Introduction
Intraosseous hemangiomas are rare, benign tumors
originating from blood vessels, constituting <1% of all bone
tumors (1). These lesions are
typically found in vertebral bodies and several reports have also
documented their occurrence in the calvarium, including the orbital
region (2,3). Although most hemangiomas are
considered benign, intraosseous epithelioid hemangiomas can exhibit
intermediate behavior with the potential for locally aggressive
features (2,4). Epidemiological data indicate a higher
incidence in females compared with males, predominantly affecting
individuals in the 40-50 year old age group (5). Intracranial hemangiomas may be
asymptomatic, but in certain instances, tumor enlargement can
result in headaches, visual disturbances (due to optic nerve
compression) and morphological changes (6).
Surgical intervention is the standard treatment when
hemangiomas cause deformity or impair the function of adjacent
organs due to their enlargement, occasionally supplemented with
sclerotherapy (7). Radiation
therapy is a crucial modality for certain types of hemangiomas,
particularly when surgical options are unfeasible or
pharmacological treatments are ineffective (8). Radiation therapy not only alleviates
symptoms but has also been reported to significantly reduce or
eradicate tumors in hemangiomas beyond the bone (9). For intracranial hemangiomas,
radiation therapy is primarily employed for lesions located in
surgically inaccessible areas or for recurrent hemangiomas
post-surgery, for example cavernous sinus hemangiomas with
fractionated radiotherapy and stereotactic radiosurgery being the
preferred technique (10,11).
Proton beam therapy (PBT), favored for its precision
and dosimetric advantages, employs the ‘Bragg peak’ of high-energy
proton beams to selectively target tumor (12). This modality is particularly
effective for tumors situated in sensitive or complex anatomical
regions, demonstrating superior treatment outcomes (13). PBT can minimize potential damage to
the temporal lobe and other critical brain structures (14), preserve renal function during
abdominal irradiation (15,16)
and protect long-term cardiopulmonary function following lung
cancer irradiation (17).
Only one case of radiation therapy for calvarial
hemangiomas has been previously, reported (18). However, there are no records of PBT
for primary calvarial hemangiomas and the optimal dosing,
therapeutic outcomes and potential adverse effects remain
undetermined. The current report presents the first case of a giant
calvarium hemangioma that was well controlled with PBT, as well as
a comprehensive literature review.
Case report
Two surgeries and the hemangioma
diagnosis
The patient was a 10-year-old girl who presented
with left eye proptosis in February 2020 at Beijing Tongren
Hospital (Beijing, China). Computed tomography (CT) and magnetic
resonance imaging (MRI) results revealed a ~50x32x65 mm tumor in
the left cranio-orbital junction. The patient subsequently
underwent a craniotomy for tumor resection and the surgical margins
were positive for tumor cells. Postoperative histopathology
findings revealed irregular proliferation of bone trabecular within
mature cortical bone, with osteoblasts visible surrounding the bone
trabeculae. The stroma was loose and rich in dilated, thin-walled
blood vessels, consistent with the features of an intraosseous
hemangioma. Following surgery, the patient received chemotherapy at
another hospital. According to our thorough review of past medical
records, the patient was treated with propranolol, cyclophosphamide
and vincristine, followed by the immunosuppressive drug sirolimus.
Propranolol dosing was weight-based, starting at 0.5 mg/kg/day and
increasing to 2 mg/kg/day, with careful monitoring of heart rate
and blood pressure. Vincristine was administered intravenously at
1.5 mg/m² weekly. Cyclophosphamide was administered at a dose of
500 mg/m². Chemotherapy was administered for 6 cycles. Sirolimus
was administered orally with a starting dose of 0.8 mg/m² per
day.
However, the MRI results in May 2022 showed
progression of the tumor. In July 2022, the patient underwent a
second craniotomy for tumor resection, 2 years after the first
surgery. Following the second craniotomy, microscopic analysis
showed new bone trabecular surrounded by osteoblasts. Irregular
vascular proliferation was observed between the trabeculae, further
supporting the suspicion of intraosseous hemangioma. The resection
had positive surgical margins for tumor cells. The final diagnosis
of intraosseous hemangioma was based on the presence of irregular
vascular proliferation between the bone trabeculae, a hallmark of
the condition. Osteoid osteoma was excluded because it usually
presents as a small, well-defined lesion with central nidus
composed of osteoid tissue and osteoblasts surrounded by reactive
sclerotic bone, which contrasted with the loose, vascularized
stroma and lack of a central nidus in the present case.
Immunohistochemistry results were: CD31(+), CD34(+), D2-40(-),
Factor VIII (F8)(+), Ki67 (3%) and EMA(-). CD31(+), CD34(+) and
F8(+) and further supported the diagnosis of hemangioma, as these
markers are typically expressed in endothelial cells, the primary
cell type in hemangiomas (19).
CD31 and CD34 are well-established markers for vascular endothelial
cells, indicating blood vessel proliferation (20). D2-40(-), a lymphatic endothelial
marker, helps exclude other vascular tumors such as lymphangiomas.
Ki67, with a low proliferation index (3%), is consistent with a
benign lesion and EMA(-) helps rule out differential diagnoses,
such as meningioma. An attempt was made to retrieve the patient's
original medical report; however, the initial hospital did not
specify the exact localization of the positive staining (that is,
nucleus, cytoplasm, or cell membrane).
Second recurrence of tumor
In August 2023, the patient presented with
subcutaneous swelling on the left side of the head and preauricular
region. The MRI findings suggested hemangioma recurrence. MRI
revealed a large blood-rich lesion measuring ~96x71x112 mm in the
left middle cranial fossa, left supraorbital wall, pterygoid,
frontal, parietal and temporal bones (Fig. 1). T1-weighted imaging (T1WI)
(Fig. 1A) showed the lesion with
heterogeneous hypointensity, while T2-weighted imaging (T2WI)
(Fig. 1B) revealed the lesion with
heterogeneous hyperintensity and linear low intensity areas.
Post-contrast T1WI images (Fig. 1C
and D) showed that the lesion was
markedly enhanced with hyperintensity extending into the left
orbit, pterygoid, frontal, parietal and temporal bone areas.
Diffusion-weighted imaging (DWI) and apparent diffusion coefficient
(ADC) maps (Fig. 1E and F) showed heterogeneous hyperintensity and
reduced ADC values. In addition, there was compression of the
adjacent brain parenchyma.
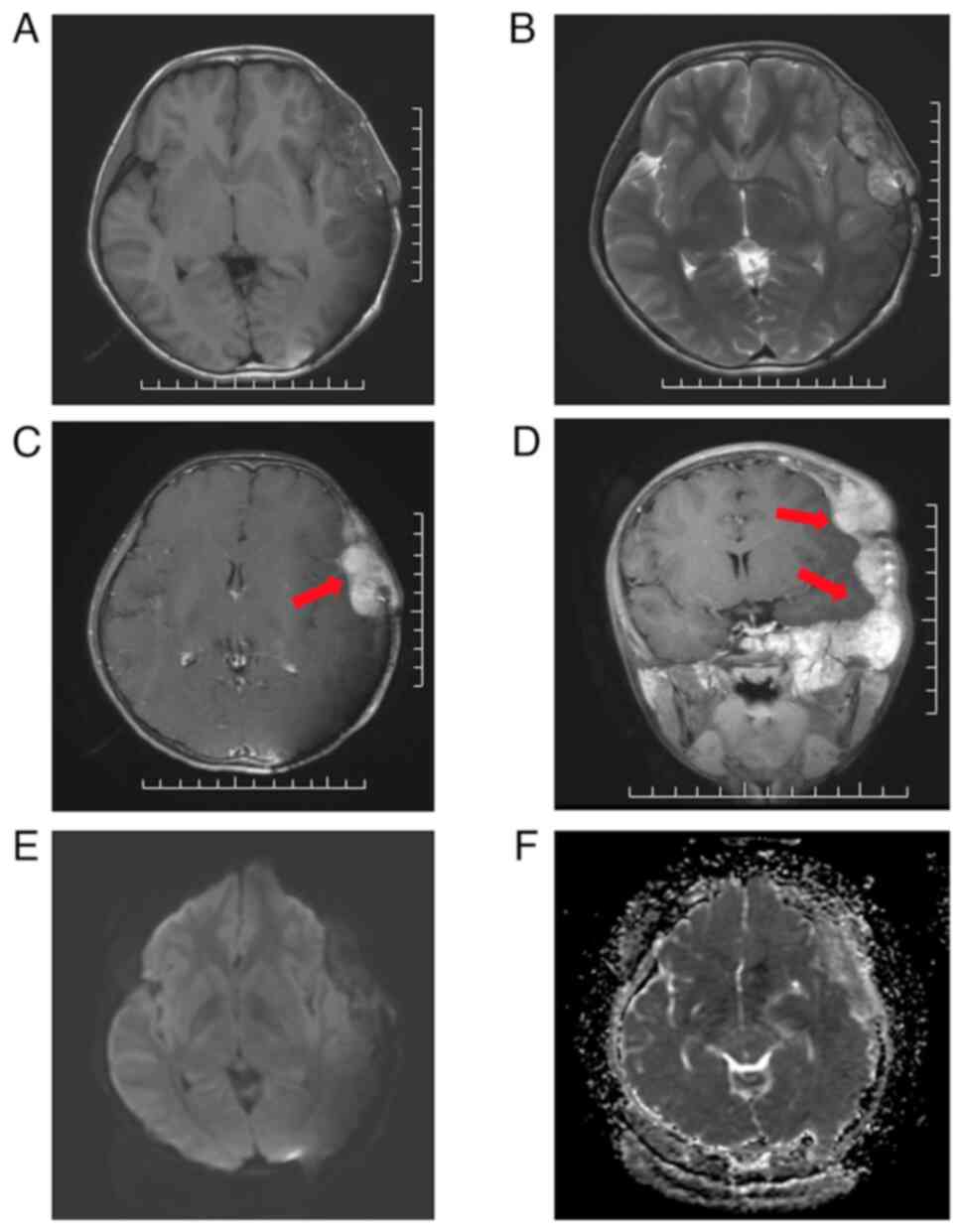 | Figure 1Cranial MRI findings prior to PBT
(scale bar, 10 mm). A large blood-rich flow-like lesion measuring
96x71x112 mm was observed in the left middle cranial fossa, left
supraorbital wall, pterygoid, frontal, parietal and temporal bones,
compressing adjacent brain parenchyma. Red arrows indicate the
tumor location. (A) T1WI: Hypointense lesion with scattered small
hyperintense areas in the temporal region. (B) T2WI: Hyperintense
lesion with linear, low-intensity areas in the temporal region. (C)
Post-contrast T1WI in axial planes: Markedly enhanced lesion with
hyperintensity. (D) Post-contrast T1WI in coronal planes: Strongly
enhanced lesion extending into the left orbit, pterygoid, frontal,
parietal and temporal bone areas. (E) DWI: Lesion with
heterogeneous hyperintensity. (F) ADC maps: Lesion with reduced ADC
values. MRI, magnetic resonance imaging; PBT, proton beam therapy;
T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; DWI,
Diffusion-weighted imaging; ADC, apparent diffusion
coefficient. |
Physical examination revealed the patient exhibited
normal development and clear consciousness. Subcutaneous swelling
was palpable on the left side of her head, left preauricular area
and left occipital region. The swellings were firm and non-tender.
The patient exhibited normal muscle strength and tone, with no
meningeal irritation signs. Brudzinski's and Kernig's signs were
negative (21). Since she had
undergone two surgeries, both of which recurred, the patient was
referred to Hebei Yizhou Cancer Hospital (Zhuozhou, China) in order
to undergo PBT as a treatment option.
The present study was performed in accordance with
the principles of the Declaration of Helsinki (2013 version).
Approval was granted by the Ethics Committee of Hebei Yizhou Cancer
Hospital (approval no. 2024LLSC03). As the patient was a minor,
written informed consent was obtained from the patient's legal
guardians.
PBT and dose distribution
PBT is an advanced form of radiotherapy that uses
protons rather than traditional X-rays (photons) to treat cancer.
The physical properties of protons allow for precise dose
deposition, with most energy being released at a specific depth in
tissue, known as the ‘Bragg peak’. This characteristic enables PBT
to deliver a highly targeted dose to the tumor while sparing
surrounding healthy tissues from unnecessary exposure, making it
particularly advantageous for treating tumors in sensitive areas,
such as the brain or near critical structures.
In the present case, the gross target volume for PBT
was defined as the contrast-enhanced region plus the tumor-adjacent
area on MRI. The clinical target volume (CTV) was defined as the
tumor-adjacent area with an additional 5-mm margin to cover
potential areas of tumor cell infiltration. Instead of including
the entire initial tumor area, the CTV encompassed only the
residual or recurrent tumor and its attachments. Finally, to
compensate for setup errors, a 3-mm margin was added to the CTV to
define the planning target volume (PTV). Pencil beam scanning (PBS)
intensity-modulated proton therapy (IMPT) is an advanced delivery
technique in PBT. In PBS, a highly focused proton beam, with a
diameter of just few mm, is scanned across the tumor in a
layer-by-layer approach, ensuring precise coverage of the tumor
volume. The intensity of the proton beam is modulated to optimize
the dose distribution within the tumor while minimizing exposure to
surrounding healthy tissue. This technique is particularly useful
for irregularly shaped or complex tumors, such as those located in
the brain. PBS IMPT was employed in the current case to precisely
deliver high radiation doses to the tumor with minimal collateral
damage to adjacent critical structures. A total prescribed dose of
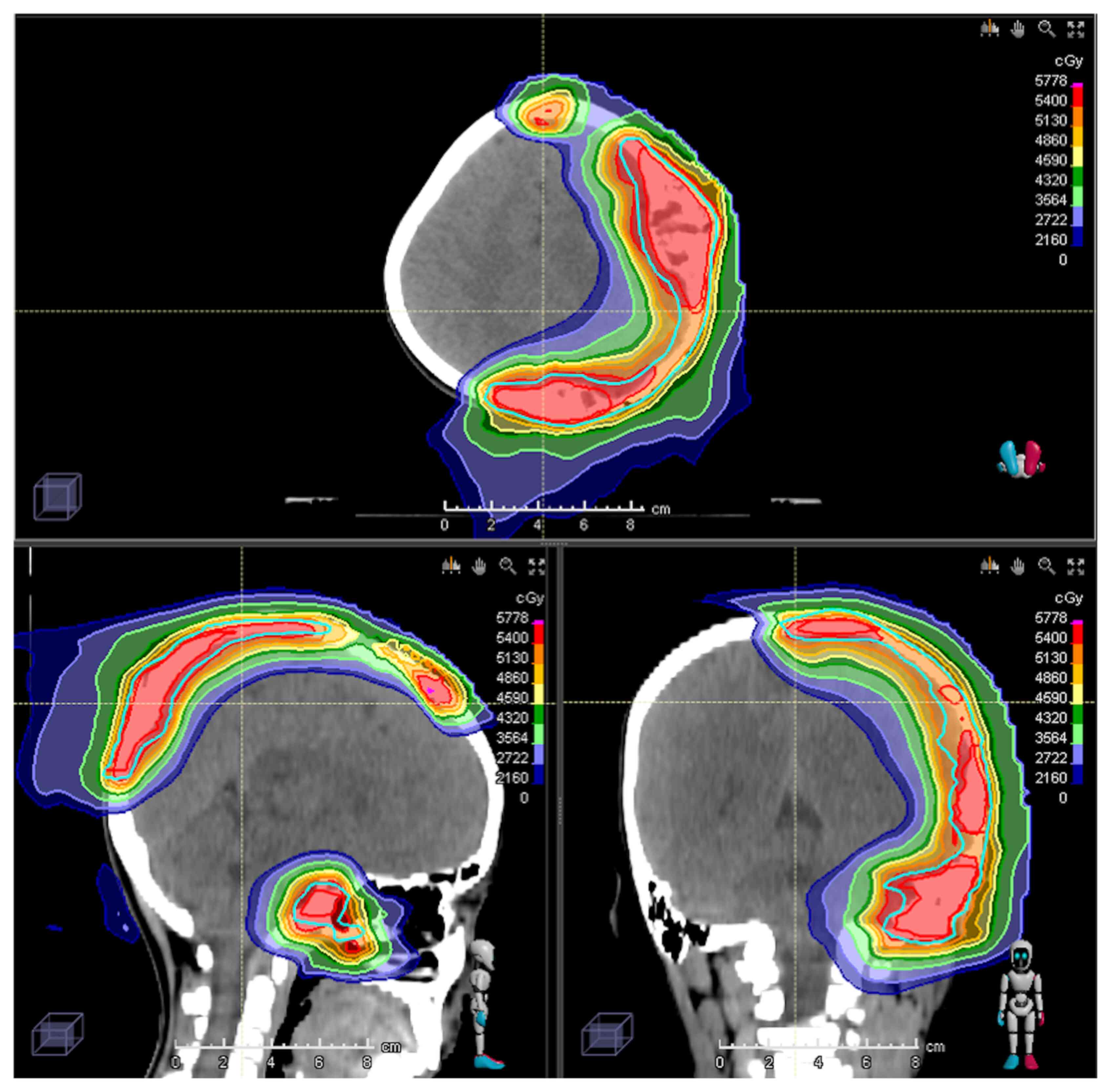
50.4 Gy in 28 fractions was delivered. The PBT dose distribution is
illustrated in Fig. 2. Regarding
organs at risk, the maximum doses were as follows: Brainstem, 50
Gy; left cochlea, 50 Gy; left lens, 5.5 Gy; optic chiasm, 42 Gy;
left optic nerve, 50 Gy; and spinal cord, 18 Gy. The patient was
positioned using a head shell and cone beam computed tomography for
alignment before each irradiation.
During PBT, acute adverse events included hair loss
in the irradiated area starting at 21.6 Gy/12 fractions, complete
hair loss at 30.6 Gy/17 fractions and red-black pigmentation at
37.8 Gy/21 fractions. Epidermal peeling occurred at 48.6 Gy/27
fractions.
Treatment outcomes and evaluation
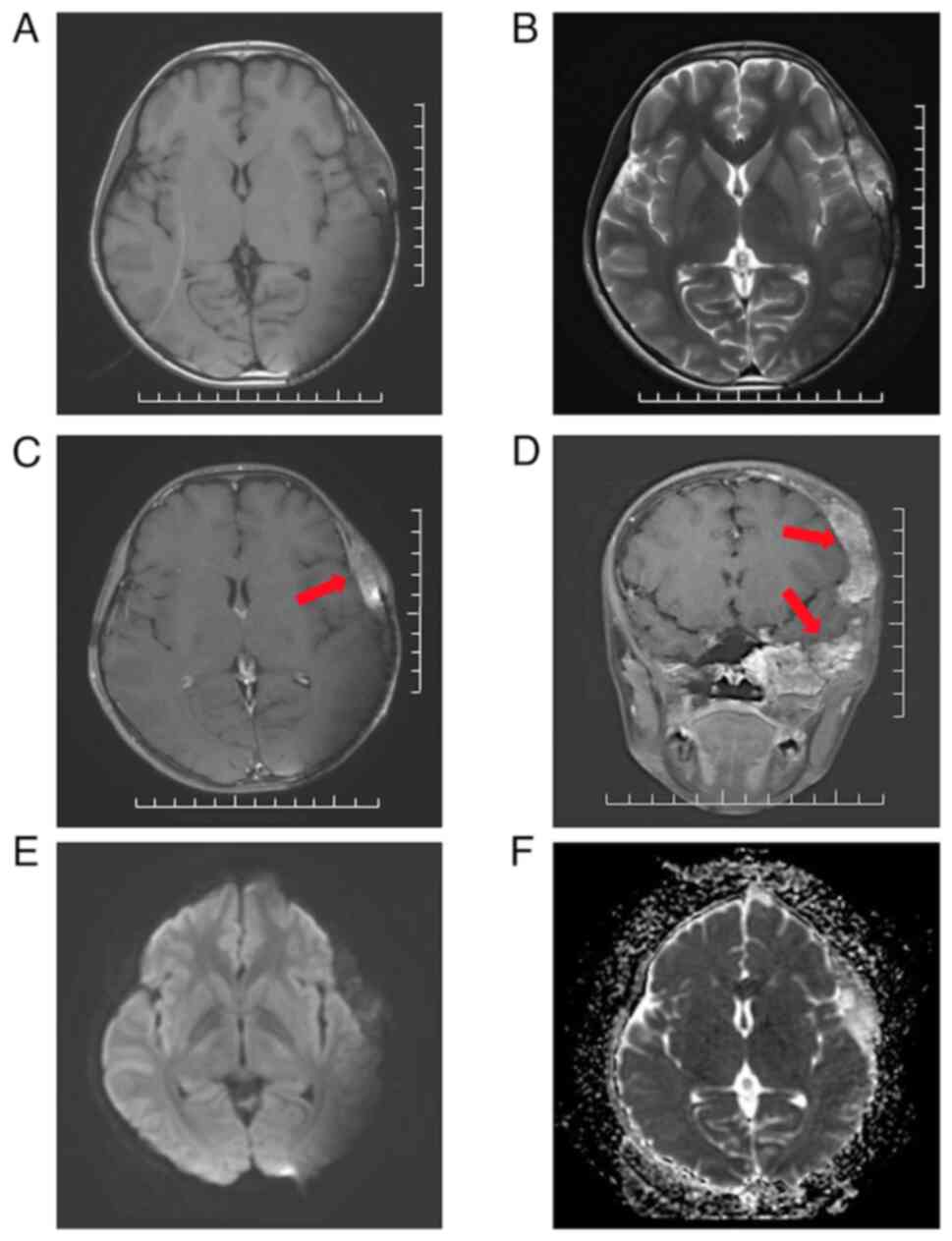
At 14 months post-PBT, the cranial MRI findings
(Fig. 3) showed a significant
reduction in tumor size from the initial measurements to 57x35x76
mm. DWI indicated markedly hypointensity in the lesion area, with
an increase in ADC values. No significant edema or necrosis was
observed 14 months after irradiation.
Based on the MRI results, the activity of the
calvarial hemangioma markedly decreased 14 months after the
completion of PBT, with tumor shrinkage, indicating effective local
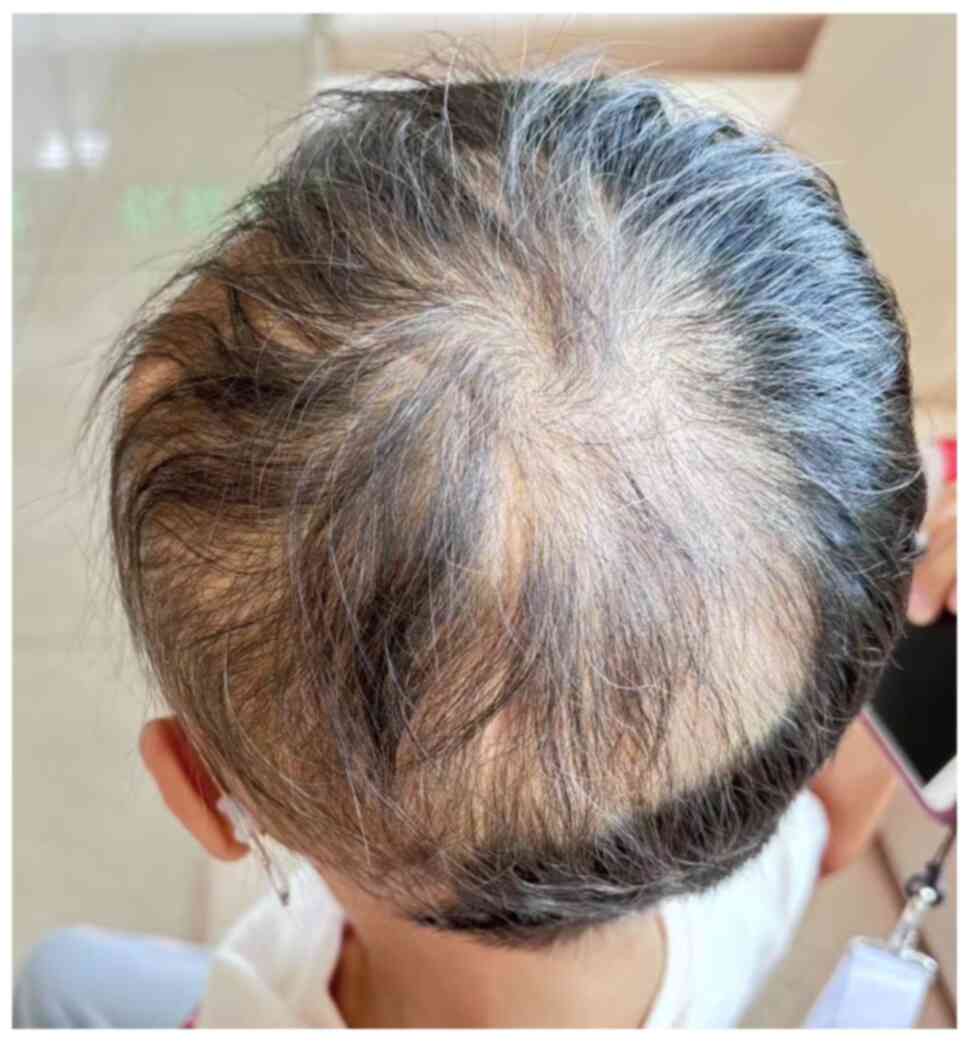
control of the hemangioma. Additionally, the patient's hair loss
symptoms had improved. An image of the patient's scalp was captured
14 months post-PBT and showed a large craniotomy scar on the left
parietal-occipital-temporal region (Fig. 4). Hair regrowth was observed in the
affected area, with the new hair appearing fine, though the density
showed a trend of gradual increase.
No neurological deficits were observed following
PBT. The patient's limb muscle strength and tone were normal,
physiological reflexes were present and no pathological signs were
detected. The complete blood count results showed a white blood
cell count of 7.00x109 cells/l, a neutrophil count of
4.66x109 cells/l, a hemoglobin level of 135 g/l, a
platelet count of 331x109 cells/l and C-reactive protein
at a level of <0.01 mg/l. Evaluation of organ function,
including liver and kidney function as well as cardiac enzymes,
revealed no abnormalities.
As the effects of PBT may continue to manifest
several years post-treatment (22), it was advised that the patient
should continue to be monitored with follow-up observations in the
future.
Discussion
Hemangiomas can be histologically classified into
three types: Cavernous, capillary and mixed, all of which are
benign tumors with no reports of malignant transformation (23). While epithelioid hemangiomas are
typically considered benign, intraosseous epithelioid hemangiomas
can exhibit intermediate behavior with the potential for locally
aggressive features (4).
Intraosseous hemangiomas can occur in any part of the body, but are
most commonly found in the vertebrae, followed by facial bones and
the skull. Within the skull, the frontal bone has the highest
prevalence, followed by the parietal and temporal bones, while the
incidence in the occipital bone is lower (24). The specific etiology of hemangiomas
remains unclear; however, a possible association with a history of
trauma has been previously suggested (25). Typically, intraosseous hemangiomas
are asymptomatic, but they can occasionally evolve into painful,
swollen areas that are palpable on the surface (26). Osseous hemangiomas are frequently
detected through imaging examinations such as CT and MRI (27,28).
However, the gold standard for diagnosis is histopathological
examination. Characteristic well-formed vascular channels and
trabecular bone surrounded by osteoblasts can be observed (23). When the tumor causes significant
pain or neurological symptoms, or poses a risk of pathological
fractures, surgical excision becomes the treatment of choice
(29). In addition, embolization
may be considered before surgery (30).
Surgical resection, including microscopic or
endoscopic approaches, is an optimal treatment for intraosseous
hemangiomas (31). In a previous
study, the recurrence of completely excised hemangiomas was
relatively rare (32). When total
resection cannot be achieved in certain cases, adjuvant gamma-knife
radiosurgery (GKRS) is occasionally conducted as an alternative.
Lee et al (33) reported
the results of GKRS for 31 cavernous sinus hemangiomas. A total of
21 patients received GKRS as a primary treatment, while 11 received
GKRS as adjuvant treatment after surgery and all patients had
>50% reduction in tumor volume at 6 months post-GKRS without
late adverse events. However, GKRS is indicated for small tumors,
whereas large tumors such as the present case are difficult to be
managed.
The efficacy of radiotherapy for large hepatic
hemangioma has been reported in previous studies. Gaspar et
al (34) reported cases
controlled through X-ray radiation therapy, while Shimizu et
al (9) reported a case of
giant hepatic hemangioma controlled by PBT. By contrast, there are
few reports on PBT for intracranial osseous hemangioma. A
literature search on PubMed using the keywords ‘Radiation therapy,
Calvarial hemangiomas’ yielded only one report on the effects of
radiation therapy on calvarial hemangiomas. Liu et al
(18) reported a case of a
50-year-old female patient who achieved local control of a
calvarial hemangioma through radiation therapy, using 6 MV X-rays
with 3D-CRT technology across 30 fractions to deliver a total of 60
Gy. However, the situation is more complex for children, as the
risk of growth disturbances and secondary tumors due to radiation
therapy must be considered while attempting to control the
tumor.
PBT, a radiation therapy method using high-energy
proton beams, is particularly suited for pediatric patients and
tumors located in complex anatomical areas due to its dosimetric
advantages (35). In standard
radiotherapy, photons release energy continuously along their path,
delivering a significant dose to both the tumor and the surrounding
healthy tissues. This increases the risk of side effects,
especially when treating tumors near critical structures. By
contrast, protons exhibit a unique property known as the ‘Bragg
peak’ where most of their energy is deposited at a specific depth
in the tissue, corresponding to the location of the tumor. Beyond
this peak, there is a rapid drop-off in dose, resulting in minimal
radiation exposure to tissues beyond the tumor. This characteristic
of PBT allows for highly targeted treatment, markedly reducing the
dose to nearby healthy tissues and critical structures, thereby
lowering the risk of long-term side effects (36).
Currently, there are no reports in the literature on
intraosseous hemangiomas being treated with PBT. For pediatric
benign tumors such as the present case and even inoperable ones,
the risks of cognitive decline, visual impairments and secondary
cancer associated with traditional radiation therapy are important
concerns. In the current case, PBT was selected due to the
proximity of the tumor to critical structures and the ability of
PBT to deliver a highly conformal dose while sparing healthy
tissue, which is critical for achieving effective local control
with minimal complications. Takizawa et al (37) reported higher predicted
Intelligence Quotient scores in children 10 years after PBT
compared with X-ray treatment. Gross et al (38) also reported generally higher scores
in neuropsychological tests conducted on pediatric patients with
brain tumors following PBT compared with those after X-ray
radiotherapy. In terms of secondary tumors, Dennis et al
(39) reported that the risk of
secondary cancer with intensity modulated radiation therapy for
intracranial tumors was 2-fold higher than that of PBT, while Sato
et al (40) reported no
observed cases of secondary malignancies in unresectable
meningiomas over a median observation period of 10.5 years after
PBT. In addition, in PBS IMPT, it is possible to set the tolerable
dose for surrounding tissues in advance. Compared with traditional
broad-beam PBT, this method allows for more concentrated
irradiation of the tumor while ensuring the dose to the CTV and
further reducing the dose to critical organs (41). The present case used this latest
technology, to ensure the patient received the most optimal dose
distribution possible, thereby minimizing the risk of secondary
cancer and cerebrovascular complications. Long-term assessments of
the effects of PBT often become apparent years after treatment,
especially for slowly growing tumors or when evaluating the
treatment's long-term benefits in reducing recurrence rates.
The current case report presented a pediatric
calvarial hemangioma that recurred following two surgeries and
chemotherapy. Pre-PBT MRI images showed the tumor was notably
large, occupying multiple functional areas within the skull and
causing notably deformity. MRI findings 14 months post-PBT showed
no progression, reduced activity and well-controlled tumor growth.
The disease reported in the present study is a rare condition and
information on effective treatment methods is limited. Given its
predominance in young females, PBT is considered an effective and
beneficial treatment option. The current case reports contribute to
the limited but growing evidence supporting the use of advanced
radiation therapies in managing rare and complex conditions such as
calvarial hemangiomas.
In conclusion, the present study represented the
first reported use of PBT to treat a calvarial hemangioma in a
pediatric patient. The successful use of PBT in the current case
highlights its potential as an effective and less invasive option
for managing rare and complex conditions such as calvarial
hemangiomas, particularly in cases where conventional treatments
have failed.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SSXQ and ZS confirm the authenticity of all the raw
data. Conceptualization was by SSXQ, validation by ZS and SSXQ,
formal analysis by YL and WeiwW and investigation by MM, YO and YJ.
JW, CL, ZW, WeiW, JZ and SSXQ were responsible for resources. JW
obtained radiotherapy dose distribution data. SZ integrated data
and visualised the treatment process. CL gathered patient data. ZW
acquired and verified radiotherapy dose distribution data. WeiWeiW
collected treatment data. JZ collected follow-up data after patient
treatment. MM and YJ were responsible for data curation. ZS and
SSXQ wrote the original draft and writing, review and editing was
by ZS, YJ, MM, YO and YL. Visualization was performed by SZ, and
supervision and project administration were performed by SSXQ. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the principles of the Declaration of Helsinki (2013 version).
Approval was granted by the Ethics Committee of Hebei Yizhou Cancer
Hospital (Zhuozhou, China; approval no. 2024LLSC03). As the patient
was a minor, written informed consent to participate was obtained
from the patient's legal guardians.
Patient consent to publication
As the patient was a minor, written informed consent
for the publication of the case report and images in Fig. 1, Fig.
2, Fig. 3 and Fig. 4 was obtained from the patient's
legal guardians.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yao K, Tang F, Min L, Zhou Y and Tu C:
Multifocal intraosseous hemangioma: A case report. Medicine
(Baltimore). 98(e14001)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Choi JS, Bae YC, Kang GB and Choi KU:
Intraosseous hemangioma of the orbit. Arch Craniofac Surg.
19:68–71. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Akhter AS, El Tecle N, Alexopoulos G,
Espinoza G and Coppens J: Intraosseous orbital cavernous hemangioma
with frontal extension and dural involvement. Cureus.
11(e4823)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ramkumar S: Epithelioid Haemangioma of
Bone: A case series and comprehensive literature review
reappraising the diagnostic classification of all epithelioid
vascular neoplasms of bone. Cureus. 13(e15371)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Powers DB, Fisher E and Erdmann D:
Zygomatic intraosseous hemangioma: Case report and literature
review. Craniomaxillofac Trauma Reconstr. 10:1–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Almeida JA, Gellen PVB, Hiramatsu DM,
Santos MAD, Bitencourt L, Marceliano EFV, Galhardi MPW,
Marceliano-Alves MF and Marques EF: Cavernous Hemangioma in the
Orbital Cavity: Case Report. Eur J Dent. 16:230–233.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ademi Abdyli R, Abdyli Y, Perjuci F, Gashi
A, Agani Z and Ahmedi J: Sclerotherapy of Intraoral Superficial
Hemangioma. Case Rep Dent. 2016(4320102)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sharma S, Kamal R and Rathi AK: Vertebral
hemangioma- the current radiation therapy perspective. Rep Pract
Oncol Radiother. 28:93–101. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shimizu S, Mizumoto M, Okumura T, Li Y,
Baba K, Murakami M, Ishida T, Nakamura M, Hiroshima Y, Iizumi T, et
al: Proton beam therapy for a giant hepatic hemangioma: A case
report and literature review. Clin Transl Radiat Oncol. 27:152–156.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Park S, Yoon SM, Lee S, Park JH, Song SY,
Lee SW, Ahn SD, Kim JH and Choi EK: Role of fractionated
radiotherapy in patients with hemangioma of the cavernous sinus.
Radiat Oncol J. 35:268–273. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ooi KH and Low SW: Fractionated
External-beam Radiation Therapy For Incompletely Resected
Intracranial Extra-axial Cavernous Haemangioma: A Case Report.
Cureus. 10(e2285)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Matsuda M, Mizumoto M, Kohzuki H, Sugii N,
Sakurai H and Ishikawa E: High-dose proton beam therapy versus
conventional fractionated radiation therapy for newly diagnosed
glioblastoma: A propensity score matching analysis. Radiat Oncol.
18(38)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Senirkentli GB, Ekinci F, Bostanci E,
Güzel MS, Dağli Ö, Karim AM and Mishra A: Proton Therapy for
Mandibula Plate Phantom. Healthcare (Basel). 9(167)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nakamura M, Mizumoto M, Saito T, Shimizu
S, Li Y, Oshiro Y, Inaba M, Hosaka S, Fukushima H, Suzuki R, et al:
A systematic review and meta-analysis of radiotherapy and particle
beam therapy for skull base chondrosarcoma: TRP-chondrosarcoma
2024. Front Oncol. 14(1380716)2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li Y, Mizumoto M, Oshiro Y, Nitta H, Saito
T, Iizumi T, Kawano C, Yamaki Y, Fukushima H, Hosaka S, et al: A
retrospective study of renal growth changes after proton beam
therapy for pediatric malignant tumor. Curr Oncol. 30:1560–1570.
2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li Y, Mizumoto M, Nitta H, Fukushima H,
Suzuki R, Hosaka S, Yamaki Y, Murakami M, Baba K, Nakamura M, et
al: Late Changes in Renal Volume and Function after Proton Beam
Therapy in Pediatric and Adult Patients: Children Show Significant
Renal Atrophy but Deterioration of Renal Function Is Minimal in the
Long-Term in Both Groups. Cancers (Basel). 16(1634)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jin Y, Shimizu S, Li Y, Yao Y, Liu X, Si
H, Sakurai H and Xiao W: Proton therapy (PT) combined with
concurrent chemotherapy for locally advanced non-small cell lung
cancer with negative driver genes. Radiat Oncol.
18(189)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu H, Chang X, Shang H, Li F, Zhou H and
Xue X: Diffuse cavernous hemangioma of the skull misdiagnosed as
skull metastasis in breast cancer patient: One case report and
literature review. BMC Cancer. 19(172)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fujii T, Zen Y, Sato Y, Sasaki M, Enomae
M, Minato H, Masuda S, Uehara T, Katsuyama T and Nakanuma Y:
Podoplanin is a useful diagnostic marker for epithelioid
hemangioendothelioma of the liver. Mod Pathol. 21:125–130.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Majchrzak K, Kaspera W, Szymaś J,
Bobek-Billewicz B, Hebda A and Majchrzak H: Markers of angiogenesis
(CD31, CD34, rCBV) and their prognostic value in low-grade gliomas.
Neurol Neurochir Pol. 47:325–331. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mehndiratta M, Nayak R, Garg H, Kumar M
and Pandey S: Appraisal of Kernig's and Brudzinski's sign in
meningitis. Ann Indian Acad Neurol. 15:287–288. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Niitsu H, Mizumoto M, Li Y, Nakamura M,
Ishida T, Iizumi T, Saito T, Numajiri H, Makishima H, Nakai K, et
al: Tumor response on diagnostic imaging after proton beam therapy
for hepatocellular carcinoma. Cancers (Basel).
16(357)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jorge MIS, Brinkmann JCB, Corchón AG and
Ocaña RA: Diagnostic challenge and management of intraosseous
mandibular hemangiomas: A case report and literature review. J
Korean Assoc Oral Maxillofac Surg. 47:321–326. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang Y, Guan J, Ma W, Li Y, Xing B, Ren Z,
Su C and Wang R: Primary Intraosseous Cavernous Hemangioma in the
Skull. Medicine (Baltimore). 95(e3069)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kirmani AR, Sarmast AH and Bhat AR: A
unique case of calvarial hemangioma. Surg Neurol Int. 7 (Suppl
14):S398–S401. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Myadam S, Kishan V, Deepa A, Shri Puja K
and Divya Rani K: Intraosseous hemangioma of the zygomatic bone: A
rare site for hemangioma. Med J Armed Forces India. 72:85–87.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ilyas M, Shah SA, Gojwari T, Rafiq S,
Ellahi I and Ganaie KH: Classic imaging features of calvarial
hemangioma-a case report. The Egyptian J Radiol Nucl Med.
49:663–665. 2018.
|
|
28
|
Geng P, Sun X and Liu J: Adopting
quaternion wavelet transform to fuse multi-modal medical images. J
Med Biol Eng. 37:230–239. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Leong S, Kok HK, Delaney H, Feeney J,
Lyburn I, Munk P and Torreggiani W: The Radiologic Diagnosis and
Treatment of Typical and Atypical Bone Hemangiomas: Current Status.
Can Assoc Radiol J. 67:2–11. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dong WK and Chang HC: A Case of Calvarial
Hemangioma in Cranioplasty Site. J Korean Neurosurg Soc.
46:484–487. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tomioka Y, Kondo K, Numahata T, Moriwaki Y
and Okazaki M: Endoscopic open rhinoplasty enables a cosmetic
approach for a rare case of intraosseous cavernous hemangioma in
the nasal bone. Auris Nasus Larynx. 47:1064–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sary A, Yavuzer R, Latfoglu O and Çelebi
MC: Intraosseous Zygomatic Hemangioma. Ann Plast Surg. 46:659–660.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee CC, Sheehan JP, Kano H, Akpinar B,
Martinez-Alvarez R, Martinez-Moreno N, Guo WY, Lunsford LD and Liu
KD: Gamma Knife radiosurgery for hemangioma of the cavernous sinus.
J Neurosurg. 126:1498–1505. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gaspar L, Mascarenhas F, da Costa MS, Dias
JS, Afonso JG and Silvestre ME: Radiation therapy in the
unresectable cavernous hemangioma of the liver. Radiother Oncol.
29:45–50. 1993.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Peters S, Frisch S, Stock A, Merta J,
Bäumer C, Blasé C, Schuermann E, Tippelt S, Bison B, Frühwald M, et
al: Proton beam therapy for pediatric tumors of the central nervous
system-experiences of clinical outcome and feasibility from the
KiProReg Study. Cancers (Basel). 14(5863)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Spiotto MT, McGovern SL, Gunn GB,
Grosshans D, McAleer MF, Frank SJ and Paulino AC: Proton
Radiotherapy to Reduce Late Complications in Childhood Head and
Neck Cancers. Int J Part Ther. 8:155–167. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Takizawa D, Mizumoto M, Yamamoto T, Oshiro
Y, Fukushima H, Fukushima T, Terunuma T, Okumura T, Tsuboi K and
Sakurai H: A comparative study of dose distribution of PBT, 3D-CRT
and IMRT for pediatric brain tumors. Radiat Oncol.
12(40)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gross JP, Powell S, Zelko F, Hartsell W,
Goldman S, Fangusaro J, Lulla RR, Smiley NP, Chang JH and Gondi V:
Improved neuropsychological outcomes following proton therapy
relative to X-ray therapy for pediatric brain tumor patients. Neuro
Oncol. 21:934–943. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dennis ER, Bussière MR, Niemierko A, Lu
MW, Fullerton BC, Loeffler JS and Shih HA: A comparison of critical
structure dose and toxicity risks in patients with low grade
gliomas treated with IMRT versus proton radiation therapy. Technol
Cancer Res Treat. 12:1–9. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sato H, Mizumoto M, Okumura T, Sakurai H,
Sakamoto N, Akutsu H, Ishikawa E and Tsuboi K: Long-term outcomes
of patients with unresectable benign meningioma treated with proton
beam therapy. J Radiat Res. 62:427–437. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kaulfers T, Lattery G, Cheng C, Zhao X,
Selvaraj B, Wu H, Chhabra AM, Choi JI, Lin H, Simone CB II, et al:
Pencil beam scanning proton bragg peak conformal FLASH in prostate
cancer stereotactic body radiotherapy. Cancers (Basel).
16(798)2024.PubMed/NCBI View Article : Google Scholar
|