Introduction
A period of >5 years has passed since the
emergence of coronavirus disease 2019 (COVID-19), yet the world
continues to grapple with its impact (1). Successive waves of infection led to
case fatality rates as high as 15% in certain populations, with
mortality estimates suggesting that the true toll may be
significantly worse than this (2,3). The
cause of this lethal disease is severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2), and its worldwide effect was classified
as a pandemic by the World Health Organization in March
2020(4). Viral transmission is via
human-to-human bodily fluid contact (droplet and/or aerosol), and
the incubation period can be as long as 14-28 days before symptoms
develop (5). Quarantine has proven
effective in protecting susceptible people and reducing
transmission rate.
The initial symptoms of most COVID-19 infections are
often non-specific, such as coughing and dyspnea, while ~20% of
patients present with gastrointestinal (GI) symptoms within the
first 2 days of infection (6).
Current interventions focus on supportive therapies, and novel
vaccines have proven effective at mitigating disease severity
(7,8).
The synergistic association between infection and
malnutrition has been established (9). As COVID-19 progresses, ~5% of
patients who become critically ill require an intensive care unit
(ICU) admission, and regulating nutritional balance in these
patients is vitally important for supportive care (10). Clinical reports have shown that the
severity of COVID-19 infection is compounded by nutritional
variables, especially for patients with GI symptoms (4). In this context, related studies have
also demonstrated that nutritional support is a promising treatment
for patients with COVID-19 and high nutritional risks (11-14).
To facilitate generalization of nutritional
interventions, the president of the American Society for Parenteral
and Enteral Nutrition (ASPEN) proposed a focused review of current
clinical nutrition practices, including nutrition assessment,
processing steps, monitoring and reassessment (15). The main points included: i) Early
EN initiation, within 24-36 h of ICU admission or 12 h of
intubation; ii) monitoring and reassessing patients during the
EN/PN regimens; and iii) early EN may not be preferential in a
subset of patients with GI symptoms; PN needing to be used early
instead. Several malnutrition screening tools, such as Nutrition
Risk Screen-2002 (NRS-2002), Global Leadership Initiative on
Malnutrition (GLIM) (13,16,17),
have gained widespread acceptance in recent years, largely due to
their clinical practicality. These tools typically incorporate
common clinical variables such as weight loss, body mass index
(BMI), fat-free mass indices (FFMI) and signs of eating
difficulties (such as appetite loss or reduced intake). The present
study included BMI and FFMI for nutritional evaluation since their
acquisitions were easy to access.
Recent studies on nutritional timing in ICU patients
with COVID-19 found mixed outcomes regarding early versus delayed
support. Some have reported that early enteral nutrition (EN)
(within 24-48 h) can lead to reduced mortality and ICU stay, while
others report equivocal findings with no clear advantage from early
or delayed support, and they do not raise any concerns about
tolerance during the acute phase (15-21).
Following ICU admission, EN could be started early
as an effective way to improve nutritional status through
nasogastric tube, even while patients are in a prone position
(12). However, after EN
initiation, refeeding syndrome (RS) can occur in patients who have
been undernourished for a substantial period of time, owing to the
wide range of metabolic and electrolyte imbalances, impaired
glucose tolerance and vitamin deficiency in these patients
(22,23). In severe cases, it can even lead to
organ failure and death. The current definition of RS is a decrease
in either serum potassium, phosphorus or magnesium level within 5
days of the reintroduction of calories (22). Based on the degree of reduction in
the levels of these nutrients, RS is classified as mild (10-20%),
moderate (20-30%) or severe (>30% or organ dysfunction resulting
from a decrease of any of the aforementioned electrolyte ions or
thiamine-deficiency) (22). It is
reasonable to speculate that a delay in nutritional support could
exacerbate RS since the period of nutrient restriction would be
prolonged. However, for most COVID-19 patients at high risk of
malnutrition, nutritional support is initiated as early as
possible. Once the starvation period is excluded as a contributing
factor, the method of nutritional intervention remains the key
determinant influencing RS incidence. As the preferred nutritional
therapy for patients with COVID-19, EN requires careful timing,
composition and monitoring of total daily intakes. However, it
remains unclear whether the timing of EN initiation influences RS
incidence or severity in critically ill patients with COVID-19. To
address this, a retrospective study was conducted on patients with
COVID-19 who were admitted to the ICU, comparing different EN
initiation times to assess their impact on RS incidence.
Patients and methods
Study population
The present study included critically ill patients
with established COVID-19 infection, confirmed by the standard
reverse transcriptase-polymerase chain reaction method, who were
admitted to Tongji Hospital (Wuhan, China) between January 1 and
December 31, 2020. Severe cases met at least one of the following
criteria: i) Shortness of breath (respiratory rate ≥30
breaths/min); ii) oxygen saturation ≤93% in resting state; iii)
partial pressure of arterial oxygen/inspired oxygen concentration
≤300 mmHg; and iv) obvious progress of lesion size by >50%
within 24-48 h, as determined by pulmonary imaging (24,25).
Study procedures
Nutritional support was given to severely ill
patients at high nutritional risk, as defined by ASPEN criteria
within 24-36 h of ICU admission (15). EN was the first line of nutrient
support for most patients, while parenteral nutrition (PN) was
administered if EN was contraindicated or insufficient. EN was
supplied with complete nutritional formula, such as whole protein
preparations or short peptide/amino acid-based preparations. PN was
provided with nutrient mixtures, including carbohydrates, fats,
proteins, vitamins and minerals. A previous study (26) showed that RS prevalence is
significantly associated withNRS-2002 scores. According to NRS-2002
scoring (27), a total of 304
cases in Tongji Hospital scored ≥3 points, representing those with
a high nutritional risk.
Nutritional support was administered as quickly as
possible following admission to the hospital. Energy requirement
was determined based on energy expenditure calculations from
indirect calorimetry measurements for each patient. Target
nutritional requirement ranges were 25-30 kcal/(kg/day) total
calories, 1.5-2.0 g/(kg/day) total protein and 7.8-11.1 mmol/l
blood glucose. After admission, EN was the preferred route of
administration. However, for patients with digestive symptoms or
elevated risk of aspiration, PN was administered within 24-48 h and
gradually shifted to EN thereafter. Once the nutritional ranges
were achieved using EN, PN was discontinued. In severe cases, EN
was administered through a nasogastric tube. Patients in a prone
position could also receive EN under the rationale of prophylaxis
for aspiration. The mean initial rate of EN therapy was 20 ml/h,
and gradually increased to 70-100 ml/h. The dosage of EN therapy
increased from 500 ml/day to 1,000 ml/day. During EN
administration, each patient's tolerance was evaluated by checking
the gastric residual volume every 4 h. If the residual volume was
>500 ml, or accompanied with severe nausea, vomiting, diarrhea
or other symptoms, EN infusion would be slowed, paused or changed
to another EN reagent. For all COVID-19 patients with malnutrition
risk, EN support was preferred for those without contraindications.
PN was only applied for those who were EN intolerant or
insufficient response from EN. To minimize the risk of RS
associated with PN, electrolyte levels were continuously monitored
throughout PN administration to ensure they remained within
relatively normal ranges prior to initiating EN.
During the nutritional support period, serum
electrolytes were measured daily. If any electrolyte levels dropped
below the following thresholds, sodium <130 mmol/l, potassium
<3.5 mmol/l, phosphorus<0.96 mmol/l and magnesium <0.70
mmol/l, then the corresponding electrolytes would be replenished as
needed and carefully monitored to ensure they remained within the
normal range. Throughout the entire course of nutritional support,
levels of thiamine and other essential vitamins (such as vitamins D
and E) were continuously monitored and promptly supplemented when
found to be deficient. RS in the present study was identified
according to the ASPEN definition as any one, two or three
electrolyte imbalances, hypophosphatemia, hypokalemia and/or
hypomagnesemia, after starting EN. A time cut-off time of 3 days
after nutritional support was selected, which was within 5 days of
reintroduction of calories.
Data collection
All patients who received EN therapy in this study
were classified into two groups based on EN initiation intervals.
The early EN (EEN) group were those with an interval from admission
to first EN therapy of ≤2 days and the late EN (LEN) group were
those with an interval from admission to first EN therapy of ≥3
days.
To ensure adequate sample size in the power
estimates, an odds ratio (OR) of 2 was assumed for the association
between LEN and RS, with a significance level (α) of 0.05
(two-sided) and a statistical power (1-β) of 0.8. Based on these
parameters (OR, α, 1-β), the calculated minimum required sample
size was 280 participants (case group, n=140; control group,
n=140). A total of 211 cases were included in the final analysis,
owing to limitations in patient volume and implementing
inclusion/exclusion criteria. The inclusion criteria were as
follows: i) Severe COVID-19, ii) NRS-2002 scores ≥3 points, iii)
receiving EN support, iv) age ≥18 years and v) no immunodeficiency
or malignant diseases. Exclusion criteria were as follows: i) Mild
or moderate COVID-19, ii) NRS-2002 scores <3 points, iii) not
needing nutritional support, or only PN support, iv) age <18
years and v) presence of immunodeficiency or malignant
diseases.
The requirement for informed consent was waived from
Tongji Hospital as this was a retrospective analysis. Data on
patient demographics, symptoms, underlying diseases and laboratory
examinations were collected from electronic medical records. Some
interventions that could potentially affect nutritional status and
RS incidence [for example, mechanical ventilation and renal
replacement therapy (RRT)], were also compared between the two
groups. Inflammatory and biochemical markers, including serum
albumin, globulin, pH and creatinine, were measured upon admission.
Additionally, serum phosphorus, potassium and magnesium levels were
assessed on admission and 3 days after EN initiation as
aforementioned.
Outcome variables
The primary outcome of the present study was the
incidence and degree of RS 3 days after EN initiation. Patients
were defined as RS-positive if they experienced a decrease below
the aforementioned thresholds in serum phosphorus, potassium or
magnesium level 3 days after EN initiation. Secondary outcomes were
3-month overall survival rate, in-hospital length of stay, ICU
length of stay, incidence of airway complications and GI
intolerance. Airway complications were defined as any episode of
vomiting, followed by desaturation and formation of a mucus plug.
Subjects who required frequent sputum suction (>2 times/h) and
had episodes of temporary desaturation caused by airway obstruction
due to sputum were defined as having a mucus plug (21). GI intolerance was defined as the
appearance of serious diarrhea, nausea, vomiting, abdominal
discomfort or GI bleeding after EN, which required therapeutic
intervention (28).
Statistical analysis
Descriptive data are presented as the mean ±
standard deviation and were confirmed by Student's t-test or
Mann-Whitney U test. Categorical variables are presented as
percentages and analyzed by the χ2 test or Fisher's
exact test. The 6-month survival curve was plotted using the
Kaplan-Meier method with the log-rank test for analysis. Receiver
operating characteristic (ROC) curves were plotted, and the area
under the curve (AUC) was calculated to analyze the potential
predictors for RS. Independent risk factors for RS were determined
using logistic regression. Variables with a P-value <0.2 in the
univariate analysis were included in the multivariate analysis.
P<0.05 was considered to indicate a statistically significant
difference. To further decrease the type I error, Bonferroni's
correction adjustment was applied if necessary. All statistical
analyses and plotting were performed using SPSS 22.0 (IBM Corp.)
and GraphPad Prism 6.0 (Dotmatics).
Results
Baseline patient demographics and
clinical variables
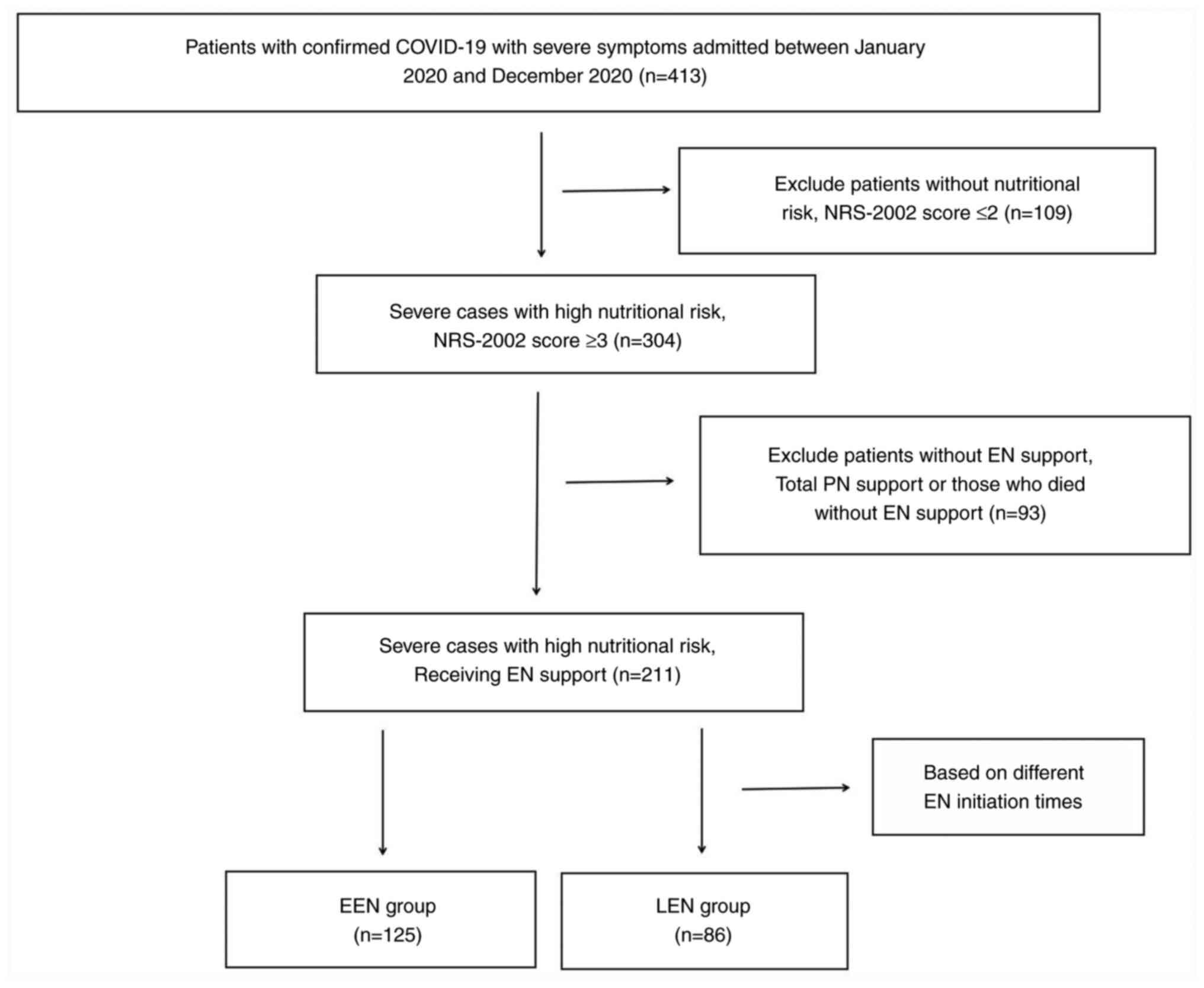
A total of 413 consecutive hospitalized patients
with severe symptoms of COVID-19 infection were evaluated at Tongji
Hospital (Wuhan, China) between January 2020 and December 2020.
Among them, 211 patients with high nutritional risk received EN
support. Of these, 122 patients required electrolyte
supplementation during the first 3 days of EN. Table I shows the key demographic and
clinical characteristics of participants based on EN initiation
intervals, with 125 patients (59.24%) assigned to the EEN group and
86 patients (40.76%) assigned to the LEN group. Patient selection
and group assignment criteria are shown in Fig. 1.
 | Table IDemographic characteristics of
patients with coronavirus disease 2019 according to EN
initiation. |
Table I
Demographic characteristics of
patients with coronavirus disease 2019 according to EN
initiation.
| Variables | All (n=211) | EEN (n=125) | LEN (n=86) | P-value |
|---|
| Age, years | 64±1.4 | 64±1.5 | 64±1.3 | 0.6974 |
| Male sex, n
(%) | 101 (47.87) | 51 (40.80) | 50 (58.14) | 0.1473 |
| Weight, kg | 60±1.6 | 59±1.6 | 60±1.5 | 0.5847 |
| Body mass index,
kg/m2 | 22±0.4 | 22±0.4 | 22±0.3 | 0.5698 |
| Fat-free mass
indices, kg/m2 | 17±0.5 | 17±0.4 | 16±0.7 | 0.5022 |
| APACHE II
scorea | 22±0.5 | 22±0.6 | 23±0.5 | 0.7872 |
| Symptoms, n
(%) | | | | |
|
Fever | 184 (87.20) | 104 (83.20) | 80 (93.02) | 0.4383 |
|
Cough | 174 (82.46) | 99 (79.20) | 75 (87.21) | 0.0788 |
|
Respiratory
distress | 103 (48.82) | 65 (52.00) | 38 (44.19) | 0.7431 |
| Underlying
diseases, n (%) | | | | |
|
Hypertension | 124 (58.77) | 73 (58.40) | 51 (59.30) | 0.9803 |
|
Diabetes
mellitus | 78 (36.97) | 42 (33.60) | 36 (41.86) | 0.4555 |
|
Chronic
cardiovascular disease | 47 (22.27) | 28 (22.40) | 19 (22.09) | 0.5838 |
|
Chronic
respiratory disease | 42 (19.91) | 24 (19.20) | 18 (20.93) | 0.4125 |
| Past history, n
(%) | | | | |
|
Smoking | 56 (26.54) | 34 (27.20) | 22 (25.58) | 0.4449 |
|
Drinking | 24 (11.37) | 16 (12.80) | 8 (9.30) | 0.4535 |
| Mechanical
ventilation, n (%) | 110 (52.13) | 70 (56.00) | 40 (46.51) | 0.4380 |
| Renal replacement
therapy, n (%) | 24 (11.37) | 16 (12.80) | 8 (9.30) | 0.1075 |
| Caloric intake,
Kcal/day | 1778±11.4 | 1773±14.3 | 1785±10.5 | 0.5063 |
| Protein intake,
g/day | 115±1.2 | 115±1.1 | 114±1.1 | 0.7304 |
| Electrolyte
supplementation from days 1 to day 3, n (%) | 132 (62.56) | 77 (61.60) | 55 (63.95) | 0.3020 |
| NRS-2002 score | 4±0.1 | 4±0.2 | 4±0.1 | 0.3897 |
| Time interval to
EN, days | 3±0.1 | 2±0.1 | 5±0.1 | 0.0413b |
The mean age of all patients was 64 years, ranging
from 46 to 92 years, and 101 patients (47.87%) were male. General
symptoms of COVID-19 included fever, cough and respiratory
distress. The most common comorbidity was hypertension (58.77% of
the entire cohort). Both mechanical ventilation and RRT, which may
be confounders for nutrition consumption, were similar between the
groups (P=0.4380 and P=0.1075, respectively). Daily mean calories
and protein intake were also not significantly different between
groups (P=0.5063 and P=0.7304, respectively), which excluded some
confounding factors for RS incidence. The nutritional state,
including weight, BMI, FFMI and NRS-2002 scores on admission, was
comparable between the two groups (all P≥0.05). The only
significant difference between the two groups was the mean time
interval to EN therapy initiation, which at 2 days in the EEN
group, was significantly lower than the interval of 5 days in the
LEN group (P=0.0413).
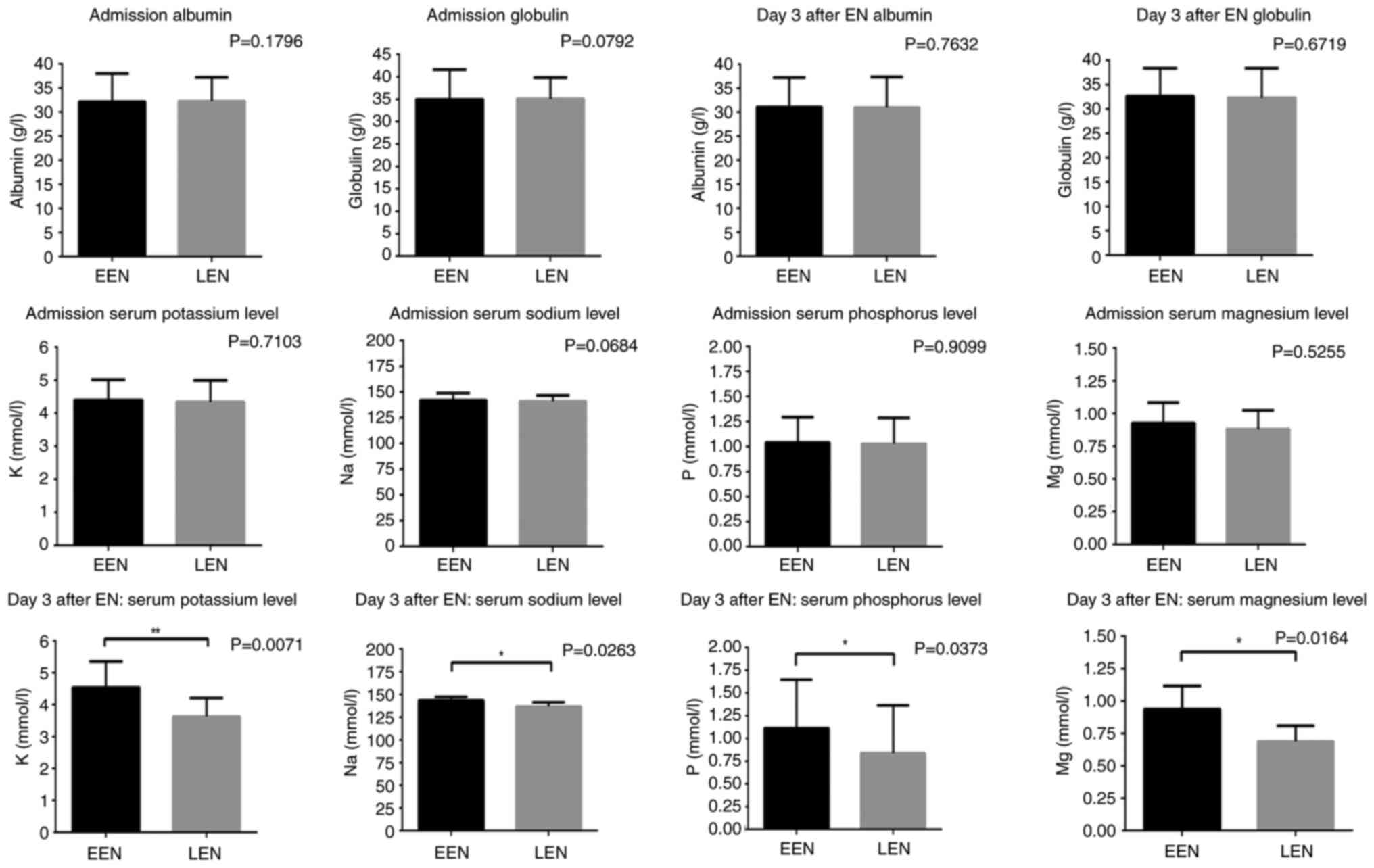
Serum electrolytes and blood
variables
Baseline laboratory results for all patients were
collected on admission and again 3 days after EN initiation
(Table II; Fig. 2). At time of admission, blood cell
counts for leukocytes, neutrophils and lymphocytes were similar
between the two groups (all P>0.05). Likewise, serum levels of
albumin, globulin, pH and creatinine were also not significantly
different between groups (all P>0.05). Both groups displayed
similar electrolyte balance, all within the acceptable range (all
P>0.05). At 3 days post-EN initiation, there was a significant
reduction in potassium, sodium, phosphorus and magnesium levels in
the LEN group compared with those in the EEN group (P=0.0071,
P=0.0263, P=0.0373 and P=0.0164, respectively), with all of these
electrolytes falling below the clinically acceptable range. In
order to decrease the type I error incidence, Bonferroni's
correction was applied. After adjustment, only the changes in serum
potassium (P=0.0213) and magnesium (P=0.0492) levels at 3 days
post-EN initiation remained statistically significant (Table II; Fig. S1).
 | Table IILaboratory examination of patients
with coronavirus disease 2019 according to EN initiation. |
Table II
Laboratory examination of patients
with coronavirus disease 2019 according to EN initiation.
| A, Admission |
|---|
| Variables | All (n=211) | EEN (n=125) | LEN (n=86) | P-value | P-value
(Bonferroni's correction) |
|---|
| Leukocytes
(x109/l) | 8.874±0.7250 | 8.901±0.7324 | 8.846±0.6977 | 0.5470 | / |
| Neutrophils
(x109/l) | 7.512±0.7158 | 7.520±0.7443 | 7.507±0.6869 | 0.6984 | / |
| Lymphocytes
(x109/l) | 0.855±0.0715 | 0.822±0.0794 | 0.881±0.0637 | 0.4631 | / |
| Albumin, g/l | 32.19±0.6886 | 32.13±0.7904 | 32.24±0.5867 | 0.1796 | / |
| Globulin, g/l | 35.03±0.7327 | 34.98±0.8993 | 35.07±0.5642 | 0.0792 | / |
| pH | 7.401±0.0039 | 7.401±0.0043 | 7.402±0.0034 | 0.7953 | / |
| Creatinine,
µmol/l | 130.5±5.2535 | 135.8±5.8310 | 124.4±4.6760 | 0.1253 | / |
| Potassium,
mmol/l | 4.372±0.0803 | 4.403±0.0834 | 4.349±0.0771 | 0.7103 | / |
| Sodium, mmol/l | 141.5±0.7088 | 142.0±0.9232 | 141.1±0.6445 | 0.0684 | / |
| Phosphorus,
mmol/l | 1.032±0.0448 | 1.039±0.0482 | 1.027±0.0394 | 0.9099 | / |
| Magnesium,
mmol/l | 0.899±0.0273 | 0.928±0.0299 | 0.882±0.0216 | 0.5255 | / |
| B, 3 days after EN
initiation |
| Variables | All (n=211) | EEN (n=125) | LEN (n=86) | P-value | P-value
(Bonferroni's correction) |
| Potassium,
mmol/l | 4.029±0.0889 | 4.540±0.1093 | 3.432±0.0683 | 0.0071a | 0.0213a |
| Sodium, mmol/l | 139.8±0.5276 | 143.6±0.5226 | 134.8±0.5287 | 0.0263a | 0.0789 |
| Phosphorus,
mmol/l | 0.944±0.0913 | 1.114±0.1023 | 0.838±0.0802 | 0.0373a | 0.1119 |
| Magnesium,
mmol/l | 0.789±0.0257 | 0.938 ± 0.0337 | 0.691 ± 0.0181 | 0.0164a | 0.0492a |
Primary and secondary outcomes
RS incidence was significantly lower in the EEN
group compared with that in the LEN group (27.20% vs. 60.47%,
P=0.0440), with RS severity higher in the LEN group, since 63.46%
of patients had ‘moderate’ RS compared with 41.18% in the EEN group
(P=0.0236) (Table III). There
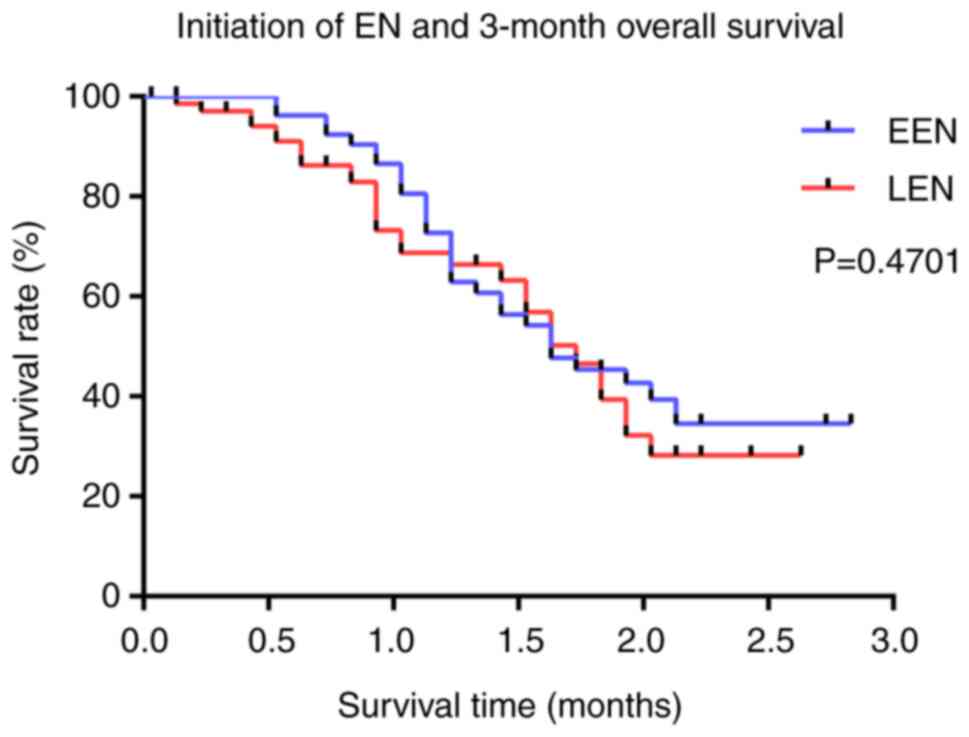
were no significant differences in overall survival between the two
groups at 3 months (P=0.4701) (Fig.
3). Notably, despite the similar mortality rates, in-hospital
and ICU stay lengths were significantly lower in the EEN group
(19.46±0.81 vs. 29.22±1.10, P<0.0001; and 17.16±1.04 vs.
22.39±1.50, P=0.0099, respectively). Moreover, the EEN group did
not experience any significant increase in risk of airway
complications or GI intolerance (P=0.0742 and P=0.3392,
respectively) (Table III).
 | Table IIIOutcomes of subjects with coronavirus
disease 2019 in the groups receiving early versus late EN. |
Table III
Outcomes of subjects with coronavirus
disease 2019 in the groups receiving early versus late EN.
| Outcomes | All (n=211) | EEN (n=125) | LEN (n=86) | P-value |
|---|
| Refeeding syndrome,
n (%) | 86 (40.76) | 34 (27.20) | 52 (60.47) | 0.0440a |
|
Mild | 39 (45.35) | 20 (58.82) | 19 (36.54) | 0.0236a |
|
Moderate | 47 (54.65) | 14 (41.18) | 33 (63.46) | |
| 3-Month mortality,
n (%) | 106 (50.24) | 69 (55.20) | 37 (43.02) | 0.4701 |
| In-hospital stay
length, days | 24.95±0.964 | 19.46±0.813 | 29.22±1.095 |
<0.0001a |
| ICU stay length,
days | 20.23±1.277 | 17.16±1.037 | 22.39±1.495 | 0.0099a |
| Airway
complications, n (%) | 82 (38.86) | 54 (43.20) | 28 (32.56) | 0.0742 |
| GI intolerance, n
(%) | 80 (37.91) | 44 (35.20) | 36 (41.86) | 0.3392 |
Prediction of RS incidence
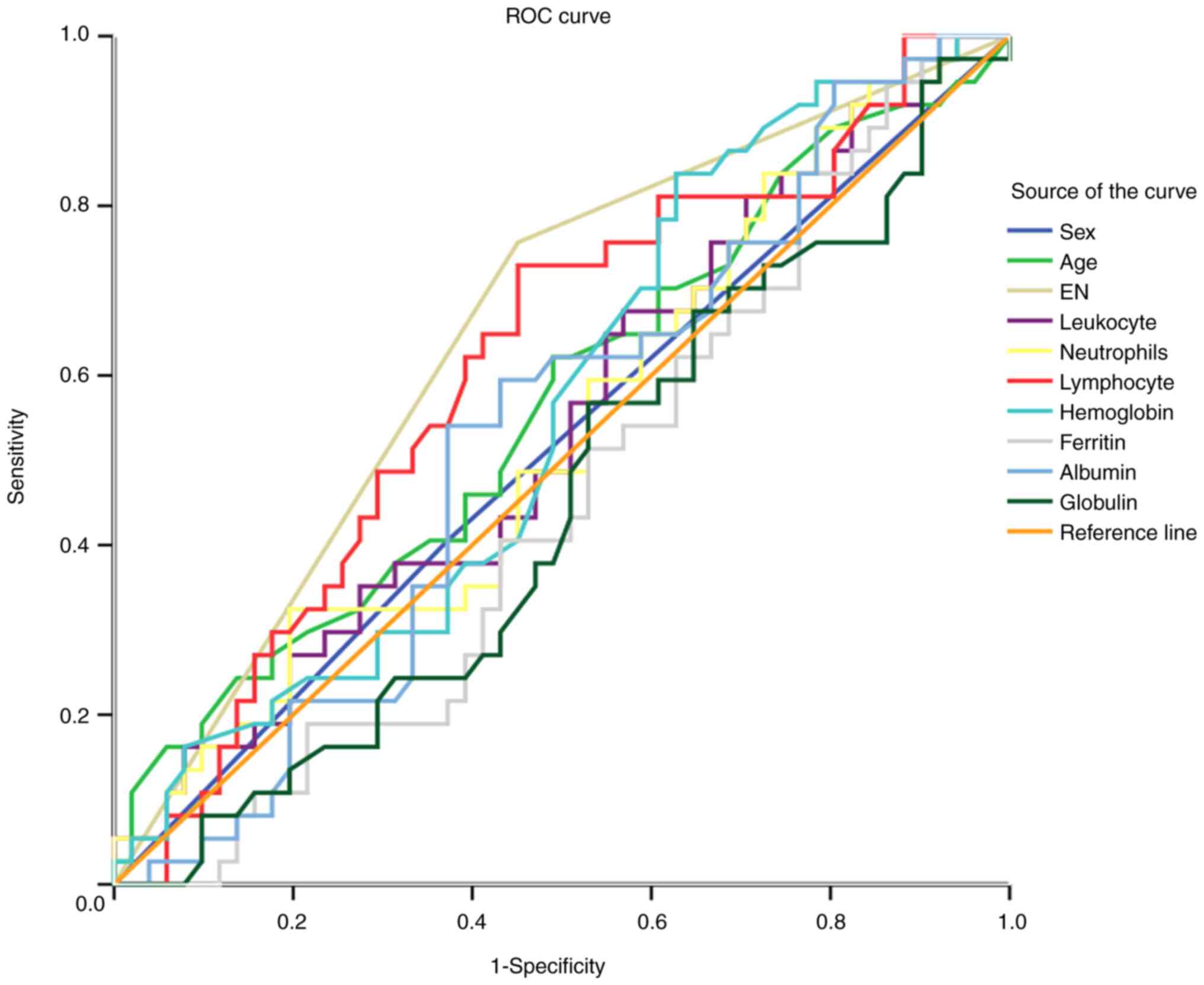
ROC curves and their associated AUCs for both the
EEN and LEN groups are shown in Fig.
4 and Table IV, respectively.
AUC values for most parameters, including age, sex, and serum
electrolytes at admission, were within the range of 0.447-0.573
(all P>0.05), which could not effectively predict RS incidence.
However, the EN initiation AUC was 0.667 (P=0.022), indicating a
reasonable predictive value for this variable.
 | Table IVROC curve of risk model for refeeding
syndrome with coronavirus disease 2019. |
Table IV
ROC curve of risk model for refeeding
syndrome with coronavirus disease 2019.
| | Asymptotic 95%
confidence interval |
|---|
| Variables | AUROC | Standard error | P-value | Lower bound | Upper bound |
|---|
| Age | 0.573 | 0.072 | 0.316 | 0.431 | 0.715 |
| Sex | 0.530 | 0.075 | 0.676 | 0.383 | 0.677 |
| EN | 0.667 | 0.072 | 0.022a | 0.526 | 0.808 |
| Admission | | | | | |
|
Potassium | 0.486 | 0.072 | 0.844 | 0.345 | 0.627 |
|
Sodium | 0.447 | 0.071 | 0.470 | 0.308 | 0.587 |
|
Phosphorus | 0.472 | 0.074 | 0.698 | 0.328 | 0.616 |
|
Magnesium | 0.470 | 0.072 | 0.685 | 0.330 | 0.611 |
Logistic regression analysis for RS
incidence
Potential independent risk factors for RS incidence
were analyzed using logistical regression. Univariate analysis
revealed that EN initiation time was associated with RS
(P<0.001). Using a P-value of <0.2 as a cut-off, age, EN
initiation, hypertension, and chronic respiratory disease were
included in the multivariate analysis. It was determined that RS
incidence was 6.530-fold higher in the LEN group compared with that
in the EEN group (OR, 6.530; confidence interval, 2.895-14.727;
P<0.001), suggesting EN initiation as an independent predictor
for RS (Table V).
 | Table VUnivariate and multivariate analysis
of risk factors related to refeeding syndrome. |
Table V
Univariate and multivariate analysis
of risk factors related to refeeding syndrome.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Sex (female vs.
male) | 1.435
(0.705-2.920) | 0.319 | | |
| Age | 1.019
(0.987-1.053) | 0.145 | 1.013
(0.975-1.053) | 0.497 |
| EN (LEN vs.
EEN) | 6.327
(2.863-13.982) |
<0.001a | 6.530
(2.895-14.727) |
<0.001a |
| Weight | 0.991
(0.964-1.020) | 0.556 | | |
| Body mass
index | 1.012
(0.899-1.139) | 0.843 | | |
| APACHE II
score | 1.013
(0.931-1.102) | 0.766 | | |
| Smoking | 0.263
(0.029-2.419) | 0.238 | | |
| Drinking | 0.871
(0.223-3.409) | 0.843 | | |
| Hypertension | 1.669
(0.824-3.377) | 0.115 | 1.781
(0.792-4.001) | 0.163 |
| Diabetes
mellitus | 1.500
(0.637-3.531) | 0.353 | | |
| Chronic
cardiovascular disease | 0.769
(0.287-2.063) | 0.602 | | |
| Chronic respiratory
disease | 2.333
(0.557-9.777) | 0.146 | 2.182
(0.454-10.494) | 0.330 |
| Mechanical
ventilation | 1.487
(0.735-3.009) | 0.269 | | |
| Calories
intake | 0.999
(0.995-1.003) | 0.550 | | |
| Protein intake | 0.989
(0.952-1.028) | 0.574 | | |
| Admission | | | | |
|
Potassium | 1.350
(0.720-2.529) | 0.350 | | |
|
Sodium | 0.969
(0.911-1.031) | 0.317 | | |
|
Phosphorus | 0.453
(0.062-3.318) | 0.436 | | |
|
Magnesium | 0.289
(0.010-8.266) | 0.468 | | |
Discussion
COVID-19 has brought immeasurable adverse health
effects to the world, including nutritional deficiency, which can
affect as many as 30% of infected patients, according to most
reports. Moreover, if such patients have protein deficiencies or
electrolyte imbalance, their mortality rate will further increase
(9). There are several ways in
which COVID-19 can adversely impact nutrition, digestion and
absorption. First, it has been shown that SARS-CoV-2 binds human
angiotensin-converting enzyme 2 (ACE-2) receptor on hepatocytes
derived from bile duct epithelial cells, thus causing liver tissue
injury via ACE-2 upregulation (6).
Second, ACE-2 receptors are also expressed on ileum and colon
enterocytes, and proliferation of these cells caused by
viral-mediated ACE-2 activation can disrupt the intestinal flora
(5). Finally, inflammatory
responses to the virus can induce dysbiosis and GI disturbance,
especially diarrhea (4). Several
studies have identified the association between nutritional risk
and mortality in critically ill patients with COVID-19. Zhao et
al (28) reported that
critical COVID-19 patients had significantly higher NRS-2002
scores, leading to longer hospital stays and a higher risk of
mortality. Furthermore, a report by Li et al (16) confirmed the prevalence of
malnutrition in elderly patients with COVID-19 in Wuhan, China. The
nutritional status of the participants was determined by a clinical
nutritionist, and the mean NRS-2002 score was 3, indicating a high
risk of malnutrition and the need for nutritional support (17).
The therapeutic benefits of nutritional support to
reduce the severity of COVID-19 illness has been described by
several recent reviews (15,18-21).
Specifically, experts from ASPEN extrapolated the results of
previous studies and recommended initiating nutritional support
within 24-36 h of admission for critically ill patients with
COVID-19(15). The expert
consensus from the European Society for Clinical Nutrition and
Metabolism suggests that nutritional support could be initiated
within 1-2 days for patients with COVID-19 undergoing mechanical
ventilation therapy (18). Recent
reports have further demonstrated the benefits of early nutritional
support in ICU patients with COVID-19. For instance, Haines et
al (19) found that early
nutrition in mechanically ventilated patients was associated with
faster weaning from mechanical ventilation and decreased length of
stay in the ICU and hospital overall. A meta-analysis by Ojo et
al (20) further concluded
that early nutritional intervention significantly reduced mortality
risk among critically ill patients with COVID-19(20). Additionally, Yuan et al
(21) reported that EEN was the
preferred method of support for elderly patients with common-type
COVID-19(21). Some studies also
suggest that non-critically ill patients would benefit from early
nutritional supplementation, particularly increased protein and
calorie intake through oral supplements (29-31).
Carbohydrates, lipids, amino acid supplements, vitamins and
minerals have been shown to reduce the risk of COVID-19 infection
and death (30). For example,
vitamin C supplementation may reduce the susceptibility to lower
respiratory tract infections, while vitamin D could induce
cathelicidins and defensins to inhibit the viral replication rate
(31).
Although EN and PN are the most commonly employed
routes of administration for nutritional support, most clinicians
prefer EN (15). Theoretical
benefits of EN include the preservation of integral mucosal
architecture, gut-associated lymphoid tissue and hepatic immune
function. EN may also reduce inflammatory responses during
nutritional input and prevent antigen leak from the gut, as
previously shown (32). A
practical nutritional guideline for patients with COVID-19,
published by Thibault et al (12), states that EN delivered within 48 h
of ICU admission should be the first-line intervention, while PN
should be prescribed if EN is contraindicated or insufficient
(12). Adequate early EN support
has contributed to improved clinical outcomes in mechanically
ventilated patients with COVID-19(33). For these reasons, EN support was
applied for the most severe COVID-19 cases in Tongji Hospital
during the study period. Patients in the EEN group received EN
support within 2 days of admission and gradually transitioned to
oral nutrition after that. For patients with digestive symptoms or
those at high risk of aspiration, PN support was initiated within
24-48 h and gradually transitioned to EN. In the present cohort, PN
was applied to all patients in the LEN group within 2 days of
admission. Although previous observations have shown that
nutritional supplements administered at an early stage are
important for enhancing host resistance to virus infection, there
is still no consensus on the best time to initiate EN (34). For patients with severe COVID-19
and high nutritional risk, it is difficult to avoid RS after EN
support, even with adequate daily electrolyte supplementation
(35). However, whether there is
an association between EN initiation time and the incidence of RS
is still unclear.
RS encompasses a range of metabolic and electrolyte
imbalances that occur due to the reintroduction or increased
provision of calories after a period of caloric restriction
(22). All patients included in
the study had a reduction of caloric intake with high nutritional
risk upon admission, therefore were at higher risk of RS. To
prevent RS in these patients, the strategy was to provide essential
electrolyte supplementation if they were below a daily threshold.
Furthermore, some measures were also taken to ensure that proper
nutrition and electrolytes were within a normal range during the PN
period, in order to ensure a smooth transition to EN support. Since
RS often occurs within 5 days of reintroduction of calories, serum
electrolytes were monitored 3 days after initiating EN to identify
RS incidence and degree of severity. A time cut-off of 3 days was
chosen since it was the median for peak incidence of the RS. The
relative electrolytes were significantly changed from the start of
monitoring to the end. Hypophosphatemia is often considered the
hallmark of this syndrome owing to the rapid egress of phosphorus
ions from the intravascular to intracellular space after EN
initiation (36). Intracellular
phosphate plays a key role in adenosine triphosphate (ATP)
production and transfer within cells, and increased consumption of
phosphate during refeeding can indirectly lead to reduced ATP and
2,3-diphosphoglycerate generation due to enhanced production of
phosphorylated intermediates, thereby causing impairments in
cardiac and respiratory functions (37). Hypokalemia and hypomagnesemia are
also of equal importance. Decreased serum potassium causes an
imbalance in the electrochemical membrane potential and disturbs
cardiac electromechanical function and nerve impulses, resulting in
abnormal cardiac rhythm and neurotransmitter release (38). Hypokalemia also causes paralysis in
the neuromuscular junction (39).
Serum magnesium is essential for activating the sodium/potassium
ATP-pump (Na+/K+-ATPase), and magnesium
deficiency could aggravate potassium loss in cardiomyocytes,
reflected by lengthening of the PR and QT intervals in an
electrocardiogram (ECG) and widening of the QRS complex, all of
which leads to an increased risk of tachyarrhythmias (40).
In the present study, serum electrolyte and blood
cell values upon hospital admission were similar between the two
groups and were within the normal ranges. Although measures were
taken to maintain electrolyte levels within normal limits before
transitioning to EN, the potential risk of RS could not be entirely
avoided. There were no differences in daily calories or protein
intake, and nutritional content and composition were similar
between groups. BMI, as a commonly used anthropometric index
applied in clinical and research settings, has well-recognized
limitations when used as a standalone marker of nutritional status.
BMI alone does not accurately reflect muscle mass and may obscure
underlying sarcopenia or malnutrition, especially in older adults
or critically ill patients (10,16).
FFMI was therefore included as another nutritional assessment
indicator for the present study. Certain confounding factors, such
as mechanical ventilation and RRT, which can potentially affect
electrolyte balances, were also excluded, since these interventions
were equally administered in both groups. At 3 days post-EN
initiation, the serum electrolyte levels were significantly lower
in the LEN group compared with those in the EEN group. Moreover,
each of these electrolytes was below clinically acceptable levels
in the LEN group, suggesting that delayed EN initiation may have
adverse effects on homeostasis. A significantly higher incidence
and severity of RS was also observed in the LEN group. It was
reasoned that 2 days was the appropriate time interval to
distinguish early from late EN initiation, as studies show that
intestinal dysbacteriosis occurs within 48 h of discontinuing
normal gut nutritional support. Later initiation of EN further
impairs integral mucosal architecture and increases the risk of
bacterial translocation (41).
Delayed EN support is a known risk factor for RS. Notably, the ROC
analysis in the present study found that the AUC for EN was 0.667,
which is favorable for RS prediction. Additionally, logistical
regression demonstrated that the risk of RS was 4-fold higher in
the LEN group compared with that in the EEN group.
Two important physiological processes occur after
initiating EN. Increased insulin secretion drives glucose into
cells, which results in increased uptake of serum phosphorus,
potassium, magnesium, calcium and thiamine. This causes
Na+/K+-ATPase to increase active exchange of
these ions across the plasma membrane (Na+ pumped out
and K+ pumped into the cell). In turn, cellular uptake
of serum phosphorus and magnesium is further increased (42). In light of the aforementioned two
effects, early nutritional support is beneficial for regulating the
body's ability to tolerate changes in the internal environment
following initiation, thereby reducing the likelihood of RS.
The association between EN initiation and mortality
in other respiratory diseases has been widely investigated. Zhong
et al (41) (2017) found
that early EN support could improve nutritional status, decrease
blood glucose fluctuations and further improve the 28-day mortality
rate in patients with acute respiratory distress syndrome (ARDS), a
common complication of severe COVID-19. Subsequently, Yan et
al (43) (2018) showed a
similar result wherein early EN support reduced the incidence of
infection, improved lung function, and reduced the duration of
mechanical ventilation and length of ICU stay in patients with
ARDS. By contrast, Peterson et al (44) (2017) suggested that early
administration of high-calorie support was associated with
increased mortality in patients with ARDS, while initiating support
after day 8 decreased the risk. An important finding in the present
study was that EEN support was significantly associated with
decreased length of stay in the hospital and in the ICU, without
affecting the 3-month overall survival rate. During the course of
treatments, all patients with severe COVID-19 receive similar
therapies, including respiratory and circulatory monitoring,
electrolyte supplementation and use of glucocorticoids. After
excluding these treatment confounding factors, EEN support showed
no effect on long-term mortality. The patients in the LEN group had
worse GI symptoms, characterized by severe nausea, vomiting and
diarrhea. Given that it takes some time to transition from PN to EN
to ensure EN efficacy and safety, it is not unexpected to see that
the patients in the LEN group took a longer time for proper GI
function, or that they required longer stays in the ICU and in the
hospital.
Finally, to evaluate the safety of early EN
initiation in the present study, the incidence rates of airway
complications and GI intolerance were evaluated. According to an
earlier report, nearly 20% of critically ill patients who received
EN during non-invasive positive pressure ventilation developed at
least one adverse event, including pneumonia and progressive
respiratory failure (43). Efforts
to provide early EN can be further impaired by GI intolerance and
bleeding (44). In the present
study, the incidence of airway complications and GI intolerance
were similar between the two groups, in part owing to the excellent
quality of patient care and regular monitoring provided by the
hospital staff. Collectively, these findings indicate that early EN
can be safely initiated for patients with severe COVID-19 and high
nutritional risk.
The present study has several limitations. First,
only the NRS-2002 clinical scoring tool was used, which, although
validated for identifying malnutrition, does not provide a formal
diagnosis or severity grading. A more recent and globally endorsed
tool named the GLIM criteria offers a standardized diagnostic
framework based on etiological and phenotypic criterion (45), but this was not applied and
measured in the present study, since we could not obtain
comprehensive data regarding the participants' weight losses over
the previous 6-month period. Its inclusion in future studies could
improve diagnostic consistency and enable severity classification.
Second, the retrospective single-center design may introduce
selection bias and limit the generalizability of the present
findings. The observed association between EN interval timing and
RS incidence reflects association, not causation. Moreover, the
study failed to reach a sufficient calculated sample size owing to
limitations in patient volume and implementing inclusion/exclusion
criteria, and as a result, adjustment with Bonferroni's correction
was only applied to a significantly certain criterion
(electrolytes), since it was the most important variable for RS
incidence. Furthermore, due to limited sample sizes within certain
subgroups, stratified analyses were not performed in the current
study, and this may bring about poor stability for the results. To
better address the issue of insufficient sample size, a large,
prospective study is needed to confirm these findings, provided
that patient safety is ensured. Third, the present study population
was limited to patients with severe COVID-19 with NRS scores ≥ 3,
excluding those with milder symptoms or lower nutritional risk. As
a result, the potential benefit of early EN in less severe cases
remains unclear. Fourth, there was no way to determine how long
participants had been undernourished prior to admission, despite
all presenting with high nutritional risk. Additionally, to meet
baseline caloric needs, patients in the LEN group received PN
before EN. This introduced a key limitation, as PN is a known risk
factor for RS owing to its high carbohydrate content and potential
for electrolyte shifts. Therefore, the association between delayed
EN and increased RS incidence may be confounded by prior PN
exposure rather than EN timing alone. The present study design did
not permit full adjustment for this variable, and future studies
should control for PN use either through stratification or
multivariable analysis or compare early PN and EN cohorts
separately to reduce this bias. The lack of propensity score
matching or covariate adjustment must all be acknowledged, as this
may have introduced bias. Causal inference would be strengthened
with larger studies that incorporate these methods. It is also
plausible that routine use of thiamine, multivitamins and
electrolyte management among each patient in their daily lives may
have reduced RS risk, although the extent of this effect could not
be fully assessed in the present retrospective analysis. Lastly,
only 3-month survival rate was recorded in this cohort, and it may
not have been sufficient to adequately differentiate mortality risk
between the EEN and LEN groups. Longer-term outcomes should be
evaluated in future studies.
In conclusion, EN initiation within 2 days after
hospital admission was associated with reduced RS incidence and
decreased length of stay in the hospital and in the ICU, although
this therapy had no effect on overall survival rate at 3 months
post-infection. These findings suggest that early initiation of EN
support is recommended to reduce RS incidence for severely ill
patients with COVID-19 and high nutritional risk if they do not
have EN contraindications.
Supplementary Material
Laboratory examinations 3 days after
EN initiation after adjustment with Bonferroni’s correction. EN,
enteral nutrition; EEN, early EN; LEN, late EN.
*P<0.05.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the COVID-19 Rapid Response
Research Project of Huazhong University of Science and Technology
(grant no. 2020kfyXGYJ049).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LX contributed to the conception and design of the
research. XR contributed to the acquisition and analysis of the
data. LX and XR contributed to the interpretation of the data. SL
contributed to the statistical analysis. XR and SL confirm the
authenticity of all the raw data. SL agreed to be accountable for
all aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. LX and SL drafted the manuscript and
revised it critically for important intellectual content. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was a retrospective study, so the
requirement for informed consent was waived by Tongji Hospital
Ethics Committee (Wuhan, China). The requirement for ethical
approval was also waived by the ethics committee, and clinical
research related to the severe corona virus disease 2019 was
strongly encouraged by Tongji Hospital Affiliated to Tongji Medical
College of Huazhong University of Science and Technology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang J, Yu M, Tong S, Liu LY and Tang LV:
Predictive factors for disease progression in hospitalized patients
with coronavirus disease 2019 in Wuhan, China. J Clin Virol.
127(104392)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cambon L, Bergeron H, Castel P, Ridde V
and Alla F: When the worldwide response to the COVID-19 pandemic is
done without health promotion. Glob Health Promot. 28:3–6.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
McDermott KT, Perry M, Linden W, Croft R,
Wolff R and Kleijnen J: The quality of COVID-19 systematic reviews
during the coronavirus 2019 pandemic: An exploratory comparison.
Syst Rev. 13(126)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aguila EJT, Cua IHY, Fontanilla JAC, Yabut
VLM and Causing MFP: Gastrointestinal manifestations of COVID-19:
Impact on nutrition practices. Nutr Clin Pract. 35:800–805.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Aguila EJT, Cua IHY, Dumagpi JEL,
Francisco CPD, Raymundo NTV, Sy-Janairo MLL, Cabral-Prodigalidad
PAI and Lontok MAD: COVID-19 and its effects on the digestive
system and endoscopy practice. JGH Open. 4:324–331. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J,
Li P, Hu B, Wang J, Hu C, et al: Clinical characteristics of
COVID-19 patients with digestive symptoms in Hubei, China: A
descriptive, cross-sectional, multicenter study. Am J
Gastroenterol. 115(766)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Haq EU, Yu J and Guo J: Frontiers in the
COVID-19 vaccines development. Exp Hematol Oncol.
9(24)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kumar V, Srinivasan V and Kumara S: Smart
vaccine manufacturing using vovel biotechnology platforms: A study
during COVID-19. J Comput Inf Sci Eng. 22(040903)2022.
|
|
9
|
Hakeem R and Sheikh MA: Beyond
transmission: Dire need for integration of nutrition interventions
in COVID-19 pandemic-response strategies in Developing Countries
like Pakistan. Pak J Med Sci. 36(COVID19-S4):S85–S89.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Martindale R, Patel JJ, Taylor B, Arabi
YM, Warren M and Mcclave SA: Nutrition therapy in critically Ill
patients with coronavirus disease 2019. JPEN J Parenter Enteral
Nutr. 44:1174–1184. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chapple LS, Fetterplace K, Asrani V,
Burrell A, Cheng AC, Collins P, Doola R, Ferrie S, Marshall AP and
Ridley EJ: Nutrition management for critically and acutely unwell
hospitalised patients with coronavirus disease 2019 (COVID-19) in
Australia and New Zealand. Aust Crit Care. 33:399–406.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thibault R, Seguin P, Tamion F, Pichard C
and Singer P: Nutrition of the COVID-19 patient in the intensive
care unit (ICU): A practical guidance. Crit Care.
24(447)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Stachowska E, Folwarski M, Jamioł-Milc D,
Maciejewska D and Skonieczna-Żydecka K: Nutritional support in
coronavirus 2019 disease. Medicina (Kaunas). 56(289)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang L and Liu Y: Potential interventions
for novel coronavirus in China: A systematic review. J Med Virol.
92:479–490. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wells Mulherin D, Walker R, Holcombe B and
Guenter P: ASPEN report on nutrition support practice processes
with COVID-19: The first response. Nutr Clin Pract. 35:783–791.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li T, Zhang Y, Gong C, Wang J, Liu B, Shi
L and Duan J: Prevalence of malnutrition and analysis of related
factors in elderly patients with COVID-19 in Wuhan, China. Eur J
Clin Nutr. 74:871–875. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cederholm T, Jensen GL, Correia MITD,
Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R,
Blaauw R, Coats AJS, et al: GLIM criteria for the diagnosis of
malnutrition-A consensus report from the global clinical nutrition
community. J Cachexia Sarcopenia Muscle. 10:207–217.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Barazzoni R, Bischoff SC, Breda J,
Wickramasinghe K, Krznaric Z, Nitzan D, Pirlich M and Singer P:
endorsed by the ESPEN Council. ESPEN expert statements and
practical guidance for nutritional management of individuals with
SARS-CoV-2 infection. Clin Nutr. 39:1631–1638. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Haines K, Parker V, Ohnuma T,
Krishnamoorthy V, Raghunathan K, Sulo S, Kerr KW, Besecker BY,
Cassady BA and Wischmeyer PE: Role of early enteral nutrition in
mechanically ventilated COVID-19 patients. Crit Care Explor.
4(e0683)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ojo O, Ojo OO, Feng Q, Boateng J, Wang X,
Brooke J and Adegboye ARA: The effects of enteral nutrition in
critically Ill patients with COVID-19: A systematic review and
meta-analysis. Nutrients. 14(1120)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yuan J, Guan Y, Zhao Z, Shen J, Tan D,
Zhao F, Ge L, Xie R and Li T: Enteral nutrition therapy for elderly
patients with common-type COVID-19, a retrospective study based on
medical records. Int Health. 17:678–684. 2025.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Da Silva JSV, Seres DS, Sabino K, Adams
SC, Berdahl GJ, Citty SW, Cober MP, Evans DC, Greaves JR, Gura KM,
et al: ASPEN consensus recommendations for refeeding syndrome. Nutr
Clin Pract. 35:178–195. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Meira APC, Santos COD, Lucho CLC,
Kasmirscki C and Silva FM: Refeeding syndrome in patients receiving
parenteral nutrition is not associated to mortality or length of
hospital stay: A retrospective observational study. Nutr Clin
Pract. 36:673–678. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu Z, Cai T, Fan L, Lou K, Hua X, Huang Z
and Gao G: Clinical value of immune-inflammatory parameters to
assess the severity of coronavirus disease 2019. Int J Infect Dis.
95:332–339. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, Li
B, Song X and Zhou X: Prognostic value of interleukin-6, C-reactive
protein, and procalcitonin in patients with COVID-19. J Clin Virol.
127(104370)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pourhassan M, Cuvelier I, Gehrke I,
Marburger C, Modreker MK, Volkert D, Willschrei HP and Wirth R:
Risk factors of refeeding syndrome in malnourished older
hospitalized patients. Clin Nutr. 37:1354–1359. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kogo M, Nagata K, Morimoto T, Ito J, Sato
Y, Teraoka S, Fujimoto D, Nakagawa A, Otsuka K and Tomii K: Enteral
nutrition is a risk factor for airway complications in subjects
undergoing noninvasive ventilation for acute respiratory failure.
Resp Care. 62:459–467. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao X, Li Y, Ge Y, Shi Y, Lv P, Zhang J,
Fu G, Zhou Y, Jiang K, Lin N, et al: Evaluation of nutrition risk
and its association with mortality risk in severely and critically
Ill COVID-19 patients. JPEN J Parenter Enteral Nutr. 45:32–42.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Caccialanza R, Laviano A, Lobascio F,
Montagna E, Bruno R, Ludovisi S, Corsico AG, Di Sabatino A,
Belliato M, Calvi M, et al: Early nutritional supplementation in
non-critically ill patients hospitalized for the 2019 novel
coronavirus disease (COVID-19): Rationale and feasibility of a
shared pragmatic protocol. Nutrition. 74(110835)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Grant WB, Lahore H, Mcdonnell SL, Baggerly
CA, French CB, Aliano JL and Bhattoa HP: Evidence that vitamin D
supplementation could reduce risk of influenza and COVID-19
infections and deaths. Nutrients. 12(988)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rozga M, Cheng FW, Moloney L and Handu D:
Effects of micronutrients or conditional amino acids on
COVID-19-related outcomes: An evidence analysis center scoping
review. J Acad Nutr Diet. 121:1354–1363. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ohbe H, Jo T, Matsui H, Fushimi K and
Yasunaga H: Early enteral nutrition in patients undergoing
sustained neuromuscular blockade: A propensity-matched analysis
using a nationwide inpatient database. Crit Care Med. 47:1072–1080.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Melchers M, Hubertine Hermans AJ, Hulsen
SB, Kehinde Kouw IW and Hubert van Zanten AR: Individualised energy
and protein targets achieved during intensive care admission are
associated with lower mortality in mechanically ventilated COVID-19
patients: The COFEED-19 study. Clin Nutr. 42:2496–2492.
2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Seres DS, Valcarcel M and Guillaume A:
Advantages of enteral nutrition over parenteral nutrition. Therap
Adv Gastroenterol. 6:157–167. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Alexander J, Tinkov A, Strand TA, Alehagen
U, Skalny A and Aaseth J: Early nutritional interventions with
zinc, selenium and vitamin D for raising anti-viral resistance
against progressive COVID-19. Nutrients. 12(2358)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ribeiro AC, Dock-Nascimento DB, Silva JM
Jr, Caporossi C and Aguilar-Nascimento JE: . Hypophosphatemia and
risk of refeeding syndrome in critically ill patients before and
after nutritional therapy. Rev Assoc Med Bras (1992). 66:1241–1246.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ponzo V, Pellegrini M, Cioffi I, Scaglione
L and Bo S: The refeeding syndrome: A neglected but potentially
serious condition for inpatients. A narrative review. Intern Emerg
Med. 16:49–60. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Matthews-Rensch K, Capra S and Palmer M:
Systematic review of energy initiation rates and refeeding syndrome
outcomes. Nutr Clin Pract. 36:153–168. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
De Silva A and Nightingale JMD: Refeeding
syndrome: Physiological background and practical management.
Frontline Gastroenterol. 11:404–409. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yoshida M, Izawa J, Wakatake H, Saito H,
Kawabata C, Matsushima S, Suzuki A, Nagatomi A, Yoshida T, Masui Y
and Fujitani S: Mortality associated with new risk classification
of developing refeeding syndrome in critically ill patients A
cohort study. Clin Nutr. 40:1207–1213. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhong C, Ji C, Dai Z, Fu K, Wen X and Pan
H: Effect of early enteral nutrition standardized treatment on
blood glucose and prognosis in acute respiratory distress syndrome
patients with mechanical ventilation. Zhonghua Wei Zhong Bing Ji
Jiu Yi Xue. 29:1133–1137. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
42
|
Ghaddar R, Chartrand J, Benomar A,
Jamoulle O, Taddeo D, Frappier JY and Stheneur C: Excessive
laboratory monitoring to prevent adolescent's refeeding syndrome:
Opportunities for enhancement. Eat Weight Disord. 25:1021–1027.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Guo Y, Cheng J and Li Y: Influence of
enteral nutrition initiation timing on curative effect and
prognosis of acute respiratory distress syndrome patients with
mechanical ventilation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue.
30:573–577. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Peterson SJ, Lateef OB, Freels S, Mckeever
L, Fantuzzi G and Braunschweig CA: Early exposure to recommended
calorie delivery in the intensive care unit is associated with
increased mortality in patients with acute respiratory distress
syndrome. JPEN J Parenter Enteral Nutr. 42:739–747. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yildirim M, Halacli B, Yuce D, Gunegul Y,
Ersoy EO and Topeli A: Assessment of admission COVID-19 associated
hyperinflammation syndrome score in critically-III COVID-19
patients. J Intensive Care Med. 38:70–77. 2023.PubMed/NCBI View Article : Google Scholar
|