Introduction
Despite the routine use of immunosuppressive
prophylaxis, ~50% of patients develop acute graft-vs.-host disease
(aGVHD) following allogeneic hematopoietic stem cell
transplantation (allo-HSCT) (1).
Among these, 14-36% of patients will develop severe aGVHD (2), leading to early post-transplant
mortality (3,4). The non-relapse mortality rate by
response days 14, 28 and 56 for those who do not achieve complete
remission has been reported as ~49, 53 and 69%, respectively
(4). The first-line treatment for
aGVHD is systemic corticosteroid therapy; however, 35-50% of
patients are refractory to corticosteroid treatment (5-7).
The long-term prognosis for patients with steroid-refractory
(SR)-aGVHD remains poor, with survival rates of 5-30% (8). Several second-line treatments for
SR-aGVHD have already been developed, including mycophenolate
mofetil (MMF), a selective inhibitor of the Janus kinase pathway
(ruxolitinib), IL-2 receptor antagonists (IL-2RAs), antitumor
necrosis factor (TNF-α) antibodies, extracorporeal photopheresis
(ECP) and mesenchymal stromal cells (MSCs) (5,9-15).
Consensus on the optimal second-line therapy for SR-aGVHD has not
been achieved for decades due to the lack of randomized controlled
trials (RCTs) for second-line treatments.
Sirolimus (rapamycin) and second-generation
mammalian target of rapamycin (mTOR) inhibitors (temsirolimus and
everolimus) inhibit T-cell proliferation, promote the production of
regulatory T cells (Tregs) and suppress dendritic cell function,
thereby exerting immunosuppressive effects (16). Further, mTOR inhibitors have been
proven to maintain the graft-vs.-leukemia (GVL) effect (17). Besides their effects on Tregs,
sirolimus and its analogs display direct antitumor activity against
several malignancies, such as advanced renal cell carcinoma,
multiple myeloma, non-Hodgkins lymphoma and myelodysplastic
syndrome (18). Sirolimus exerts
dose-dependent immunomodulatory effects on CD8+ memory T
cells in vivo exposed to viral and bacterial pathogens, such
as acute lymphocytic choriomeningitis virus, modified vaccinia
virus Ankara and Listeria (19-22).
Therefore, in addition to its immunosuppressive properties,
sirolimus has antifungal, antiviral and antineoplastic
properties.
Sirolimus-based regimens for the prophylaxis of
aGVHD have exhibited promising results over the past decade,
including lower rates of aGVHD and treatment-related mortality in
patients undergoing allo-HSCT (23-35).
These regimens can markedly reduce the relapse rate and improve
survival in patients with lymphoma undergoing allo-HSCT with
reduced-intensity conditioning regimens (24). The use of sirolimus in the
first-line therapy of aGVHD has been previously evaluated,
demonstrating similar initial treatment efficacy as prednisone
(36). However, the data on its
potential role in SR-aGVHD are scarce. The present meta-analysis
aimed to systematically review current evidence on the use of mTOR
inhibitors for the treatment of SR-aGVHD.
Materials and methods
Inclusion/exclusion criteria
The present meta-analysis was performed on published
papers with complete data. The severity of aGVHD was graded
according to organ staging of aGVHD and overall aGVHD grading
according to Keystone Consensus 1994 criteria (5). The inclusion criteria were as
follows: i) Studies on patients with SR-aGVHD with no age,
ethnicity or sex restrictions; ii) patients diagnosed with SR-aGVHD
after allo-HSCT; iii) patients who received mTOR inhibitor-based
therapy as treatment for SR-aGVHD; and iv) studies published in
English. Reviews, case reports, animal models, cell lines, letters,
duplicate publications and conference or meeting abstracts without
available data were excluded. Each study was independently reviewed
for inclusion and exclusion criteria by two investigators.
Discrepancies were resolved by consensus.
Literature search
The literature search was conducted according to the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
guidelines (37). Relevant
publications from December 2001 to September 2024 were searched in
the PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Embase
(https://www.embase.com/) databases. The key words
were ‘mTOR inhibitor’ or ‘sirolimus’ or ‘rapamycin’ or ‘everolimus’
and ‘steroid refractory’ or ‘steroid resistant’ or ‘corticosteroid
refractory’ and ‘acute graft vs. host disease’ or ‘aGVHD’. The
search results were limited to humans and studies published in
English. The search results included the overall response rate
(ORR), complete response rate (CRR), chronic GVHD (cGVHD) incidence
and overall survival (OS) rate in patients with SR-aGVHD treated
with mTOR inhibitors.
Data extraction and quality
assessment
All studies reported response rates. The main
outcomes observed after treatment with mTOR inhibitors were ORR,
CRR, cGVHD, OS and infection-related complications. Furthermore,
the efficacy of this treatment was evaluated 1 month after
treatment. Different studies had different time points. Therefore,
the time frame for the evaluation of response rate at 1 month after
treatment was prolonged; that is, the earliest studies evaluated it
at 2 weeks, whereas the latest studies evaluated it at 6 weeks
after treatment with mTOR inhibitors. All these studies were
included in the present analysis. Incomplete data were recorded as
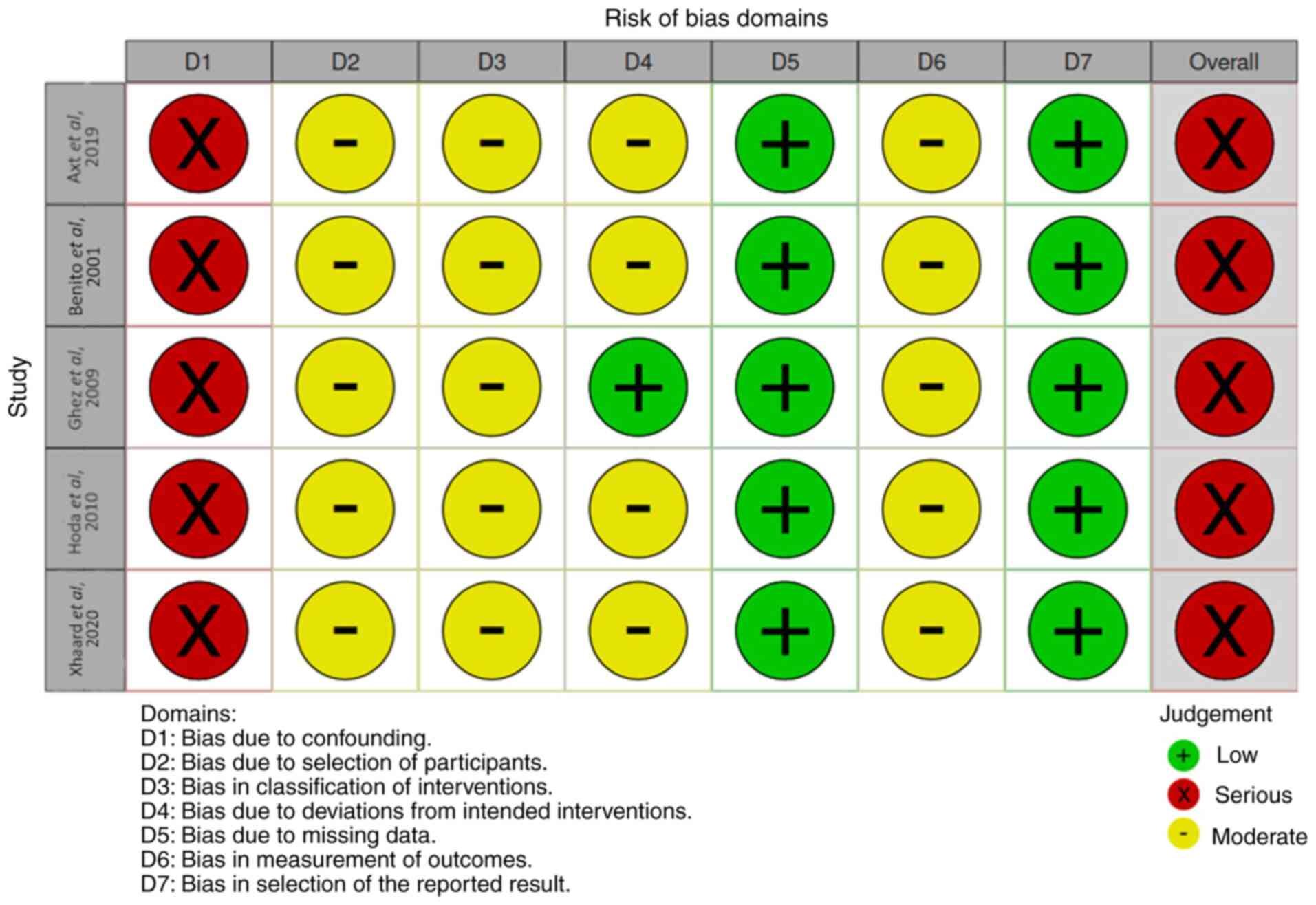
‘not available’. A formal quality assessment was conducted using
the Risk Of Bias In Non-randomized Studies of Interventions tool
(https://github.com/mcguinlu/robvis)
(Fig. 1).
Statistical analysis
All statistical calculations were conducted with the
‘meta’ package version 4.18-0 of R software (version 4.0.5; R
Development Core Team). The present study assessed statistical
heterogeneity between studies using the I2 statistic and
Cochran's Q-test. A random-effects model was used and a P-value
<0.10(38).
Results
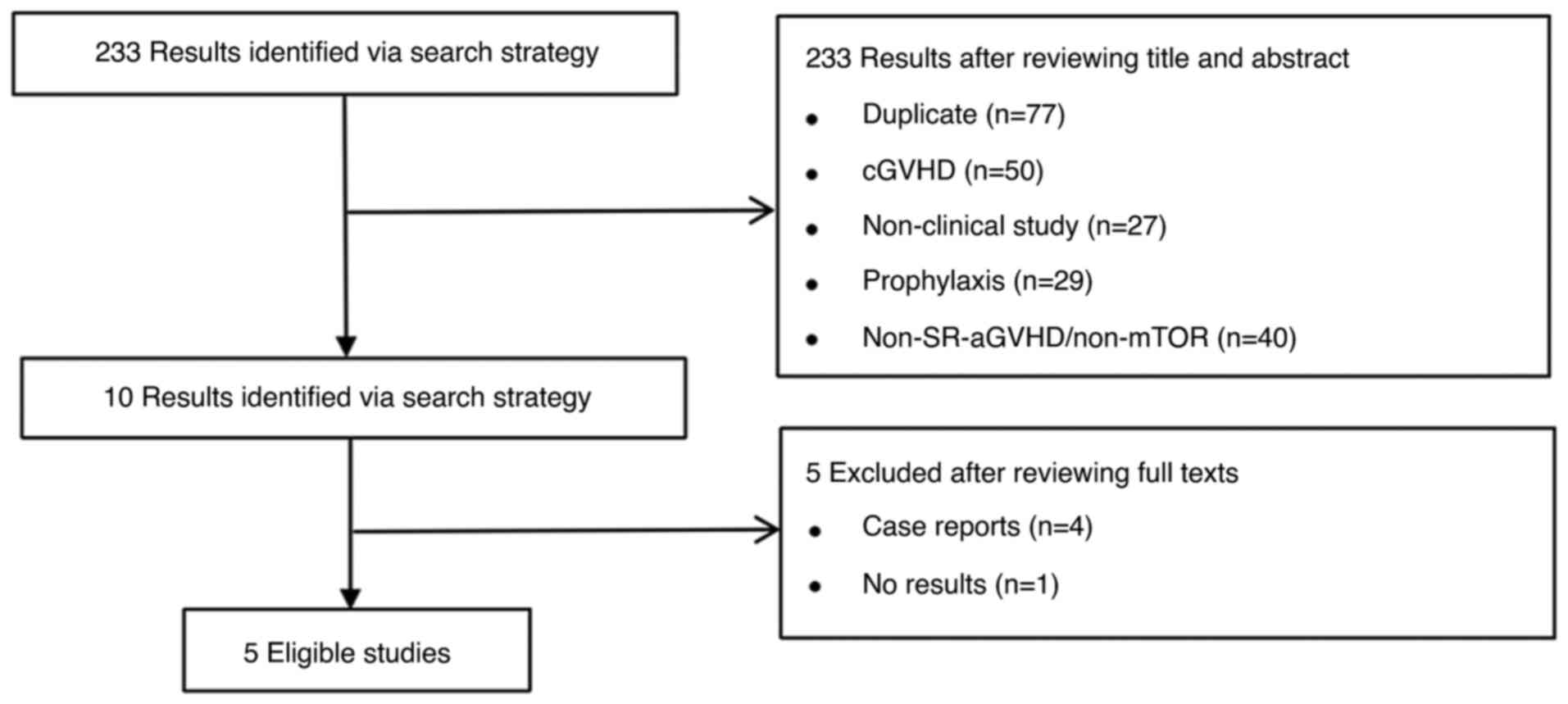
In total, 5 studies on treatment with mTOR
inhibitors (Fig. 2) for SR-aGVHD
were identified from the database search results (39-43),
which included 134 patients (Table
I). mTOR inhibitors (sirolimus, n=99; everolimus, n=35) were
only available as an oral formula; they were used as second-line
therapy for 87 patients with SR-aGVHD after initiating steroid
treatment at a dose of 2 mg/kg/day (n=53) (40,41,43)
and 1 mg/kg/day (n=34) (42).
However, 29 patients with SR-aGVHD received an mTOR inhibitor as an
alternative therapy after the failure of second-line treatment
(41,43). The second-line immunosuppressant
therapies before administering mTOR inhibitors included anti-CD25
monoclonal antibodies (daclizumab or inolimomab; n=18) and others
[anti-thymocyte globulin (ATG), n=5; anti-IL2, n=3; anti-TNF, n=2;
and ECP, n=1] (41,43). Previously introduced
immunosuppressive agents such as cyclosporine A and tacrolimus were
maintained after administering mTOR inhibitors (41-43).
 | Table IMain characteristics of five studies
on mTOR inhibitors-based treatment. |
Table I
Main characteristics of five studies
on mTOR inhibitors-based treatment.
| First author,
year | Axt et al,
2019 | Benito et
al, 2001 | Ghez et al,
2009 | Hoda et al,
2010 | Xhaard et
al, 2019 |
|---|
| (Refs.) | (39) | (40) | (41) | (42) | (43) |
| Study design | Retrospective | Prospective | Retrospective | Retrospective | Prospective |
| Number of
patients | 15 | 21 | 22 | 34 | 42 |
|
Second-line
treatment, n | 15 | 21 | 1 | 34 | 31 |
|
Third-line
treatment, n | 0 | 0 | 18 | 0 | 11 |
|
Beyond
third-line treatment, n | 0 | 0 | 3 | 0 | 0 |
| mTOR inhibitor
administration | | | | | |
|
Sirolimus,
n | 15 | 21 | 22 | 34 | 7 |
|
Everolimus,
n | 0 | 0 | 0 | 0 | 35 |
|
Loading dose
of sirolimus | NA | 15
mg/m2, 1 day | 2-4
mg/m2/day, days 1-5 | 6 mg/day (range,
3-8 days) | 4-8 mg/day, days
1-2 |
|
Loading dose
of everolimus | NA | NA | NA | NA | 1-2 mg, bid |
|
Maintenance
dose of sirolimus | NA | 4-5
mg/m2/day | 2-4 mg/day | 1-2 mg/day | NA |
|
Blood trough
levels (range), ng/ml | NA | 17.0-31.8 | 7-13 | 4-12 | 4-8 |
|
Median
treatment length (range), days | NA | NA (1-14) | 61 (27-150) | NA | NA |
|
Second-line
treatment (range), days | NA | NA (1-14) | NA | NA | 35 (5-971) |
|
Third-line
treatment (range), days | NA | NA | NA | NA | 19 (7-238) |
|
Initiation
time after aGVHD (range), days | NA | 37 (19-78) | 34 (7-177) | 80 (9-255) | 12 (4-144) |
| HLA matching,
n | | | | | |
|
MRD | NA | 2 | 8 | 12 | NA |
|
mMRD | NA | 4 | 0 | 0 | NA |
|
MUD | NA | 7 | 5 | 22 | NA |
|
mMUD | NA | 8 | 9 | 0 | NA |
| aGVHD grade, n | | | | | |
|
I | 0 | 0 | 0 | 2 | 0 |
|
II | 0 | 0 | 6 | 15 | 7 |
|
III | 15 | 10 | 12 | 8 | 24 |
|
IV | 0 | 11 | 4 | 8 | NA |
| Organ involvement
of aGVHD, n (%) | | | | | |
|
Skin | NA | 16 (76.2) | 22 (100.0) | 13 (38.2) | 12 (28.6) |
|
Gut | NA | 10 (47.6) | 7 (31.8) | 27 (79.4) | 27 (64.3) |
|
Liver | NA | 13 (61.9) | 2 (8.0) | 4 (11.8) | 5 (11.9) |
| Response, n
(%) | | | | | |
|
ORR | 8 (53.3) | 12 (57.0) | 20 (90.9) | 26 (76.0) | NA |
|
Second-line
treatment | NA | NA | 20 (90.9) | NA | 15 (48.5) |
|
Third-line
treatment | NA | NA | NA | NA | 3 (27.0) |
|
Refractory
to IL-2RAs | NA | NA | 17 (94.0) | NA | NA |
|
Refractory
to others | NA | NA | NA | NA | 3 (27.0) |
|
CRR | NA | NA | 19 (86.0) | NA | NA |
|
Second-line
treatment | NA | 5 (28.0) | NA | 15 (44.0) | 13 (42.0) |
|
Third-line
treatment | NA | NA | NA | NA | 1 (9.0) |
|
Refractory
to IL-2RAs | NA | NA | 14 (78.0) | NA | NA |
|
Refractory
to others | NA | NA | NA | NA | 1 (9.0) |
|
ORR at 1
month | NA | 12 (57.0) | NA | NA | NA |
|
CRR at 1
month | NA | 5 (23.8) | 19 (86.0) | NA | NA |
|
Median time
to CR, days (range) | NA | NA | 8 (5-15) | NA | NA |
|
Second-line
treatment, days (range) | NA | NA | NA | NA | 16.5 (10-51) |
| Outcomes | | | | | |
|
1-year
overall survival rate, % | NA | 33 | NA | 44 | 42 |
|
3-year
overall survival rate, % | NA | NA | 41 | NA | 32 |
|
Median
follow-up, months | NA | 8.7 | 6.8 | 7.0 | 12.0 |
| Adverse events, n
(%) | | | | | |
|
Cytopenias | NA | 5 (23.8) | 14 (65.0) | NA | NA |
|
Interruption
due to cytopenias | NA | 2 (9.5) | 0 (0.0) | NA | 3 (10.0) |
|
TA-TMA | NA | 10 (47.6) | 10 (45.0) | 7 (21.0) | NA |
|
Interruption
due to TA-TMA | NA | 2 (9.5) | 5 (22.5) | NA | 2 (7.0) |
|
Hyperlipidemia | NA | 14 (66.7) | 4 (18.0) | 15 (44.1) | NA |
|
Infection, n
(%) | | | | | |
|
Bacteria | NA | 8 (38.1) | 13 (60.0) | NA | 28 (66.7) |
|
Viruses | NA | 3 (14.3) | NA | NA | 24 (57.1) |
|
Cytomegalovirus
reactivation | NA | NA | 9 (40.9) | NA | 14 (33.3) |
|
Fungi | NA | 3 (14.3) | 5 (22.7) | NA | 11 (26.2) |
| cGVHD incidence,
% | NA | 90.0 | 52.9 | 44.0 | NA |
Administration of mTOR inhibitors
The present study data reported heterogeneity in the
doses of mTOR inhibitors used by patients. A loading dose of
sirolimus was administered in 4 studies (40-43).
The loading dose was associated with weight and the combination of
antifungal agents, such as voriconazole, itraconazole and
posaconazole, which was followed by a maintenance dose adjusted to
serum trough levels in the therapeutic range. mTOR inhibitors were
indefinitely continued at the discretion of the treating physician
(41-43)
rather than for a fixed duration, as reported in the study by
Benito et al (40). mTOR
inhibitors were discontinued due to their adverse effects,
unresponsiveness or progression of aGVHD (40).
Patients were divided into the following four groups
based on the blood trough levels (target concentration) and the
median duration of treatment with mTOR inhibitors: i) High target
concentration (17-31.8 ng/ml) during a short course (14 days;
range, 1-14 days) in the study by Benito et al (40); ii) appropriate target concentration
(7-13 ng/ml) during the long course (61 days; range, 27-150 days)
in the study by Ghez et al (41); iii) a wide range of target
concentration (4-12 ng/ml) in the study by Hoda et al
(42); and iv) and low target
concentration (4-8 ng/ml) in 35 days with a wide range of 5-971
days in second-line therapy and 19 days (range, 7-238) in
third-line therapy (43) (Table I).
Response after treatment with mTOR
inhibitors
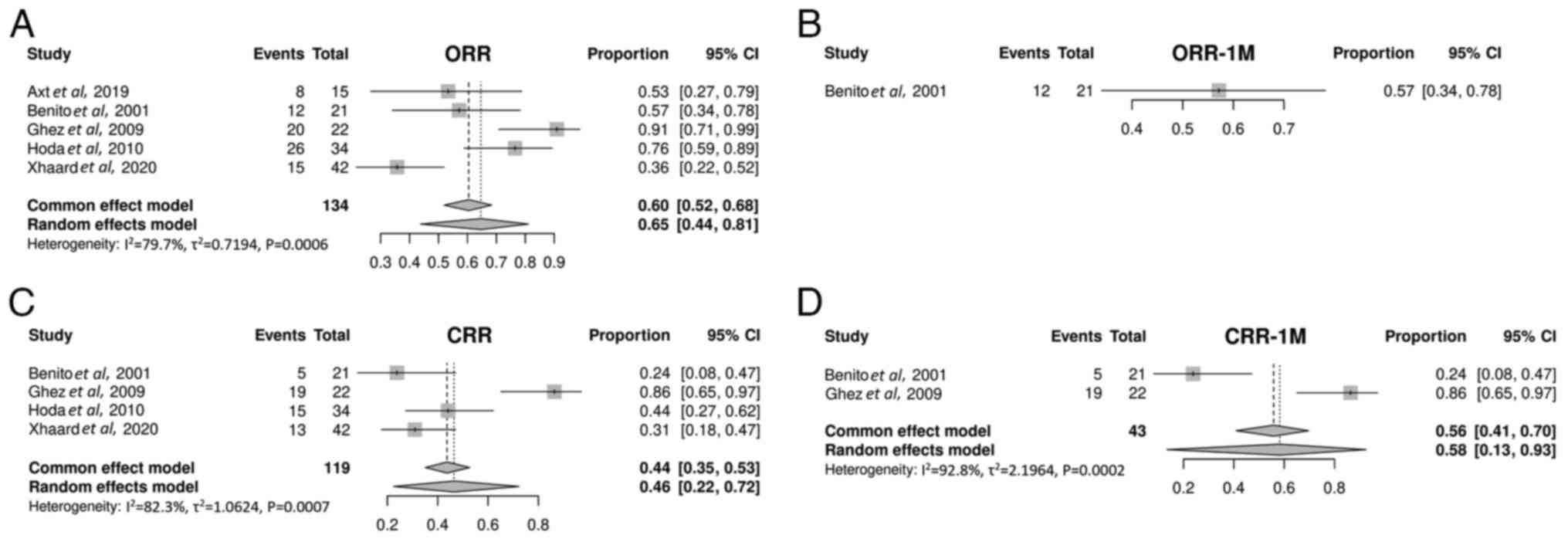
The ORR was 65% (95% CI, 44-81%; Fig. 3A) in patients with SR-aGVHD treated
with mTOR inhibitors. Only 1 study was included in the ORR analysis
at 1 month after treatment (40).
The ORR at 1 month was 57% (95% CI, 34-78%; Fig. 3B). A single study was excluded from
the CRR analysis after treatment, due to insufficient data
(39). The CRR was 46% (95% CI,
22-72%; Fig. 3C). A total of 2
studies were included in the CRR analysis at 1 month after
treatment (40,41). The CRR at 1 month was 58% (95% CI,
13-93%; Fig. 3D).
Overall, 2 studies in the meta-analysis included 29
patients using mTOR inhibitors as a third-line treatment for
SR-aGVHD (41,43). Ghez et al (41) examined the optimal response and
reported a 78% CRR within 8 days (range, 5-15 days), which was
markedly higher compared with the 9% reported in the study by
Xhaard et al (43). It was
also higher compared with that for mTOR inhibitors used as
second-line therapy (27% of CRR within 16.5 days; range, 10-51
days) in the study reported by Xhaard et al (43). Response was observed within at
least 9 days in the study by Benito et al (40). In the study by Hoda et al
(42), the median organ-specific
time-to-best responses were as follows: i) Skin within 4 weeks
(range, 1-8 weeks); ii) gastrointestinal organs within 4.5 weeks
(range, 1-6 weeks); and iii) liver within 3 weeks (range, 2-4
weeks).
Impact factors for response to mTOR
inhibitors
The highest ORR (94%) and CRR (78%) were observed in
refractory patients with SR-aGVHD receiving mTOR inhibitors as
third-line treatment combined with IL-2RAs (41). The response rate was markedly
higher compared with that of the other second-line treatments,
including ATG, anti-IL2, anti-TNF and ECP (27% of ORR and 9% of
CRR) (43). The response was also
higher compared with that for mTOR inhibitors alone (15-76% ORR and
28-44% CRR) as the second-line treatment against SR-aGVHD (9,40,42,43).
No notable difference in CRR (42 vs. 44%) was
observed between patients with SR-aGVHD treated with mTOR
inhibitors as second-line salvage therapy refractory to 2 mg/kg of
glucocorticoids (43) and those
with treatment failure using 1 mg/kg of steroids (42). CRR did not vary markedly according
to the organ involvement and overall aGVHD grade at the time of
salvage with mTOR inhibitors (42,43).
Regarding the initiation time of mTOR inhibitors after aGVHD, the
CRR was similar (44 vs. 42%) between the prolonged (42) and timely initiation (43) [80 days (range, 9-255) vs. 12 days
(range, 4-144 days)]. The CRR was markedly different (28 vs. 86%)
even for a similar initiation time [37 days (range, 19-78 days) vs.
34 days (range, 7-177 days)] (40,41)
(Table I), suggesting no impact of
initiation time on the complete response to mTOR inhibitors.
Pharmacokinetic influence on response
to mTOR inhibitors
The best response, including the highest CRR of 78%
and the shortest time to CR within 8 days (range, 5-15 days), was
observed in the study by Ghez et al (41), indicating the possible synergistic
effect of anti-CD25 antibodies and rapamycin at an appropriate
target concentration (7-13 ng/ml). The worst response of mTOR
inhibitors in the study by Xhaard et al (43) might be attributed to lower serum
trough levels (target concentration, 4-8 ng/ml). The CRR (42%) in
the low-dose (target concentration, 4-8 ng/ml) group (43) was similar to that in the 4-12 ng/ml
blood trough level group (44%) after administering mTOR inhibitors
as second-line therapy (42).
Definitive results of other studies also confirmed that the
therapeutic levels were achieved above the target concentration of
4 ng/ml (42,43). Considering the worst response at
the lower serum trough levels (target concentration, 4-8 ng/ml)
(43), 7-13 ng/ml should be
recommended as an appropriate serum trough level in the future.
Dose escalation was usually permitted based on drug toxicity and
response, with 21 patients achieving high blood trough levels
(target concentration, 17-31.8 ng/ml). However, in the study by
Benito et al (40),
rapamycin had to be discontinued early due to its inefficacy and
toxicity; this reflected that higher-dose mTOR inhibitors did not
enhance the curative effect but had more toxicity. The CR to mTOR
inhibitors (41-43)
was markedly improved in the long-course group (no fixed duration)
compared with that in the short-course group (14 days) (40). The time needed to achieve CR after
administering mTOR inhibitors was at least 1-2 weeks, with an
extensive range and a maximum time of 8 weeks (40-42).
Therefore, prolonged therapy was more likely to be effective. The
dosing should be considered while analyzing the response and
toxicity in the future.
Other impact factors for response to
mTOR inhibitors
The present study demonstrated that all baseline
characteristics including organ damage, grading of mTOR inhibitor
rescue treatment, time to start mTOR inhibitors after steroid
treatment or initial dose of glucocorticoids, did not influence the
achievement of CR after mTOR inhibitor treatment, except for the
prior and combined therapy, serum trough level and treatment course
(Fig. 3). This was similar to the
report from a single center (42).
mTOR inhibitors (oral only) displayed notable intestinal absorption
even in patients with gastrointestinal aGVHD (40-42).
Adverse events after treatment with
mTOR inhibitors. Transplant-associated thrombotic microangiopathy
(TA-TMA)
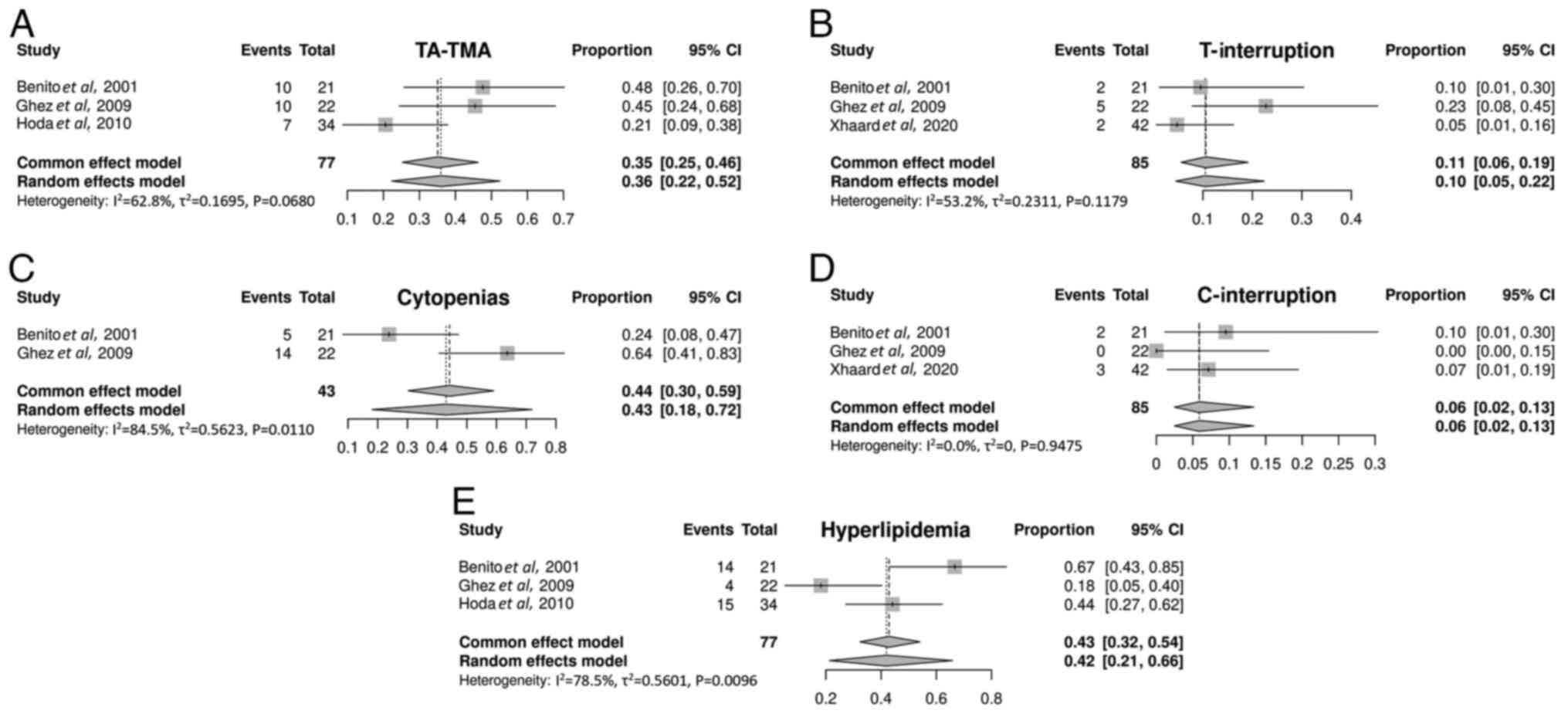
TA-TMA occurred in 36% (95% CI, 22-52%) of patients
with SR-aGVHD. The incidence of TA-TMA varied, which was likely due
to the lack of a consensus definition for TA-TMA (44). Treatment with mTOR inhibitors was
interrupted in 10% (95% CI, 5-22%) of the patients due to the
persistence of TA-TMA. Furthermore, the median time to TA-TMA onset
after rapamycin use was 24 days, with a range of 12-60 days. TA-TMA
also occurred in certain patients at the start of rapamycin
treatment (41). Therapy was
successfully managed by reducing the dose or discontinuing
cyclosporine A or tacrolimus. TA-TMA was resolved to a certain
extent after interrupting treatment with mTOR inhibitors (Fig. 4A and B) (41-43).
Myelosuppression. The myelotoxic effect of
rapamycin was observed in 43% (95% CI, 18-72%) of patients. Also,
6% (95% CI, 2-13%) of patients discontinued mTOR inhibitor
treatment due to cytopenia. The median decrease in absolute
neutrophil count was 57% and the median decrease in platelet count
was 61% within 30 days of sirolimus treatment initiation (42). The blood cell counts were restored
upon discontinuing treatment with mTOR inhibitors (Fig. 4C and D) (40).
Hyperlipidemia. Hyperlipidemia attributable
to rapamycin was also a frequent complication (40-42),
observed in 42% of patients (95% CI, 21-66%). No treatment
interruption was required (Fig.
4E).
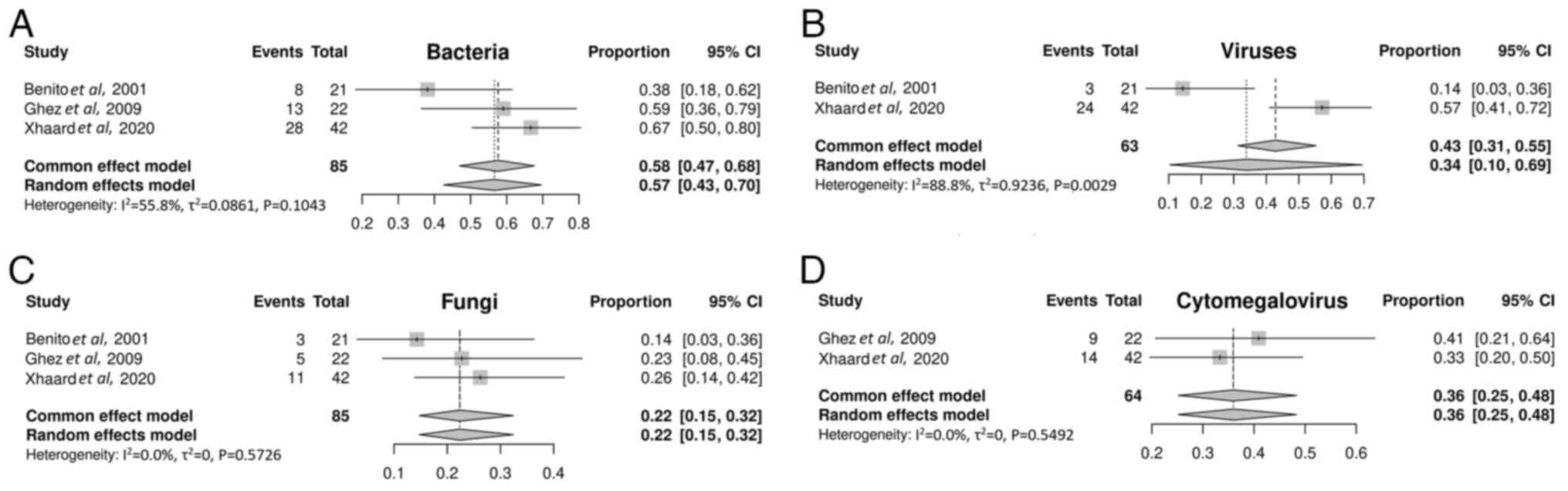
Infection. A total of 3 studies (40,41,43)
included in the meta-analysis investigated the incidence of
infections after mTOR inhibitor treatment. The incidence of
bacterial, viral and fungal infections was 57% (95% CI, 43-70%;
Fig. 5A), 34% (95% CI, 10-69%;
Fig. 5B) and 22% (95% CI, 15-32%;
Fig. 5C), respectively. The
cytomegalovirus (CMV) reactivation rate was 36% (95% CI, 25-48%;
Fig. 5D).
cGVHD and survival
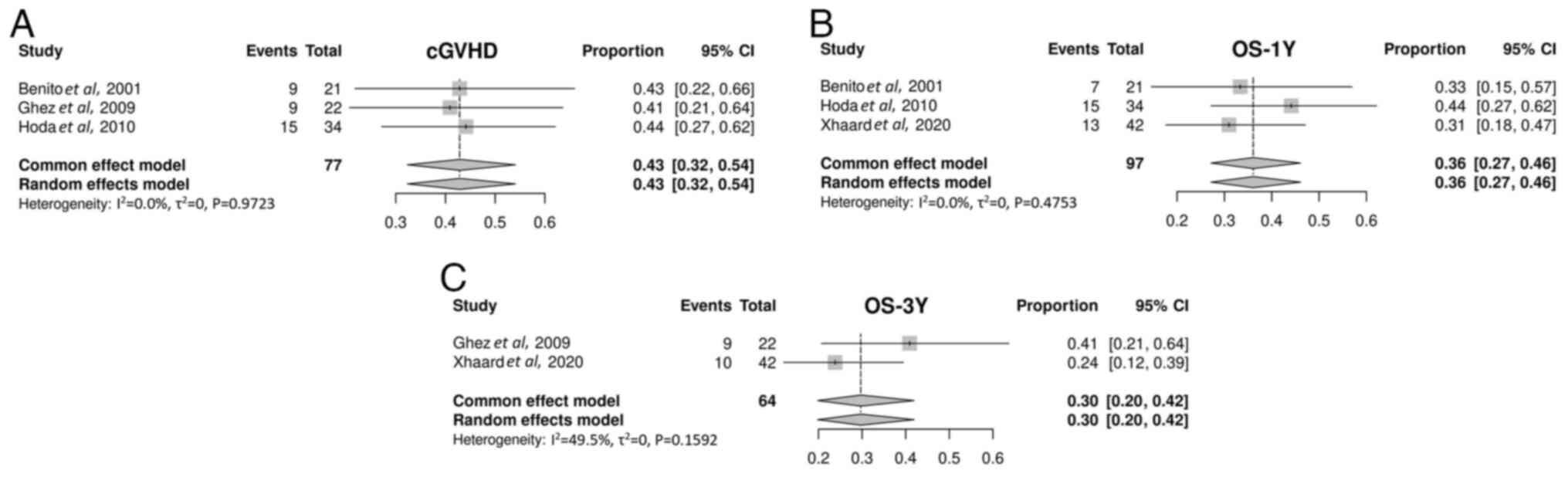
A total of 3 studies on mTOR inhibitors were
included to explore cGVHD (40-42).
The cGVHD incidence after treatment with mTOR inhibitors was 88%
(95% CI, 32-99%; Fig. 6A).
Overall, 4 studies on mTOR inhibitors were enrolled to examine
survival (40-43).
A single study (39) was excluded
as it did not report OS data. Of these, 3 studies on mTOR
inhibitors were included in the 1-year OS analysis (40,42,43).
The 1-year OS rate for treatment with mTOR inhibitors was 36% (95%
CI, 27-46%; Fig. 6B). Only 2
studies on mTOR inhibitors were included in the 3-year OS analysis
(41,43). The 3-year OS rate for mTOR
inhibitors was 29% (95% CI, 19-41; Fig. 6C).
Discussion
The present systematic review summarized the current
literature, including 5 studies on the use of mTOR inhibitors for
the treatment of patients with SR-aGVHD. Furthermore, the present
study was novel in demonstrating the feasibility of using mTOR
inhibitors for SR-aGVHD therapy. The present study data evaluated
134 patients and the outcomes revealed that mTOR inhibitors used as
salvage therapy exerted notable activity against SR-aGVHD, with a
marked complete remission rate of 46%, a partial remission rate of
18% and 1- to 3-year OS from 36 to 29% in patients with SR-aGVHD.
mTOR inhibitors induced markedly higher CRR compared with the CD52
antibody alemtuzumab (27.8%; 5/18) (45), tocilizumab (an IL-6 receptor
antagonist; 33.3%; 2/6) (46),
α1-antitrypsin (35%; 14/40) (47),
etanercept (a recombinant human soluble TNF-α receptor fusion
protein; 30.7%; 4/13) (48) and
begelomab (anti-CD26 monoclonal antibody; 10.7%; 3/28) (49), compared with the preliminary
results from a small series of patients with SR-aGVHD. The findings
on mTOR inhibitor treatment were compared with the present
meta-analysis results evaluating other treatments in patients with
SR-aGVHD, achieving a lower CRR with ruxolitinib (56%) (11) and basiliximab (55%), which was
identified as the most effective among the IL-2RAs for SR-aGVHD
(13). mTOR inhibitors were not
notably enhanced compared with ECP (50) and MSCs (50), with pooled CRRs for SR-aGVHD of
69.0 and 73.1% in the meta-analysis, respectively. Non-conventional
treatments such as ECP and MSCs had the potential for SR-aGVHD
therapy but were less feasible due to higher technical requirements
and costs.
The highest CRR (78%) was achieved in patients
receiving mTOR inhibitors as salvage therapy for SR-aGVHD and
undergoing allo-HSCT. This was probably due to both the potential
synergistic effect of mTOR inhibitors plus IL-2RAs (16) and an appropriate rapamycin dose
(serum trough levels, 7-13 ng/ml). Due to the limited sample size,
the present study conclusions should be drawn with caution although
the outcome was promising. In the future, a prospective RCT can be
designed for verification and in vitro experiments can be
conducted to explore the possible synergistic effects of the two
drugs used in combination. However, mTOR inhibitor treatment was
interrupted with a high incidence of TA-TMA (22.5%) upon
cyclosporine A prophylaxis due to the cumulative toxicity of mTOR
inhibitors and calcineurin inhibitors (41). Although sirolimus also exhibited
synergistic effects with tacrolimus, the lower serum trough levels
(4-8 ng/ml) were associated with suboptimal efficacy (42% of CRR)
(35). However, this regimen
demonstrated a lower rate of mTOR inhibitor discontinuation (7%)
(43).
The preliminary results of the study by Benito et
al (40) indicated that
sirolimus was extremely toxic at high serum trough levels (17-31.8
ng/ml), leading to frequent TA-TMA (47.6%) and cytopenia (23.8%).
Besides the appropriate dose, the present study data emphasized the
importance of prolonged treatment with mTOR inhibitors, as the
T-cell response was also associated with the treatment duration
(19). Switching CNI to MMF should
be managed first for the long-term response of patients to
rapamycin in the future (41). A
total of 6% of the patients with SR-aGVHD after treatment with mTOR
inhibitors developed severe cytopenia leading to treatment
interruption (Fig. 4D). The
hematological toxicity might limit the use of the combination with
ruxolitinib and methotrexate.
The non-relapse mortality of SR-aGVHD is high
(60-85%) (17), with an estimated
2-year survival rate of 17% (51)
and a 4-year survival rate of 15% (52). Compared with the aforementioned
findings, mTOR inhibitor salvage therapy depended on notably
increased survival in SR-aGVHD, with 1- to 3-year OS ranging from
36 to 29%. Infection-related mortality (46%) was high in patients
with SR-aGVHD (52). The incidence
of bacterial infections (56%) and CMV reactivation (35%) after mTOR
inhibitor-based therapy for SR-aGVHD was significantly lower
compared with that after anti-cytokine treatment for SR-aGVHD (74
and 65%, respectively) (52). This
might be because rapamycin enhanced the pathogen-specific
CD8+ T-cell response (21) and improved CMV-specific T-cell
function (53) and the outcome of
CMV complications (54).
Ruxolitinib offers a novel immunotherapeutic option
for TA-TMA complicated by GVHD but is associated with cytopenia and
CMV reactivation (55.6 and 33.3%, respectively) (55). Although ruxolitinib can also
preserve GVL/graft-vs.-tumor (GVT) function (56), it is less favorable compared with
sirolimus, which has antifungal, antiviral and antineoplastic
properties. The incidence of infectious events can reach up to 73%
using other immunosuppressive agents (particularly MMF), especially
in the context of impaired GVL/GVT function, despite no increase in
the incidence of TA-TMA or cytopenia (57).
In summary, the present study results confirmed that
mTOR inhibitors displayed potent activity against SR-aGVHD and may
be used alone or in combination with other targeted therapies (such
as anti-CD25 monoclonal antibodies and ATG), despite biases in
non-controlled studies, heterogeneity in the quality of studies and
variability among patients receiving immunosuppressive agents.
However, the generalizability of the present meta-analysis was
limited by multiple factors, primarily heterogeneity stemming from
variations in study designs, conduct and analysis processes,
thereby compromising the accuracy of the present study findings.
Furthermore, several biases might have been introduced, including
disparities in medical resources across different time periods or
regions. Furthermore, most studies included in the meta-analysis
were retrospective with relatively small sample sizes. Therefore,
the efficacy and safety of mTOR inhibitors in patients with
SR-aGVHD require validation in further prospective RCTs comparing
these inhibitors with other treatments. Therapeutic strategies of
combined treatment regimens with a balance between efficacy and
side effects are key to maximizing therapeutic efficacy while
minimizing drug side effects. Future studies should also focus on
the impact of rapamycin and other immunosuppressive agents on
T-cell response.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Shanxi Province (grant no. 202103021224001) and the
Science and Technology Program Project of Taiyuan City (grant no.
202239).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YG and ZL confirm the authenticity of all the raw
data. YG curated the data, provided project administration,
validated the data and wrote the original draft. ZL devised the
methodology, provided project administration and wrote the original
draft. YiqZ searched the literature data and assisted with data
analysis. ZP, JS, YinZ and SC contributed to data collection and
analysis. ZG curated the data, conducted the investigation,
reviewed the manuscript and acquired funding for the present study.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anasetti C, Logan BR, Lee SJ, Waller EK,
Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A,
Couban S, et al: Peripheral-blood stem cells versus bone marrow
from unrelated donors. N Engl J Med. 367:1487–1496. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Al-Kadhimi Z, Gul Z, Chen W, Smith D,
Abidi M, Deol A, Ayash L, Lum L, Waller EK, Ratanatharathorn V and
Uberti J: High incidence of severe acute graft-versus-host disease
with tacrolimus and mycophenolate mofetil in a large cohort of
related and unrelated allogeneic transplantation patients. Biol
Blood Marrow Transplant. 20:979–985. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Justiz Vaillant AA, Modi P and Mohammadi
O: Graft-versus-host disease. In: StatPearls. StatPearls
Publishing, Treasure Island, FL, 2025.
|
|
4
|
Levine JE, Logan B, Wu J, Alousi AM, Ho V,
Bolaños-Meade J and Weisdorf D: Blood and Marrow Transplant
Clinical Trials Network. Graft-versus-host disease treatment:
Predictors of survival. Biol Blood Marrow Transplant. 16:1693–1699.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Martin PJ, Rizzo JD, Wingard JR, Ballen K,
Curtin PT, Cutler C, Litzow MR, Nieto Y, Savani BN, Schriber JR, et
al: First- and second-line systemic treatment of acute
graft-versus-host disease: Recommendations of the American society
of blood and marrow transplantation. Biol Blood Marrow Transplant.
18:1150–1163. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Westin JR, Saliba RM, De Lima M, Alousi A,
Hosing C, Qazilbash MH, Khouri IF, Shpall EJ, Anderlini P, Rondon
G, et al: Steroid-refractory acute GVHD: Predictors and outcomes.
Adv Hematol. 2011(601953)2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Malard F, Huang XJ and Sim JPY: Treatment
and unmet needs in steroid-refractory acute graft-versus-host
disease. Leukemia. 34:1229–1240. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zeiser R and Blazar BR: Acute
graft-versus-host disease-biologic process, prevention, and
therapy. N Engl J Med. 377:2167–2179. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mohty M, Holler E, Jagasia M, Jenq R,
Malard F, Martin P, Socié G and Zeiser R: Refractory acute
graft-versus-host disease: A new working definition beyond
corticosteroid refractoriness. Blood. 136:1903–1906.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wölfl M, Qayed M, Benitez Carabante MI,
Sykora T, Bonig H, Lawitschka A and Diaz-de-Heredia C: Current
prophylaxis and treatment approaches for acute graft-versus-host
disease in haematopoietic stem cell transplantation for children
with acute lymphoblastic leukaemia. Front Pediatr.
9(784377)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Baccelli F, Gottardi F, Muratore E,
Leardini D, Grasso AG, Gori D, Belotti T, Prete A and Masetti R:
Ruxolitinib for the treatment of acute and chronic
graft-versus-host disease in children: A systematic review and
individual patient data meta-analysis. Bone Marrow Transplant.
59:765–776. 2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Inagaki J, Kodama Y, Fukano R, Noguchi M
and Okamura J: Mycophenolate mofetil for treatment of
steroid-refractory acute graft-versus-host disease after pediatric
hematopoietic stem cell transplantation. Pediatr Transplant.
19:652–658. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shen MZ, Li JX, Zhang XH, Xu LP, Wang Y,
Liu KY, Huang XJ, Hong SD and Mo XD: Meta-analysis of interleukin-2
receptor antagonists as the treatment for steroid-refractory acute
graft-versus-host disease. Front Immunol. 12(749266)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Greinix HT, Ayuk F and Zeiser R:
Extracorporeal photopheresis in acute and chronic
steroid-refractory graft-versus-host disease: An evolving treatment
landscape. Leukemia. 36:2558–2566. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moiseev IS, Morozova EV, Bykova TA, Paina
OV, Smirnova AG, Dotsenko AA, Borzenkova ES, Galimov AN,
Gudognikova YV, Ekushov KA, et al: Long-term outcomes of
ruxolitinib therapy in steroid-refractory graft-versus-host disease
in children and adults. Bone Marrow Transplant. 55:1379–1387.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ferrer IR, Araki K and Ford ML:
Paradoxical aspects of rapamycin immunobiology in transplantation.
Am J Transplant. 11:654–659. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Olivieri A and Mancini G: Current
approaches for the prevention and treatment of acute and chronic
GVHD. Cells. 13(1524)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Teachey DT, Grupp SA and Brown VI:
Mammalian target of rapamycin inhibitors and their potential role
in therapy in leukaemia and other haematological malignancies. Br J
Haematol. 145:569–580. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Araki K, Turner AP, Shaffer VO, Gangappa
S, Keller SA, Bachmann MF, Larsen CP and Ahmed R: mTOR regulates
memory CD8 T-cell differentiation. Nature. 460:108–112.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pearce EL, Walsh MC, Cejas PJ, Harms GM,
Shen H, Wang LS, Jones RG and Choi Y: Enhancing CD8 T-cell memory
by modulating fatty acid metabolism. Nature. 460:103–107.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ferrer IR, Wagener ME, Robertson JM,
Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP and Ford ML:
Cutting edge: Rapamycin augments pathogen-specific but not
graft-reactive CD8+ T cell responses. J Immunol. 185:2004–2008.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dufour M, Dormond-Meuwly A, Demartines N
and Dormond O: Targeting the mammalian target of rapamycin (mTOR)
in cancer therapy: Lessons from past and future perspectives.
Cancers (Basel). 3:2478–2500. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pidala J, Kim J, Jim H, Kharfan-Dabaja MA,
Nishihori T, Fernandez HF, Tomblyn M, Perez L, Perkins J, Xu M, et
al: A randomized phase II study to evaluate tacrolimus in
combination with sirolimus or methotrexate after allogeneic
hematopoietic cell transplantation. Haematologica. 97:1882–1889.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Armand P, Kim HT, Sainvil MM, Lange PB,
Giardino AA, Bachanova V, Devine SM, Waller EK, Jagirdar N, Herrera
AF, et al: The addition of sirolimus to the graft-versus-host
disease prophylaxis regimen in reduced intensity allogeneic stem
cell transplantation for lymphoma: A multicentre randomized trial.
Br J Haematol. 173:96–104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Al Malki MM, Gendzekhadze K, Yang D,
Mokhtari S, Parker P, Karanes C, Palmer J, Snyder D, Forman SJ,
Nademanee A and Nakamura R: Long-term outcome of allogeneic
hematopoietic stem cell transplantation from unrelated donor using
tacrolimus/sirolimus-based GvHD prophylaxis: Impact of HLA
mismatch. Transplantation. 104:1070–1080. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ceberio I, Devlin SM, Sauter C, Barker JN,
Castro-Malaspina H, Giralt S, Ponce DM, Lechner L, Maloy MA,
Goldberg JD and Perales MA: Sirolimus, tacrolimus and low-dose
methotrexate based graft-versus-host disease prophylaxis after
non-ablative or reduced intensity conditioning in related and
unrelated donor allogeneic hematopoietic cell transplant. Leuk
Lymphoma. 56:663–670. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang L, Gu Z, Zhai R, Li D, Zhao S, Luo L,
Zhao X, Wei H, Pang Z, Wang L, et al: The efficacy and safety of
sirolimus-based graft-versus-host disease prophylaxis in patients
undergoing allogeneic hematopoietic stem cell transplantation: A
meta-analysis of randomized controlled trials. Transfusion.
55:2134–2141. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sandmaier BM, Kornblit B, Storer BE,
Olesen G, Maris MB, Langston AA, Gutman JA, Petersen SL, Chauncey
TR, Bethge WA, et al: Addition of sirolimus to standard
cyclosporine plus mycophenolate mofetil-based graft-versus-host
disease prophylaxis for patients after unrelated non-myeloablative
haemopoietic stem cell transplantation: A multicentre, randomised,
phase 3 trial. Lancet Haematol. 6:e409–e418. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Al-Kadhimi Z, Gul Z, Rodriguez R, Chen W,
Smith D, Mitchell A, Abidi M, Ayash L, Deol A, Lum L, et al:
Anti-thymocyte globulin (thymoglobulin), tacrolimus, and sirolimus
as acute graft-versus-host disease prophylaxis for unrelated
hematopoietic stem cell transplantation. Biol Blood Marrow
Transplant. 18:1734–1744. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Alyea EP, Li S, Kim HT, Cutler C, Ho V,
Soiffer RJ and Antin JH: Sirolimus, tacrolimus, and low-dose
methotrexate as graft-versus-host disease prophylaxis in related
and unrelated donor reduced-intensity conditioning allogeneic
peripheral blood stem cell transplantation. Biol Blood Marrow
Transplant. 14:920–926. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pulsipher MA, Wall DA, Grimley M, Goyal
RK, Boucher KM, Hankins P, Grupp SA and Bunin N: A phase I/II study
of the safety and efficacy of the addition of sirolimus to
tacrolimus/methotrexate graft versus host disease prophylaxis after
allogeneic haematopoietic cell transplantation in paediatric acute
lymphoblastic leukaemia (ALL). Br J Haematol. 147:691–699.
2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cutler C, Logan B, Nakamura R, Johnston L,
Choi S, Porter D, Hogan WJ, Pasquini M, MacMillan ML, Hsu JW, et
al: Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD
prophylaxis after matched, related donor allogeneic HCT. Blood.
124:1372–1377. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shayani S, Palmer J, Stiller T, Liu X,
Thomas SH, Khuu T, Parker PM, Khaled SK, Forman SJ and Nakamura R:
Thrombotic microangiopathy associated with sirolimus level after
allogeneic hematopoietic cell transplantation with
tacrolimus/sirolimus-based graft-versus-host disease prophylaxis.
Biol Blood Marrow Transplant. 19:298–304. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ziakas PD, Zervou FN, Zacharioudakis IM
and Mylonakis E: Graft-versus-host disease prophylaxis after
transplantation: A network meta-analysis. PLoS One.
9(e114735)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cutler C and Antin JH: Sirolimus for GVHD
prophylaxis in allogeneic stem cell transplantation. Bone Marrow
Transplant. 34:471–476. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pidala J, Hamadani M, Dawson P, Martens M,
Alousi AM, Jagasia M, Efebera YA, Chhabra S, Pusic I, Holtan SG, et
al: Randomized multicenter trial of sirolimus vs prednisone as
initial therapy for standard-risk acute GVHD: the BMT CTN 1501
trial. Blood. 135:97–107. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. Open Med. 3:e123–e130.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Borenstein M, Hedges LV, Higgins JP and
Rothstein HR: A basic introduction to fixed-effect and
random-effects models for meta-analysis. Res Synth Methods.
1:97–111. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Axt L, Naumann A, Toennies J, Haen SP,
Vogel W, Schneidawind D, Wirths S, Moehle R, Faul C, Kanz L, et al:
Retrospective single center analysis of outcome, risk factors and
therapy in steroid refractory graft-versus-host disease after
allogeneic hematopoietic cell transplantation. Bone Marrow
Transplant. 54:1805–1814. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Benito AI, Furlong T, Martin PJ, Anasetti
C, Appelbaum FR, Doney K, Nash RA, Papayannopoulou T, Storb R,
Sullivan KM, et al: Sirolimus (rapamycin) for the treatment of
steroid-refractory acute graft-versus-host disease.
Transplantation. 72:1924–1929. 2001.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ghez D, Rubio MT, Maillard N, Suarez F,
Chandesris MO, Delarue R, Deau-Fischer B, Varet B, Hermine O and
Buzyn A: Rapamycin for refractory acute graft-versus-host disease.
Transplantation. 88:1081–1087. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hoda D, Pidala J, Salgado-Vila N, Kim J,
Perkins J, Bookout R, Field T, Perez L, Ayala E, Ochoa-Bayona JL,
et al: Sirolimus for treatment of steroid-refractory acute
graft-versus-host disease. Bone Marrow Transplant. 45:1347–1351.
2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xhaard A, Launay M, Sicre de Fontbrune F,
Michonneau D, Sutra Del Galy A, Coman T, Pagliuca S, Dhedin N,
Robin M, Peffault bde Latour R and Socie G: A monocentric study of
steroid-refractory acute graft-versus-host disease treatment with
tacrolimus and mTOR inhibitor. Bone Marrow Transplant. 55:86–92.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Young JA, Pallas CR and Knovich MA:
Transplant-associated thrombotic microangiopathy: Theoretical
considerations and a practical approach to an unrefined diagnosis.
Bone Marrow Transplant. 56:1805–1817. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Schub N, Günther A, Schrauder A, Claviez
A, Ehlert C, Gramatzki M and Repp R: Therapy of steroid-refractory
acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow
Transplant. 46:143–147. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Drobyski WR, Pasquini M, Kovatovic K,
Palmer J, Douglas Rizzo J, Saad A, Saber W and Hari P: Tocilizumab
for the treatment of steroid refractory graft-versus-host disease.
Biol Blood Marrow Transplant. 17:1862–1868. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Magenau JM, Goldstein SC, Peltier D,
Soiffer RJ, Braun T, Pawarode A, Riwes MM, Kennel M, Antin JH,
Cutler CS, et al: α1-Antitrypsin infusion for treatment
of steroid-resistant acute graft-versus-host disease. Blood.
131:1372–1379. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Busca A, Locatelli F, Marmont F, Ceretto C
and Falda M: Recombinant human soluble tumor necrosis factor
receptor fusion protein as treatment for steroid refractory
graft-versus-host disease following allogeneic hematopoietic stem
cell transplantation. Am J Hematol. 82:45–52. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bacigalupo A, Angelucci E, Raiola AM,
Varaldo R, Di Grazia C, Gualandi F, Benedetti E, Risitano A, Musso
M, Zallio F, et al: Treatment of steroid resistant acute graft
versus host disease with an anti-CD26 monoclonal
antibody-Begelomab. Bone Marrow Transplant. 55:1580–1587.
2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Abu-Dalle I, Reljic T, Nishihori T, Antar
A, Bazarbachi A, Djulbegovic B, Kumar A and Kharfan-Dabaja MA:
Extracorporeal photopheresis in steroid-refractory acute or chronic
graft-versus-host disease: Results of a systematic review of
prospective studies. Biol Blood Marrow Transplant. 20:1677–1686.
2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Han LJ, Wang Y, Fan ZP, Huang F, Zhou J,
Fu YW, Qu H, Xuan L, Xu N, Ye JY, et al: Haploidentical
transplantation compared with matched sibling and unrelated donor
transplantation for adults with standard-risk acute lymphoblastic
leukaemia in first complete remission. Br J Haematol. 179:120–130.
2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
García-Cadenas I, Rivera I, Martino R,
Esquirol A, Barba P, Novelli S, Orti G, Briones J, Brunet S,
Valcarcel D and Sierra J: Patterns of infection and
infection-related mortality in patients with steroid-refractory
acute graft versus host disease. Bone Marrow Transplant.
52:107–113. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bak S, Tischer S, Dragon A, Ravens S, Pape
L, Koenecke C, Oelke M, Blasczyk R, Maecker-Kolhoff B and
Eiz-Vesper B: Selective effects of mTOR inhibitor sirolimus on
Naïve and CMV-specific T cells extending its applicable range
beyond immunosuppression. Front Immunol. 9(2953)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Marty FM, Bryar J, Browne SK, Schwarzberg
T, Ho VT, Bassett IV, Koreth J, Alyea EP, Soiffer RJ, Cutler CS, et
al: Sirolimus-based graft-versus-host disease prophylaxis protects
against cytomegalovirus reactivation after allogeneic hematopoietic
stem cell transplantation: A cohort analysis. Blood. 110:490–500.
2007.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yang W, Zhu G, Qin M, Li Z, Wang B, Yang J
and Wang T: The effectiveness of ruxolitinib for acute/chronic
graft-versus-host disease in children: A retrospective study. Drug
Des Devel Ther. 15:743–752. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Choi J, Cooper ML, Alahmari B, Ritchey J,
Collins L, Holt M and DiPersio JF: Pharmacologic blockade of
JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia
effect. PLoS One. 9(e109799)2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Onishi C, Ohashi K, Sawada T, Nakano M,
Kobayashi T, Yamashita T, Akiyama H and Sakamaki H: A high risk of
life-threatening infectious complications in mycophenolate mofetil
treatment for acute or chronic graft-versus-host disease. Int J
Hematol. 91:464–470. 2010.PubMed/NCBI View Article : Google Scholar
|