Introduction
Acute Q fever is a zoonotic disease caused by the
intracellular bacterium Coxiella burnetii; it typically
presents with a sudden onset of high fever, severe headache and
myalgia, often accompanied by pneumonia or hepatitis. Transmission
to humans primarily occurs through inhalation of contaminated
aerosols from infected animals, though tick bites may also serve as
a transmission route (1-4),
albeit less commonly documented.
Recent studies suggest that C. burnetii may
have originated from tick endosymbionts, specifically Coxiella-like
endosymbionts (CLEs), which share similar 16S rRNA gene sequences
with C. burnetii (1). This
genetic similarity poses a significant challenge in accurately
identifying the source of infection. When ticks are found to carry
CLEs that closely resemble C. burnetii, it can lead to the
erroneous conclusion that the infection is not tick-borne. This
misinterpretation occurs as the genetic markers used to identify
C. burnetii may also detect these symbiotic bacteria,
leading to false-negative results for C. burnetii when it
is, in fact, present (5).
Such complications in distinguishing between C.
burnetii and its genetically similar endosymbionts can result
in underestimating the role of ticks in transmitting Q fever.
Consequently, patients who contract Q fever through tick bites
might not be correctly identified, especially if the diagnostic
methods fail to differentiate between these bacteria. This
underscores the importance of using highly specific and sensitive
diagnostic tools, such as targeted next-generation sequencing
(tNGS), which can provide a more accurate identification of the
pathogens involved. Although the probability of contracting Q fever
through tick bites is lower compared with contracting the disease
through airborne transmission, ticks are recognized by the
scientific community as a potential vector (3,4).
Documenting this case underscores the necessity for heightened
clinical vigilance regarding the potential for tick-borne
transmission of Q fever and demonstrates the effectiveness of tNGS
in accurately diagnosing such infections. This case also serves as
a reminder of the importance of considering tick-borne pathogens in
patients with compatible clinical presentations and potential tick
exposure.
Case report
A 26-year-old male internal medicine resident
physician at Guang'anmen Hospital (Beijing, China) presented to the
Emergency Department in May 2024 (day 0) with a severe headache,
persistent high fever, chills and significant nausea. The patient
reported that these symptoms/complaints began following a hiking
trip to Baiguzha Mountain in the Xiaowutai Nature Reserve
(Zhangjiakou, China) earlier that month. Approximately 3 days
before the hospital visit (day-3), the patient's condition suddenly
deteriorated with a severe headache, high fever of 39.5˚C, chills,
sweating and nausea. The patient performed a self-initiated
complete blood count and C-reactive protein (CRP) test, revealing a
slightly elevated CRP of 13.66 mg/l, with otherwise normal
parameters. Suspecting severe influenza, the patient began
self-treatment with loxoprofen sodium, moxifloxacin (0.4 g) and
oseltamivir (75 mg). Despite this, the symptoms persisted and the
headache worsened significantly, prompting the patient's visit to
the Emergency Department by 9 p.m.
At the Emergency Department, the patient reported
persistent high fever, chills and nausea, and the patient's
headache had become unmanageable. Initial laboratory tests 2 days
post-admission (day 2) showed mildly elevated aspartate
aminotransferase (AST) as well as direct and indirect bilirubin,
decreased potassium and sodium levels, and elevated lactate
(Table I). High-resolution chest
CT and viral tests for influenza and COVID-19 were negative (data
not shown), so the symptoms were initially attributed to stress and
dehydration, considering the patient's own earlier negative tests.
However, over the next three days, the patient's symptoms did not
improve with fluid and electrolyte correction (Fig. 1A and B). The patient's condition continued to
deteriorate, with fluctuating fevers between 38.6 and 40.4˚C and
persistent headaches. The patient's body weight had dropped from
70.2 to 67.1 kg over the 3 days before admission to the hospital.
Despite receiving daily intravenous fluids and electrolytes, the
patient's liver enzymes continued to rise and electrolytes remained
imbalanced. Urgent lab tests also supported this deterioration
(Fig. 1C and D).
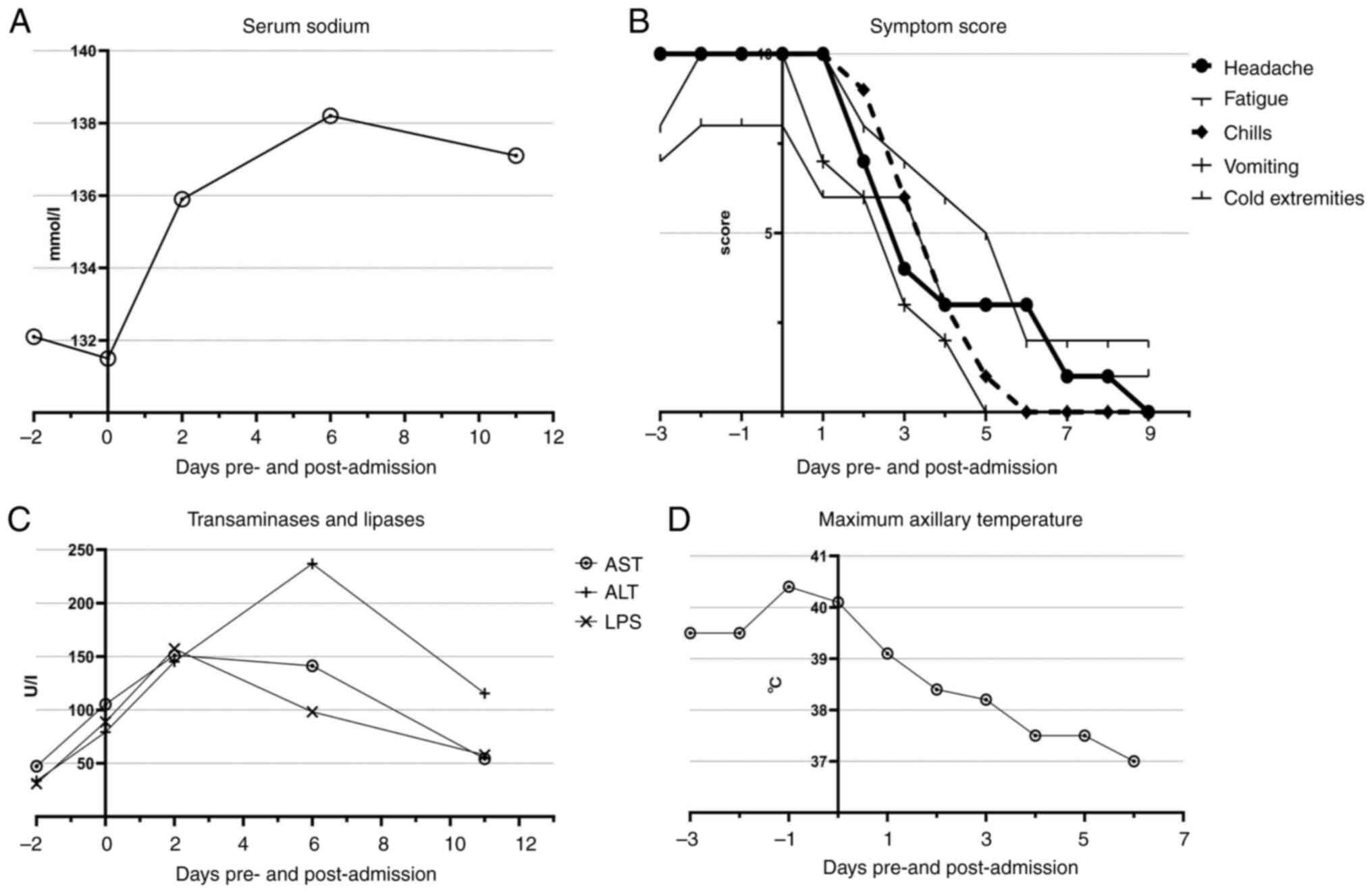 | Figure 1(A) Symptom scores (headache,
fatigue, chills, vomiting, cold extremities) from day-3 to day 9,
rated on a scale of 0-10. (B) Daily maximum axillary temperature of
the patient from day -3 to day 9, ranging from 37-40.4˚C. (C) Serum
sodium levels from day -2 to day 12. (D) Levels of AST, ALT and LPS
from day -2 to day 12, showing liver function recovery. AST,
aspartate aminotransferase; ALT, alanine aminotransferase; LPS,
lipopolysaccharide. |
 | Table ILaboratory tests and symptom scores
of the patient over time. |
Table I
Laboratory tests and symptom scores
of the patient over time.
| | Laboratory
tests | |
|---|
| | Serum sodium,
mmol/l | Aspartate
aminotransferase, U/l | Alanine
aminotransferase, U/l | Lipase, U/l | Symptom scores
(subjective scale) |
|---|
| Days pre- and post-
admission | Detection
value | Reference
value | Detection
value | Reference
value | Detection
value | Reference
value | Detection
value | Reference
value | Temperature,
˚C | Headache
(0-10) | Fatigue (0-10) | Chills (0-10) | Vomiting
(0-10) | Cold extremities
(0-10) |
|---|
| -3 | | 137-147 | | 13-35 | | 7-40 | | 5.6-51.3 | 39.5 | 10 | 8 | 10 | 10 | 7 |
| -2 | 132.1 | | 47.2 | | 33.6 | | 30.9 | | 39.5 | 10 | 10 | 10 | 10 | 8 |
| -1 | | | | | | | | | 40.4 | 10 | 10 | 10 | 10 | 8 |
| 0 | 131.5 | | 105.2 | | 79.2 | | 88.8 | | 40.1 | 10 | 10 | 10 | 10 | 8 |
| 1 | | | | | | | | | 39.1 | 10 | 10 | 10 | 7 | 6 |
| 2 | 135.9 | | 151.1 | | 145.1 | | 157.2 | | 38.4 | 7 | 8 | 9 | 6 | 6 |
| 3 | | | | | | | | | 38.2 | 4 | 7 | 6 | 3 | 6 |
| 4 | 138.2 | | | | | | | | 37.5 | 3 | 6 | 3 | 2 | 3 |
| 5 | | | | | | | | | 37.5 | 3 | 5 | 1 | 0 | 3 |
| 6 | | | 141.2 | | 236.8 | | 98.1 | | 37 | 3 | 2 | 0 | 0 | 3 |
| 7 | | | | | | | | | | 1 | 2 | 0 | 0 | 1 |
| 8 | | | | | | | | | | 1 | 2 | 0 | 0 | 1 |
| 9 | | | | | | | | | | 0 | 2 | 0 | 0 | 1 |
| 10 | | | | | | | | | | | | | | |
| 11 | 137.1 | | 54.3 | | 115.5 | | 57.7 | | | | | | | |
Upon presentation, while examining the patient due
to unrelenting headaches and persistent high fever, the Emergency
Department physician noticed significant conjunctival edema
(Fig. 2). The physician learned
that the patient had returned home from the hike and slept directly
under an air conditioner set to 24˚C. The next morning, the patient
developed a mild headache and a body temperature of 37.6˚C, which
the patient attributed to possible cold exposure or air
conditioning. Given the patient's recent exposure to air
conditioning, the combination of respiratory and gastrointestinal
symptoms and the presence of hyponatremia, the physician initially
suspected Legionella infection. Legionella
pneumophila, often associated with air conditioning systems,
can present with these symptoms and is known to cause hyponatremia
(6-8).
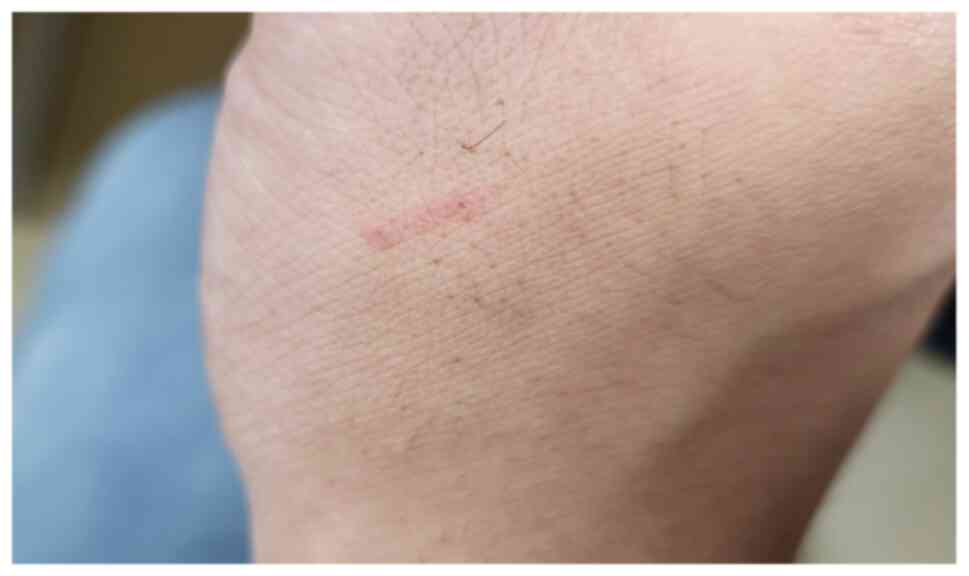
During the examination, the physician discovered two
small lesions on the patient's right hand with a 4-mm red mark
between them, which the patient had not noticed (Fig. 3). Upon further questioning, the
patient recalled brushing off a small insect during his hike, later
identified as a longhorned tick (Haemaphysalis longicornis)
based on online images and local reports (9) confirming the tick's presence in the
area. Despite the rarity of tick-borne infections leading to acute
illnesses in China (10), this
finding led the physician to consider tick-borne infections and
order both metagenomic NGS (mNGS) and tNGS to identify potential
pathogens.
The tNGS assay (11), performed at Sanway Clinical
Laboratories, used the tNGS 296 PLUS panel, which screens for 296
pathogens. DNA was extracted using the Pathogen Target Gene
Detection Kit (cat. no. #sx0010; Sansure Biotech Inc.), and the
quality was assessed by capillary electrophoresis on the Qsep400
system. Sequencing was carried out on a GenoLab M platform
(GeneMind Biosciences, Co., Ltd.) with a single-end read length of
75 base pairs, using the GenoLab M Sequencing Kit V3.0 (cat. no.
FCM-D SE075-D; GeneMind Biosciences, Co., Ltd.). The final library
was loaded at 4000 pM, measured by a Qubit Fluorometer. Data
analysis included fastp for quality control, BWA for alignment,
Samtools for SNP calling and BLAST for pathogen identification.
The tNGS results, available later that day,
identified 43 sequences of Coxiella burnetii, diagnosing
acute Q fever (Table II). In
addition to Coxiella burnetii, other microorganisms were
also detected, including 44 reads of Burkholderia cepacia,
40 reads of Candida parapsilosis and 7 reads of SARS-CoV-2.
However, these organisms were considered unlikely to be the
causative agents of the patient's symptoms due to their lower
pathogenic relevance in the given clinical context and
epidemiological background. Specifically, Burkholderia
cepacia is primarily associated with immunocompromised patients
or those with chronic lung disease, neither of which applied to
this patient, and it is often a colonizer rather than a true
pathogen in non-immunocompromised hosts (12). Furthermore, Candida
parapsilosis and SARS-CoV-2 had very low reads and were not
consistent with the patient's presenting symptoms. To confirm the
presence of Coxiella burnetii, the tNGS results were further
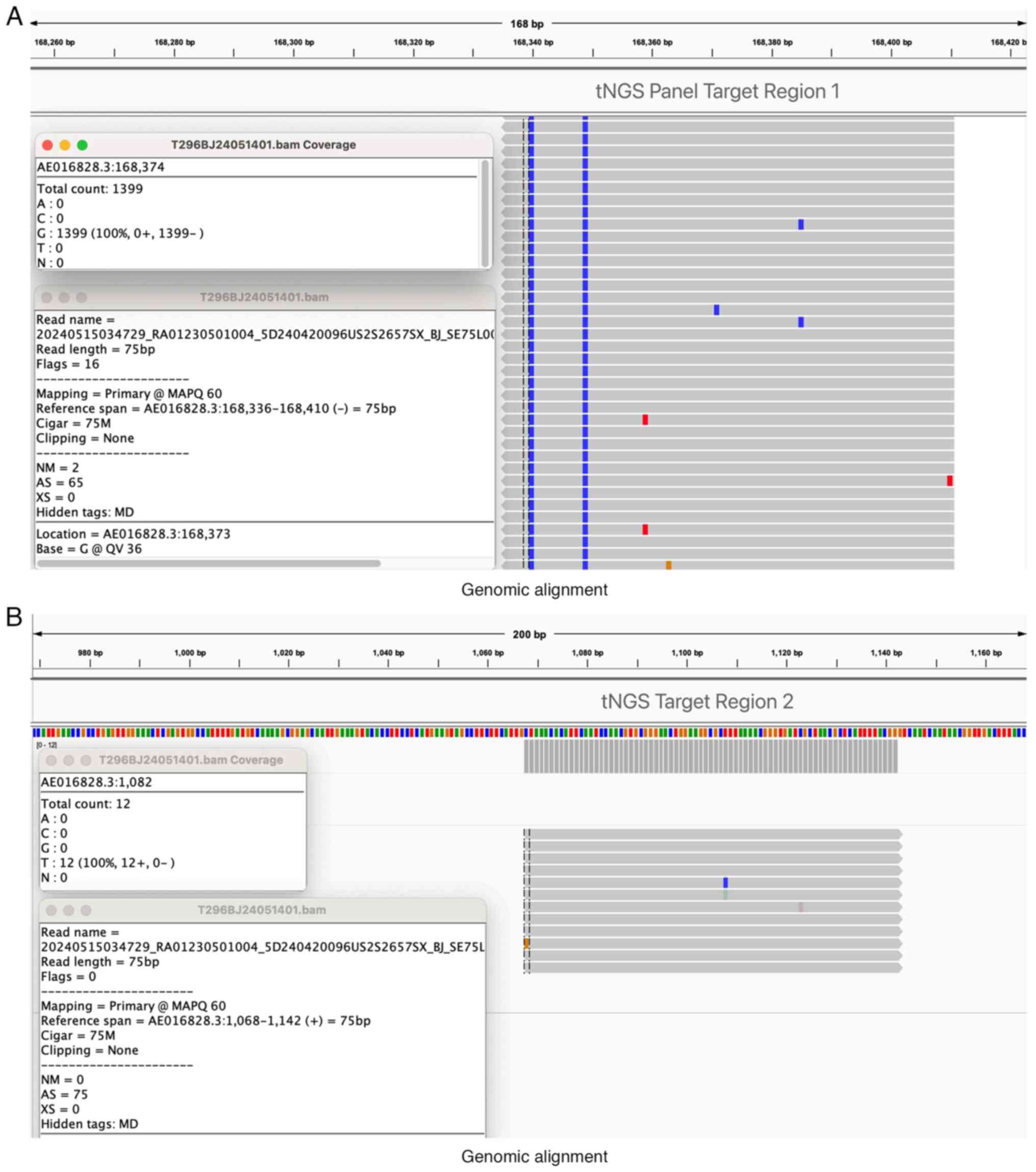
supported by two genomic alignment maps (Fig. 4A and B), which illustrate the alignment of the
patient's sequenced reads with the Coxiella burnetii
reference genome. The genomic alignment maps (Fig. 4A and B) were generated using Integrative
Genomics Viewer version 2.18.4 software (Broad Institute), which is
commonly used for visualizing sequencing data aligned to a
reference genome. These results provide strong evidence of
Coxiella burnetii as the primary pathogen responsible for
the patient's acute symptoms.
 | Table IISpecies detected from the targeted
next-generation sequencing analysis of the patient's venous blood
sample, including Coxiella burnetii and other
microorganisms. |
Table II
Species detected from the targeted
next-generation sequencing analysis of the patient's venous blood
sample, including Coxiella burnetii and other
microorganisms.
| Species name | Reads, n |
|---|
| Total | 34,647 |
| Coxiella
burnetii | 43 |
| Burkholderia
cepacia | 44 |
| Candida
parapsilosis | 40 |
| Severe acute
respiratory syndrome coronavirus 2 | 7 |
The mNGS results on day 1 confirmed the presence of
Staphylococcus haemolyticus (relative abundance, 0.17%) and
9 sequences of Coxiella burnetii (relative abundance,
0.05%). Of note, the patient had undergone QFR-IgM
[immunofluorescence assay (IFA)] testing and blood cultures the day
before, both of which returned negative results after the tNGS
results were obtained.
The timely tNGS results led to the initiation of
doxycycline and glutathione for liver protection on the evening of
day 0 (Table III). Upon
retrospective examination, the patient reported the highest
intensity of symptoms, including headache, chills and anorexia from
day -3 to day 0, with gradual improvement following the initiation
of doxycycline. The patient experienced the highest recorded
temperatures of 40.4˚C on day -1 and 40.1˚C on day 0, which
gradually decreased after day 1. The patient's treatment regimen
evolved over time. Initially, the patient self-administered oral
moxifloxacin for 3 days prior to admission (from day -3 to day -1).
Upon presenting to the Emergency Department on day 0, the treatment
was adjusted to intravenous moxifloxacin from days 0 to day 2 to
enhance the anti-infective effect. The patient also received
potassium citrate and electrolytes to correct imbalances, and pain
was managed using loxoprofen and oxycodone-acetaminophen.
 | Table IIIDaily treatment regimen from day-3 to
day 9, including medication names, dosages, frequencies and stop
dates. |
Table III
Daily treatment regimen from day-3 to
day 9, including medication names, dosages, frequencies and stop
dates.
| Days pre- and
post-admission | Treatment | Frequency | Stop date |
|---|
| Day-3 | Moxifloxacin 0.4
g | 1 | Day-1 |
| | Oseltamivir 75
mg | 2 | ST |
| | Loxoprofen 60
mg | 2 | Day-2 |
| Day-2 | Oxycodone 5 mg and
acetaminophen 325 mg tablet | 2 | ST |
| | 0.9% NS 500 ml | 1 | Day 0 |
| | 5% Dextrose 500
ml | 1 | ST |
| | Vitamin C 1 g,
vitamin B6 200 mg | 2 | Day 0 |
| Day-1 | Oxycodone 5 mg and
acetaminophen 325 mg tablet | 3 | ST |
| | 5% Dextrose 1,000
ml | 1 | ST |
| Day 0 | IV Moxifloxacin 250
ml: 0.4 g | 1 | Day 3 |
| | Doxycycline 100
mg | 2 | Day 21 |
| | Loxoprofen 60
mg | 3 | ST |
| | IV Glutathione 2.4
g | 1 | Day 3 |
| Day 3 | Oral silybin
meglumine 0.2 g | 3 | Day 21 |
Due to severe nausea and anorexia, the patient did
not consume any oral nutrition for several days, relying on
intravenous fluids for hydration and electrolyte balance. Oral
intake resumed as the patient's symptoms improved. By day 6, the
patient's AST and alanine aminotransferase (ALT) levels had peaked
and then began to normalize gradually (Table I). Potassium and sodium levels
continued to fluctuate, requiring ongoing supplementation. Imaging
and diagnostic tests showed no significant findings, but the tNGS
results on day 0 were pivotal in diagnosing acute Q fever. The
diagnosis was confirmed by mNGS the following day, revealing
Coxiella burnetii sequences. By day 11, as determined in a
follow-up examination, the patient's laboratory indicators and
symptoms had significantly improved. On that day, the IFA test
reported positive results. The patient continued with oral
doxycycline and liver protection medication for 24 days, while
monitoring their condition independently.
Discussion
Acute Q fever, caused by Coxiella burnetii,
poses a diagnostic challenge due to its nonspecific symptoms and
underreporting in certain regions, including China (13). This case underscores the vital role
of tNGS in diagnosing Q fever, particularly when traditional
methods are inconclusive.
The patient's initial presentation of mild headache
and low-grade fever was nonspecific, contributing to a delay in
identifying the underlying, more serious infection. Over the next
few days, the symptoms progressed to severe headache, high fever
(exceeding 40˚C) and gastrointestinal disturbances, including
vomiting and chills. These clinical features, along with abnormal
laboratory findings initially prompted differential diagnoses that
included viral infections and systemic inflammatory responses.
However, despite empirical treatments aimed at symptom control, the
patient's high fever persisted and his headache remained severe,
indicating the need for further diagnostic exploration.
The patient's symptom scores were highest between
day-3 and day 0, particularly in terms of headache and fatigue.
Despite initial symptomatic treatment, the severity of symptoms did
not abate, suggesting that an infectious etiology was at play. By
day 1, the persistence of these symptoms, combined with the
patient's laboratory results and recent travel history, led to a
reassessment of the differential diagnosis. Q fever, caused by
Coxiella burnetii, frequently presents with a spectrum of
non-specific symptoms, including flu-like illness, acute hepatitis
and chronic fatigue syndrome (14,15),
all of which were observed in the patient of the present study. The
patient's symptom onset 10 days after potential exposure is
consistent with the known median incubation period for acute Q
fever, which ranges between 7 to 32 days post-exposure (16). Male gender and recent travel to
rural areas, where tick exposure may occur, further supported the
clinical suspicion of Q fever in this case (17).
Following the confirmation of the diagnosis using
tNGS, doxycycline therapy was initiated immediately. The patient's
maximum axillary temperature declined rapidly post-treatment, with
the fever subsiding completely after 5 days. In addition, within 2
days, symptoms such as chills and vomiting had significantly
improved, as evidenced by the reduced symptom severity reported by
the patient. The patient's electrolyte balance was restored by day
6 and serum sodium levels returned to within the normal range
(Fig. 1C). Furthermore, despite a
transient increase in liver enzymes following treatment initiation,
timely intervention with glutathione for liver protection resulted
in a steady decline in both ALT and AST levels, as shown in
Fig. 1D.
Given the patient's presentation, several potential
causes for the key symptoms, including headache, elevated liver
enzymes and overall clinical deterioration, needed to be
considered. Below, the differential diagnoses for these symptoms
are being discussed. Severe headache is a common symptom of Q
fever, but oseltamivir, which the patient had self-administered, is
also associated with headache as a mild side effect. However,
oseltamivir-induced headaches are typically mild and transient
(18). In the present case, the
persistence and intensity of the headache, along with the patient's
other systemic symptoms, made Q fever the more likely cause. This
symptom, combined with the presence of fever and a known history of
tick exposure, led to the prioritization of infectious causes over
medication-induced effects.
Loxoprofen and oxycodone-acetaminophen are both
known to cause hepatic injury and elevated liver enzymes; the
typical onset of drug-induced liver damage occurs at least 24 h
post-ingestion, often requiring sustained use to reach a critical
threshold (19). However, in the
patient of the present study, the elevated ALT and AST levels were
detected before loxoprofen use. Therefore, the hepatic
abnormalities were more likely due to the underlying Q fever
infection.
Glutathione (GSH) plays a crucial role in cellular
defense against oxidative stress, particularly during infection,
but it is well-established that GSH may lower zinc levels by
promoting the utilization of zinc in cellular repair and
antioxidant processes. Given zinc's vital role in maintaining
immune function, including T-cell activation, cytokine production
and neutrophil activity, a reduction in zinc availability can
impair the body's ability to fight infections (20). This connection suggests that during
the acute phase of infections such as Q fever, early administration
of GSH could theoretically exacerbate the condition by reducing
zinc levels. However, in the present case, the rapid progression of
liver damage and the patient's response to doxycycline were
carefully weighted. Despite the potential risks, the preservation
of liver function was prioritized by administering GSH early in the
treatment to mitigate hepatic damage. The balance between infection
control with doxycycline and hepatic protection with GSH appears to
have been beneficial, as demonstrated by the patient's improved
clinical course.
The use of tNGS in the present case was pivotal.
Unlike traditional methods such as serology and culture, which are
often time-consuming and may lack sensitivity, high-throughput
sequencing technologies such as tNGS and mNGS offer rapid,
comprehensive and unbiased pathogen identification (21-23).
tNGS in particular provides several advantages over mNGS and
traditional methods. Firstly, tNGS focuses on specific pathogens,
allowing for higher sensitivity and faster results compared to
mNGS, which screens for all potential pathogens and requires
extensive data analysis (24-28).
The high sensitivity and specificity of tNGS make it an invaluable
tool in the diagnosis of Q fever, particularly given its
exceptional ability to detect atypical pathogens with high accuracy
(29-32).
This is particularly crucial in regions where the disease is rare
and clinicians may not readily consider it in their differential
diagnoses (33). In the patient of
the present study, tNGS identified 43 sequences of C.
burnetii within a short turnaround time, leading to a prompt
diagnosis of acute Q fever. The tNGS296 PLUS panel, used in the
present case, is designed to detect 296 pathogens that are suitable
for emergency screening. This targeted approach facilitated the
rapid identification of C. burnetii and demonstrated the
efficiency of tNGS in an acute clinical setting. mNGS results,
which corroborated the presence of C. burnetii (9 sequences)
and also detected Staphylococcus haemolyticus (relative
abundance, 0.17%), took longer to process and analyze. Secondly,
the rapid identification of C. burnetii through tNGS allowed
for the timely initiation of doxycycline therapy, which was pivotal
in the patient's recovery. Traditional diagnostic methods, which
often take several days to weeks to yield results, would have
delayed the initiation of appropriate treatment. This case
illustrates how tNGS can directly impact clinical decision-making
by providing fast and accurate pathogen identification, thereby
improving patient outcomes in acute infectious diseases. Thirdly,
tNGS has demonstrated higher accuracy in detecting low-abundance
pathogens due to its targeted approach (34-36).
This is particularly important in cases of rare or emerging
infections where pathogen loads may be low or when patients have
been pre-treated with antibiotics, which can reduce the pathogen
load detectable by traditional methods. Furthermore, this case also
highlights a less common route of Q fever transmission, emphasizing
the need for clinicians to consider tick-borne transmission in
patients with compatible symptoms and exposure history. The
identification of C. burnetii in a patient with a recent
tick bite, a less documented transmission route in China, adds to
the growing body of evidence supporting the role of ticks in Q
fever epidemiology. Furthermore, tNGS can provide insights into the
genetic diversity of pathogens, such as C. burnetii,
revealing information on virulence factors, resistance patterns and
epidemiological trends (28,37).
These insights are valuable for understanding disease outbreaks and
tailoring appropriate public health responses. The use of tNGS296
PLUS in this case not only facilitated a rapid and accurate
diagnosis but also underscored the potential of tNGS panels in
enhancing the clinical management of infectious diseases.
Traditional diagnostic methods such as indirect IFA
and ELISA, while useful, have notable limitations. These methods
can take several days to weeks to yield results and may not detect
early-stage infections due to the reliance on antibody presence,
which may not be detectable in the initial stages of the disease
(38,39). IFA, although considered the primary
method for diagnosing acute Q fever, was outperformed by tNGS in
terms of both sensitivity and speed in this case (40-42).
PCR, another common diagnostic tool, offers rapid and specific
pathogen detection but is limited to known targets and may have
lower sensitivity in blood samples (43). tNGS, on the other hand, provides a
targeted approach that ensures high sensitivity and quick
diagnosis, crucial for timely clinical decision-making (33). By contrast, mNGS offers broad
pathogen detection without pre-set targets, making it useful for
identifying unknown or unexpected pathogens. However, mNGS is
expensive, requires complex data analysis and may not consistently
outperform traditional methods in certain contexts, such as
diagnosing suspected pneumonia in immunocompromised patients
(35,44). Compared to mNGS, tNGS focuses on
specific pathogens, offering high sensitivity and rapid diagnosis,
making it a highly effective tool for timely clinical
decision-making (Table IV). mNGS
offers broad pathogen detection without the need for pre-set
targets, making it highly versatile, but it requires expensive
equipment, complex data analysis and specialized expertise. On the
other hand, tNGS is designed for high sensitivity and rapid
diagnosis, focusing on specific pathogens, which makes it
advantageous for time-sensitive clinical applications. However, the
limitations of tNGS include narrower coverage and a dependency on
pre-set targets, which restricts its ability to detect unexpected
pathogens. Importantly, the diagnostic performance of NGS is not
compromised by the empirical use of antibiotics prior to sampling
(45,46). In addition, high-throughput
sequencing technologies like tNGS and mNGS provide comprehensive
and precise insights into the virulence, resistance patterns and
epidemiological trends of C. burnetii (47). In fact, preliminary successes have
already been achieved in using NGS to diagnose Q fever in China
(48-52).
 | Table IVComparison of diagnostic methods,
outlining the advantages and disadvantages of isolation and
culture, serological tests (IFA, CFT, ELISA), staining, PCR, mNGS,
tNGS and cfDNA NGS. |
Table IV
Comparison of diagnostic methods,
outlining the advantages and disadvantages of isolation and
culture, serological tests (IFA, CFT, ELISA), staining, PCR, mNGS,
tNGS and cfDNA NGS.
| | Serological
tests | |
|---|
| Method | Isolation and
culture | IFA | CFT | ELISA | Staining | PCR | mNGS | tNGS | cfDNA NGS |
|---|
| Advantages | Direct pathogen
acquisition; useful for phenotypic/genotypic characterization | High sensitivity
and specificity; differentiates acute and chronic infections | Reference test;
used in some official guidelines | High sensitivity
and specificity; suitable for large-scale screening | Quick and
inexpensive; initial pre-sumptive diagnosis | High sensitivity
and specificity; rapid results; multiple gene | Broad pathogen
detection; no pre-set target needed; high sensitivity targets | High sensitivity;
focused on specific pathogens; rapid diagnosis | Early diagnosis;
non-invasive; high sensitivity and specificity; reduces need for
invasive procedures |
| Disadvantages | Complex, costly,
time-consuming; requires BSL-3 lab | Not suitable for
early acute infection; requires specialized equipment and
skills | Lower sensitivity;
interference from anti-complementary activity | Potential for false
positives/negatives; requires specific antigens | Low specificity;
further confirmation needed | Complex data
analysis; limited to known targets; lower sensitivity in blood
samples | Expensive; complex
data analysis; requires specialized expertise | Narrow coverage;
limited to pre-set targets | Expensive; complex
data analysis; limited availability; requires specialized
expertise |
Due to the nonspecific clinical presentation of
acute Q fever, which includes high fever, severe headache, chills
and gastrointestinal disturbances, its differential diagnosis
encompasses various infectious and non-infectious diseases, such as
bacterial and viral infections, and systemic inflammatory
responses, particularly Legionnaires disease. The causative agent
of Q fever, Coxiella burnetii, is closely related to
Legionella species and both can cause severe atypical
pneumonia, making clinical differentiation particularly challenging
(1,53,54).
The present case underscores the significant
advantages of integrating tNGS into emergency clinical practice,
particularly for diagnosing rare infectious diseases such as Q
fever. By providing rapid and accurate pathogen identification,
tNGS facilitates timely clinical decision-making, allowing for
earlier and more targeted therapeutic interventions. We advocate
for the broader application of tNGS in emergency settings,
particularly in regions where rare infectious diseases may not be
readily considered.
This case also raises several questions for future
research. One area of interest is the role of ticks in the
epidemiology of Q fever. While aerosol transmission from livestock
is well documented, the contribution of tick-borne transmission to
the overall incidence of Q fever remains less clear. In China,
there is no sufficient evidence to suggest that Q fever is commonly
transmitted to humans through tick bites (55). However, individual case reports
have documented instances of Q fever following tick bites in China
(36). In addition, numerous other
infectious diseases are transmitted through tick bites (56-60),
making them a significant infection factor that cannot be ignored.
Further studies are needed to elucidate the prevalence of C.
burnetii in tick populations and the risk factors associated
with tick bites in endemic areas.
Another area for research is the optimization of
tNGS protocols for the detection of C. burnetii and other
intracellular pathogens. Current studies indicate that the design
of primers is critical to the sensitivity and specificity of tNGS
assays, yet significant advancements are still needed in this area
(61-63).
Improving these aspects could enhance the utility of tNGS in
clinical diagnostics and epidemiological surveillance. In addition,
exploring the genetic diversity of C. burnetii strains
through tNGS could provide insight into the pathogen's virulence,
resistance patterns and epidemiological trends (44). While tNGS shows promise, mNGS has
not consistently demonstrated statistical superiority over
traditional methods in certain contexts, such as in diagnosing
suspected pneumonia in immunocompromised patients, and may have
lower sensitivity for specific pathogens such as Aspergillus
species (41). Therefore, focused
research on tNGS protocol optimization is essential to maximize its
clinical application.
Further research into the pathophysiology of acute Q
fever is also warranted, particularly its association with
hyponatremia. Although reliable evidence to definitively link acute
Q fever to hyponatremia appears to be lacking, this patient
exhibited persistent hyponatremia before doxycycline treatment. A
1998 study reported hyponatremia in 28.2% of Q fever cases
(64), suggesting a potential
underlying mechanism worth exploring. It may be hypothesized that,
similar to Legionella infections (65,66),
C. burnetii may induce increased secretion of antidiuretic
hormone, leading to enhanced sodium excretion. The close
phylogenetic relationship between C. burnetii and
Legionella supports this speculation. However, the mechanism
of hyponatremia was not further investigated in this case.
In conclusion, the present case of acute Q fever
diagnosed through tNGS underscores the diagnostic challenges posed
by the disease and the potential of advanced molecular techniques
to overcome these challenges. The integration of tNGS into clinical
practice can facilitate the rapid and accurate diagnosis of
infectious diseases, leading to timely and effective treatment.
Further research into the epidemiology of Q fever and the
optimization of tNGS protocols will enhance our understanding and
management of this complex disease. Further research and clinical
awareness are needed to improve the recognition and management of Q
fever, particularly in endemic regions. Future studies should also
focus on the cost-effectiveness and feasibility of implementing
tNGS in routine clinical practice, particularly in resource-limited
settings.
Acknowledgements
The authors would like to express their sincere
gratitude to Sanway Clinical Laboratories Inc., for their
professional support regarding NGS technology, which was crucial
for the completion of this manuscript. The tNGS assay used in the
present study was performed at Sanway Clinical Laboratories, Inc.
Their expertise and assistance greatly contributed to the quality
of the research. The authors would also like to extend special
thanks to Dr Jun Zhou, Dr Yanqing Guo and Dr Yu Zhang from Sanway
Clinical Laboratories for their critical role in conducting the
tNGS296 Plus genomic comparison for this patient. Their guidance in
interpreting the comparison results was invaluable to thte
team.
Funding
Funding: This project was supported by the Science and
Technological Innovation Project of China Academy of Chinese
Medical Sciences Innovation Fund (grant no. CI2021A02902) and
High-Level Chinese Medical Hospital Promotion Project (grant no.
HLCMHPP2023091).
Availability of data and materials
The data generated in this study are available from
the corresponding author upon reasonable request. The sequencing
data generated in the present study may be found in the NCBI
Sequence Read Archive under accession number SRX26312195,
associated with BioProject PRJNA1169003 and BioSample SAMN44063895,
or at the following URL: https://www.ncbi.nlm.nih.gov/sra/?term=SRX26312195.
Authors' contributions
ZJ was the primary investigator of this study. ZJ
performed the data analysis, drafted the initial manuscript, and
coordinated the clinical and laboratory information used in the
study. ZY, YY, YT and XZ contributed equally as the second authors.
ZY assisted in collecting clinical information and interpreting the
patient's data, YY participated in both data collection and
manuscript preparation, and YT was involved in laboratory testing
and data interpretation. XZ provided supervision for the overall
study design and guided the interpretation of results. XL, YB, LZ,
JY, RM, YG, LH and YW contributed to the patient's clinical
management, data collection and manuscript editing. YY also
provided English language revisions. All authors read and approved
the final manuscript. Furthermore, ZJ and ZY confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent has been obtained from the
patient to publish the information and images included in this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Duron O, Sidi-Boumedine K, Rousset E,
Moutailler S and Jourdain E: The importance of ticks in Q fever
transmission: What has (and has not) been demonstrated? Trends
Parasitol. 31:536–552. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pacheco RC, Echaide IE, Alves RN, Beletti
ME, Nava S and Labruna MB: Coxiella burnetii in ticks
Argentina. Emerg Infect Dis. 19:344–346. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Borawski K, Dunaj J, Czupryna P, Pancewicz
S, Świerzbińska R, Żebrowska A and Moniuszko-Malinowska A:
Assessment of Coxiella burnetii presence after tick bite in
north-eastern Poland. Infection. 48:85–90. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dehhaghi M, Kazemi Shariat Panahi H,
Holmes EC, Hudson BJ, Schloeffel R and Guillemin GJ: Human
tick-borne diseases in Australia. Front Cell Infect Microbiol.
9(3)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Celina SS and Cerný J: Coxiella
burnetii in ticks livestock pets and wildlife: A mini-review.
Front Vet Sci. 9(1068129)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sopena N, Force L, Pedro-Botet ML,
Barrufet P, Sauca G, García-Núñez M, Tolchinsky G, Capdevila JA and
Sabrià M: Sporadic and epidemic community legionellosis: Two faces
of the same illness. Eur Respir J. 29:138–142. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cunha BA: The clinical diagnosis of
Legionnaires' disease: The diagnostic value of combining
non-specific laboratory tests. J Infect. 56:395–398.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Carratalà J and Garcia-Vidal C: An update
on Legionella. Curr Opin Infect Dis. 23:152–157.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu X, Zheng J, Wang W, Wei W and Li K:
Diurnal questing activity of Haemaphysalis longicornis
(Acari: Ixodidae) nymphs in Xiaowutai mountain area. Chin J
Zoonoses. 35:779–784. 2019.
|
|
10
|
Zhu B, Lang S, Bian Y, et al: Epidemic
risk and response measures of novel tick-borne infectious diseases
in China. Chin Front Health Quarantine. 47:431–435. 2024.
|
|
11
|
Souche E, Beltran S, Brosens E, Belmont
JW, Fossum M, Riess O, Gilissen C, Ardeshirdavani A, Houge G, van
Gijn M, et al: Recommendations for whole genome sequencing in
diagnostics for rare diseases. Eur J Hum Genet. 30:1017–1021.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Manno G, Dalmastri C, Tabacchioni S,
Vandamme P, Lorini R, Minicucci L, Romano L, Giannattasio A,
Chiarini L and Bevivino A: Epidemiology and clinical course of
Burkholderia cepacia complex infections, particularly those
caused by different Burkholderia cenocepacia strains, among
patients attending an Italian cystic fibrosis center. J Clin
Microbiol. 42:1491–1497. 2004.PubMed/NCBI View Article : Google Scholar : El-Mahallawy HS, Lu
G, Kelly P, Xu D, Li Y, Fan W and Wang C: Q fever in China: A
systematic review, 1989-2013. Epidemiol Infect 143: 673-681,
2015.
|
|
13
|
Woldehiwet Z: Q fever (coxiellosis):
Epidemiology and pathogenesis. Res Vet Sci. 77:93–100.
2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lai CH, Huang CK, Chin C, Chung HC, Huang
WS, Lin CW, Hsu CY and Lin HH: Acute Q fever: An emerging and
endemic disease in southern Taiwan. Scand J Infect Dis. 40:105–110.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Todkill D, Fowler T and Hawker JI:
Estimating the incubation period of acute Q fever, a systematic
review. Epidemiol Infect. 146:665–672. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jaltotage B, Ali U, Dorai-Raj A, Rankin J,
Sanfilippo F and Dwivedi G: Q fever endocarditis: A review of local
and all reported cases in the literature. Heart Lung Circ.
30:1509–1515. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang X, Chen H, Han D and Wu W: Clinical
usefulness of metagenomic next-generation sequencing for Rickettsia
and Coxiella burnetii diagnosis. Eur J Clin Microbiol Infect
Dis. 42:681–689. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Antipov EA and Pokryshevskaya EB: The
effects of adverse drug reactions on patients' satisfaction:
Evidence from publicly available data on Tamiflu (oseltamivir). Int
J Med Inform. 125:30–36. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Greig SL and Garnock-Jones KP: Loxoprofen:
A review in pain and inflammation. Clin Drug Investig. 36:771–781.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shankar AH and Prasad AS: Zinc and immune
function: The biological basis of altered resistance to infection.
Am J Clin Nutr. 68 Suppl):447S–463S. 1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ranganath N, Khodadadi RB and Abu Saleh
OM: Karius with a Q: Role for microbial cell-free DNA
next-generation sequencing in diagnosis of acute Q fever. Open
Forum Infect Dis. 10(ofac666)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huang C, Ding H, Lin Y, Zhang Z, Fang X,
Chen Y, Chen Y, Zhang C, Li W, Zhang W and Huang Z: Diagnosis of
Coxiella burnetii prosthetic joint infection using mNGS and
ptNGS: A case report and literature review. Orthop Surg.
15:371–376. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Li S, Tong J, Li H, Mao C, Shen W, Lei Y
and Hu P: L. pneumophila infection diagnosed by tNGS in a
lady with lymphadenopathy. Infect Drug Resist. 16:4435–4442.
2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li J, Zhang L, Yang X, Wang P, Feng L, Guo
E and Chen Y: Diagnostic significance of targeted next-generation
sequencing in central nervous system infections in neurosurgery of
pediatrics. Infect Drug Resist. 16:2227–2236. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ye J, Huang K, Xu Y, Chen N, Tu Y, Huang
J, Shao L, Kong W, Zhao D and Xie Y: Clinical application of
nanopore-targeted sequencing technology in bronchoalveolar lavage
fluid from patients with pulmonary infections. Microbiol Spectr.
12(e0002624)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shaikh A and Rodrigues C: What's new in
the molecular diagnosis of childhood tuberculosis? J Pediat Infect
Dis J. 42:e377–e379. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nafea AM, Wang Y, Wang D, Salama AM, Aziz
MA, Xu S and Tong Y: Application of next-generation sequencing to
identify different pathogens. Front Microbiol.
14(1329330)2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ding L, Zhao Y, Li X, Wang R, Li Y, Tang
X, Sun B and He H: Early diagnosis and appropriate respiratory
support for Mycoplasma pneumoniae pneumonia associated acute
respiratory distress syndrome in young and adult patients: A case
series from two centers. BMC Infect Dis. 20(367)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shi Y, Chen J, Shi X, Hu J, Li H, Li X,
Wang Y and Wu B: A case of chlamydia psittaci caused severe
pneumonia and meningitis diagnosed by metagenome next-generation
sequencing and clinical analysis: A case report and literature
review. BMC Infect Dis. 21(621)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Huang T, Chen Y, Zhang J, He R, Qu D, Ye Q
and Chen X: Rapid and accurate diagnosis of brain abscess caused by
Nocardia asiatica with a combination of Ziehl-Neelsen staining and
metagenomics next-generation sequencing. Eur J Neurol. 28:355–357.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yue R, Wu X, Li T, Chang L, Huang X and
Pan L: Early detection of Legionella pneumophila and
Aspergillus by mNGS in a critically ill patient with
Legionella pneumonia after extracorporeal membrane
oxygenation treatment: Case report and literature review. Front Med
(Lausanne). 8(686512)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jin X, Li J, Shao M, Lv X, Ji N, Zhu Y,
Huang M, Yu F, Zhang C, Xie L, et al: Improving suspected pulmonary
infection diagnosis by bronchoalveolar lavage fluid metagenomic
next-generation sequencing: A multicenter retrospective study.
Microbiol Spectr. 10(e0247321)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mitchell SL and Simner PJ: Next-generation
sequencing in clinical microbiology: Are we there yet? Clin Lab
Med. 39:405–418. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gaston DC, Miller HB, Fissel JA, Jacobs E,
Gough E, Wu J, Klein EY, Carroll KC and Simner PJ: Evaluation of
metagenomic and targeted next-generation sequencing workflows for
detection of respiratory pathogens from bronchoalveolar lavage
fluid specimens. J Clin Microbiol. 60(e0052622)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hong HL, Flurin L, Thoendel MJ, Wolf MJ,
Abdel MP, Greenwood-Quaintance KE and Patel R: Targeted versus
shotgun metagenomic sequencing-based detection of microorganisms in
sonicate fluid for periprosthetic joint infection diagnosis. Clin
Infect Dis. 76:e1456–e1462. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bianconi I, Aschbacher R and Pagani E:
Current uses and future perspectives of genomic technologies in
clinical microbiology. Antibiotics (Basel). 12(1580)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Guatteo R, Seegers H, Taurel AF, Joly A
and Beaudeau F: Prevalence of Coxiella burnetii infection in
domestic ruminants: A critical review. Vet Microbiol. 149:1–16.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Körner S, Makert GR, Ulbert S, Pfeffer M
and Mertens-Scholz K: The prevalence of Coxiella burnetii in
hard ticks in Europe and their role in Q fever transmission
revisited-A systematic review. Front Vet Sci.
8(655715)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ghanem-Zoubi N, Mustafa-Hellou M, Zahran
M, Gazit L, Shalaginov R, Dabaja-Younis H and Szwarcwort M: The
integration of Coxiella burnetii PCR testing in serum into
the diagnostic algorithm of suspected acute Q fever in an endemic
setting. J Clin Microbiol. 62(e0170323)2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Navaei H: Q fever: Etiology diagnosis and
treatment. J Zoonotic Dis. 7:260–274. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ullah Q, Jamil T, Saqib M, Iqbal M and
Neubauer H: Q fever-a neglected zoonosis. Microorganisms.
10(1530)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Diseko LJ, Tsotetsi-Khambule AM, Onyiche
TE, Ramatla T, Thekisoe O and Gcebe N: Coxiella burnetii
infections from animals and ticks in South Africa: A systematic
review. Vet Res Commun. 48:19–28. 2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Peng JM, Du B, Qin HY, Wang Q and Shi Y:
Metagenomic next-generation sequencing for the diagnosis of
suspected pneumonia in immunocompromised patients. J Infect.
82:22–27. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin
W, Yao Y, Su Y, Huang Y, Wang M, et al: Microbiological diagnostic
performance of metagenomic next-generation sequencing when applied
to clinical practice. Clin Infect Dis. 67 (Suppl 2):S231–S240.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Duan H, Li X, Mei A, Li P, Liu Y, Li X, Li
W, Wang C and Xie S: The diagnostic value of metagenomic
next-generation sequencing in infectious diseases. BMC Infect Dis.
21(62)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Massung RF, Cutler SJ and Frangoulidis D:
Molecular typing of Coxiella burnetii (Q fever). In: Toman
R, Heinzen R, Samuel J and Mege JL (eds). Coxiella burnetii: Recent
Advances and New Perspectives in Research of the Q Fever Bacterium.
Advances in Experimental Medicine and Biology. Vol. 984. Springer,
Dordrecht, pp381-396, 2012.
|
|
47
|
Kondo M, Dalai SC, Venkatasubrahmanyam S,
Eisenberg N, Robinson BD, Westblade LF and Marks KM: Diagnosis and
genotyping of Coxiella burnetii endocarditis in a patient
with prosthetic pulmonary valve replacement using next-generation
sequencing of plasma microbial cell-free DNA. Open Forum Infect
Dis. 6(ofz242)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Huang J, Wang R, Gao C, Lü Y, Cao Z, Deng
S and Yue C: A case of tick-transmitted Q fever in Lishui, China
diagnosed by next-generation sequencing. J Int Med Res.
49(3000605211025398)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xing F, Ye H, Deng C, Sun L, Yuan Y, Lu Q,
Yang J, Lo SKF, Zhang R, Chen JHK, et al: Diverse and atypical
manifestations of Q fever in a metropolitan city hospital: Emerging
role of next-generation sequencing for laboratory diagnosis of
Coxiella burnetii. PLoS Negl Trop Dis.
16(e0010364)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gao Y, Che L, Wang Z, Niu J, Wei W, Song M
and Liu Q: A case report of autochthonous Q fever with pneumonia
and hepatitis in northeastern China. Biosaf Health. 3:179–182.
2021.
|
|
51
|
Li D, Liu H, Liu M, Chang C, Zhao X, Yu H,
Yan L, Han H and Yu XJ: Delayed diagnosis of acute Q fever, China.
Emerg Infect Dis. 28:2580–2582. 2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang D, Zhang L, Cai Z and Liu Y:
Diagnosis of acute Q fever in a patient by using metagenomic
next-generation sequencing: A case report. Infect Drug Resist.
16:1923–1930. 2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Parker NR, Barralet JH and Bell AM: Q
fever. Lancet. 367:679–688. 2006.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Eldin C, Mélenotte C, Mediannikov O, Ghigo
E, Million M, Edouard S, Mege JL, Maurin M and Raoult D: From Q
fever to Coxiella burnetii infection: A paradigm change.
Clin Microbiol Rev. 30:115–190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wu XB, Na RH, Wei SS, Zhu JS and Peng HJ:
Distribution of tick-borne diseases in China. Parasit Vectors.
6(119)2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yu Z, Wang H, Wang T, Sun W, Yang X and
Liu J: Tick-borne pathogens and the vector potential of ticks in
China. Parasit Vectors. 8(24)2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Li H, Zheng YC, Ma L, Jia N, Jiang BG,
Jiang RR, Huo QB, Wang YW, Liu HB, Chu YL, et al: Human infection
with a novel tick-borne Anaplasma species in China: A surveillance
study. Lancet Infect Dis. 15:663–670. 2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang ZD, Wang B, Wei F, Han SZ, Zhang L,
Yang ZT, Yan Y, Lv XL, Li L, Wang SC, et al: A new segmented virus
associated with human febrile illness in China. N Engl J Med.
380:2116–2125. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Jiang BG, Jia N, Jiang JF, Zheng YC, Chu
YL, Jiang RR, Wang YW, Liu HB, Wei R, Zhang WH, et al: Borrelia
miyamotoi infections in humans and ticks Northeastern China. Emerg
Infect Dis. 24:236–241. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hysom DA, Naraghi-Arani P, Elsheikh M,
Carrillo AC, Williams PL and Gardner SN: Skip the alignment:
Degenerate, multiplex primer and probe design using K-mer matching
instead of alignments. PLoS One. 7(e34560)2012.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hugerth LW, Wefer HA, Lundin S, Jakobsson
HE, Lindberg M, Rodin S, Engstrand L and Andersson AF: DegePrime, a
program for degenerate primer design for broad-taxonomic-range PCR
in microbial ecology studies. Appl Environ Microbiol. 80:5116–5123.
2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhang XA, Ma YD, Zhang YF, Hu ZY, Zhang
JT, Han S, Wang G, Li S, Wang X, Tang F, et al: A new
orthonairovirus associated with human febrile illness. N Engl J
Med. 391:821–831. 2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Xia H, Zhang Z, Luo C, Wei K, Li X, Mu X,
Duan M, Zhu C, Jin L, He X, et al: MultiPrime: A reliable and
efficient tool for targeted next-generation sequencing. Imeta.
2(e143)2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Caron F, Meurice JC, Ingrand P, Bourgoin
A, Masson P, Roblot P and Patte F: Acute Q fever pneumonia: A
review of 80 hospitalized patients. Chest. 114:808–813.
1998.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Schuetz P, Haubitz S, Christ-Crain M,
Albrich WC, Zimmerli W and Mueller B: ProHOSP Study Group.
Hyponatremia and anti-diuretic hormone in Legionnaires' disease.
BMC Infect Dis. 13(585)2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Bellew S, Grijalva CG, Williams DJ,
Anderson EJ, Wunderink RG, Zhu Y, Waterer GW, Bramley AM, Jain S,
Edwards KM and Self WH: Pneumococcal and Legionella urinary
antigen tests in community-acquired pneumonia: Prospective
evaluation of indications for testing. Clin Infect Dis.
68:2026–2033. 2019.PubMed/NCBI View Article : Google Scholar
|