Introduction
Chronic lower back pain (CLBP) is a widespread and
debilitating condition that notably affects the quality of life of
individuals and contributes to increased healthcare utilization
(1). Globally, CLBP remains one of
the leading causes of disability, with substantial socioeconomic
impacts, including direct medical costs and the loss of
productivity (2). Low back pain is
increasingly becoming a major public health concern, with an
estimated global lifetime prevalence of 70-85%. Despite its
widespread occurrence, effective management remains a challenge
(3), the underlying mechanisms of
CLBP are often complex and multifactorial, making effective
management challenging. Pharmacological interventions, such as
non-steroidal anti-inflammatory drugs (NSAIDs), are commonly used
but are often associated with limited efficacy and adverse side
effects, underscoring the need for alternative approaches (4-6).
The management of chronic lower back pain often
begins with the use of NSAIDs. However, prolonged use of NSAIDs can
lead to adverse effects, such as gastrointestinal complications
(7-9).
As a result, there is an increasing focus on investigating adjunct
therapies that can alleviate pain with fewer adverse reactions. For
instance, massage therapy (10)
has been acknowledged for its effectiveness in decreasing pain and
enhancing functionality in individuals with CLBP, ultimately
improving the mental and emotional health of patients (11,12).
Among the adjunct therapy options, botanical
extracts (including Chinese herbs and essential oils), especially
those applied topically, have shown promise due to their potential
analgesic and anti-inflammatory properties. For instance, studies
have indicated the effectiveness of substances such as capsaicin in
managing pain through desensitization mechanisms, which do not
induce notable systemic side effects (7,13-16).
Furthermore, combining massage with botanical treatments, such as
herbal compresses, has been traditionally used to amplify
therapeutic benefits through mechanisms that increase local blood
flow and provide thermal and aromatherapy benefits (12).
Given the limitations of current pharmacological
therapies and the potential of integrative approaches, the primary
aim of the present study was to evaluate the therapeutic potential
of botanical extract-based interventions for managing CLBP. The
present meta-analysis assessed evidence from clinical trials
investigating the effects of plant-based treatments, including
Chinese herbs and essential oils, and their application in
conjunction with manual therapies such as massage. By analyzing the
efficacy of different administration routes and treatment
durations, the present study addressed critical questions regarding
the optimal use of botanical therapies for pain relief and
functional improvement.
Materials and methods
Data sources and selection
criteria
In the present study, a thorough search for
randomized controlled trials (RCTs) assessing botanical extract
interventions for individuals with CLBP was conducted. This
comprehensive search encompassed multiple databases, including
PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www-embase-com.sw.lib.csmu.edu.tw:8443/search/quick),
Cochrane Library (https://www-cochranelibrary-com.sw.lib.csmu.edu.tw:8443/)
and Web of Science (https://www-webofscience-com.sw.lib.csmu.edu.tw:8443/wos/woscc/basic-search),
from database inception to July 2024. Search terms including
‘Chinese herbs’, ‘plant extracts’, ‘botanicals’, ‘essential oils’,
‘aromatherapy’, ‘lower back pain’, ‘chronic lower back pain’,
‘random’, ‘randomized’ and ‘randomised’ were used, focusing
specifically on clinical trials involving human participants. The
methodology strictly followed the Preferred Reporting Items for
Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17). All identified publications were
carefully reviewed, including an analysis of their bibliographies
to uncover additional relevant studies. Selection was limited to
studies published in English and excluded case reports, technical
reports, conference papers, review articles, letters, editorials
and laboratory research.
Study selection
The screening and assessment of studies were
independently conducted by two researchers, CCC and JYH, with a
third researcher, RYT, verifying the accuracy of the process. To
ensure comprehensive analysis, printed copies of all pertinent
articles were acquired and scrutinized. The details of the study
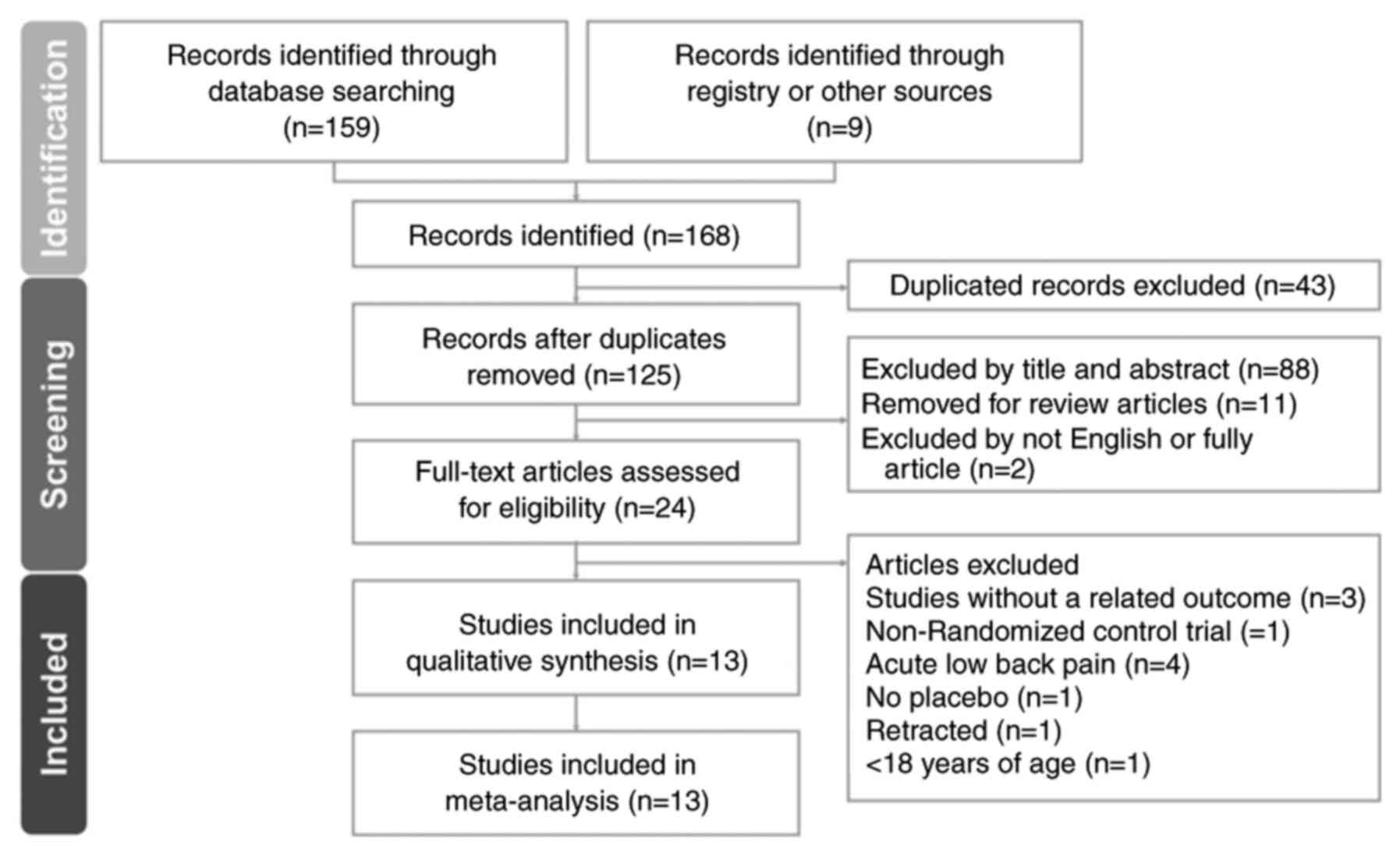
selection process are illustrated in the PRISMA flow diagram
presented in Fig. 1.
Data extraction
Data extraction was independently conducted by two
authors, SYL and RYT, using a standardized form in accordance with
the protocols outlined in the Cochrane Handbook (18). The extracted data included key
information such as the names of the study authors, year and
country of publication, inclusion criteria for participants,
participant demographics (including the total number and age
range), study design, details of the interventions studied and the
outcomes, as well as the methods used for their assessment.
Outcomes
The primary outcome of the present study was the
evaluation of pain scales. The secondary outcomes included the type
of plant extracts used, types of treatments, duration of treatment
and impact of plant extracts on lumbar flexion, as well as their
effect on walking duration.
Quality assessment
In total, two independent researchers, CCC and SYL,
evaluated the potential biases in the included studies using the
Cochrane Collaboration's Risk of Bias tool (18) for assessing methodological quality.
Any discrepancies in the assessments were resolved through joint
discussions with a third reviewer, RYT, to achieve consensus in the
final evaluation. Studies were determined to have a high risk of
bias if they demonstrated issues in ≥1 domains specified by this
assessment framework.
Statistical analysis
Data from each selected study were quantitatively
analyzed using standard mean difference (SMD) and 95% confidence
intervals (CIs) to compare outcomes between the intervention and
control (placebo) groups. These SMD values were pooled using a
random-effects model to account for inter-study variability.
Statistical analyses were performed using Comprehensive
Meta-Analysis software (version 3.0; Biostat, Inc.). Heterogeneity
among the studies was assessed using the I2 statistic,
with values >50% indicating significant heterogeneity.
Publication bias was evaluated through funnel plots and Egger's
regression test. P<0.05 was used to indicate a statistically
significant difference. Additionally, subgroup analyses were
conducted to identify potential sources of heterogeneity and
sensitivity analyses were performed by systematically excluding
individual studies to verify the robustness of the overall
results.
Results
Study selection and characteristics of
the included patients
The study screening and selection process is
outlined in Fig. 1. Initially,
four databases (PubMed, Embase, Cochrane Library and Web of
Science) were explored and an additional source, the ‘related
articles’ feature in PubMed, was utilized, which resulted in the
identification of 168 articles. Upon the removal of duplicates, 125
articles were subjected to title and abstract screening, leading to
the exclusion of 101 articles. A thorough full-text review of the
remaining 24 articles led to the further exclusion of 11 articles
due to the study outcomes being unrelated to the present research
focus (19-21),
lacking a placebo (22),
addressing acute low back pain (23-26),
being a non-RCT (27), including
individuals aged ≤18 years (28)
or being retracted (29).
Consequently, the present meta-analysis ultimately comprised 13
articles (7,13,30-40).
All the included articles were published in the English language.
Table SI provides an overview of
the key characteristics of the trials under consideration,
including participant demographics and study methodologies. The 13
trials were published from 1999-2024 and encompassed a total of
1,739 participants, with the number of participants per trial
varying from 20-320. Among these selected RCTs, 9 utilized a
double-blind approach, 1 was single-blind, 1 was assessor-blind and
2 did not specify the blinding method.
Quality assessment
The risk of bias assessment using the Cochrane
Collaboration’s tool for evaluation identified a range of
methodological strengths and weaknesses across the 13 included
studies. Overall, most studies exhibited a low risk of bias in
several domains. However, some concerns were noted, particularly in
the domains of deviations from the intended interventions, the
measurement of the outcome and missing outcome data. In total, 4
studies showed notable quality issues, including a high risk of
bias in certain domains (33-36).
Most studies (10/13) were RCTs, demonstrating generally low risk in
this domain. Fig. S1 provides a
detailed visual representation of these assessments. Fig. S1A illustrates the risk of bias for
each study across all domains, while Fig. S1B summarizes the proportion of
studies with different levels of bias across the domains. Overall,
this figure highlights the methodological rigor and the areas that
needed improvement within the included studies. In summary, while
the overall methodological quality of the included studies was
moderate to high, specific areas such as the adherence to
intervention protocols and the management of outcome data require
careful consideration in future research.
Impact of the botanical extract on
CLBP
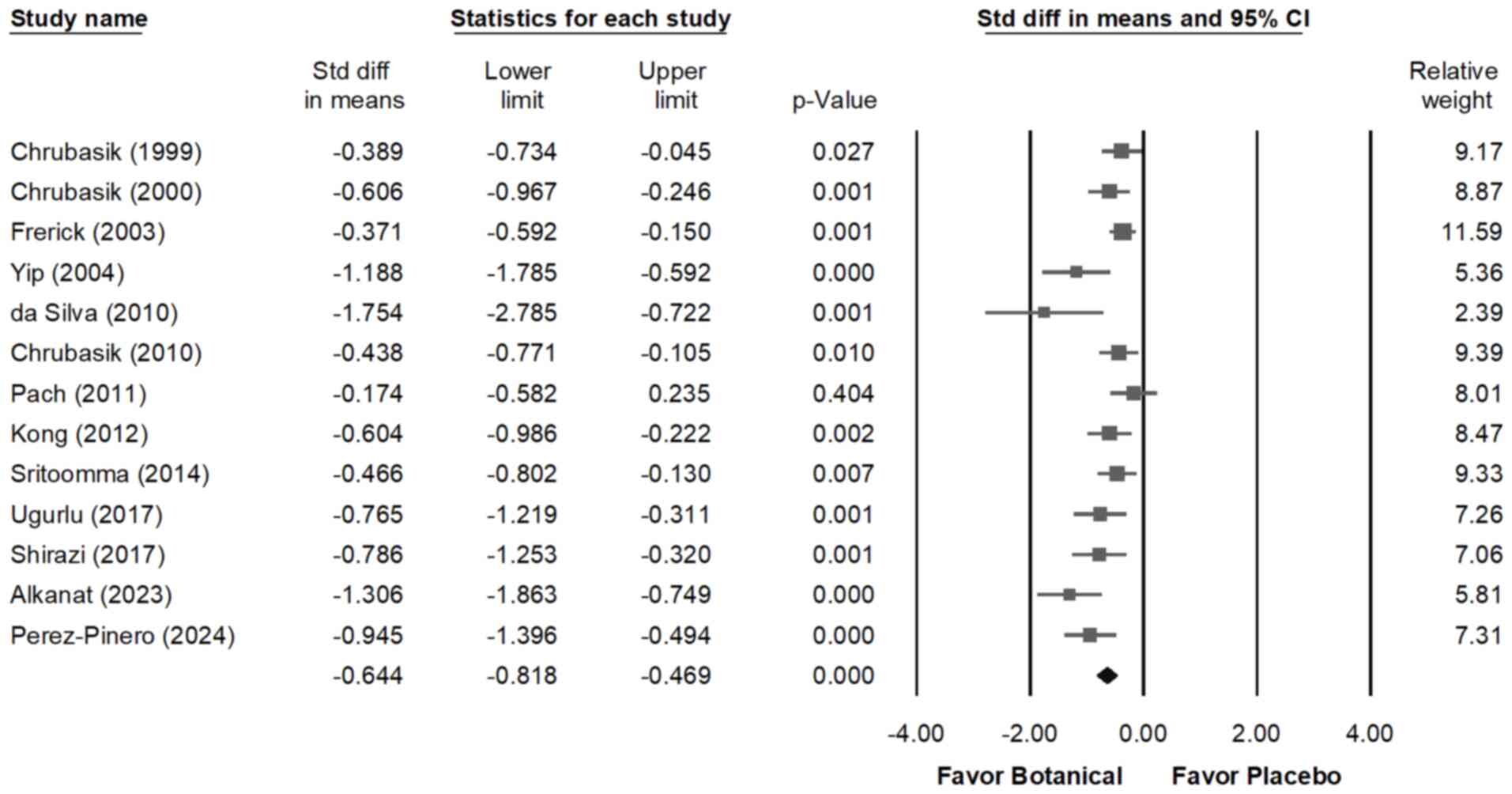
The forest plot shown in Fig. 2 demonstrates that all interventions
led to a reduction in pain compared with the placebo. In patients
with CLBP, the interventions moderately decreased the pain scores
(overall random effect, -0.644; 95% CI, -0.818 to -0.469; P=0.000)
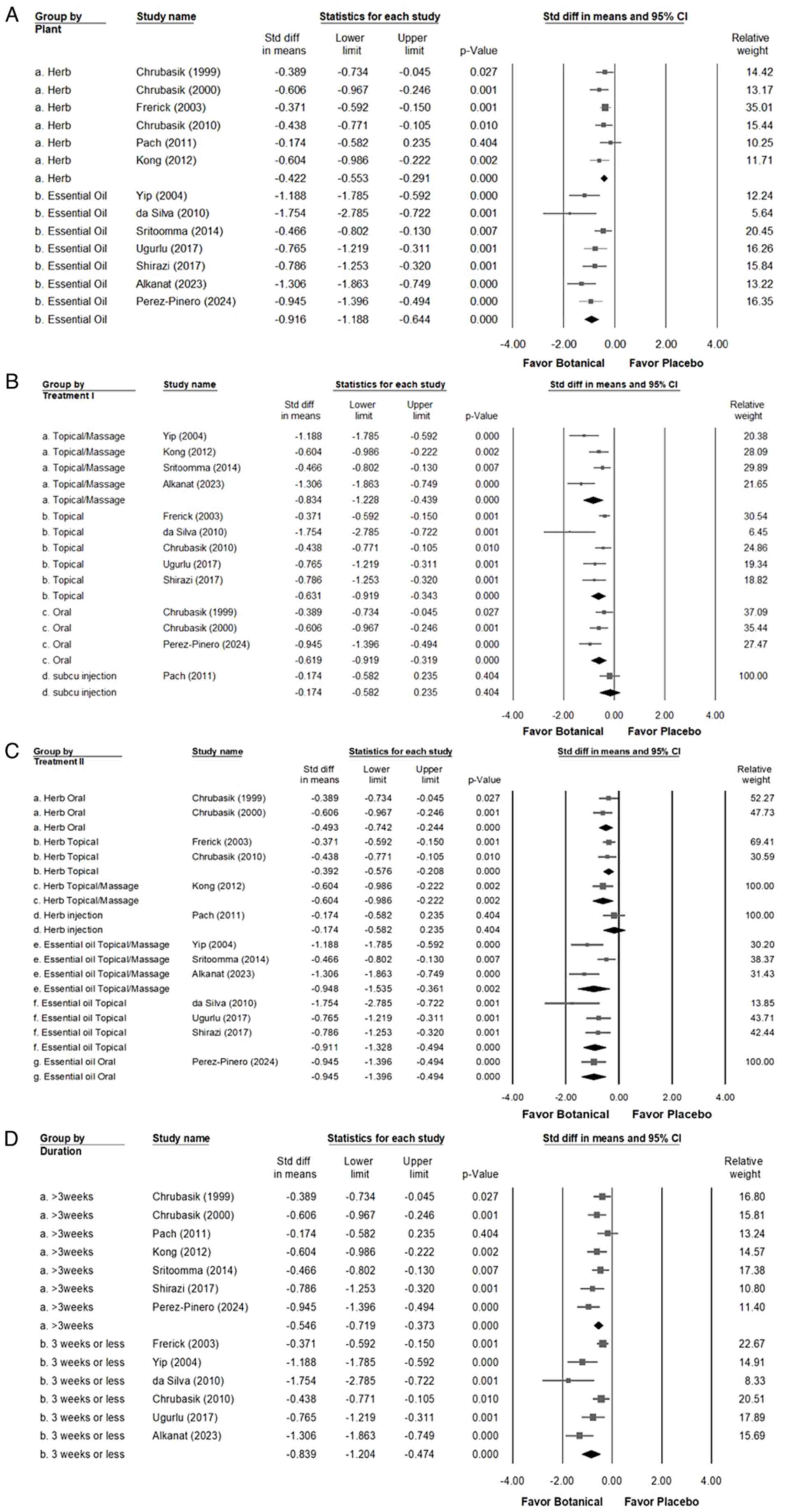
(I²=58.454%; P=0.004). Notably, as illustrated in Fig. 3A, interventions with Chinese herbs
(overall random effect, -0.422; 95% CI, -0.553 to -0.291; P=0.000)
(I²=0.000%; P=0.617) or essential oils (overall random effect,
-0.916; 95% CI, -1.188 to -0.644; P<0.001) (I²=50.607%; P=0.059)
alleviated CLBP compared with the placebo, with both small and
large effects observed respectively, with a trend indicating that
essential oils may be more effective than Chinese herbs. As
detailed in Fig. 3B, the analysis
of administration routes indicated that groups treated with
botanical extracts experienced significantly lower pain levels
compared with the placebo group across various applications:
Topical application (overall random effect, -0.631; 95% CI, -0.919
to -0.343; P<0.001) (I²=59.308%; P=0.043), topical application
combined with massage (overall random effect, -0.834; 95% CI,
-1.228 to -0.439; P<0.001) (I²=67.271%; P=0.027), oral
administration (overall random effect, -0.619; 95% CI, -0.919 to
-0.319; P<0.001) (I²=45.796%; P=0.158) and subcutaneous
injection (overall random effect, -0.174; 95% CI, -0.582 to 0.235;
P=0.404) (I²=0.000%; P>0.999). These findings suggest that
topical applications combined with massage offered simpler and
safer therapeutic approaches compared with oral administration and
were more effective in alleviating pain. As shown in Fig. 3C, combining the results of Fig. 3A and B and further analyzing the treatment
routes revealed that oral administration (overall random effect,
-0.493; 95% CI, -0.742 to -0.244; P<0.001) (I²=0.000%; P=0.394)
or topical use (overall random effect, -0.392; 95% CI, -0.576 to
-0.208; P<0.001) (I²=0%; P=0.743) of herbal medicine produced a
small effect in alleviating CLBP. By contrast, the topical
application combined with massage of Chinese herbs resulted in a
moderate effect (overall random effect, -0.604; 95% CI, -0.986 to
-0.222; P=0.002) (I²=0%; P=>0.999). However, topical use
(overall random effect, -0.911; 95% CI, -1.328 to -0.494;
P<0.001) (I²=36.494%; P=0.207), topical application combined
with massage (overall random effect, -0.948; 95% CI, -1.535 to
-0.361; P=0.002) (I²=76.703%; P=0.014) and oral administration
(overall random effect, -0.945; 95% CI, -1.396 to -0.494;
P<0.001) (I²=0.000%; P>0.999) of essential oil significantly
alleviated CLBP. As shown in Fig.
3D, the analysis of treatment duration revealed that using
botanical extracts for >3 weeks (overall random effect, -0.546;
95% CI, -0.719 to -0.373; P<0.001) (I²=28.760%; P=0.209) or for
<3 weeks (overall random effect, -0.839; 95% CI, -1.204 to
-0.474; P<0.001) (I²=75.275%; P=0.001) resulted in a moderate
reduction in pain compared with the placebo.
Sensitivity analysis and impact of
botanical extract on CLBP
To assess the robustness of the findings, a
one-study removal sensitivity analysis was conducted by
systematically excluding each study one at a time and recalculating
the overall effect estimate. As illustrated in Fig. 4, the results showed that no single
study significantly altered the overall pooled effect, confirming
the stability of the meta-analysis findings. The effect sizes
remained within the confidence intervals across all iterations,
indicating that the primary results were not driven by any specific
study.
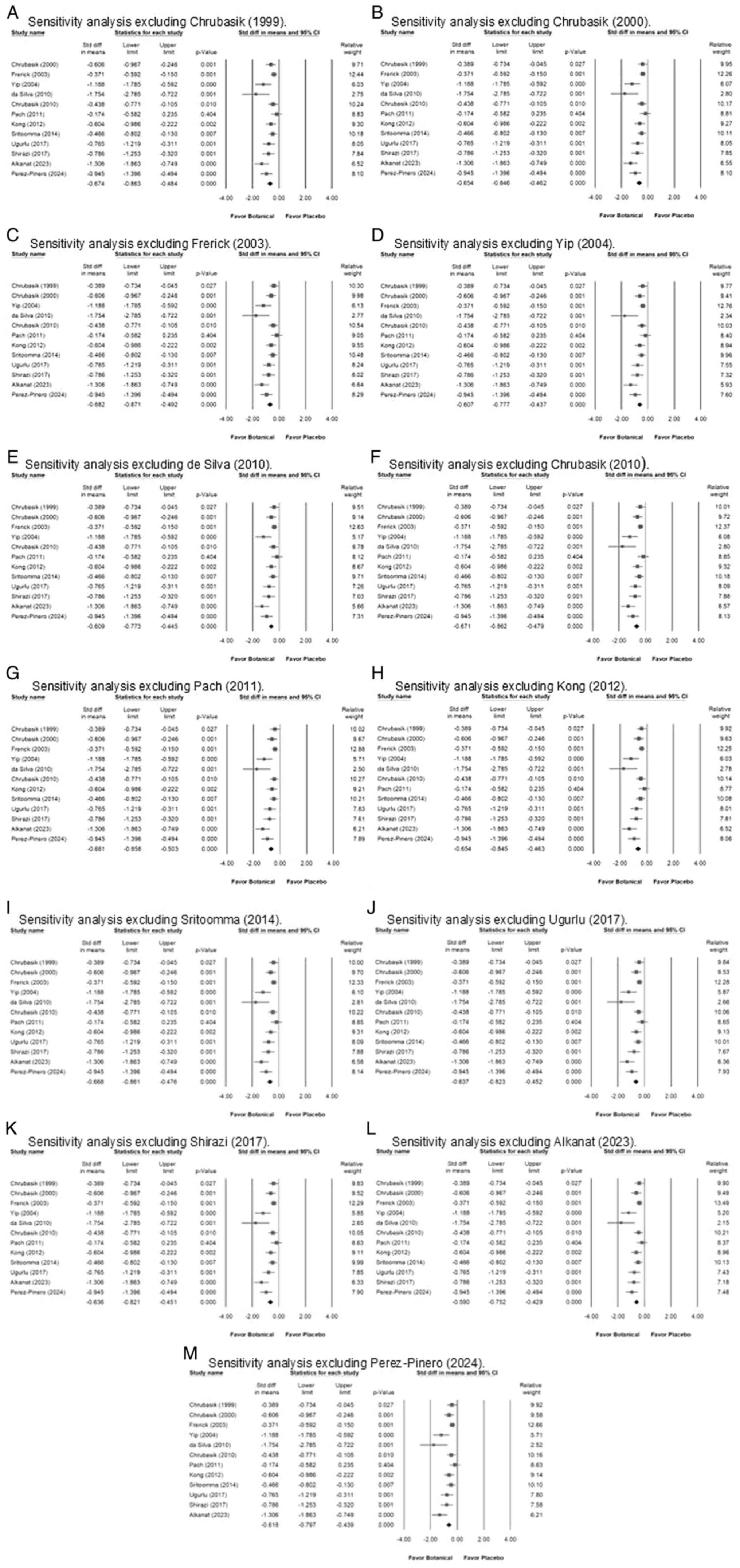 | Figure 4Forest plots depicting the
sensitivity analysis using the one-study removal method, where each
of the thirteen included studies was sequentially excluded.
Exclusion of the study by (A) Chrubasik (1999), (B) Chrubasik
(2000), (C) Frerick (2003), (D) Yip (2004), (E) de Silva (2010),
(F) Chrubasik (2010), (G) Pach (2011), (H) Kong (2012), (I)
Sritoomma (2014), (J) Ugurlu (2107), (K) Shirazi (2017), (L)
Alkanat (2023) and (M) Perez-Pinero (2024). The overall
random-effect estimate remained consistent, confirming that the
study's conclusions are not influenced by the inclusion or
exclusion of any single study. |
Impact of botanical extract on lumbar
flexion and walking time
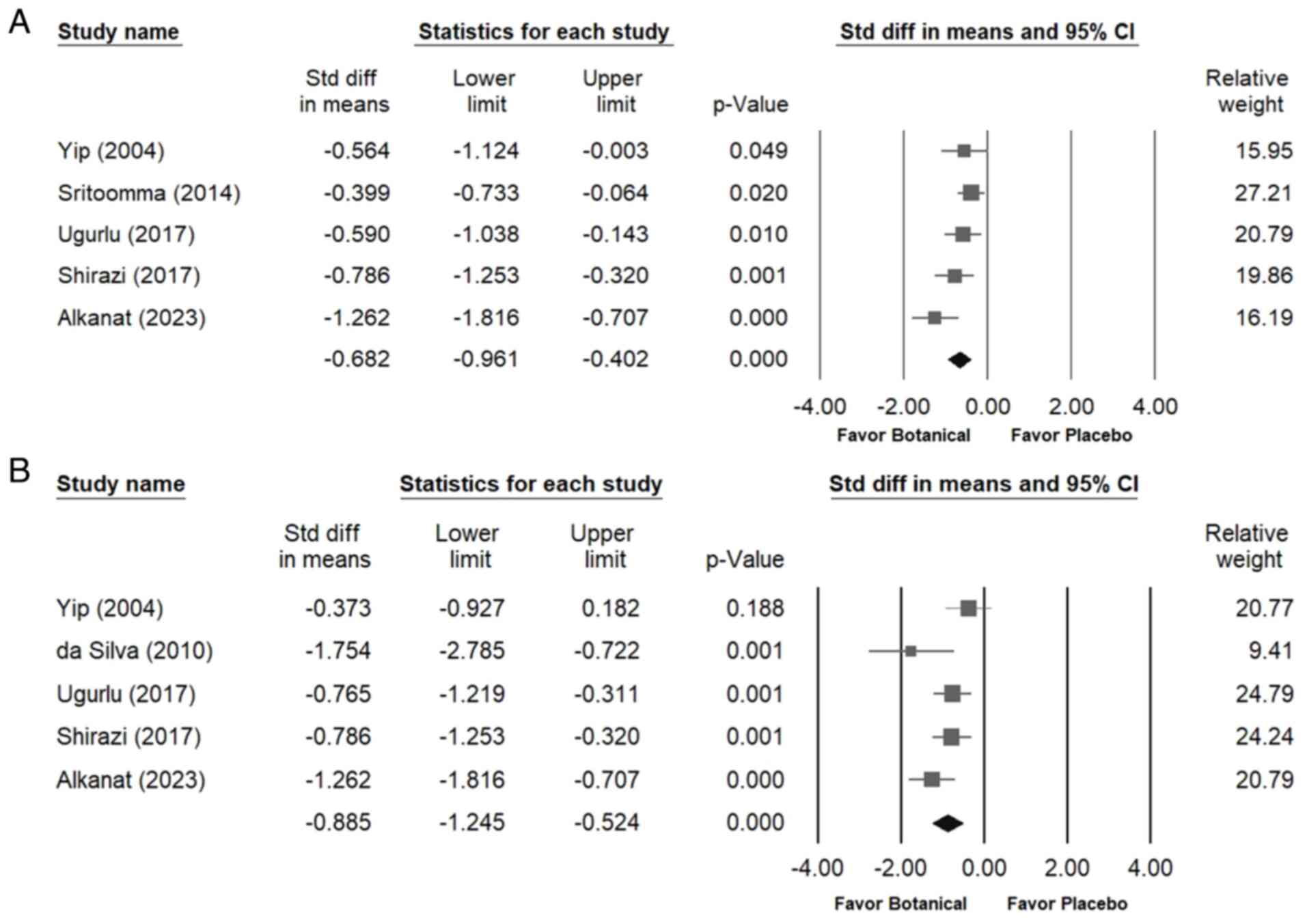
The forest plots shown in Fig. 5 demonstrate that botanical extracts
provided a moderate improvement in walking time (overall random
effect, -0.682; 95% CI, -0.961 to -0.402; P<0.001) (I²=45.428%;
P=0.119) and significantly enhanced lumbar flexion (overall random
effect, -0.885; 95% CI, -1.245 to -0.524; P<0.001) (I²=50.733%;
P=0.087) compared with the placebo.
Publication bias
Egger's regression analysis provided evidence of
significant publication bias within the dataset (P=0.00064). A
funnel plot showing the SMDs for the efficacy of botanical
interventions in reducing CLBP is presented in Fig. S2A. Additionally, Fig. S2B corresponds to the sensitivity
analysis shown in Fig. 4G and
illustrates the results of Egger's regression analysis (P=0.00002).
In conclusion, these findings raise concerns about the credibility
of the meta-analysis.
Discussion
The present systematic review and meta-analysis,
based on a thorough evaluation of both recent and established
research, highlighted the therapeutic effects of botanical
interventions on CLBP. These findings indicated that botanical
extracts significantly improved the management of chronic
musculoskeletal pain, improving lumbar flexion and extending the
walking time for individuals with CLBP. These interventions may
offer an effective alternative to conventional pharmacological
methods, which often coincide with notable adverse effects. The
results of the present study also aligned with clinical guidelines
for chronic low back pain management in primary care (41) for managing CLBP, which recommend
prioritizing non-pharmacological treatments as the first-line
therapy.
The present study addressed a critical gap in the
existing literature by providing a comprehensive analysis of
botanical extracts for the management of CLBP. While previous
meta-analyses have predominantly focused on single interventions,
such as the application of topical essential oils (42,43)
for musculoskeletal disorders, they have largely overlooked the
broader therapeutic landscape of botanical-based treatments.
Earlier studies primarily examined the analgesic properties of
individual essential oils, including lavender (32), peppermint (44) and rosemary (35), assessing their short-term
pain-relieving effects through massage or direct skin absorption.
However, these investigations did not explore the synergistic
effects of botanical combinations, nor did they assess the varying
efficacy of different administration routes. By adopting a more
integrative approach, the present meta-analysis evaluated a diverse
range of botanical therapies, encompassing both essential oils and
herbal extracts, such as capsaicin, ginger and Traditional Chinese
Medicinal formulations. Unlike previous studies that focused solely
on isolated interventions, the present study examined the
collective impact of these botanical treatments, investigating
their effectiveness when applied topically, integrated with massage
or administered orally. Moreover, it considered key factors that
may influence therapeutic outcomes, including treatment duration
and mode of application, offering a more nuanced understanding of
the mechanisms underlying botanical interventions in pain relief
and functional improvement. By addressing these aspects, the
present study advanced the body of evidence supporting
non-pharmacological strategies for pain management. It highlighted
the potential of botanical therapies not only as adjuncts to
conventional treatments but also as effective standalone
interventions with minimal adverse effects. These findings
reinforce the growing shift toward integrative and personalized
approaches in chronic pain management, underscoring the need for
further research to elucidate the long-term efficacy and optimal
application of botanical extracts for musculoskeletal
disorders.
The inclusion of 13 RCTs involving 1,739 patients
provided a statistically robust dataset for analysis. The use of a
random-effects model accounted for inter-study variability and the
results demonstrated consistent and clinically significant
findings. Heterogeneity was moderate and was addressed through
subgroup and sensitivity analyses, confirming the reliability of
the primary outcomes. The sample size used was sufficient for
detecting meaningful differences, as reflected by the significant
reductions in pain scores and improvements in functional outcomes
observed across various intervention methods.
Various botanical extracts, such as capsaicin, have
demonstrated significant reductions in pain scores, likely due to
their analgesic and anti-inflammatory properties (14). The anti-inflammatory and analgesic
mechanisms underlying the effects of botanical extracts have a
crucial role in their therapeutic potential for CLBP. Essential
oils, such as lavender, exert their analgesic effects by modulating
neurotransmitters, such as glutamate, which are key to pain
perception pathways (31).
Capsaicin, a bioactive compound, acts through transient receptor
potential cation channel subfamily V member 1 activation, leading
to the depletion of pro-inflammatory neuropeptides, such as
substance P and calcitonin gene-related peptide, ultimately
reducing nociceptive signaling (45). The active compounds of ginger,
including 6-gingerol and 6-shogaol, inhibit pro-inflammatory
cytokines (such as TNF-α and IL-6) and suppress NF-κB activation,
mitigating inflammation and oxidative stress (46). These mechanisms not only highlight
the biological plausibility of botanical interventions but also
provide a strong foundation for exploring their long-term benefits
in pain management. The application methods, including topical,
with massage or oral, offer flexibility in treatment plans to suit
individual patient needs and preferences. This is supported by
research from Frerick et al (7) and Mason et al (15,16),
which emphasized the minimal systemic side effects and localized
action of topical agents such as capsaicin. Additionally, the
findings of the present study aligned with those of Kroenke et
al (47), who advocate for a
stepped-care approach to chronic pain management that includes
topical analgesics, particularly when other treatments are
ineffective. This strategy not only addresses pain but also
minimizes the negative side effects associated with systemic
medications. Furthermore, incorporating aromatic ginger oil into
massage therapy has demonstrated significant potential, augmenting
the efficacy of conventional massage techniques in alleviating pain
and enhancing functional outcomes in patients with CLBP (36). This observation is consistent with
prior research on the analgesic and anti-inflammatory effects of
ginger, which have been shown to be beneficial for various
musculoskeletal disorders, including knee osteoarthritis, neck
pain, non-specific lower back pain and pregnancy-related lower back
pain (15,16,47).
In the present study, the administration route
analysis further substantiated the effectiveness of botanical
extracts, with all application modes showing significant benefits
compared with the placebo. This is particularly relevant for
clinical practice, where the customization of treatment based on
patient-specific factors is crucial for optimizing therapeutic
outcomes. The findings from the subgroup analyses in the present
study also emphasized the importance of considering individual
differences in response to treatment, which can guide more
personalized and effective management strategies for CLBP.
Additionally, the results of the present study suggested that
combining botanical treatments with manual therapies can amplify
their benefits. This synergistic effect not only reduces pain but
also addresses the underlying inflammatory processes, contributing
to a holistic treatment strategy that aligns with both patient
needs and the complexities of CLBP. The present study demonstrated
a trend indicating a greater efficacy of essential oils compared
with Chinese herbs, which may be attributed to the potent and
pleasant aromas of essential oils. A study has shown that essential
oils can significantly improve pain and functionality in
musculoskeletal conditions, aligning with these findings (10). Additionally, the integration of
essential oils into massage therapy has been shown to enhance
therapeutic outcomes by increasing local blood flow and providing
both thermal and aromatherapy benefits (12). This dual action likely contributes
to the superior pain relief observed with essential oils in the
present study.
In the present meta-analysis, 1 RCT explored the use
of subcutaneous injections of botanical extracts for CLBP but found
no significant difference in pain reduction or functional
improvement compared with the control group. This contrasts with
the overall study findings, where topical and oral applications
showed more consistent efficacy. The route of administration may
explain this discrepancy; subcutaneous injections may not achieve
the localized anti-inflammatory and analgesic effects observed with
topical treatments, which interact more directly with sensory
receptors (48,49). Additionally, the small sample size
and the potential variability in the bioavailability of the
botanical extract may have affected the results. While subcutaneous
administration may warrant further study, current evidence supports
the superiority of topical and oral botanical treatments for
managing CLBP.
Despite the promising findings, several limitations
of the present meta-analysis should be acknowledged. First,
although some analyses, such as the duration of botanical extract
use, showed low heterogeneity, others exhibited significant
variability. For instance, the heterogeneity for the use of
essential oils in topical application combined with massage and for
durations <3 weeks was notably high, indicating that the results
may be influenced by differences in study populations,
methodologies or massage techniques. Second, Egger's regression
analysis indicated significant publication bias, suggesting that
the effects of botanical extracts on CLBP may be overestimated due
to the selective publication of positive results. Additionally,
there were inconsistencies in the blinding methods among the
included studies. Only 9/13 studies utilized a double-blind
approach, while others employed single-blind, assessor-blind or
unspecified methods. This lack of uniformity in the study design
could impact the reliability of the findings. Furthermore, 4
studies demonstrated significant quality issues and there were
moderate concerns about the risk of bias across five distinct
areas, which raises questions regarding the robustness of the
conclusions drawn from these studies. A total of 4 studies had
unclear outcome measurements, which could affect the interpretation
of the results. The accurate and consistent measurement of outcomes
is crucial for reliable meta-analysis conclusions. Moreover, the
meta-analysis included 13 RCTs, which may limit the
generalizability of the findings. More high-quality, large-scale
RCTs are required to strengthen the evidence for the use of
botanical extracts in treating CLBP. Additionally, the studies
included in the present analysis did not provide long-term
follow-up data, limiting the understanding of the sustained effects
of botanical extracts on CLBP. In addition, to assess publication
bias, the present study utilized Egger's regression test and
visually inspected funnel plots. While it can be acknowledged that
additional methods, such as the trim-and-fill method, could provide
further adjustments for small-study effects, they were not employed
in the present analysis. This is an area that should be addressed
in future research to enhance the robustness of these findings.
Moreover, the specific types and formulations of botanical extracts
varied across studies. This variability makes it challenging to
draw definitive conclusions about the most effective type or
preparation of botanical extract for CLBP. The comparative
evaluation of essential oils, capsaicin and ginger could not be
performed in the present study due to the limited number of
head-to-head RCTs directly comparing these interventions.
Additionally, the included studies exhibited considerable
variability in terms of study design, intervention protocols,
dosages and outcome measures, which limited the feasibility of
conducting a reliable indirect comparison. A network meta-analysis
requires a more interconnected dataset with sufficient overlap
between studies, which was not available in the present study.
However, this meta-analysis offers distinct advantages by
incorporating both essential oils and herbal extract-based
therapies, providing a more comprehensive evaluation of botanical
interventions for CLBP management. By assessing different
administration routes and treatment durations, the present study
offered practical insights into personalized treatment strategies.
Additionally, these findings highlighted the potential benefits of
combining botanical treatments with manual therapies, such as
massage, which may further enhance therapeutic outcomes. Future
research should aim to address these limitations by standardizing
botanical extract formulations, conducting larger and
higher-quality RCTs with long-term follow-up data and further
investigating the mechanisms underlying botanical interventions.
Exploring the potential interactions between botanical treatments
and manual therapies may also contribute to the development of more
effective and integrative approaches for CLBP management.
Moreover, current research on botanical therapies
for CLBP primarily focuses on their efficacy and safety in
isolation, without direct comparisons to conventional
pharmacological treatments. A Cochrane review has suggested that
certain herbal medicines, such as devil's claw (Harpagophytum
procumbens) and white willow bark (Salix alba), may
offer greater pain relief when compared with a placebo, with
adverse effects generally limited to mild and transient
gastrointestinal discomfort or skin irritation (50). A systematic review indicated that
NSAIDs and opioids provide short-term pain relief for non-specific
CLBP but are associated with a higher incidence of adverse effects
compared with a placebo. However, this review did not include
direct comparisons with botanical therapies (51). Similarly, while some studies have
explored the effectiveness of complementary therapies, such as
acupuncture and herbal medicine, for chronic non-specific lower
back pain, the evidence remains inconclusive, and direct
comparisons with standard pharmacological treatments are lacking
(42,52). Beyond their efficacy, concerns
persist regarding the long-term safety and cost-effectiveness of
botanical treatments. Regarding cost-effectiveness, research has
shown that interdisciplinary rehabilitation, exercise, acupuncture,
spinal manipulation and cognitive-behavioral therapy are
cost-effective for sub-acute or chronic low back pain (50). However, there is insufficient
evidence on the cost-effectiveness of botanical therapies compared
with conventional pharmacological treatments (53). To address these limitations, future
research should prioritize long-term safety assessments by
conducting RCTs with extended follow-up periods, which will allow
for a more thorough evaluation of delayed adverse effects. Economic
evaluations comparing the direct costs, such as medication expenses
and hospitalizations, with indirect costs, including productivity
loss and quality of life impact, will be essential for determining
their real-world applicability. Standardization of botanical
formulations should also be a key area of focus to ensure
consistency in composition and therapeutic effects across studies,
thereby improving the reproducibility and reliability of clinical
outcomes. Another critical aspect that requires attention is the
methodological rigor of clinical trials, particularly regarding
blinding and placebo control. Many trials involving botanical
therapies face challenges in maintaining blinding due to the
distinct sensory characteristics of herbal preparations. Future
studies should adopt more sophisticated blinding techniques, such
as the use of indistinguishable placebo formulations, to minimize
bias and strengthen the reliability of the evidence. By addressing
these gaps, future investigations can provide more comprehensive
insights into the clinical utility, safety, and economic viability
of botanical therapies for CLBP. This will ultimately contribute to
a stronger evidence base for their potential role in evidence-based
treatment strategies.
In conclusion, the present meta-analysis highlighted
the potential of botanical extracts as effective and safe
alternatives for managing CLBP, providing pain relief and
functional improvements with minimal adverse effects. To strengthen
these findings, larger RCTs with standardized botanical
formulations, consistent blinding methods and long-term follow-up
are needed to confirm the sustained benefits of these
interventions. Additionally, future research should investigate the
cost-effectiveness of botanical therapies to support their role in
reducing dependence on conventional pharmacological treatments.
While the present study offered an overview of the individual
effects of various botanical extracts, future network meta-analyses
should provide a more robust framework for directly comparing their
relative effectiveness. Such analyses could enable a comprehensive
ranking of these interventions, offering valuable insights to guide
clinical decision-making and addressing current gaps in the
literature.
Supplementary Material
Assessment of methodological quality
of the included trials. (A) Risk of bias for each included study.
(B) The overall summary of bias of the ten studies.
Funnel plot that summarizes the
results from all the included studies. The lines usually depict the
confidence intervals around the effect estimates, showing the range
where the true effect size is likely to fall. The circles denote
individual studies included in the meta.analysis, with their size
possibly reflecting the weight or sample size of each study; larger
circles signify studies with greater weight or larger sample sizes.
The diamond symbol represents the overall effect estimate from the
meta.analysis. The center of the diamond indicates the pooled
effect size, while the width of the diamond illustrates the
confidence interval for this estimate. (A) The 13 included
randomized controlled trials. (B) Corresponds to the sensitivity
analysis from Fig. 4G.
Characteristics of included
studies.
Acknowledgements
Not applicable.
Funding
Funding: This research was funded by the Chung Shan Medical
University (grant no. CSMU-INT-112-22).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RYT was responsible for study conception, data
curation, investigation (gathering and analyzing primary data, and
conducting experiments), visualization of results as figures, and
writing, reviewing and editing the manuscript. CCC was responsible
for study conception and data curation. SYL was responsible for
investigation (gathering and analyzing primary data, and conducting
experiments) and visualization of results as figures. JYU assisted
in identifying RCTs and abstract screening. YH assisted in
identifying RCTs and figure production. RYT and CCC confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Quittan M: Management of back pain.
Disabil Rehabil. 24:423–434. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gaskin DJ and Richard P: The economic
costs of pain in the United States. J Pain. 13:715–724.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kebede A, Abebe SM, Woldie H and Yenit MK:
Low back pain and associated factors among primary school teachers
in mekele city, North Ethiopia: A cross-sectional study. Occup Ther
Int. 2019(3862946)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Katz JN: Lumbar disc disorders and
low-back pain: Socioeconomic factors and consequences. J Bone Joint
Surg Am. 88 (Suppl 2):S21–S24. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Miedema HS, Chorus AM, Wevers CW and van
der Linden S: Chronicity of back problems during working life.
Spine (Phila Pa 1976). 23:2021–2029. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gatchel RJ, Polatin PB and Mayer TG: The
dominant role of psychosocial risk factors in the development of
chronic low back pain disability. Spine (Phila Pa 1976).
20:2702–2709. 1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Frerick H, Keitel W, Kuhn U, Schmidt S,
Bredehorst A and Kuhlmann M: Topical treatment of chronic low back
pain with a capsicum plaster. Pain. 106:59–64. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Laine L: Gastrointestinal effects of
NSAIDs and coxibs. J Pain Symptom Manage. 25 (2 Suppl):S32–S40.
2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Singh G: Recent considerations in
nonsteroidal anti-inflammatory drug gastropathy. Am J Med.
105:31S–38S. 1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lakhan SE, Sheafer H and Tepper D: The
effectiveness of aromatherapy in reducing pain: A systematic review
and meta-analysis. Pain Res Treat. 2016(8158693)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Adams R, White B and Beckett C: The
effects of massage therapy on pain management in the acute care
setting. Int J Ther Massage Bodywork. 3:4–11. 2010.PubMed/NCBI
|
|
12
|
Boonruab J, Damjuti W, Niempoog S and
Pattaraarchachai J: Effectiveness of hot herbal compress versus
topical diclofenac in treating patients with myofascial pain
syndrome. J Tradit Complement Med. 9:163–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chrubasik S, Weiser T and Beime B:
Effectiveness and safety of topical capsaicin cream in the
treatment of chronic soft tissue pain. Phytother Res. 24:1877–1885.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Keitel W, Frerick H, Kuhn U, Schmidt U,
Kuhlmann M and Bredehorst A: Capsicum pain plaster in chronic
non-specific low back pain. Arzneimittelforschung. 51:896–903.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mason L, Moore RA, Derry S, Edwards JE and
McQuay HJ: Systematic review of topical capsaicin for the treatment
of chronic pain. BMJ. 328(991)2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mason L, Moore RA, Edwards JE, Derry S and
McQuay HJ: Topical NSAIDs for chronic musculoskeletal pain:
Systematic review and meta-analysis. BMC Musculoskelet Disord.
5(28)2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hutton B, Salanti G, Caldwell DM, Chaimani
A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen
JP, et al: The PRISMA extension statement for reporting of
systematic reviews incorporating network meta-analyses of health
care interventions: Checklist and explanations. Ann Intern Med.
162:777–784. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Sterne JAC, Savović J, Page MJ, Elbers RG,
Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge
SM, et al: RoB 2: A revised tool for assessing risk of bias in
randomised trials. BMJ. 366(l4898)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Krivoy N, Pavlotzky E, Chrubasik S,
Eisenberg E and Brook G: Effect of salicis cortex extract on human
platelet aggregation. Planta Med. 67:209–212. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Luo X, Wang P, Li Z, Liang D, Huang F, Liu
J, Chen X, Hu G, Fang S and Zhang H: Evaluation of a granulated
formula for the nerve root type and vertebral artery type of
cervical spondylosis: A multicenter, single-blind, randomized,
controlled, phase III clinical trial. J Tradit Chin Med.
37:193–200. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hughes CM, Liddle SD, Sinclair M and
McCullough JEM: The use of complementary and alternative medicine
(CAM) for pregnancy related low back and/ or pelvic girdle pain: An
online survey. Complement Ther Clin Pract. 31:379–383.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Laosee O, Sritoomma N, Wamontree P,
Rattanapan C and Sitthi-Amorn C: The effectiveness of traditional
Thai massage versus massage with herbal compress among elderly
patients with low back pain: A randomised controlled trial.
Complement Ther Med. 48(102253)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Giannetti BM, Staiger C, Bulitta M and
Predel HG: Efficacy and safety of comfrey root extract ointment in
the treatment of acute upper or lower back pain: Results of a
double-blind, randomised, placebo controlled, multicentre trial. Br
J Sports Med. 44:637–641. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chrubasik S, Model A, Black A and Pollak
S: A randomized double-blind pilot study comparing Doloteffin and
Vioxx in the treatment of low back pain. Rheumatology (Oxford).
42:141–148. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stam C, Bonnet MS and van Haselen RA: The
efficacy and safety of a homeopathic gel in the treatment of acute
low back pain: A multi-centre, randomised, double-blind comparative
clinical trial. Br Homeopath J. 90:21–28. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chrubasik S, Künzel O, Model A, Conradt C
and Black A: Treatment of low back pain with a herbal or synthetic
anti-rheumatic: A randomized controlled study. Willow bark extract
for low back pain. Rheumatology (Oxford). 40:1388–1393.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Han X, Eggett DL and Parker TL: Evaluation
of the health benefits of a multivitamin, multimineral, herbal,
essential oil-infused supplement: A pilot trial. J Diet Suppl.
15:153–160. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fischer AM, Holmes P, Bahar YZ, Vacca S,
Goldberg S and Gold MA: Aroma acupoint therapy for symptom
management with adolescent patients: Early experiences from
school-based health centers. Med Acupunct. 32:287–292.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lo WC, Chen YT and Chen CC: The effects of
elgucare on degenerated intervertebral disc-induced low back pain
and disc regeneration: A clinical trial. Comput Math Methods Med.
2021(5824956)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chrubasik S, Eisenberg E, Balan E,
Weinberger T, Luzzati R and Conradt C: Treatment of low back pain
exacerbations with willow bark extract: A randomized double-blind
study. Am J Med. 109:9–14. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yip YB and Tse SHM: The effectiveness of
relaxation acupoint stimulation and acupressure with aromatic
lavender essential oil for non-specific low back pain in Hong Kong:
A randomised controlled trial. Complement Ther Med. 12:28–37.
2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
da Silva AG, de Sousa CPG, Koehler J,
Fontana J, Christo AG and Guedes-Bruni RR: Evaluation of an extract
of Brazilian arnica (Solidago chilensis Meyen, Asteraceae) in
treating lumbago. Phytother Res. 24:283–287. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Alkanat HÖ, Özdemir Ü and Kulaklı F: The
effects of massage with frankincense and myrrh oil in chronic low
back pain: A three-arm randomised controlled trial. Explore (NY).
19:761–767. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shirazi M, Mohebitabar S, Bioos S,
Yekaninejad MS, Rahimi R, Shahpiri Z, Malekshahi F and Nejatbakhsh
F: The effect of topical rosa damascena (rose) oil on
pregnancy-related low back pain: A randomized controlled clinical
trial. J Evid Based Complementary Altern Med. 22:120–126.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sritoomma N, Moyle W, Cooke M and O'Dwyer
S: The effectiveness of Swedish massage with aromatic ginger oil in
treating chronic low back pain in older adults: A randomized
controlled trial. Complement Ther Med. 22:26–33. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Uğurlu M, Aksekili MAE, Alkan BM, Kara H
and Çağlar C: Effects of artcure diffusional patch application on
pain and functional status in lumbar disc herniation patients: A
prospective randomized controlled study. Turk J Med Sci.
47:874–882. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chrubasik S, Junck H, Breitschwerdt H,
Conradt C and Zappe H: Effectiveness of Harpagophytum extract WS
1531 in the treatment of exacerbation of low back pain: A
randomized, placebo-controlled, double-blind study. Eur J
Anaesthesiol. 16:118–129. 1999.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kong LJ, Fang M, Zhan HS, Yuan WA, Tao JM,
Qi GW and Cheng YW: Chinese massage combined with herbal ointment
for athletes with nonspecific low back pain: A randomized
controlled trial. Evid Based Complement Alternat Med.
2012(695726)2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pérez-Piñero S, Muñoz-Carrillo JC,
Echepare-Taberna J, Luque-Rubia AJ, Millán Rivero JE, Muñoz-Cámara
M, Díaz Silvente MJ, Valero Merlos E, Ávila-Gandía V, Caturla N, et
al: Dietary supplementation with plant extracts for amelioration of
persistent myofascial discomfort in the cervical and back regions:
A randomized double-blind controlled study. Front Nutr.
11(1403108)2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pach D, Brinkhaus B, Roll S, Wegscheider
K, Icke K, Willich SN and Witt CM: Efficacy of injections with
Disci/Rhus toxicodendron compositum for chronic low back pain-a
randomized placebo-controlled trial. PLoS One.
6(e26166)2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pillastrini P, Gardenghi I, Bonetti F,
Capra F, Guccione A, Mugnai R and Violante FS: An updated overview
of clinical guidelines for chronic low back pain management in
primary care. Joint Bone Spine. 79:176–185. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bakó E, Fehérvári P, Garami A, Dembrovszky
F, Gunther EE, Hegyi P, Csupor D and Böszörményi A: Efficacy of
topical essential oils in musculoskeletal disorders: Systematic
review and meta-analysis of randomized controlled trials.
Pharmaceuticals (Basel). 16(144)2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li CP, Tsai RY, Yu JT, Huang SC, Liu FL
and Lin SY: Efficacy of plant extract in chronic low back pain: A
meta-analysis. Tungs' Med J. 18:92–101. 2024.
|
|
44
|
Mahendran G and Rahman LU: Ethnomedicinal,
phytochemical and pharmacological updates on peppermint (Mentha x
piperita L.)-A review. Phytother Res. 34:2088–2139. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gupta A, Joshi R, Dewangan L, Shah K, Soni
D, Patil UK and Chauhan NS: Capsaicin: Pharmacological applications
and prospects for drug designing. J Pharm Pharmacol.
(rgae150)2024.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
46
|
Li S, Cao J, Yang Z, Jin S, Yang L and
Chen H: Licorice and dried ginger decoction inhibits inflammation
and alleviates mitochondrial dysfunction in chronic obstructive
pulmonary disease by targeting siglec-1. Int Immunopharmacol.
146(113789)2025.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kroenke K, Krebs EE and Bair MJ:
Pharmacotherapy of chronic pain: A synthesis of recommendations
from systematic reviews. Gen Hosp Psychiatry. 31:206–219.
2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Coderre TJ and Fulas OA: Topical drug
delivery in the treatment of chronic pain: A review. Chron Pain
Manag. 8(152)2024.
|
|
49
|
Sawynok J: Topical and peripherally acting
analgesics. Pharmacol Rev. 55:1–20. 2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gagnier JJ, Oltean H, van Tulder MW,
Berman BM, Bombardier C and Robbins CB: Herbal medicine for low
back pain: A cochrane review. Spine (Phila Pa 1976). 41:116–133.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kuijpers T, van Middelkoop M, Rubinstein
SM, Ostelo R, Verhagen A, Koes BW and van Tulder MW: A systematic
review on the effectiveness of pharmacological interventions for
chronic non-specific low-back pain. Eur Spine J. 20:40–50.
2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Rubinstein SM, van Middelkoop M, Kuijpers
T, Ostelo R, Verhagen AP, de Boer MR, Koes BW and van Tulder MW: A
systematic review on the effectiveness of complementary and
alternative medicine for chronic non-specific low-back pain. Eur
Spine J. 19:1213–1228. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lin CWC, Haas M, Maher CG, Machado LAC and
van Tulder MW: Cost-effectiveness of guideline-endorsed treatments
for low back pain: A systematic review. Eur Spine J. 20:1024–1038.
2011.PubMed/NCBI View Article : Google Scholar
|