Introduction
The gastrointestinal epithelium serves as a barrier
between the external environment and the internal milieu of the
body. This barrier is crucial for preventing the translocation of
bacteria and toxins into systemic circulation (1). Intestinal permeability occurs via two
main pathways: Paracellular and transcellular. The transcellular
pathway involves the passage of molecules through the epithelial
cells, via processes such as endocytosis, vesicular transport, and
exocytosis, and is regulated by membrane transporters and channels.
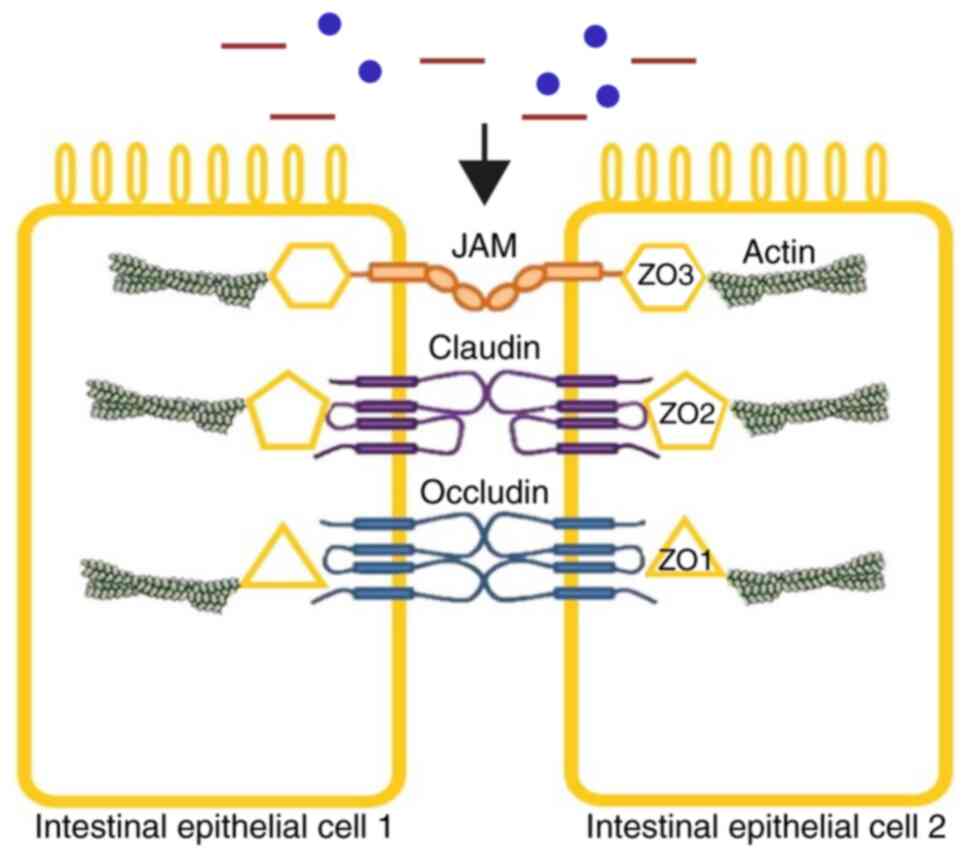
Paracellular permeability is governed by tight junction (TJ)
proteins; TJs consist of several complex membrane proteins,
including claudins, occludin and zonula occludens (ZO) (Fig. 1). Epithelial TJs can be dynamically
modulated by various signals, including humoral and neural factors
that engage multiple cellular pathways, and alterations in the
expression of these proteins are implicated in various diseases
such as inflammatory bowel and celiac disease, and irritable bowel
syndrome (2,3).
Intestinal barrier function is influenced by
numerous factors such as diet, stress, microbiota and drugs.
Increased intestinal permeability has been associated with
gastrointestinal disorders, including celiac disease, inflammatory
bowel disease, irritable bowel syndrome and food allergies, as well
as systemic conditions such as schizophrenia, multiple sclerosis,
diabetes mellitus and sepsis (4).
The mucosal barrier is essential for human health, and several
strategies have been developed to strengthen this barrier. One such
strategy is to maintain intestinal integrity through enteral
nutrition, which refers to the delivery of nutrients directly into
the gastrointestinal tract via oral intake or feeding tubes
(nasogastric or gastrostomy tubes). Additional approaches include
the use of probiotics and prebiotics to enhance TJ stability and
support beneficial microbiota composition, as well as dietary
fibers and short-chain fatty acids (5). Enteral nutrition preserves mucosal
structure by providing luminal nutrients that stimulate epithelial
cell turnover, enhance TJ protein expression, and support local
immune function. This strategy is commonly implemented in clinical
and perioperative settings to prevent intestinal atrophy and
maintain barrier integrity. Developing strategies such as this is
important as compromised intestinal integrity can lead to
endotoxemia and a proinflammatory state (6).
Bile and pancreatic secretions are important for
digestion, particularly for fatty acid absorption, and contribute
to cholesterol homeostasis. Furthermore, bile contributes to
intestinal barrier function by modulating glucose and lipid
metabolism, and may influence enterocyte proliferation and
apoptosis (7). The present study
aimed to evaluate the impact of the presence of luminal nutrients
(food) and biliopancreatic secretions on intestinal integrity to
guide clinical strategies in settings where maintaining intestinal
barrier function is critical.
Materials and methods
Animals
A total of 30 adult male Sprague-Dawley rats (8-10
weeks; weight, 240-380 g) were housed under controlled conditions
(22˚C; 50-60% humidity; 12-h light/dark cycle) and fed a standard
diet (DSA Agrifood Products Inc.) with free access to both food and
water for 10 days prior to surgery.
Animal groups and surgical design
Rats were randomly divided into three groups (n=10
each): i) Group 1 (control), laparotomy + two jejunal enterotomies
and re-anastomoses; ii) group 2, biliopancreatic diversion (BP)
with separated food and biliopancreatic secretion segments; and
iii) group 3, jejunal bypass (JP) with food and biliary-deficient
isolated jejunal segments.
Anaesthesia was induced with ketamine (40 mg/kg) and
xylazine (10 mg/kg), and the skin was prepped with 10%
povidone-iodine. Sterile conditions were maintained throughout.
Prophylactic cefazolin (60 mg/kg) and subcutaneous morphine (1
mg/kg) were administered. All laparotomies were midline.
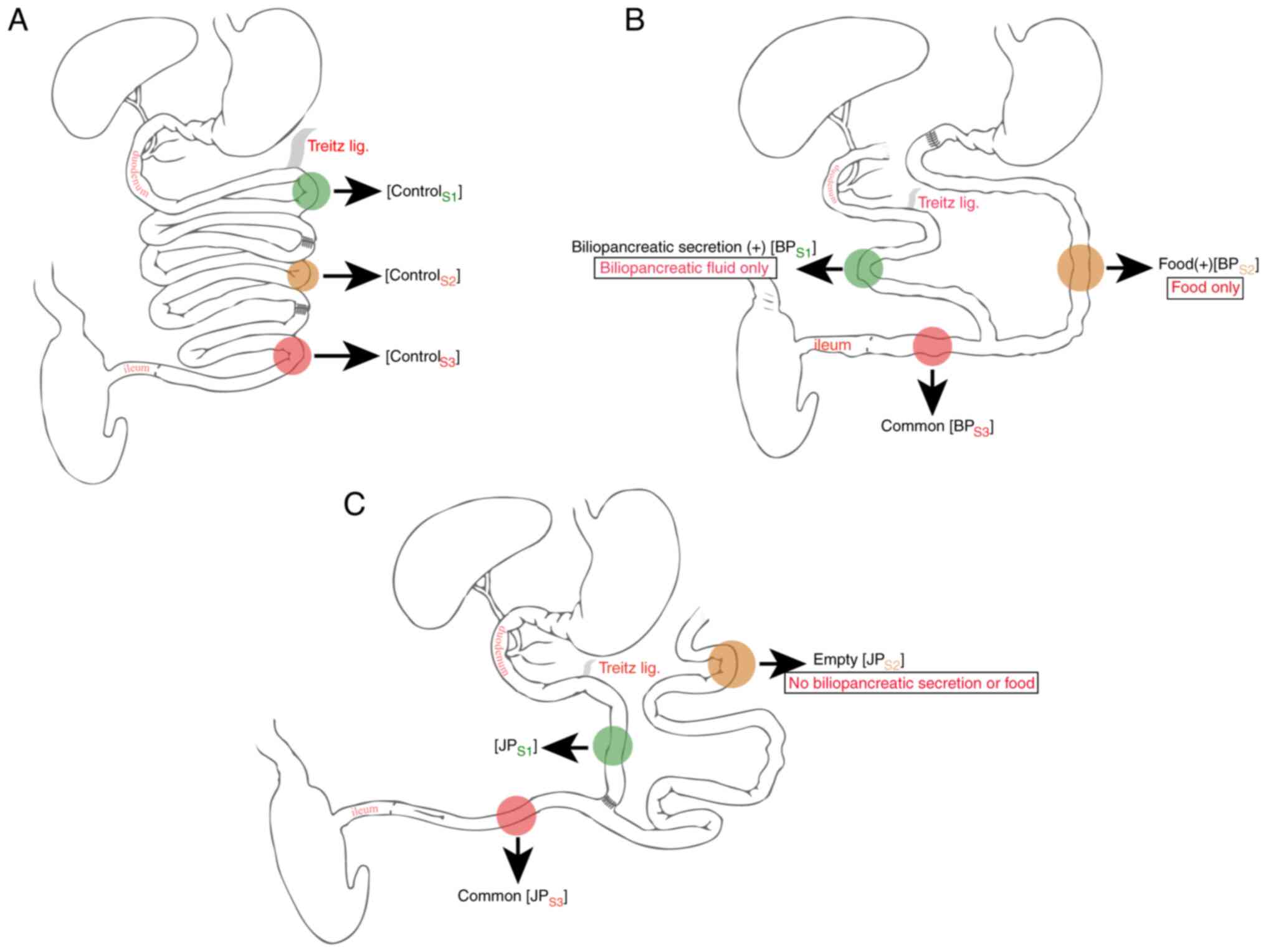
In group 1 (control), the jejunum was transected 30
and 90 cm distal to the ligament of Treitz, then re-anastomosed
using 6-0 polydioxanone sutures (Fig.
2A). The abdomen was irrigated with saline and closed using
standard techniques. In group 2 (BP), the duodenum was transected
and closed; the jejunum was divided 20 cm from Treitz. The distal
jejunum was anastomosed to the duodenum and the proximal segment
was reconnected 40 cm downstream (Figs. 2B-3A). Anastomotic integrity was tested
before closure (Video S1). In
group 3 (JP), the jejunum was transected 30 cm from Treitz, and the
proximal and distal ends were reconnected 40 cm apart (Fig. 2C). The bypassed segment was closed
and fixed to the abdominal wall.
Once surgery was complete, the surgical sites were
disinfected with chloramphenicol and iodine. Postoperatively, rats
recovered for 30 min before being returned to individual cages. On
day 1, a standard diet and water (with 100 mg/kg acetaminophen) was
resumed. Body weights were recorded every 3 days. On day 24,
animals were re-anesthetized using ketamine (40 mg/kg) and xylazine
(10 mg/kg). Euthanasia was performed via exsanguination through
portal vein puncture under deep anaesthesia. Cessation of heartbeat
and respiration were used to confirm the death of all animals.
Sample collection
Blood samples were collected from the portal vein at
the time of sacrifice. Intestinal tissue samples were then
harvested immediately. Samples intended for microbiological
analysis were processed fresh, while tissues for
immunohistochemistry were fixed in formalin and embedded in
paraffin blocks prior to staining. Samples were collected for
histopathological and microbiological examination as follows:
Control group (n=10): ControlS1, 10 cm from the ligament
of Treitz; controlS2, 50 cm from the ligament of Treitz;
controlS3, 80 cm from the ligament of Treitz (Fig. 2A). Group 2, BP (n=9):
BPS1, 10 cm from the ligament of Treitz, biliopancreatic
secretion (+); BPS2, 30 cm from the gastrojejunostomy,
food (+); BPS3, 20 cm from the jejunojejunostomy,
representing the common limb through which both food and
biliopancreatic secretions pass (Fig.
2B). Group 3, JP (n=8): JPS1, 10 cm from the
ligament of Treitz; JPS2, 20 cm from the stump of the
blind loop, biliopancreatic secretion (-) and food (-);
JPS3, 10 cm from the jejuno-jejunostomy, common
intestinal limb (i.e., the segment where luminal contents and
biliopancreatic secretions mix) (Fig.
2C). Analyses of histopathological and immunohistochemical
findings were performed, comparing the intestinal segment samples
to other segments within the same group and also to those of the
control group (Fig. S1A).
Histopathology and
immunohistochemistry
For histopathological examination, tissue samples
were fixed in 10% neutral buffered formalin at room temperature for
24 h, embedded in paraffin blocks, and sectioned at 3 µm thickness.
Sections were stained with hematoxylin (4 min) and eosin (30 sec)
at room temperature. The stained slides were examined under a light
microscope (Olympus SL-50). Villus height/crypt depth ratios were
evaluated (2:1=atrophic, 5:1=normal) based on literature standards
(8). Intraepithelial lymphocytes
(IELs) counting was performed on H&E-stained sections. For each
sample, four randomly selected high-power fields (x400
magnification) were evaluated. The number of intraepithelial
lymphocytes was manually counted and expressed as the number of
IELs/100 epithelial cells (≤20=normal, >20=high). IELs were
identified as small, round intraepithelial cells with darkly
stained, round nuclei and minimal cytoplasm. Neutrophils were
excluded from IEL counts based on their characteristic multilobed
nuclei and lighter cytoplasmic staining. Tissue samples were also
stained for the TJ proteins occludin, claudin-1 and ZO-3. All
immunohistochemistry examinations were performed on
paraffin-embedded sections. Following deparaffinization, endogenous
peroxidase activity was blocked with 3% hydrogen peroxide for 5 min
for 5 min at room temperature. Non-specific binding was blocked
using 5% normal goat serum (Sigma-Aldrich; Merck KGaA; cat. no.
G9023) for 30 min at room temperature. Antigen retrieval was
performed by boiling the sections in citrate buffer for claudin-1
or EDTA buffer for occludin and ZO-3 at 95˚C for 20 min. Sections
were incubated with primary rabbit monoclonal antibodies at ~95˚C
against claudin-1 (Abcam, cat. no. ab140349, 1:200), occludin
(Abcam, ab168986, 1:150), and ZO-3 (Abcam, ab191143, 1:200) for 20
min. Detection was performed using the Leica HRP-conjugated
detection kit (DS9800, New Castle, UK), followed by sequential
incubation with 3% hydrogen peroxide (10 min at room temperature)
and DAB (6 min at room temperature). Slides were counterstained
with hematoxylin for 1-2 min at room temperature. Skin, kidney, and
intestinal tissue were used as positive controls for claudin-1,
occludin, and ZO-3, respectively.
Results were scored for staining intensity of
occludin as weak (400x), moderate (100x), or strong (40x). Results
were scored for staining intensity of ZO-3 as weak (400x), moderate
(200x), or strong (40x). Results were scored for staining pattern
of claudin-1 as weak (≤1/3 of villi) or strong (>1/3 of villi)
(9) Staining intensity was
evaluated semi-quantitatively based on the brown DAB chromogenic
signal and the proportion of positively stained epithelial cells.
All histopathological and immunohistochemical samples were
initially evaluated by a pathologist. Subsequently, the samples
were blindly reviewed by another pathologist.
Microbiological and biochemical
analyses
After laparotomy and blood sampling, the intestinal
segments were excised and immediately placed into pre-weighed
sterile containers containing 0.9% normal saline. The containers
were weighed again to determine the tissue mass by difference. Each
tissue sample was then homogenized using a sterile tissue
homogenizer in saline at a 1:10 (w/v) ratio. Ten-fold serial
dilutions (10-¹-10-5) were prepared, and 100
µl from each dilution was plated on blood agar and Endo agar. The
plates were incubated aerobically at 37˚C for 24 h, after which
colony numbers were counted. Bacterial load was expressed as
colony-forming units per gram of tissue (CFU/g). Portal blood was
sampled from the portal vein, as demonstrated in Fig. 3B, for the measurement of plasma
lipopolysaccharide (LPS) and citrulline levels. Plasma was isolated
from heparinized portal blood samples and stored at -80˚C until
analysis. Plasma concentrations of lipopolysaccharides were
measured using an ELISA kit (Elabscience, cat. no: E-EL-0025), and
plasma citrulline levels were measured using an ELISA kit
(MyBioSource; cat. no. MBS2600386), according to the manufacturers'
instructions.
Statistical analysis
Statistical analyses were performed using SPSS
Statistics version 23 (IBM Corp.). Proportions of histopathological
and immunohistochemical data (ordinal variables) were presented
using cross-tabulations. χ² or Fisher's exact test, as appropriate,
was used to compare the proportions in the different groups. The
results of descriptive analyses are presented as the mean and
standard deviation for weight and biochemical variables. The
Kruskal-Wallis test was used to compare those parameters. P<0.05
was considered to indicate a statistically significant
difference.
Results
Macroscopic findings
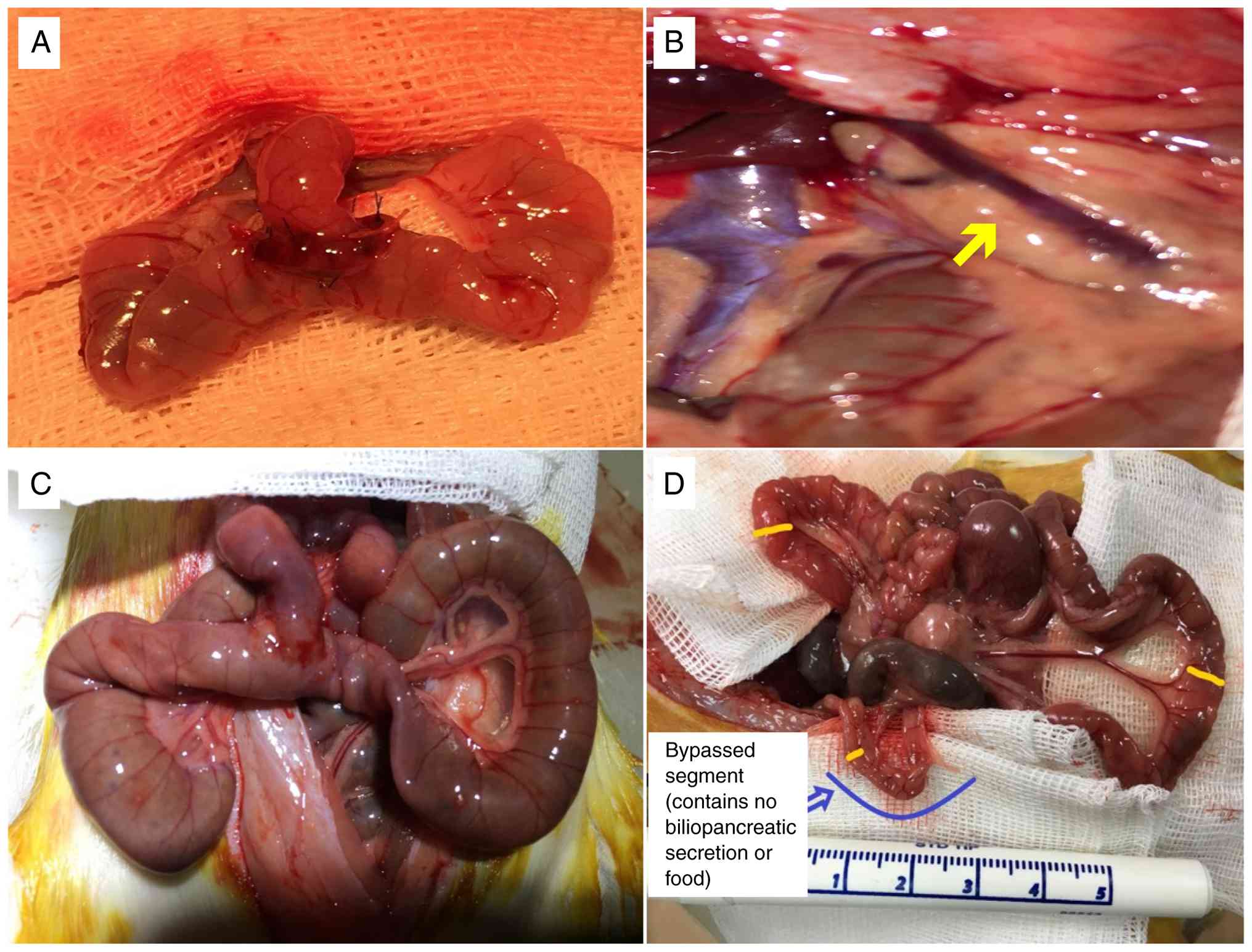
Three animals (two from the JP group and one from
the BP group) died spontaneously prior to day 24. Necropsy revealed
major intra-abdominal complications consistent with leakage and
infection, as the cause of death. These deaths were excluded from
the final histopathological and biochemical analyses. Overall, no
other major intra-abdominal complications (such as abscess or fluid
accumulation) were observed in the animals used for analysis. Some
animals had mild adhesions between bowel loops, but no obstruction
or stenosis was detected. Gross intestinal atrophy was most evident
in segments deprived of both food and biliopancreatic secretions
(Fig. 3D).
Weight changes
All animals gained weight postoperatively, with no
significant differences between the groups (Fig. S1B). The mean weights prior to
euthanasia were 404±54 in the Control group, 361±65 g in the BP
group, and 397±50 g in the JP group.
Villus/crypt ratio
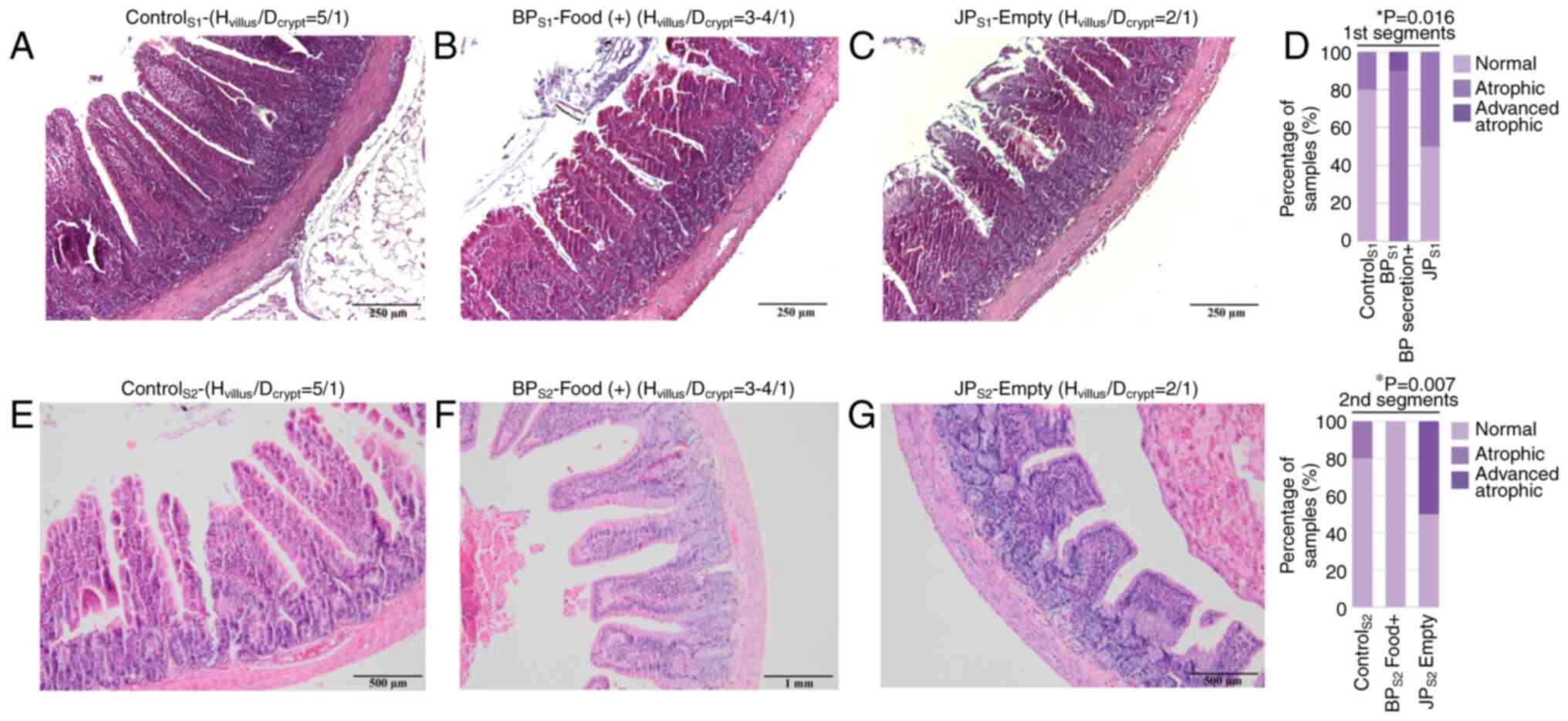
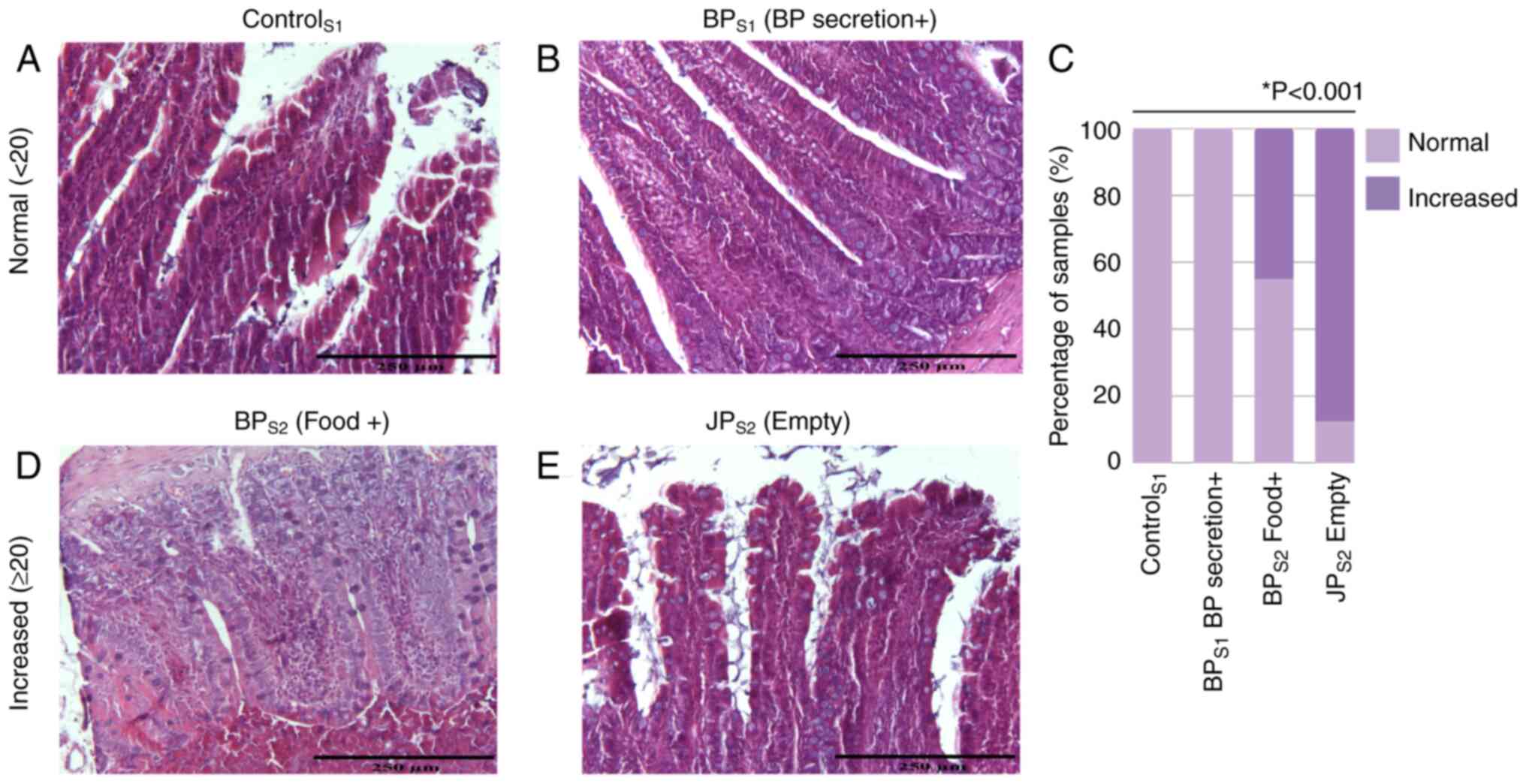
Villus/crypt ratios were assessed to evaluate
mucosal integrity across intestinal segments, and representative
histological images are presented in Fig. 4A-C, E-F. When villus height/crypt
depth ratios of the first segments (S1) in all groups were
compared, a significant decrease in villus height/crypt depth ratio
was observed in the BPS1 segment, where only
biliopancreatic secretions were present. When the villus
height/crypt depth ratio of the second segments (S2) of all groups
were compared, the most severe atrophy occurred in JPS2
(no food or bile; Fig. 4D). No
significant differences were found in the third segments (common
limb; S3).
IEL count
IEL counts were assessed based on the number of
intraepithelial lymphocytes per 100 epithelial cells as described
(Fig. 5A, B, D-E). IEL counts were
compared across segments with differing exposure to luminal
contents: Control S1 (food + biliopancreatic secretions), BPS1
(biliopancreatic secretions only), BPS2 (food only), and JPS2
(neither food nor biliopancreatic secretions). IEL counts were
significantly higher in the segments lacking biliopancreatic
secretions (BPS2 and JPS2), with the highest counts observed in
JPS2 (Fig. 5C). No significant
differences were observed in the S3 (common limb) segments when
comparing the Control, BP, and JP groups, as this region receives
both luminal nutrients and biliopancreatic secretions in all
models.
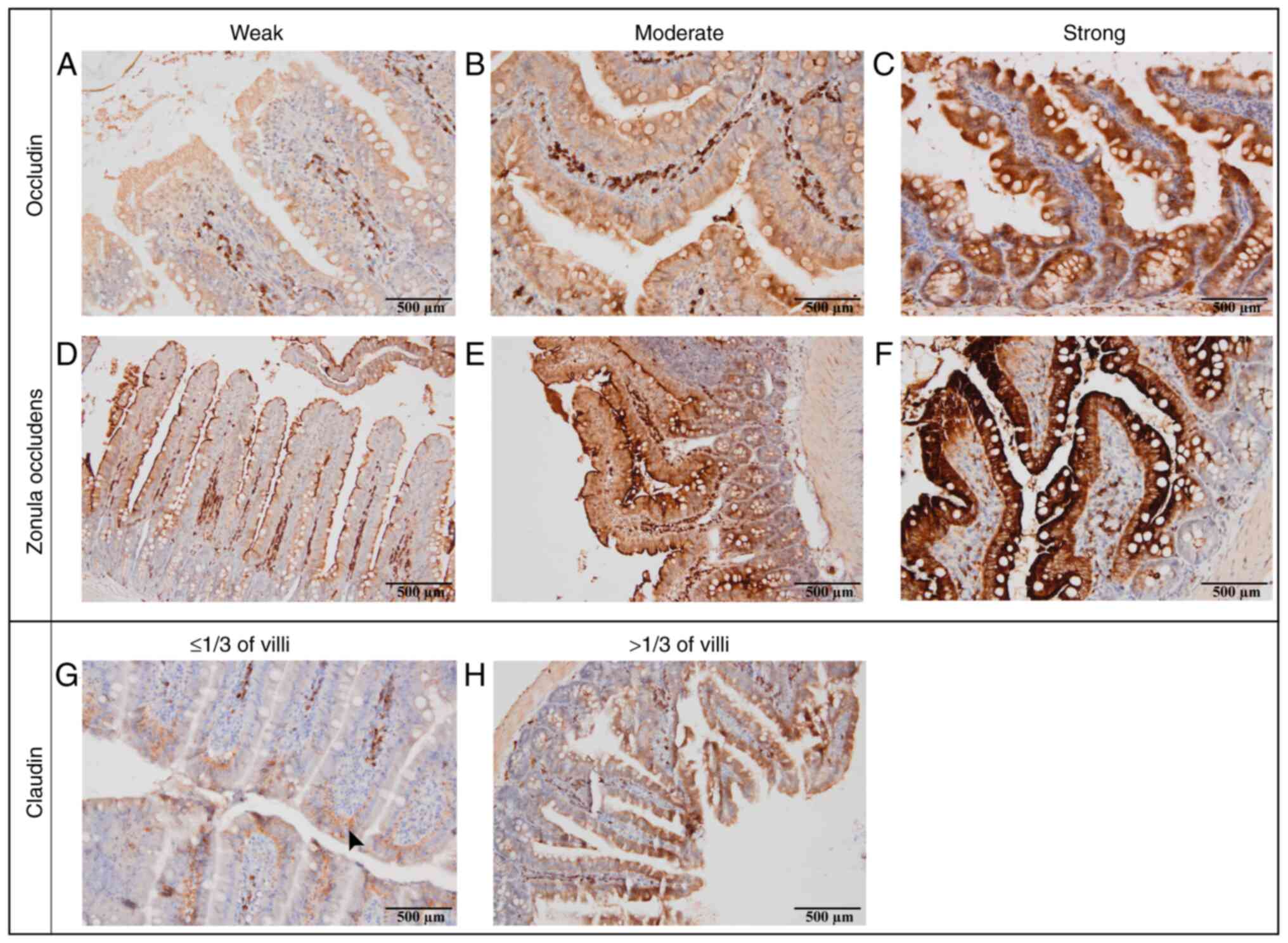
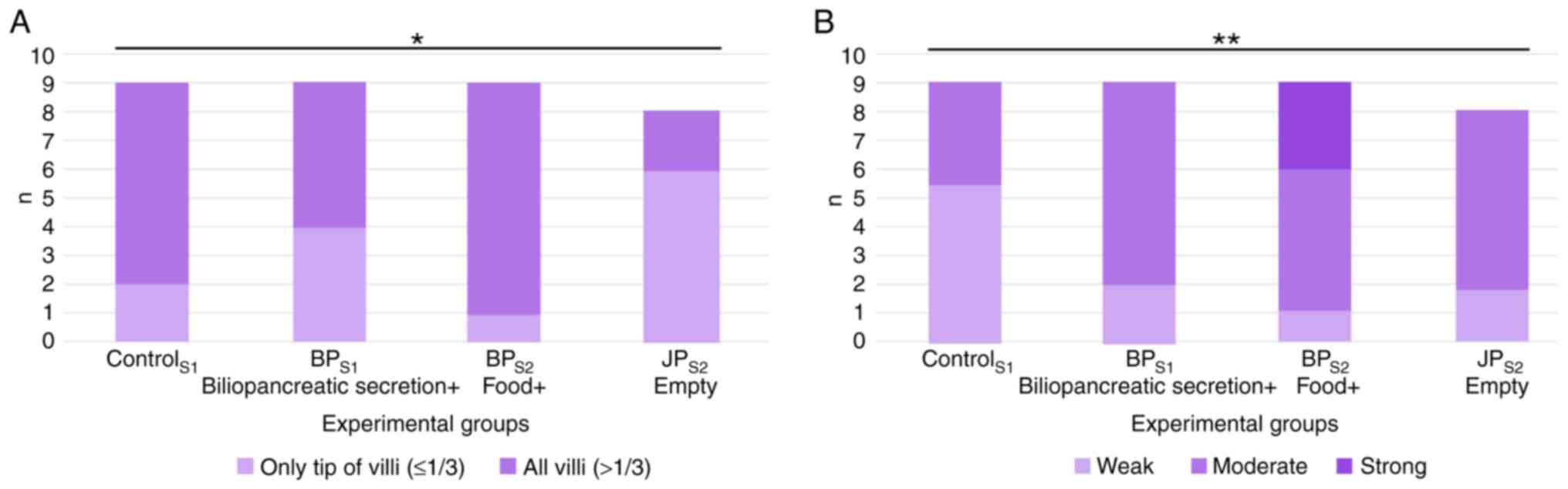
TJ protein expression
The staining intensity and distribution patterns of
occludin, ZO-3, and claudin-1 were evaluated according to the
semi-quantitative criteria shown in Fig. 6A-H (weak, moderate or strong
staining for occludin and ZO-3; and ≤1/3 vs. >1/3 villus height
for claudin-1). No statistically significant changes in ZO-3
expression were observed across any segment. The results
demonstrated slight variations in BPS1 and
BPS2, but this did not reach statistical
significance.
For claudin-1, when the first segments (S1) across
all groups were compared, no significant differences in claudin-1
staining patterns were observed. Expression levels of claudin-1
were significantly reduced in intestinal JPS2 segment
when compared with expression in the control group (Fig. 7A). No differences were noted in the
third segments (S3). In the S1 segment, occludin staining intensity
was significantly higher in the JP group compared with the Control
and BP groups. By contrast, no significant differences were
observed among the groups in the S2 and S3 segments. Compared with
the control segment (S1, where both food and biliopancreatic
secretions were present), occludin staining intensity was slightly
increased in the BPS1 segment (bile only) and the JPS2 segment
(neither food nor bile). The highest occludin expression was
observed in the BPS2 segment, where only food was present (Fig. 7B).
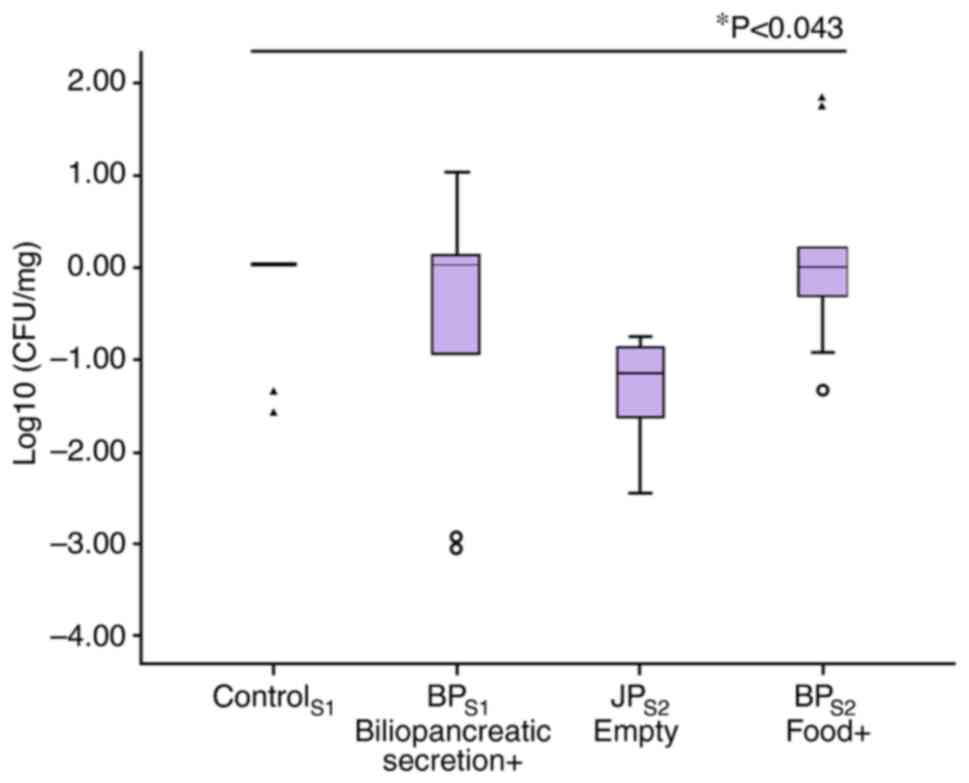
Jejunal aerobic bacteria
Compared with control group, bacteria population in
related segments on values of log10(CFU/mg) had no noticeable
change in segment where only bile passed, an increase in segment
where only food passed, a decrease in segment which included
neither food nor bile (Fig. 8).
The difference among them was recorded statistically
significant.
Plasma LPS and citrulline
Plasma LPS and citrulline levels represent one
measurement per animal obtained from portal blood and are reported
as group mean values. Plasma LPS levels did not differ
significantly between groups. Plasma citrulline levels were highest
in controls (8±2.0 nmol/ml) and lowest in JP group segments (6±1.7
nmol/ml), but this difference was not significant (Fig. S1C).
Discussion
The largest microorganism reserve in the human body
is found in the gastrointestinal tract (10). The physical, chemical and immune
barrier functions of the gastrointestinal system prevent bacteria
from spreading and invading the systemic circulation (11). TJ proteins between intestinal cells
serve a major role in the regulation of intestinal permeability;
notably, altered intestine permeability is associated with
endotoxemia and may be involved in the pathogenesis of a number of
diseases, including inflammatory bowel disease, celiac disease,
type 2 diabetes, obesity, and non-alcoholic fatty liver disease
(12). The effects of enteral
nutrition on intestinal integrity have been widely studied; the
literature consistently highlights enteral nutrition in maintaining
intestinal structure and function due to its physiological
engagement of the gut (13,14).
However, to the best of our knowledge, there are limited studies on
the individual effects of bile and pancreatic secretion or
food.
In the present study an experimental rat model was
used, jejunum segments were surgically created through which
biliopancreatic secretions, food or both did not pass. The
intestinal segments were long enough to prevent malnutrition as a
confounding factor. Consequently, there were no significant changes
in body weight among the groups throughout the experimental
period.
The present study showed that occludin expression,
which has a key regulatory role among TJ proteins, was
significantly affected depending on the presence of food within the
lumen. Specifically, the staining intensity of occludin was
increased in the segment where only food passed (BPS2), indicating
that direct luminal nutrient exposure was associated with enhanced
occludin expression. The present finding is supported by another
experimental study, which showed that occludin increases with
enteral nutrition compared with parenteral nutrition (15). Oral nutrients are key in building
up the gastrointestinal system from the beginning of life (16). The segment receiving only luminal
nutrients (BPS2) demonstrated the strongest occludin staining,
supporting the concept that direct exposure to dietary content
helps preserve TJ integrity throughout the lifespan. A decrease in
the expression of TJ proteins is associated with necrotizing
enterocolitis in the neonatal period, highlighting the importance
of proper nutrition in the intestines (17).
Claudin-1 is a TJ protein found in numerous tissues
besides the intestine, including the epidermal TJs in skin, the
bile canalicular membrane in the liver, and the distal renal
tubules in the kidney. These distributions highlight its broader
role in maintaining epithelial barrier function across organ
systems (18,19). Its expression has been shown to be
decreased in enterocytes in a number of conditions including
allergies, pancreatitis, cholangitis, colon cancer and inflammatory
bowel disease (20). In the
present study, claudin-1 expression was decreased in the absence of
food, and the decrease was more pronounced in the absence of both
biliopancreatic secretions and food. However, no change was
observed in the absence of biliopancreatic secretions alone.
Identifying the components of food that are most important for
improving the condition of TJ proteins will require further studies
(21).
In the present study, the expression of ZO-3 was
examined; ZO-3 is a member of the ZO protein family, which
functions as a cytoplasmic scaffold linking transmembrane TJ
proteins to the actin cytoskeleton (22,23).
No statistically significant differences in ZO-3 expression among
the experimental groups were observed, suggesting that this
structural protein may remain relatively stable in response to
luminal changes such as the absence of food or biliopancreatic
secretions. Similarly, in a high-fat diet mouse model by Murakami
et al (15) ZO-1 expression
remained unchanged, and no alterations were reported in the small
intestine, supporting the idea that ZO proteins are more resistant
to such changes. Collectively, these findings support the notion
that members of the ZO protein family, particularly ZO-1 and ZO-3,
may exhibit greater resistance to environmental or dietary
perturbations compared with transmembrane TJ components.
The intestinal mucosa can adapt in response to
different stimuli; this adaptation is key for survival in different
conditions including short bowel syndrome and after bariatric
surgery (24). In the present
study, it was observed that after the surgical procedures, the
animals in the experimental groups lost weight for 4-5 days and
regained weight after this period. Therefore, preserving the weight
of the animals and avoiding malnutrition are strengths of the
present study. In the study by Taqi et al (25), weight loss and reduced mucosal
growth were observed, particularly in segments deprived of luminal
nutrients. In contrast, in our study, animal body weights were
preserved throughout the experimental period, preventing
malnutrition and reducing its potential confounding effects. In
addition, citrulline levels, which were used as an indicator of
functional enterocyte mass, did not differ between the groups. This
suggests that functional enterocyte mass was preserved across the
groups, helping to prevent malnutrition and its systemic effects.
Although segment-specific structural and TJ protein differences
were detected, the overall functional integrity of the intestine
was maintained across groups. The intestinal segments through which
food passed demonstrated gross enlargement (increased segment
diameter and wall thickness). This macroscopic change may represent
an adaptive response, helping to compensate for the bypassed
segments that receive reduced luminal stimulation.
Mucosal integrity and growth are mediated by
hormonal, neural, immune and mechanical signals (26). A reduction in mucosal integrity and
barrier function is recognized as an early step preceding villous
atrophy, as the breakdown of TJs increases permeability and
compromises epithelial turnover. Mucosal atrophy is characterized
by morphological changes of villus height, crypt depth, surface
area and the number of epithelial cells (27). In the present study, it was shown
that there was notable atrophy in the segments through which
biliopancreatic secretions and food did not pass. The atrophy was
less notable if either food or biliopancreatic secretions were
present. The findings of the present study showed that food may
induce enhanced adaptation compared with biliopancreatic secretions
in terms of morphological features.
The interaction between the intestinal microflora
and the host has been a subject of research in numerous studies
(28,29). The current study presented that
intestinal bacterial content significantly decreased in the
segments through which food and biliopancreatic secretions did not
pass. On the other hand, there was an increasing trend in the
bacterial populations of the intestinal segments that had food but
not biliopancreatic secretions [BPS2-food(+)] compared within the
four-segment (Control S1, BP S1, BP S2, and JP S2). The effects of
food and different diets on intestinal microbiota have been
intensively explored in other studies and it has been shown that
diet is one of the major factors affecting the microbiota (30,31).
The present study has some limitations: i) The
effects of food and biliopancreatic secretions on intestinal
morphology were examined; however, the individual effects of bile
and pancreatic secretions were not determined; and ii) the focus
was only on the aerobic population and other bacterial subgroups
were not studied. Therefore future research should explore these
areas further.
In conclusion, the present study demonstrated that
biliopancreatic secretions and food regulate intestinal morphology,
IEL count and the levels of TJ proteins, including occludin and
claudin-1. Similarly, the intestinal microbiota was shown to be
affected by food and gastrointestinal secretions. The present
findings indicated that food has a more prominent role than
gastrointestinal secretions in maintaining the morphology and
integrity of the gastrointestinal tract.
Supplementary Material
Representative immunohistochemical
images from all experimental groups and all intestinal segments
(S1-S3) stained for occludin, ZO-1 and claudin-1 (27 images total).
These images demonstrate staining intensity and localization
patterns along the villi, including segments that were not shown in
the main figures. (B) Weight change of animals during the study
period. Body weights were recorded every three days for all groups.
No significant weight loss was observed in the intervention groups
compared with the control group throughout the study duration. (C)
Plasma citrulline and lipopolysaccharide (LPS) levels.
Completion of gastrojejunostomy and
jejunojejunostomy anastomoses by hand-sewn technique in a rat
model. The video demonstrates the final stages of both anastomoses
and includes intraoperative assessment of anastomotic patency and
integrity.
Supplementary Video
Acknowledgements
This study was presented as an oral presentation at
the 41st ESPEN Congress, Krakow, Poland.
Funding
Funding: The present study was supported by the Hacettepe
University Scientific Research Unit (grant no: THD-2016-9077;
project number: 9077).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
OC performed the investigation, analyzed data and
wrote the original draft of the manuscript. AH performed
experiments, data interpretation and formal analysis, and assisted
in manuscript drafting and revision. BŞ and FA performed
experiments. CS analyzed and interpreted data. OA conceived and
designed the study, supervised the research process, interpreted
data, and critically revised the manuscript for important
intellectual content. All authors have read and approved the final
manuscript. OC and AH confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Hacettepe University (approval no. 15/54-03; Ankara,
Turkey).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ghosh SS, Wang J, Yannie PJ and Ghosh S:
Intestinal barrier dysfunction, LPS translocation, and disease
development. J Endocr Soc. 4(bvz039)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Macura B, Kiecka A and Szczepanik M:
Intestinal permeability disturbances: Causes, diseases and therapy.
Clin Exp Med. 24(232)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fung KY, Fairn GD and Lee WL:
Transcellular vesicular transport in epithelial and endothelial
cells: Challenges and opportunities. Traffic. 19:5–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Neurath MF, Artis D and Becker C: The
intestinal barrier: A pivotal role in health, inflammation, and
cancer. Lancet Gastroenterol Hepatol. 10:573–592. 2025.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rose EC, Odle J, Blikslager AT and Ziegler
AL: Probiotics, prebiotics and epithelial tight junctions: A
promising approach to modulate intestinal barrier function. Int J
Mol Sci. 22(6729)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xu F, Lu G and Wang J: Enhancing sepsis
therapy: The evolving role of enteral nutrition. Front Nutr.
11(1421632)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fleishman JS and Kumar S: Bile acid
metabolism and signaling in health and disease: Molecular
mechanisms and therapeutic targets. Signal Transduct Target Ther.
9(97)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Serra S and Jani PA: An approach to
duodenal biopsies. J Clin Pathol. 59:1133–1150. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Resnick MB, Konkin T, Routhier J, Sabo E
and Pricolo VE: Claudin-1 is a strong prognostic indicator in stage
II colonic cancer: A tissue microarray study. Mod Pathol.
18:511–518. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sender R, Fuchs S and Milo R: Revised
estimates for the number of human and bacteria cells in the body.
PLoS Biol. 14(e1002533)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yu LC, Wang JT, Wei SC and Ni YH:
Host-microbial interactions and regulation of intestinal epithelial
barrier function: From physiology to pathology. World J
Gastrointest Pathophysiol. 3:27–43. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Horowitz A, Chanez-Paredes SD, Haest X and
Turner JR: Paracellular permeability and tight junction regulation
in gut health and disease. Nat Rev Gastroenterol Hepatol.
20:417–432. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Villalona G, Price A, Blomenkamp K,
Manithody C, Saxena S, Ratchford T, Westrich M, Kakarla V,
Pochampally S, Phillips W, et al: No gut no gain! enteral bile acid
treatment preserves gut growth but not parenteral
nutrition-associated liver injury in a novel extensive short bowel
animal model. JPEN J Parenter Enteral Nutr. 42:1238–1251.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen R, Yin W, Gao H, Zhang H and Huang Y:
The effects of early enteral nutrition on the nutritional statuses,
gastrointestinal functions, and inflammatory responses of
gastrointestinal tumor patients. Am J Transl Res. 13:6260–6269.
2021.PubMed/NCBI
|
|
15
|
Shen TY, Qin HL, Gao ZG, Fan XB, Hang XM
and Jiang YQ: Influences of enteral nutrition combined with
probiotics on gut microflora and barrier function of rats with
abdominal infection. World J Gastroenterol. 12:4352–4358.
2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Al-Sadi R, Khatib K, Guo S, Ye D, Youssef
M and Ma T: Occludin regulates macromolecule flux across the
intestinal epithelial tight junction barrier. Am J Physiol
Gastrointest Liver Physiol. 300:G1054–G1064. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Garg PM, Denton MX, Ravisankar S, Herco M,
Shenberger JS and Chen YH: Tight junction proteins and intestinal
health in preterm infants. J Neonatal Perinatal Med. 18:409–418.
2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu AS: Claudins and the kidney. J Am Soc
Nephrol. 26:11–19. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Németh Z, Szász AM, Tátrai P, Németh J,
Gyorffy H, Somorácz A, Szíjártó A, Kupcsulik P, Kiss A and Schaff
Z: Claudin -1, -2, -3, -4, -7, -8, and -10 protein expression in
biliary tract cancers. J Histochem Cytochem. 57:113–121.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yuan B, Zhou S, Lu Y, Liu J, Jin X, Wan H
and Wang F: Changes in the expression and distribution of claudins,
increased epithelial apoptosis, and a mannan-binding
lectin-associated immune response lead to barrier dysfunction in
dextran sodium sulfate-induced rat colitis. Gut Liver. 9:734–740.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Bertrand J, Ghouzali I, Guerin C,
Bôle-Feysot C, Gouteux M, Déchelotte P, Ducrotté P and Coëffier M:
Glutamine restores tight junction protein claudin-1 expression in
colonic mucosa of patients with diarrhea-predominant irritable
bowel syndrome. JPEN J Parenter Enteral Nutr. 40:1170–1176.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fanning AS, Jameson BJ, Jesaitis LA and
Anderson JM: The tight junction protein ZO-1 establishes a link
between the transmembrane protein occludin and the actin
cytoskeleton. J Biol Chem. 273:29745–29753. 1998.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Alizadeh A, Akbari P, Garssen J,
Fink-Gremmels J and Braber S: Epithelial integrity, junctional
complexes, and biomarkers associated with intestinal functions.
Tissue Barriers. 10(1996830)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Feris F, McRae A, Kellogg TA, McKenzie T,
Ghanem O and Acosta A: Mucosal and hormonal adaptations after
Roux-en-Y gastric bypass. Surg Obes Relat Dis. 19:37–49.
2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Taqi E, Wallace LE, de Heuvel E, Chelikani
PK, Zheng H, Berthoud HR, Holst JJ and Sigalet DL: The influence of
nutrients, biliary-pancreatic secretions, and systemic trophic
hormones on intestinal adaptation in a Roux-en-Y bypass model. J
Pediatr Surg. 45:987–995. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jacobson A, Yang D, Vella M and Chiu IM:
The intestinal neuro-immune axis: Crosstalk between neurons, immune
cells, and microbes. Mucosal Immunol. 14:555–565. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sugita K, Kaji T, Yano K, Matsukubo M,
Nagano A, Matsui M, Murakami M, Harumatsu T, Onishi S, Yamada K, et
al: The protective effects of hepatocyte growth factor on the
intestinal mucosal atrophy induced by total parenteral nutrition in
a rat model. Pediatr Surg Int. 37:1743–1753. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Afzaal M, Saeed F, Shah YA, Hussain M,
Rabail R, Socol CT, Hassoun A, Pateiro M, Lorenzo JM, Rusu AV and
Aadil RM: Human gut microbiota in health and disease: Unveiling the
relationship. Front Microbiol. 13(999001)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tremaroli V and Bäckhed F: Functional
interactions between the gut microbiota and host metabolism.
Nature. 489:242–249. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yu J, Wu Y, Zhu Z and Lu H: The impact of
dietary patterns on gut microbiota for the primary and secondary
prevention of cardiovascular disease: A systematic review. Nutr J.
24(17)2025.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Singh RK, Chang HW, Yan D, Lee KM, Ucmak
D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, et al:
Influence of diet on the gut microbiome and implications for human
health. J Transl Med. 15(73)2017.PubMed/NCBI View Article : Google Scholar
|