Introduction
Nail discoloration is an abnormal change in nail
(toenail) color that can provide important clues to potential
systemic and skin diseases. The causes of nail discoloration are
mainly divided into internal factors and external factors,
including infection, systemic disease, trauma and drug influence.
White nail is the most common nail discoloration; white nail can be
divided into true white nail, white spot and false white nail
(1). It is caused by the change of
the structure of the nail substance itself, the pathological change
of the nail bed, and the superficial invasion and dissemination of
fungi. Blue nail beds can be caused by cyanosis, hereditary
acrotelangiectasia, antimalarial drugs, argyria and glomus tumors,
and the use of systemic drugs can also cause blue nails (2).
Minocycline is a tetracycline antibiotic commonly
used to treat acne and rosacea. Common side effects of minocycline
associated with pigment include the following: Minocycline may
affect the development of bones and teeth in children, causing
teeth to turn yellow or brown. There have been numerous reports of
patients taking minocycline for a period of time and their nails
changed from the normal light color to gray or blue (2).
Pigmentation caused by minocycline can be divided
into five types, among which the ‘blue nail’ phenomenon caused by
minocycline is considered to be type II pigmentation (3). However, in this case, the report of
minocycline-related tinea is significantly different from the
traditional type II pigmentation, which is manifested as a snowy
mountain of nail bed changes, i.e., the unique clinical
characteristics of blue gray pigmentation and bright white nail bed
changes, providing new phenotypic characteristics for
minocycline-related nail bed pigment changes and contributing to
further research on its pathogenesis and clinical diagnosis.
Case report
In July 2022, a 19-year-old male presented to the
Department of Dermatology with facial acne vulgaris, featuring
hyperplastic nasal scars, atrophic scars and persistent papular
lesions. The patient took minocycline, a medication prescribed by
the attending physician, twice a day at 50 mg each time, totaling
100 mg per day, and maintained this dosage for 1 month. During the
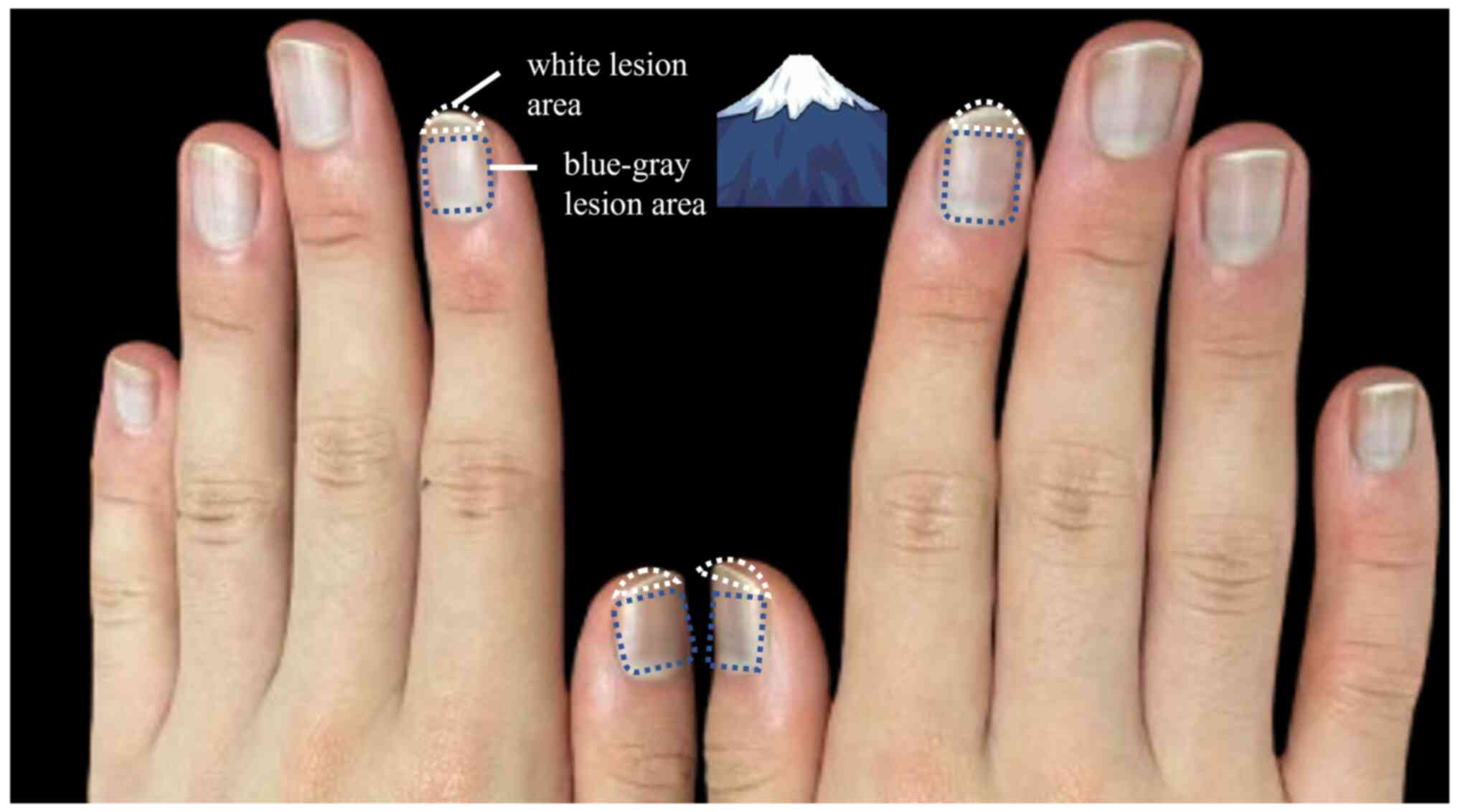
1-month treatment, the patient's nails developed a unique
appearance, ‘snow mountain nails’ (Fig. 1), with a blue-gray pigmentation
near the nail bed. There was also a distinct bright white nail
change at the free end, where parallel bright white stripes were
visible on the nail bed, complementing the blue-gray area to form a
unique bicolor change. It is worth noting that the patient did not
have abnormal pigmentation of the skin, teeth or toenails during
the course of the disease. When white nails appeared at the free
end of the nail, the treatment plan was adjusted. Minocycline was
discontinued, and the patient was instructed by the attending
physician to take isotretinoin soft capsules orally twice a day at
a dose of 10 mg to treat the acne. The abnormal nail appearance did
not improve until 2 months after the initial medication was
stopped. Biochemical tests and complete blood count analyses
conducted before and after minocycline treatment revealed an
abnormal elevation in plateletcrit at 2 weeks post-administration,
increasing from 0.256 to 0.303% (reference range: 0.108-0.303%).
All other results were within the normal ranges. Routine blood
tests were performed using the Sysmex XN-10 automatic hematology
analyzer (Sysmex Corporation). Biochemistry tests were conducted
with the Abbott Alinity C automatic biochemistry analyzer (Abbott).
Dry biochemistry tests were carried out using the Ortho Vitros 5600
automatic biochemistry and immunoassay analyzer (QuidelOrtho
Corporation). The patient was followed up every 2 weeks, where a
physical examination was conducted. Throughout the follow-up
period, the dosage of isotretinoin was adjusted based on the
patient's facial acne condition and nail status. At 2 months after
discontinuation of the medication, the color of the nail had
gradually improved: The blue-gray pigmentation range of the nail
bed was obviously reduced and the color changed from dark to light.
The free end of the bright white nail was gradually blurred, but
certain residual color changes were still observed. At 2 years
post-discontinuation of the medication, the patient's nail color
improved, with the bluish-gray and white tones gradually
disappearing, but the nails still did not regain their normal
appearance. The patient has been consistently taking isotretinoin
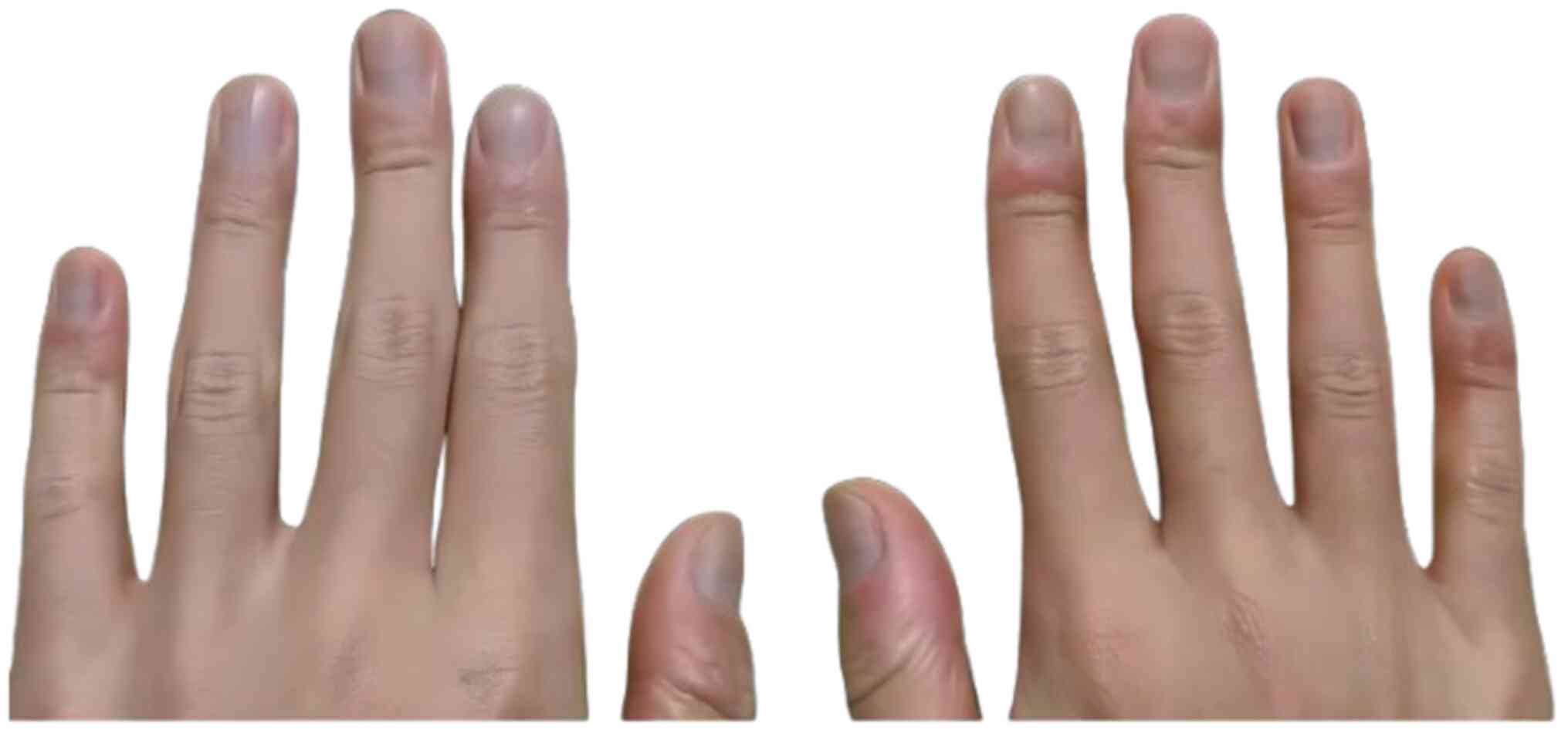
for acne treatment ever since. The periungual erythema and swelling
of the right hand may be associated with a chronic low-grade
inflammatory response triggered by the deposition of
minocycline-iron chelate complexes (Fig. 2).
Discussion
Blue-gray nail pigmentation is affected by a variety
of factors, such as infectious factors, including green nail
syndrome caused by bacterial infection, which can be identified by
direct fungal microscopy or positive culture, and lesions usually
start at the nail margin and gradually expand to the entire nail
(4). If Pseudomonas
aeruginosa is detected in nail culture, green nail syndrome
caused by bacterial infection can be diagnosed. Furthermore, nail
discoloration also requires a differential diagnosis with other
causes. For example, mechanical injury may lead to subungual
bleeding (appearing bluish-black), while prolonged exposure to
chemicals may directly cause nail discoloration (5).
Systemic diseases such as liver disease can cause
white nails. One study found that 82% of patients with ‘alcohol,
necrosis and cholangitis’ cirrhosis had a red or brown distal nail
bed that included <20% of the total nail length, kidney failure
leading to half nails, cardiovascular disease leading to pale
nails, and in addition, nail bed edema in lymphedema (1). Pale nail bed in severe anemia, scar
changes after whole-skin electron beam irradiation and selenium
deficiency can also cause obvious leukonychia (6). Drug reactions from long-term
medication use can turn nails yellow or black (7). The most common genetic factor is
autosomal dominant inheritance; for example, patients with
congenital leukonychia typically have milky white nails (1).
Minocycline, as a broad-spectrum antibiotic, is
commonly used to treat bacterial infections, particularly those
associated with acne and chlamydia. However, some side effects may
occur with the use of minocycline, among which pigmentation and
nail discoloration are the more common skin and appearance changes.
Long-term use of minocycline can cause pigmentation of nails, skin
and sclera without affecting function. Discontinuation is one of
the treatments for blue nails. In one study, it was found that nail
pigmentation can be resolved after discontinuation of oral
minocycline and the resolution time varies from 1 month to 3 years
(2). In the 2024 treatment
guidelines for common acne, it is stated that oral minocycline is a
commonly used medication for treating moderate to severe acne and
the patient in this case does not fall into the prohibited
population category (8). During
the 16-week treatment period for inflammatory skin lesions
associated with rosacea, the treatment success rate for the 40 mg
dose of minocycline was 66%, which was significantly higher than
the 11.5% in the placebo group, and it also demonstrated a
significant advantage in reducing the number of inflammatory
lesions (9). Compared to
tetracycline, minocycline has a stronger antibacterial effect
(10).
There are five types of pigmentation caused by
minocycline: Type I develops blue-black discoloration in previously
inflamed areas, such as acne secondary scarring; Type II skin has
blue-gray pigmentation in normal areas; Type III is a rare diffuse
mud-brown pigmentation, aggravated in sun-exposed areas; Type IV
appears with bluish-gray spots on the scarred area of the back; and
Type V is associated with hyperpigmentation and subcutaneous
involvement and is considered a progression of Types I and II to
subcutaneous infiltration. The ‘blue nail’ phenomenon caused by
minocycline is considered type II pigmentation. The incidence of
type II and III pigmentation increases with the cumulative dose of
minocycline (3). The
minocycline-associated nail pigmentation reported in the present
case showed significant differences from traditional type II
pigmentation. In addition to the blue-gray pigmentation of the nail
bed, the presentation was also uniquely accompanied by a bright
white change of the free end of the nail and the cross-distribution
of bright white stripes on the nail bed, and with the blue-gray
area near the nail root, it exhibited a sharp two-color contrast.
This unique nail manifestation not only enriches the scope of the
clinical phenotype of minocycline-associated pigmentation, but also
provides new ideas and research directions for further exploration
of its pathogenesis and diagnostic value.
The blue-gray pigmentation effect of minocycline is
mainly related to its metabolic process in vivo and its
interaction with tissues. It usually occurs in patients taking high
doses and using it for a longer period of time. Minocycline itself
can be deposited in the basal layer of the skin by chelating with
iron to form a stable complex, and minocycline can also affect
melanin production in the skin or interact directly with the
melanin synthesis mechanism, resulting in increased local
pigmentation. The chelation of minocycline with iron may accumulate
within the cuticle of the nail, and due to the high protein and
metal ions in the nail, the binding of minocycline with these
components may cause nail discoloration. In one study, it was
reported that patient who used minocycline for a long time may have
blue or gray pigmentation of their skin (11). The metabolism of minocycline in the
body may cause the drug to bind to metal ions in the skin, causing
this type of pigmentation change. A patient who had taken
minocycline for 4 years also experienced diffuse blue-gray
pigmentation on the face and ears (12). This pigmentation may occur not only
in the skin, but discoloration of the nails may also be observed,
another one of the more common side effects of minocycline. After
taking minocycline for several months, the patient developed a
significant discoloration of the nails, which changed from a normal
light color to gray or blue. Masuyama et al (13) found that after 10 weeks of
minocycline use, a patient's nail bed turned grayish-blue and its
thickness increased to 9 mm. This phenomenon is usually associated
with the accumulation of drugs. A 66-year-old male patient had
persistent blue-gray discoloration of the nails during the
treatment of rosacea with minocycline for 22 years (3). This blue nail was likely to be
misdiagnosed as a change in nail bed color related to heart
disease, and further cardiac-related tests were needed (3). It may take several months for the
nail color to recover after stopping the drug. Long-term use of
minocycline may lead to pigmentation when the cumulative dose
exceeds the threshold of 100 grams (3). This pigmentation is dose-dependent
and a daily dose of 100 mg for patients falls within the low-dose
range, which may also be one of the reasons for the regression of
‘snow mountain nail’. In addition to the blue-gray pigmentation
near the nail base of the nail bed, there is also a significant
bright white nail change at the free end. This comprehensive nail
change provides a new clinical feature for drug-induced nail
lesions, and its pathogenesis and diagnostic significance need to
be further studied. The formation of white stripes on the nail
plate may be due to the indirect effect of minocycline on the local
blood supply or inflammation of the nail bed during the
anti-inflammatory treatment of acne, which leads to a color change
of the nail plate (13). The
formation and deposition of iron-minocycline chelates may affect
the normal function of nail bed keratinocytes, including keratin
production and metabolism, making nails look pale. In addition, the
long-term use of minocycline may reactively interfere with local
melanin production and distribution, or it may be a rare
manifestation related to individual differences or local
environmental factors such as light exposure, inflammation and
metabolic status. In the present case, the clinical manifestations
improved markedly following discontinuation of minocycline,
supporting the hypothesis that the occurrence of ‘snow mountain
nails’ represents an adverse reaction associated with minocycline
therapy. However, the persistent bluish discoloration at the
proximal nail bed is likely attributable to the slow degradation of
minocycline-iron complexes deposited within the keratinized layer
and superficial dermis, resulting in residual blue-gray
pigmentation. Under these circumstances, the coexistence of
proximal bluish pigmentation and distal whitish striations did not
resolve after drug withdrawal or improved circulation, thereby
excluding hypoperfusion or systemic hypoxemia as potential
underlying mechanisms. This observation may further indicate the
persistence of local inflammatory activity, which could account for
the erythematous swelling observed over the dorsal aspect of the
interphalangeal joints.
In conclusion, side effects of minocycline, a common
antibiotic used to treat acne, include pigmentation of the nails
and other areas, but are generally reversible. To the best of our
knowledge, the present study reports the first case of a ‘snow
mountain nail’ characterized by blue-gray pigmentation near the
root of the nail accompanied by bright white changes at the free
end and parallel bright white stripes, which was different from the
blue pigmentation caused by minocycline reported in the past.
Although it was only a single case report, the clinical
manifestations of this case were relatively important, and this is
highly likely to be a new type of minocycline-based disease.
Therefore, the present case was reported for reference. This
clinical manifestation not only expands the phenotype spectrum of
minocycline-associated pigmentation, but also provides a new clue
for clinicians to monitor drug-related pigmentation during
minocycline use. It provides an important reference for further
study on the pathogenesis and diagnostic value of drug-related nail
lesions.
It was further confirmed that minocycline-induced
nail discoloration was improvable by withdrawal of minocycline and
detailed follow-up observation. The nail changes gradually returned
to normal after drug withdrawal, suggesting that this phenomenon
was an adverse reaction caused by minocycline. Case observations in
the literature suggest that such pigment alterations may involve
the chelation of minocycline and iron and their interference with
melanin production and metabolism, and are closely related to
inter-individual differences or local inflammatory states. The
report of this case not only provides important clues for the
diagnosis and differential diagnosis of minocycline-related
hyperpigmentation, but also highlights the importance of focusing
on hyperpigmentation of parts such as nails during minocycline
treatment. Future studies can further explore the pathogenesis,
diagnostic criteria and application value of ‘snow mountain nail’
in the monitoring of adverse drug reactions.
Acknowledgements
Not applicable.
Funding
Funding: The study was supported by the National Natural Science
Foundation of China (grant no. 82473556), Huadong Hospital Elite
Talent (grant no. JYRC2204) and the Clinical Research Plan of SHDC
(grant no. SHDC22025306).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
As the attending physician for this patient, LS was
the first to observe the ‘Snow Mountain Nail’ phenomenon. KL
performed a literature review and revised and supplemented the
manuscript. YTQ conducted the biopsy and initially analyzed the
medical record data. WJZ was responsible for the language editing
of the article. YTQ and WJZ confirm the authenticity of all the raw
data. KL, WJZ, YTQ and LS were involved in the conception of the
study and the interpretation of the data, and have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of case information and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iorizzo M, Starace M and Pasch MC:
Leukonychia: What can white nails tell us? Am J Clin Dermatol.
23:177–193. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hwang JK and Lipner SR: Blue nail
discoloration: Literature review and diagnostic algorithms. Am J
Clin Dermatol. 24:419–441. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Varghese K, Dykstra J and Bisbee E:
Minocycline-induced hyperpigmentation of nails. Cureus.
15(e38640)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reynolds RV, Yeung H, Cheng CE,
Cook-Bolden F, Desai SR, Druby KM, Freeman EE, Keri JE, Stein Gold
LF, Tan JKL, et al: Guidelines of care for the management of acne
vulgaris. J Am Acad Dermatol. 90:1006.e1–1006.e30. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tsianakas A, Pieber T, Baldwin H,
Feichtner F, Alikunju S, Gautam A, Shenoy S, Singh P and Sidgiddi
S: Minocycline Extended-release comparison with doxycycline for the
treatment of rosacea: A randomized, Head-to-Head, clinical trial. J
Clin Aesthet Dermatol. 14:16–23. 2021.PubMed/NCBI
|
|
6
|
Asadi A, Abdi M, Kouhsari E, Panahi P,
Sholeh M, Sadeghifard N, Amiriani T, Ahmadi A, Maleki A and Gholami
M: Minocycline, focus on mechanisms of resistance, antibacterial
activity, and clinical effectiveness: Back to the future. J Glob
Antimicrob Resist. 22:161–174. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Taniguchi Y and Yamamoto H: Clinical
images: Methotrexate-induced melanonychia. Arthritis Rheumatol: Jul
28, 2025 (Epub ahead of print).
|
|
8
|
Forouzan P and Cohen PR: Fungal
viridionychia: Onychomycosis-induced chloronychia caused by Candida
parapsilosis-associated green nail discoloration. Cureus.
13(e20335)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gregoriou S, Platsidaki E, Sidiropoulou P
and Rigopoulos D: Nails with bloodstained discoloration. BMJ.
375(n1951)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hasunuma N, Umebayashi Y and Manabe M:
True leukonychia in Crohn disease induced by selenium deficiency.
JAMA Dermatol. 150:779–780. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ricardo JW, Shah K, Minkis K and Lipner
SR: Blue Skin, Nail, and Scleral Pigmentation Associated with
Minocycline. Case Rep Dermatol. 14:239–242. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang P, Farmer JP and Rullo J:
Minocycline-induced hyperpigmentation. JAMA Dermatol.
57(992)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Masuyama T, Branch J, Ishizuka K, Uchida
R, Otsuki T, Ie K and Okuse C: Minocycline-induced blue nails. Am J
Med. 137:e190–e191. 2024.PubMed/NCBI View Article : Google Scholar
|