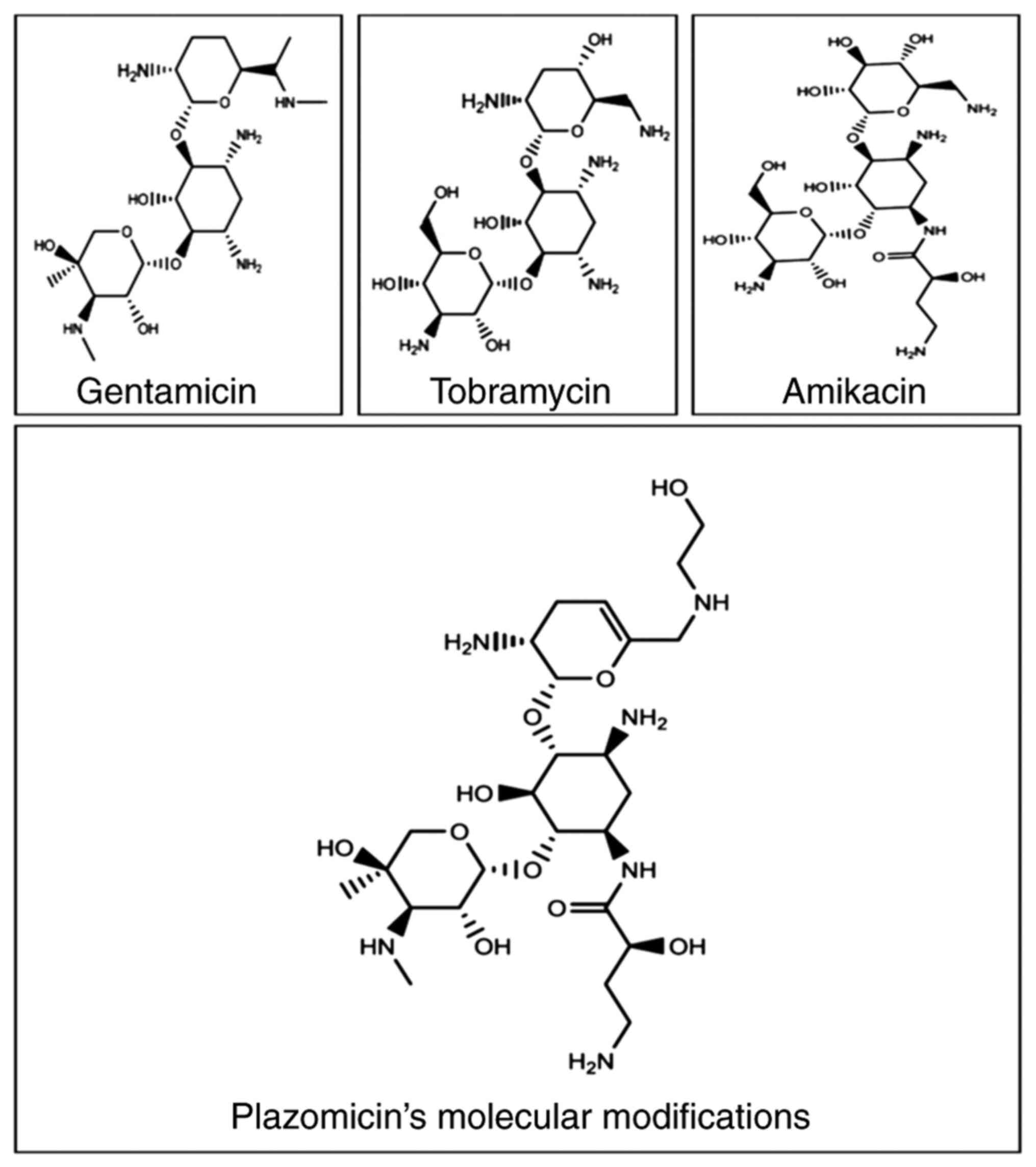
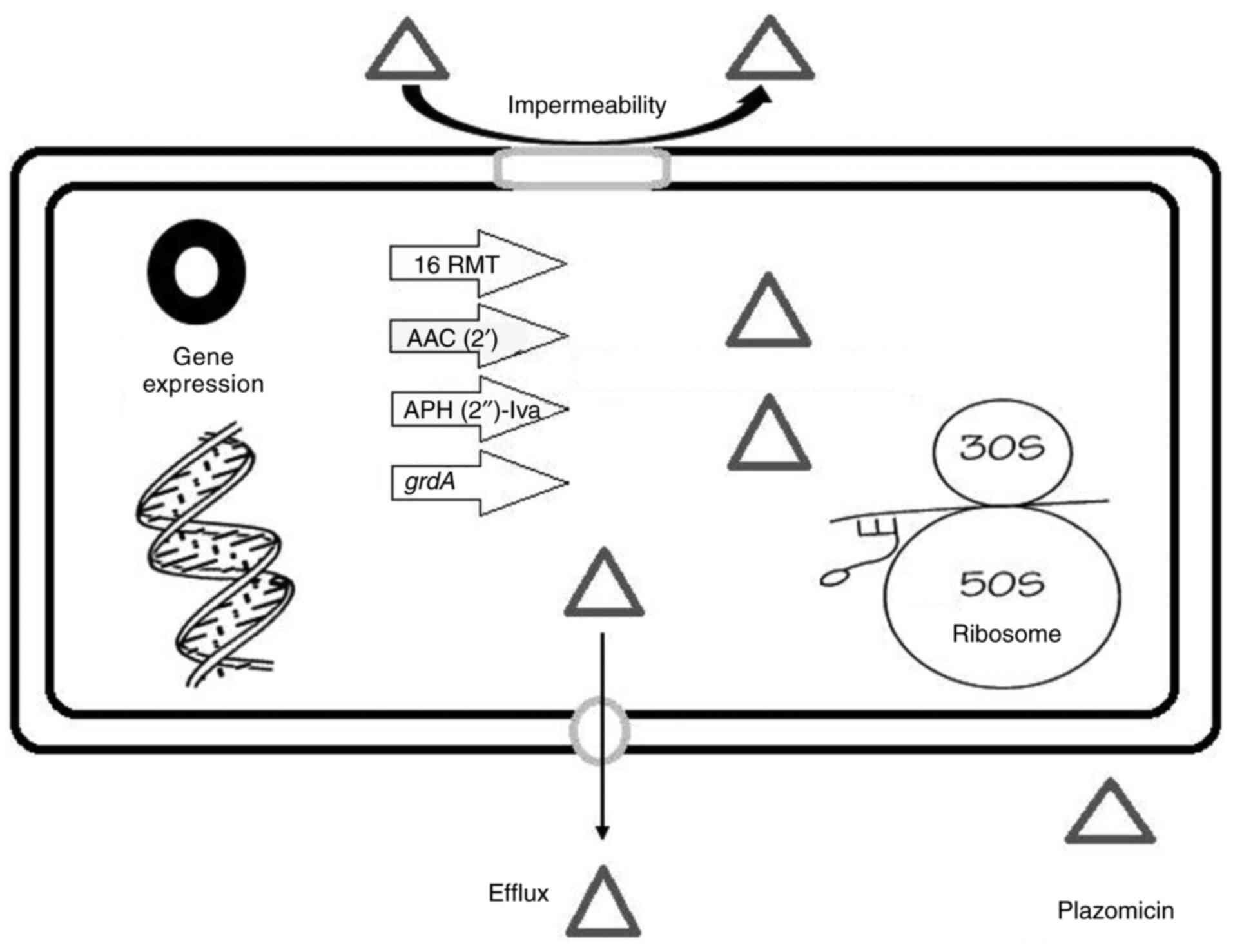
|
1
|
Schatz A, Bugie E and Waksman SA:
Streptomycin, a substance exhibiting antibiotic activity against
gram-positive and Gram-negative bacteria. 1944. Clin Orthop Relat
Res. 437:3–6. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Weinstein MJ, Luedemann GM, Oden EM and
Wagman GH: Gentamicin, a new broad-spectrum antibiotic complex.
Antimicrob Agents Chemother (Bethesda). 161:1–7. 1963.PubMed/NCBI
|
|
3
|
Kluge RM, Standiford HC, Tatem B, Young
VM, Greene WH, Schimpff SC, Calia FM and Hornick RB: Comparative
activity of tobramycin, amikacin, and gentamicin alone and with
carbenicillin against Pseudomonas aeruginosa. Antimicrob
Agents Chemother. 6:442–446. 1974.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reynolds AV, Hamilton-Miller JM and
Brumfitt W: Newer aminoglycosides-amikacin and tobramycin: An
in-vitro comparison with kanamycin and gentamicin. Br Med J.
3:778–780. 1974.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Degtyareva NN, Gong C, Story S, Levinson
NS, Oyelere AK, Green KD, Garneau-Tsodikova S and Arya DP:
Antimicrobial activity, AME resistance, and A-site binding studies
of anthraquinone-neomycin conjugates. ACS Infect Dis. 3:206–215.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Thy M, Timsit JF and de Montmollin E:
Aminoglycosides for the treatment of severe infection due to
resistant Gram-negative pathogens. Antibiotics (Basel).
12(860)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
World Health Organization (WHO): Deaths
due to AMR estimated to reach 10 million people by 2050. WHO,
Geneva, 2024. https://www.who.int/indonesia/news/detail/20-08-2024-deaths-due-to-amr-estimated-to-reach-10-million-people-by-2050--ministry-of-health-and-who-launch-national-strategy.
Accessed August 31, 2025.
|
|
8
|
Coppola N, Maraolo AE, Onorato L, Scotto
R, Calò F, Atripaldi L, Borrelli A, Corcione A, De Cristofaro MG,
Durante-Mangoni E, et al: Epidemiology, mechanisms of resistance
and treatment algorithm for infections due to carbapenem-resistant
Gram-negative bacteria: An expert panel opinion. Antibiotics
(Basel). 11(1263)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Alfieri A, Di Franco S, Donatiello V,
Maffei V, Fittipaldi C, Fiore M, Coppolino F, Sansone P, Pace MC
and Passavanti MB: Plazomicin against multidrug-resistant bacteria:
A scoping review. Life (Basel). 12(1949)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rice LB: Federal funding for the study of
antimicrobial resistance in nosocomial pathogens: No ESKAPE. J
Infect Dis. 197:1079–1081. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Ramirez MS and Tolmasky ME: Aminoglycoside
modifying enzymes. Drug Resist Updat. 13:151–171. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Doi Y, Wachino JI and Arakawa Y:
Aminoglycoside resistance: The emergence of acquired 16S ribosomal
RNA methyltransferases. Infect Dis Clin North Am. 30:523–537.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Castanheira M, Deshpande LM, Woosley LN,
Serio AW, Krause KM and Flamm RK: Activity of plazomicin compared
with other aminoglycosides against isolates from European and
adjacent countries, including Enterobacteriaceae molecularly
characterized for aminoglycoside-modifying enzymes and other
resistance mechanisms. J Antimicrob Chemother. 73:3346–3354.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Castanheira M, Davis AP, Serio AW, Krause
KM and Mendes RE: In vitro activity of plazomicin against
Enterobacteriaceae isolates carrying genes encoding
aminoglycoside-modifying enzymes most common in US Census
divisions. Diagn Microbiol Infect Dis. 94:73–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Walkty A, Karlowsky JA, Baxter MR, Adam
HJ, Boyd D, Bharat A, Mulvey MR, Charles M, Bergevin M and Zhanel
GG: Frequency of 16S ribosomal RNA methyltransferase detection
among Escherichia coli and Klebsiella pneumoniae
clinical isolates obtained from patients in Canadian hospitals
(CANWARD, 2013-2017). Diagn Microbiol Infect Dis. 94:199–201.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nikaido H and Takatsuka Y: Mechanisms of
RND multidrug efflux pumps. Biochim Biophys Acta. 1794:769–781.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aggen JB, Armstrong ES, Goldblum AA, Dozzo
P, Linsell MS, Gliedt MJ, Hildebrandt DJ, Feeney LA, Kubo A, Matias
RD, et al: Synthesis and spectrum of the neoglycoside ACHN-490.
Antimicrob Agents Chemother. 54:4636–4642. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eljaaly K, Alharbi A, Alshehri S, Ortwine
JK and Pogue JM: Plazomicin: A novel aminoglycoside for the
treatment of resistant Gram-negative bacterial infections. Drugs.
79:243–269. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Haidar G, Alkroud A, Cheng S, Churilla TM,
Churilla BM, Shields RK, Doi Y, Clancy CJ and Nguyen MH:
Association between the presence of aminoglycoside-modifying
enzymes and in vitro activity of gentamicin, tobramycin, amikacin,
and plazomicin against Klebsiella pneumoniae carbapenemase-
and extended-spectrum-β-lactamase-producing enterobacter species.
Antimicrob Agents Chemother. 60:5208–5214. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Thwaites M, Hall D, Shinabarger D, Serio
AW, Krause KM, Marra A and Pillar C: Evaluation of the bactericidal
activity of plazomicin and comparators against multidrug-resistant
Enterobacteriaceae. Antimicrob Agents Chemother. 62:e00236–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Karaiskos I, Lagou S, Pontikis K, Rapti V
and Poulakou G: The ‘old’ and the ‘new’ antibiotics for MDR
Gram-negative pathogens: For whom, when, and how. Front Public
Health. 7(151)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saravolatz LD and Stein GE: Plazomicin: A
new aminoglycoside. Clin Infect Dis. 70:704–709. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Castanheira M, Davis AP, Mendes RE, Serio
AW, Krause KM and Flamm RK: In vitro activity of plazomicin against
Gram-negative and gram-positive isolates collected from U.S.
Hospitals and comparative activities of aminoglycosides against
carbapenem-resistant Enterobacteriaceae and isolates carrying
carbapenemase genes. Antimicrob Agents Chemother. 62:e00313–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Walkty A, Karlowsky JA, Baxter MR, Adam HJ
and Zhanel GG: In vitro activity of plazomicin against
Gram-negative and gram-positive bacterial pathogens isolated from
patients in Canadian hospitals from 2013 to 2017 as part of the
CANWARD surveillance study. Antimicrob Agents Chemother.
63:e02068–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cox G, Ejim L, Stogios PJ, Koteva K,
Bordeleau E, Evdokimova E, Sieron AO, Savchenko A, Serio AW, Krause
KM and Wright GD: Plazomicin retains antibiotic activity against
most aminoglycoside modifying enzymes. ACS Infect Dis. 4:980–987.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sonousi A, Sarpe VA, Brilkova M, Schacht
J, Vasella A, Böttger EC and Crich D: Effects of the 1-N-(4-Amino-2
S-hydroxybutyryl) and 6'-N-(2-Hydroxyethyl) substituents on
ribosomal selectivity, cochleotoxicity, and antibacterial activity
in the sisomicin class of aminoglycoside antibiotics. ACS Infect
Dis. 4:1114–1120. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Armstrong ES and Miller GH: Combating
evolution with intelligent design: The neoglycoside ACHN-490. Curr
Opin Microbiol. 13:565–573. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Garneau-Tsodikova S and Labby KJ:
Mechanisms of resistance to aminoglycoside antibiotics: Overview
and perspectives. Medchemcomm. 7:11–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lebeaux D, Chauhan A, Létoffé S, Fischer
F, de Reuse H, Beloin C and Ghigo JM: pH-mediated potentiation of
aminoglycosides kills bacterial persisters and eradicates in vivo
biofilms. J Infect Dis. 210:1357–1366. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Parajuli NP, Mandava CS, Pavlov MY and
Sanyal S: Mechanistic insights into translation inhibition by
aminoglycoside antibiotic arbekacin. Nucleic Acids Res.
49:6880–6892. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Clinical and Laboratory Standards
Institute (CLSI): M100Ed33. Performance standards for antimicrobial
susceptibility testing. CLSI, Wayne, PA, 2023.
|
|
32
|
US Food and Drug Administration:
Antibacterial susceptibility test interpretive criteria 2019.
https://www.fda.gov/drugs/development-resources/antibacterial-susceptibility-test-interpretive-criteria.
Accessed June 6, 2025.
|
|
33
|
The United States Committee on
Antimicrobial Susceptibility Testing (USCAST): Breakpoint tables
for interpretation of MIC and zone diameter results. Version 5.3,
July 2019. https://app.box.com/s/cru62wluz4qq64vd5izk41wle9ss172x.
Accessed June 6, 2025.
|
|
34
|
Sader HS, Mendes RE, Kimbrough JH, Kantro
V and Castanheira M: Impact of the recent clinical and laboratory
standards institute breakpoint changes on the antimicrobial
spectrum of aminoglycosides and the activity of plazomicin against
multidrug-resistant and carbapenem-resistant enterobacterales from
United States medical centers. Open Forum Infect Dis.
10(ofad058)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Clinical and Laboratory Standards
Institute (CLSI): M100Ed32. Performance standards for antimicrobial
susceptibility testing. CLSI, Wayne, PA, 2022.
|
|
36
|
Earle W, Bonegio RGB, Smith DB and
Branch-Elliman W: Plazomicin for the treatment of
multidrug-resistant Klebsiella bacteraemia in a patient with
underlying chronic kidney disease and acute renal failure requiring
renal replacement therapy. BMJ Case Rep. 14(e243609)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ramirez MS and Tolmasky ME: Amikacin:
Uses, resistance, and prospects for inhibition. Molecules.
22(2267)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Clark JA and Burgess DS: Plazomicin: A new
aminoglycoside in the fight against antimicrobial resistance. Ther
Adv Infect Dis. 7(2049936120952604)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hikal AF, Zhao S, Foley S and Khan AA: The
newly identified grdA gene confers high-level plazomicin resistance
in Salmonella enterica serovars. Antimicrob Agents
Chemother. 69(e0160624)2025.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cass R, Kostrub CF, Gotfried M, Rodvold K,
Tack KJ and Bruss J: A double-blind, randomized, placebo-controlled
study to assess the safety, tolerability, plasma pharmacokinetics
and lung penetration of intravenous plazomicin in healthy subjects.
In: Proceedings of the 23rd European Congress of Clinical
Microbiology and Infectious Diseases (ECCMID). ECCMID, Berlin,
poster 1637, 2013.
|
|
41
|
U.S. Food and Drug Administration and
Center for Drug Evaluation and Research: Zemdri Approval Letter
Reference ID: 4282864. U.S. Food and Drug Administration, Silver
Spring, MD, USA, 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210303orig1s000lbl.pdf.
Accessed June 6, 2025.
|
|
42
|
Choi T, Seroogy JD, Sanghvi M and Dhuria
SV: 1400 Mass balance, metabolism, and excretion of
(14C)-plazomicin in healthy human subjects. Open Forum Infect Dis.
5 (Suppl 1)(S431)2018.
|
|
43
|
Zemdri (plazomicin) (package insert).
South Achaogen, Inc., San Francisco, CA, 2021. https://zemdri.com/assets/pdf/Prescribing-Information.pdf.
Accessed June 6, 2025).
|
|
44
|
Connolly LE, Riddle V, Cebrik D, Armstrong
ES and Miller LG: A multicenter, randomized, double-blind, phase 2
study of the efficacy and safety of plazomicin compared with
levofloxacin in the treatment of complicated urinary tract
infection and acute pyelonephritis. Antimicrob Agents Chemother.
62:e01989–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wagenlehner FME, Cloutier DJ, Komirenko
AS, Cebrik DS, Krause KM, Keepers TR, Connolly LE, Miller LG,
Friedland I and Dwyer JP: EPIC Study Group. Once-daily plazomicin
for complicated urinary tract infections. N Engl J Med.
380:729–740. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
McKinnell JA, Dwyer JP, Talbot GH,
Connolly LE, Friedland I, Smith A, Jubb AM, Serio AW, Krause KM and
Daikos GL: CARE Study Group. Plazomicin for infections caused by
carbapenem-resistant Enterobacteriaceae. N Engl J Med. 380:791–793.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jospe-Kaufman M, Siomin L and Fridman M:
The relationship between the structure and toxicity of
aminoglycoside antibiotics. Bioorg Med Chem Lett.
30(127218)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Drugs and Lactation Database
(LactMed®) (Internet). National Institute of Child
Health and Human Development, Bethesda, MD, 2006. https://www.ncbi.nlm.nih.gov/books/NBK513053/.
Accessed June 6, 2025.
|
|
49
|
Başaran SN and Öksüz L: Newly developed
antibiotics against multidrug-resistant and carbapenem-resistant
Gram-negative bacteria: Action and resistance mechanisms. Arch
Microbiol. 207(110)2025.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Edson RS and Terrell CL: The
aminoglycosides. Mayo Clin Proc. 74:519–528. 1999.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Paul M, Carrara E, Retamar P, Tängdén T,
Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S,
et al: European society of clinical microbiology and infectious
diseases (ESCMID) guidelines for the treatment of infections caused
by multidrug-resistant Gram-negative bacilli (endorsed by European
society of intensive care medicine). Clin Microbiol Infect.
28:521–547. 2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gilchrist M and Seaton RA: Outpatient
parenteral antimicrobial therapy and antimicrobial stewardship:
Challenges and checklists. J Antimicrob Chemother. 70:965–970.
2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Choi T, Komirenko AS, Riddle V, Kim A and
Dhuria SV: No effect of plazomicin on the pharmacokinetics of
metformin in healthy subjects. Clin Pharmacol Drug Dev. 8:818–826.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gall J, Choi T, Riddle V, Van Wart S,
Gibbons JA and Seroogy J: A phase 1 study of intravenous plazomicin
in healthy adults to assess potential effects on the QT/QTc
interval, safety, and pharmacokinetics. Clin Pharmacol Drug Dev.
8:1032–1041. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
No authors listed. Plazomicin (Zemdri)-a
new aminoglycoside antibiotic. Med Lett Drugs Ther. 60:180–182.
2018.PubMed/NCBI
|
|
56
|
Althobaiti H, Seoane-Vazquez E, Brown LM,
Fleming ML and Rodriguez-Monguio R: Disentangling the cost of
orphan drugs marketed in the United States. Healthcare (Basel).
11(558)2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Clancy CJ, Potoski BA, Buehrle D and
Nguyen MH: Estimating the treatment of carbapenem-resistant
Enterobacteriaceae infections in the United States using antibiotic
prescription data. Open Forum Infect Dis. 6(ofz344)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hawkey PM, Warren RE, Livermore DM,
McNulty CAM, Enoch DA, Otter JA and Wilson APR: Treatment of
infections caused by multidrug-resistant Gram-negative bacteria:
report of the British society for antimicrobial
chemotherapy/healthcare infection society/British infection
association joint working party. J Antimicrob Chemother. 73 (Suppl
3):iii2–iii78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Theuretzbacher U and Paul M: Developing a
new antibiotic for extensively drug-resistant pathogens: The case
of plazomicin. Clin Microbiol Infect. 24:1231–1233. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
United States Securities and Exchange
Commission (SEC 2019). Achaogen 10K fiscal year 2018. https://www.sec.gov/Archives/edgar/data/1301501/000156459019010412/akao-10k_20181231.htm.
Accessed September 05, 2025.
|
|
61
|
Wagenlehner F, Caballero VR, Maheshwari V,
Biswas A, Saini P, Quevedo J, Polifka J, Ruiz L and Cure S:
Efficacy of treatment options for complicated urinary tract
infections including acute pyelonephritis: A systematic literature
review and network meta-analysis. J Comp Eff Res.
14(e240214)2025.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Leibovici L, Vidal L and Paul M:
Aminoglycoside drugs in clinical practice: An evidence-based
approach. J Antimicrob Chemother. 63:246–251. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Abd El-Aziz Gadallah M, El-Sayed WM,
Hussien MZ, Elheniedy MA and Maxwell SY: In-vitro activity of
plazomicin, meropenem-vaborbactam, and omadacycline against
carbapenem-resistant Gram-negative isolates in Egypt. J Chemother.
13:205–218. 2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Thabit AK, Fatani DF, Bamakhrama MS,
Barnawi OA, Basudan LO and Alhejaili SF: Antibiotic penetration
into bone and joints: An updated review. Int J Infect Dis.
81:128–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Castanheira M, Deshpande LM, Hubler CM,
Mendes RE, Serio AW, Krause KM and Flamm RK: Activity of plazomicin
against Enterobacteriaceae isolates collected in the United States
including isolates carrying aminoglycoside-modifying enzymes
detected by whole genome sequencing. Open Forum Infect Dis. 4
(Suppl 1)(S377)2017.
|
|
66
|
Mougakou E, Mastrogianni E, Kyziroglou M
and Tziomalos K: The role of novel antibiotics in the management of
diabetic foot infection. Diabetes Ther. 14:251–263. 2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ray S, Flemming LK, Scudder CJ, Ly MA,
Porterfield HS, Smith RD, Clark AE, Johnson JK and Das S:
Comparative phenotypic and genotypic antimicrobial susceptibility
surveillance in Achromobacter spp. through whole genome
sequencing. Microbiol Spectr. 13(e0252724)2025.PubMed/NCBI View Article : Google Scholar
|