Introduction
Sickle cell disease (SCD) is an autosomal recessive
hematologic disorder caused by a single nucleotide mutation,
leading to the production of abnormal hemoglobin S (HbS). First
described by Dr Herrick in 1910, this mutation replaces glutamic
acid with valine in the β-globin chain, altering red blood cell
shape and function. Under hypoxic or acidic conditions, these cells
sickle, triggering vaso-occlusive crises, hemolysis and multi-organ
dysfunction (1).
SCD comprises several genotypes, with sickle cell
anemia (HbSS) being the most prevalent (70%), followed by
hemoglobin SC disease (HbSC) and sickle β-thalassemia
(HbS/β-thalassemia). The severity of symptoms varies, with HbSS
being the most severe. HbSC results from inheriting an HbS allele
from one parent and hemoglobin C from the other, typically causing
a milder course. In HbS/β-thalassemia, severity depends on the
degree of β-globin production (1).
Globally, SCD remains a major health challenge,
particularly in malaria-endemic regions, where the sickle cell
trait provides a survival advantage against malaria. Each year,
>500,000 infants are born with SCD, with the highest prevalence
in Sub-Saharan Africa, India, the Mediterranean and the Middle East
(2,3).
The disease's pathophysiology is driven by HbS
polymerization, which stiffens red blood cells, reducing
flexibility and increasing their tendency to block small vessels.
This leads to ischemia, inflammation and endothelial dysfunction.
Chronic hemolysis releases free hemoglobin into the circulation,
depleting nitric oxide (NO), which worsens vasoconstriction and
vascular injury. Adhesion molecules such as selectins further
contribute to vaso-occlusion and disease progression (4).
SCD manifests through acute and chronic
complications. The hallmark is vaso-occlusive crises, characterized
by severe pain, anemia, fatigue, jaundice and increased
susceptibility to infections, particularly from encapsulated
bacteria like Streptococcus pneumoniae and Haemophilus
influenzae. Long-term complications include pulmonary
hypertension, left-sided heart disease, stroke, renal impairment,
avascular necrosis and growth delays (1). Among the most severe complications is
acute chest syndrome (ACS), an acute lung injury presenting with
respiratory distress, fever and hypoxia. It is a leading cause of
morbidity and mortality in SCD and can be triggered by infections,
pulmonary vasoconstriction and fat embolism. Management includes
oxygen therapy, hydration, analgesics, broad-spectrum antibiotics
and, in severe cases, blood transfusions (1,5).
Diagnosing asthma in patients with SCD presents a
unique clinical challenge due to overlapping respiratory
manifestations. Symptoms such as wheezing, cough and
dyspnea-typical of asthma-are also common during vaso-occlusive
crises and episodes of ACS, both of which are prevalent in SCD.
These overlapping features can lead to misclassification or delayed
diagnosis. Furthermore, pulmonary function testing may reveal
restrictive or obstructive patterns in SCD that do not clearly
align with classic asthma profiles, further complicating
differentiation (6). Additionally,
chronic airway inflammation due to SCD itself may mimic or mask
asthma, and standard biomarkers such as elevated IgE or
eosinophilia may be variably expressed (6), necessitating a multifaceted
diagnostic approach incorporating spirometry, detailed history and
longitudinal symptom monitoring (6).
Asthma is a common comorbidity in SCD, further
complicating disease management. It exacerbates airway
inflammation, increasing the risk of hypoxia, vaso-occlusive events
and ACS. Patients with SCD and asthma or recurrent wheezing have
higher rates of painful crises, stroke and mortality (7). The mechanisms linking asthma and ACS
include chronic airway inflammation, heightened vulnerability to
respiratory infections and exacerbation of pulmonary complications
(6).
Cohort studies and biomarker research have
emphasized the immunological and pulmonary complexity in patients
with both SCD and asthma. It has been demonstrated that increased
eosinophilic airway inflammation, as evidenced by elevated
fractional exhaled NO (FeNO) and sputum eosinophils, is
significantly associated with higher rates of ACS in children with
SCD (8). Furthermore, convergence
points within key inflammatory signaling pathways have been
identified. For instance, the activation of signal transducer and
activator of transcription 6 by interleukin-4 (IL-4) and IL-13, a
well-established mechanism in asthma, was highlighted.
Additionally, the nuclear factor κ-light-chain-enhancer of
activated B cells (NF-κB) pathway, which plays a central role in
regulating immune responses, was found to be active in both SCD and
asthma. These shared molecular pathways suggest potential
overlapping mechanisms of inflammation between the two conditions
(9).
Recognizing ACS as a significant complication of SCD
highlights the need for further research into its risk factors and
optimal management. The link between asthma and ACS is particularly
critical. Thus, to the best of our knowledge, in the present study,
the first meta-analysis was conducted to quantify ACS risk in
patients with SCD and asthma.
Materials and methods
General
This systematic review and meta-analysis was
conducted in accordance with the Preferred Reporting Items for
Systematic reviews and Meta-Analyses (PRISMA) guidelines (10) and the Meta-analysis Of
Observational Studies in Epidemiology guidelines (11). This systematic review has been
registered in the International Prospective Register of Systematic
Reviews (PROSPERO; https://www.crd.york.ac.uk/prospero/) with ID no.
CRD420251012367.
Search strategy
Articles were searched in the electronic databases
PubMed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane library
(https://www.cochrane.org/) and Scopus
(https://www.scopus.com/home.uri?zone=header&origin=sbrowse),
as well as gray literature, including conference proceedings, from
inception until the 4th of March, 2025. No restriction regarding
sample size, study setting or publication language was imposed.
MeSH terms were used for both intervention (asthma) and outcome
(ACS), along with free-text words. The Boolean operators ‘OR’ and
‘AND’ were also used. The detailed search strategy applied in
PubMed is presented in Table SI.
Equivalent search terms and Boolean logic were adapted and applied
appropriately to the Cochrane Library, Scopus and gray literature
sources to ensure consistency across databases.
Eligibility criteria
Studies enrolling patients with SCD and asthma of
any age with asthma, compared with patients with SCD without
asthma, assessing ACS episodes, were searched for. Case reports,
case series, previous meta-analyses (if any), editorials, opinion
papers and narrative reviews were excluded.
PRISMA screening process
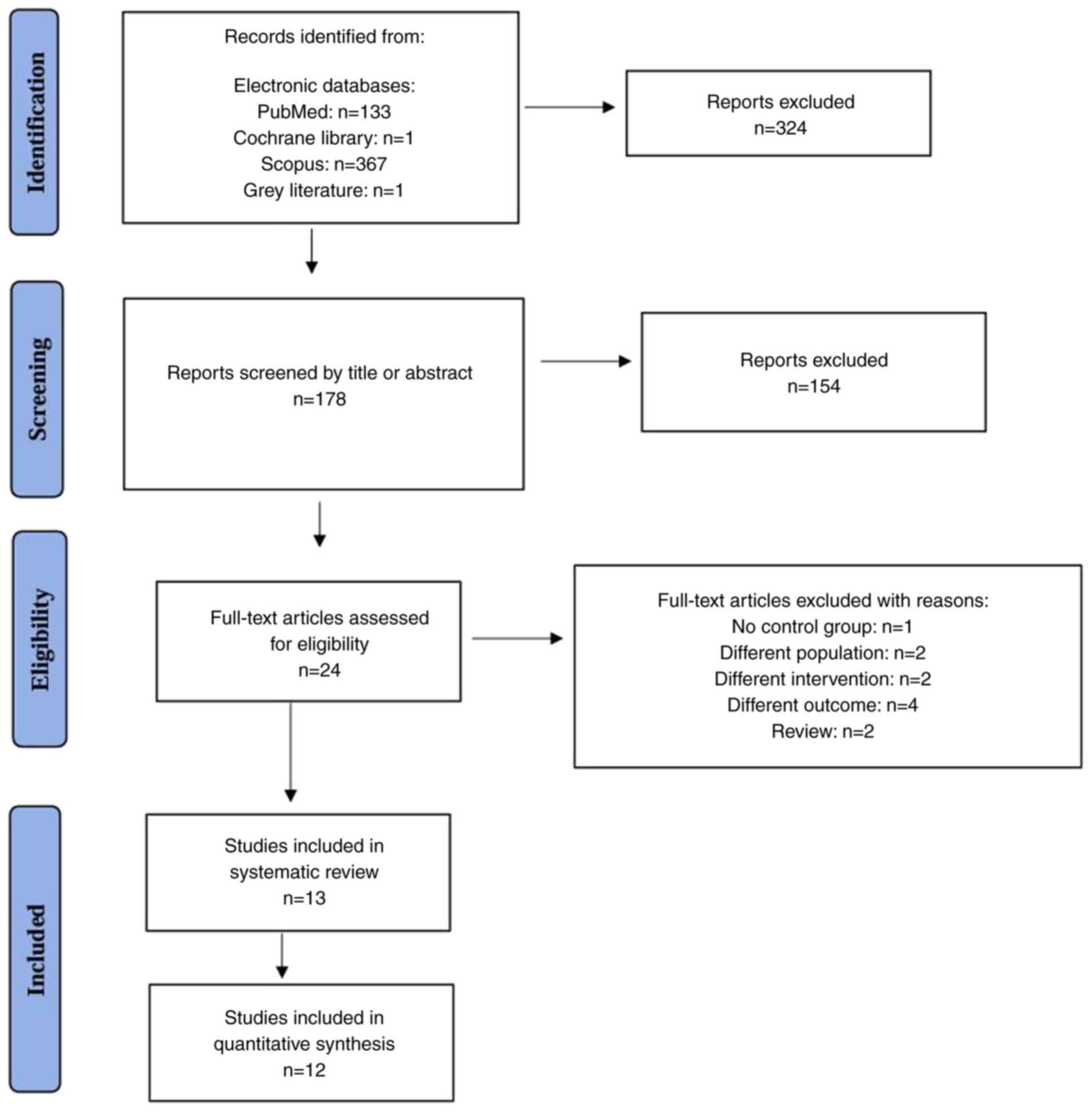
A total of 501 records were identified from
electronic databases: PubMed (n=133), Cochrane Library (n=1),
Scopus (n=367) and grey literature (n=1). After removing
duplicates, 178 records remained for screening.
During the screening process, 178 records were
reviewed based on titles and abstracts and 154 were excluded. This
left 24 full-text articles for eligibility assessment. Of these 24
full-text articles, 11 were excluded for the following reasons: 1
lacked a control group; 2 included a different intervention (i.e.,
they evaluated asthma treatment efficacy without specifically
assessing its association with ACS); 2 included populations without
confirmed SCD; 4 examined outcomes other than ACS (e.g., general
hospitalization rates or pulmonary function indices); and 2 were
narrative review articles rather than original research.
Ultimately, 13 studies were included in the systematic review and
12 were included in the quantitative synthesis (meta-analysis).
The flowchart of the study selection process is
illustrated in Fig. 1.
Quality assessment
The Newcastle-Ottawa Scale (NOS) (12) was used by two independent reviewers
(T-VK, KD) to assess the quality of the included observational
studies. The included studies were evaluated based on the following
broad perspectives: Study groups, group comparability and
ascertainment of either the exposure or outcomes of interest.
According to the NOS, studies are evaluated across three domains:
Selection (maximum 4 stars), comparability (maximum 2 stars) and
outcome/exposure (maximum 3 stars), for a total of up to 9 stars. A
higher number of stars indicates higher methodological quality and
lower risk of bias. Studies scoring 7-9 stars were considered high
quality, 4-6 stars moderate quality and <4 stars low quality.
The individual scores for each study are presented in Tables SII and SIII. Divergent views among reviewers
were settled through debate, consensus or arbitration by a third
senior reviewer (VEG).
Data extraction
A total of three independent reviewers (TVK, KD,
VEG) extracted the data from the eligible reports. Relevant
information was extracted and recorded in a data collection form
developed in Excel© (Microsoft Corp.). Extracted
information included the following: First author, year of study
conduction, country of origin, study sample size, key clinical
outcome (ACS), diagnostic method of asthma and ACS.
Data synthesis and analysis
The study aimed to assess the major clinical
endpoint (ACS) representing a dichotomous variable; thus, the risk
ratio (RR) with 95% confidence intervals (CI) was estimated. To
generate the pooled estimates of the outcome, the Mantel-Haenszel
random-effects formula was implemented. The I2 statistic
was used for the evaluation of the extent to which statistical
heterogeneity in a meta-analysis is due to differences among
studies. Low heterogeneity was present if I2 was between
0 and 25%, moderate if I2 was between 25 and 50%, or
high if I2 was >75%. Forest plots were created for a
visual representation of the presence and nature of statistical
heterogeneity (13). All analyses
were performed with the Meta-Mar software (version 3.5.1) (14) and P<0.05 was considered to
indicate statistical significance.
Results
Characteristics of the included
studies
A detailed description of characteristics of the
included studies (8,15-26)
is provided in Table I. In cases
where the number of episodes of ACS per patient per year was
available, appropriate calculations were made in order to determine
the number of events in the asthma group and in the non-asthma
group. The inclusion of the study by Strunk et al (22) in the meta-analysis was not
possible, as the number of ACS episodes per group was unknown.
However, it should be noted that the result of this study (patients
with SCD and asthma had a higher risk of ACS) was similar to that
of the present meta-analysis. Regarding the outcome ACS, data were
combined from 12 studies with a total of 2,269 enrolled subjects,
of which 497 (21.9%) had asthma. Overall, 725 episodes of ACS were
recorded. Among patients with SCD and asthma, 270 out of 497
(54.3%) experienced at least one episode of ACS. By contrast, the
proportion of ACS among patients with SCD without asthma was
significantly lower, at 25.7%.
 | Table ICharacteristics of the included
studies. |
Table I
Characteristics of the included
studies.
| First author,
year | Design | Country | SCD type | Age | A/NA subjects | Follow-up | ACS episodes | (Refs.) |
|---|
| De, 2023 | Retrospective | USA | HbSS 85.5%, HbSC
9.1%, HbSB 3.6%, HbS/HPFH 1.8%, | 3-18 y | 16/39 | 2 y | A: 13 NA: 27 | (8) |
| Boyd, 2004 | Retrospective
case-control | USA | HbSS HbSβ HbSC | 2-21 y | 31/108 | - | A: 22 NA: 41 | (15) |
| Knight-Madden,
2005 | Retrospective | Jamaica | HbSS | 5-10 y | 38/42 | - | A: 38 NA: 3 | (16) |
| Boyd, 2006 | Prospective | USA | HbSS | <6 m at
entry | 49/242 | 11.7 y | A: 19 NA: 48 | (17) |
| Sylvester,
2007 | Retrospective | UK | HbSS | <18 y | 12/153 | - | A: 6 NA: 27 | (18) |
| Bartram, 2008 | Retrospective | UK | HbSS HbSC | 1-15 y | 16/47 | - | A: 10 NA: 12 | (24) |
| Bernaudin,
2008 | Retrospective | France | HbSS | 0-18 y | 25/272 | 6-7 y | A: 18 NA: 118 | (25) |
| An, 2011 |
Cross-sectional | North America,
Europe | HbSS 94%, HbSβο
6% | 5-14 y | 140/381 | - | A: 27 NA: 46 | (19) |
| Glassberg,
2012 | Retrospective | USA | HbSS 68.4%, HbSβο
10.5%, HbSβ+ 7.9%, HbSC 13.2% | 6 m-67.5 y | 48/214 | - | A: 16 NA: 37 | (20) |
| Intzes, 2013 | Retrospective | USA | HbSS 79.6%, HbSβο
3.2%, HbSβ+ 14.1%, HbSC 30.2% HbSα 1.1% | 5-18 y | 58/61 | - | A: 45 NA: 9 | (21) |
| Strunk, 2014 | Prospective | USA, UK | HbSS HbSβ | 4-18 y | 53/134 | 4.61±1.16 y | IRR=2.21 (CI 95%
1.31-3.76) | (22) |
| Pahl, 2016 | Retrospective | USA | HbSS HbSβο HbSβ+
HbSC | 2-21 y | 29/73 | - | A: 23 NA: 17 | (26) |
| Bafunyembaka,
2024 | Retrospective
nested case control | French Guiana | HbSS HbSβ+
HbSC | 6 m-18 y | 35/140 | - | A: 33 NA: 70 | (23) |
Meta-analysis
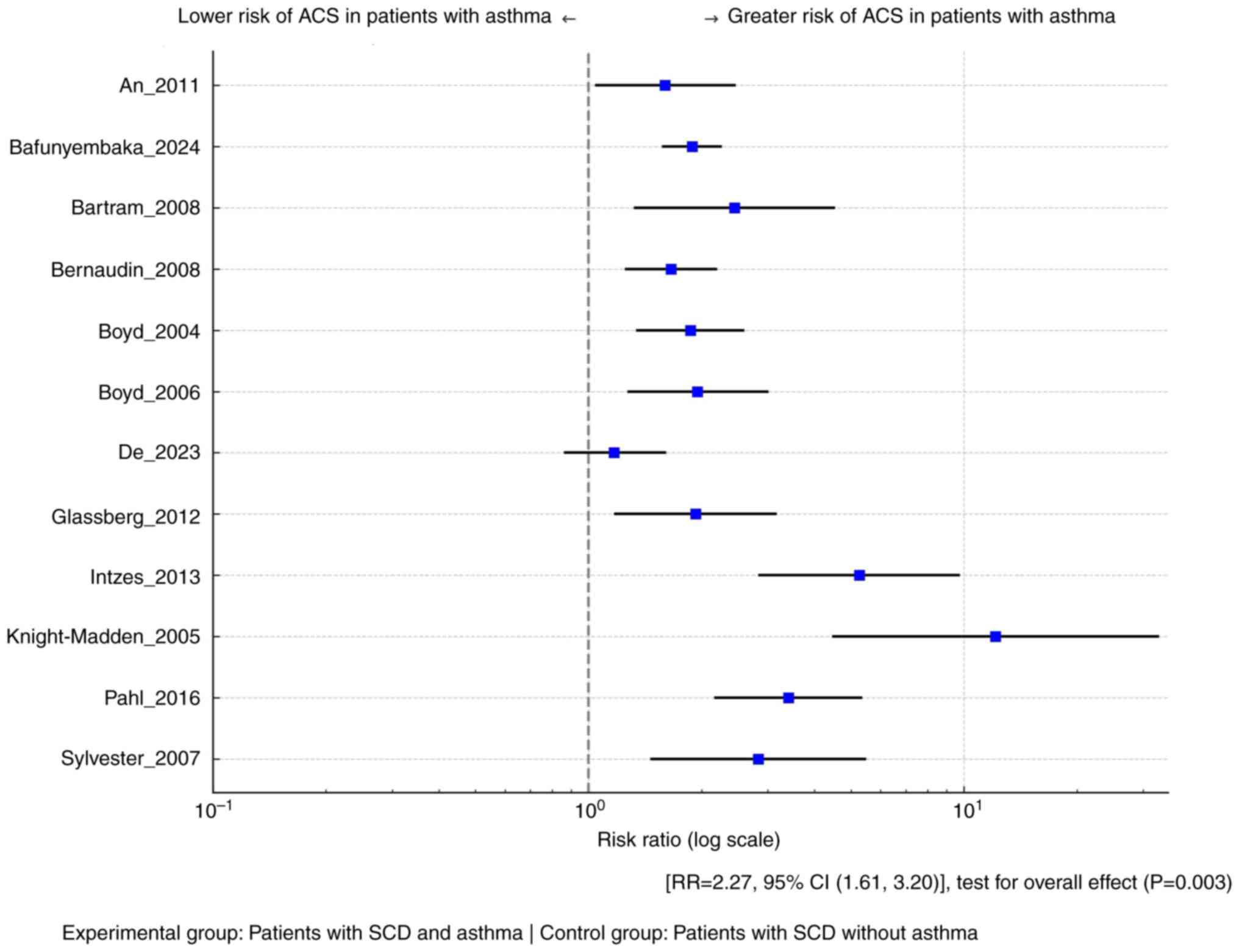
As presented in Fig.
2, it was demonstrated that asthma is associated with a
significantly higher risk for ACS in patients with SCD [RR=2.27,
95% CI (1.61, 3.20)], with the test for the overall effect
confirming statistical significance (P=0.0003). The heterogeneity
of the included studies was substantial (I2=74%). The
funnel plot is provided in Fig.
S1.
Subgroup and sensitivity analysis
Subgroup analysis revealed that studies with a
prospective design exhibited no heterogeneity (I²=0%), indicating
that the overall heterogeneity observed in the meta-analysis
originated primarily from retrospective studies. To further explore
this, a sensitivity analysis was performed by excluding the studies
by Knight-Madden et al (16) and Intzes et al (21), which contributed substantially to
heterogeneity in the retrospective group. This exclusion reduced
the I² of the retrospective subgroup from 85.5 to 68%, confirming
their disproportionate influence on variability. Following these
exclusions, 10 studies remained, comprising 401 participants in the
asthma (experimental) group and 1,669 in the non-asthma (control)
group. Using a Mantel-Haenszel random-effects model, the pooled RR
for ACS was 1.88 (95% CI: 1.59-2.24), demonstrating a significantly
increased risk in the asthma group (P<0.05). Moderate
heterogeneity was detected (I²=52%, P=0.03), indicating that just
over half of the observed variation among studies was due to real
differences rather than chance (Figs.
S2 and S3).
Discussion
To our knowledge, this is the first systematic
evaluation and meta-analysis of the association between asthma and
ACS episodes. A notable systematic review and qualitative analysis
of the existing evidence was presented in 2016 by DeBaun and Strunk
(6), who performed a literature
search in PubMed investigating the association between asthma and
ACS in children with SCD. The authors concluded that asthma is
common in children with SCD, leading to higher rates of severe
vaso-occlusive pain and ACS and indicating that there is strong
justification for routinely assessing asthma risk factors and
symptoms at each clinic visit and that spirometry should complement
respiratory histories to detect and monitor lower airway
obstruction and treatment responses. Three years later, Willen
et al (27) reported on the
relationship between asthma and ACS in their review article,
focusing on three important studies (17,19,22).
More specifically, the study by Willen et al (27) found that a physician's diagnosis of
asthma in children with SCD is significantly associated with
increased incidence of both vaso-occlusive pain episodes and ACS.
The analysis of three major cohorts including 1,685 children
demonstrated a 1.89-fold increased risk of ACS [incidence rate
ratio=1.89; 95% CI: 1.61-2.22; P<0.001] among those with asthma.
The authors emphasized that pulmonary symptoms-whether due to
asthma or asthma-like features-substantially contribute to SCD
morbidity and warrant routine respiratory screening and specialist
referral (27).
The present study demonstrates a statistically
significant association between asthma and an increased risk of ACS
in patients with SCD, with a pooled RR of 2.27 (95% CI: 1.31-3.20;
P=0.0003). By synthesizing all available observational data, this
meta-analysis provides the first quantitative estimate of ACS risk
in patients with asthma and SCD, contributing valuable evidence to
the existing literature.
This finding is particularly important given the
complex interplay between asthma and the pulmonary complications
associated with SCD. The underlying mechanisms for this association
warrant further exploration. Understanding the potential
pathogenetic relationship between these two conditions is crucial
for improving clinical outcomes and guiding management
strategies.
Asthma and SCD exhibit several similarities
regarding the immunological factors linked to their disease states.
Both conditions lead to inflammation and airway hyperreactivity,
affect vulnerability to respiratory infections and necessitate
targeted interventions to alleviate their associated complications
(7,28). The link between asthma and ACS in
patients with SCD can be possibly unraveled through several
interrelated mechanisms, including inflammation, hypoxemia,
oxidative stress and vascular complications (7).
Asthma is a chronic respiratory condition
characterized by persistent inflammation that activates a range of
immune cell types, including eosinophils, mast cells and T
lymphocytes. This inflammatory reaction produces cytokines and
chemokines, which enhance bronchial hyperreactivity and airway
obstruction, imitating or intensifying ACS symptoms, including
dyspnea and chest pain. SCD also has higher levels of endothelial
activation markers, such as intercellular adhesion molecule-1 and
vascular cell adhesion molecule-1. Shared inflammatory pathways
between SCD and asthma may result in an asthma-like phenotype in
patients with SCD, potentially increasing the likelihood of
developing asthma. Exacerbation of airway inflammation caused by
asthma may significantly increase the possibility of developing ACS
in patients with SCD who are already at risk of pulmonary
complications due to vaso-occlusive crises and impaired gas
exchange (8,29).
Endothelial activation and oxidative stress are
critical processes that connect sickled red blood cells with
vaso-occlusion. Sickle cells bind to endothelium integrins,
inducing damage via reactive oxygen species and sustaining
additional endothelial activation. This mechanism causes the influx
of monocytes and neutrophils, which contributes to increased cell
adhesion in patients with SCD. The combination of sickling and
inflammation can worsen vascular integrity, increasing the risk of
an ACS. SCD is characterized by elevated levels of pro-inflammatory
cytokines, such as IL-3 and granulocyte-macrophage
colony-stimulating factor (GM-CSF), as well as steady-state levels
of tumor necrosis factor α (TNF-α) and other cytokines. Increased
neutrophil counts contribute to acute lung injury by forming
neutrophil extracellular traps, which worsens pulmonary functioning
and infection susceptibility (9).
Asthma may exacerbate SCD-related vascular issues.
Inflammation and oxidative stress may cause endothelial
dysfunction, which is a precursor to atherosclerosis and acute
coronary events. In patients with SCD, the combination of sickling
and inflammation can worsen vascular integrity, increasing the risk
of ACS. Furthermore, asthma can cause hemodynamic changes such as
increased heart rate and blood pressure fluctuations, which can
contribute to cardiovascular stress. Asthma can increase acute
coronary events in patients with SCD, whose cardiovascular systems
are already stressed by chronic hemolysis and vaso-occlusive crises
(30).
Although the present discussion outlines
inflammation, hypoxemia, oxidative stress and vascular
complications as potential mechanisms linking asthma to ACS in
patients with SCD, a deeper exploration of molecular pathways can
provide clearer targets for intervention. Inflammatory mediators
play a central role in both asthma and SCD-related pulmonary
events. In asthma, allergen-induced activation of type 2 T-helper
(Th2) cells leads to the release of interleukins such as IL-4, IL-5
and IL-13, promoting eosinophilic inflammation, mucus production
and airway hyperresponsiveness (9,28).
In patients with SCD, a parallel increase in pro-inflammatory
cytokines such as IL-3, TNF-α and GM-CSF is observed (9,30),
which contributes to neutrophil activation and adhesion to the
endothelium, further exacerbating vascular occlusion. These
cytokines converge on NF-κB, a key transcription factor regulating
inflammatory gene expression. Activation of NF-κB in both airway
epithelial cells and endothelial cells promotes a pro-inflammatory
milieu conducive to ACS onset (9).
Furthermore, oxidative stress from repeated hemolysis in SCD
diminishes NO bioavailability, impairing vasodilation and
amplifying endothelial injury, while concurrently asthma-associated
hypoxemia further aggravates pulmonary vasoconstriction (30). Collectively, these overlapping
pathways-particularly those involving IL-5 and GM-CSF signaling in
eosinophils and neutrophils-highlight actionable targets such as
anti-IL-5 or NF-κB inhibitors.
It is important to consider how age influences the
risk of ACS among patients with both asthma and SCD. The majority
of included studies focused on pediatric populations, with most
participants being children or adolescents. Evidence suggests that
the risk of ACS is highest in early childhood and tends to decline
with age (6). This may be due to
developmental changes in lung structure, immune response maturation
and a reduced frequency of upper respiratory infections, which are
common triggers for both asthma exacerbations and ACS in younger
children. Boyd et al (17)
conducted a prospective study with a median follow-up of 11.7
years, while Bernaudin et al (25) reported follow-up durations of 6-7
years in a large pediatric cohort. These extended observation
periods enabled the identification of asthma-related complications
well into adolescence and early adulthood. The findings from these
cohorts support the hypothesis that younger children with SCD and
coexisting asthma are particularly susceptible to ACS. This
increased vulnerability likely stems from age-related physiological
factors, including heightened airway hyperresponsiveness,
underdeveloped immune regulation and narrower airway calibers,
which interact with the pro-inflammatory and vaso-occlusive state
characteristic of SCD.
Incorporating recent molecular evidence also
broadens the implications of the present findings. The work by
Habib et al (28) and De
et al (8) supports a
precision medicine approach, where monitoring Th2 cytokines and
airway eosinophilic activity may guide tailored interventions in
patients with SCD with asthma. This is crucial in light of the
emerging evidence that biologics such as anti-IL-5 or anti-IL-13
agents (e.g., mepolizumab, dupilumab) not only reduce asthma
exacerbations but may also minimize inflammatory triggers of ACS
(28). Similarly, Pahl and Mullen
(26) reported differential ACS
risk based on genotype (HbSS vs. HbSC), suggesting that genetic
stratification should be combined with the asthma phenotype for
effective risk assessment and personalized treatment planning.
The present results underscore the importance of
comprehensive management of asthma in patients with SCD. While the
study demonstrates a significant association between asthma and
ACS, it is important to acknowledge potential limitations.
Substantial heterogeneity of the studies included in the present
meta-analysis was noted. Variations in study design should be
highlighted, as some of the studies were cohort studies, either
prospective or retrospective, and others were case-control and
cross-sectional studies. The retrospective design frequently lacks
complete access to risk factor data and comorbidities. Population
variability was also present, as the studies enrolled participants
with different characteristics, such as age (ranging from infancy
to elderly) and geographic contexts. Furthermore, the duration of
asthma diagnosis was different among studies, which can also lead
to varied outcomes. Of course, random variation should be
acknowledged, as some heterogeneity may be due to random chance,
particularly in the present study, where the number of the pooled
studies is relatively small. Nonetheless, the present findings
should be highlighted, even if they need to be verified in future
large-scale prospective, well-designed studies.
One of the primary strengths of the present
meta-analysis is its comprehensive approach to synthesizing
existing research. By systematically reviewing numerous studies in
the SCD field, the precision of the estimates was increased, which
enhances the reliability of the conclusions drawn in the present
study. This large sample size allows for more robust
generalizations. In addition, the inclusion of diverse study
designs and populations strengthens the external validity of the
present findings. By aggregating data from various settings, it is
possible to better understand the broader implications of the
results and their applicability across different demographic
groups. Another significant advantage of this meta-analysis is the
rigorous methodology employed throughout the research process. A
comprehensive literature search was conducted, adhering to
established guidelines for meta-analyses. This careful selection
process helped ensure that the studies included were of high
quality and relevant to the research question. Furthermore, the use
of advanced statistical techniques allowed for the assessment of
heterogeneity and publication bias, providing a more nuanced
understanding of the data. By aggregating results from various
studies, a more unified perspective on the topic was offered, which
is beneficial for both researchers and practitioners. This
synthesis not only reinforces existing evidence but also highlights
areas where further research is needed, guiding future
investigations in the fields. While this meta-analysis provides
strong evidence of a significant association between asthma and
increased risk of ACS in patients with SCD, the generalizability of
these findings may be influenced by variations in demographic and
geographic factors. Most of the included studies were conducted in
specific populations, often in high-income countries with
predominantly African or African-American cohorts. Future research
should aim to validate these findings across diverse ethnic and
geographic groups to ensure broader applicability. A well-designed,
multicenter, prospective cohort study encompassing diverse clinical
settings and populations would offer a more comprehensive
understanding of how asthma modifies ACS risk in the global SCD
population. Such an approach would also allow the evaluation of
potential interactions between genetic, environmental and
socio-economic determinants that may influence disease severity and
comorbidity burden.
Furthermore, although this review suggests a
biological interplay between asthma and ACS, the causal mechanisms
remain inadequately understood. The overlap in clinical
presentation between asthma exacerbations and early ACS episodes
adds to the diagnostic complexity and may contribute to
under-recognition or misclassification. To explore the underlying
pathophysiology in greater depth, future studies should incorporate
translational approaches, including animal models and in
vitro experiments. These models can help delineate the specific
roles of airway inflammation, eosinophilic activity, oxidative
stress and endothelial dysfunction in the pathogenesis of ACS among
individuals with coexisting asthma and SCD. Additionally,
population-based interventional studies, such as randomized
controlled trials evaluating asthma-specific therapies (e.g.,
inhaled corticosteroids or leukotriene inhibitors) in patients with
SCD, would be valuable in determining whether asthma management can
effectively reduce the incidence or severity of ACS episodes.
Another important consideration to be addressed in
future studies is the possible inconsistency of the asthma
diagnostic criteria, ranging from self-reported asthma history to
physician-diagnosed cases without standardized pulmonary function
testing, which can lead to misclassification and impact the
strength of the observed association between asthma and ACS. To
enhance the validity and reproducibility of future findings, it is
essential that unified and clearly defined asthma diagnostic and
evaluation criteria are employed. Ideally, studies should
incorporate objective measures such as spirometry, bronchodilator
responsiveness and biomarkers of airway inflammation to ensure
accurate asthma classification. Standardizing asthma diagnosis will
not only reduce methodological bias but also facilitate more robust
cross-study comparisons and meta-analyses, ultimately contributing
to a clearer understanding of the interplay between asthma and ACS
in SCD.
Although subgroup or meta-regression analyses could
potentially help identify sources of heterogeneity, the current
meta-analysis was limited by the small number of included studies
and the incomplete reporting of relevant stratifying variables,
such as age, region, gender and genotype. These limitations
restricted the feasibility and statistical power of conducting such
additional analyses. However, the significant heterogeneity
observed highlights the need for future research that incorporates
more detailed and standardized demographic and clinical data,
enabling more granular subgroup analyses. Large-scale, multicenter
cohort studies with harmonized reporting standards would be
particularly valuable for disentangling the influence of various
factors on the relationship between asthma and ACS in patients with
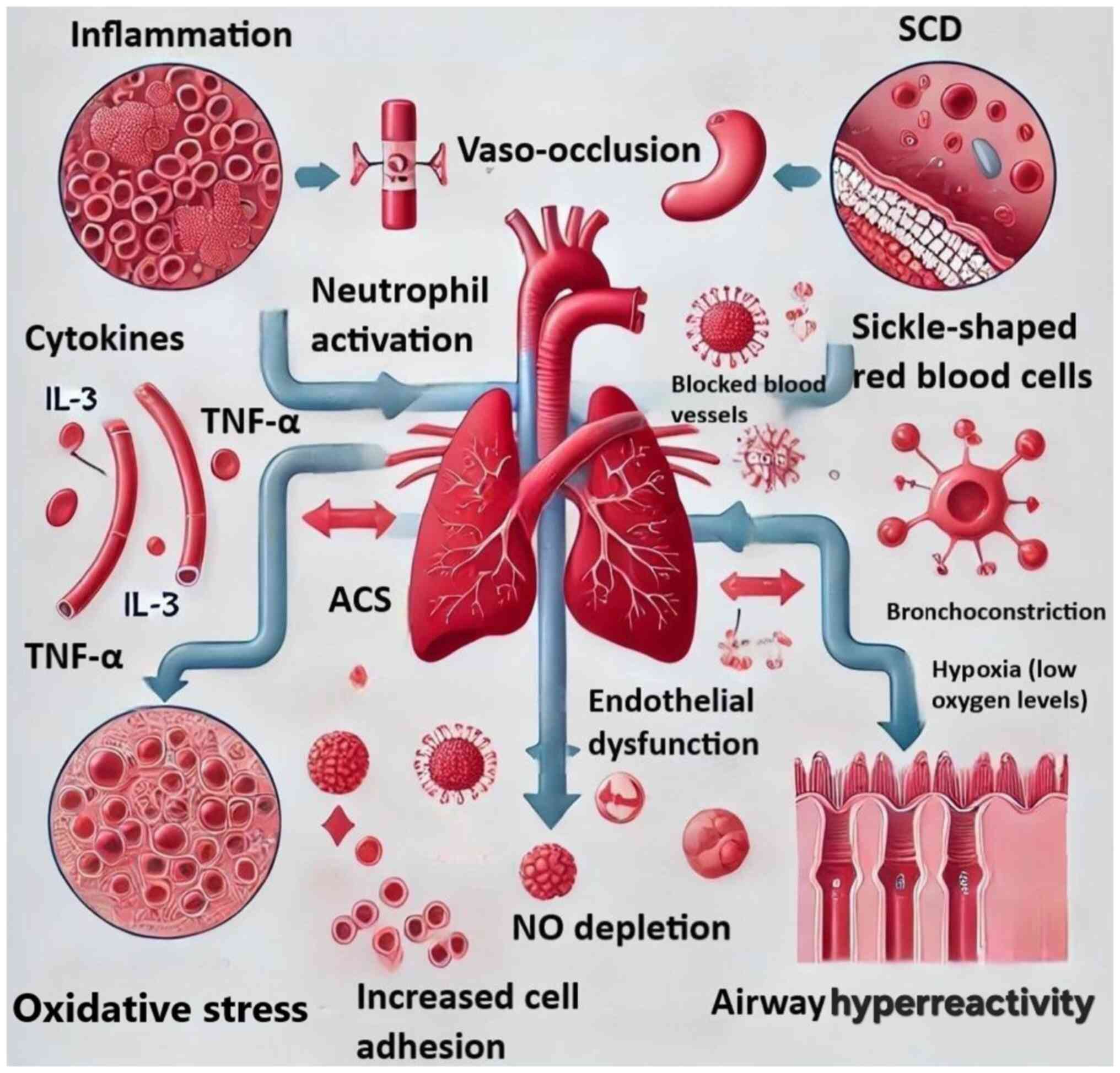
SCD. The convergence of inflammatory mediators, endothelial
dysfunction and oxidative stress represents a mechanistic continuum
linking asthma and ACS in SCD. By visually consolidating these
interactions, Fig. 3 serves as a
conceptual tool for clinicians and researchers evaluating
therapeutic targets such as IL-5 inhibition or NF-κB pathway
modulation.
Although the association between asthma and
increased risk of ACS in SCD is well established, future research
should focus on elucidating the underlying pathophysiological
mechanisms and developing targeted interventions. Chronic hemolysis
and NO depletion in SCD contribute to endothelial dysfunction,
while Th2-driven airway inflammation in asthma-characterized by
elevated IL-4, IL-5 and IL-13-promotes eosinophilic infiltration,
mucus overproduction and airway hyperreactivity (6,17,25).
These converging pathways exacerbate pulmonary vascular occlusion,
leading to ACS. Furthermore, asthma-related hypoxemia may amplify
sickling and microvascular adhesion, further increasing pulmonary
complications (8,9).
To improve outcomes, future clinical studies should
evaluate whether biomarker-guided asthma management (e.g., FeNO or
eosinophil counts) reduces ACS incidence in SCD. Investigations
into leukotriene receptor antagonists and biological therapies
targeting IL-5 or IL-4/IL-13 signaling are also warranted, as these
may modulate the shared inflammatory environment. Additionally,
integrated treatment strategies combining disease-modifying
therapies such as hydroxyurea with optimized asthma control should
be explored to determine their synergistic potential in preventing
ACS.
A key limitation of this meta-analysis is the
presence of substantial heterogeneity (I²=74%) across the included
studies. Although subgroup and sensitivity analyses were performed,
more advanced statistical approaches such as meta-regression were
not conducted. Such analyses could have provided deeper insights
into potential sources of heterogeneity, including age
distribution, sickle cell genotypes, asthma diagnostic methods and
regional or ethnic differences among study populations (13,15,17).
Future meta-analyses incorporating these variables could help
clarify their individual contributions and improve the precision of
pooled estimates.
Another important limitation is the inconsistency in
asthma diagnostic criteria across the included studies. While
certain studies relied on self-reported asthma history, others used
physicians' diagnosis, and only a minority incorporated objective
assessments such as spirometry, bronchodilator responsiveness or
airway inflammatory biomarkers (16,18,21).
This variability increases the likelihood of diagnostic
misclassification, which could have influenced the observed
association between asthma and acute chest syndrome. Standardizing
diagnostic approaches in future research-ideally using objective
pulmonary function tests and validated biomarkers-will be essential
to improve the reliability and reproducibility of results.
A further limitation of this meta-analysis is that
it focused exclusively on the risk of ACS occurrence without
evaluating how asthma may influence the severity of ACS episodes.
Clinically relevant outcomes such as the duration of
hospitalization, need for intensive care unit admission,
requirement for mechanical ventilation or mortality were not
consistently reported across studies and, therefore, could not be
included in the pooled analysis (17,20,25).
Incorporating these severity-related endpoints in future research
would provide a more comprehensive understanding of the clinical
impact of asthma in patients with sickle cell disease and better
inform risk stratification and management strategies.
Another methodological limitation concerns the
assessment of publication bias. Although a funnel plot was
presented, no quantitative statistical tests, such as Egger's
regression test or Begg's rank correlation test, were performed to
evaluate the potential presence of small-study effects (13,14).
The absence of these tests limits the objectivity of the
publication bias assessment and may leave residual uncertainty
regarding the robustness of the pooled estimates. Future
meta-analyses should incorporate both graphical and statistical
methods for publication bias evaluation to strengthen the validity
of their conclusions.
Finally, although subgroup analyses based on study
design were performed, the statistical power of these analyses was
not assessed or reported. This omission raises concerns that some
non-significant findings may reflect insufficient power rather than
a true absence of association (12,15,19).
Future studies should predefine subgroup analyses and ensure
adequate sample sizes to detect clinically meaningful differences,
thereby minimizing the risk of false-negative results and enhancing
the interpretability of subgroup findings.
In conclusion, the association between asthma and
ACS in patients with SCD highlights the need for heightened
awareness and targeted management of asthma in this population. By
addressing asthma as a significant comorbidity, healthcare
providers can potentially mitigate the risk of ACS and improve the
overall health and quality of life for individuals with SCD. The
present results have clinical implications, as careful evaluation
by a respiratory medicine specialist should be included in patient
monitoring and assessment, so that asthma is diagnosed early and is
appropriately treated. The significant association between asthma
and increased risk for ACS in patients with SCD highlights a
critical area for clinical attention and further research.
Supplementary Material
Funnel plot assessing publication
bias. Funnel plot of standard error against risk ratio for the
included studies examining the association between asthma and acute
chest syndrome in patients with sickle cell disease. Each green dot
represents an individual study. The symmetrical distribution of
studies around the central line suggests low likelihood of
publication bias. The vertical dashed line indicates the overall
pooled effect estimate, while the triangle represents the 95%
confidence boundaries. The white and darker grey triangles indicate
different confidence regions. The white triangle represents the 95%
confidence region, showing where study results are expected to lie
if there is no publication bias and only random variation around
the true effect. The darker grey regions represent wider confidence
limits, such as 99% or 90% intervals, providing additional context
for how study results should be distributed with varying levels of
precision. Notably, Knight-Madden et al (16) appears as an outlier, contributing
to observed heterogeneity.
Subgroup analysis by study design
(prospective vs. retrospective). Forest plot comparing the risk of
ACS in patients with sickle cell disease and asthma vs. those
without asthma, stratified by study design. The pooled RR in
prospective studies was 1.85 (95% CI: 1.58-2.17) with no
heterogeneity (I2=0%), while retrospective studies
showed a higher pooled RR of 2.56 (95% CI: 1.72-3.82) and
substantial heterogeneity (I2=85.5%). The prediction
interval (0.93 to 5.38) suggests variability in future study
outcomes. The red line indicates the overall pooled effect. These
results support the robustness of the asthma-ACS association, while
highlighting study design as a contributor to heterogeneity. MH,
Mantel-Haenszel; df, degrees of freedom; RR, risk ratio; ACS, acute
chest syndrome.
Sensitivity analysis excluding
high-heterogeneity studies. Forest plot showing the association
between asthma and ACS in patients with sickle cell disease
following the exclusion of the studies by Knight-Madden et
al (16) and Intzes et
al (21), which were
identified as major contributors to heterogeneity. The remaining 10
studies (401 experimental vs. 1,669 control participants) yielded a
pooled risk ratio of 1.88 (95% CI: 1.59-2.24; P<0.05),
indicating a significant association between asthma and increased
ACS risk. Heterogeneity was reduced to moderate levels
(I2=52%), suggesting improved consistency across
studies. The prediction interval (1.17 to 3.02) reinforces the
robustness of the association. MH, Mantel-Haenszel; df, degrees of
freedom; ACS, acute chest syndrome.
Search strategy applied in
PubMed.
NOS quality assessment form regarding
included studies.
NOS quality assessment form regarding
included studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
KD and VEG conceptualized the study. KD, VEG, TVK,
AC and DAS made a substantial contribution to data interpretation
and analysis and wrote and prepared the draft of the manuscript. KD
and VEG analyzed the data and provided critical revisions. KD, TVK,
DAS, AC and VEG confirmed the authenticity of all the raw data. All
authors contributed to manuscript revision, and read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, the AI tool
ChatGPT was used to improve the readability and language of the
manuscript, and subsequently, the authors revised and edited the
content produced by the AI tool as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Rees DC, Williams TN and Gladwin MT:
Sickle-cell disease. Lancet. 376:2018–2031. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kochhar M and McGann PT: Sickle cell
disease in India: Not just a mild condition. Br J Haematol.
206:380–381. 2025.PubMed/NCBI View Article : Google Scholar
|
|
3
|
GBD 2021 Sickle Cell Disease
Collaborators. Global, regional, and national prevalence and
mortality burden of sickle cell disease, 2000-2021: A systematic
analysis from the global burden of disease study 2021. Lancet
Haematol. 10:e585–e599. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sundd P, Gladwin MT and Novelli EM:
Pathophysiology of sickle cell disease. Annu Rev Pathol.
14:263–292. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tentolouris A, Stafylidis C, Siafarikas C,
Dimopoulou MN, Makrodimitri S, Bousi S, Papalexis P, Damaskos C,
Trakas N, Sklapani P, et al: Favorable outcomes of patients with
sickle cell disease hospitalized due to COVID-19: A report of three
cases. Exp Ther Med. 23(338)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
DeBaun MR and Strunk RC: The intersection
between asthma and acute chest syndrome in children with
sickle-cell anaemia. Lancet. 387:2545–2553. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lunt A, Sturrock SS and Greenough A:
Asthma and the outcome of sickle cell disease. Expert Opin Orphan
Drugs. 6:733–740. 2018.
|
|
8
|
De A, Williams S, Yao Y, Jin Z, Brittenham
GM, Kattan M, Lovinsky-Desir S and Lee MT: Acute chest syndrome,
airway inflammation and lung function in sickle cell disease. PLoS
One. 18(e0283349)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Samarasinghe AE and Rosch JW: Convergence
of inflammatory pathways in allergic asthma and sickle cell
disease. Front Immunol. 10(3058)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: an updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of observational studies in epidemiology: A
proposal for reporting. Meta-analysis of observational studies in
epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wells GA, Shea B, O'Connell D, O'Connell
D, Peterson J, Welch Losos M, Tugwell P, Ga SW, Zello G and
Petersen J: The Newcastle-Ottawa Scale (NOS) for assessing the
quality of nonrandomized studies in meta-analyses. American Academy
of General Practice, Kansas City, MO, 2000.
|
|
13
|
Deeks JJ, Higgins JPT and Altman DG:
Analyzing data and undertaking meta-analyses. Chapter 9: In:
Cochrane Handbook for Systematic Reviews of Interventions. Higgins
JPT and Green S (eds). The Cochrane Collaboration, London,
2011.
|
|
14
|
Meta-Mar. Meta Mar: A tool for
meta-analysis. Available from https://www.meta-mar.com/.
|
|
15
|
Boyd JH, Moinuddin A, Strunk RC and DeBaun
MR: Asthma and acute chest in sickle-cell disease. Pediatr
Pulmonol. 38:229–232. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Knight-Madden JM, Forrester TS, Lewis NA
and Greenough A: Asthma in children with sickle cell disease and
its association with acute chest syndrome. Thorax. 60:206–210.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Boyd JH, Macklin EA, Strunk RC and DeBaun
MR: Asthma is associated with acute chest syndrome and pain in
children with sickle cell anemia. Blood. 108:2923–2927.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sylvester KP, Patey RA, Broughton S,
Rafferty GF, Rees D, Thein SL and Greenough A: Temporal
relationship of asthma to acute chest syndrome in sickle cell
disease. Pediatr Pulmonol. 42:103–106. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
An P, Barron-Casella EA, Strunk RC,
Hamilton RG, Casella JF and DeBaun MR: Elevation of IgE in children
with sickle cell disease is associated with doctor diagnosis of
asthma and increased morbidity. J Allergy Clin Immunol.
127:1440–1446. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Glassberg JA, Chow A, Wisnivesky J,
Hoffman R, Debaun MR and Richardson LD: Wheezing and asthma are
independent risk factors for increased sickle cell disease
morbidity. Br J Haematol. 159:472–479. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Intzes S, Kalpatthi RV, Short R and Imran
H: Pulmonary function abnormalities and asthma are prevalent in
children with sickle cell disease and are associated with acute
chest syndrome. Pediatr Hematol Oncol. 30:726–732. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Strunk RC, Cohen RT, Cooper BP, Rodeghier
M, Kirkham FJ, Warner JO, Stocks J, Kirkby J, Roberts I, Rosen CL,
et al: Wheezing symptoms and parental asthma are associated with a
physician diagnosis of asthma in children with sickle cell anemia.
J Pediatr. 164:821–826.e1. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bafunyembaka G, Nacher M, Maniassom C,
Birindwa AM and Elenga N: Asthma is an independent risk factor for
acute chest syndrome in children with sickle cell disease in French
Guiana. Children (Basel). 11(1541)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bartram JL, Fine-Goulden MR, Green D,
Ahmad R and Inusa BP: Asthma in pediatric sickle cell acute chest
syndrome: In an inner City London hospital. Blood.
112(2481)2008.
|
|
25
|
Bernaudin F, Strunk RC, Kamdem A, Arnaud
C, An P, Torres M, Delacourt C and DeBaun MR: Asthma is associated
with acute chest syndrome, but not with an increased rate of
hospitalization for pain among children in France with sickle cell
anemia: A retrospective cohort study. Haematologica. 93:1917–1918.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pahl K and Mullen CA: Original research:
Acute chest syndrome in sickle cell disease: Effect of genotype and
asthma. Exp Biol Med (Maywood). 241:745–758. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Willen SM, Rodeghier M and DeBaun MR:
Asthma in children with sickle cell disease. Curr Opin Pediatr.
31:349–356. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Habib N, Pasha MA and Tang DD: Current
understanding of asthma pathogenesis and biomarkers. Cells.
11(2764)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gomez E and Morris CR: Asthma management
in sickle cell disease. Biomed Res Int. 2013(604140)2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Miller AC and Gladwin MT: Pulmonary
complications of sickle cell disease. Am J Respir Crit Care Med.
185:1154–1165. 2012.PubMed/NCBI View Article : Google Scholar
|