawa Scale assessment results.
Results of the network meta-analysis of objective response rate.
Results of network meta-analysis of disease control rate.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key Project of Medical and Health Science and Technology Plan of Xiangyang Municipal Science and Technology Bureau (grant no. 2022 YL 49 A).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
All authors participated in the design process, discussion of the study and manuscript writing. WW, FL and YZ designed the search strategy and performed the search. FL, SQ, YJ and YZ collected the data and participated in the data analysis. WW and YZ confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ: Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 134:783–791. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Berek JS and Bast RC Jr: Epithelial ovarian cancer. In: Holland-Frei Cancer Medicine. 6th edition, BC Decker, 2003.
|
|
3
|
Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, Myers T, Taskiran C, Robison K, Mäenpää J, et al: Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: Placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol. 39:1842–1855. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rinne N, Christie EL, Ardasheva A, Kwok CH, Demchenko N, Low C, Tralau-Stewart C, Fotopoulou C and Cunnea P: Targeting the PI3K/AKT/mTOR pathway in epithelial ovarian cancer, therapeutic treatment options for platinum-resistant ovarian cancer. Cancer Drug Resist. 4:573–595. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gaona-Luviano P, Medina-Gaona LA and Magaña-Pérez K: Epidemiology of ovarian cancer. Chin Clin Oncol. 9(47)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kuroki L and Guntupalli SR: Treatment of epithelial ovarian cancer. BMJ. 371(m3773)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kurnit KC, Fleming GF and Lengyel E: Updates and new options in advanced epithelial ovarian cancer treatment. Obstet Gynecol. 137:108–121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lheureux S, Braunstein M and Oza AM: Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin. 69:280–304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dasari S and Bernard Tchounwou P: Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. 740:364–378. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Falzone L, Bordonaro R and Libra M: SnapShot: Cancer chemotherapy. Cell. 186:1816–1816.e1811. 2023.
|
|
11
|
Zhang C, Xu C, Gao X and Yao Q: Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics. 12:2115–2132. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang H, Li Y, Wang Y, Shang X, Yan Z, Li S and Bao W: Cisplatin-induced PANDAR-Chemo-EVs contribute to a more aggressive and chemoresistant ovarian cancer phenotype through the SRSF9-SIRT4/SIRT6 axis. J Gynecol Oncol. 35(e13)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, Villegas E, Jacquemont C, Farrugia DJ, Couch FJ, et al: Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 451:1116–1120. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shen DW, Pouliot LM, Hall MD and Gottesman MM: Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 64:706–721. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen Z, Xiao Z, Tian W, Li Z and Wu T: Two enhances the Cisplatin sensitivity of cervical cancer cells via suppression of c-MET expression. Iran J Public Health. 52:1476–1486. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xia L, Peng J, Lou G, Pan M, Zhou Q, Hu W, Shi H, Wang L, Gao Y, Zhu J, et al: Antitumor activity and safety of camrelizumab plus famitinib in patients with platinum-resistant recurrent ovarian cancer: Results from an open-label, multicenter phase 2 basket study. J Immunother Cancer. 10(e003831)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Trillsch F, Mahner S, Czogalla B, Rottmann M, Chekerov R, Braicu EI, Oskay-Öczelik G, Wimberger P, Richter R and Sehouli J: Primary platinum resistance and its prognostic impact in patients with recurrent ovarian cancer: An analysis of three prospective trials from the NOGGO study group. J Gynecol Oncol. 32(e37)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, Richardson GE, Sessa C, Yonemori K, Banerjee S, et al: Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): An open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 22:1034–1046. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Oronsky B, Ray CM, Spira AI, Trepel JB, Carter CA and Cottrill HM: A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol. 34:1–7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wolford JE, Bai J, Moore KN, Kristeleit R, Monk BJ and Tewari KS: Cost-effectiveness of niraparib, rucaparib, and olaparib for treatment of platinum-resistant, recurrent ovarian carcinoma. Gynecol Oncol. 157:500–507. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Chen LM, Cristea M, DeRosa M, Eisenhauer EL, et al: Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 19:191–226. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
McMullen M, Madariaga A and Lheureux S: New approaches for targeting platinum-resistant ovarian cancer. In: Seminars in cancer biology Elsevier, pp167-181, 2021.
|
|
23
|
Nero C, Ciccarone F, Pietragalla A, Duranti S, Daniele G, Salutari V, Carbone MV, Scambia G and Lorusso D: Ovarian cancer treatments strategy: Focus on PARP inhibitors and immune check point inhibitors. Cancers (Basel). 13(1298)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Eskander RN and Tewari KS: Incorporation of anti- angiogenesis therapy in the management of advanced ovarian carcinoma-mechanistics, review of phase III randomized clinical trials, and regulatory implications. Gynecol Oncol. 132:496–505. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen M, Wang X and Zhang J: Efficacy and safety of apatinib in platinum-resistant recurrent ovarian cancer. China Modern Doctor. 57:74–77, 81. 2019.
|
|
26
|
Chen W, Li Z, Zheng Z and Wu X: Efficacy and safety of low-dose apatinib in ovarian cancer patients with platinum-resistance or platinum-refractoriness: A single-center retrospective study. Cancer Med. 9:5899–5907. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chu C and Huang Q: Efficacy and safety of apatinib in the chemotherapy of recurrent platinum resistant ovarian cancer. Chin J N Drugs. 29:299–302. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Geng A, Yang H, Wang Z and Wu C: Apatinib plus paclitaxel versus paclitaxel monotherapy for platinum-resistant recurrent ovarian cancer treatment: A retrospective cohort study. J Clin Pharm Ther. 47:2264–2273. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Miao M, Deng G, Luo S, Zhou J, Chen L, Yang J, He J, Li J, Yao J, Tan S and Tang J: A phase II study of apatinib in patients with recurrent epithelial ovarian cancer. Gynecol Oncol. 148:286–290. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin Y, Qin S, Li Z, Yang H, Fu W, Li S, Chen W, Gao Z, Miao W, Xu H, et al: Apatinib vs placebo in patients with locally advanced or metastatic, radioactive iodine-refractory differentiated thyroid cancer: The REALITY randomized clinical trial. JAMA Oncol. 8:242–250. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang Q, Chu C, Tang J and Dai Z: Efficacy and safety of apatinib combined with etoposide in patients with recurrent platinum-resistant epithelial ovarian cancer: A retrospective study. J Cancer. 11:5353–5358. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lan CY, Wang Y, Xiong Y, Li JD, Shen JX, Li YF, Zheng M, Zhang YN, Feng YL, Liu Q, et al: Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): A phase 2, single-arm, prospective study. Lancet Oncol. 19:1239–1246. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pinilla-Ibarz J, Cortes J and Mauro MJ: Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: Definitions and clinical implications. Cancer. 117:688–697. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
United States Department of Health Human Services: Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, 2017.
|
|
35
|
Higgins JP, Savović J, Page MJ, Elbers RG and Sterne JA: Assessing risk of bias in a randomized trial. Cochrane Handbook for systematic reviews of interventions, pp205-228, 2019.
|
|
36
|
Fei-Ling A, Kui-Ru H, Yu-Lin S, Yu-Tong W, Jian Z, Xue-Wei W and Xia W: Quality assessment of cohort studies literature on Chinese smoking by using Newcastle-Ottawa-Scale. Chin J Dis Control Prevention. 25:722–729. 2021.
|
|
37
|
Fahmy O, Fahmy UA, Alhakamy NA and Khairul-Asri MG: Single-port versus Multiple-port Robot-assisted radical prostatectomy: A systematic review and Meta-analysis. J Clin Med. 10(5723)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
van Valkenhoef G and Kuiper J: Gemtc: Network meta-analysis using Bayesian methods. R Package version 0.8-8, 2020.
|
|
39
|
Treister-Goltzman Y and Peleg R: Fibromyalgia and mortality: A systematic review and meta-analysis. RMD Open. 9(e003005)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rücker G, Schwarzer G, Krahn U, König J and Schwarzer MG: Package ‘netmeta’. Network Meta-analysis using Frequentist Methods (Version 07-0). 1:08–02. 2015.
|
|
41
|
Shim SR, Kim SJ, Lee J and Rücker G: Network meta-analysis: Application and practice using R software. Epidemiol Health. 41(e2019013)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lai Z, Liang R, Wu Q and Zhang J: Short-term efficacy of apatinib in advanced ovarian cancer after failure of second-line treatment. J Pract Oncol. 33:309–312. 2018.
|
|
43
|
Sun S, Zhai D and Yu C: Clinical study on the efficacy of apatinib treatment for advanced ovarian cancer after second-line chemotherapy failure. Ann Oncol. 29(viii347)2018.
|
|
44
|
Li JZA, Jiang Q, Zheng F and Zhu H: Efficacy and safety of apatinib treatment in platinum-resistant recurrent epithelial ovarian cancer: A real world study. Drug Des Devel Ther. 13:3913–3918. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang M, Liu X, Zhang C, Liao F, Li Z, Luo X, Sun Y and Chen C: A study of efficacy and safety with apatinib or apatinib combined with chemotherapy in recurrent/advanced ovarian cancer patients. Cancer Manag Res. 11:8869–8876. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sun L, Liu X, Yu H, Wang K and Liu W: Evaluation of efficacy and safety of apatinib for patients with recurrent epithelial ovarian cancer. Chin J Clin Oncol. 46:627–630. 2019.
|
|
47
|
Xia X and Zhao W: Apatinib monotherapy in patients with advanced ovarian cancer after platinum resistance: A prospective, exploratory study. Int J Gynecological Cancer. 29(A542)2019.
|
|
48
|
Miao Y, Liu H, Yi Q, et al: Clinical efficacy of Apatinib combined with intraperitoneal injection of cisplatin in treatment of elderly advanced ovarian cancer patients with malignant ascites. Anti Tumor Pharmacy. 9:291–295. 2019.
|
|
49
|
Zhang J, Li A, Jiang Q, Zheng F and Zhu H: Efficacy and safety of apatinib treatment in platinum-resistant recurrent epithelial ovarian cancer: A real world study. Drug Des Devel Ther. 13:3913–3918. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang W, Ma X, Zhang Q and Tan Y: Clinical efficacy of apatinib combined with tegiol in the treatment of platinum-resistant or platinum-refractory advanced ovarian cancer. J Baotou Med Coll. 35:33–34, 36. 2019.
|
|
51
|
Li Y, Jin X and Wang H, Liu Y and Wang H: Clinical efficacy of irinotecan combined with apatinib in the treatment of Platinum-and Taxane-resistant advanced ovarian cancer. J Med Res. 49:136–140. 2020.
|
|
52
|
Li Y, Liu J, Li J and Piao J: Efficacy of apatinib combined with etoposide in the treatment of patients with platinum-resistant recurrent ovarian cancer. J Chin J Primary Med Pharmacy. 27:2453–2457. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Qiu M, Wu J, Ye X, Zhang Q and Yin J: Clinical efficacy and prognosis of chemotherapy regimen of apatinib combined with paclitaxel in the treatment of advanced ovarian cancer. Int J Clin Exp Med. 13:191–199. 2020.
|
|
54
|
Yan Z, Gu YY, Hu XD, Zhao Q, Kang HL, Wang M, Duan W and Guan Y: Clinical outcomes and safety of apatinib monotherapy in the treatment of patients with advanced epithelial ovarian carcinoma who progressed after standard regimens and the analysis of the VEGFR2 polymorphism. Oncol Lett. 20:3035–3045. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yuan Y, Shi H and Xing L: Clinical observation of apatinib mesylate combined with etoposide capsules in the treatment of platinum-resistant recurrent and metastatic ovarian cancer. World Latest Med Information. 20:214–215. 2020.
|
|
56
|
Song X and Wang X: Analysis of the effect of apatinib mesylate in the treatment of platinum-resistant or refractory ovarian cancer. Chin Remedies Clin. 21:1185–1187. 2021.
|
|
57
|
Wang Z, Huang Y, Long L, Zhou L, Huang Y, Gan L, Pu A, Li S and Xie R: Apatinib treatment efficiently delays biochemical-only recurrent ovarian cancer progression. J Ovarian Res. 14(91)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Guo J, Liu D, Wang N, et al: Effect of apatinib on platinum resistance in patients with ovarian cancer after recurrence. J Anhui Med Pharmaceutical J. 26:603–606. 2022.
|
|
59
|
Pan Z, Luo Z, He H, Chen Y, Zhao B, Yang Z and Li L: Efficacy and safety of apatinib in the treatment of advanced platinum-resistant recurrent epithelial ovarian cancer: A retrospective study. J Clin Oncol. 40(e17594)2022.
|
|
60
|
Tang J, Han Z, Liu W, Wang HM, Zhao Y, Ge Y and Qin XB: Application of apatinib combined with pemetrexed in treatment of platinum-resistant recurrent ovarian cancer. Chi J Sch Doctor. 36:171–173, 188. 2022.
|
|
61
|
Wang T, Tang J, Yang H, Yin R, Zhang J, Zhou Q, Liu Z, Cao L, Li L, Huang Y, et al: Effect of Apatinib Plus Pegylated liposomal doxorubicin vs Pegylated liposomal doxorubicin alone on platinum-resistant recurrent ovarian cancer: The APPROVE randomized clinical trial. JAMA Oncol. 8:1169–1176. 2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wu Y and Chen L: Efficacy and safety of apatinib mesylate combined with etoposide in the treatment of platinum-resistant recurrent ovarian cancer. Eval Analysis Drug Use Hosp China. 22:1326–1329, 1333. 2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Yan J and He G: Analysis of the clinical efficacy of apatinib combined with paclitaxel monotherapy in patients with recurrent platinum-resistant ovarian cancer. China Practical Med. 17:133–135. 2022.
|
|
64
|
Pan Z, Luo Z, He H, Chen Y, Zhao B, Yang Z and Li L: Observation of the therapeutic effect of apatinib in advanced platinum-resistant recurrent epithelial ovarian cancer. J Ovarian Res. 16(44)2023.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Yang H, Geng A, Wang Z and Wu C: Efficacy and safety of apatinib combined with liposomal doxorubicin or paclitaxel versus liposomal doxorubicin or paclitaxel monotherapy in patients with recurrent platinum-resistant ovarian cancer. J Obstet Gynaecol Res. 49:1611–1619. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhu F and Zhuang L: Evaluation of the efficacy and safety of apatinib combined with chemotherapy in patients with platinum-sensitive recurrent ovarian cancer. Anti Tumor Pharmacy. 12:521–527. 2022.
|
|
67
|
Wang J, Shen Y, Chen J, Chen X, Guan Q, Liu Q, Xu J, Xu Y, Zhang B, Zhang H, et al: 202TiP A single-arm, open, multicenter and exploratory clinical study of fluzopari combined with apatinib in pts with platinum-sensitive relapsed ovarian cancer first-line treated with a PARP inhibitor. Ann Oncol. 33 (Suppl 9)(S1513)2022.
|
|
68
|
Wang Z, Chen W, Zuo L, Xu M, Wu Y, Huang J, Zhang X, Li Y, Wang J, Chen J, et al: The Fibrillin-1/VEGFR2/STAT2 signaling axis promotes chemoresistance via modulating glycolysis and angiogenesis in ovarian cancer organoids and cells. Cancer Commun (Lond). 42:245–265. 2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Guo J, Yue H, Wang Y, Du X, Saoudi M and Syarif A: Evaluation preparation of apatinib-loaded polymer nanoparticles and its effect in the treatment of advanced ovarian cancer. J Nanosci Nanotechnol. 21:1212–1219. 2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Liu G, Wang C, He Y and Mingyan E: Application effect of apatinib in patients with failure of standard treatment for advanced malignant tumours. BMC Pharmacol Toxicol. 20(61)2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Li L, Zheng J, Liu Z, Huang Y, Xiao J, Wang S, Yu Q, Zhang Q, Hu X, Zhao W, et al: Pre-treatment 18F-RGD uptake may predict adverse events during Apatinib Antiangiogenic therapy. Clin Oncol (R Coll Radiol). 34:e238–e245. 2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wu Z and Fang H: Efficacy of paclitaxel and S-1 combined with apatinib in the conversion therapy for unresectable advanced gastric cancer. J BUON. 26:1485–1490. 2021.PubMed/NCBI
|
|
73
|
Deng W, Wang J and Shi X: Value of low-dose apatinib combined with first-line chemotherapy in the conversion therapy of advanced gastric cancer. China J Modern Med. 33:20–24. 2023.
|
|
74
|
Xu X, Ye M, Zhao E, Li L and Meng Y: Efficacy and safety of apatinib in the treatment of advanced ovarian cancer: A meta-analysis. Chin J Health Care Med. 23:634–637. 2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Xu X: Comparison of the efficacy and safety of Bevacizumab and apatinib in the treatment of recurrent cervical cancer. Pract J Cancer. 37:679–682. 2022.
|
|
76
|
Narod SA and Offit K: Prevention and management of hereditary breast cancer. J Clin Oncol. 23:1656–1663. 2005.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Lasta JL, Groto AD and Brandalize APC: Assessment of medical knowledge toward genetic testing for individuals with hereditary breast and ovarian cancer syndrome in Brazil. Prev Med Rep. 35(102356)2023.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Daly MB, Pal T, Berry MP, Buys SS, Dickson P, Domchek SM, Elkhanany A, Friedman S, Goggins M, Hutton ML, et al: Genetic/Familial High-risk assessment: Breast, ovarian, and pancreatic, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 19:77–102. 2021.PubMed/NCBI View Article : Google Scholar
|




















