|
1
|
Anasetti C, Logan BR, Lee SJ, Waller EK,
Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A,
Couban S, et al: Peripheral-blood stem cells versus bone marrow
from unrelated donors. N Engl J Med. 367:1487–1496. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Al-Kadhimi Z, Gul Z, Chen W, Smith D,
Abidi M, Deol A, Ayash L, Lum L, Waller EK, Ratanatharathorn V and
Uberti J: High incidence of severe acute graft-versus-host disease
with tacrolimus and mycophenolate mofetil in a large cohort of
related and unrelated allogeneic transplantation patients. Biol
Blood Marrow Transplant. 20:979–985. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Justiz Vaillant AA, Modi P and Mohammadi
O: Graft-versus-host disease. In: StatPearls. StatPearls
Publishing, Treasure Island, FL, 2025.
|
|
4
|
Levine JE, Logan B, Wu J, Alousi AM, Ho V,
Bolaños-Meade J and Weisdorf D: Blood and Marrow Transplant
Clinical Trials Network. Graft-versus-host disease treatment:
Predictors of survival. Biol Blood Marrow Transplant. 16:1693–1699.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Martin PJ, Rizzo JD, Wingard JR, Ballen K,
Curtin PT, Cutler C, Litzow MR, Nieto Y, Savani BN, Schriber JR, et
al: First- and second-line systemic treatment of acute
graft-versus-host disease: Recommendations of the American society
of blood and marrow transplantation. Biol Blood Marrow Transplant.
18:1150–1163. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Westin JR, Saliba RM, De Lima M, Alousi A,
Hosing C, Qazilbash MH, Khouri IF, Shpall EJ, Anderlini P, Rondon
G, et al: Steroid-refractory acute GVHD: Predictors and outcomes.
Adv Hematol. 2011(601953)2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Malard F, Huang XJ and Sim JPY: Treatment
and unmet needs in steroid-refractory acute graft-versus-host
disease. Leukemia. 34:1229–1240. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zeiser R and Blazar BR: Acute
graft-versus-host disease-biologic process, prevention, and
therapy. N Engl J Med. 377:2167–2179. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mohty M, Holler E, Jagasia M, Jenq R,
Malard F, Martin P, Socié G and Zeiser R: Refractory acute
graft-versus-host disease: A new working definition beyond
corticosteroid refractoriness. Blood. 136:1903–1906.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wölfl M, Qayed M, Benitez Carabante MI,
Sykora T, Bonig H, Lawitschka A and Diaz-de-Heredia C: Current
prophylaxis and treatment approaches for acute graft-versus-host
disease in haematopoietic stem cell transplantation for children
with acute lymphoblastic leukaemia. Front Pediatr.
9(784377)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Baccelli F, Gottardi F, Muratore E,
Leardini D, Grasso AG, Gori D, Belotti T, Prete A and Masetti R:
Ruxolitinib for the treatment of acute and chronic
graft-versus-host disease in children: A systematic review and
individual patient data meta-analysis. Bone Marrow Transplant.
59:765–776. 2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Inagaki J, Kodama Y, Fukano R, Noguchi M
and Okamura J: Mycophenolate mofetil for treatment of
steroid-refractory acute graft-versus-host disease after pediatric
hematopoietic stem cell transplantation. Pediatr Transplant.
19:652–658. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shen MZ, Li JX, Zhang XH, Xu LP, Wang Y,
Liu KY, Huang XJ, Hong SD and Mo XD: Meta-analysis of interleukin-2
receptor antagonists as the treatment for steroid-refractory acute
graft-versus-host disease. Front Immunol. 12(749266)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Greinix HT, Ayuk F and Zeiser R:
Extracorporeal photopheresis in acute and chronic
steroid-refractory graft-versus-host disease: An evolving treatment
landscape. Leukemia. 36:2558–2566. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moiseev IS, Morozova EV, Bykova TA, Paina
OV, Smirnova AG, Dotsenko AA, Borzenkova ES, Galimov AN,
Gudognikova YV, Ekushov KA, et al: Long-term outcomes of
ruxolitinib therapy in steroid-refractory graft-versus-host disease
in children and adults. Bone Marrow Transplant. 55:1379–1387.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ferrer IR, Araki K and Ford ML:
Paradoxical aspects of rapamycin immunobiology in transplantation.
Am J Transplant. 11:654–659. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Olivieri A and Mancini G: Current
approaches for the prevention and treatment of acute and chronic
GVHD. Cells. 13(1524)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Teachey DT, Grupp SA and Brown VI:
Mammalian target of rapamycin inhibitors and their potential role
in therapy in leukaemia and other haematological malignancies. Br J
Haematol. 145:569–580. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Araki K, Turner AP, Shaffer VO, Gangappa
S, Keller SA, Bachmann MF, Larsen CP and Ahmed R: mTOR regulates
memory CD8 T-cell differentiation. Nature. 460:108–112.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pearce EL, Walsh MC, Cejas PJ, Harms GM,
Shen H, Wang LS, Jones RG and Choi Y: Enhancing CD8 T-cell memory
by modulating fatty acid metabolism. Nature. 460:103–107.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ferrer IR, Wagener ME, Robertson JM,
Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP and Ford ML:
Cutting edge: Rapamycin augments pathogen-specific but not
graft-reactive CD8+ T cell responses. J Immunol. 185:2004–2008.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dufour M, Dormond-Meuwly A, Demartines N
and Dormond O: Targeting the mammalian target of rapamycin (mTOR)
in cancer therapy: Lessons from past and future perspectives.
Cancers (Basel). 3:2478–2500. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pidala J, Kim J, Jim H, Kharfan-Dabaja MA,
Nishihori T, Fernandez HF, Tomblyn M, Perez L, Perkins J, Xu M, et
al: A randomized phase II study to evaluate tacrolimus in
combination with sirolimus or methotrexate after allogeneic
hematopoietic cell transplantation. Haematologica. 97:1882–1889.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Armand P, Kim HT, Sainvil MM, Lange PB,
Giardino AA, Bachanova V, Devine SM, Waller EK, Jagirdar N, Herrera
AF, et al: The addition of sirolimus to the graft-versus-host
disease prophylaxis regimen in reduced intensity allogeneic stem
cell transplantation for lymphoma: A multicentre randomized trial.
Br J Haematol. 173:96–104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Al Malki MM, Gendzekhadze K, Yang D,
Mokhtari S, Parker P, Karanes C, Palmer J, Snyder D, Forman SJ,
Nademanee A and Nakamura R: Long-term outcome of allogeneic
hematopoietic stem cell transplantation from unrelated donor using
tacrolimus/sirolimus-based GvHD prophylaxis: Impact of HLA
mismatch. Transplantation. 104:1070–1080. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ceberio I, Devlin SM, Sauter C, Barker JN,
Castro-Malaspina H, Giralt S, Ponce DM, Lechner L, Maloy MA,
Goldberg JD and Perales MA: Sirolimus, tacrolimus and low-dose
methotrexate based graft-versus-host disease prophylaxis after
non-ablative or reduced intensity conditioning in related and
unrelated donor allogeneic hematopoietic cell transplant. Leuk
Lymphoma. 56:663–670. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang L, Gu Z, Zhai R, Li D, Zhao S, Luo L,
Zhao X, Wei H, Pang Z, Wang L, et al: The efficacy and safety of
sirolimus-based graft-versus-host disease prophylaxis in patients
undergoing allogeneic hematopoietic stem cell transplantation: A
meta-analysis of randomized controlled trials. Transfusion.
55:2134–2141. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sandmaier BM, Kornblit B, Storer BE,
Olesen G, Maris MB, Langston AA, Gutman JA, Petersen SL, Chauncey
TR, Bethge WA, et al: Addition of sirolimus to standard
cyclosporine plus mycophenolate mofetil-based graft-versus-host
disease prophylaxis for patients after unrelated non-myeloablative
haemopoietic stem cell transplantation: A multicentre, randomised,
phase 3 trial. Lancet Haematol. 6:e409–e418. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Al-Kadhimi Z, Gul Z, Rodriguez R, Chen W,
Smith D, Mitchell A, Abidi M, Ayash L, Deol A, Lum L, et al:
Anti-thymocyte globulin (thymoglobulin), tacrolimus, and sirolimus
as acute graft-versus-host disease prophylaxis for unrelated
hematopoietic stem cell transplantation. Biol Blood Marrow
Transplant. 18:1734–1744. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Alyea EP, Li S, Kim HT, Cutler C, Ho V,
Soiffer RJ and Antin JH: Sirolimus, tacrolimus, and low-dose
methotrexate as graft-versus-host disease prophylaxis in related
and unrelated donor reduced-intensity conditioning allogeneic
peripheral blood stem cell transplantation. Biol Blood Marrow
Transplant. 14:920–926. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pulsipher MA, Wall DA, Grimley M, Goyal
RK, Boucher KM, Hankins P, Grupp SA and Bunin N: A phase I/II study
of the safety and efficacy of the addition of sirolimus to
tacrolimus/methotrexate graft versus host disease prophylaxis after
allogeneic haematopoietic cell transplantation in paediatric acute
lymphoblastic leukaemia (ALL). Br J Haematol. 147:691–699.
2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cutler C, Logan B, Nakamura R, Johnston L,
Choi S, Porter D, Hogan WJ, Pasquini M, MacMillan ML, Hsu JW, et
al: Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD
prophylaxis after matched, related donor allogeneic HCT. Blood.
124:1372–1377. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shayani S, Palmer J, Stiller T, Liu X,
Thomas SH, Khuu T, Parker PM, Khaled SK, Forman SJ and Nakamura R:
Thrombotic microangiopathy associated with sirolimus level after
allogeneic hematopoietic cell transplantation with
tacrolimus/sirolimus-based graft-versus-host disease prophylaxis.
Biol Blood Marrow Transplant. 19:298–304. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ziakas PD, Zervou FN, Zacharioudakis IM
and Mylonakis E: Graft-versus-host disease prophylaxis after
transplantation: A network meta-analysis. PLoS One.
9(e114735)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cutler C and Antin JH: Sirolimus for GVHD
prophylaxis in allogeneic stem cell transplantation. Bone Marrow
Transplant. 34:471–476. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pidala J, Hamadani M, Dawson P, Martens M,
Alousi AM, Jagasia M, Efebera YA, Chhabra S, Pusic I, Holtan SG, et
al: Randomized multicenter trial of sirolimus vs prednisone as
initial therapy for standard-risk acute GVHD: the BMT CTN 1501
trial. Blood. 135:97–107. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
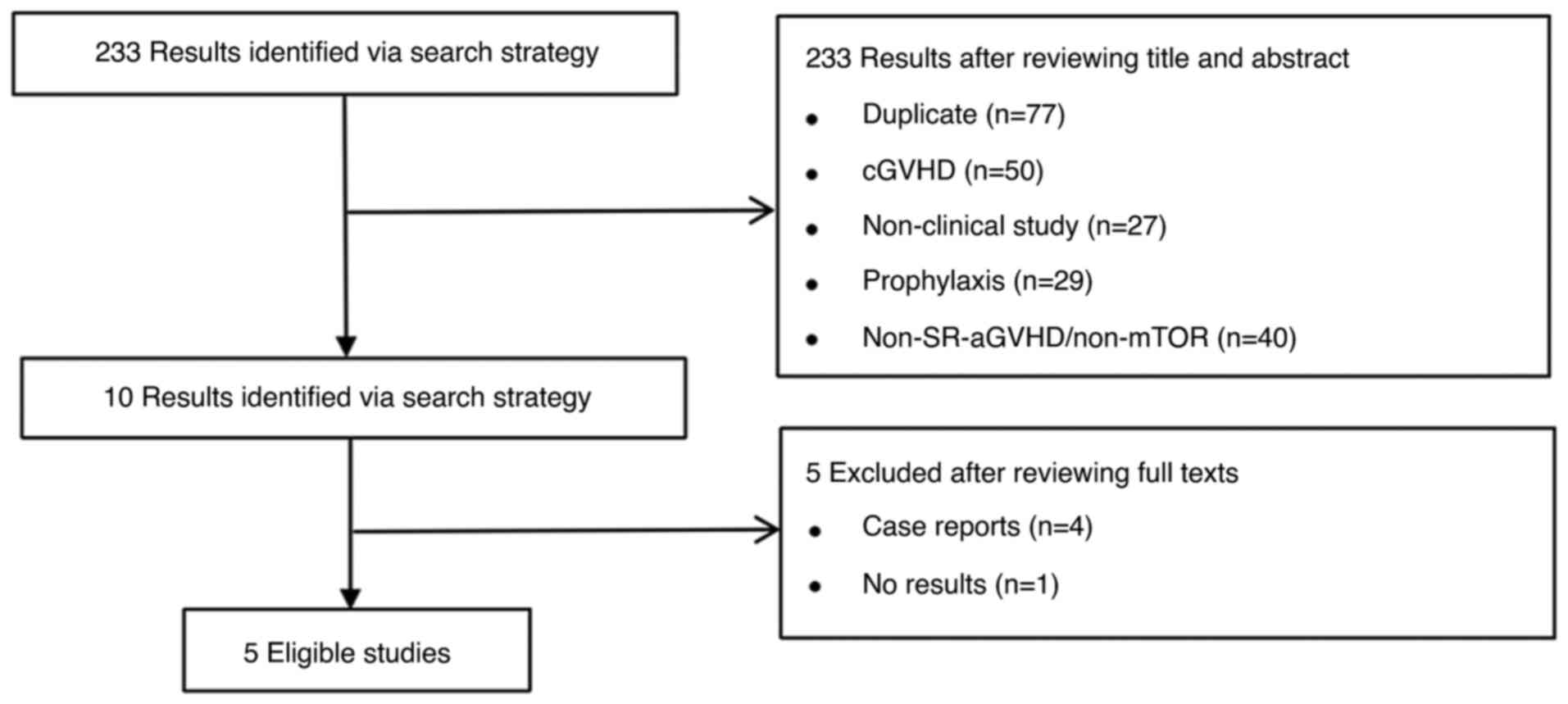
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. Open Med. 3:e123–e130.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Borenstein M, Hedges LV, Higgins JP and
Rothstein HR: A basic introduction to fixed-effect and
random-effects models for meta-analysis. Res Synth Methods.
1:97–111. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
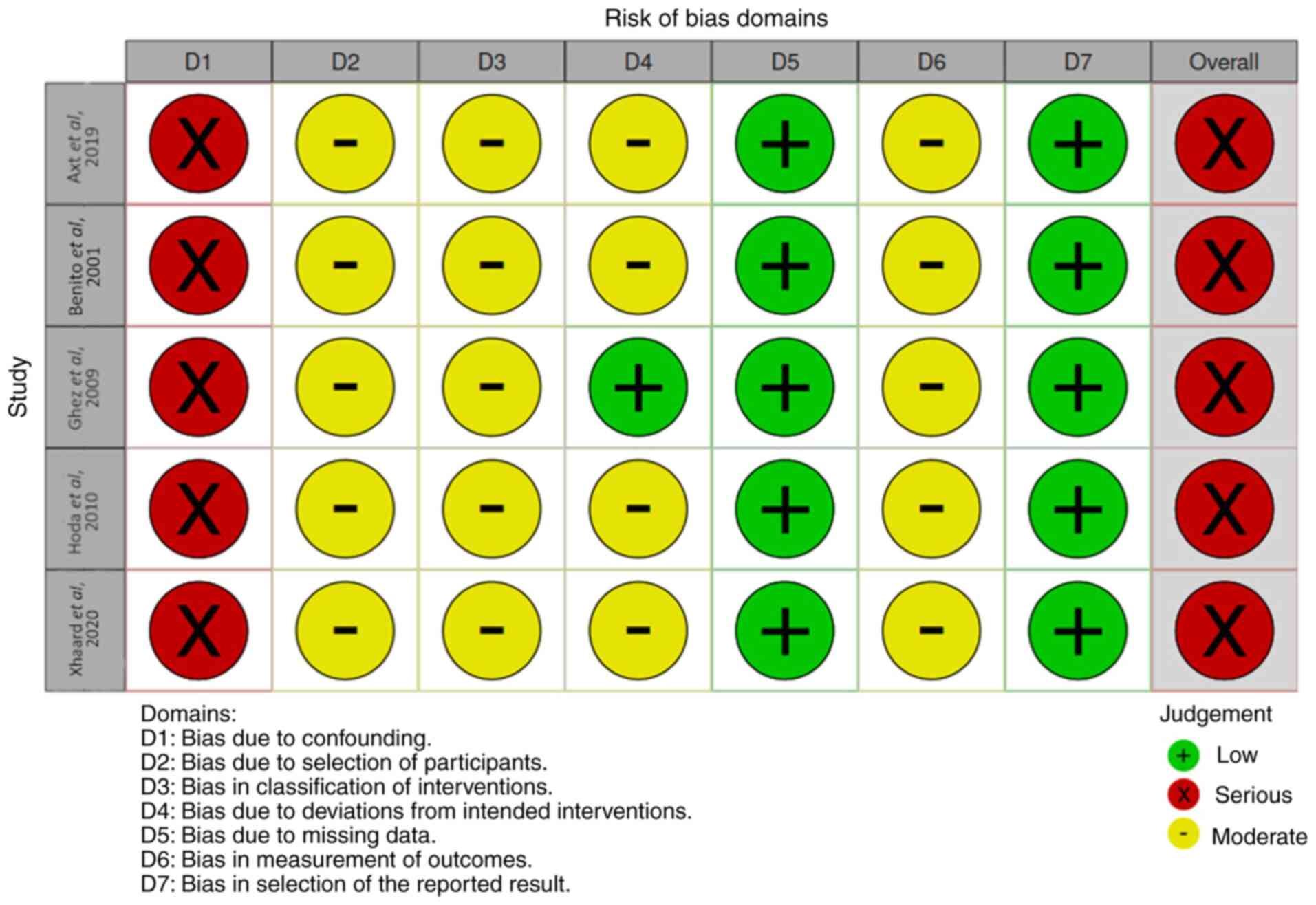
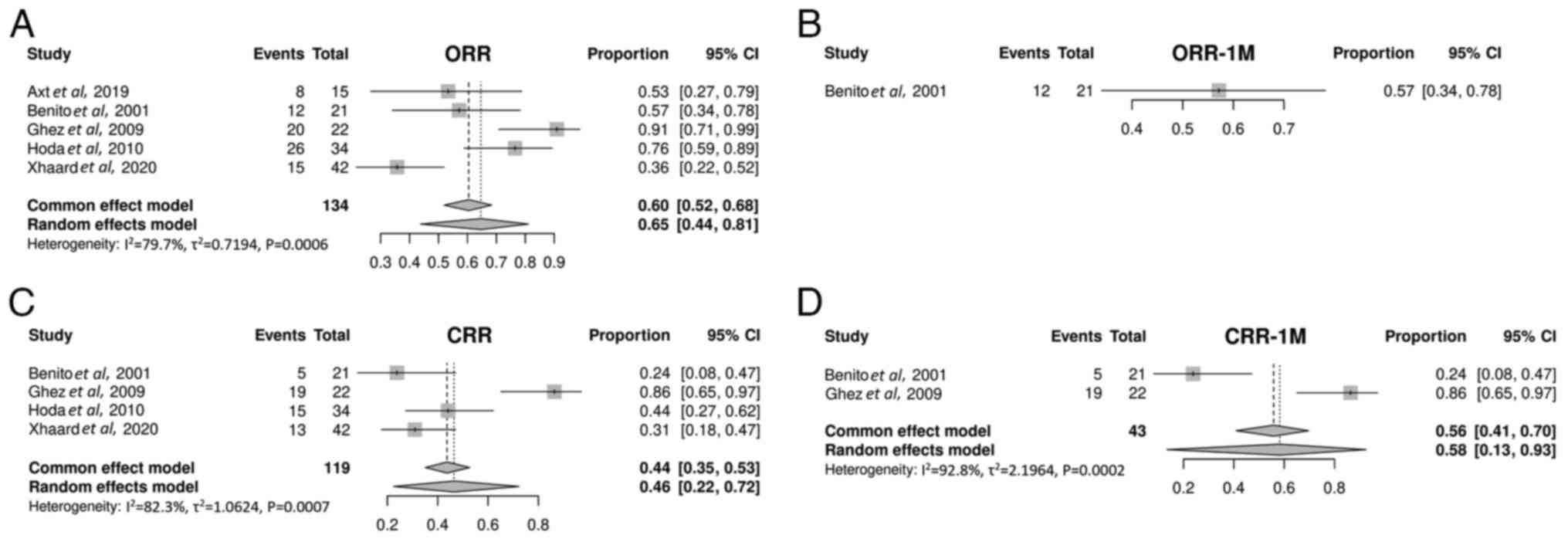
Axt L, Naumann A, Toennies J, Haen SP,
Vogel W, Schneidawind D, Wirths S, Moehle R, Faul C, Kanz L, et al:
Retrospective single center analysis of outcome, risk factors and
therapy in steroid refractory graft-versus-host disease after
allogeneic hematopoietic cell transplantation. Bone Marrow
Transplant. 54:1805–1814. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
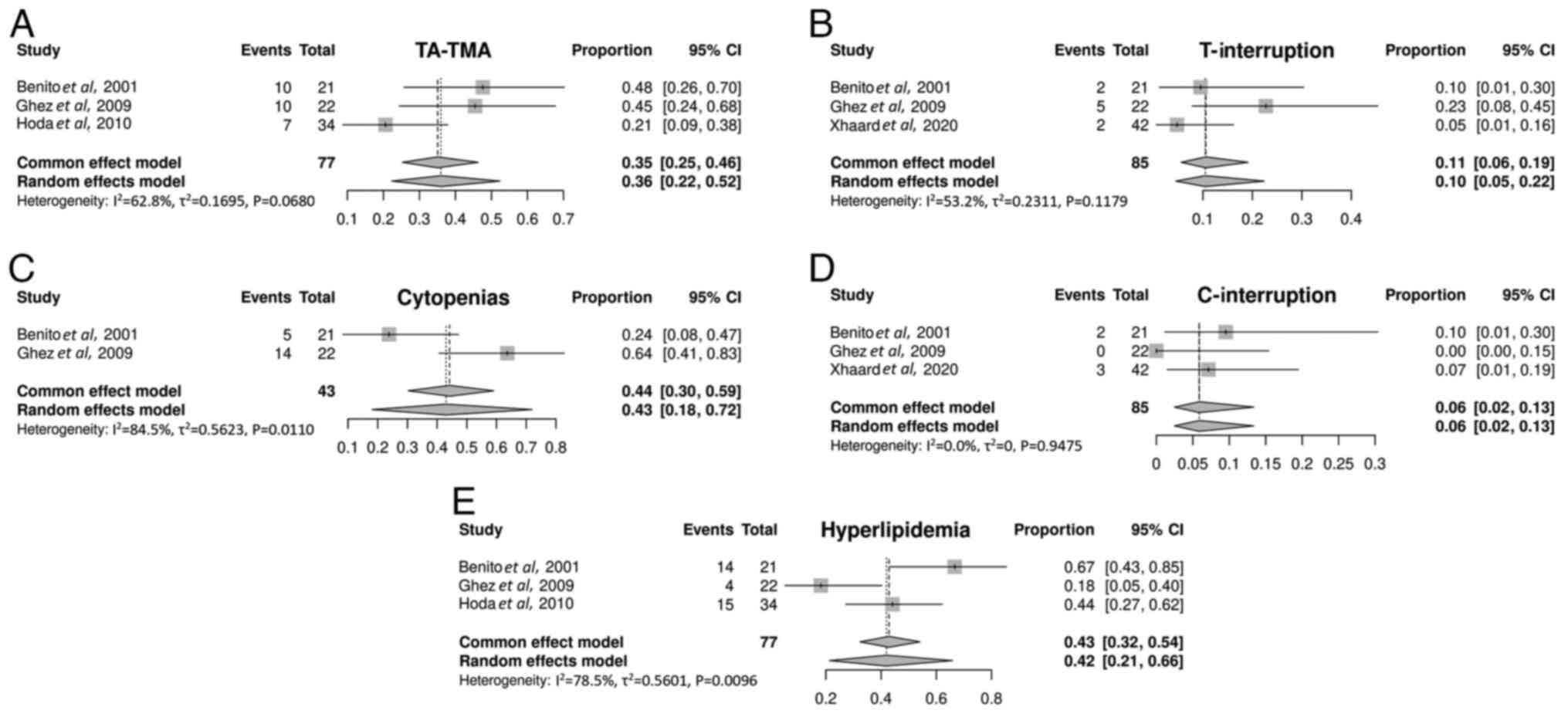
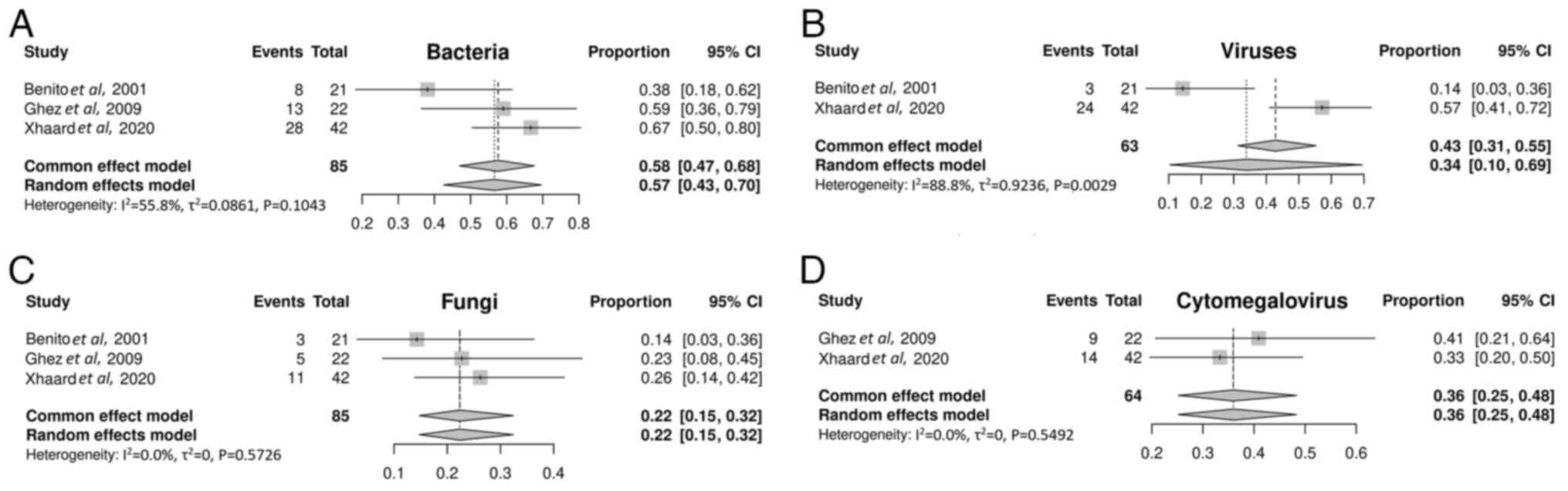
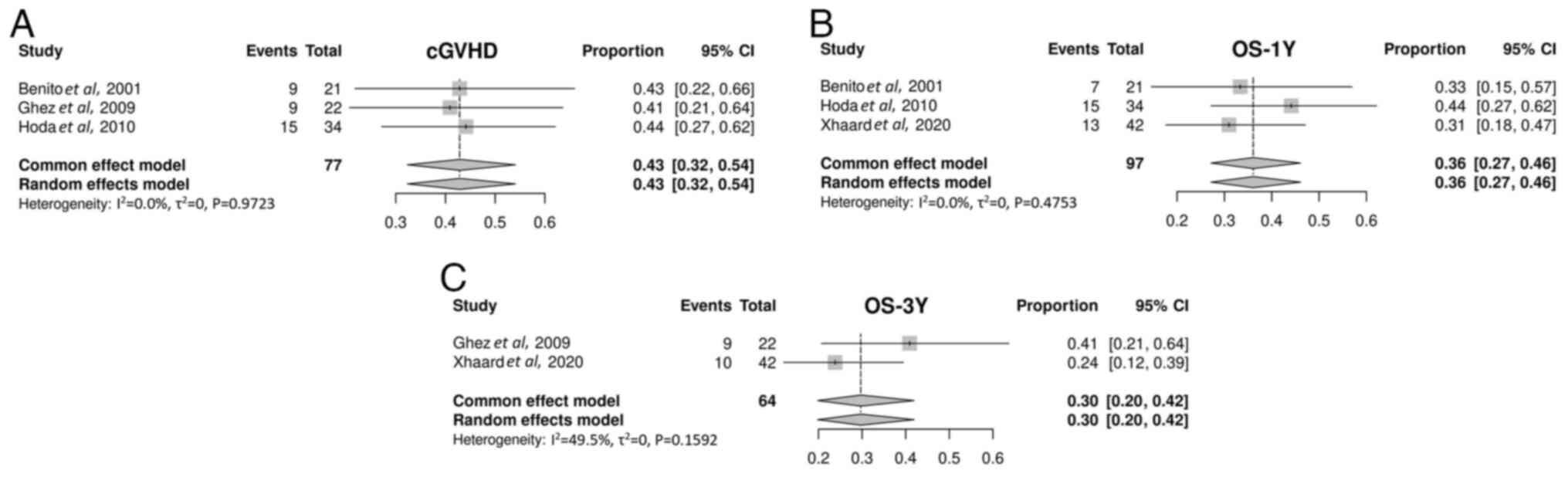
Benito AI, Furlong T, Martin PJ, Anasetti
C, Appelbaum FR, Doney K, Nash RA, Papayannopoulou T, Storb R,
Sullivan KM, et al: Sirolimus (rapamycin) for the treatment of
steroid-refractory acute graft-versus-host disease.
Transplantation. 72:1924–1929. 2001.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ghez D, Rubio MT, Maillard N, Suarez F,
Chandesris MO, Delarue R, Deau-Fischer B, Varet B, Hermine O and
Buzyn A: Rapamycin for refractory acute graft-versus-host disease.
Transplantation. 88:1081–1087. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hoda D, Pidala J, Salgado-Vila N, Kim J,
Perkins J, Bookout R, Field T, Perez L, Ayala E, Ochoa-Bayona JL,
et al: Sirolimus for treatment of steroid-refractory acute
graft-versus-host disease. Bone Marrow Transplant. 45:1347–1351.
2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xhaard A, Launay M, Sicre de Fontbrune F,
Michonneau D, Sutra Del Galy A, Coman T, Pagliuca S, Dhedin N,
Robin M, Peffault bde Latour R and Socie G: A monocentric study of
steroid-refractory acute graft-versus-host disease treatment with
tacrolimus and mTOR inhibitor. Bone Marrow Transplant. 55:86–92.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Young JA, Pallas CR and Knovich MA:
Transplant-associated thrombotic microangiopathy: Theoretical
considerations and a practical approach to an unrefined diagnosis.
Bone Marrow Transplant. 56:1805–1817. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Schub N, Günther A, Schrauder A, Claviez
A, Ehlert C, Gramatzki M and Repp R: Therapy of steroid-refractory
acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow
Transplant. 46:143–147. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Drobyski WR, Pasquini M, Kovatovic K,
Palmer J, Douglas Rizzo J, Saad A, Saber W and Hari P: Tocilizumab
for the treatment of steroid refractory graft-versus-host disease.
Biol Blood Marrow Transplant. 17:1862–1868. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Magenau JM, Goldstein SC, Peltier D,
Soiffer RJ, Braun T, Pawarode A, Riwes MM, Kennel M, Antin JH,
Cutler CS, et al: α1-Antitrypsin infusion for treatment
of steroid-resistant acute graft-versus-host disease. Blood.
131:1372–1379. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Busca A, Locatelli F, Marmont F, Ceretto C
and Falda M: Recombinant human soluble tumor necrosis factor
receptor fusion protein as treatment for steroid refractory
graft-versus-host disease following allogeneic hematopoietic stem
cell transplantation. Am J Hematol. 82:45–52. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bacigalupo A, Angelucci E, Raiola AM,
Varaldo R, Di Grazia C, Gualandi F, Benedetti E, Risitano A, Musso
M, Zallio F, et al: Treatment of steroid resistant acute graft
versus host disease with an anti-CD26 monoclonal
antibody-Begelomab. Bone Marrow Transplant. 55:1580–1587.
2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Abu-Dalle I, Reljic T, Nishihori T, Antar
A, Bazarbachi A, Djulbegovic B, Kumar A and Kharfan-Dabaja MA:
Extracorporeal photopheresis in steroid-refractory acute or chronic
graft-versus-host disease: Results of a systematic review of
prospective studies. Biol Blood Marrow Transplant. 20:1677–1686.
2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Han LJ, Wang Y, Fan ZP, Huang F, Zhou J,
Fu YW, Qu H, Xuan L, Xu N, Ye JY, et al: Haploidentical
transplantation compared with matched sibling and unrelated donor
transplantation for adults with standard-risk acute lymphoblastic
leukaemia in first complete remission. Br J Haematol. 179:120–130.
2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
García-Cadenas I, Rivera I, Martino R,
Esquirol A, Barba P, Novelli S, Orti G, Briones J, Brunet S,
Valcarcel D and Sierra J: Patterns of infection and
infection-related mortality in patients with steroid-refractory
acute graft versus host disease. Bone Marrow Transplant.
52:107–113. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bak S, Tischer S, Dragon A, Ravens S, Pape
L, Koenecke C, Oelke M, Blasczyk R, Maecker-Kolhoff B and
Eiz-Vesper B: Selective effects of mTOR inhibitor sirolimus on
Naïve and CMV-specific T cells extending its applicable range
beyond immunosuppression. Front Immunol. 9(2953)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Marty FM, Bryar J, Browne SK, Schwarzberg
T, Ho VT, Bassett IV, Koreth J, Alyea EP, Soiffer RJ, Cutler CS, et
al: Sirolimus-based graft-versus-host disease prophylaxis protects
against cytomegalovirus reactivation after allogeneic hematopoietic
stem cell transplantation: A cohort analysis. Blood. 110:490–500.
2007.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yang W, Zhu G, Qin M, Li Z, Wang B, Yang J
and Wang T: The effectiveness of ruxolitinib for acute/chronic
graft-versus-host disease in children: A retrospective study. Drug
Des Devel Ther. 15:743–752. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Choi J, Cooper ML, Alahmari B, Ritchey J,
Collins L, Holt M and DiPersio JF: Pharmacologic blockade of
JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia
effect. PLoS One. 9(e109799)2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Onishi C, Ohashi K, Sawada T, Nakano M,
Kobayashi T, Yamashita T, Akiyama H and Sakamaki H: A high risk of
life-threatening infectious complications in mycophenolate mofetil
treatment for acute or chronic graft-versus-host disease. Int J
Hematol. 91:464–470. 2010.PubMed/NCBI View Article : Google Scholar
|